Abstract
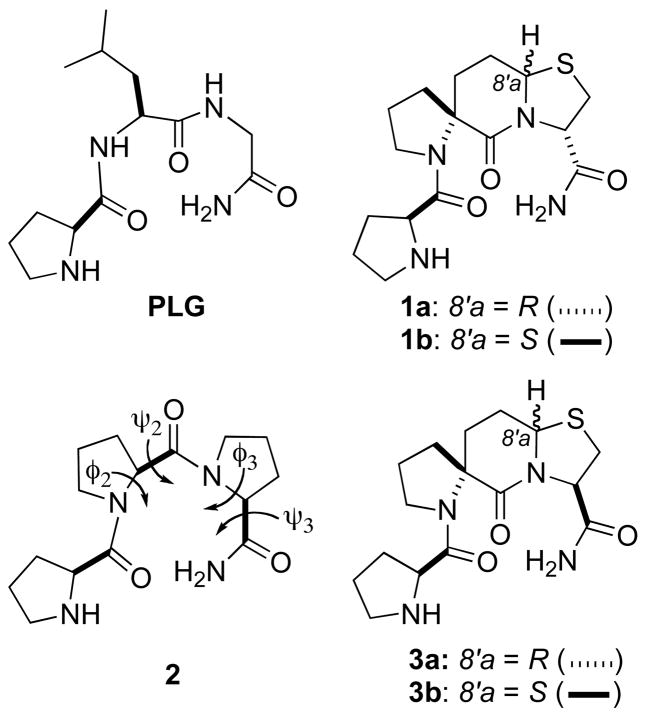
Type II β-turn mimics and polyproline II helix mimics based upon diastereoisomeric 5.6.5 spiro bicyclic scaffolds have provided two pairs of Pro-Leu-Gly-NH2 (PLG) peptidomimetics with contrasting dopamine receptor modulating activities. Compounds 1a and 3a were found to be positive allosteric modulators of the dopamine receptor, while the corresponding diastereoisomeric compounds 1b and 3b provided the first PLG peptidomimetics with the ability to decrease the binding of agonists to the dopamine receptor. The positive allosteric modulating activity of 3a supported the hypothesis that a polyproline II helix conformation is the bioactive conformation for the PLG analogue Pro-Pro-Pro-NH2. The results also show that a change in the bridgehead chirality of the 5.6.5 scaffold brings about opposite effects in terms of the modulation of the dopamine receptor.
Introduction
L-Prolyl-L-leucyl-glycinamide (PLGa), an endogenous neuropeptide, allosterically modulates the dopamine D2 and D4 receptor subtypes.1–3 PLG increases the affinity of the high-affinity state of the dopamine receptor for agonists and it increases the ratio of the dopamine receptor in the high-affinity state, which is coupled to G-proteins.1,4 PLG also prevents and reverses drug-induced dopamine receptor super sensitivity caused by dopamine receptor antagonists.5–7
Structure activity studies wherein the φ and ψ angles of PLG are constrained suggest that the bioactive conformation of PLG is a type II β-turn.8,9 Peptidomimetic 1a (Figure 1), which possesses the highly rigid 5.6.5 spiro bicyclic lactam type II β-turn mimic, was found to be significantly more potent than PLG in the 3[H]spiroperidol/N-propylnorapomorphine (NPA) competition binding assay and in the 6-hydroxydopamine lesioned model of Parkinson’s disease.9 This PLG peptidomimetic also increased, to a more significant degree than PLG, the ratio of dopamine receptors in the high-affinity state. The results with this highly constrained PLG peptidomimetic provided strong support for the hypothesis that the bioactive conformation of PLG is a type II β-turn.
Figure 1.
Structures of Pro-Leu-Gly-NH2 (PLG), Pro-Pro-Pro-NH2 (2), and PLG peptidomimetics with 5.6.5 spiro bicyclic scaffolds designed to mimic either a type II β-turn (1a and 1b) or a polyproline II helix (3a and 3b).
Interestingly, the triproline compound Pro-Pro-Pro-NH2 (2, Figure 1), also showed activity in increasing apomorphine induced rotations in the 6-hydroxydopamine lesioned rat and in increasing the ratio of dopamine receptors in the high affinity state.10 Since 2 cannot exist as a type II β-turn, its activity either calls into question the hypothesis that the bioactive conformation of PLG is a type II β-turn or it implies that 2 can assume a different conformation that still places the important pharmacophore groups in relatively the same topographical space as do the PLG peptidomimetics designed to mimic a type II β-turn. In this paper, we report the synthesis of Pro-Pro-Pro-NH2 peptidomimetics 3a and 3b (Figure 1) possessing the highly constrained 5.6.5 spiro bicyclic lactam scaffold designed to mimic a polyproline II helix. This gave a pair of compounds with the ability to either enhance or decrease the binding of the agonist NPA to dopamine receptors depending upon the chirality of the scaffold bridgehead carbon. In light of the differential activities of 3a and 3b, the bridgehead epimer of 1a, 1b (Figure 1), was also synthesized and evaluated.
Design Rationale
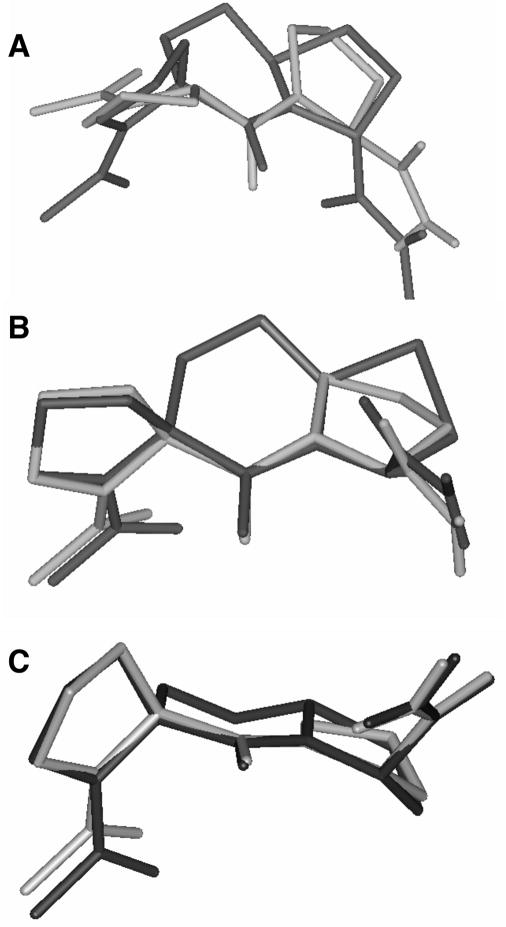
It is well established that a string of L-prolines in a polypeptide can assume either a polyproline I (PPI)- or a polyproline II (PPII) helix.11 In a PPI helix conformation, the Pro-Pro amide bonds are all-cis, whereas in the more predominant PPII helix conformation all-trans amide bonds exist. The PPII helix is a left-handed helix with three residues per turn. An overlay of Ac-Pro-Pro-NH2 in a PPII helix conformation and the 5.6.5 type II β-turn spiro bicyclic scaffold of 1a (Figure 2A) shows that there is a good overlay of the backbone atoms and that the carboxamide NH2 moieties of both project in the same relative area of space. However, the two carbonyl groups of the carboxamide moieties are oriented in opposite directions. If one compares the torsion angles of a PPII helix with those of the 5.6.5 spiro bicyclic scaffold of 1a (Table 1) one sees that the φ2 and ψ2 angles are similar between the two, while the respective φ3 angles have about the same magnitude, but are opposite in sign. Thus, a molecule with the opposite stereochemistry at the spiro bicyclic scaffold carbon possessing the carboxamide moiety would give a PPII helix mimic. This is illustrated by the overlays of Ac-Pro-Pro-NH2 in a PPII helix conformation with the 5.6.5 spiro bicyclic frameworks of the proposed polyproline II helix PLG peptidomimetics 3a and 3b (Figure 2B and 2C). Previously, Witter et al.12 reported on the use of the spiro bicyclic scaffold as a potential mimic of a polyproline II helix.
Figure 2.
Overlay of Ac-Pro-Pro-NH2 in a PPII conformation (grey) with: (A) the 5.6.5 spiro bicyclic scaffold of 1a (black); (B) the 5.6.5 spiro bicyclic scaffold of 3a (black); (C) the 5.6.5 spiro bicyclic scaffold of 3b (black).
Table 1.
Comparison of the torsion angles of 5.6.5 spiro bicyclic scaffolds with those of a polyproline II helix.
| System | φ2 | ψ2 | φ3 | ψ3 |
|---|---|---|---|---|
| Polyproline II helix | −80 | 155 | −80 | 155 |
| 1a Spiro bicyclic scaffolda | −56 | 133.7 | 97.3 | −19.9 |
| 1b Spiro bicyclic scaffoldb | −54 | 159 | 41 | −82 |
| 3a Spiro bicyclic scaffoldc | −48.5 | 136.2 | −64.3 | 160.5 |
| 3b Spiro bicyclic scaffoldd | −50.0 | 148.4 | −82.8 | 162.8 |
Data obtained from ref. 9 and is based upon a X-ray structure of this scaffold.
Values were calculated using the Amber89 force field in MOE to do the optimizations with 0.001 kcal/mol Angstrom as the gradient. The relatively large difference in φ3 between 1a and 1b is likely due to the difference in puckering of the thiazolidine ring brought about by the difference in bridgehead stereochemistry. The ψ3 torsion angle is not constrained in these scaffolds and thus is expected to vary.
Values are from the X-ray structure of the precursor to 3a, compound 12a.
Values are from the X-ray structure of the precursor to 3b, compound 12b.
Synthesis
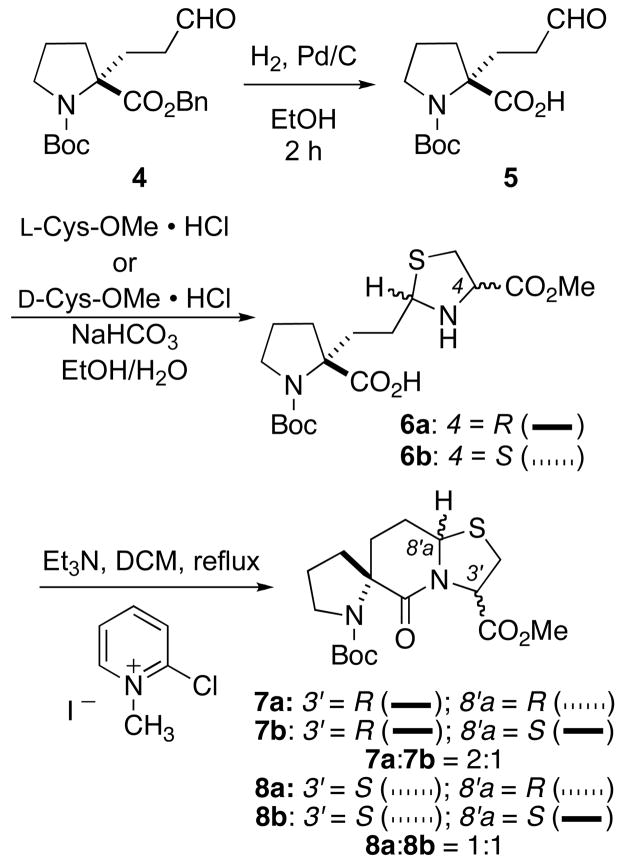
Proline aldehyde derivative 4 served as the starting material for the synthesis of 3a and 3b (Scheme 1). We previously reported the synthesis of this material through a modification of Seebach’s oxazolidinone methodology.9,13 The debenzylation of 4 posed some problems initially, as the use of solvents like benzene or EtOAc resulted in incomplete debenzylation, while the use of MeOH resulted in internal acetal formation. However, when the debenzylation of 4 was carried out in 95% EtOH with 10% Pd/C as the catalyst, complete debenzylation was observed and acetal formation was minimized if the reaction was worked up immediately and the product carried directly onto the next reaction. Prolonged exposure of 5 to the alcohol solvent resulted in acetal formation. Addition of a base like Et3N to the reaction further ensured prevention of the acid-catalyzed acetal formation during debenzylation.
Scheme 1.
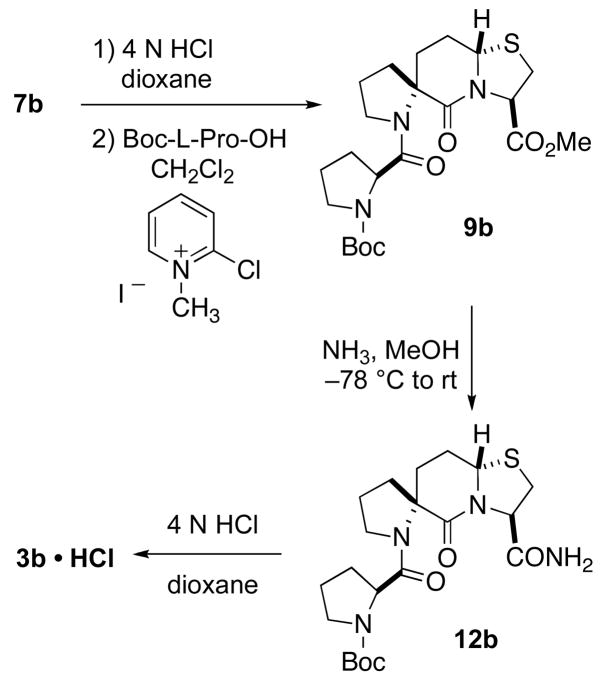
Carboxy aldehyde 5 was then condensed with either L-Cys-OMe·HCl or D-Cys-OMe·HCl to give a diastereomeric mixture of thiazolidines 6a or 6b, respectively. Mukaiyama’s reagent, 2-chloro-1-methyl pyridinium iodide, facilitated the cyclization of thiazolidines 6a to give a mixture of diastereoisomers 7a and 7b in a 2:1 ratio. These diastereoisomers were separable by column chromatography. Isomer 7a was crystallized from Et2O/EtOAc and its absolute stereochemistry at the bridgehead position was determined by X-ray crystallography to have the R configuration (see Figure 1 in Supporting Information).14 Likewise, cyclization of 6b with Mukaiyama’s reagent gave the diastereoisomers 8a and 8b,9 which were separated through column chromatography, in a 1:1 ratio.
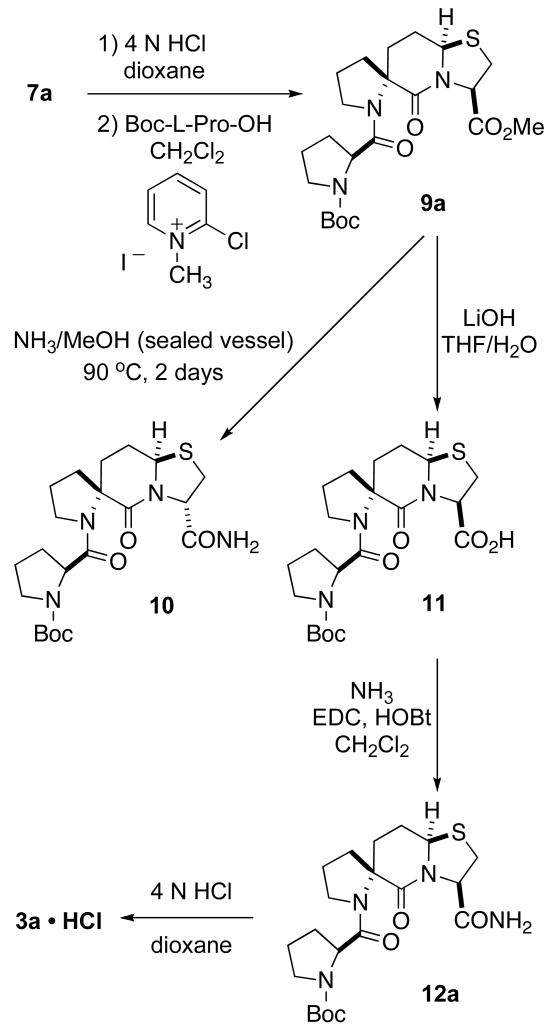
Spiro bicyclic ester 7a was treated with HCl/dioxane to remove the tert-butoxycarbonyl group and the resulting hydrochloride salt was initially coupled to Boc-L-Pro-OH using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide/1-hydroxybenzotriazole (EDC/HOBt) to give 9a in about a 50% yield. Replacing EDC/HOBt with Mukaiyama’s reagent increased the yield of 9a to 70 % (Scheme 2). The conversion of ester 9a to its corresponding amide posed a problem, however. Treatment of 9a with a methanolic solution of ammonia at room temperature overnight did not provide the desired amide 12a. When the reaction mixture was heated to 90 °C in a sealed vessel amidation resulted, but so did epimerization at the carbon possessing the carboxamide moiety thereby giving the known epimeric spiro bicyclic lactam amide 10.9 The epimerization was slow because even after two days only 50% of the starting material had been converted to amide 10. In order to resolve this problem, 9a was first hydrolyzed to acid 11 with LiOH in THF/H2O. The coupling of 11 with ammonia via activation with EDC/HOBt resulted in the formation of amide 12a. Removal of the tert-butoxycarbonyl protecting group from 12a with HCl/dioxane provided the desired polyproline PLG peptidomimetic 3a as its hydrochloride salt.
Scheme 2.
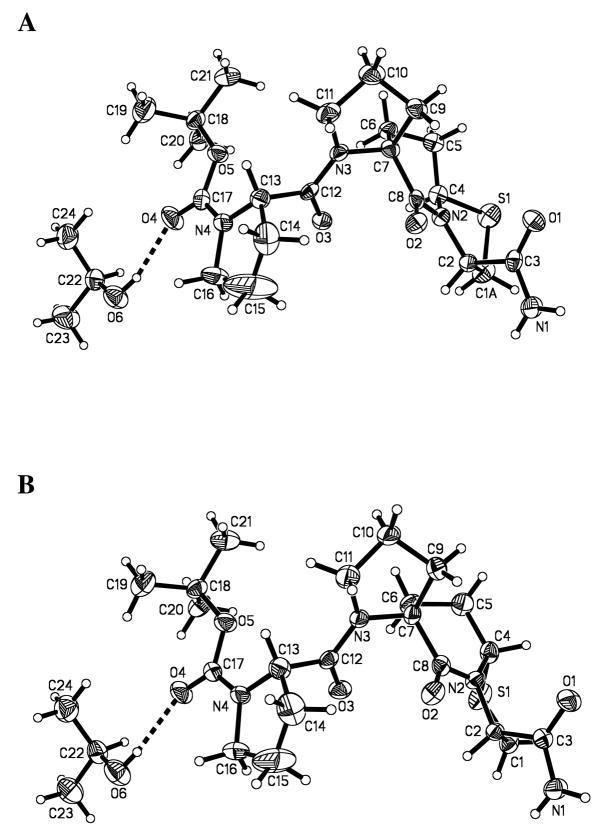
Spiro bicyclic ester 7b was coupled to Boc-L-Pro-OH with the aid of 2-chloro-1-methyl pyridinium iodide to give 9b (Scheme 3). In contrast with 9a, the ammonolysis of diastereoisomer 9b proceeded smoothly in methanolic ammonia solution at room temperature to give 12b in a 77% yield after purification. The final product, 3b • HCl, was obtained by removal of the tert-butoxycarbonyl group with HCl/dioxane. The assignment of the bridgehead stereochemistry of 3a and 3b was based upon the X-ray crystal structures of the corresponding precursors 12a and 12b (Figures 3)14 The purity of 3a and 3b was confirmed with HPLC.
Scheme 3.
Figure 3.
ORTEP drawings of the X-ray crystal structures of 12a (A) and 12b (B).
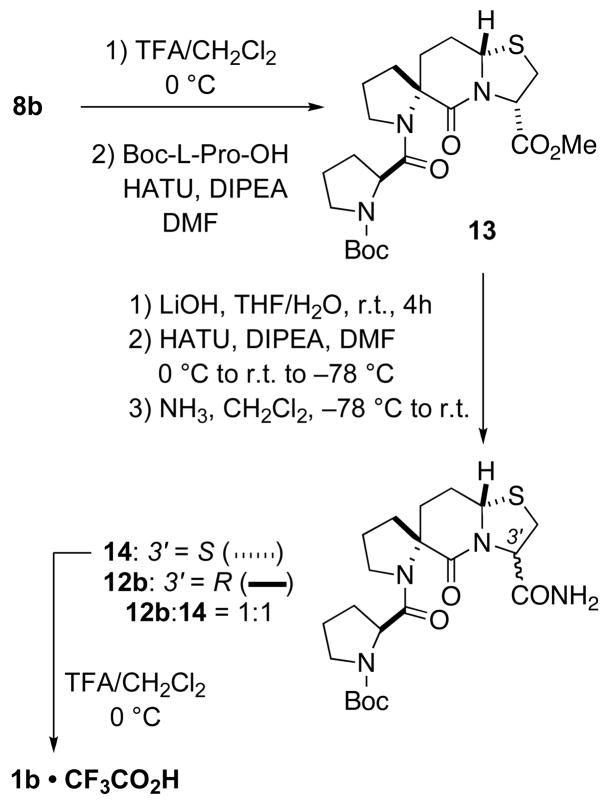
The synthesis of 1b was achieved from 8b as outlined in Scheme 4. Treatment of 8b with trifluoroacetic acid to remove the N-tert-butoxycarbonyl group gave the trifluoroacetate salt, which was coupled to Boc-L-Pro-OH using O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HATU) as the coupling reagent. This provided 13 in a 79% yield. Initially, ammonolysis of 13 was tried with a saturated solution of ammonia in dichloromethane/methanol first at room temperature then followed by heating to 55 °C for 2 days (in the presence of 10 mol% DMAP on the third day). These conditions only resulted in partial conversion of 13 to a 1:1 mixture of carboxamides 14 and 12b. This difficulty in ammonolysis was analogous to that seen with 9a, another isomer where the bridgehead hydrogen and carboxamide group are in a trans relationship. We therefore hydrolyzed the methyl ester and then coupled the resulting acid to ammonia using HATU as the coupling reagent. Although complete conversion was obtained, a 1:1 mixture of 14 and 12b was obtained once again, indicating epimerization at the 3′ position. It was determined by further experimentation that this competing reaction occurred during the ester hydrolysis step. Attempting ester hydrolysis under cold conditions (−5 °C to 0 °C) only served to reduce the rate of the main hydrolysis reaction. Diastereoisomers 12b and 14 were separated through column chromatography. Removal of the tert-butoxycarbonyl protecting group from 14 with TFA/CH2Cl2 provided the desired PLG peptidomimetic 1b as its trifluoroacetate salt, the purity of which was confirmed by HPLC analysis.
Scheme 4.
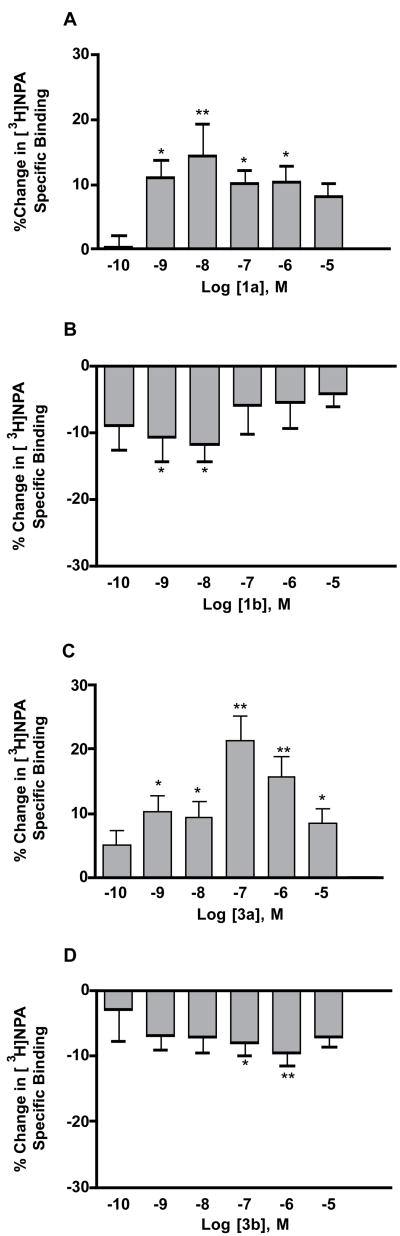
Compounds 1a, 1b, 3a and 3b were tested in a tritiated N-propylnorapomorphine ([3H]NPA) binding assay with bovine striatal dopamine D2 receptors.1 Each PLG peptidomimetic, at various concentrations, was incubated with the membrane preparation containing [3H]NPA and the percent change in [3H]NPA binding was measured. The results are depicted in Figure 4A–D for 1a, 1b, 3a and 3b, respectively. Both compound 1a and 3a behaved like PLG and typical PLG peptidomimetics, wherein they enhanced the binding of [3H]NPA to isolated dopamine D2 receptors and they exhibited bell-shaped dose response curves. The maximum effect for 1a was at a concentration of 10−8 M where it significantly enhanced the binding of [3H]NPA binding by 14.6 ± 3.9%. In contrast, PLG peptidomimetic 3a maximally enhanced [3H]NPA binding by 21.4 ± 6.1% at a concentration of 10−7 M.
Figure 4.
Modulation of [3H]NPA binding to dopamine D2 receptors by PLG peptidomimetics 1a (A), 1b (B), 3a (C), and 3b (D). Data represent the percent change in specific [3H]NPA binding relative to the control value when the indicated concentration of peptidomimetic was added directly to the assay buffer. Results are the mean ± SEM of five separate experiments carried out in triplicate. The data were analyzed by a one way analysis of variance followed by a Dunnett’s post-hoc test. *Significantly different (p<0.05) from the control value. ** Significantly different (p<0.01) from the control value.
Interestingly, the diastereoisomers of 1a and 3a, spiro bicyclic compounds 1b and 3b, respectively, decreased the binding of [3H]NPA to isolated dopamine D2 receptors. Spiro bicyclic derivative 1b decreased the binding of [3H]NPA to dopamine D2 receptors by 11.5 ± 3.1 % at a concentration of 10−8 M, while 3b, at a concentration of 10−6 M, decreased the binding of [3H]NPA to dopamine D2 receptors by 9.2 ± 3.4%. Like the positive allosteric modulators, both 1b and 3b possessed bell-shaped dose response curves.
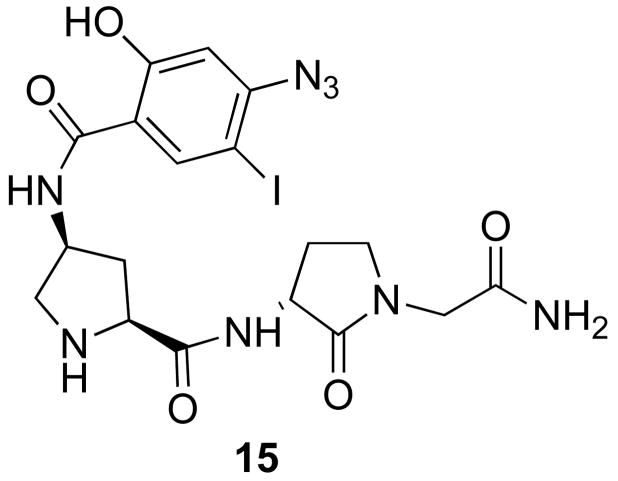
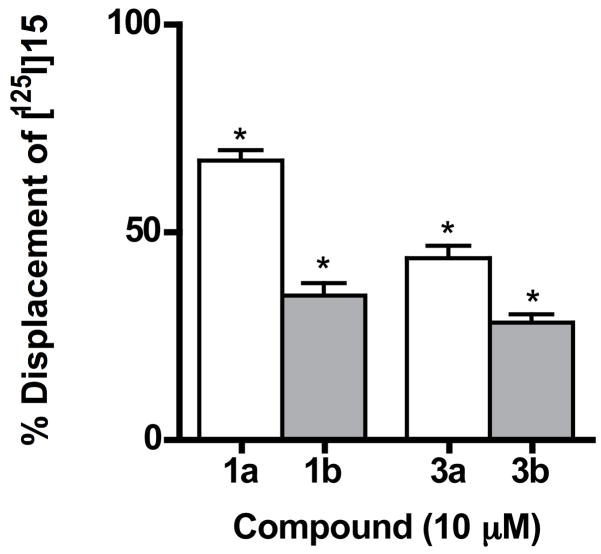
The spiro bicyclic PLG peptidomimetics 1a, 1b, 3a and 3b were also evaluated for their ability to displace [125I]15 (Figure 5) in a competition binding assay. PLG peptidomimetic 15 and its derivatives were previously shown to be positive modulators of the dopamine receptor and it was developed as a radioligand for the PLG allosteric modulatory site on the dopamine D2 receptor.3 The results depicted in Figure 6 show that all four spiro bicyclic PLG peptidomimetics are able to displace [125I]15 from its binding site.
Figure 5.
PLG peptidomimetic employed as the radioligand in the competition binding assay for the PLG binding site on the dopamine D2 receptor.
Figure 6.
Displacement of [125I]15 from the PLG allosteric modulatory site on the dopamine D2 receptor by analogues 1a, 1b, 3a, and 3b. Data represent the percent displacement of [125I]15 from its binding site on bovine striatal membranes. Values graphed are relative to controls in which maximum specific binding was calculated as the amount of [125I]15 bound in the presence and absence of 10 mM PLG. Results are the mean ± SEM of five separate experiments carried out in triplicate. The data were analyzed by a one way analysis of variance followed by a Dunnett’s post-hoc test. *Significantly different (p<0.05) from the control value.
Discussion
An earlier study showed that Pro-Pro-Pro-NH2 (2) possessed significant activity in enhancing the binding of NPA, a dopamine receptor agonist, to the dopamine D2 receptor.10 Also, we previously showed and have confirmed in the present study that the 5.6.5 spiro bicyclic peptidomimetic of PLG, 1a, possesses significant activity in enhancing the binding of the dopamine receptor agonist, NPA, to the dopamine D2 receptor.9 Since the highly constrained 5.6.5 spiro bicyclic scaffold of 1a has been shown to be an excellent mimic of a type II β-turn9 and since Pro-Pro-Pro-NH2 (2) cannot exist in a type II β-turn, we postulated that 2 could assume a conformation that puts the pharmacophore groups required for dopamine receptor modulating activity in positions similar to those occupied by the corresponding pharmacophore groups of PLG peptidomimetics when they are in a type II β-turn conformation. Molecular modeling suggested that a polyproline II helix conformation might be one such conformation, as the overlay of a polyproline II helix with the 5.6.5 spiro bicyclic type II β-turn scaffold showed that the backbone atoms and the carboxamide NH2 groups occupy the same relative area of space (Figure 2A). The only difference between the two was that the carbonyl groups were projected in the opposite directions.
A highly constrained 5.6.5 spiro bicyclic scaffold that could mimic a polyproline II helix was designed and incorporated into the Pro-Pro-Pro-NH2 structure to give peptidomimetics 3a and 3b to test our hypothesis, The dihedral angles obtained from the X-ray analysis of 12a and 12b indicated that the synthesized molecules possessing such a 5.6.5 spiro bicyclic scaffold form a polyproline II helix conformation (Table 1). Furthermore, the PLG-like biological activity profile of 3a indicated that Pro-Pro-Pro-NH2 constrained in a polyproline II helix conformation is capable of activating the allosteric binding site with which PLG and its peptidomimetics interact. Previous work by us13 has shown that constraining Pro-Pro-Pro-NH2 and Pro-Leu-Pro-NH2 in a type VI β-turn conformation also leads to molecules with the ability to allosterically modulate dopamine receptors in a positive manner. Although the polyproline II helix and type VI β-turn conformations possess different ψ3 and ω torsion angles, their other torsion angles are similar and both place the NH2 group of the carboxamide moiety in the same relative topological space that the NH2 group occupies in PLG peptidomimetics constrained in a type II β-turn conformation.
The ability of 1b and 3b to decrease the binding of the dopamine receptor agonist NPA to dopamine D2 receptors represents a novel finding with respect to PLG peptidomimetics. However, this is not the first report of the negative modulation of the dopamine receptor, since homocysteine recently has been shown to reduce the affinity of the dopamine D2 receptor for agonists.15 The fact that 1b and 3b, like 1a and 3a, are able to displace [125I]15 in a competition binding assay suggests that 1b and 3b are interacting with the same PLG allosteric modulatory site on the dopamine D2 receptor with which 1a and 3a interact.3 However, in contrast to 1a and 3a, they have a negative effect on dopamine receptor agonist binding. The structural basis behind the activity of 1b and 3b is not known at present. Clearly the structural difference between 1a and 1b and between 3a and 3b is the chirality of the bridgehead carbon atom. This difference in bridgehead chirality has an effect on the possible conformations of the spiro bicyclic scaffolds, particularly the bicyclic thiazolidines-lactam portion. For example, modeling studies suggest that isomer 3b can exist in two conformations differing in the pucker of the lactam ring, which consequently affects the pucker of the thiazolidine ring and therefore the positions of the β-methylene group and the carboxamide moiety. Such conformations are not seen in modeling of 3a. We postulate that such conformational differences between 3a and 3b and also between 1a and 1b may produce different conformational changes at the PLG allosteric binding site upon binding thereby causing different conformational effects at the orthosteric site to which NPA binds. The design and synthesis of conformationally constrained analogues of such conformations are underway to test this hypothesis.
In summary, type II β-turn mimics and polyproline II helix mimics based upon diastereoisomeric 5.6.5 spiro bicyclic scaffolds have provided two pairs of PLG peptidomimetics with contrasting dopamine receptor modulating activities. Compounds 1a and 3a were found to be positive allosteric modulators of the dopamine receptor, while the corresponding diastereoisomeric compounds 1b and 3b provided the first PLG peptidomimetics with the ability to decrease the binding of the dopamine receptor agonist NPA to dopamine D2 receptors. The activity of 3a supports our hypothesis that a polyproline II helix conformation is the bioactive conformation for the PLG analogue Pro-Pro-Pro-NH2. These results also provide additional support for the concept that conformationally different molecules are able to serve as allosteric modulators of the dopamine receptor as long as these conformations place key pharmacophores in the correct region of space. Finally, that a change in the bridgehead chirality of the 5.6.5 scaffold brings about opposite effects in terms of the modulation of the dopamine receptor will make these peptidomimetics important ligands in future studies aimed at delineating the molecular mechanism behind the allosteric modulation of the dopamine receptor.
Experimental Section
Compound Purity
The purity of compounds 1b, 3a, and 3b is >98%.
Methyl [3′ R-(3′β,6′α,8′aα)]-1-(tert-Butoxycarbonyl)-5′-oxospiro[pyrrolidine-2,6′-thiazolidino[3,2-a]piperidine]-3′-carboxylate (7a) and Methyl [3′ R-(3′ β,6′ α,8′ a β)]-1-(tert-Butoxycarbonyl)-5′-oxospiro[pyrrolidine-2,6′-thiazolidino[3,2-a]piperidine]-3′-carboxylate (7b)
Aldehyde benzyl ester 4 (3.3 g, 9.1 mmol) was dissolved in 95% EtOH in a Parr bottle purged with argon. An equivalent of Et3N (1.3 mL, 9.1 mmol) and 10% wt Pd/C (200 mg) were added and the reaction was hydrogenated on a Parr shaker at 50 psi for 2 h. The reaction mixture was filtered and the Pd residue rinsed with EtOAc. The reaction mixture was concentrated and then redissolved in CH2Cl2. This solution was filtered through an Acrodisc to remove any residual catalyst. The filtrate was concentrated under vacuum. The residue was dissolved in toluene, which was then removed under vacuum. This process was repeated with toluene and then done with CH2Cl2 to give 5 as a white foam, which was then carried on to the next reaction. Minimum contact with EtOH is essential to prevent acetal formation.
Carboxy aldehyde 5 was dissolved in 1:1 EtOH/H2O (30 mL). The reaction mixture was cooled to −15 °C and NaHCO3 (764 mg, 9.1 mmol) was added. The mixture was stirred for 15 min and to this mixture was added L-Cys-OMe·HCl in one portion. The pH of the reaction was adjusted to around 7 with NaHCO3. The reaction was stirred overnight. The next day, the reaction mixture was concentrated to remove EtOH, the pH of the aqueous layer was adjusted to 6 with 1N HCl and extracted with EtOAc (2 × 50 mL). NaCl was added to the aqueous layer and the solution extracted with CHCl3 (2 × 50 mL). The pH was always adjusted to 6 between the extractions. The organic extracts were combined and washed with NaCl solution, dried over MgSO4 and concentrated under vacuum. This reaction gave a 1:1 mixture of diastereomeric thiazolidines (6) as a white foam in 80 % crude yield. No further purification was carried out.
The thiazolidine diastereomers were dissolved in dry CH2Cl2 (200 mL). 2-Chloro-1-methyl pyridinium iodide (Mukaiyama’s reagent, 1.91 g, 7.3mmol) and Et3N (2.54 mL, 18.3 mmol) were added to the solution and the reaction mixture was heated at reflux overnight. The reaction mixture was cooled and then extracted with 1M citric acid, water, NaHCO3, water and brine. The organic layer was dried over MgSO4 and concentrated under vacuum to give an orange oil consisting of diastereomers with the stereochemistry varying at the bridgehead carbon. The crude material was purified by column chromatography using 1:1 to 1:2 hexanes/EtOAc. The diastereomers 7a and 7b were obtained in the ratio of 2:1 in a 70 % yield. Diastereomer 7a was crystallized from EtOAc/Et2O and the stereochemistry at the bridgehead was determined by X-ray crystallography.
7a
mp 131–132 °C; [α]D − 40 (c 0.95, CHCl3); TLC Rf 0.38 (EtOAc/hexanes, 2:1); 1H and 13C NMR show the presence of two rotamers about the carbamate bond in a ratio 1.5:1. 1H NMR (300 MHz, CDCl3) δ 5.0 (dd, 0.5 H, J = 3.3 and 11.3 Hz, 8′a-CH); 4.77–4.86 (m, 1.5 H, 3′-CH and 8′a-CH), 3.74 and 3.76 (s, 3 H, OCH3), 3.29 –3.58 (m, 3 H, 5-CH2, 2′-CH2), 3.13 (dd, 1H, J = 12.75 and 20.25 Hz, 2′-CH2), 2.56–2.66 (m, 1 H, 8′-CH2), 2.21–2.41 (m, 2 H, 3-CH2 and 7′-CH2), 1.77–2.04 (m, 5 H, 4-CH2, 8′-CH2, 3-CH2, 7′-CH2), 1.40 and 1.42 (s, 9 H, Boc CH3); 13C NMR (75 MHz, CDCl3) δ 171.4, 171.2, 170.3, and 169.9 (CO2CH3 and 5′-C=O), 153.4 and 154.1 (Boc C=O), 79.8 and 80.3 (Boc C(CH3)3), 64.9 and 65.1 (6′-C), 64.0 and 64.2 (8′a-C), 61.7 and 61.9 (3′-C), 52.8 and 52.9 (OCH3), 48.6 and 48.9 (5-C), 39.0 and 39.8 (3-C), 33.3 and 33.4 (2′-C), 32.6 and 33.7 (8′-C), 28.8 and 28.9 (Boc CH3), 27.4 and 27.5 (7′ C), 23.1 and 23.8 (4-C); ESI HRMS m/z 393.1460 [M+Na]+, (C17H26N2O5S + Na+) requires 393.1460.
7b
(oil); [α]D −154.5 (c 1.42, CHCl3); TLC Rf 0.39 (EtOAc/hexanes, 2:1); 1H and 13C NMR show the presence of two rotamers about the carbamate bond. 1H NMR (300 MHz, CDCl3) δ 5.29–5.35 (m, 1H, 3′-CH), 4.90 (t, 1H, J = 6.45 Hz, 8′a-CH), 3.74 (s, 3H, OCH3), 3.41–3.69 (m, 2H, 5-CH2), 3.29 –3.34 (m, 1H, 2′-CH2), 3.15–3.19 (m, 1H, 2′-CH2), 2.56–2.72 (m, 1H, 8′-CH2), 2.18–2.35 (m, 2H, 3-CH2 and 7′-CH2), 1.76–1.97 (m, 5H, 4-CH2, 8′-CH2, 3-CH2, 7′-CH2), 1.42 (s, 9H, Boc CH3); 13C NMR (75 MHz, CDCl3) δ 22.7 and 23.2 (4-C), 26.6 and 28 (7′ C), 29 (Boc CH3), 32.3 and 32.3 (2′-C), 33.8 (8′-C), 41.2 and 42.2 (3-C), 48.6 and 48.8 (5-C), 53.0 (OCH3), 61.2 and 61.8 (3′-C), 61.2 (8′a-C), 64.1 and 64.4 (6′-C), 79.6 and 80.6 (Boc C=O), 154.0 and 154.3 (Boc C=O), 170.8, 170.9, 171 (CO2CH3 and 5′-C=O); ESI HRMS m/z 393.1460 [M+Na]+, (C17H26N2O5S + Na+) requires 393.1460.
Methyl [3′ R-(3′ β,6′ α,8′ a α)]-1-[[1-(tert-Butoxycarbonyl)-2(S)-pyrrolidinyl]carbonyl]-5′-oxospiro[pyrrolidine-2,6′-thiazolidino[3,2-a]piperidine]-3′-carboxylate (9a)
Spiro bicyclic ester 7a (500 mg, 1.35 mmol) was dissolved in 5 mL 4N HCl/dioxane. The reaction was stirred under argon for 1 h and then it was concentrated under vacuum. The residue twice was dissolved in CH2Cl2 and then stripped of solvent. The white foam that was obtained was stored under vacuum for a couple of hours before being carried on to the next step without purification.
Boc-L-Pro-OH (580 mg, 2.7 mmol) was dissolved in CH2Cl2 under argon in a flask cooled in an ice-bath. N-Methyl morpholine (1.04 mL, 9.45 mmol) was added to the reaction, followed by 2-chloro-1-methyl pyridinium iodide (709 mg, 2.7 mmol). The reaction mixture was stirred for 15 min followed by addition of the spiro bicyclic ester hydrochloride. The reaction then was stirred overnight. The next day a catalytic amount of 4-DMAP (10 mg) was added and the reaction was stirred for another 2 h. EtOAc (75 mL) was then added to the reaction mixture and this solution was washed with water, 1M citric acid solution, NaHCO3, water and brine. The organic layer was then dried over Na2SO4 and concentrated under vacuum to give the crude product which was purified in a 70% yield by silica gel column chromatography (6.5 × 3 cm) using EtOAc/MeOH (20:1) as the eluent. mp 123–125 °C; [α]D −87.2 (c 1, 1H and 13C NMR showed the presence of two rotamers CHCl3); TLC Rf 0.63 (CH2Cl2/MeOH, 10:1); about the carbamate bond in a ratio around 1:1. 1H NMR (300 MHz, CDCl3) δ 5.00 (dt, 1H, J = 3 and 10.8 Hz, 8′-CH), 4.80 (dd, 1 H, J = 4.2 and 7.5 Hz, 3′-CH), 4.41 (dd, J = 3 and 8.4 Hz, 0.5 H, Pro αCH), 4.31 (dd, J = 3.3 and 8.1 Hz, 0.5 H, Pro α-CH), 3.80–3.86 (m, 0.5H, Pro δ-CH2), 3.69 (s, 3H, OCH3), 3.28–3.59 (m, 4.5H, 2′-CH2, 5-CH2, and Pro δ-CH2), 3.08 (dd, 1H, J = 4.2 and 12.6 Hz, 2′-CH2), 2.53–2.68 (m, 1H, 8′-CH2), 1.70–2.34 (m, 11 H, 8′-CH2, Pro γ-CH2, Pro β-CH2, 4-CH2, 3-CH2, 7-CH2), 1.37 and 1.41 (s, 9 H, Boc CH3); 13C NMR (75 MHz, CDCl3) δ 171.3, 171, 170.5, 170.3, and 170.2 (C=O), 153.7 and 154.5 (Boc C=O), 79.5 and 79.6 (Boc C(CH3)3), 66.3 (6′-C), 63.8 (8′ a-C), 61.7 (3′-C), 58.3 (Pro α-C), 52.8 (OCH3), 47, 47.2, 48.4 and 48.5 (Pro δ-C and 5-C), 37.7 and 37.8 (3-C), 33.2 (2′-C), 32 and 32.2 (8′-C), 29.7 (Pro β-C), 28.7 and 28.8 (Boc CH3), 27.5 (7′-C), 23.9, 24.5 and 24.6 (Pro γ-C and 4-C), ESI HRMS m/z 490.1997 [M+Na]+, (C22H33N3O6S + Na+) requires 490.1988.
Methyl [3′ R-(3′ β,6′ α,8′ a β)]-1-[[1-(tert-Butoxycarbonyl)-2(S)-pyrrolidinyl]carbonyl]-5′-oxospiro[pyrrolidine-2,6′-thiazolidino[3,2-a]piperidine]-3′-carboxylate (9b)
Spiro bicyclic ester 7b (440 mg, 1.2 mmol) was treated in the same manner as that described above for the conversion of 7a into 9a. The product 9b still showed traces of impurities despite chromatographic purification. TLC Rf 0.72 (CH2Cl2/MeOH, 10:1); 1H and 13C NMR show the presence of two rotamers about the carbamate bond in a ratio around 1:1. 1H NMR (300 MHz, CDCl3) δ 5.29 (dd, 1 H, J = 7.05 and 12.75 Hz, 3′-CH), 4.87 (td, 1 H, J = 3.6 and 8.7 Hz, 8′ a-CH), 4.43 (dd, J = 3 and 7.8 Hz, 0.5 H, Pro α-CH), 4.33 (dd, 0.5 H, J = 3.45 and 8.25 Hz, Pro α-CH), 3.81–3.86 (m, 0.5 H, Pro δ-CH2), 3.72 and 3.73 (s, 3H, OCH3), 3.27–3.69 (m, 4.5 H, 2′-CH2, 5-CH2, and Pro δ-CH2), 3.12 (dq, J = 3.6 and 5.1 Hz, 1H, 2′-CH2), 2.59–2.67 (m, 1H, 8′-CH2), 1.71–2.50 (m, 11H, 8′-CH2, Pro γ-CH2, Pro β-CH2, 4-CH2, 3-CH2, 7-CH2), 1.40 and 1.42 (s, 9H, Boc CH3); 13C NMR (75 MHz, CDCl3) δ 171.3, 171, 170.8, 170.3, and 170.1 (C=O), 153.8 and 154.6 (Boc C=O), 79.4 and 79.6 (Boc C(CH3)3), 65.3 (6′-C), 62.1 and 62.3 (8′ a-C), 61.9 and 62.0 (3′-C), 58.4 (Pro α-C), 52.9 (OCH3), 47.1, 47.3 and 48.2 (Pro δ-C and 5-C), 40.8 and 41 (3-C), 34.5 and 34.8 (8′ a-C), 31.9 and 32.0 (2′-C), 29.0 and 29.9 (Pro β-C), 28.8 (Boc CH3), 28.3 (7′-C), 23.9, 24.1 and 24.7 (Pro γ-C and 4-C); ESI HRMS m/z 490.2003 [M+Na]+, (C22H33N3O6S + Na+) requires 490.1988.
[3′ R-(3′ β,6′ α,8′ a α)]-1-[[1-(tert-Butoxycarbonyl)-2(S)-pyrrolidinyl]carbonyl]-5′-oxospiro[pyrrolidine-2,6′-thiazolidino[3,2-a]piperidine]-3′-carboxamide (12a)
Spiro bicyclic compound 9a (320 mg, 0.68 mmol) was dissolved in 10 mL THF/H2O (3:1). LiOH (49 mg, 2.18 mmol) was added and the reaction was stirred overnight. The next day the reaction was concentrated under vacuum and the aqueous layer was extracted with Et2O (5 mL). The aqueous layer was then acidified to pH 3 with citric acid whereupon it was extracted with CH2Cl2 (3 × 50 mL). The organic layers were combined and washed with brine and dried over MgSO4. After removal of the solvent under reduced pressure, the spiro bicyclic free acid 11 was obtained as a solid (250 mg).
Carboxylic acid 11 (250 mg, 0.55 mmol) was stirred with HOBt (76 mg, 0.55 mmol) in CH2Cl2 at −78 °C under nitrogen. EDC·HCl (105 mg, 0.55 mmol) was added to this mixture and the reaction was stirred for 15 min. The reaction was warmed to room temperature till the solution cleared and then it was recooled. A solution of NH3 in CH2Cl2 was added to this reaction mixture and the reaction was stirred at −78 °C for 2 h and then stirred overnight at room temperature. The next day the reaction was concentrated under vacuum to remove the excess ammonia. The reaction mixture was then partitioned between CH2Cl2 and water. The CH2Cl2 layer was washed with NaHCO3 solution, water and brine, dried over MgSO4 and concentrated under vacuum. The residue was purified on a normal phase Redisep column (40 g) on a Combiflash Retrieve system to give 100 mg of product. The product was crystallized from isopropyl alcohol to give white crystals suitable for X-ray studies. mp 221 °C (dec); TLC Rf 0.56 (CH2Cl2/MeOH 5:1); [α]D −95.8 (c 0.72, CHCl3).
1D NOESY studies were carried out to delineate the relative stereochemistry by NMR. Both the 3′-H and the 8′ a-H protons were irradiated in separate experiments and NOE enhancements observed in each case. The experiments confirmed that the bridgehead proton was cis to the α-proton of the thiazolidine ring. 1H and 13C NMR showed the presence of two rotamers about the carbamate bond in a 1:1 ratio. 1H NMR (300 MHz, CDCl3) δ 6.46 (br s, 1 H, CONH2), 5.74 (br s, 1 H, CONH2), 4.99 (dt, 1 H, J = 3 and 10.8 Hz, 8′ a-CH), 4.69–4.70 (apparent doublet, 1 H, 3′-CH), 4.42 (dd, 0.5 H, J = 3 and 8.4 Hz, Pro αCH), 4.32 (dd, 0.5 H, J = 3.3 and 8.1 Hz, Pro α-CH), 3.84–3.89 (m, 0.5 H, Pro δ-CH2), 3.73–3.78 (m, 0.5 H, Pro δ-CH2), 3.20–3.59 (m, 5 H, 2′-CH2, 5-CH2, and Pro δ-CH2), 2.48–2.62 (m, 1 H, 8′-CH2), 1.75–2.32 (m, 11 H, 8′-CH2, Pro γ-CH2, Pro β-CH2, 4-CH2, 3-CH2, 7-CH2), 1.38 and 1.41 (s, 9 H, Boc CH3); 13C NMR (75 MHz, CDCl3) δ 172.5, 172.3, 171.5, 171.4, 171.1, 170.8 (C=O), 153.7 and 154.5 (Boc C=O), 79.5 and 79.7 (Boc C(CH3)3), 66.5 (6′-C), 64.5 and 64.6 (8′ a-C), 62.8 and 62.9 (3′-C), 58.2 (Pro α-C), 47, 47.2, 48.3 and 48.4 (Pro δ-C and 5-C), 37.7 and 37.8 (3-C), 32.9 and 33.0 (2′-C), 32.2 and 32.4 (8′-C), 29.7 (Pro β-C), 28.7 and 28.8 (Boc CH3), 27.6 (7′-C), 23.9, 24.6 and 24.7 (Pro γ-C and 4-C); ESI HRMS m/z 475.1996 [M+Na]+, (C21H32N4O5S + Na+) requires 475.1991; Anal (C21H32N4O5S) C, H, N, S.
[3′ R-(3′ β,6′ α,8′ a β)]-1-[[1-(tert-Butoxycarbonyl)-2(S)-pyrrolidinyl]carbonyl]-5′-oxospiro[pyrrolidine-2,6′-thiazolidino[3,2-a]piperidine]-3′-carboxamide (12b)
Spiro bicyclic methyl ester 9b (200 mg, 0.43 mmol) was dissolved in a saturated solution of methanolic ammonia that had been cooled to −78 °C in a flask fitted with a balloon. The reaction was stirred overnight as it was allowed to warm to room temperature. The solvent was removed under vacuum and the residue was twice dissolved in CH2Cl2 and the solvent then removed under reduced pressure. The reaction went in quantitative yield. If needed, the product can be purified on a Combiflash column by eluting with EtOAc, followed by CH2Cl2/EtOAc/MeOH (20:3:5) and then finally by CH2Cl2/MeOH (5:1). The product was crystallized slowly from isopropyl alcohol to give 150 mg of a white crystalline solid. The stereochemistry of this molecule was confirmed through X-ray studies. mp 232 °C (dec); TLC Rf 0.56 (CH2Cl2/MeOH, 5:1); [α]D −200.5 (c 1.08, CHCl3); 1H NMR (300 MHz, CDCl3) δ 6.69 and 6.83 (br s, 1 H, CONH2), 5.51 (br s, 1 H, CONH2), 5.02–5.09 (dd, 1 H, J = 7.2 and 4.2 Hz, 3′-CH), 4.78–4.86 (m, 1 H, 8′ a-CH), 4.42–4.45 (dd, J = 3 and 7.8 Hz, 0.5 H, Pro α-CH), 4.31–4.35 (dd, J = 4.2 and 9.6 Hz, 0.5 H, Pro α-CH), 3.32–3.88 (m, 5 H, 2′-CH2, 5-CH2, and Pro δ-CH2), 3.18–3.29 (m, 1 H, 2′-CH2), 2.70–2.81 (m, 1 H, 8′-CH2), 1.73–2.45 (m, 11 H, 8′-CH2, Pro γ-CH2, Pro β-CH2, 4-CH2, 3-CH2, 7-CH2), 1.40 and 1.43 (s, 9 H, Boc CH3); 13C NMR (75 MHz, CDCl3) δ 172.0, 171.9, 171.6, 171.4, 171.1, and 170.9 (C=O), 153.8 and 154.6 (Boc C=O), 79.5 and 79.7 (Boc C(CH3)3), 65.5 and 65.6 (6′-C), 62.7 and 62.8 (8′ a-C), 62.1 (3′-C), 58.4 and 58.5 (Pro α-C), 47.1, 47.4, 48.2 and 48.4 (Pro δ-C and 5-C), 39.7 and 39.4 (3-C), 32.4 and 32.5 (8′-C), 31.4 and 30.9 (2′-C), 29.9 (Pro β-C), 28.7 and 28.8 (Boc CH3), 26.0 and 26.5 (7′-C), 24.0, 24.2 and 24.7 (Pro γ-C and 4-C); ESI HRMS m/z 475.2004 [M+Na]+, (C12H32N4O5S + Na+) requires 475.1991; Anal (C21H32N4O5S) C, H, N, S.
[3 ′R-(3 ′β,6 ′α,8 ′aα)]-1-[2(S)-Pyrrolidinylcarbonyl]-5′-oxospiro[pyrrolidine-2,6′-thiazolidino[3,2-a]piperidine]-3′-carboxamide Hydrochloride (3a•HCl)
To the spirocycle amide 12a (70 mg, 0.15 mmol) in a flask under argon was added 5 mL 4N HCl/dioxane. The reaction was stirred for 1 h. A white precipitate formed as the reaction progressed. On removal of the solvent under vacuum, a white solid was obtained. The white solid was suspended twice in CH2Cl2 and then concentrated under vacuum to obtain a white solid. Addition of ether to the flask followed by mildly boiling the solid in ether and then decanting the ether layer led to removal of impurities in the product. This procedure had to be repeated 4–5 times to ensure complete removal of the impurities and this resulted in 15 mg of pure product as a white solid. The purity of the product was confirmed by HPLC using 250 mm × 10 mm Phenomenex LunaPrep C18 column with acetonitrile/H2O (99:1) and acetonitrile/MeOH (99:1) as the eluting systems. The product possessed a tR of 6.96 min and 7.25 min, respectively, in the two systems. TLC Rf 0.25 (2-propanol/NH4OH, 5:1); [α]D −118.4 (c 0.57, CH3OH). 1H NMR (300 MHz, CD3OD) δ 5.01 (d, 1H, J = 10.5 Hz, 8′ a-CH), 4.60 (d, 1H, J = 7.2, 3′-CH), 4.53 (m, 1H, Pro α-CH), 3.73–3.80 (m, 1H, Pro δ-CH2), 3.43–3.53 (m, 2H, Pro δ-CH2, 2′-CH2), 3.29–3.42 (m, 2H, 5-CH), 3.10 (d, 1H, J = 12 Hz, 2′-CH), 2.44–2.50 (m, 2H, 8′-CH2, 3-CH2), 1.94–2.30 (m, 10H, Pro β-CH2, Pro γ-CH2, 7′-CH2, 8′-CH2, 3-CH2, 4-CH2); 13C NMR (75 MHz, CD3OD) δ 174.2, 171.5, 167.2 (C=O); 68.2 (6′-C), 65.4 (8′ a-C), 63.9 (3′-C), 60.5 (Pro α-C), 49.6 (Pro δ-CH2), 47.7 (5-C), 38.5 (3-C), 34.7 (2′-C), 32.1 (8′-C), 28.9 (Pro β-CH2), 27.3 (7′-C), 25.2, 25.1 (4-C and Pro γ-C); ESI HRMS m/z 353.1647 [M]+; (C16H25N4O3S)+ requires 353.1642.
[3 ′R-(3′β,6 ′α,8′aβ)]-1-[2(S)-Pyrrolidinylcarbonyl]-5′-oxospiro[pyrrolidine-2,6′-thiazolidino[3,2-a]piperidine]-3′-carboxamide Hydrochloride (3b•HCl)
Spiro bicyclic amide 12b (70 mg, 0.15 mmol) was treated in the same manner as described above for the preparation of 3a to give 20 mg of pure 3b as a white solid. The purity of the product was confirmed by HPLC using a 250 mm × 10 mm Phenomenex LunaPrep C18 column with acetonitrile/H2O (99:1) and acetonitrile/MeOH (99:1) as the eluting systems. The product possessed a tR = 7.20 min and 7.08 min, respectively, in the two systems. TLC Rf 0.34 (2-propanol/NH4OH, 5:1); [α]D −174.5 (c 0.31, CH3OH); 1H NMR (300 MHz, CD3OD) δ 5.01 (dd, 1H, J = 4.5 and 8.4 Hz, 8′ a-CH), 4.88–4.95 (m, 1H, 3′-CH), 4.49–4.52 (m, 1H, Pro α-CH), 3.73–3.80 (m, 1H, Pro δ-CH2), 3.52–3.60 (m, 1H, Pro δ-CH2), 3.31–3.42 (m, 3H, 2′-C, 5-CH), 3.15 (dd, 1H, J = 7.5 and 11.7 Hz, 2′-CH), 1.96–2.59 (m, 12 H, Pro β-CH2, Pro γ-CH2, 7′-CH2, 8′-CH2, 3-CH2, 4-CH2); 13C NMR (75 MHz, CD3OD) δ 174.3, 171.7, and 167.5 (C=O), 67.3 (6′-C), 64.8 and 64.5 (3′-C and 8′ a-C), 60.7 (Pro α-C), 49.3 (Pro δ-C), 47.7 (5-C), 40.9 (3-C), 34.4 (8′-C), 33.4 (2′-C), 29.1 (Pro β-CH2), 27.5 (7′-C), 25.2, 24.9 (Pro γ-C and 4-C); ESI HRMS m/z 353.1640 [M]+; (C16H25N4O3S)+ requires 353.1642.
Methyl [3′S-(3′α,6′α,8′aβ)]-1-[[1-(tert-Butoxycarbonyl)-2(S)-pyrrolidinyl]carbonyl]-5′ oxospiro[pyrrolidine-2,6′-thiazolidino[3,2-a]piperidine]-3′-carboxylate (13)
Trifluoroacetic acid (2.5 mL, 34 mmol) was added to an ice-cooled anhydrous dichloromethane solution of the spirocycle (313 mg, 0.85 mmol, 1.0 equiv.). The final concentration of TFA in dichloromethane was 20–25%. The reaction was monitored by thin layer chromatography (TLC) using 25% ethyl acetate in hexanes. After 45–60 minutes, the starting material was consumed completely. Solvent and excess TFA were removed in vacuo. The pale-yellow colored oil was used in the next step without further purification.
Boc-L-proline (220 mg, 1.0 mmol, 1.2 equiv.) was dissolved in anhydrous DMF (5 mL). The solution was cooled in an ice-salt bath, followed by the addition of DIPEA (0.2 mL, 1.3 mmol) and HATU (388 mg, 1.0 mmol). The solution was stirred under argon at this temperature for 30 min. In another 10 mL flask, the deprotected amine from the first step was suspended in anhydrous DMF (5mL) and the trifluoroacetate salt neutralized with DIPEA (0.2 mL, 1.3 mmol). The clear solution obtained was added to the activated acid. The mixture was stirred under argon for 20 h after which the solvent was removed in vacuo. The residue was dissolved in EtOAc and washed sequentially with 10% citric acid, saturated NaHCO3, and brine solutions. The organic layer was dried over anhydrous MgSO4, filtered and concentrated in vacuo to give a yellow-colored oil which was purified by flash chromatography starting with 100% CH2Cl2 and increasing the polarity in discrete steps to 4% MeOH in CH2Cl2 to give the purified compound as a colorless oil in 79% yield. TLC Rf 0.65 (CH2Cl2/MeOH, 10:1); [α]D −40.7 (c 0.43, CDCl3); 1H and 13C NMR spectra indicate presence of two rotameric forms of the molecule about the carbamate bond in a 1:1 ratio. 1H NMR (300 MHz, CDCl3) δ 4.80–4.74 (m, 1H, 8′ a-CH), 4.68–4.65 (m, 1H, 3′-CH), 4.43 (app t, 0.5 H, J = 4.8, 9.9 Hz, Pro α-CH), 4.30 (dd, 0.5H, J = 1.2, 5.7 Hz, Pro α-CH), 3.86–3.78 (AB qt. 0.5 H, J = 9, 15 Hz, Pro δ-CH2), 3.74–3.49 (m, 2.5H, Pro δ-CH2, 5-CH2), 3.67 (s, OCH3), 3.39–3.26 (m, 2H, Pro δ-CH2, 2′-CH2), 3.15–3.09 (dd, 1H, J = 4.5, 12.6 Hz, 2′-CH2), 2.68–2.52 (m, 2H, 8′ CH2), 2.12–1.84 (m, 9H, Pro β-CH2, Pro γ-CH2, 3-CH2, 4-CH2, 7′-CH2), 1.80–1.70 (m, 1H, 7′-CH2), 1.39 and 1.37 (s, 9H, Boc-CH3); 13C NMR (75 MHz, CDCl3) δ 171.4, 171.1, 170.8, and 170.6 (amide and ester C=O), 154.7 and 153.9 (Boc C=O), 79.5 and 79.3 (Boc C(CH3)3), 65.0 and 64.9 (6′-C), 63.6, 63.5, 63.4 (8′ a-C and 3′-C), 58.3 (Pro α-C), 52.6 and 52.5 (OCH3), 48.1 (Pro δ-C), 47.2 and 46.9 (5-C), 42.0 and 41.9 (3-C), 37.0 (8′-C), 33.1(2′-C), 29.8 (Pro β-C), 28.9 and 28.8 (7′-C), 28.7 and 28.6 (Boc CH3), 24.3, 23.8 and 23.8, 23.6 (Pro γ-C and 4-C); ESI HRMS m/z 490.1978 [M+Na]+, (C22H33N3O6S+ Na+) requires 490.1988.
[3′S-(3′α,6′α,8′aβ)]-1-[[1-(tert-Butoxycarbonyl)-2(S)-pyrrolidinyl]carbonyl]-5′-oxospiro[pyrrolidine-2,6′-thiazolidino[3,2-a]piperidine]-3′-carboxamide (14)
A solution of the ester (240 mg, 0.5 mmol) in 3:1 THF-water was cooled to −5 °C in an ice-salt bath. Lithium hydroxide (37 mg, 1.5 mmol) was added at this temperature. The final molarity of LiOH was 0.5 M. The reaction was gradually warmed to room temperature. Ester hydrolysis was determined to be complete in less than 4 hours by thin layer chromatography. Two distinct spots were observed by TLC, indicative of possible epimerization of the thiazolidine stereocenter. The mixture of carboxylic acids was used in the subsequent coupling step.
The carboxylic acid obtained (211 mg, 0.5 mmol) was dissolved in anhydrous DMF (3 mL) in the presence of DIPEA (0.12 mL, 0.8 mmol, 1.6 equiv.) and the solution cooled to −5 °C in an ice-salt bath. HATU (179 mg, 0.5 mmol) was added to the solution which was then warmed to room temperature for 20 minutes. The pale-yellow colored solution obtained was cooled to −78 °C and a saturated solution of ammonia in anhydrous CH2Cl2 was added (8–10 mL). The solution turned into a bright canary yellow color. An empty balloon was fitted onto the flask via an adaptor and the reaction warmed to room temperature over 1 h. The reaction mixture was stirred at ambient temperature for 24 h, following which an additional amount of ammonia in CH2Cl2 (3–4 mL) was added. Following another 24 h, the solvent and excess ammonia were removed in vacuo. The residue was dissolved in EtOAc and washed sequentially with 10% citric acid, saturated NaHCO3, and brine solutions. The organic layer was dried over anhydrous MgSO4, filtered and concentrated to give a pale yellow oil which was purified by flash chromatography using gradient elution (100% CH2Cl2 first and increasing to 20 % MeOH in CH2Cl2) to give an overall 85% yield of diastereomeric carboxamides (1:1 ratio) differing in stereochemistry about the 3′ carbon.
TLC Rf 0.49 (CH2Cl2/MeOH, 10:1)]; [α]D −35.8 (c 0.81, CDCl3); 1H and 13C NMR spectra indicate presence of two rotameric forms of the molecule about the carbamate bond in a 1:1 ratio. 1H NMR (300 MHz, CDCl3, gCOSY) δ 7.49 and 7.59 (s, 1H, carboxamide NH), 5.36 (br. s, 1H, carboxamide NH), 4.81–4.75 (m, 2H, 8′ a-CH and 3′-CH), 4.45–4.41 (dd, 0.5H, J = 3.0 and 8.1 Hz, Pro α-CH), 4.37–4.33 (dd, 0.5H, J = 3.0 and 8.7 Hz, Pro α-CH), 3.98–3.91 (ddd, 0.5H, J = 3.9, 8.1, and 9.3 Hz, Pro δ-CH), 3.79–3.72 (ddd, 0.5H, J = 3.0, 7.8, and 8.7 Hz, Pro δ-CH), 3.62–3.22 (m, 5H, Pro δ-CH, 5-CH2, 2′-CH2), 2.69–2.39 (m, 1H, 8′-CH), 2.33–1.79 (m, 11H, Pro β-CH2, Pro γ-CH2, 3-CH2, 4-CH2, 7′-CH2, 8′-CH), 1.43 and 1.41 (s, 9H, Boc-CH3); 13C NMR (75 MHz, CDCl3, HMQC) δ 172.5, 172.3, 172.2, 172.1, 170.5, and 170.2 (amide C=O), 154.8 and 153.9 (Boc C=O), 79.7 and 79.5 (Boc C(CH3)3), 64.5, 64.4, 64.1, 63.9 (6′-C, 8′ a-C and 3′-C), 58.1 and 58.0 (Pro α-C), 47.96 and 47.94 (Pro δ-C), 47.1 and 46.9 (5-C), 41.2 and 41.1 (3-C), 38.1 and 38.0 (8′-C), 33.9 and 33.8 (2′-C), 29.5 (Pro β-C), 28.7 and 28.6 (Boc C(CH3)3), 28.1 and 27.8 (7′-C), 24.6, 24.5, 23.5 (Pro γ-C and 4-C); ESI HRMS m/z 475.1987 [M+Na]+, (C21H32N4O5S+ Na+) requires 475.1991.
[3′ S-(3′ α,6′ α,8′ a β)]-1-[2(S)-Pyrrolidinylcarbonyl]-5′-oxospiro[pyrrolidine-2,6′-thiazolidino[3,2-a]piperidine]-3′-carboxamide Hydrochloride (1b)
The Boc-protected molecule (50 mg, 0.1 mmol) was dissolved in 1–2 mL anhydrous CH2Cl2 and cooled to −5 °C using an ice-salt bath. Trifluoroacetic acid (0.2 mL, 2.2 mmol) was added drop-wise to this solution, which was stirred at that temperature for 45–60 minutes. The starting material was completely consumed at this point (as determined by thin layer chromatography). Solvent and excess TFA were removed in vacuo. The colorless oil obtained was suspended in CH2Cl2 and solvent removed in the rotary evaporator. This process was repeated several times until a semi-solid material was obtained. This material was triturated with ether and ether decanted to remove any soluble impurities. A white foam/semi-solid weighing 45 mg was obtained, indicating quantitative yield. A solution of the molecule in 4:1 acetonitrile-water was subjected to HPLC analysis on a Supelco Ascentis® C18 column (5μm particle size, 250 mm × 10 mm dimension), using an isocratic 98:2 acetonitrile/0.1% TFA aq. solution. A retention time of 3.75 min. was obtained (100% compared to a blank composed of 4:1 acetonitrile-water); TLC Rf 0.53 (2-propanol/NH4OH, 4:1); [α]D −11.5 (c 0.13, CD3OD); 1H NMR (300 MHz, CDCl3) δ 4.89–4.84 (dd, 1H, J = 2.4 and 10.8 Hz, 8′ a-CH), 4.56 (d, 1H, J = 6.6 Hz, 3′-CH), 4.47–4.42 (m, 1H, Pro α-CH), 3.72–3.65 (m, 1H, Pro δ-CH2), 3.49–3.41 (m, 1H, Pro δ-CH2),3.34–3.14 (m, 4H, 2′-CH2, 5-CH2), 2.43–2.26 (m, 3H, 7′-CH, 8′-CH2), 2.15–1.86 (m, 9H, Pro β-CH2, Pro γ-CH2, 3-CH2, 4-CH2, 7′-CH); 13C NMR (75 MHz, CDCl3, HMQC) d 174.8, 171.6, 168.5 (amide C=O), 66.7 (6′-C), 65.9, 65.1 (8′ a-C and 3′-C), 60.5 (Pro α-C), 49.2 (Pro δ-C), 47.8 (5-C), 41.9 (3-C), 38.0 (8′-C), 34.3 (2-C), 29.1(Pro β-C), 28.7 (7′-C), 25.3, 25.0 (Pro γ-C and 4-C); ESI HRMS m/z 353.1627 [M+H]+, (C16H25N4O3S+ H+) requires 353.1642.
Dopamine D2 Receptor Binding Assay
The effect of compounds 1a, 1b, 3a, and 3b on [3H]NPA binding in striatal membranes was analyzed using receptor binding studies. Tritiated NPA was synthesized through a custom order with Perkin-Elmer (Boston, MA) and provided with a specific activity of 43.1 Ci/mmol. Binding assays were carried out as previously described,1,4 wherein 100 μg of striatal membranes was prepared and incubated with the compounds at concentrations indicated in the presence of [3H]NPA. The mixture was incubated in a total 500 μL volume of assay buffer (50 mM Tris-HCl, 5 mM KCl, 4 mM MgCl2, 1 mM ethylenediaminetetraacetic acid (EDTA) and 120 mM NaCl (pH 7.6)) for 1h at 25 °C and the incubation then terminated by rapid filtration. Radioactivity-bound filter disks were placed in plastic scintillation vials containing 5 mL scintillation fluid and were counted in a Beckman LS5000 liquid scintillation counter (Model LS5 KTA). Specific binding was defined as the difference between the radioactivity bound in absence and presence of 10 mM dopamine.
Radiolabeling of 15 with [125I] and Competitive Receptor Binding Assay
To iodinate 15 two iodo beads (Pierce, Rockford, IL) were washed with 1 mL of phosphate buffer saline for 5 min twice. One mg of the non-iodinated precursor of 153 was incubated with the iodo beads and 10 μL (1mCi) of Na125I for 15 min at room temperature with periodic shaking. The mixture was then transferred to the pre-equilibrated column (a C18 (18%) column (Canadian Life Science, Peterborough, ON) was equilibrated twice with 5 mL of MeOH and twice with 5 mL of distilled water). The column was washed five times with 1 mL of distilled water to remove unbound iodine, then washed with 1 mL of 25%, 50% and 100% methanol sequentially with each fraction being collected for incorporation analysis. Flow was collected and 2 μL of each collection was counted for radioactivity. Specific activity of the material was 20 Ci/mmol.
Competition binding assays were performed in triplicates using [125I]15. Striatal membranes (100 μg) were incubated with 10 μM of the peptidomimetics (1a, 1b, 3a, or 3b) and 0.1 μM of [125I]15. The mixture was incubated at 4 °C for 8 h prior to rapid filtration and counting radioactivity (as described above). Specific binding was defined as the difference between the radioactivity bound in absence and presence of 10 mM PLG.
Supplementary Material
Acknowledgments
This work was supported in part by a N.I.H. grant (NS20036) to R.L.J. We thank Victor G Young, Jr. of the Department of Chemistry X-Ray Crystallographic Laboratory for the X-ray crystallographic data on compounds 7a, 12a, and 12b. We thank Drs. Elizabeth Amin, Yuk Sham, Ashish P. Vartak, Richard Wood and the Biomedical Modeling, Simulation, and Design Laboratory of the Minnesota Supercomputing Institute for their help with the molecular modeling.
Footnotes
Abbreviations: EDC, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide; HATU, O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate; HOBt, 1-hydroxybenzotriazole; NPA, N-propylnorapomorphine; PLG, L-prolyl-L-leucyl-glycinamide; PPI, polyproline I; PPII, polyproline II.
Supporting information Available: ORTEP drawing of the X-ray crystal structure of 7a. This material is available free of charge over the internet at http://pubs.acs.org.
References
- 1.Srivastava LK, Bajwa SB, Johnson RL, Mishra RK. Interaction of L-Prolyl-L-leucyl glycinamide with Dopamine D2 Receptor: Evidence for Modulation of Agonist Affinity States in Bovine Striatal Membranes. J Neurochem. 1988;50:960–968. doi: 10.1111/j.1471-4159.1988.tb03005.x. [DOI] [PubMed] [Google Scholar]
- 2.Verma V, Mann A, Costain W, Pontoriero G, Castellano JM, Skoblenick K, Gupta SK, Pristupa Z, Niznik HB, Johnson RL, Nair VD, Mishra RK. Modulation of Agonist Binding to Human Dopamine Receptor Subtypes by L-Prolyl-L-Leucyl-Glycinamide and a Peptidomimetic Analogue. J Pharmacol Exp Ther. 2005;315:1228–1236. doi: 10.1124/jpet.105.091256. [DOI] [PubMed] [Google Scholar]
- 3.Fisher A, Mann A, Verma V, Thomas N, Mishra RK, Johnson RL. Design and Synthesis of Photoaffinity-Labeling Ligands of the L-Prolyl-L-leucylglycinamide Binding Site Involved in the Allosteric Modulation of the Dopamine Receptor. J Med Chem. 2006;49:307–317. doi: 10.1021/jm050644n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baures PW, Ojala WH, Gleason WB, Mishra RK, Johnson RL. Design, Synthesis, X-ray Analysis, and Dopamine Receptor-modulating Activity of Mimics of the “C5” Hydrogen-bonded Conformation in the Peptidomimetic 2-Oxo-3(R)-[(2(S)-pyrrolidinylcarbonyl)amino]-1-pyrrolidineacetamide. J Med Chem. 1994;37:3677–3683. doi: 10.1021/jm00048a003. [DOI] [PubMed] [Google Scholar]
- 5.Chiu S, Paulose CS, Mishra RK. Neuroleptic Drug-induced Dopamine Receptor Supersensitivity: Antagonism by L-Prolyl-L-leucyl-glycinamide. Science. 1981;214:121–1262. doi: 10.1126/science.6117947. [DOI] [PubMed] [Google Scholar]
- 6.Bhargava HN. Effect of MIF and Cyclo(Leu-Gly) on Supersensitivity of Dopamine Receptors in Brain Induced by Haloperidol. Neuropharmacology. 1984;23:439–444. doi: 10.1016/0028-3908(84)90252-1. [DOI] [PubMed] [Google Scholar]
- 7.Rajakumar G, Naas F, Johnson RL, Chiu S, Yu KL, Mishra RK. Down-regulation of haloperidol-induced Striatal Dopamine Receptor Supersensitivity by Active Analogs of L-Prolyl-L-leucyl-glycinamide (PLG) Peptides. 1987;8:855–861. doi: 10.1016/0196-9781(87)90072-6. [DOI] [PubMed] [Google Scholar]
- 8.Genin MJ, Mishra RK, Johnson RL. Dopamine Receptor Modulation by a Highly Rigid Spiro Bicyclic Peptidomimetic of Pro-Leu-Gly-NH2. J Med Chem. 1993;36:3481–3483. doi: 10.1021/jm00074a032. [DOI] [PubMed] [Google Scholar]
- 9.Khalil EM, Ojala WH, Pradhan A, Nair VD, Gleason WB, Mishra RK, Johnson RL. Design, Synthesis and Dopamine Receptor Modulating Activity of Spirobicyclic Peptidomimetics of L-Prolyl-L-leucyl-glycinamide. J Med Chem. 1999;42:628–637. doi: 10.1021/jm980525q. [DOI] [PubMed] [Google Scholar]
- 10.Baures PW, Pradhan A, Ojala WH, Gleason WB, Mishra RK, Johnson RL. Synthesis and Dopamine Receptor Modulating Activity of Unsubstituted and Substituted Triproline Analogues of L-Prolyl-L-leucyl-glycinamide (PLG) Bioorg Med Chem Lett. 1999:2349–2352. doi: 10.1016/s0960-894x(99)00386-8. [DOI] [PubMed] [Google Scholar]
- 11.Loomis RE, Tseng CC, Bergey EJ, Levine MJNMR. Computer Analyses of a Nonapeptide Found in a Human Salivary Proline-rich Glycoprotein. Int J Peptide Protein Res. 1988;32:130–140. doi: 10.1111/j.1399-3011.1988.tb00673.x. [DOI] [PubMed] [Google Scholar]
- 12.Witter DJ, Famiglietti SJ, Cambier JC, Castelhano AL. Design and Synthesis of SH3 Domain Binding Ligands: Modifications of the Consensus Sequence XPpXp. Bioorg Med Chem Lett. 1998;8:3137–3142. doi: 10.1016/s0960-894x(98)00577-0. [DOI] [PubMed] [Google Scholar]
- 13.Vartak A, Skoblenick K, Thomas N, Mishra RK, Johnson RL. Allosteric Modulation of the Dopamine Receptor by Conformationally Constrained Type VI β-Turn Peptidomimetics of Pro-Leu-Gly-NH2. J Med Chem. 2007;50:6725–6729. doi: 10.1021/jm070895r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CCDC 665023, 665024, and 665025 contain the supplementary crystallographic data for compounds 12a, 12b, and 7a, respectively. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif
- 15.Agnati LF, Ferre S, Genedani S, Leo G, Guidolin D, Filaferro M, Carriba P, Casado V, Lluis C, Franco R, Woods AS, Fuxe K. Allosteric Modulation of Dopamine D2 Receptors by Homocysteine. J Proteome Res. 2006;5:3077–3083. doi: 10.1021/pr0601382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.