Abstract
The extracellular microenvironment plays a significant role in controlling cellular behavior. Identification of appropriate biomaterials that support cellular attachment, proliferation and, most importantly in the case of human embryonic stem cells, lineage-specific differentiation is critical for tissue engineering and cellular therapy. In addition to growth factors and morphogenetic factors known to induce lineage commitment of stem cells, a number of scaffolding materials, including synthetic and naturally-derived biomaterials, have been utilized in tissue engineering approaches to direct differentiation. This review focuses on recent emerging findings and well-characterized differentiation models of human embryonic stem cells. Additionally, we also discuss about various strategies that have been used in stem cell expansion.
Keywords: Embryonic stem cells, Biomaterials, Microenvironment, Differentiation, Self-renewal, Biomimetic
1. Introduction
Stem cells have been touted to play a pivotal role in treating debilitating diseases and tissue damages. Human pluripotent stem cells have been derived from the inner cell mass of the blastocyst (embryonic stem cells) and the fetal primordial gonadal ridge (embryonic germ cells) whereas mesenchymal stem cells (MSCs) have been isolated from various adult tissues [1–5]. Recent advances in stem cell biology have also enabled generation of pluripotent cell populations from both fetal and adult mouse fibroblasts through reprogramming by defined factors or by cell–cell fusion [6–10]. MSCs are thought to be a self-renewing population of cells that can give rise to differentiated cells found in adult tissues. Bone marrow derived-MSCs are currently undergoing clinical trials for cardiac and orthopedic applications. However, MSCs ability to proliferate and differentiate decreases with age of the donor and culture time [11,12]. In contrast, embryonic stem cells are immortal and could potentially provide unlimited numbers of cells for regenerative medicine (cell and tissue based therapies) [13–16].
The potential of embryonic stem cells to differentiate into almost all cell types, in addition to providing unlimited number of cells, has stirred interest in their use as an integral part of modern clinical treatment. Additionally, stem cells are being used to understand the complex molecular and cellular events occurring during early development, disease progression, epigenetics, and pathophysiology [17–19]. For example, ES cell lines with genetic disease markers have been generated through nuclear transfer technology to study the underlying cause of disease and diseases progression [19]. Pluripotent stem cells could also be used in drug development, screening, and toxicology [20,21].
Perhaps the most exciting of all applications of stem cells could be their use in cell replacement therapies and regenerative medicine. The chronic shortage of organ transplants in conjunction with the limitation of artificial implants (prostheses) has intensified research in cell and tissue based therapies. The key advantage of cell and tissue therapy over pharmacological therapies to treating debilitating diseases and abnormalities is that the former offers “living biological replacements” while the latter merely provides a palliative solution. However, before stem cell-based therapies could be transferred from the “bench to the bedside”, many fundamental biological and engineering challenges need to be overcome that include: controlling the self-renewal of stem cells, directing the lineage/tissue-specific stem cell differentiation, in vivo delivery, and integration to the host milieu.
In this review, we summarize various strategies that have been used in the last decade to address the above-mentioned issues in the field of stem cell-based regenerative medicine. In particular, we will be discussing the pivotal role biomaterials play in maintaining the undifferentiated state of ES cells during their expansion, lineage-specific differentiation, and in vivo transplantation. Unlike the first generation biomaterials which were extensively used as artificial implants, current research in biomaterial has mainly focused on developing “custom-designed, bio-interactive” materials with pre-encoded instructive signals to modulate various cellular functions such as self-renewal and morphogenesis.
2. Expansion of stem cells
2.1. Self-renewal of embryonic stem cells
Pluripotent embryonic cells require a co-culture environment for their self-renewal in monolayer expansion, conventionally achieved by culturing on a layer of feeder cells. The key components that regulate the self-renewal of murine ES cells (mESCs) have been deciphered and they are largely dependent on two key signaling pathways involving LIF and BMP [22]. However, the factors involved in human embryonic stem cell (hESC) self-renewal have still not been elucidated, although significant progress has been made in recent years.
The first successful isolation of hESCs by Thomson et al. used mouse embryonic fibroblasts as the feeder layer [1]. Concerns about exposure of hESCs to xenogenic products have precluded their widespread use in human clinical applications. This concern was substantiated by a recent study where Martin et al. showed that hESCs cultured in the presence of animal products express the non-human sialic acid, N-glycolylneuraminic acid (NeuGc) [23]. Over the last decade, various other alternatives have been identified to support hESC self-renewal. For instance, many studies have explored several alternative cell sources as feeders to support hESC culture in monolayer and thereby limiting cross-species contaminations, which includes human embryo derived fibroblasts, foreskin fibroblast, and adult bone marrow cells [24–27]. However, cell and tissue therapy required maintenance of large quantities of undifferentiated hESCs, and therefore usage of feeder cells to support hESCs expansion may not be an optimal solution. Additionally, the use of animal-based products as well as feeder cells may introduce batch-to-batch variations. Therefore, the ideal culture method for hESCs-based cell and tissue therapy would be a defined culture free of animal components and feeder layer.
2.2. Biomaterial supported feeder-free culture of ESCs
ES cells that are free of foreign proteins and antigens would hold promise as an untapped cell source for multiple clinical and biotechnological applications. In the case of mouse ES cells, the feeder layer can be easily replaced by exogenous supplementation of BMP and LIF [22]. Various groups have utilized biomaterial support to expand mESCs in vitro in conjunction with LIF [28,29]. For instance, Nur et al. used a polyamide-based 3D nanofibrillar porous matrix (Ultra-Web)™ to support ex vivo expansion of mESCs in a LIF-based medium [29]. Their results showed enhanced proliferation and self-renewal of ESCs cultured on the above nanofibrillar matrix compared to tissue culture dishes [29]. However, supplementation with LIF or BMP does not work for human ES cells, and therefore developing feeder-free culture condition for hESCs expansion has been an active research area.
Xu et al. reported the first successful feeder-free culture of hESCs [30]. In this study, hESCs were grown on culture dishes coated with various biologically active materials such as laminin, collagen, and Matrigel™ with 100% MEF conditioned medium supplemented with serum replacement and growth factors such as bFGF. The authors demonstrated that a synergistic action of ECM-based biomaterial along with MEF conditioned medium (MEF-CM) supports hES cells up to 130 population doublings in their undifferentiated state without any karyotypic changes. The ES cells cultured on these ECM-based biomaterials had a doubling time of 31–33 h comparable to that of ESCs grown on feeder layers. In contrast, cells grown on gelatin-coated dishes in MEF-CM showed poor survival. Additionally, all the surviving cells differentiated within the first passage. Although the above study was successful in expanding the hESCs in a feeder-free condition, it still required MEF-CM. Similarly Mohr et al. used Matrigel-coated microwells for maintaining the undifferentiated hESC for weeks in MEF-CM without passaging [31].
Other studies that excluded conditioned medium for feeder-free ESC cultures used various growth factor “cocktails” in addition to culture dishes coated with natural ECM-based biomaterials [32,33]. Yao et al. developed a chemically defined medium to achieve self-renewal hESCs while growing on Matrigel-coated dishes [34]. The advantage of using chemically defined medium is that it eliminates influence of unknown components from the growth factor cocktail. The use of cell-based ECM components, however, poses threat of pathogen transmission. Therefore, it would be advisable to use synthetic ECM-based support, which would offer numerous advantages such as risk free environment, ease of scale up, and control over biochemical and biomechanical properties.
Synthetic polymer matrices have been evaluated as cell culture substrates and may provide a fully-defined microenvironment for hESC propagation, as naturally-derived substrates may have undefined composition and batch-to-batch inconsistencies [35]. Li et al. reported the use of synthetic ECM-based hydrogels matrices for supporting the self-renewal of hESCs for a short period of time [36]. In this study, the authors used a semi-interpenetrating network consisting of poly(N-isopropylacrylamide-co-acrylic acid) and synthetic peptide chains as the matrix. This hydrogel system was able to support the self-renewal of hESCs in the presence of MEF-CM. A recent study by Gerecht et al. examined the potential of using synthetic three-dimensional cultures for maintaining the undifferentiated state of hESCs [37]. Stem cell colonies were encapsulated within hyaluronic acid (HA) hydrogels and cultured in MEF-CM. This culture system maintained the undifferentiated state of hESCs for 30 days. Unlike HA hydrogels, dextran hydrogels cultured under identical conditions induced differentiation of encapsulated hESC colonies suggesting that HA may play a regulatory role in maintaining the self-renewal of hESCs. Although the aforementioned studies were successful in demonstrating the potential of synthetic artificial matrix in maintaining the undifferentiated state of hES cells during their ex vivo expansion, they still required MEF-CM. The use of MEF-CM as well as animal products (i.e. undefined culture condition) limits their viability as a cell source for regeneration therapies.
The development of defined large-scale culture conditions that can maintain the undifferentiated state of ES cells is still a grand challenge. Few chemically defined culture conditions have been reported in literature [38,39]. Ludwig et al. recently reported a chemically defined medium condition that is free of serum and animal components for maintaining the undifferentiated state of hESCs in presence of ECM-based components [38]. The same culture conditions also supported the derivation of hESCs under feeder-free culture conditions. Researches that aim to develop defined culture conditions have started exploiting combinatorial chemistry approaches to screen small chemical molecules to replace animal components of the culture medium. For instance, Chen et al. reported the beneficial effect of small chemical molecules such as 3,4-dihydropyrimido[4,5-d]pyrimidine (and its anolgue SC1) in retaining the undifferentiated state of murine ES cells in chemically defined culture conditions (free of feeder cells, serum, and LIF) [40]. So far, no such small molecules have been reported in the literature that promotes self-regulation of hESCs over a prolonged period of time. A fundamental understanding of the cascade of molecular events that regulate self-renewal of stem cell could lead to the development of novel biomaterials that can maintain the undifferentiated state of hESCs during ex vivo expansion in defined conditions.
3. Directed stem cell differentiation
Differentiation of the ES cells prior to transplantation is very critical, because undifferentiated ES cells may cause teratoma formation in vivo. The potential use of ES cells to replace functional loss of particular tissues may depend on efficient differentiation protocols to derive tissue-specific progenitor cells without any detrimental in vivo side effects. By manipulating the culture conditions in which ES cells differentiate, it has been possible to control and restrict the differentiation pathways and thereby generate cultures enriched in lineage-specific precursors in vitro. However, commitment and long-term engraftment of these cells in vivo for functional tissue regeneration are challenging. In addition, the intrinsic biologic difference between somatic cells and hESC-derived somatic cells may exist. In a recent report, Mauck et al. suggested stem cell-based engineered tissues are likely be inferior tissue [41]. The amount and mechanical properties of engineered cartilage using MSCs were inferior to engineered cartilage utilizing chondrocytes under the same condition [41]. It is necessary to design and develop culture conditions that promote homogeneous and enhanced differentiation of ES cells to yield functional tissues.
3.1. Differentiation of embryonic stem cells
3.1.1. In vitro embryoid body formation
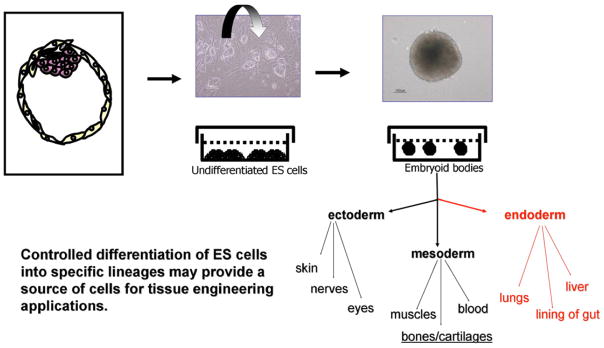
Different strategies have been utilized to induce in vitro differentiation of ES cells. ES cells spontaneously differentiate into derivatives of three-embryonic germ layers: mesoderm, endoderm, and ectoderm via formation of embryoid bodies (EBs) upon removal of factors that maintain the undifferentiated or pluripotent state of stem cells [42]. Creation of EBs is usually the first step for differentiation of ES cells (Fig. 1). Differentiation of EBs into particular cell lineages has been extensively studied due to current technical challenges in achieving their homogenous and efficient differentiation [43]. Most of the methods for differentiation of EBs utilize the following: 1) EB formation from suspension culture, 2) plating EBs on tissue culture plates coated with gelatin, and 3) supplementation with growth factors or differentiation-inducing factors [44,45]. A number of studies have shown that differentiation of ES cell through EBs parallels embryonic development, in which EBs recapitulate early embryonic developmental phases [44,46]. Therefore EBs can be utilized instead of embryo or whole animals to study effects of small molecules and/or biological agents on the early human development [47]. Different methodologies have been utilized for EB formation, which includes dissociated suspension culture [48], methylcellulose culture [49,50], hanging drop culture [45,51], spinner flask [52,53], bioreactor culture [54], and microwell technology [31,55].
Fig. 1.
Differentiation scheme of ES cells via formation of EBs. Controlled differentiation of ES cells into specific lineages may provide a source of cells for tissue engineering applications.
Suspension methods usually give rise to heterogeneous cell clusters. Additionally, this method allows spontaneous aggregation of EBs. Initial cell density has been shown to play an important role in EB formation. The heterogeneous size and shape of EBs resulting from suspension culture usually influence their differentiation potential [56]. In contrast, hanging drop methods provide relatively uniform EB size. However, this method is technically challenging due to the need for large number of EBs for characterization. Methyl cellulose culture employs formation of EBs of a clonal origin from a single cell [43]. Recently, other methods such as those using round-bottom 96-well plate and conical tubes have been adopted to form EBs from predetermined numbers of ES cells [57,58]. This method results in generation of EBs with uniform size distribution.
For the production of large numbers of EBs, stirred-suspension culture using spinner flasks and bioreactors have been utilized [52–54,59]. Enhanced growth of EBs in spinner flasks has been reported due to effective nutrient and oxygen supply [53]. In addition to stirred-suspension culture for scalable production of EBs, other methods such as transferring EBs to stirred-suspension culture or ES cells encapsulated in size-specified-suspension culture have been utilized to form efficient EBs [59,60]. These strategies have been shown to be advantageous compared to conventional suspension method due to their ability to control EB–EB interactions [59]. Each of these methods has its own characteristic features that influences EB formation and subsequent differentiation. However, a recent report comparing the efficiency between suspension, hanging drop, and methylcellulose method on hematopoietic differentiation showed no differences among these methods [49].
One factor that may influence the lineage commitment and differentiation is the size of EBs. Heterogeneity and difficulties in reproducing EB size often results in heterogeneous differentiation and commitment [61]. For example, due to differences in oxygen tension, nutrient gradient across EBs, and mechanical and force dependent stimulation, smaller EBs might preferentially differentiate along one particular germ cell lineage compared to others. Our unpublished data demonstrates that there is a size dependent chondrogenic lineage differentiation of EBs. To this end, microfabrication technology has been utilized to create EBs with uniform size and shape. Karp et al. used microwells with hydrophilic poly(ethylene glycol) (PEG) coating as templates to initiate the formation of homogenous EBs [61]. Here the size and shape of the EBs were defined by the geometry of the microwells [61]. In addition to the formation of homogenous EBs, microwell culture also promotes formation of hESC colonies with a defined size, and this method could be used to form monodisperse EBs. Mohr et al. demonstrated a microwell system to constrain hESC growth and facilitate generation of undifferentiated cell aggregates that could be easily passaged or differentiated in suspension [31,62].
3.1.2. 2D vs. 3D differentiation of ES cells
Stem cell differentiation is context dependent. Even though EBs have a three-dimensional structure, terminal differentiation of EBs is conducted in 2D culture (tissue culture plates). Most of the studies investigating stem cell differentiation have been performed on 2D plates coated with various biomaterials. Precise spatial and temporal presentation of factors directing the stem cell differentiation is extremely important to achieve homogeneous and efficient differentiation. ES differentiation in 2D cultures does not mimic the physiological (in vivo) environment and may result in inefficient and heterogeneous differentiation. Indeed, significant differences were found in the differentiation profile of ESCs when cultured in a 3D environment vs. 2D [63,64]. Three-dimensional cultures in the form of pellets alone is sufficient to induce selective differentiation of embryonic-derived cells [65,66]. Maintenance and differentiation of EBs in three-dimensional culture may promote cell–cell interactions, entrapment of secreted extracellular matrix, and maintenance of spherical cellular morphologies [66,67]. In addition, 3D culture and differentiation of ES cells provide structural support for higher order tissue organization and remodeling. We have previously demonstrated that TGF-β1 and BMP-2 have a significant impact on chondrogenic differentiation of mouse embryonic stem cells and they act by different mechanisms to regulate chondrogenic cell fate depending on 2D vs. 3D culture of EBs [64] (Fig. 2). The heterogeneous nature of EBs may result in variable response of cells to exogenous factors, and therefore further optimization of EB culture strategies to guide stem cell behavior and differentiation is required.
Fig. 2.
Culture condition dependent effects of growth factors on the chondrogenic differentiation of EBs. BMP-2 promoted aggrecan expression of EBs that were cultured in tissue culture plates (2D) while TGF-β1 promoted aggrecan expression in hydrogel culture (3D).
3.1.3. Derivation of progenitor cells from ES cells
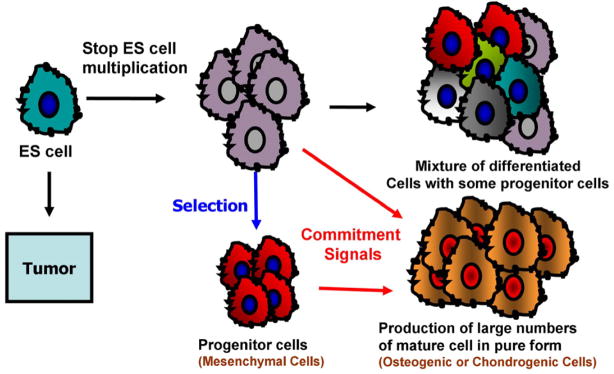
Advancements in stem cell biology have enabled the use of EBs to produce unlimited numbers of specialized progenitor cell populations for stem cell-based therapy. One hypothesis for such applications is that partially differentiated or tissue-restricted progenitor cells can be isolated from the ES cells, purified through cell selection, and expanded in vitro to generate adequate progenitor cell populations before they can be used safely and effectively in clinical applications (Fig. 3). ES cells differentiate into multiple mature somatic cell types, presumably via precursor cells, when the appropriate stimuli are applied. For instance, mesodermal progenitor cells were isolated by fluorescence-activated cell sorting (FACS) after EB stimulation with BMP [14]. Moreover, multipotent hematopoietic progenitor cells [68,69], cardiac progenitor cells [62,70], endothelial progenitor cells [71], and neuronal progenitor cells [72–74] have been isolated and characterized. Recently, Lu et al. have reported an efficient and reproducible method for generating large numbers of such bipotential progenitors (hemangioblasts) from hESCs using an in vitro differentiation system [75]. In addition to obtaining a relatively high purity of progenitor cells by separation methods such as FACS and magnetic affinity cell sorting, stage-specific growth factor treatment or genetically altering the ES cells may greatly enhance their differentiation into desired progenitor lineages.
Fig. 3.
Spontaneous differentiation of hESCs leads to heterogeneous cell population. However, application of commitment signals (in forms of soluble factors and culture conditions) would enable the selection of progenitor cell population which would later lead to the production of larger number of mature cells in pure form.
MSCs are generated from a number of different developmental origins [76]. Recent advances in stem cell biology have identified mesenchymal progenitor cell populations that can undergo multi-lineage differentiation into different mesenchymal tissues such as cartilage, fat, and bone [16,77,78]. These cells efficiently engrafted when injected into SCID mouse and did not show any in vivo tumorigenic potential [78]. A similar strategy has been applied to isolate clinically applicable mesenchymal precursor cells from hESCs without feeder support and EB formation [79]. MSCs have been derived from a number of different adult tissues, such as bone marrow, deciduous teeth, adipose tissue, umbilical cord blood, and synovium [4,80–83]. MSCs have the capability to differentiate into mesodermal lineages, such as adipogenic, osteogenic, chondrogenic, and myogenic cells and can potentially be utilized as a cell source for musculoskeletal tissue regeneration [84,85]. However, there are potential limitations when using MSCs for tissue engineering and repair. These limitations include the relatively low frequency with which these cells occur in the marrow stroma and donor site morbidity. Additionally, the self-renewal and proliferative capacity of MSCs is limited and decreases with age [11]. The greater proliferative capacities of hESC-derived mesenchymal cells compared to human MSCs and lack of teratoma formation in vivo highlight their significant potential for tissue engineering and regenerative medicine applications [78] [unpublished data]. hESC-derived progenitor cells such as mesenchymal cells may have potential application in tissue regeneration and provide a tool for elucidating the mechanism of lineage commitment specification from ES cells, which may provide a platform for efficiently generating specialized transplantable cells for clinical applications. Plating density of cells and tissue culture substrates may influence the progenitor cell differentiation potentials. Therefore, efficient culture conditions optimal for maintenance and selection of hESC-derived progenitors need to be developed [86].
3.1.4. Growth factors
The first screening of growth factors to understand the differentiation of hESCs was conducted by Schuldiner et al. in 2000 [87]. They investigated a number of different growth factors, and depending upon their type (biological activity), the EBs differentiate selectively into mesodermal, endodermal, or ectodermal lineages. However, these studies did not result in homogeneous differentiation of ES cells. Hence, the current challenge is to find an optimized combination of these various cytokines and growth factors that would bias differentiation specifically towards a desired lineage. An added complication is that many of these cytokines and growth factors exert non-specific pleiotropic effects on stem cell differentiation, which is most likely due to the activation of multiple intracellular signaling pathways by each individual cytokine or growth factor. Therefore controlled release and delivery of growth factors responsible for different stages of differentiation may provide instructive signals for guided differentiation of ES cells. For example, chondrogenesis is an orchestrated molecular and cellular process during embryogenesis [88]. This is a multi-step process that occurs with mesenchymal cell recruitment, migration, proliferation, and condensation [89]. This complex process is controlled by cellular interactions with the surrounding matrix, growth and differentiation factors, and other environmental factors that initiate or suppress cellular signaling pathways and transcription of specific genes in a temporal–spatial manner. A number of morphogenetic factors including hedgehog proteins (Hhgs), Wnt proteins, Notch ligands, members of TGF-β superfamily of growth factors, or FGFs, have been implicated to play important roles in controlling the regulation of these transcription factors in musculoskeletal tissue development. TGF-β1 is among the earliest signals in chondrogenic condensation, and has been shown to stimulate the synthesis of fibronectin, which in turn regulates N-CAM [90]. BMPs play an important role during bone morphogenesis by initiating chondro-progenitor cell determination and differentiation [91]. BMP has also been shown to regulate the later stages of chondrocytes maturation and terminal differentiation to the hypertrophic phenotype [90].
In order to mimic development, biomaterials with different growth factor delivery systems can be utilized to mimic embryogenic process. Delivery of growth factors for morphogenesis and differentiation of stem cells have been successfully verified in various biomaterials [92]. Growth factors have been covalently modified on the surface of biomaterials to provide pro-growth and pro-survival factors which may enhance long-term in vivo survival and differentiation of stem cells [93,94]. Growth factors released from biomaterials via diffusion, cell-mediated proteolysis, or in response to mechanical stimuli have been successfully incorporated for in vitro and in vivo regenerative applications [92,95]. Controlled delivery of multiple growth factors via different release kinetics has been shown to promote enhanced differentiation and tissue formation. To induce prolonged effects of growth factors, Hung et al. attempted bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4) [96]. Additionally, a combination of growth factors has been used within a biomimetic environment for enhanced tissue formation [97]. Incorporation of growth factors in biomaterials will be an important way to regulate cell differentiation and enhance functionality of differentiated cells by providing adequate signaling and concentration of growth factors.
3.1.5. Morphogenetic factors
Increasingly, morphogenetic factors that play regulatory roles during embryogenesis and morphogenesis are being studied for stem cell culture and differentiation. In addition to utilizing biological factors through exogenous treatment, cell-secreted morphogenetic factors can be utilized to modulate differentiation signaling pathways leading to commitment and tissue formation. For example, during endochondral ossification, cartilage serves as a morphological template for blood vessel invasion and MSC recruitment for future bone [88]. Recent studies have indicated that morphogenetic signals from chondrocytes regulate multiple steps of chondrogenesis and endochondral ossification during skeletal differentiation [98–100]. These studies have implicated that mechanisms of endochondral ossification of condensing mesenchyme can be regulated by cell-secreted morphogenetic factors. Gerstenfeld et al. demonstrated that the morphogenetic factors secreted by chondrocytes can regulate MSC differentiation, and we recently showed that these factors promote osteogenic as well as chondrogenic potential of MSCs in a micromass-culture [99,101]. In addition to modulating MSC differentiation, the morphogenetic factors from chondrocytes selectively modulated chondrogenic differentiation of hESCs when co-cultured [102]. In addition to chondrocytes, embryonic calvarial [103], embryonic limb bud [104], and articular perichondrial cells [105] have also been reported to have stimulatory effects on chondrogenesis, and these cells may be utilized to direct the differentiation of ES cells to the chondrogenic lineage. In addition to musculoskeletal lineages, endothelial cell- or astrocyte-conditioned medium promoted neuronal as well as glial differentiation of ES cells [106]. Neural stem cell-conditioned medium has also been shown to be a crucial reagent for inducing neural differentiation of ES cells [107]. Co-culture with two or more distinct cell population results in complex and functionally vascularized tissue, which was in part contributed by humoral factors secreted by adjacent cells [108].
A recent report by Sze et al. demonstrated that hESC-derived mesenchymal stem cells secrete morphogenetic factors that have the potential to act as paracrine modulators for tissue repair and regeneration in cardiovascular, hematopoietic and skeletal diseases [109]. These observations indicate that cell–cell interactions, in the form of secreted morphogenetic factors, can be utilized to regulate the commitment and differentiation of stem cells and possibly play a significant role in tissue regeneration applications. Secreted factors within conditioned media may be suitable for prolonged expansion of ES cells for guided tissue-specific differentiation before transplantation.
Numbers of studies have shown that co-culture and co-transplantation of different cell types could be a promising therapeutic approach to regenerate fully functional tissues. Levenberg et al. achieved significantly higher vascularization of engineered tissues with enhanced cell viability by co-transplantation of hESC-derivatives with endothelial cells and MEFs [108]. However, it is difficult to regulate cell–cell interactions in vivo, thereby complicating their usage. In addition to contact-mediated signals from neighboring cells, stem cells respond to neighboring cells through soluble factor-mediated cell–cell interactions. The autocrine and paracrine factors secreted by differentiated cell types in proximity with stem cells may lead a direct and efficient transduction of molecular signals that may result in expression of organ specific markers. Recently, our lab demonstrated that the co-culture system utilizing bilayered hydrogel may provide 3D co-culture system to investigate soluble factor-mediated differentiation and commitment of stem cells in vivo (Fig. 4). Given the inefficient commitment and homogenous differentiation protocols, utilizing morphogenetic factors from differentiated cells via co-culture may provide innovative tissue culture methods for directing and generating cartilaginous tissue from stem cells. Elucidating an efficient cellular microenvironment for differentiation and tissue formation of ESC-derived cells will have significant impact on regeneration applications utilizing stem cells.
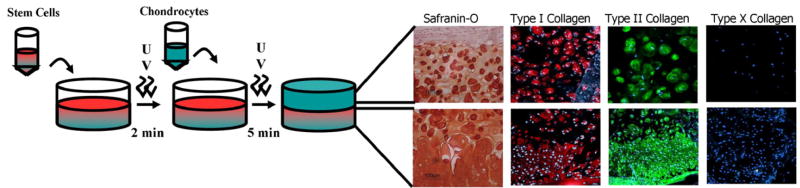
Fig. 4.
Schematic representation of the multi-layering techniques for co-culture. Bottom layer (stem cells) is polymerized for 2 min to achieve a partially gelled consistency. Upon application of another layer with chondrocytes–polymer suspension, the entire construct is exposed to UV for 5 min to ensure complete polymerization of all layers.
3.1.6. Small molecules
In addition to growth factors and cell-secreted morphogenetic factors, the fate of stem cells can be regulated by small cell-permeable molecules such as dexamethasone, vitamin C, sodium pyruvate, thyroid hormones, prostaglandin E2, dibutryl cAMP, concavalin A, vanadate, and retinoic acids. Recently, new biomolecules, in the form of small molecules, have been investigated as a repertoire of differentiation-inducing factors to alter stem cell fates [110,111]. Ding et al. screened a variety of small molecules for their ability to modulate differentiation of ES cells into various tissue-specific cells [112]. Such small molecules play important roles during embryogenesis and may be utilized to direct or control the differentiation process of ES cells. For example, retinoic acid (RA) enhances expression of neural crest and reduces mesodermal differentiation, which are also found to be alternative mesenchymal origin [113,114]. Sodium butyrate treatment resulted in hepatocyte-like cells expressing glycolytic phenotype [115]. Thyroid hormones, steroid derivatives of cholesterol metabolism, have also been implicated as potent differentiation factors [116,117]. In addition, our recent findings indicate that glucosamine has chondrogenic effects on embryonic stem cells [118]. These findings point towards the importance of cell-permeable small molecule-mediated biological signals in guiding the commitment of ES cells toward specific 3D tissue development in vitro. As the identification of these molecules and their roles in stem cell biology becomes well understood, they can be incorporated into tissue engineering scaffold design so as to harness their beneficial effects for lineage-specific differentiation and tissue development.
3.1.7. Mechanical factors
During tissue development and remodeling, the cells are subjected to various forms of mechanical stimulation, which in turn shapes and regulate a large array of physiological processes. Upon mechanical stimulation, cells convert these mechanical signals into biochemical responses through a mechanism termed as mechano-transduction [119]. Cells interact with their surroundings by ECM receptors such as intergrins, laminin receptors, syndecans, and MMPs. Specifically, the ECM dynamics and matrix stiffness are translated into cytoskeletal tension mediated through integrin–ECM interactions [120]. Integrin signaling is principally mediated via focal adhesion kinases. Integrin-mediated cell responses modulate a number of intracellular signals affecting cellular responses that may cooperatively mediate SMADs, Rho GTPases, ERK and many other down stream signaling pathways affecting transcriptional and epigenetic changes [121]. This integrin-mediated adhesion signaling exerts its effects by cooperating with soluble factors to regulate Rho GTPases and the generation of actin cytoskeletal tension [122].
In terms of hESCs differentiation, response to mechanical stimulation is largely unknown. Recent study by Schmelter et al. found that embryonic stem cells utilize reactive oxygen species as transducers for mechanical strain-induced cardiovascular differentiation [123]. Conversely, Huang et al. indicated that hESC differentiation into vascular cells could be appropriately modulated by shear force [124]. Embryonic stem cells differentiated into vascular wall cells under in vitro pulsatile flow loading [124]. Cyclic strain induces vascular smooth muscle cell differentiation from murine embryonic mesenchymal progenitor cells [125]. Interestingly, a study by Saha et al. concluded that hESC differentiation was inhibited by biaxial cyclic strain [126]. However, in this study mechanical strain was not sufficient to inhibit differentiation when differentiation medium was applied. In unconditioned medium, hESCs cultured under strain differentiated at the same rate as cells cultured in the absence of strain.
Mechanical responsiveness of ES cells indicates that mechanical properties of a scaffold or culture surface can regulate differentiation of stem cells. Differentiated cells such as fibroblasts, muscle cells, neurons, and epithelial cells exert different levels of traction forces on the substrates when they are plated and sense the stiffness of the substrates, resulting in various cellular morphologies and force balance between cells and the substrates. Indeed, a recent study by Engler et al. demonstrated that MSC differentiation can be modulated by varying mechanical properties of substrates [127]. Microfabrication approaches to engineering model cellular micro-environment to decouple various cell adhesion groups showed that the degree of adhesion, cell spreading, and focal adhesion plays a pivotal role in regulating the commitment of MSCs to osteogenic, chondrocytic, and adipogenic fates [128]. In addition, mechanical strain plays a role in stem cells differentiation and proliferation, and the effects of the strain were dependent on the orientation with respect to the strain axis [129]. Moreover, mechanotransduction through electrical stimulation [130], pulsatile flow loading [131], and dynamic compression can be utilized to direct the differentiation of ES cells. These results confirm that physical factors, through regulation of cellular tension, or biophysical cues, sensed by the cells appear to play significant roles in the lineage commitment process.
3.2. Biomaterials as instructive extracellular microenvironments for controlled differentiation
3.2.1. Surface chemistry
Substrates with varying surface chemistry and topography induce diverse cellular responses [132,133]. Substrate surface properties (e.g. hydrophobic/hydrophilic properties) play an important role in protein adsorption kinetics and their folded conformation, which in turn influence cellular activities [133,134]. Additionally, exclusive studies have been carried out to decorate the biomaterial surface with bioactive molecules in a precise manner to enhance their bioactivity [135]. These surface engineered biomaterials having active receptor binding domains can be used to modulate the integrin-signaling pathway in a defined manner to control stem cell fate. Various studies have demonstrated the role of surface chemistry as well as topography on osteogenic differentiation of cells as the surface properties have a prominent effect on biomineralization and structure of the formed hydroxyapatites [133,136,137]. Brodbeck et al. recently demonstrated that the hydrophilicity of the substrate surface can also have an impact on apoptosis as the hydrophilic surfaces produced a decreased expression of proinflammatory cytokines [138]. Surface engineering approaches that alter the substrate surface properties including topography could be used as a powerful tool in directing the ES cell–matrix interactions and their subsequent differentiation.
3.2.2. Naturally-derived biomaterials
The extracellular microenvironment plays a significant role in controlling cellular behavior [139–141]. In addition to providing structural stability for developing tissues, scaffolds with desirable biochemical and biophysical cues can direct cellular behavior and function. Matrix composition has an important role in ES differentiation and influences their behavior towards preferred lineages. A number of natural materials have been used to support the differentiation for hESCs that include agarose, alginate, hyaluronic acid, gelatin, fibrin glue, collagen derivatives, and acellular tissue matrices. Collagen is the main component of native ECM and cells interact with collagen through integrin binding-mediated interactions. Collagen has long been utilized as a natural biomaterial due to its low immunogenicity. Three-dimensional collagen gel has been used to support ES cell-derived endothelial cells [142] and neurite outgrowth [143]. High concentrations of collagen gel inhibited EB apoptosis and enhanced differentiation [140]. Addition of fibronectin to the collagen gel preferentially stimulated ES cell differentiation into endothelial cells, leading to vascularization, while addition of laminin favored ES cell differentiation into beating cardiomyocytes [140]. Gelatin is a porous denatured collagen scaffold, and it has been used for tissue engineering applications due to its biocompatibility [144–146]. Hyaluronan is a high molecular weight polymer having disaccharide unit glucuronic acid and acetyl-glucosamine. HA binds specifically to proteins in the ECM, on the cell surface, and within the cellular cytosol, and thus has roles in a number of different physiological roles such cartilage matrix stabilization, angiogenesis, cell mobility, inflammation regulation, and growth factor action [147]. In addition, developmental roles of HA include regulation of gene expression, signaling, and proliferation. Therefore, HA-based materials can be utilized to regulate stem cell differentiation [148]. Currently, HA-based biomaterials have been utilized to support differentiation of stem cells in combination with growth factors or other ECM components [147,149].
Matrigel contains multiple extracellular matrix components and growth factors that may promote cell attachment and differentiation. It has been used to support endothelial differentiation of ESCs [150] and for promoting the development of glandular- and tubular-like structures from differentiating ES cells. Matrigel is less adhesive than collagen and has been shown to support efficient aggregation of ES cells and further differentiation into mesoderm and endoderm lineages [151]. However, Matrigel contains a series of unknown proteins, and therefore may not be an appropriate microenvironment for lineage-specific differentiation of ES cells. Another biomaterial that has been explored for stem cell differentiation is alginate. Alginate is derived from seaweed, and in presence of a divalent cation such as Ca2+, forms an ionically crosslinked hydrogel. Alginate-based hydrogels in combination with oligochitosan have been shown to support ES cells growth [152]. Additionally, alginate hydrogels demonstrated to be conducive for ES cell differentiation into hepatic lineage [153,154]. Three-dimensional porous alginate scaffolds promoted generation of vascularized embryoid bodies from human embryonic stem cells [155]. Alginate has also been investigated for in vivo delivery of ES-derived beating myocardial tissues [156].
Decellularized matrix components have been utilized as tissue engineering scaffolds. These decellularized matrices are biodegradable and bioresorbable, and provide efficient scaffolds for cell seeding. Decellularized matrix components have also been shown to enhance matrix accumulation [157]. Recently, acellular tissue matrices or decellularized tissues were utilized to direct the differentiation of ES cells [158]. The individual matrix components as well as complex matrices influence differentiation of ES cells. Philp et al. demonstrated that ECM components can promote the differentiation of ES cells into cells and structures that are similar to the tissue from which the matrix is derived [158].
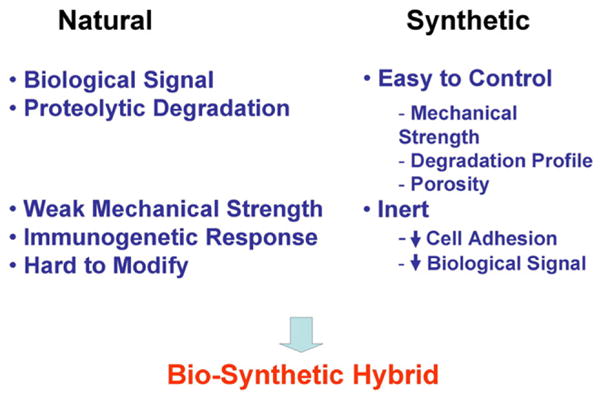
Overall, natural biomaterials may provide efficient adhesion sites for attachment and a wide range of biological signals. Even though these natural scaffolds have been utilized for differentiation and attachment of hESCs, use of naturally-derived biomaterials has been limited to in vitro differentiation application of ES cells due to their weak mechanical properties and regulatory/manufacturing difficulties.
3.2.3. Synthetic and biosynthetic biomaterials
Recent tissue engineering studies have indicated that 3-dimensional (3D) synthetic scaffolds are suitable for ESC-based tissue engineering applications [64,66,108,159,160]. As shown in Fig. 5, synthetic hydrogels such as poly(ethylene glycol) (PEG) can provide a 3D support to encapsulated EBDs undergoing chondrogenic differentiation [64]. Hydrogels have been extensively utilized as a three-dimensional support for stem cells. Hydrogels are 3D networks of hydrophilic polymers that imbibe a large quantity of water as well as biological fluids [161,162]. PEG-based hydrogels are ideal as tissue engineering scaffolds due to their high water content, elasticity, biocompatibility, and their ability to permit diffusion of nutrients and bioactive molecules [163,164]. PEG-based hydrogels, however, are bio-inert and do not interact with the cells and therefore various cell-interacting components need to be incorporated to improve their bioactivity (Fig. 6). These components include extracellular matrix molecules, small peptides, and glycoproteins [160–164]. In addition to allowing suitable mechanical properties to support tissue formation, synthetic scaffolds allow incorporation of biological signals mimicking, the natural ECMs for regulating complex morphogenetic processes in regeneration and tissue formation [135,160,166–169]. Synthetic scaffolds with bioactivity may provide physical cues for cell orientation and spreading, which are critical for hESC differentiation and tissue formation (Fig. 7). Biomaterials provide a three-dimensional environment and should ideally degrade as cells deposit extracellular matrix. A number of polymers have been microfabricated to develop bioactive, biodegradable, porous, mechanically supportive scaffolds for stem cell differentiation and tissue formation both in vitro and in vivo. One of the advantages of utilizing completely synthetic biomaterial is that their properties — mechanical strength, porosity, degradation profile, and biologically active sites —can be molecularly tailored. Most commonly used synthetic biomaterials are poly(ethylene glycol) (PEG), poly(vinyl alcohol) (PVA), poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA), poly(hydroxyl ethyl methacrylate) (PHEMA), and poly(anhydride). Novel synthetic biomaterials may allow hESC adhesion and guided differentiation toward a desired lineage. Anderson et al. demonstrated the use of array-based technology to study polymer–cell interactions [165]. Using a highly modified fluid handling system, they deposited in triplicate 576 different combinations of 25 different acrylate, diacrylate, dimethacrylate and triacrylate monomers with radical initiators onto a layer of poly(hydroxyethyl methacrylate) (pHEMA) and investigated hESC response to the synthetic polymers in terms of attachment, proliferation, and differentiation [170]. Such array-based technologies may enable the screening of biomaterials that can be used as a scaffold component to direct stem cell fate and differentiation commitment.
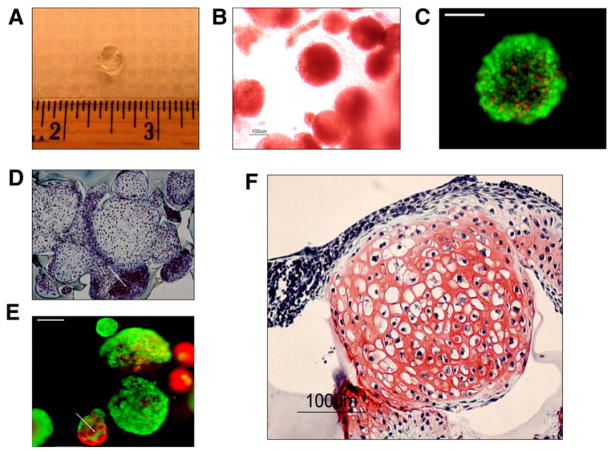
Fig. 5.
Gross image of an acellular poly(ethylene oxide)-diacrylate (PEGDA) photopolymerizing hydrogels (A). Inverted light microscopy image of embryoid bodies (EBs) in PEGDA hydrogel system immediately after photoencapsulation. EBs maintained their round morphologies as well as cell–cell contacts (dotted line) (B). Viability during photoencapsulation was assessed by live–dead assay immediately after encapsulation (C). Encapsulated EBs showed heterogeneous nature as noncartilaginous EBs demonstrated little or no matrix around the cells (D). Smaller EBs often contained large numbers of dead cells, as evidenced by live–dead cell viability assay (E). Basophilic extracellular matrix (ECM) deposition characteristic of neocartilage was promoted by transforming growth factor (TGF)-β1 treatment (F).
Fig. 6.
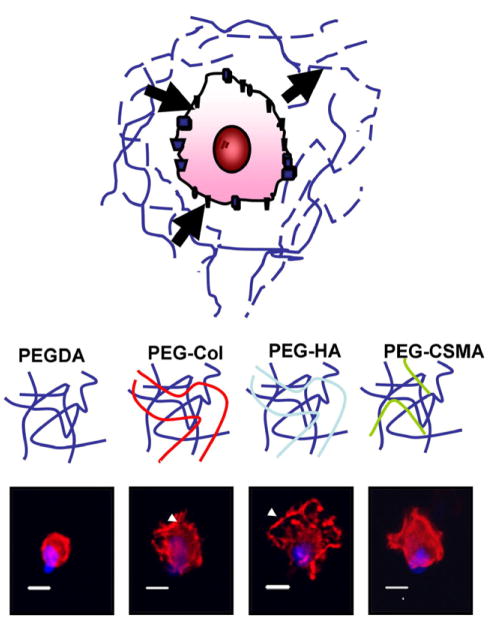
Bio-synthetic scaffolds combine suitable mechanical properties and biological signals that mimic the natural ECM for stem cell-based tissue engineering.
Fig. 7.
The extracellular microenvironment plays a significant role in controlling cellular behavior. PEG-based hydrogels can mimic natural ECMs both biochemically and biophysically by polymerizing the hydrogels with exogenous ECM components. Distinct cellular morphologies were induced by the various extracellular microenvironments. Actin: Phalloidin (Red), Nucleus: DAPI (Blue). Bar=10 μm.
Ideal scaffolds for stem cell differentiation should provide a microenvironment where adhesive moieties are expressed in a spatial and temporal manner to control cellular behaviors. Incorporation of cell adhesive ligands into biomaterials can mediate specific receptor–ligand interactions leading to activation of receptor-mediated signaling pathways to control cellular behavior and differentiation. Several cell adhesive ligands such as Arg-Gly-Asp (RGD), Tyr-Ile-Gly-Ser-Arg (YIGSR), or Ile-Lys-Val-Ala-Val (IKVAV) have been incorporated into PEG-based hydrogels to enhance the cell–matrix interactions [160,171]. For instance, the ability of RGD to enhance cell–matrix interaction resulted in enhanced chondrogenic differentiation of hESCs [160]. Recently, Yang et al. found that there is an optimal RGD concentration to promote osteogenic differentiation [164]. Other than cell adhesive ligands that may directly interact with cell membrane receptors, biological domains that can interact with secreted ECM components have been attached to improve the bioactivity of PEG-based hydrogels. Synthetic collagen mimetic peptide (CMP) was integrated into PEG to interact with cell-secreted collagen to create a microenvironment reminiscent of native tissue. These peptides may also interact with various cell-secreted morphogenetic and growth factors.
The mechanisms of ECM remodeling are an important aspect of tissue morphogenesis since degradation rate affects the ECM production. The degradation pattern of synthetic scaffolds can be tuned by varying monomer properties, composition, and crosslinking density. Degradation in such synthetic scaffolds occurs mainly through hydrolysis and is not controlled by cellular activities. To this end, several groups have developed biomimetic scaffolds with enzyme-mediated degradation sites [135,172–174]. These biomimetic scaffolds generally contain enzyme sensitive peptide sequences within the scaffold structure that are responsive to MMP or other cell-mediated enzyme activities.
In addition to hydrogels, porous biodegradable polymer scaffolds have also been used to support ES cells. Levenberg et al. generated complex tissues from embryonic cells utilizing PLLA/PLGA polymer scaffolds [159]. Recently, we utilized hydroxyapatite-doped PLLA/PLGA sponges as scaffolds for bone tissue engineering using human embryonic stem cells [unpublished data]. Our preliminary data indicate that the mechanism of endochondral bone formation from mesenchymal precursor cells can be modulated by scaffold properties, thus demonstrating the importance of scaffold properties on the modulation of hESCs differentiation and tissue formation.
3.2.4. Nano-biomaterials
Natural ECM is constructed through self-assembly of many nanofibrillar proteins secreted by cells, e.g., collagen fibrils. The normal cell environment is comprised of a complex network of extracellular matrix molecules with nano–micro scale dimensions. Though the aforementioned biomimetic hydrogels and porous scaffolds have been fairly successful in providing 3D structural support to cells, they fail to mimic the spatial dimensions of the ECM. In addition, cellular phenotype and differentiation can be profoundly influenced by the diameter of fibrous scaffolds [175]. To this end, the latest efforts in scaffold research have focused on developing biomaterials with nano-structures [176].
Self-assembling amphilic peptides that form nanofibers have been utilized to control cellular activities such as cell adhesion, differentiation, and homing of other cells [177,178]. Self-assembly of these peptides can be controlled at physiological pH by altering salt concentration. Silva et al. demonstrated that neural progenitor cells can be selectively differentiated into neurons over astrocytes by laminin epitope IKVAV containing self-assembled nanofibers [179,180]. In another study, murine ES cells were encapsulated in Puramatrix™ for osteogenic differentiation [181].
In addition to self-assembly, nanofibers can be created through electrospinning. The advantage of electrospinning technique is its ability to produce continuous nano- or macro fibers with high surface area to volume ratio and an interconnected porous structure. Electro-spun nanofibers have been utilized for stem cell culture to enhance proliferation and self-renewal of stem cells [29]. Recently, nanotechnology has provided ways for functionalizing these nanofibers with bioactive factor (drugs, proteins, or nucleic acids), which may direct the differentiation of ESCs [182].
3.2.5. Micro/patterned biomaterials
During embryogenesis and tissue formation, cells are tightly regulated by their microenvironment, which are composed of surrounding cells, soluble factors, and ECM molecules. Cellular communication between cells and ECM occurs via variety of signaling pathways, which may result in paracrine and autocrine signaling, gap junction communication, and juxtacrine signaling. Understanding and interconnecting these complex networks of signaling pathways during differentiation may enable us to design appropriate biosynthetic microenvironment for efficient and reproducible differentiation protocols. Microfabrication technology has enabled us to selectively study different components of cell–cell and cell–matrix interactions, polarized cell adhesion, cell differentiation in response to surface texture, cell migration, mechanotransduction, and cell response to gradient effects of surface-bound ligands in a precisely controlled manner [183–185]. In addition, the development of bioMEMS devices comprised of microfluidics channels has been utilized for high-throughput analysis of number of different factors with minimal reagent consumption. Microfabricated platform can be used to monitor commitment and differentiated state of individual cells through patterning arrays of cells and tracking them via clonal analysis [186,187]. Microcontact printing is a popular and convenient technique where a pattern is transferred from an elastomeric stamp to a solid substrate by conformal contact. Through patterning the cells on geometrically define shapes; cell-shape dependent signaling pathways that may regulate the fate decision of individual cell can be achieved.
Effect of autocrine signaling mediated by proximity and cell–cell contact plays an important role in differentiation of stem cells. Novel methods of generating spatially oriented co-cultures have been developed using layer-by-layer deposition of bioactive molecules, and these systems may be useful to understand cell–cell contact-mediated commitment of ES cell differentiation [188]. Spatial and temporal distribution of signaling molecules is tightly controlled during morphogenesis and they may elicit unique responses to ES cell differentiation. In general, utilizing microfabrication technology to control of cell-microenvironment such as cell–cell, cell–matrix, and cell-soluble factors may provide powerful tools to investigate factors that regulate ES cell fate and commitment.
4. Conclusion
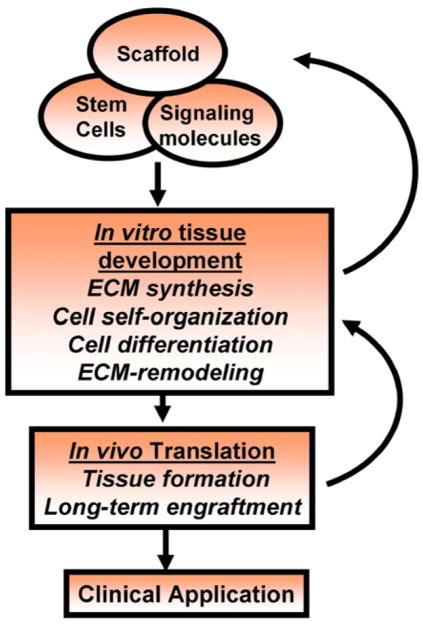
In summary, while hESCs still remain an ethically debatable source of cells, hESCs are potentially powerful tools to be used for therapeutic application of tissue regeneration. However, we have little understanding of the microenvironment specified molecular mechanisms and signaling pathways leading to efficient differentiation and tissue formation. A number of differentiation factors and biomaterials have been investigated to provide appropriate micro-environments for commitment and differentiation of stem cells. Given the complexity of stem cell fate control systems, much can still be learned through observations of in vitro high-throughput analysis of these factors (Fig. 8). As engineers lean more about how the microenvironment directs stem cell fate decisions, these factors can be incorporated into culture conditions to better control ESC growth and differentiation.
Fig. 8.
Tissue engineering scheme of utilizing stem cells. Basic building blocks of tissue engineering include scaffolds, bioactive factors, along with stem cells. Stem cell differentiation, cell-self organization, ECM synthesis, and remodeling would result tissues due to close interactions between the cells, scaffolds, and bioactive factors. However, long-term in vivo engraftment and tissue maintenance would be critical before applying the engineered tissues for clinical applications.
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “Emerging Trends in Cell-Based Therapeutics”.
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ, Blumenthal PD, Huggins GR, Gearhart JD. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci U S A. 1998;95:13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multi-lineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 6.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 7.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Cowan CA, Atienza J, Melton DA, Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309:1369–1373. doi: 10.1126/science.1116447. [DOI] [PubMed] [Google Scholar]
- 10.Tada M, Takahama Y, Abe K, Nakatsuji N, Tada T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr Biol. 2001;11:1553–1558. doi: 10.1016/s0960-9822(01)00459-6. [DOI] [PubMed] [Google Scholar]
- 11.Fehrer C, Lepperdinger G. Mesenchymal stem cell aging. Exp Gerontol. 2005;40:926–930. doi: 10.1016/j.exger.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Stolzing A, Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell. 2006;5:213–224. doi: 10.1111/j.1474-9726.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 13.Hegert C, Kramer J, Hargus G, Muller J, Guan K, Wobus AM, Muller PK, Rohwedel J. Differentiation plasticity of chondrocytes derived from mouse embryonic stem cells. J Cell Sci. 2002;115:4617–4628. doi: 10.1242/jcs.00171. [DOI] [PubMed] [Google Scholar]
- 14.Nakayama N, Duryea D, Manoukian R, Chow G, Han CY. Macroscopic cartilage formation with embryonic stem-cell-derived mesodermal progenitor cells. J Cell Sci. 2003;116:2015–2028. doi: 10.1242/jcs.00417. [DOI] [PubMed] [Google Scholar]
- 15.Barberi T, Studer L. Mesenchymal cells. Methods Enzymol. 2006;418:194–208. doi: 10.1016/S0076-6879(06)18012-X. [DOI] [PubMed] [Google Scholar]
- 16.Barberi T, Willis LM, Socci ND, Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2:e161. doi: 10.1371/journal.pmed.0020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishikawa S, Jakt LM, Era T. Embryonic stem-cell culture as a tool for developmental cell biology. Nat Rev Mol Cell Biol. 2007;8:502–507. doi: 10.1038/nrm2189. [DOI] [PubMed] [Google Scholar]
- 18.Jakobsson L, Kreuger J, Claesson-Welsh L. Building blood vessels—stem cell models in vascular biology. J Cell Biol. 2007;177:751–755. doi: 10.1083/jcb.200701146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menendez P, Bueno C, Wang L. Human embryonic stem cells: a journey beyond cell replacement therapies. Cytotherapy. 2006;8:530–541. doi: 10.1080/14653240601026654. [DOI] [PubMed] [Google Scholar]
- 21.Rohwedel J, Guan K, Hegert C, Wobus AM. Embryonic stem cells as an in vitro model for mutagenicity, cytotoxicity and embryotoxicity studies: present state and future prospects. Toxicol In Vitro. 2001;15:741–753. doi: 10.1016/s0887-2333(01)00074-1. [DOI] [PubMed] [Google Scholar]
- 22.Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 23.Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 24.Amit M, Margulets V, Segev H, Shariki K, Laevsky I, Coleman R, Itskovitz-Eldor J. Human feeder layers for human embryonic stem cells. Biol Reprod. 2003;68:2150–2156. doi: 10.1095/biolreprod.102.012583. [DOI] [PubMed] [Google Scholar]
- 25.Hovatta O, Mikkola M, Gertow K, Stromberg AM, Inzunza J, Hreinsson J, Rozell B, Blennow E, Andang M, Ahrlund-Richter L. A culture system using human foreskin fibroblasts as feeder cells allows production of human embryonic stem cells. Hum Reprod. 2003;18:1404–1409. doi: 10.1093/humrep/deg290. [DOI] [PubMed] [Google Scholar]
- 26.Cheng L, Hammond H, Ye Z, Zhan X, Dravid G. Human adult marrow cells support prolonged expansion of human embryonic stem cells in culture. Stem Cells. 2003;21:131–142. doi: 10.1634/stemcells.21-2-131. [DOI] [PubMed] [Google Scholar]
- 27.Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20:933–936. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- 28.Harrison J, Pattanawong S, Forsythe JS, Gross KA, Nisbet DR, Beh H, Scott TF, Trounson AO, Mollard R. Colonization and maintenance of murine embryonic stem cells on poly(alpha-hydroxy esters) Biomaterials. 2004;25:4963–4970. doi: 10.1016/j.biomaterials.2004.01.054. [DOI] [PubMed] [Google Scholar]
- 29.Nur EKA, Ahmed I, Kamal J, Schindler M, Meiners S. Three-dimensional nanofibrillar surfaces promote self-renewal in mouse embryonic stem cells. Stem Cells. 2006;24:426–4233. doi: 10.1634/stemcells.2005-0170. [DOI] [PubMed] [Google Scholar]
- 30.Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 31.Mohr JC, de Pablo JJ, Palecek SP. 3-D microwell culture of human embryonic stem cells. Biomaterials. 2006;27:6032–6042. doi: 10.1016/j.biomaterials.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Beattie GM, Lopez AD, Bucay N, Hinton A, Firpo MT, King CC, Hayek A. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2005;23:489–495. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
- 33.Amit M, Shariki C, Margulets V, Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol Reprod. 2004;70:837–845. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- 34.Yao S, Chen S, Clark J, Hao E, Beattie GM, Hayek A, Ding S. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A. 2006;103:6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ilic D. Culture of human embryonic stem cells and the extracellular matrix microenvironment. Regen Med. 2006;1:95–101. doi: 10.2217/17460751.1.1.95. [DOI] [PubMed] [Google Scholar]
- 36.Li YJ, Chung EH, Rodriguez RT, Firpo MT, Healy KE. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J Biomed Mater Res A. 2006;79:1–5. doi: 10.1002/jbm.a.30732. [DOI] [PubMed] [Google Scholar]
- 37.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:11298–11303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, Crandall LJ, Daigh CA, Conard KR, Piekarczyk MS, Llanas RA, Thomson JA. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 39.Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 40.Chen S, Do JT, Zhang Q, Yao S, Yan F, Peters EC, Scholer HR, Schultz PG, Ding S. Self-renewal of embryonic stem cells by a small molecule. Proc Natl Acad Sci U S A. 2006;103:17266–17271. doi: 10.1073/pnas.0608156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthr Cartil. 2006;14:179–189. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Weitzer G. Embryonic stem cell-derived embryoid bodies: an in vitro model of eutherian pregastrulation development and early gastrulation. Handb Exp Pharmacol. 2006:21–51. [PubMed] [Google Scholar]
- 43.Kurosawa H. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103:389–398. doi: 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- 44.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- 45.Hopfl G, Gassmann M, Desbaillets I. Differentiating embryonic stem cells into embryoid bodies. Methods Mol Biol. 2004;254:79–98. doi: 10.1385/1-59259-741-6:079. [DOI] [PubMed] [Google Scholar]
- 46.Desbaillets I, Ziegler U, Groscurth P, Gassmann M. Embryoid bodies: an in vitro model of mouse embryogenesis. Exp Physiol. 2000;85:645–651. [PubMed] [Google Scholar]
- 47.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 48.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 49.Dang SM, Kyba M, Perlingeiro R, Daley GQ, Zandstra PW. Efficiency of embryoid body formation and hematopoietic development from embryonic stem cells in different culture systems. Biotechnol Bioeng. 2002;78:442–453. doi: 10.1002/bit.10220. [DOI] [PubMed] [Google Scholar]
- 50.Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13:473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hescheler J, Fleischmann BK, Lentini S, Maltsev VA, Rohwedel J, Wobus AM, Addicks K. Embryonic stem cells: a model to study structural and functional properties in cardiomyogenesis. Cardiovasc Res. 1997;36:149–162. doi: 10.1016/s0008-6363(97)00193-4. [DOI] [PubMed] [Google Scholar]
- 52.Carpenedo RL, Sargent CY, McDevitt TC. Rotary suspension culture enhances the efficiency, yield and homogeneity of embryoid body differentiation. Stem Cells. 2007;9:2224–2234. doi: 10.1634/stemcells.2006-0523. [DOI] [PubMed] [Google Scholar]
- 53.Cameron CM, Hu WS, Kaufman DS. Improved development of human embryonic stem cell-derived embryoid bodies by stirred vessel cultivation. Biotechnol Bioeng. 2006;94:938–948. doi: 10.1002/bit.20919. [DOI] [PubMed] [Google Scholar]
- 54.Dang SM, Zandstra PW. Scalable production of embryonic stem cell-derived cells. Methods Mol Biol. 2005;290:353–364. doi: 10.1385/1-59259-838-2:353. [DOI] [PubMed] [Google Scholar]
- 55.Khademhosseini A, Ferreira L, Blumling J, III, Yeh J, Karp JM, Fukuda J, Langer R. Co-culture of human embryonic stem cells with murine embryonic fibroblasts on microwell-patterned substrates. Biomaterials. 2006;27:5968–5977. doi: 10.1016/j.biomaterials.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 56.Wartenberg M, Gunther J, Hescheler J, Sauer H. The embryoid body as a novel in vitro assay system for antiangiogenic agents. Lab Invest. 1998;78:1301–1314. [PubMed] [Google Scholar]
- 57.Kurosawa H, Imamura T, Koike M, Sasaki K, Amano Y. A simple method for forming embryoid body from mouse embryonic stem cells. J Biosci Bioeng. 2003;96:409–411. doi: 10.1016/S1389-1723(03)90148-4. [DOI] [PubMed] [Google Scholar]
- 58.Koike M, Kurosawa H, Amano Y. A round-bottom 96-well polystyrene plate coated with 2-methacryloyloxyethyl phosphorylcholine as an effective tool for embryoid body formation. Cytotechnology. 2005;47:3–10. doi: 10.1007/s10616-005-3743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dang SM, Gerecht-Nir S, Chen J, Itskovitz-Eldor J, Zandstra PW. Controlled, scalable embryonic stem cell differentiation culture. Stem Cells. 2004;22:275–282. doi: 10.1634/stemcells.22-3-275. [DOI] [PubMed] [Google Scholar]
- 60.Schroeder M, Niebruegge S, Werner A, Willbold E, Burg M, Ruediger M, Field LJ, Lehmann J, Zweigerdt R. Differentiation and lineage selection of mouse embryonic stem cells in a stirred bench scale bioreactor with automated process control. Biotechnol Bioeng. 2005;92:920–933. doi: 10.1002/bit.20668. [DOI] [PubMed] [Google Scholar]
- 61.Karp JM, Yeh J, Eng G, Fukuda J, Blumling J, Suh KY, Cheng J, Mahdavi A, Borenstein J, Langer R, Khademhosseini A. Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab Chip. 2007;7:786–794. doi: 10.1039/b705085m. [DOI] [PubMed] [Google Scholar]
- 62.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka H, Murphy CL, Murphy C, Kimura M, Kawai S, Polak JM. Chondrogenic differentiation of murine embryonic stem cells: effects of culture conditions and dexamethasone. J Cell Biochem. 2004;93:454–462. doi: 10.1002/jcb.20171. [DOI] [PubMed] [Google Scholar]
- 64.Hwang NS, Kim MS, Sampattavanich S, Baek JH, Zhang Z, Elisseeff J. Effects of three-dimensional culture and growth factors on the chondrogenic differentiation of murine embryonic stem cells. Stem Cells. 2006;24:284–291. doi: 10.1634/stemcells.2005-0024. [DOI] [PubMed] [Google Scholar]
- 65.Kim MS, Hwang NS, Lee J, Kim TK, Leong K, Shamblott MJ, Gearhart J, Elisseeff J. Musculoskeletal differentiation of cells derived from human embryonic germ cells. Stem Cells. 2005;23:113–123. doi: 10.1634/stemcells.2004-0110. [DOI] [PubMed] [Google Scholar]
- 66.Liu H, Roy K. Biomimetic three-dimensional cultures significantly increase hematopoietic differentiation efficacy of embryonic stem cells. Tissue Eng. 2005;11:319–330. doi: 10.1089/ten.2005.11.319. [DOI] [PubMed] [Google Scholar]
- 67.Liu H, Lin J, Roy K. Effect of 3D scaffold and dynamic culture condition on the global gene expression profile of mouse embryonic stem cells. Biomaterials. 2006;27:5978–5989. doi: 10.1016/j.biomaterials.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Yates F, Naveiras O, Ernst P, Daley GQ. Embryonic stem cell-derived hematopoietic stem cells. Proc Natl Acad Sci U S A. 2005;102:19081–19086. doi: 10.1073/pnas.0506127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma F, Wang D, Hanada S, Ebihara Y, Kawasaki H, Zaike Y, Heike T, Nakahata T, Tsuji K. Novel method for efficient production of multipotential hematopoietic progenitors from human embryonic stem cells. Int J Hematol. 2007;85:371–379. doi: 10.1532/IJH97.06203. [DOI] [PubMed] [Google Scholar]
- 70.Baba S, Heike T, Yoshimoto M, Umeda K, Doi H, Iwasa T, Lin X, Matsuoka S, Komeda M, Nakahata T. Flk1(+) cardiac stem/progenitor cells derived from embryonic stem cells improve cardiac function in a dilated cardiomyopathy mouse model. Cardiovasc Res. 2007;76:119–131. doi: 10.1016/j.cardiores.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 71.Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schuldiner M, Eiges R, Eden A, Yanuka O, Itskovitz-Eldor J, Goldstein RS, Benvenisty N. Induced neuronal differentiation of human embryonic stem cells. Brain Res. 2001;913:201–205. doi: 10.1016/s0006-8993(01)02776-7. [DOI] [PubMed] [Google Scholar]
- 73.Carpenter MK, Inokuma MS, Denham J, Mujtaba T, Chiu CP, Rao MS. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 2001;172:383–397. doi: 10.1006/exnr.2001.7832. [DOI] [PubMed] [Google Scholar]
- 74.Brokhman I, GamarnikZiegler L, Pomp O, Aharonowiz M, Reubinoff BE, Goldstein RS. Peripheral sensory neurons differentiate from neural precursors derived from human embryonic stem cells. Differentiation. doi: 10.1111/j.1432-0436.2007.00196.x. In Press. [DOI] [PubMed] [Google Scholar]
- 75.Lu SJ, Feng Q, Caballero S, Chen Y, Moore MA, Grant MB, Lanza R. Generation of functional hemangioblasts from human embryonic stem cells. Nat Methods. 2007;4:501–509. doi: 10.1038/nmeth1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takashima Y, Era T, Nakao K, Kondo S, Kasuga M, Smith AG, Nishikawa SI. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007;129:1377–1388. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 77.Olivier EN, Rybicki AC, Bouhassira EE. Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem Cells. 2006;24:1914–1922. doi: 10.1634/stemcells.2005-0648. [DOI] [PubMed] [Google Scholar]
- 78.Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642–648. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- 79.Lian Q, Lye E, Suan Yeo K, Khia Way Tan E, Salto-Tellez M, Liu TM, Palanisamy N, El Oakley RM, Lee EH, Lim B, Lim SK. Derivation of clinically compliant MSCs from CD105+, CD24-differentiated human ESCs. Stem Cells. 2007;25:425–436. doi: 10.1634/stemcells.2006-0420. [DOI] [PubMed] [Google Scholar]
- 80.Jo YY, Lee HJ, Kook SY, Choung HW, Park JY, Chung JH, Choung YH, Kim ES, Yang HC, Choung PH. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13:767–773. doi: 10.1089/ten.2006.0192. [DOI] [PubMed] [Google Scholar]
- 81.Guilak F, Awad HA, Fermor B, Leddy HA, Gimble JM. Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology. 2004;41:389–399. [PubMed] [Google Scholar]
- 82.Lee MW, Yang MS, Park JS, Kim HC, Kim YJ, Choi J. Isolation of mesenchymal stem cells from cryopreserved human umbilical cord blood. Int J Hematol. 2005;81:126–130. doi: 10.1532/ijh97.a10404. [DOI] [PubMed] [Google Scholar]
- 83.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharma B, Elisseeff JH. Engineering structurally organized cartilage and bone tissues. Ann Biomed Eng. 2004;32:148–159. doi: 10.1023/b:abme.0000007799.60142.78. [DOI] [PubMed] [Google Scholar]
- 85.Elisseeff J, Puleo C, Yang F, Sharma B. Advances in skeletal tissue engineering with hydrogels. Orthod Craniofac Res. 2005;8:150–161. doi: 10.1111/j.1601-6343.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- 86.Islam MQ, Islam K, Sharp CA. Epigenetic reprogramming of nonreplicating somatic cells for long-term proliferation by temporary cell–cell contact. Stem Cells Dev. 2007;16:253–268. doi: 10.1089/scd.2006.0094. [DOI] [PubMed] [Google Scholar]
- 87.Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2000;97:11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 89.Cancedda R, Castagnola P, Cancedda FD, Dozin B, Quarto R. Developmental control of chondrogenesis and osteogenesis. Int J Dev Biol. 2000;44:707–714. [PubMed] [Google Scholar]
- 90.Han F, Gilbert JR, Harrison G, Adams CS, Freeman T, Tao Z, Zaka R, Liang H, Williams C, Tuan RS, Norton PA, Hickok NJ. Transforming growth factor-beta1 regulates fibronectin isoform expression and splicing factor SRp40 expression during ATDC5 chondrogenic maturation. Exp Cell Res. 2007;313:1518–1532. doi: 10.1016/j.yexcr.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pogue R, Lyons K. BMP signaling in the cartilage growth plate. Curr Top Dev Biol. 2006;76:1–48. doi: 10.1016/S0070-2153(06)76001-X. [DOI] [PubMed] [Google Scholar]
- 92.Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res. 2005;20:848–857. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 93.Fan VH, Tamama K, Au A, Littrell R, Richardson LB, Wright JW, Wells A, Griffith LG. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells. 2007;25:1241–1251. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- 94.Suzuki Y, Tanihara M, Suzuki K, Saitou A, Sufan W, Nishimura Y. Alginate hydrogel linked with synthetic oligopeptide derived from BMP-2 allows ectopic osteoinduction in vivo. J Biomed Mater Res. 2000;50:405–409. doi: 10.1002/(sici)1097-4636(20000605)50:3<405::aid-jbm15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 95.Kaigler D, Wang Z, Horger K, Mooney DJ, Krebsbach PH. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J Bone Miner Res. 2006;21:735–744. doi: 10.1359/jbmr.060120. [DOI] [PubMed] [Google Scholar]
- 96.Huang YC, Simmons C, Kaigler D, Rice KG, Mooney DJ. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4) Gene Ther. 2005;12:418–426. doi: 10.1038/sj.gt.3302439. [DOI] [PubMed] [Google Scholar]
- 97.Murphy WL, Peters MC, Kohn DH, Mooney DJ. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21:2521–2527. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 98.Gerstenfeld LC, Barnes GL, Shea CM, Einhorn TA. Osteogenic differentiation is selectively promoted by morphogenetic signals from chondrocytes and synergized by a nutrient rich growth environment. Connect Tissue Res. 2003;44:85–91. [PubMed] [Google Scholar]
- 99.Gerstenfeld LC, Cruceta J, Shea CM, Sampath K, Barnes GL, Einhorn TA. Chondrocytes provide morphogenic signals that selectively induce osteogenic differentiation of mesenchymal stem cells. J Bone Miner Res. 2002;17:221–230. doi: 10.1359/jbmr.2002.17.2.221. [DOI] [PubMed] [Google Scholar]
- 100.Solursh M, Reiter RS. The enhancement of in vitro survival and chondrogenesis of limb bud cells by cartilage conditioned medium. Dev Biol. 1975;44:278–287. doi: 10.1016/0012-1606(75)90398-x. [DOI] [PubMed] [Google Scholar]
- 101.Hwang NS, Varghese S, Puleo C, Zhang Z, Elisseeff J. Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. J Cell Physiol. 2007;212:281–284. doi: 10.1002/jcp.21052. [DOI] [PubMed] [Google Scholar]
- 102.Vats A, Bielby RC, Tolley N, Dickinson SC, Boccaccini AR, Hollander AP, Bishop AE, Polak JM. Chondrogenic differentiation of human embryonic stem cells: the effect of the micro-environment. Tissue Eng. 2006;12:1687–1697. doi: 10.1089/ten.2006.12.1687. [DOI] [PubMed] [Google Scholar]
- 103.Wong M, Tuan RS. Interactive cellular modulation of chondrogenic differentiation in vitro by subpopulations of chick embryonic calvarial cells. Dev Biol. 1995;167:130–147. doi: 10.1006/dbio.1995.1012. [DOI] [PubMed] [Google Scholar]
- 104.Elder SH. Conditioned medium of mechanically compressed chick limb bud cells promotes chondrocyte differentiation. J Orthop Sci. 2002;7:538–543. doi: 10.1007/s007760200096. [DOI] [PubMed] [Google Scholar]
- 105.Di Nino DL, Long F, Linsenmayer TF. Regulation of endochondral cartilage growth in the developing avian limb: cooperative involvement of perichondrium and periosteum. Dev Biol. 2001;240:433–442. doi: 10.1006/dbio.2001.0471. [DOI] [PubMed] [Google Scholar]
- 106.Zhang JQ, Yu XB, Ma BF, Yu WH, Zhang AX, Huang G, Mao GFF, Zhang XM, Wang ZC, Li SN, Lahn BT, Xiang AP. Neural differentiation of embryonic stem cells induced by conditioned medium from neural stem cell. NeuroReport. 2006;17:981–986. doi: 10.1097/01.wnr.0000227977.60271.ca. [DOI] [PubMed] [Google Scholar]
- 107.Bentz K, Molcanyi M, Hess S, Schneider A, Hescheler J, Neugebauer E, Schaefer U. Neural differentiation of embryonic stem cells is induced by signalling from non-neural niche cells. Cell Physiol Biochem. 2006;18:275–286. doi: 10.1159/000097674. [DOI] [PubMed] [Google Scholar]
- 108.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA, Mulligan RC, D’Amore PA, Langer R. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 109.Sze SK, de Kleijn DP, Lai RC, Tan EK, Zhao H, Yeo KS, Low TY, Lian Q, Lee CN, Mitchell W, El Oakley RM, Lim SK. Elucidating the secretion proteome of human ESC-derived mesenchymal stem cells. Mol Cell Proteomics. 2007;10:1680–1689. doi: 10.1074/mcp.M600393-MCP200. [DOI] [PubMed] [Google Scholar]
- 110.Ding S, Schultz PG. A role for chemistry in stem cell biology. Nat Biotechnol. 2004;22:833–840. doi: 10.1038/nbt987. [DOI] [PubMed] [Google Scholar]
- 111.Ding S, Schultz PG. Small molecules and future regenerative medicine. Curr Top Med Chem. 2005;5:383–395. doi: 10.2174/1568026053828402. [DOI] [PubMed] [Google Scholar]
- 112.Ding S, Wu TY, Brinker A, Peters EC, Hur W, Gray NS, Schultz PG. Synthetic small molecules that control stem cell fate. Proc Natl Acad Sci U S A. 2003;100:7632–7637. doi: 10.1073/pnas.0732087100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee GS, Kochhar DM, Collins MD. Retinoid-induced limb malformations. Curr Pharm Des. 2004;10:2657–2699. doi: 10.2174/1381612043383728. [DOI] [PubMed] [Google Scholar]
- 114.Kawaguchi J, Mee PJ, Smith AG. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone. 2005;36:758–769. doi: 10.1016/j.bone.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 115.Sharma NS, Shikhanovich R, Schloss R, Yarmush ML. Sodium butyrate-treated embryonic stem cells yield hepatocyte-like cells expressing a glycolytic phenotype. Biotechnol Bioeng. 2006;94:1053–1063. doi: 10.1002/bit.20936. [DOI] [PubMed] [Google Scholar]
- 116.Wakita R, Izumi T, Itoman M. Thyroid hormone-induced chondrocyte terminal differentiation in rat femur organ culture. Cell Tissue Res. 1998;293:357–364. doi: 10.1007/s004410051127. [DOI] [PubMed] [Google Scholar]
- 117.Siebler T, Robson H, Shalet SM, Williams GR. Dexamethasone inhibits and thyroid hormone promotes differentiation of mouse chondrogenic ATDC5 cells. Bone. 2002;31:457–464. doi: 10.1016/s8756-3282(02)00855-4. [DOI] [PubMed] [Google Scholar]
- 118.Hwang NS, Varghese S, Theprungsirikul P, Canver A, Elisseeff J. Enhanced chondrogenic differentiation of murine embryonic stem cells in hydrogels with glucosamine. Biomaterials. 2006;27:6015–6023. doi: 10.1016/j.biomaterials.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 119.Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 120.Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 121.Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schmelter M, Ateghang B, Helmig S, Wartenberg M, Sauer H. Embryonic stem cells utilize reactive oxygen species as transducers of mechanical strain-induced cardiovascular differentiation. FASEB J. 2006;20:1182–1184. doi: 10.1096/fj.05-4723fje. [DOI] [PubMed] [Google Scholar]
- 124.Huang H, Nakayama Y, Qin K, Yamamoto K, Ando J, Yamashita J, Itoh H, Kanda K, Yaku H, Okamoto Y, Nemoto Y. Differentiation from embryonic stem cells to vascular wall cells under in vitro pulsatile flow loading. J Artif Organs. 2005;8:110–118. doi: 10.1007/s10047-005-0291-2. [DOI] [PubMed] [Google Scholar]
- 125.Riha GM, Wang X, Wang H, Chai H, Mu H, Lin PH, Lumsden AB, Yao Q, Chen C. Cyclic strain induces vascular smooth muscle cell differentiation from murine embryonic mesenchymal progenitor cells. Surgery. 2007;141:394–402. doi: 10.1016/j.surg.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 126.Saha S, Ji L, de Pablo JJ, Palecek SP. Inhibition of human embryonic stem cell differentiation by mechanical strain. J Cell Physiol. 2006;206:126–137. doi: 10.1002/jcp.20441. [DOI] [PubMed] [Google Scholar]
- 127.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 128.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 129.Kurpinski K, Chu J, Hashi C, Li S. Anisotropic mechanosensing by mesenchymal stem cells. Proc Natl Acad Sci U S A. 2006;103:16095–16100. doi: 10.1073/pnas.0604182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yamada M, Tanemura K, Okada S, Iwanami A, Nakamura M, Mizuno H, Ozawa M, Ohyama-Goto R, Kitamura N, Kawano M, Tan-Takeuchi K, Ohtsuka C, Miyawaki A, Takashima A, Ogawa M, Toyama Y, Okano H, Kondo T. Electrical stimulation modulates fate determination of differentiating embryonic stem cells. Stem Cells. 2007;25:562–570. doi: 10.1634/stemcells.2006-0011. [DOI] [PubMed] [Google Scholar]
- 131.Taqvi S, Roy R. Influence of scaffold physical properties and stromal cell coculture on hematopoietic differentiation of mouse embryonic stem cells. Biomaterials. 2006;27:6024–6031. doi: 10.1016/j.biomaterials.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 132.Ito Y. Surface micropatterning to regulate cell functions. Biomaterials. 1999;20:2333–2342. doi: 10.1016/s0142-9612(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 133.Keselowsky BG, Collard DM, Garcia AJ. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc Natl Acad Sci U S A. 2005;102:5953–5957. doi: 10.1073/pnas.0407356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Garcia AJ, Vega MD, Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell. 1999;10:785–798. doi: 10.1091/mbc.10.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shin H, Jo S, Mikos AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353–4364. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 136.Murphy WL, Hsiong S, Richardson TP, Simmons CA, Mooney DJ. Effects of a bone-like mineral film on phenotype of adult human mesenchymal stem cells in vitro. Biomaterials. 2005;26:303–310. doi: 10.1016/j.biomaterials.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 137.Murphy WL, Mooney DJ. Bioinspired growth of crystalline carbonate apatite on biodegradable polymer substrata. J Am Chem Soc. 2002;124:1910–1917. doi: 10.1021/ja012433n. [DOI] [PubMed] [Google Scholar]
- 138.Brodbeck WG, Patel J, Voskerician G, Christenson E, Shive MS, Nakayama Y, Matsuda T, Ziats NP, Anderson JM. Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vivo. Proc Natl Acad Sci U S A. 2002;99:10287–10292. doi: 10.1073/pnas.162124199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol. 2002;14:633–639. doi: 10.1016/s0955-0674(02)00364-2. [DOI] [PubMed] [Google Scholar]
- 140.Battista S, Guarnieri D, Borselli C, Zeppetelli S, Borzacchiello A, Mayol L, Gerbasio D, Keene DR, Ambrosio L, Netti PA. The effect of matrix composition of 3D constructs on embryonic stem cell differentiation. Biomaterials. 2005;26:6194–6207. doi: 10.1016/j.biomaterials.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 141.Streuli C. Extracellular matrix remodelling and cellular differentiation. Curr Opin Cell Biol. 1999;11:634–640. doi: 10.1016/s0955-0674(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 142.McCloskey KE, Gilroy ME, Nerem RM. Use of embryonic stem cell-derived endothelial cells as a cell source to generate vessel structures in vitro. Tissue Eng. 2005;11:497–505. doi: 10.1089/ten.2005.11.497. [DOI] [PubMed] [Google Scholar]
- 143.Lin L, Isacson O. Axonal growth regulation of fetal and embryonic stem cell-derived dopaminergic neurons by Netrin-1 and Slits. Stem Cells. 2006;24:2504–2513. doi: 10.1634/stemcells.2006-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ponticiello MS, Schinagl RM, Kadiyala S, Barry FP. Gelatin-based resorbable sponge as a carrier matrix for human mesenchymal stem cells in cartilage regeneration therapy. J Biomed Mater Res. 2000;52:246–255. doi: 10.1002/1097-4636(200011)52:2<246::aid-jbm2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 145.Tielens S, Declercq H, Gorski T, Lippens E, Schacht E, Cornelissen M. Gelatin-based microcarriers as embryonic stem cell delivery system in bone tissue engineering: an in-vitro study. Biomacromolecules. 2007;8:825–832. doi: 10.1021/bm060870u. [DOI] [PubMed] [Google Scholar]
- 146.Rosenthal A, Macdonald A, Voldman J. Cell patterning chip for controlling the stem cell microenvironment. Biomaterials. 2007;28:3208–3216. doi: 10.1016/j.biomaterials.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lisignoli G, Cristino S, Piacentini A, Toneguzzi S, Grassi F, Cavallo C, Zini N, Solimando L, Mario Maraldi N, Facchini A. Cellular and molecular events during chondrogenesis of human mesenchymal stromal cells grown in a three-dimensional hyaluronan based scaffold. Biomaterials. 2005;26:5677–5686. doi: 10.1016/j.biomaterials.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 148.Ventura C, Maioli M, Asara Y, Santoni D, Scarlata I, Cantoni S, Perbellini A. Butyric and retinoic mixed ester of hyaluronan. A novel differentiating glycoconjugate affording a high throughput of cardiogenesis in embryonic stem cells. J Biol Chem. 2004;279:23574–34579. doi: 10.1074/jbc.M401869200. [DOI] [PubMed] [Google Scholar]
- 149.Rhodes NP, Srivastava JK, Smith RF, Longinotti C. Metabolic and histological analysis of mesenchymal stem cells grown in 3-D hyaluronan-based scaffolds. J Mater Sci Mater Med. 2004;15:391–395. doi: 10.1023/b:jmsm.0000021108.74004.7e. [DOI] [PubMed] [Google Scholar]
- 150.Ruhnke M, Ungefroren H, Zehle G, Bader M, Kremer B, Fandrich F. Long-term culture and differentiation of rat embryonic stem cell-like cells into neuronal, glial, endothelial, and hepatic lineages. Stem Cells. 2003;21:428–436. doi: 10.1634/stemcells.21-4-428. [DOI] [PubMed] [Google Scholar]
- 151.Chen SS, Fitzgerald W, Zimmerberg J, Kleinman HK, Margolis L. Cell–cell and cell–extracellular matrix interactions regulate embryonic stem cell differentiation. Stem Cells. 2007;25:553–561. doi: 10.1634/stemcells.2006-0419. [DOI] [PubMed] [Google Scholar]
- 152.Willenberg BJ, Hamazaki T, Meng FW, Terada N, Batich C. Self-assembled copper-capillary alginate gel scaffolds with oligochitosan support embryonic stem cell growth. J Biomed Mater Res A. 2006;79:440–450. doi: 10.1002/jbm.a.30942. [DOI] [PubMed] [Google Scholar]
- 153.Maguire T, Davidovich AE, Wallenstein EJ, Novik E, Sharma N, Pedersen H, Androulakis IP, Schloss R, Yarmush M. Control of hepatic differentiation via cellular aggregation in an alginate microenvironment. Biotechnol Bioeng. 2007;98:631–644. doi: 10.1002/bit.21435. [DOI] [PubMed] [Google Scholar]
- 154.Maguire T, Novik TE, Schloss R, Yarmush M. Alginate-PLL microencapsulation: effect on the differentiation of embryonic stem cells into hepatocytes. Biotechnol Bioeng. 2006;93:581–591. doi: 10.1002/bit.20748. [DOI] [PubMed] [Google Scholar]
- 155.Gerecht-Nir S, Cohen S, Ziskind A, Itskovitz-Eldor J. Three-dimensional porous alginate scaffolds provide a conducive environment for generation of well-vascularized embryoid bodies from human embryonic stem cells. Biotechnol Bioeng. 2004;88:313–320. doi: 10.1002/bit.20248. [DOI] [PubMed] [Google Scholar]
- 156.Leor J, Gerecht-Nir S, Cohen S, Miller L, Holbova R, Ziskind A, Shachar M, Feinberg MS, Guetta E, Itskovitz-Eldor J. Human embryonic stem cell transplantation to repair the infarcted myocardium. Heart. 2007;93:1278–1284. doi: 10.1136/hrt.2006.093161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Datta N, Pham QP, Sharma U, Sikavitsas VI, Jansen JA, Mikos AG. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci U S A. 2006;103:2488–2493. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Philp D, Chen SS, Fitzgerald W, Orenstein J, Margolis L, Kleinman HK. Complex extracellular matrices promote tissue-specific stem cell differentiation. Stem Cells. 2005;23:288–296. doi: 10.1634/stemcells.2002-0109. [DOI] [PubMed] [Google Scholar]
- 159.Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci U S A. 2003;100:12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hwang NS, Varghese S, Zhang Z, Elisseeff J. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng. 2006;12:2695–2706. doi: 10.1089/ten.2006.12.2695. [DOI] [PubMed] [Google Scholar]
- 161.Alsberg E, Anderson KW, Albeiruti A, Rowley JA, Mooney DJ. Engineering growing tissues. Proc Natl Acad Sci U S A. 2002;99:12025–12030. doi: 10.1073/pnas.192291499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315–4323. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 163.Hubbell JA. Biomaterials in tissue engineering. Biotechnology (N Y) 1995;13:565–576. doi: 10.1038/nbt0695-565. [DOI] [PubMed] [Google Scholar]
- 164.Yang F, Williams CG, Wang DA, Lee H, Manson PN, Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26:5991–5998. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 165.Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat Biotechnol. 2004;22:863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 166.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 167.Varghese S, Elisseeff JH. Hydrogels for musculoskeletal tissue engineering. Polym Regen Med. 2006:95–144. [Google Scholar]
- 168.Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51:164–171. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 169.Bryant SJ, Bender RJ, Durand KL, Anseth KS. Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnol Bioeng. 2004;86:747–755. doi: 10.1002/bit.20160. [DOI] [PubMed] [Google Scholar]
- 170.Lee HJ, Lee JS, Chansakul T, Yu C, Elisseeff JH, Yu SM. Collagen mimetic peptide-conjugated photopolymerizable PEG hydrogel. Biomaterials. 2006;27:5268–5276. doi: 10.1016/j.biomaterials.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 171.Weber LM, Hayda KN, Haskins K, Anseth KS. The effects of cell–matrix interactions on encapsulated beta-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials. 2007;28:3004–3011. doi: 10.1016/j.biomaterials.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 172.Lutolf MP, Raeber GP, Zisch AH, Tirelli N, Hubbell JA. Cell-responsive synthetic hydrogels. Adv Mater. 2003;15:888–892. [Google Scholar]
- 173.Raeber GP, Lutolf MP, Hubbell JA. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys J. 2005;89:1374–1388. doi: 10.1529/biophysj.104.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, Hubbell JA. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21:513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 175.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 176.Yim EK, Leong KW. Significance of synthetic nanostructures in dictating cellular response. Nano Med. 2005;1:10–21. doi: 10.1016/j.nano.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 177.Zhao X, Zhang S. Molecular designer self-assembling peptides. Chem Soc Rev. 2006;35:1105–1110. doi: 10.1039/b511336a. [DOI] [PubMed] [Google Scholar]
- 178.Rajagopal K, Schneider JP. Self-assembling peptides and proteins for nanotechnological applications. Curr Opin Struct Biol. 2004;14:480–486. doi: 10.1016/j.sbi.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 179.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 180.Niece KL, Hartgerink JD, Donners JJ, Stupp SI. Self-assembly combining two bioactive peptide-amphiphile molecules into nanofibers by electrostatic attraction. J Am Chem Soc. 2003;125:7146–7147. doi: 10.1021/ja028215r. [DOI] [PubMed] [Google Scholar]
- 181.Garreta E, Genove E, Borros S, Semino CE. Osteogenic differentiation of mouse embryonic stem cells and mouse embryonic fibroblasts in a three-dimensional self-assembling peptide scaffold. Tissue Eng. 2006;12:2215–2227. doi: 10.1089/ten.2006.12.2215. [DOI] [PubMed] [Google Scholar]
- 182.Garreta E, Gasset D, Semino C, Borros S. Fabrication of a three-dimensional nanostructured biomaterial for tissue engineering of bone. Biomol Eng. 2007;24:75–80. doi: 10.1016/j.bioeng.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 183.Pirone DM, Chen CS. Strategies for engineering the adhesive microenvironment. J Mammary Gland Biol Neoplasia. 2004;9:405–417. doi: 10.1007/s10911-004-1410-z. [DOI] [PubMed] [Google Scholar]
- 184.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Park TH, Shuler ML. Integration of cell culture and microfabrication technology. Biotechnol Prog. 2003;19:243–253. doi: 10.1021/bp020143k. [DOI] [PubMed] [Google Scholar]
- 186.Tourovskaia A, Figueroa-Masot X, Folch A. Differentiation-on-a-chip: a microfluidic platform for long-term cell culture studies. Lab Chip. 2005;5:14–19. doi: 10.1039/b405719h. [DOI] [PubMed] [Google Scholar]
- 187.Chin VI, Taupin P, Sanga S, Scheel J, Gage FH, Bhatia SN. Microfabricated platform for studying stem cell fates. Biotechnol Bioeng. 2004;88:399–415. doi: 10.1002/bit.20254. [DOI] [PubMed] [Google Scholar]
- 188.Fukuda J, Khademhosseini A, Yeh J, Eng G, Cheng J, Farokhzad OC, Langer R. Micropatterned cell co-cultures using layer-by-layer deposition of extracellular matrix components. Biomaterials. 2006;27:1479–1486. doi: 10.1016/j.biomaterials.2005.09.015. [DOI] [PubMed] [Google Scholar]