Summary
The Rad1-Rad9-Hus1 (9-1-1) complex serves a dual role as a DNA-damage sensor in checkpoint signaling and as a mediator in DNA repair pathway. However, the intercellular mechanisms that regulate 9-1-1 complex are poorly understood. Jab1, the fifth component of the COP9 signalosome complex, plays a central role in the degradation of multiple proteins and is emerging as an important regulator in cancer development. Here, we tested the hypothesis that Jab1 controls the protein stability of the 9-1-1 complex via the proteosome pathway. We provide evidence that Jab1 physically associates with the 9-1-1 complex. This association is mediated through direct interaction between Jab1 and Rad1, one of the subunits of 9-1-1 complex. Importantly, Jab1 causes the translocation of the 9-1-1 complex from the nucleus to the cytoplasm, mediating rapid degradation of the 9-1-1 complex via 26S proteasome. Furthermore, Jab1 significantly suppresses checkpoint signaling activation, DNA synthesis recovery from blockage and cell viability after replication stresses such as UV exposure, γ radiation and hydroxyurea treatment. These results suggest that Jab1 is an important regulator for 9-1-1 protein stability control in cells, which may provide novel information on the involvement of Jab1 in checkpoint and DNA repair signaling in response to DNA damage.
Keywords: Jab1, protein degradation, 9-1-1 complex, checkpoint, DNA damage
Introduction
Maintenance of genomic stability relies on the accurate duplication of the genome and continuous monitoring of its integrity. To ensure the stability of their genomes, cells activate complex signaling networks, known as checkpoint signaling pathways, in response to DNA damage and replication stress.1, 2 The first step in the initiation of the checkpoint pathway is recognition of the DNA damage. The Rad9, Hus1, and Rad1 (using Schizosaccharomyces pombe nomenclature) orthologs, which form the Rad9–Hus1–Rad1 (9–1–1) complex, are critical in the initiation of cellular responses to DNA damage.
The 9-1-1 complex is the checkpoint counterpart of PCNA, a homotrimer with a ring-like structure. PCNA subunits assemble into a toroidal clamp complex that is loaded around DNA where PCNA tethers DNA-metabolizing enzymes to the site of ongoing DNA replication.3, 4 Although the Rad9, Rad1, and Hus1 proteins have little sequence homology to PCNA, or to one another, molecular modeling suggested that they form a PCNA-like structure and are loaded on DNA at sites of damage as a clamp complex. Like PCNA, the 9-1-1 complex interacts with a potential clamp loader, the Rad17-RFC complex, which is composed of the checkpoint protein Rad17 and the four small RFC subunits.5, 6 In response to genotoxic damage, the 9-1-1 complex is loaded around DNA by the Rad17-containing clamp loader. The DNA-bound 9-1-1 complex then facilitates ATR-mediated phosphorylation and activation of Chk1, a protein kinase that regulates S-phase progression, G2/M arrest, and replication fork stabilization.7, 8 Therefore, 9-1-1 serves as DNA damage sensors for transducing the damage signal to downstream signal transduction cascades.
Moreover, 9-1-1 plays a direct role as a mediator and platform for DNA repair. 9-1-1 stimulates enzymes involved in nearly every step of the one patch base excision repair (LP-BER) pathway. There is a direct interaction of 9-1-1 with the base excision repair pol, pol β, and the 9-1-1 had a specific stimulatory effect on pol β activity.9 Similar physical and functional interactions with 9-1-1 complex were identified for human Fen1,10 MutY homolog of Schizosaccharomyces pomb11 and DNA ligase I12, which are major players in DNA repair pathway. Indeed, inactivation or downregulation of Rad1, Hus1 or Rad9 contributes to increased frequency of spontaneous chromosomal aberrations, morphological transformation and cancer.13–16 However, the intercellular mechanisms that regulate 9-1-1 complex level in mammalian cells are poorly understood. hHus1 is an unstable protein, whereas Rad1 protects hHus1 from ubiquitination and degradation in cytoplasm,17 indicating that ubiquitin-proteasome pathway actively regulates 9-1-1 complex level in mammalian cells.
Jun-activation domain binding protein 1 (Jab1) was originally described as a transcriptional co-activator of AP1 proteins (especially c-Jun and Jun D).18 Jab1 is also termed CSN5 as it is the fifth component of the COP9 signalosome complex (CSN),19 which was initially discovered in Arabidopsis over a decade ago and has been shown to comprise eight core subunits in mammals. These subunits bear remarkable homology to the 19S lid of the 26S proteasome and the translation initiation complex eIF3 and are currently postulated to play a largely undetermined role in protein degradation. Most importantly, the Jab1/CSN5 has been found to play a critical role in the degradation of multiple proteins that are known regulators of disease progression in diverse cancers, including p27Kip1,20 p5321 and Smad4.22 Recent studies also determined that Jab1 functions as a nuclear exporter and inducer of cytoplasmic degradation for several proteins including p53, p27, capsid of West Nile virus, and Smad4/7 proteins. These data suggest that Jab1/CSN5 may be an important regulator in cancer development. Recently, knockout or mutational studies in a variety of organisms indicate that the Jab1/CSN5 is involved in cell cycle progression, radiation sensitivity, genome stability, and cell survival23–25. However, detailed molecular mechanisms by which Jab1 participates in DNA damage checkpoints pathway in cells are unclear.
In this report, we present evidence that that Jab1 interacts with 9-1-1 complex, induces the complex nuclear export and regulates their protein stability via the 26S proteosome pathway in cells. We also found that Jab1 suppresses checkpoint signaling activation and DNA synthesis recovery from blockage after replication stresses. These observations suggest that Jab1 regulates checkpoint and replication signaling via controlling 9-1-1 complex stability.
Results
Jab-1 interacts with the 9-1-1 complex
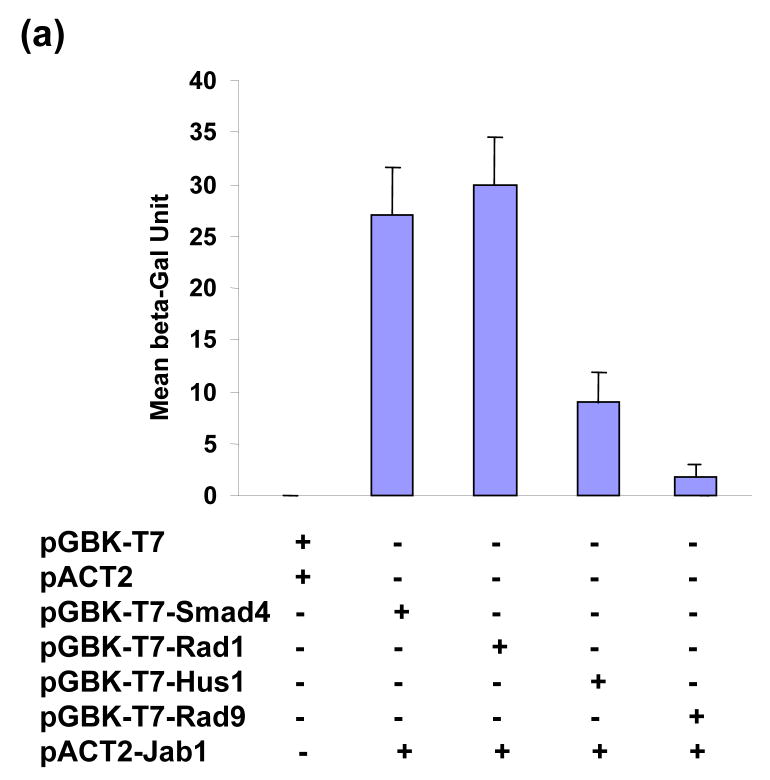
Previous studies indicate that the Jab1/CSN5 is involved in cell cycle progression, checkpoint activation, genome stability, and cell survival.23–25 To characterize the mechanisms underline these phenomenons, we performed a yeast two-hybrid screening and attempted to find Jab1-interacting proteins that are actively involved in checkpoint pathway. The entire Jab1 protein was fused in frame to the GAL4 DNA binding domain as a bait. Using this bait, a pretransformed human breast cancer cDNA library was screened as described in Materials and Methods. DNA sequence analysis identified 28 positive clones, one of which encoded human Hus1 which is one of the subunits of DNA damage sensor complex 9-1-1. To further quantify the interaction of Jab1 with Hus1 and examine whether Jab1 interacts with the other two components of 9-1-1 complex, a liquid β-gal assay was performed (Figure 1(a)). Jab1 strongly interacts with Rad1, and the interaction affinity is as high as the positive control, which shows the interaction of Jab1 with Smad4.22 Jab1 has a relatively weaker interaction with Hus1 and a very minimum interaction with Rad9. To assess the association of Jab1/CSN5 with 9-1-1 complex in mammalian cells, we performed co-immunoprecipitation experiments using total cell lysates prepared from human PANC-1 cells, pancreatic cancer cells. Western blotting analysis of the immunoprecipitates with anti-Jab1/CSN5 antibody revealed the presence of Jab1/CSN5 in the anti-Rad1, Hus1 and Rad9 immunoprecipitates (Figure 1(b), lane 2s). In the reciprocal experiments, Jab1 antibody was able to co-immunoprecipitate Rad1, Hus1 and Rad9 (Figure 1(c), lane 2s). As controls, preimmune antibodies did not immunoprecipitate Jab1/CSN5, Rad1, Hus1 and Rad9 (lane 1s of Figure 1(b) and 1(c)). The results indicate that Jab1 is associated with 9-1-1 complex in PANC-1 cells. To further examine whether the interaction is direct or which subunit(s) mediate(s) the interaction, in vitro pull-down assays were performed. After co-immunoprecipitation reaction with anti-Rad1, Hus1 or Rad9 antibodies, the immunoprecipitates were incubated with increasing salt concentration of buffer to remove endogenous binding proteins. As shown in Figure 1(d), incubation with 0.9M of NaCl eliminated almost all the co-immunoprecipitated other subunits of 9-1-1 complex and Jab1/CSN5. Then the high salt concentration buffer washed Rad1, Hus1 or Rad9 immunoprecipitates individually incubated with purified GST-Jab1 protein. The direct binding of GST-Jab1 with Rad1, but not Hus1 or Rad9, was observed (Figure 1(e)). No significant association was observed between Rad1 with GST. Taken together, the results suggest that Jab1/CSN5 interacts with 9-1-1 complex in human cells, and the interaction may be mediated by Rad1.
Figure 1.
Jab1 interacts with 9-1-1 complex. (a) Jab1 interacts with Rad1 and Hus1 in yeast. Intact Jab1 cDNA were fused with the Gal4 DNA-binding domain and transformed in yeast with indicated cDNAs in prey plasmids. The interactions were quantified by a liquid β-gal assay. (b) and (c) Jab1 interacts with Rad1 and Hus1 in mammalian cells. PANC-1 cells were incubated overnight. Pre-immune antibody (lane 1) or antibody specifically against Rad1, Hus1 or Rad9 (lane 2) were used to immunoprecipitate the endogenous proteins from the total cell lysates respectively, and the immunocomplex was detected by western blotting using anti-Jab1 antibody (b). The expression levels of Jab1, Rad1, Hus1 and Rad9 in cells were also detected, as indicated in the lower panels. Reversely, pre-immune antibody (lane 1) or antibody specifically against Jab1 (lane 2) were used to immunoprecipitate the endogenous proteins from the total cell lysates, and the immunocomplex was detected by western blotting using anti-Rad1, Hus1 or Rad9 antibody respectively (c). The expression levels of Jab1, Rad1, Hus1 and Rad9 in cells were also detected, as indicated in the lower panels. (d) Total cell lysates were immunoprecipitated with anti-Rad1 antibody. The immunoprecipitates were then washed with increasing salt concentrations of buffer. After washing, the remaining bound proteins were detected by anti-Hus1 and anti-Jab1 antibody. (e) Jab1 interacts with Rad1 and Hus1 in vitro. PANC-1 cells were incubated overnight. Antibodies specifically against Hus1, Rad1 or Rad9 were used to immunoprecipitate the endogenous proteins, and the immunocomplex was washed with the buffer containing 0.9M NaCl, and incubated with either GST (1st panel) or GST-Jab1 (2nd panel). The immunoprecipitates were then detected by western blotting using antibody specifically against GST. The expression levels of Rad1, Hus1 and Rad9 in cells were also detected, as indicated in the lower panels.
Jab1 degrades the 9-1-1 complex via the proteosome pathway
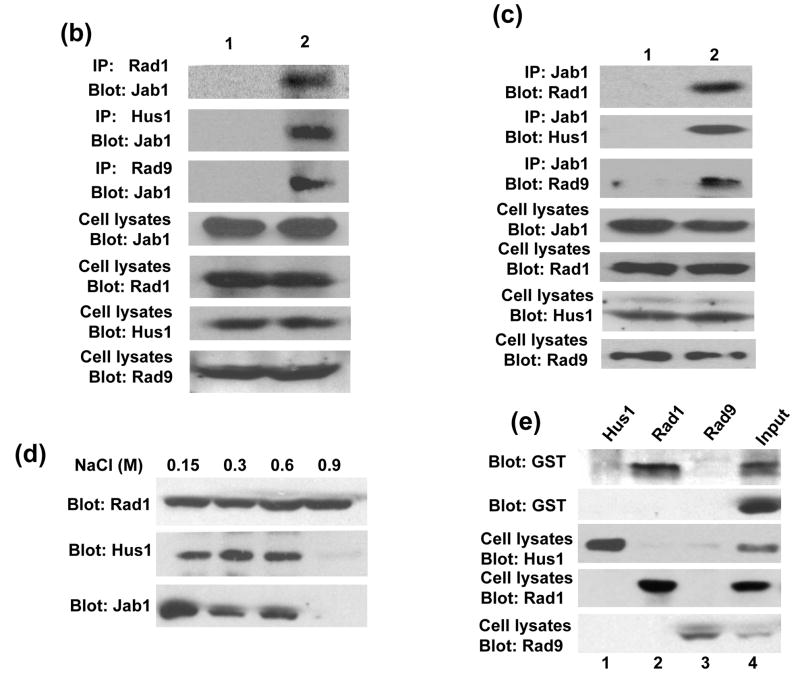
Jab1/CSN5 has been found to induce the degradation of multiple proteins that are known regulators of disease progression in diverse cancers.20–22 Thus, it is possible that Jab1/CSN5 also induces 9-1-1 complex degradation through direct interaction with the complex. We then examined whether the interaction affects Rad1, Hus1 and Rad9 protein levels in cells. A Jab1/CSN5 expression plasmid was co-transfected with Myc-tagged Rad1, Hus1 and Rad9 in 293T cells, and the protein levels of 9-1-1 were measured by western blotting with an antibody against Rad1, Hus1 or Rad9. Figure 2(a) demonstrates that the expression of Jab1 reduced the protein levels of all the three components in 9-1-1 (lane 2) compared with empty vector transfection control (lane 1). Endogenous β-actin protein level remained unchanged as a loading control. We then examined whether Jab1/CSN5 overexpression also affects the endogenous 9-1-1 protein level in three other cell lines. As expected, Jab1/CSN5 overexpression by retrovirus delivery significantly reduced endogenous Rad1, Hus1 and Rad9 protein level in all three cell lines, PANC-1, Miapaca-2 and HeLa (Figure 2(b)). These results suggest that Jab1/CSN5 indeed downregulates 9-1-1 protein complex.
Figure 2. Jab1 destabilizes the 9-1-1 complex.
(a) Jab1 downregulates overexpressed 9-1-1 in 293T cells. Myc-tagged Rad1, Hus1 and Rad9 were cotransfected with either empty vector (lane 1) or HA-tagged Jab1 in 293T cells. Forty hours after transfection, cell lysates were prepared and subjected to SDS-PAGE/immunoblot analysis with anti-Myc, anti-HA or anti-β-actin antibodies. (b) Jab1 downregulates endogenous 9-1-1 in PANC-1, MiaPaCa-2 and HeLa cells. Cells were infected with retrovirus containing pMSCVneo-GFP or pMSCVneo-HA-Jab1. Cell extracts were analyzed by immunoblotting using antibodies against Rad1, Hus1, Rad9, HA and β-actin. (c) siGFP efficiently eliminated GFP expression in PANC-1 cells. PANC-1 were infected by retrovirus containing pMSCVneo/U6 (empty vector, i) or pMSCVneo/U6/GFP (siGFP, ii), and GFP expression plasmid was transfected in these cells. Green light representing GFP expression. (d) siJab1 elevated 9-1-1 protein levels. PANC-1 cells were infected with virus containing siGFP or three siJab1s individually. Cells were harvested and expression levels of Jab1, Rad1, Hus1, Rad9 and β-actin were measured by Western blot analysis.
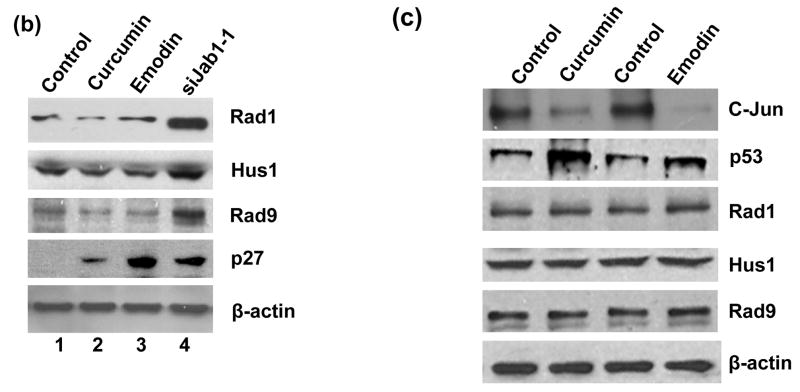
To confirm that Jab1/CSN5 is essential for controlling 9-1-1 protein stability in cells, we employed a RNAi approach to silence Jab1/CSN5 gene in PANC-1 cells. Because the efficiency of transient transfection of siRNA into pancreatic cancer cells is very low, we generated three retrovirus constructs that contain hairpin loop-based siRNA vector that effectively targets the Jab1/CSN5 transcript at three different coding regions (siJab1-1, siJab1-2 and siJab1-3). siGFP, a retrovirus irrelevant siRNA control, was also generated. High infection efficiencies of the siRNA in PANC-1 cells were yielded as indicated by the diminished green light by siGFP (Figure 2(c)). When the three Jab1/CSN5 siRNAs were introduced into PANC-1 cells individually, Jab1/CSN5 level was markedly decreased, and the protein level of Rad1, Hus1 and Rad9 were all elevated accordingly (Figure 2(d)).
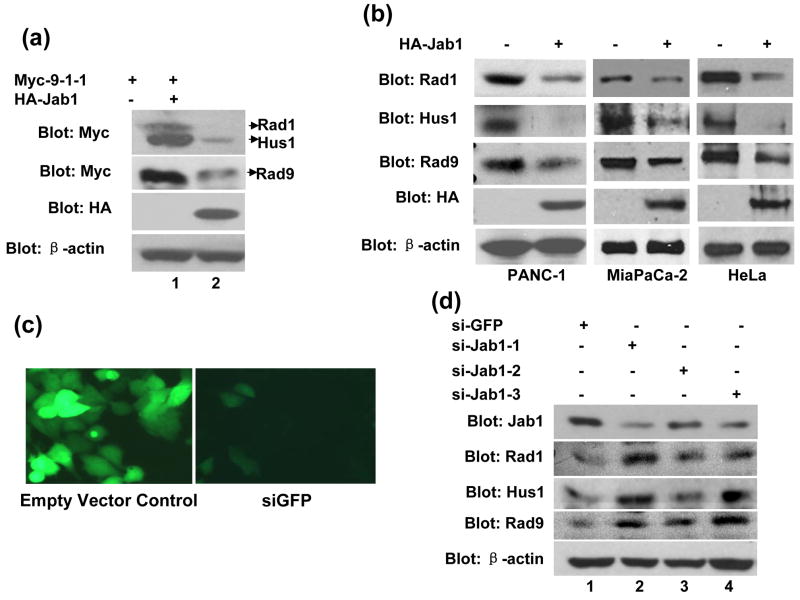
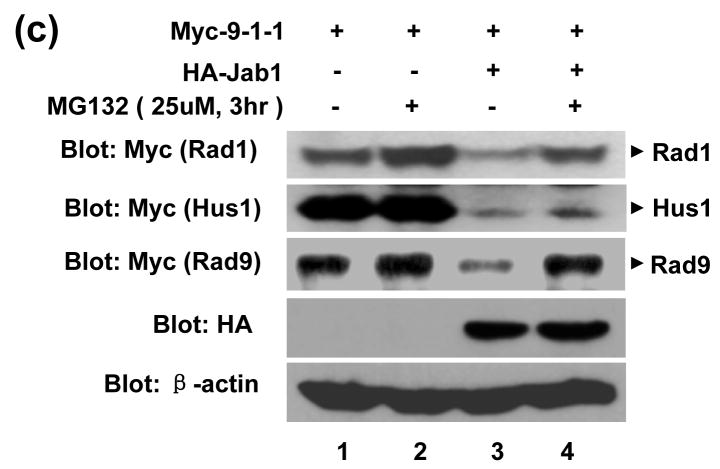
To investigate whether Jab1/CSN5-induced 9-1-1 protein downregulation is due to protein degradation, the turnover of 9-1-1 protein was also examined using the cycloheximide (CHX) chase assays. As shown in Figure 3(a), Rad1 and Hus1 are very stable with the protein level almost unchanged at 8hrs after CHX treatment. The degradation of Rad9 was visible at 2, 4, and 8hrs after CHX treatment. Jab1/CSN5 overexpression significantly accelerated the protein degradation of Rad1 and Hus1, but has weaker effect on Rad9 (Figure 3(a) and 3(b)). One possibility of Jab1 overexpression has marginal effect on Rad9 is that Rad9 may degrades very fast with endogenous Jab1 in 293T cells, and the further degradation of Rad9 by overexpressed Jab1 may not that dramatic to be shown. To evaluate if 26S proteasome pathway is responsible for the decreased 9-1-1 steady-state levels, the proteasome inhibitor MG132 were added to the cells transfected with Rad1/Hus1/Rad9 and/or Jab1 expression plasmids. Jab1-induced down-regulation of Rad1 and Rad9 was significantly inhibited by MG-132 (Figure 3(c), 1st and 3rd panel). Obviously, the degradation of these two proteins is mediated by the 26S proteasome. The inhibitory effect of MG-132 on Jab1-induce Hus1 degradation seems much weaker (Figure 3(c), 2nd panel), indicating that other degradation mechanisms, besides proteasome pathway, may also be involved.
Figure 3. The degradation of 9-1-1 complex by Jab1 is through proteasome pathway.
(a) Jab1 decreased 9-1-1 protein degradation rate. Myc-tagged Rad1, Hus1 and Rad9 were co-transfected with empty vector or HA-Jab1 expression vector in 293T cells. After 40 hours, cycloheximide (80 μg/mL) was added to the culture, and the whole cell extracts were prepared at 0, 1, 2, 4, and 8 hours and assayed by Western blotting with an anti-Myc antibody to detect 9-1-1 expression levels. (b) The intensity of the bands in (a) was quantitated by phosphorimaging and plotted relative to the amount present at time 0. (c) Proteasome inhibitor increased Jab1-downregulated 9-1-1 protein levels. Myc-tagged Rad1, Hus1 and Rad9 were co-transfected with empty vector or HA-Jab1 expression vector in 293T cells. Cells were incubated with or without the proteasome inhibitor, MG132 (25 μmol/L), for 3 hours. Extracts were assayed by Western blotting with Myc antibody to detect Rad1, Hus1 and Rad9.
Jab1 induces nuclear export of the 9-1-1 complex
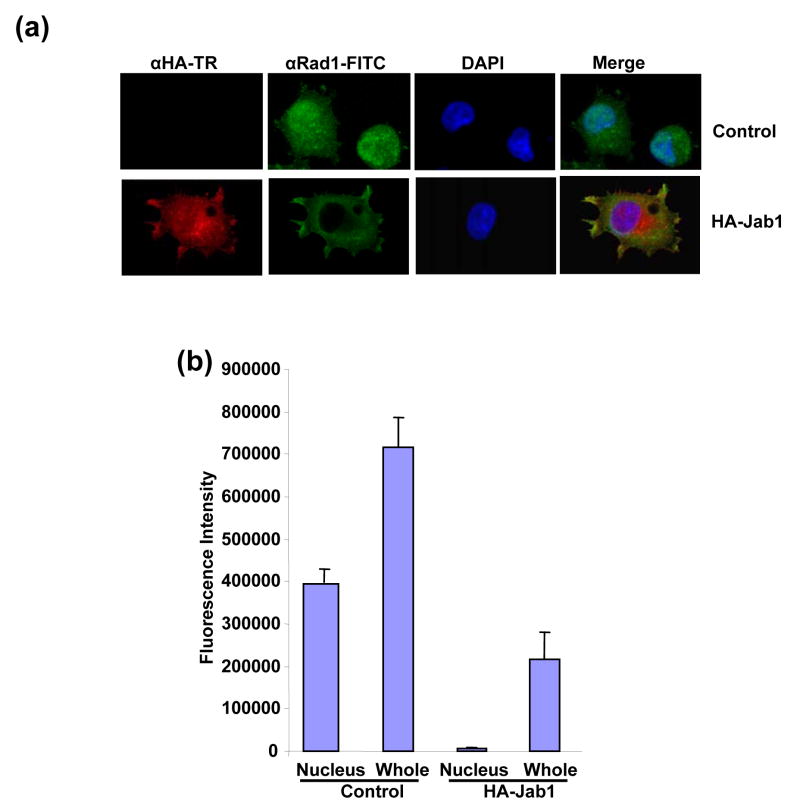
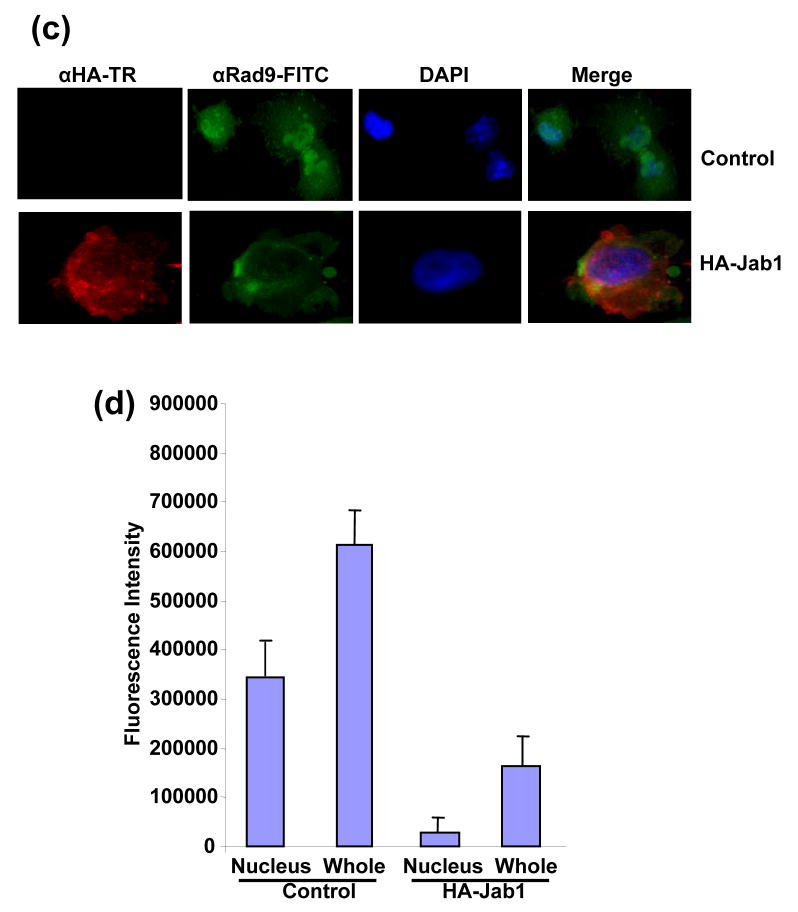
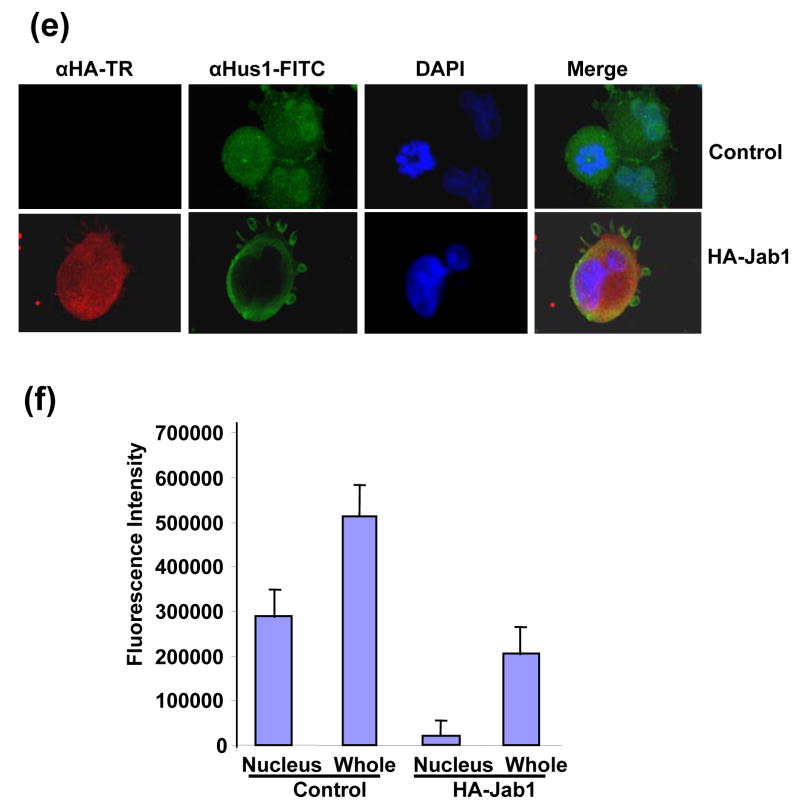
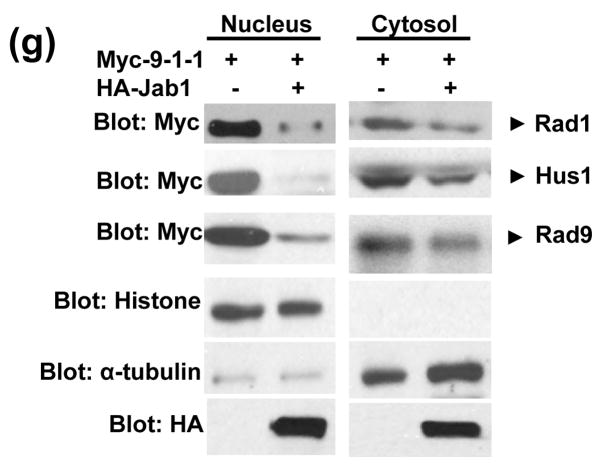
Since Jab1/CSN5 contains the nuclear export signal and is involved in the translocation of protein substrates from the nucleus to the cytoplasm and further induces their protein degradation,20, 26 we then tested whether 9-1-1 complex can be exported by Jab1/CSN5 overexpression in PANC-1 cells. Rad1, Rad9 and Hus1 reside both in the cytosol and nucleus with relatively stronger fluorescence intensity in nucleus (Figure 4(a), 4(c) or 4(e) upper panels showing in green and 4(b), 4(d) and 4(f)). Co-expression of HA-Jab1 markedly decreased the Rad1-, Rad9- and Hus1-specific immunofluorescent signals. A twice increased exposure of the cell sample revealed ~95%, ~85% and ~91% of the Rad1, Rad9 and Hus1 signals in the cytoplasm respecitively (Figure 4(a), 4(c) and 4(f) bottom panels showing in green and 4(b), 4(d) and 4(f)). Moreover, Jab1/CSN5 colocalized with Rad1, Rad9 and Hus1 (Figure 4(a) 4(c) and 4(e) bottom panel). In order to confirm the results in another cell system, cell fractionation assays were performed using 293T cells. Thee reduction of 9-1-1 protein level induced by Jab1 overexpression was dramatic in nucleus, but much milder in the cytosol (Figure 4(e)). The results indicate that Jab1/CSN5 interacts with 9-1-1 and translocates the complex from the nucleus to the cytoplasm. Similar as the degradation of p27kip1, Jab1/CSN5 may accelerate 9-1-1 degradation by bringing it to the degradation machinery in the cytoplasm.
Figure 4. Subcellular localization of 9-1-1 in the presence and absence of Jab1.
(a, c and e) Jab1 translocates 9-1-1 proteins from nucleus to cytoplasm in PANC-1 cells analyzed by fluorescence colocalization. PANC-1 cells were infected with retrovirus containing empty vector (pSMCVneo, Control) or pSMCVneo-HA-Jab1. After 48h, cells were assayed for endogenous Rad1 (a), Rad9 (c) or Hus1 (e) and ectopically expressed Jab1 (HA-Jab1) using monoclonal anti-Rad1 (a), -Rad9 (c) or -Hus1 (e) and polyclonal HA antibodies followed by FITC (Green) and Texas Red (TR, Red) labeled secondary antibodies, respectively. Nuclei were conterstained with DAPI (blue). The images were overlapped (Merge) to determine colocolization. (b, d and f) The fluorescence intensity of both the nuclear region and the total region of the cells were quantitated. For each treatment, 100 cells in three different slides were analyzed. The ratio of nuclei fluorescence intensity: whole cell fluorescence intensity was calculated and expressed as percentage ± SDE. (g) Jab1 translocates 9-1-1 proteins from nucleus to cytoplasm in 293T cells analyzed by cell fractionation. Myc-tagged Rad1, Hus1 and Rad9 were cotransfected with either empty vector (lane 1 and 3) or HA-tagged Jab1 (lane 2 and 4) in 293T cells. After 48h, cytoplasmic and nuclear fractions were prepared and separated on SDS-PAGE gels, and then probed with anti-Myc antibodies, respectively. Histone and α-tubulin level were detected as a nucleus marker and a cytosol marker. HA-Jab1 expression level was also detected with anti-HA antibody.
Inhibitors of CSN were not able to elevate 9-1-1 protein level
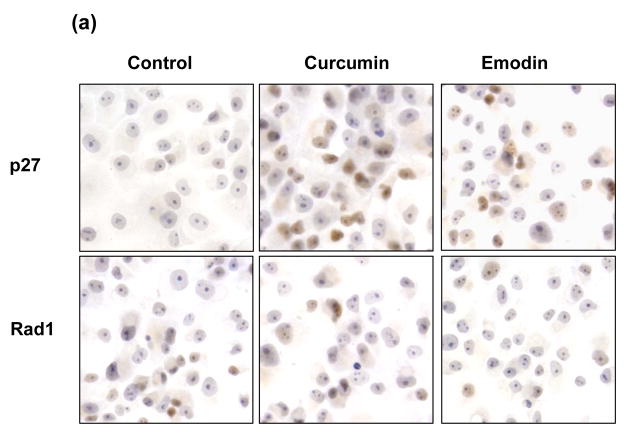
Jab1/CSN5 is one of the subunit of CSN complex. To distinguish whether Jab1 exert its effects on degrading 9-1-1 complex in the large CSN complex or in its free form/small Jab1 complex, curcumin and emodin, two inhibitors of CSN,21, 27 were used. Rad1, Hus1 and Rad9 levels were not affected by these two inhibitors (Figure 5(a) and 5(b)). As a control, knockdown Jab1 using siRNA elevated 9-1-1 protein level. It is known that p53 and c-Jun are the direct substrates of CSN-associated kinases, and the phosphorylation of these two proteins leads to degradation of p53 but stabilization of c-Jun towards the Ub system.21, 27 Therefore, to verify whether curcumin and emodin we used really inhibit CSN activity, the stabilities of p53 and c-Jun after curcumin and emodin treatment were detected in HeLa cells. Consistent with previous report28, both curcumin and emodin elevated p53 a protein level, but significantly downregulated c-Jun level (Figure 5(c)). Collectively, these results indicate that the large CSN complex may not involved in Jab1-mediated 9-1-1 protein degradation, and Jab1 may exert its effects in its free form or in small Jab1 complex.
Figure 5. Rad1 protein level was not elevated by the inhibitors of CSN.
(a) Curcumin and emodin did not affect 9-1-1 levels in PANC-1 cells analyzed by immunohistochemistry. PANC-1 cells were treated with vehicle control, 10μM curcumin or 20μM emodin. The expression of p27 and Rad1 were detected by immunohistochemistrical analysis. The brown color represents positive signal. (b) Curcumin and emodin did not affect 9-1-1 levels in PANC-1 cells analyzed by Western blot. PANC-1 cells were infected with retroviruses containing siGFP (Control, lane 1, 3, 5 and 7) or siJab1-1 (lane 4). Cells were then treated with vehicle control (lane 1 and 4), 10μM curcumin (lane 2) or 20μM emodin (lane 3). The expression level of Rad1, Hus1, Rad9, p27 and β-actin were detected by Western blotting using specific antibodies. (c) Curcumin and emodin changed the stability of c-Jun and p53, but did not affect 9-1-1 levels in HeLa cells. Cells were treated with vehicle control (lane 1 and 3), 10μM curcumin (lane 2) or 20μM emodin (lane 4). The expression level of c-Jun, p53, Rad1, Hus1, Rad9 and β-actin were detected by Western blotting using specific antibodies.
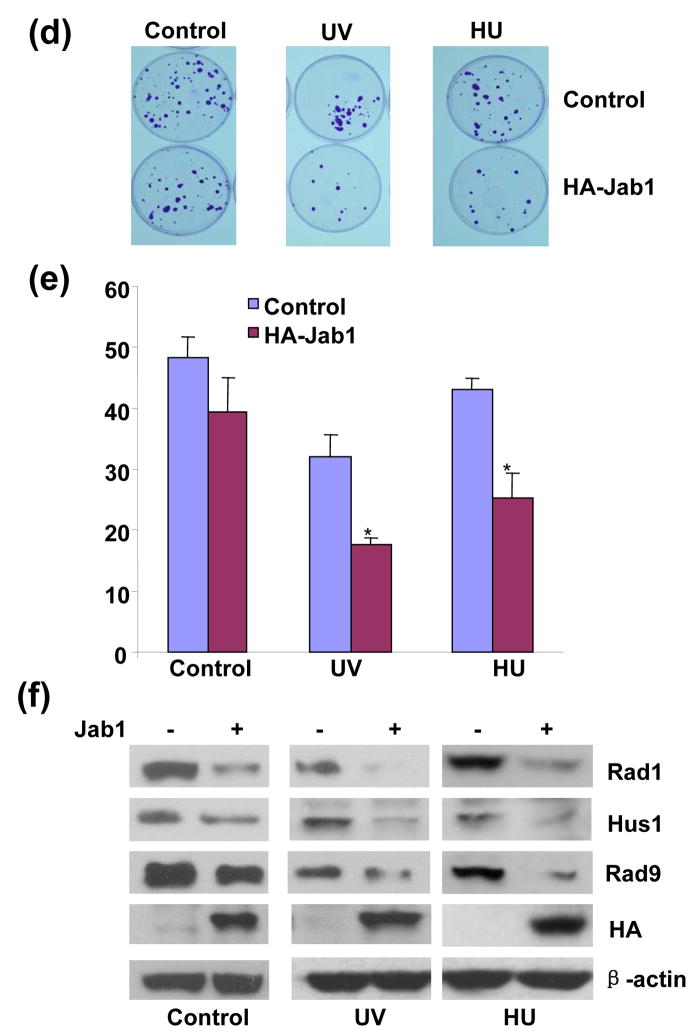
Jab1-induced 9-1-1 degradation suppresses checkpoint signaling activation and disrupts DNA synthesis recovery from blockage
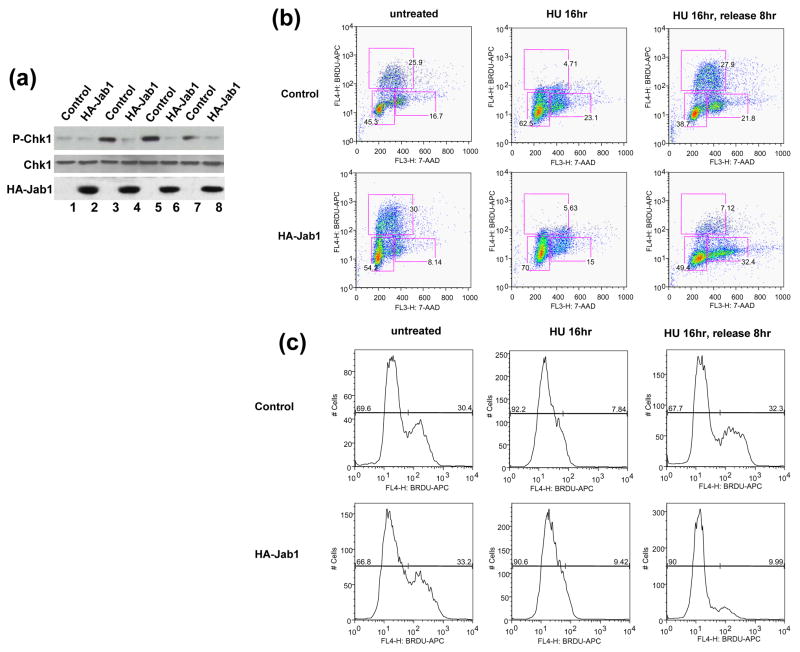
Components of 9-1-1 are essential for the activation of the ATR-dependent downstream targets. Specifically, phosphorylation of Chk1 has been shown to serve as indicators of 9-1-1-mediated checkpoint activation in response to genotoxic stress.14, 15 We therefore examined whether Jab1-induced 9-1-1 protein degradation affects ATR-dependent Chk1 phosphorylation. Consistent with previous reports by others,14, 15 treatment of cells with ultraviolet (UV), γ-irradiation (IR) and replication inhibitor hydroxyurea (HU) induced Chk1 phosphorylation on Ser 345. Jab1 overexpression significantly suppressed Chk1 phosphorylation at Ser 345 induced by these replication stresses (Figure 6(a)). 9-1-1 complex is also critical in S-phase checkpoint activation. Loss of one of the components contributes to retarded recovery of DNA synthesis from replication blockage mediated by replication blockers.14 To test whether Jab1-induced 9-1-1 degradation also affects DNA synthesis recovery from replication blockage, BrdU pulse labeling was conducted at different time points to monitor DNA synthesis. S-phase cells were equally distributed in control and Jab1 overexpressed cells without HU treatment (Figure 6b, untreated). DNA synthesis was dramatically inhibited after 16hr HU treatment in both control and Jab1 overexpressed cells (Figure 6(b) and 6(c), HU 16hr). At 8hr after HU release, approximately 26% of control cells progressed through S phase, whereas only 9% of Jab1-overexpressed cells progressed into S phase (Figure 6(b) and 6(c), the percentage shown on the figure is one of the representative data). The results indicate that consistent with the loss of 9-1-1 components, Jab1 induced the retardation of the efficient recovery from DNA synthesis blockage. To examine the potential consequence of Jab1-suppressed DNA synthesis recovery from blockage, we tested whether Jab1 overexpression could also reduce cell viability after treatment with replication blockers. Cell viability, as indicated by cell colony assays, was significantly lower in Jab1-overexpressed cells in both UV- and HU-treated groups (Figure 6(d) and 6(e)). However, Jab1 did not change cell viability in non-treated control group (Figure 6(d) and 6(e)). Besides 9-1-1 complex, many proteins, including p27, Smad4…, can be degraded by Jab1. 20–22 Degradation of these proteins which suppress cell proliferation may promote cell proliferation and compensate the effect of 9-1-1 degradation-caused reduction of cell proliferation in normal condition. That may explain why Jab1 had no effect on colony formation without UV irradiation or hydroxyurea treatment. However, Jab1-suppressed DNA synthesis recovery from blockage due to 9-1-1 degradation was so dramatic after UV irradiation or hydroxyurea treatment that cell viability significantly affected under these conditions. To verify the correlation between these effects of Jab1 on cell viability with 9-1-1 protein level, parallel experiments using the aliquots of the cells from the above experiments were conducted to detect the expression level of 9-1-1 protein. Jab1-mediated cell viability changes after UV- and HU-treatment is tightly correlated with the lowered 9-1-1 protein level in cells (Figure 6(f)). Taken together, these results suggest that Jab-mediated 9-1-1 degradation suppresses checkpoint signaling activation, disrupts DNA synthesis recovery from blockage and reduces cell survival after replication stresses.
Figure 6. Jab1 suppresses checkpoint signaling activation and disrupts DNA synthesis recovery from blockage.
(a) Jab1 inhibited UV- or HU-induced Chk1 phosphorylation. PANC-1 cells were infected with retroviruses containing empty pSMCVneo vector (Control, lane 1, 3, 5 and 7) or pSMCVneo-HA-Jab1 (HA-Jab1, lane 2, 4, 6 and 8). Cells were then treated with 50J/m2 UV irradiation (lane 3 and 4), 3mM HU for 16hrs (lane 5 and 6) or 20Gy γ-radiation (land 7 and 8). Cell lysates were subjected to Western blot analysis using phosphor-Ser345-specific Chk1 antibody (upper panel), antibody recognizes total Chk1 protein (middle panel) and antibody against HA to recognize ectopically expressed Jab1 (bottom panel). (b) Jab1 inhibited DNA synthesis recovery from replication blockage. PANC-1 cells were infected with retroviruses containing empty pSMCVneo vector (Control) or pSMCVneo-HA-Jab1 (HA-Jab1). Cells were then subjected to 16hr of DNA synthesis block by HU (7.5mM), and released into fresh growth media and pulse labeled with BrdU at indicated time points prior to fixation. After immunostaining with APC-conjugated anti-BrdU antibody and 7-AAD staining, BrdU incorporation and DNA contents were analysed by FACS. Representative data set from three independent experiments is shown. A total of 10,000 events are shown in each sample. (c) Histogram of BrdU-positive cells in (b) showing S-phase recovery and progression of control and Jab1-infected cells after exposure to HU. (d–f) Jab1 suppressed cell viability after UV or HU stimulation. PANC-1 cells infected with retrovirus containing pSMCVneo empty vector (control) or pSMCVneo-HA-Jab1 (HA-Jab1) were treated with 50J/m2 UV irradiation or 3mM HU for 16hrs, and then colony formation was assayed. The colonies were stained with 2% crystal violet (d). (e) is quantitive data of (d). Data points and error bars were derived from three or more independent experiments with duplicate transfections. (f) are the parallel experiments showing expression levels of Rad1, Hus1, Rad9, HA-Jab1 and β-actin of the cells from corresponding experimental groups in (d) by Western blot analysis.
Discussion
Jab1, one of the subunits of CSN, is aberrantly upregulated in several human malignant cancers29–31 and is associated with DNA fidelity, chromosomal stability, cell cycle control and DNA repair.23–25 This gives rise to an interesting question: what is the molecular basis for the function of Jab1/CSN in DNA damage checkpoint and DNA repair pathway? Here we demonstrated that Jab1 directly interacts with and induces the protein rapid degradation of 9-1-1 checkpoint complex, loss or downregulation of which leads to checkpoint inactivation of checkpoint signaling, DNA replication blockage and enhanced chromosomal aberrations. Thus, our current study may provide novel mechanism by which Jab1 is involved in replication stress agent-induced checkpoint responses in cells. In addition, inactivation or downregulation of 9-1-1 complex leads to enhanced chromosomal aberrations, morphological transformation and cancer. According to the progression model of tumorigenesis, cancer is the result of a multi-step process in which cells successively accumulate mutations in key genes that control cell growth,32, 33 and instability of the genome is an important contributor to heritable and somatic genetic changes that drive tumorigenic processes.34, 35 The work described here may also provide novel evidence on the role of Jab1 in the tumorigenesis.
We demonstrate that Jab1 interacts with the 9-1-1 complex in vitro and in yeast and in mammalian cells. However, the in vitro pull-down assays indicate that Rad1 is the subunit that directly binds to Jab1. Rad1, Hus1 and Rad9 strongly interact with each other and form a complex, thus the interaction of Jab1 with Hus1 and Rad9 from the immunoprecipitation assays and their weaker interaction in yeast may be indirectly through Rad1. At protein level, the 9-1-1 complex was found to be regulated by the ubiquitin-proteasome pathway (Hirai et al., 2004). It has been shown that hHus1 is an unstable protein that is actively degraded, whereas hRad1 protects hHus1 from degradation in the cytoplasm.17 Consistently, the study by Bao S. et al provides supportive evidence that loss of Rad1 causes destabilization of Rad9 and Hus1 and consequent disintegration of the sliding-clamp complex.14 On the basis of our work and the studies described above, we reasoned that Jab1 may destabilize Rad1 protein first, and the degraded Rad1 loses its protective effect for Hus1 and Rad9 and causes protein rapid degradation of the whole complex. Jab1 contains a typical leucine-rich nuclear export signal (NES) sequence and induces cytoplasmic translocation of protein substrates such as p27 for their subsequent phosphorylation and degradation in the cytoplasm.20, 26 Our immunofluorescence data also show that Jab1 display a similar effect on 9-1-1 and mediate the nuclear export of 9-1-1 complex. This may explain why Jab1 overexpression induces 9-1-1 rapid degradation in cells.
The CSN as a whole complex was previously reported to negatively regulate protein degradation of the DNA damage-binding protein (DDB2) mediated by a cullin-based E3 ligase. Knockdown of Jab1/CSN5 reduced the repair activity of DDB2 by ~50%.36 In addition to being associated with the large CSN complex, Jab1 was also found to be a monomeric form or associated with a smaller non-CSN complex in various species.37, 38 Each CSN subunit seems to have its own unique function39 in addition to being a component of the CSN complex. A distinguishing feature of Jab1 is that it is able to mediate the nuclear export and degradation of several nuclear proteins in its free form or in a small complex. Our work demonstrates that Jab1 transports 9-1-1 from the nucleus to cytosol and degrades the complex, indicating that Jab1 may exert its effect on 9-1-1 degradation in its free form or as a small complex. In addition, curcumin and emodin, two inhibitors that previously reported to inhibit the CSN-associated kinase activity and CSN-mediated protein degradation, 21, 27 were not able to elevate 9-1-1 protein level. Therefore, it is unlikely that the large CSN complex is involved in Jab1-mediated 9-1-1 protein degradation. However, it is still unclear how 9-1-1 nuclear exclusion induced by CSN complex-independent Jab1 leads to the 26S proteasome degradation of the proteins. Investigating whether ubiquitin pathway is involved in the process and further identify the specific ubiquitin ligase for 9-1-1 protein degradation in the future studies will help to better understand the regulatory mechanisms by which 9-1-1 protein stability is controlled in cells.
In summary, we have shown here that Jab1 interacts with 9-1-1 complex in vitro, in yeast and in mammalian cells. We also demonstrated that Jab1 induces 9-1-1 complex nuclear export and mediates their protein degradation via the 26S proteosome pathway. Thus, Jab1 suppresses 9-1-1-mediated checkpoint signaling activation and DNA synthesis recovery from blockage after replication stresses.
Materials and Methods
Expression plasmids
For yeast two-hybrid screening, the open reading frame region of the human Jab1 gene was amplified by PCR and subcloned into plasmid pGBKT7 (Clontech), generating the bait plasmid, pGBKT7-Jab1. For the GST-fusion Jab1 cDNA, the amplified fragment of Jab1 was subcloned into mammalian glutathione S-transferase (GST) expression vector. The sequences of the PCR-generated portion of all constructs were verified by DNA sequencing. The retrovirus plasmid pMSCVneo-HA-Jab1 was constructed by PCR amplifying HA-Jab1 using pCDNA3-HA-Jab1 as the template and subcloned into the SalI and BamHI sites of the pMSCV vector. siGFP and siJab1 plasmids were generated by using BS/U6 vector.40 Briefly, a 22-nucleotide oligonucleotide corresponding to nucleotides 106 to 127 of GFP or nucleotides 122 to 142, 209 to 229 or 234 to 254 of the human Jab1 coding region was first inserted into the BS/U6 vector. The inverted motif that contains the six-nucleotide spacer and five Ts (oligo 2) was then subcloned into intermediate plasmid to generate BS/U6/GFP and BS/U6/Jab1. For cloning into retrovectors, the U6 promoter region plus the siRNA cassette was cloned into a retrovirus vector Δ U3.
Two-hybrid library screening
A full-length Jab1 coding sequence was cloned into pGBK-T7 (Clontech) to generate the pGBK-T7-Jab1 bait plasmid. The human breast cancer pACT2 cDNA library (Clontech) was screened with the pGBKT7-Jab1 bait plasmid according to the manufacturer’s instructions (Clontech). To further confirm the interactions between Jab1 and Hus1, Rad1 or Rad9, each of which inserted into pGBK-T7 was cotransformed into yeast cells with pACT2-Jab1, and β-gal activity was then detected.
Virus infection
Generation and titration of retroviral supernatants was performed as described.41 PANC-1 will be infected with retrovirus vector containing pMSCVneo, pMSCVneo/HA-Jab1, pMSCVneo/U6-GFP (siGFP) or pMSCVneo/U6-Jab1 (siJab1) as described above. For infection, virus containing supernatant in the presence of 4 μg/ml polybrene (Sigma) will be added in the culture medium. Six days postinfection, the efficiency will be assayed by immunoblotting.
Immunoprecipitation, Western Blotting Analysis, Protein Stability Assays and GST pull-down assay
Immunoprecipitation, Western blotting analysis, protein stability assays and GST pull-down assay were performed as described previously.22, 42 All blots were developed by the enhanced chemiluminescence technique (Amersham, Little Chalfont, UK). The density of the bands was quantitated using Amersham Pharmacia Biotech Storm System and image analysis software. To measure the rate of degradation of Rad1, Hus1 and Rad9, cells were treated with 80 μg/ml of cycloheximide 48 hours after transfection to prevent further protein synthesis. Whole cell extracts were prepared from samples taken at different time points, and the amounts of the Rad1, Hus1 and Rad9 were determined by Western blotting. For GST pull-down assay, Rad1, Hus1 or Rad9 immunoprecipitates were prepared, followed by incubation with protein G-agarose beads. The immunoprecipitates were then washed three times with high salt concentration of buffer (15mM Tris-Cl, pH 7.5, 0.9mM NaCl, 0.1% NP-40) to wash off the bound other proteins. An equivalent amount of purified GST or GST–Jab1 was preincubated with different immunoprecipitates for 1hr at 4°C. The beads were then washed, and bound proteins were eluted by boiling in 2X SDS buffer for 5 min before loading onto a SDS-PAGE gel and blotted to nitrocellulose. Bounded GST-Jab1 was detected by immunoblotting with antibody against GST.
Immunofluorescence
Cells were fixed with 100% methanol, permeabilized with 0.5% Triton X-100, and blocked in 2% bovine serum albumin in TBS containing 0.1% Tween 20. Cells were then incubated overnight with antibodies. Rad1/Rad9 was visualized by immunostaining with mouse antibody against Rad1/Rad9 and goat anti–mouse FITC–conjugated IgG (Amersham Biosciences). Ectopically expressed HA-Jab1 was visualized by immunostaining with rabbit antibody against HA and goat anti–rabbit Texas Red-conjugated IgG (Amersham Biosciences). The nucleus was counterstained with DAPI. Digital pictures were taken with an Olympus, IX TRINOC camera under Olympus, IX70 Inverted Research Microscope (Olympus) with objective lenses of Hoffman Modulation Contrast®, HMC 10 LWD PL FL, 0.3NA ∝/1, OPTICS INC at room temperature, and proceeded with MagnaFire® SP imaging software (Optronics). The fluorescence intensity of both the nuclear region and the total region of the cells were quantitated. For each treatment, 100 cells in three different slides were analyzed. The ratio of nuclei fluorescence intensity: whole cell fluorescence intensity was calculated and expressed as percentage±SDE.
Cell fractionation
Cells were washed and scraped into Cavatation buffer (5 mM HEPES, pH 7.4, 3mM MgCl2, 1 mM EGTA, 250mM sucrose) with protease inhibitors. Cells were lysed by a Cavatation bomb (Parr Instrument Company, Moline, IL). Cell lysates were then centrifuged at 1000 × g for 1 min. The pellet was washed twice in cavatation buffer and used as “nuclear” fraction. The supernatant was centrifuged again at 100,000g for 30 min at 4°C to separate into soluble (Cytosol) and membrane fraction. Protein concentrations were detected and balanced, and samples were diluted into 4× SDS sample buffer before incubating in a boiling water bath.
Clonogenic Assay
The cells were plated at a density of 6 × 105 cells per plate in 10-cm dishes 24hr prior to transfection. After 48hr of transfection, cells were seeded into 6-cm diameter tissue culture dishes at 1,200 cells per dish. After 10 days of culture, the colonies were stained with 2% crystal violet, and cell numbers were determined in a parallel experiment. Only colonies containing more than 30 cells were counted.
Immunofluorescent staining of BrdU incorporation and flow cytometric analysis
The cells were plated at a density of 1×105 cells per well in 6 well plates 24hr prior to virus infection. After 24hr of infection, cells were treated with HU for 16hr and release into fresh growth media for additional 6hr. Cells were then harvested and stained using APC BrdU Flow kit according to the manufacture’s instruction (BD Pharmingen, San Diego, CA). The stained cells were analyzed on a FACScan instrument using CellQuest software (Becton Dickinson). No less than 10,000 cells were scanned from each sample.
Acknowledgments
We thank Dr. Mario Ascoli for the HA-Jab1 expression plasmid and Dr. Hong-gang Wang for Myc-tagged hRad1, hHus1 and hRad9 constructs. Dr. Yang Shi kindly provided the BS/U6 vector. This work was supported by the National Institutes of Health (grants CA112942-01 to M.W., DK53757 to X.C., and CA101955-01 to S.M.V).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 2.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 3.Caspari T, Dahlen M, Kanter-Smoler G, Lindsay HD, Hofmann K, Papadimitriou K, Sunnerhagen P, Carr AM. Characterization of Schizosaccharomyces pombe Hus1: a PCNA-related protein that associates with Rad1 and Rad9. Mol Cell Biol. 2000;74:1254–1262. doi: 10.1128/mcb.20.4.1254-1262.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.St Onge RP, Udell CM, Casselman R, Davey S. The human G2 checkpoint control protein hRAD9 is a nuclear phosphoprotein that forms complexes with hRAD1 and hHUS1. Mol Biol Cell. 1999;10:1985–1995. doi: 10.1091/mbc.10.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiomi Y, Shinozaki A, Nakada D, Sugimoto K, Usukura J, Obuse C, dTsurimoto T. Clamp and clamp loader structures of the human checkpoint protein complexes, Rad9-1-1 and Rad17-RFC. Genes Cells. 2002;7:861–868. doi: 10.1046/j.1365-2443.2002.00566.x. [DOI] [PubMed] [Google Scholar]
- 6.Griffith JD, Lindsey-Boltz LA, Sancar A. Structures of the human Rad17-replication factor C and checkpoint Rad 9-1-1 complexes visualized by glycerol spray/low voltage microscopy. J Biol Chem. 2002;277:15233–15236. doi: 10.1074/jbc.C200129200. [DOI] [PubMed] [Google Scholar]
- 7.Parrilla-Castellar ER, Arlander SJ, Karnitz L. Dial 9-1-1 for DNA damage: the Rad9-Hus1-Rad1 (9-1-1) clamp complex. DNA Repair. 2004;3:1009–1014. doi: 10.1016/j.dnarep.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 8.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 9.Toueille M, El-Andaloussi N, Frouin I, Freire R, Funk D, Shevelev I, Friedrich-Heineken E, Villani G, Hottiger MO, Hubscher U. The human Rad9/Rad1/Hus1 damage sensor clamp interacts with DNA polymerase beta and increases its DNA substrate utilisation efficiency: implications for DNA repair. Nucleic Acids Res. 2004;32:3316–3324. doi: 10.1093/nar/gkh652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Brandt P, Rossi ML, Lindsey-Boltz L, Podust V, Fanning E, Sancar A, Bambara RA. The human Rad9-Rad1-Hus1 checkpoint complex stimulates flap endonuclease 1. Proc Natl Acad Sci U S A. 2004;101:16762–16767. doi: 10.1073/pnas.0407686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi G, Chang DY, Cheng CC, Guan X, Venclovas C, Lu AL. Physical and functional interactions between MutY glycosylase homologue (MYH) and checkpoint proteins Rad9-Rad1-Hus1. Biochem J. 2006;400:53–62. doi: 10.1042/BJ20060774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Lindsey-Boltz LA, Sancar A, Bambara RA. Mechanism of stimulation of human DNA ligase I by the Rad9-rad1-Hus1 checkpoint complex. J Biol Chem. 2006;281:20865–20872. doi: 10.1074/jbc.M602289200. [DOI] [PubMed] [Google Scholar]
- 13.Weiss RS, Enoch T, Leder P. Inactivation of mouse Hus1 results in genomic instability and impaired responses to genotoxic stress. Genes Dev. 2000;14:1886–1898. [PMC free article] [PubMed] [Google Scholar]
- 14.Bao S, Lu T, Wang X, Zheng H, Wang LE, Wei Q, Hittelman WN, Li L. Disruption of the Rad9/Rad1/Hus1 (9-1-1) complex leads to checkpoint signaling and replication defects. Oncogene. 2004;23:5586–5593. doi: 10.1038/sj.onc.1207753. [DOI] [PubMed] [Google Scholar]
- 15.Dang T, Bao S, Wang XF. Human Rad9 is required for the activation of S-phase checkpoint and the maintenance of chromosomal stability. Genes Cell. 2005;10:287–295. doi: 10.1111/j.1365-2443.2005.00840.x. [DOI] [PubMed] [Google Scholar]
- 16.Pandita RK, Sharma GG, Laszlo A, Hopkins KM, Davey S, Chakhparonian M, Gupta A, Wellinger RJ, Zhang J, Powell SN, Roti JL, Lieberman HB, Pandita TK. Mammalian Rad9 plays a role in telomere stability, S- and G2-phase-specific cell survival, and homologous recombinational repair. Mol Cell Biol. 2006;26:1850–1864. doi: 10.1128/MCB.26.5.1850-1864.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirai I, Sasaki T, Wang HG. Human hRad1 but not hRad9 protects hHus1 from ubiquitin-proteasomal degradation. Oncogene. 2004;23:5124–5130. doi: 10.1038/sj.onc.1207658. [DOI] [PubMed] [Google Scholar]
- 18.Claret FX, Hibi M, Dhut S, Toda T, Karin M. A new group of conserved coactivators that increase the specificity of AP-1 transcription factors. Nature. 1996;383:453–457. doi: 10.1038/383453a0. [DOI] [PubMed] [Google Scholar]
- 19.Wei N, Deng XW. The COP9 signalosome. Annu Rev Cell Dev Biol. 2003;19:261–286. doi: 10.1146/annurev.cellbio.19.111301.112449. [DOI] [PubMed] [Google Scholar]
- 20.Tomoda K, Kubota Y, Kato J. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature. 1999;398:160–165. doi: 10.1038/18230. [DOI] [PubMed] [Google Scholar]
- 21.Bech-Otschir D, Kraft R, Huang X, Henklein P, Kapelari B, Pollmann C, Dubiel W. COP9 signalosome-specific phosphorylation targets p53 to degradation by the ubiquitin system. EMBO J. 2001;20:1630–1639. doi: 10.1093/emboj/20.7.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan M, Cao X, Wu Y, Bai S, Wu L, Shi X, Wang N, Cao X. Jab1 antagonizes TGF-beta signaling by inducing Smad4 degradation. EMBO Rep. 2002;3:71–76. doi: 10.1093/embo-reports/kvf024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doronkin S, Djagaeva I, Beckendorf SK. CSN5/Jab1 mutations affect axis formation in the Drosophila oocyte by activating a meiotic checkpoint. Development. 2002;129:5053–64. doi: 10.1242/dev.129.21.5053. [DOI] [PubMed] [Google Scholar]
- 24.Doronkin S, Djagaeva I, Beckendorf SK. The COP9 signalosome promotes degradation of Cyclin E during early Drosophila oogenesis. Dev Cell. 2003;4:699–710. doi: 10.1016/s1534-5807(03)00121-7. [DOI] [PubMed] [Google Scholar]
- 25.Tomoda K, Yoneda-Kato N, Fukumoto A, Yamanaka S, Kato JY. Functions of Jab1 are required for early embryonic development and growth potential in mice. J Biol Chem. 2004;279:43013–43018. doi: 10.1074/jbc.M406559200. [DOI] [PubMed] [Google Scholar]
- 26.Tomoda K, Kubota Y, Arata Y, Mori S, Maeda M, Tanaka T, Yoshida M, Yoneda-Kato N, Kato JY. The cytoplasmic shuttling and subsequent degradation of p27Kip1 mediated by Jab1/CSN5 and the COP9 signalosome complex. J Biol Chem. 2002;277:2302–2310. doi: 10.1074/jbc.M104431200. [DOI] [PubMed] [Google Scholar]
- 27.Uhle S, Medalia O, Waldron R, Dumdey R, Henklein P, Bech-Otschir D, Huang X, Berse M, Sperling J, Schade R, Dubiel W. Protein kinase CK2 and protein kinase D are associated with the COP9 signalosome. EMBO J. 2003;22:1302–1312. doi: 10.1093/emboj/cdg127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park MJ, Kim EH, Park IC, Lee HC, Woo SH, Lee JY, Hong YJ, Rhee CH, Choi SH, Shim BS, Lee SHSI. Curcumin inhibits cell cycle progression of immortalized human umbilical vein endothelial (ECV304) cells by up-regulating cyclin-dependent kinase inhibitor, p21WAF1/CIP1, p27KIP1 and p53. Int J Oncol. 2002;21:379–83. [PubMed] [Google Scholar]
- 29.Sui L, Dong Y, Ohno M, Watanabe Y, Sugimoto K, Tai Y, Tokuda M. Jab1 expression is associated with inverse expression of p27(kip1) and poor prognosis in epithelial ovarian tumors. Clin Cancer Res. 2001;7:4130–4135. [PubMed] [Google Scholar]
- 30.Korbonits M, Chahal HS, Kaltsa G, Jordan S, Urmanova Y, Khalimova Z, Harris PE, Farrell WE, Claret FX, Grossman AB. Expression of phosphorylated p27(Kip1) protein and Jun activation domain-binding protein 1 in human pituitary tumors. J Clin Endocrinol Metab. 2002;87:2635–2643. doi: 10.1210/jcem.87.6.8517. [DOI] [PubMed] [Google Scholar]
- 31.Fukumoto A, Ikeda N, Sho M, Tomoda K, Kanehiro H, Hisanaga M, Tsurui Y, Tsutsumi M, Kato JY, Nakajima Y. Prognostic significance of localized p27Kip1 and potential role of Jab1/CSN5 in pancreatic cancer. Oncol Rep. 2004;11:277–284. [PubMed] [Google Scholar]
- 32.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 33.Mangray S, King TC. Molecular pathobiology of pancreatic adenocarcinoma. Front Biosci. 1998;3:D1148–D1160. doi: 10.2741/a351. [DOI] [PubMed] [Google Scholar]
- 34.Nowak MA, Komarova NL, Sengupta A, Jallepalli PV, Shih IeM, Vogelstein B, Lengauer C. The role of chromosomal instability in tumor initiation. Proc Natl Acad Sci U S A. 2002;99:16226–16231. doi: 10.1073/pnas.202617399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajagopalan H, Lengauer C. Aneuploidy and cancer. Nature. 2004;432:338–341. doi: 10.1038/nature03099. [DOI] [PubMed] [Google Scholar]
- 36.Groisman R, Polanowska J, Kuraoka I, Sawada J, Saijo M, Drapkin R, Kisselev AF, Tanaka K, Nakatani Y. The ubiquitin ligase activity in the DDB2 and CSA complexes is differentially regulated by the COP9 signalosome in response to DNA damage. Cell. 2003;113:357–367. doi: 10.1016/s0092-8674(03)00316-7. [DOI] [PubMed] [Google Scholar]
- 37.Freilich S, Oron E, Kapp Y, Nevo-Caspi Y, Orgad S, Segal D, Chamovitz DA. The COP9 signalosome is essential for development of Drosophila melanogaster. Curr Biol. 1999;9:1187–1190. doi: 10.1016/S0960-9822(00)80023-8. [DOI] [PubMed] [Google Scholar]
- 38.Kwok SF, Solano R, Tsuge T, Chamovitz DA, Ecker JR, Matsui M, Deng XW. Arabidopsis homologs of a c-Jun coactivator are present both in monomeric form and in the COP9 complex, and their abundance is differentially affected by the pleiotropic cop/det/fus mutations. Plant Cell. 1998;10:1779–1790. doi: 10.1105/tpc.10.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bemis L, Chan DA, Finkielstein CV, Qi L, Sutphin PD, Chen X, Stenmark K, Giaccia AJ, Zundel W. Distinct aerobic and hypoxic mechanisms of HIF-alpha regulation by CSN5. Genes Dev. 2004;18:739–744. doi: 10.1101/gad.1180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sui G, Soohoo C, Affar EB, Gay F, Shi Y, Forrester WC, Shi Y. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci USA. 2002;99:5515–5520. doi: 10.1073/pnas.082117599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hetttihewa LM. Immunization of retrovirus-transfected dendritic cells induces specific cytotoxic T lymphocytes for two distinct malarial peptides presented by Kd molecule. Int Immunopharmacol. 2003;3:1401–1411. doi: 10.1016/S1567-5769(03)00137-1. [DOI] [PubMed] [Google Scholar]
- 42.Wu X, Shell SM, Zou Y. Interaction and colocalization of Rad9/Rad1/Hus1 checkpoint complex with replication protein A in human cells. Oncogene. 2005;24:4728–4735. doi: 10.1038/sj.onc.1208674. [DOI] [PMC free article] [PubMed] [Google Scholar]