Abstract
Background
To further clarify and/or develop calcium and vitamin D as chemopreventive agents against colorectal cancer in humans, understand the mechanisms by which these agents reduce risk for the disease, and develop ‘treatable’ biomarkers of risk for colorectal cancer, we conducted a pilot, randomized, double-blind, placebo-controlled, 2×2 factorial clinical trial to test the effects of calcium and vitamin D3, alone and in combination on markers of apoptosis in the normal colorectal mucosa.
Methods
Ninety-two men and women with at least one pathology-confirmed colorectal adenoma were treated with calcium 2.0 g/day or vitamin D3 800 IU/day, alone or in combination vs. placebo over six months. Overall expression and colorectal crypt distributions of Bcl-2 (an apoptosis inhibitor) and Bax (an apoptosis promoter), in biopsies of normal-appearing rectal mucosa were detected by automated immunohistochemistry and quantified by image analysis.
Results
After six months treatment, Bax expression along the full lengths of crypts increased 56% (p=0.02) in the vitamin D group, and 33% in both the calcium (p=0.31) and calcium plus vitamin D (p=0.36) groups relative to the placebo group. The vitamin D treatment effect was more pronounced in the upper 40%, or differentiation zone, of crypts (80%; p=0.01). There were no statistically significant treatment effects on Bcl-2 expression.
Conclusions
Overall, these preliminary results suggest that calcium and vitamin D, individually or together, may enhance apoptosis in the normal human colorectal epithelium, and the strongest treatment effects may be vitamin D related and in the upper sections of the colorectal crypts.
Keywords: vitamin D, calcium, apoptosis, randomized controlled trial, normal colorectal mucosa
Introduction
Despite advances in screening and treatment, mortality due to colorectal cancer, the second leading cause of cancer deaths in the US (1, 2), has declined only modestly over the past 50 years, the decline probably a result of screening and polypectomy (2). This situation recalls an analogous one with ischemic heart disease three decades ago. With the advent of biological measurements as markers of risk for the disease, including lipid profiles and blood pressures, plausible preventive interventions could be readily investigated, response to preventive treatment could be monitored, and subsequently, with individual and population control of the “biomarkers”, mortality rates from the disease began a dramatic decline which continues today (3). Using biological measurements of risk, as they have for ischemic heart disease, should likewise result in a decline in colorectal cancer incidence and mortality. Based on this vision, this study has intertwined missions of exploring the efficacy of two plausible, and evidentially well-supported dietary agents, calcium and vitamin D, on modulating plausible molecular phenotypic biomarkers of risk for colorectal neoplasia.
There is strong biologic plausibility and animal experimental and human evidence for protection against colorectal neoplasms by calcium and vitamin D. Proposed mechanisms of calcium against colorectal cancer include protection of colonocytes against bile acids and fatty acids (4, 5), direct effects on cell cycle regulation (6), and modulation of E-cadherin and β-catenin expression via the calcium-sensing receptor (CaSR) (6-8). Proposed mechanisms for vitamin D involve bile acid catabolism, direct effects on the cell cycle, growth factor signaling, and immunomodulation (4, 6, 9). Although calcium and, especially, vitamin D have pro-apoptotic effects on colonocytes in vitro and in animal models (10-15), this has not been sufficiently confirmed in humans (16-18). Higher total calcium intakes are associated with reduced risk for colorectal adenoma (19-21), and calcium supplementation reduces adenoma recurrence (22-24). Also, higher serum 25-OH-vitamin D levels in a limited number of studies were associated with reduced risk for colorectal adenoma (25, 26). However, the independent and combined anti-neoplastic effects of calcium and vitamin D in humans are unclear, and there have been no colorectal cancer-related chemoprevention trials of vitamin D individually or jointly with calcium.
There are no generally accepted pre-neoplastic biomarkers of risk for colorectal cancer. Colorectal cancer, like ischemic heart disease (IHD), is a complex, multi-factorial disease, which, like IHD will require a multi-factorial preventive approach and a panel of biomarkers to describe phenotypes from which to categorize and quantify risk. Whereas for IHD risk markers the obvious place to look was in the vascular system, an obvious place to look for risk markers for colon cancer is in the tissue in which it forms: the colorectal epithelium. Although at first glance this would appear impractical, we have shown that the clinical procedures required are similar in ease, time, invasiveness, and discomfort as a digital rectal/prostate exam or a PAP smear (27). Although a urine or blood test would be more practical, it is most likely that such surrogate marker tests can eventually be more readily and rationally developed guided by the results from studying tissue markers. Phenotypic biomarkers are attractive biomarkers since they “summarize” the result of complex interactions among genotype, gene-gene interactions, epigenetic phenomenon, environmental exposures, and gene-environment interactions. This is certainly true of lipid profiles (3), and should be no less so for the “molecular state”, or phenotype, of the colorectal epithelium.
To address these issues, we conducted a pilot, randomized, double-blind, placebo-controlled, 2 × 2 factorial chemoprevention clinical trial of supplemental calcium and vitamin D3, alone and in combination vs. placebo over six months, to estimate the efficacy of these agents on modulating the expression of apoptotic biomarkers (pro-apoptotic Bax and anti-apoptotic Bcl-2) of risk in the normal colorectal mucosa.
Patients and Methods
This study was approved by the Emory University IRB. Written informed consent was obtained from each study participant.
Participant Population
Participants were recruited from the patient population attending the Digestive Diseases Clinic of Emory University. Eligibility included age 30 – 75 years, in general good health, capable of informed consent, and a history of at least one pathology-confirmed sporadic colon or rectal adenoma within the past 36 months. Exclusions included contraindications to calcium or vitamin D supplementation or rectal biopsy procedures, and medical conditions, habits, or medication usage that would otherwise interfere with the study. Specific exclusions are listed in Supplementary Appendix 1.
Clinical Trial Protocol
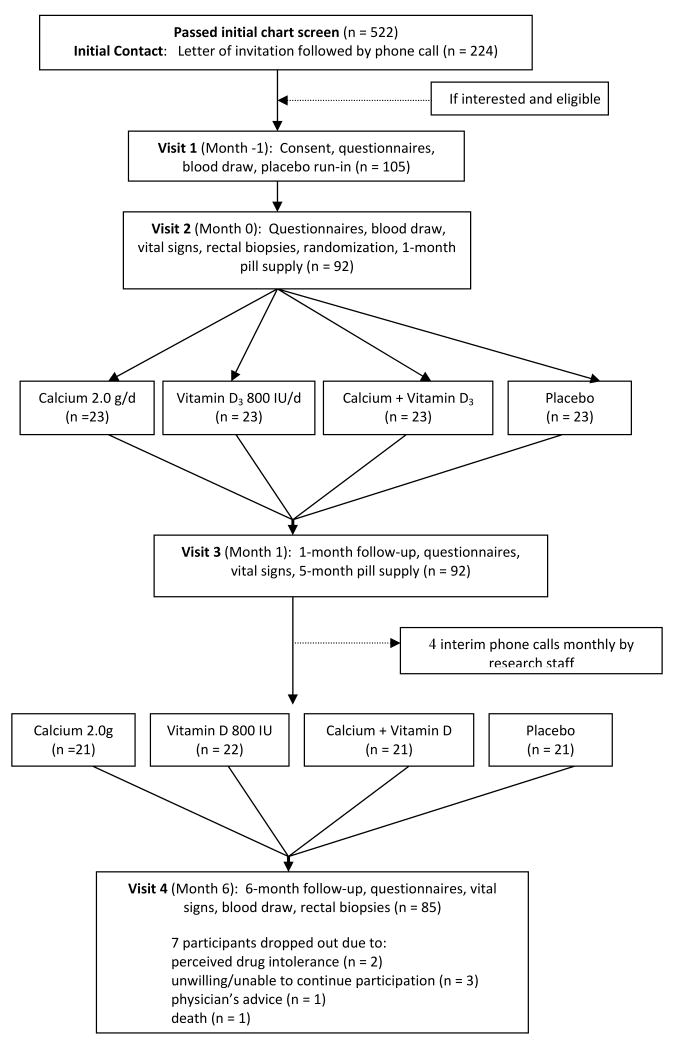
Participant recruitment and flow is depicted in Figure 1. All age-eligible practice patients diagnosed with at least one pathology-confirmed adenomatous colonic or rectal polyp within the previous 36 months were identified as potential study participants. Medical charts were screened, and potentially eligible patients were sent an introductory letter followed by a telephone interview during which willingness to participate and further eligibility was assessed, and, if appropriate, an in-person eligibility visit scheduled. During the eligibility visit, potential participants were interviewed and signed a consent form, their medication and nutritional supplement bottles were reviewed, and they completed questionnaires (on socio-demographics, medical history, medication and nutrition supplement use, lifestyle, family history, and others) and provided a blood sample. Diet was assessed with a semi-quantitative food frequency questionnaire (28). Medical and pathology records were reviewed. Those still eligible and willing to participate then entered a 30-day placebo run-in trial. Only participants without significant perceived side effects and took at least 80% of their tablets were randomized. Adherence for the run-in trial was assessed by questionnaire, interview, and pill count. Eligible participants then underwent a baseline rectal biopsy and were randomly assigned (stratified by sex and nonsteroidal anti-inflammatory drug [NSAID] use) over nine months to treatment group. Of those who passed initial chart eligibility, 42% were contacted and 20% were eligible and consented to participate.
Figure 1. Flow Diagram of a Trial of Supplemental Calcium and Vitamin D3, Alone and in Combination vs. Placebo over Six Months on Markers of Apoptosis in the Normal Colorectal Mucosa.
Participants (n=92) were randomly assigned to the following four treatments: placebo (n=23), 2.0 g elemental calcium supplementation (as calcium carbonate in equal doses twice daily) (n=23), 800 IU vitamin D3 supplementation (400 IU twice daily) (n=23), and 2.0 g elemental calcium plus 800 IU of vitamin D3 (n=23).
Study tablets were custom manufactured by Tishcon Corporation, NY, USA. The corresponding supplement and placebo pills were identical in size, appearance, and taste. The placebo was free of calcium, magnesium, vitamin D, and chelating agents.
Calcium carbonate was used for elemental calcium delivery in this trial since it was also successfully used in the Calcium Polyp Prevention adenoma recurrence (29) and Calcium and Colorectal Epithelial Cell Proliferation trials (30); it was used in most large studies using calcium long term for other reasons and therefore had the most established safety record; it is inexpensive; and it delivers more elemental calcium per tablet than other forms, thus fewer tablets are required, enhancing adherence.
Vitamin D3 was chosen as our form of vitamin D for several reasons, including avoidance of the toxicity risks associated with 1,25-(OH)2-vitamin D or 25-OH-vitamin D. Supplementation with vitamin D3 (a pro-hormone), takes advantage of natural metabolism to generate the most active moiety. Supplementation with even large doses of vitamin D3 does not increase total 1,25-(OH)2-vitamin D levels in individuals who are not vitamin D deficient (31). Multivitamins and calcium/vitamin D supplements typically provide 400 IU of vitamin D3 daily, but numerous intervention studies (reviewed in (32),(33)) show that this dose will not suppress PTH in most North American adults; however, 800 IU daily raises 25-OH-vitamin D levels toward the desired range, and leaves a substantial margin of safety, even when combined with dietary intake.
Over the six-month treatment period participants attended follow-up visits at 2 and 6 months after randomization and were contacted by telephone at monthly intervals between the second and final follow-up visits (Figure 1). At follow-up visits, pill-taking adherence was assessed by questionnaire, interview, and pill count. Adverse events were monitored by interview at each study visit and interim telephone call and two weeks after the last visit, questionnaire (included questions about hospitalizations, medical visits and diagnoses, medication changes, and symptoms) at each study visit, and by participant-initiated telephone calls, and graded according to NIH Common Toxicity Criteria and likelihood that they were study-related. Participants were instructed to remain on their usual diet and not take any nutritional supplements not in use on entry into the study. At each follow-up visit participants were interviewed and completed questionnaires. At the first and last visits all participants underwent venipuncture and a rectal biopsy procedure. Participants were asked to abstain from aspirin use for seven days prior to each biopsy visit. All visits for a given participant were scheduled at the same time of day to control for possible circadian variability in the outcome measures. Factors hypothesized to be related to the expression of apoptosis markers in normal colon mucosa (e.g., diet, medications, etc.) were assessed at baseline, several were reassessed at the first follow-up visit, and all were reassessed at the final follow-up visit. Participants did not have to be fasting for their visits and did not take a bowel cleansing preparation or enema.
Six 1-mm thick biopsy specimens were taken from the rectal mucosa 10 cm proximal to the external anal aperture through a rigid sigmoidoscope with a jumbo cup flexible endoscopic forceps mounted on a semi-flexible rod, teased off the forceps with and onto a strip of bibulous paper, then immediately placed in phosphate buffered saline and oriented under a dissecting microscope to ensure that they were not twisted or curled on the bibulous paper, and then immediately placed in 10% normal buffered formalin.
Immunohistochemistry Protocol
The biopsies in formalin were left undisturbed for at least six hours, transferred to 70% ethanol 24 hours after being placed in formalin, embedded in paraffin blocks within two weeks of the biopsy procedure, cut and stained within another four weeks, and analyzed within another four weeks. Five slides with four section levels each taken 40 microns apart were prepared for each biomarker, yielding a total of 20 levels per biomarker. Heat-mediated antigen retrieval was accomplished by steaming the slides for 40 minutes using a Pretreatment (PT) Module (Lab Vision Corp., CA) with 100× Citrate Buffer pH 6.0 (DAKO S1699, DAKO Corp., Carpinteria, CA; further referred to as DAKO). Next, immunohistochemical (IHC) processing by a labeled streptavidin-biotin method was accomplished using a DAKO Automated Stainer (DAKO). The following reagents were used: antibody (Bcl-2 antibody, Santa Cruz Biotechnology, Inc., CA, catalog no. sc-509, dilution 1:100; or Bax antibody, DAKO, catalog no. A3533, dilution 1:200) diluted with Antibody Diluent (DAKO SS0809, DAKO), LSAB2 Detection System (DAKO K0675, DAKO), DAB (DAKO K3466, DAKO), and TBS buffer (DAKO S1968, DAKO). The slides, which were not counterstained, were coverslipped automatically with a Leica CV5000 Coverslipper (Leica Microsystems, Inc., IL) and placed in opaque slide folders. In each staining batch of biopsy slides, positive and negative control slides were included. Tonsil, used as a control tissue for both apoptosis biomarkers, was processed in the same manner as the patient's tissue except that antibody diluent was used rather than primary antibody on the negative control slide.
Protocol for Quantifying Staining Density of Immunohistochemically Detected Biomarkers in Normal Colon Crypts (“Scoring”)
The method (“scoring”) used to describe and quantify various characteristics of the labeled antigens in the colon crypts was a quantitative image analysis procedure for antigens that are labeled with a wide range of intensities in gradient distributions along the crypt axis—something that cannot be done manually. The unit of analysis was the “hemicrypt”, defined as one-half of a crypt bisected from crypt base to colon lumen. A “scorable” hemicrypt was defined as an intact hemicrypt that extended from the muscularis mucosa to the colon lumen.
The major equipment and software for the image analysis procedures (“scoring”) were: personal computer, light microscope with appropriate filters and attached digital light microscope camera, digital drawing board, ImagePro Plus image analysis software (Media Cybernetics, Inc., MD), our custom developed plug-in software for colorectal crypt analysis, and Microsoft Access (Microsoft Corporation, WA). Equipment and imaging software settings were standardized. Slides were oriented in a standard manner and the section levels on the slides viewed in sequence using light microscopy at 200× magnification. The reader created a slide background correction image for the slide to be analyzed, and, focusing on the first hemicrypt, captured and transferred the image as a 16-bit per pixel grayscale image from the camera to the image analysis program. Next, the hemicrypt was analyzed by precisely tracing the borders of the hemicrypt using a digital drawing board. The program then created a crypt length line midway along the hemicrypt axis, and then drew equally spaced perpendicular lines to the crypt length line at intervals to yield segments with the average widths of normal colonocytes. Finally, the program adjusted for any background levels on the slide, measured the optical density of the labeling across the entire hemicrypt as well as within each segment, and entered the resulting data into the database automatically. Then, the reader moved to the next hemicrypt on the same or next image, section level, biopsy, and/or slide and repeated all the previously described analysis steps. The goal was to analyze a minimum of 16 hemicrypts on each of two biopsies, for a total of 32 hemicrypts.
One slide reader analyzed all of the Bax and Bcl-2 stained slides throughout the study. Blinded subsets of previously analyzed slides were resubmitted to the reader during the study to assess intra-reader reliability, which was found to range from 0.95 – 0.98 throughout.
Protocol for Measuring Serum 25-OH- and 1,25-(OH)2-Vitamin D Levels
Laboratory assays for plasma 25-OH-vitamin D and 1,25-(OH)2-vitamin D were performed by Dr. Bruce Hollis at the Medical University of South Carolina using a radioimmunoassay method as previously described (34, 35). Plasma samples for baseline and follow-up visits for all subjects were assayed together, ordered randomly, and labeled to mask treatment group, follow-up visit, and quality control replicates. The average intra-assay coefficient of variation for plasma 25-OH-vitamin D was 2.3 %, and for 1,25-(OH)2-vitamin D, 6.2 %.
Statistical Analysis
Treatment groups were assessed for comparability of characteristics at baseline and final follow-up by the Fisher's exact test for categorical variables and analysis of variance (ANOVA) for continuous variables. Slide “scoring” reliability was analyzed using intra-class correlation coefficients.
The mean density of staining for Bax, Bcl-2, and the Bax/Bcl-2 ratio in normal colon crypts was calculated for each patient at baseline and 6-months follow-up by summing all the densities from all analyzed crypts from the biopsy specimens and dividing by the number of crypts analyzed. Measures of the within-crypt distributions of the apoptotic markers (e.g., the ratio of expression in the upper 40% to the lower 60% of the crypts) were calculated for each patient by taking the mean of the biomarker densities in the upper 40% of crypts, or in the lower 60% of crypts, and constructing ratios of expression in the upper 40% to the lower 60% of crypts. We decided a priori to use as measures of the within-crypt distributions of the apoptotic markers the ratio of expression in the upper 40% (differentiation zone) to the lower 60% (proliferation zone) of the crypts, and the ratio of expression in the upper 20% (closest to colon lumen contents) to the lower 20% (furthest colon lumen contents) of the crypts because they represent the ratios of well recognized functional or exposure zones. We transformed biomarker expression density data by dividing each individual's measurement by the staining batch's mean density to adjust for possible batch effects, and then mean transformed biomarker densities were calculated for each treatment group for the baseline and 6-months follow-up visits.
Treatment effects were evaluated by assessing the differences in the transformed densities from baseline to the 6-months follow-up visit between patients in each active treatment group and the placebo group. The differences in the transformed densities from baseline to six months between each active treatment group and controls were tested with two-sided Wilcoxon exact non-parametric tests. The magnitude of the treatment effects on the biomarker staining densities and distributions were expressed as relative effects, defined as: [treatment group follow-up mean/treatment group baseline mean]/[placebo follow-up mean/placebo baseline mean]. The interpretation of the relative effect is somewhat analogous to that of an odds ratio (e.g., a relative effect of 2.0 would mean that the relative proportional change in the treatment group was twice as great as that in the placebo group). Primary analyses were based on randomization treatment assignment regardless of adherence status (intent-to-treat analysis).
The distributions of Bax and Bcl-2 batch-standardized staining densities were plotted along the colorectal crypts by normalizing each crypt to 50 sections, averaging within each section across all crypts separately for each patient, and then for each treatment group.
In sensitivity analyses, we also analyzed data without batch standardization by including batch as a covariate, and using different transformations; the results from these analyses did not differ materially from those reported.
Statistical analyses were done using SAS System software (version 9.1; SAS Institute, Inc., NC). A cutoff level of P ≤ 0.05 (2-sided) was used for assessing statistical significance.
Results
Study Participants
Treatment groups did not differ significantly on characteristics measured at baseline (Table 1) or at final follow-up (data not shown). The mean age of participants was 61 years, 64% were men, 71% were white, and 20% had a family history of colorectal cancer in a first degree relative. Most participants were non-smokers, college graduates, and overweight.
Table 1. Selected Baseline Characteristics of the Study Participants* (n = 92).
| Treatment Group | |||||
|---|---|---|---|---|---|
| Characteristics | Placebo (n = 23) |
Calcium (n = 23) |
Vitamin D (n = 23) |
Calcium + vitamin D (n = 23) |
P-value** |
| Demographics | |||||
| Age, years | 58.5 (8.2) | 61.9 (8.2) | 60.2 (8.1) | 62.1 (7.5) | 0.39 |
| Men (%) | 70 | 70 | 70 | 70 | 1.00 |
| White (%) | 74 | 83 | 65 | 61 | 0.39 |
| College graduate (%) | 65 | 61 | 57 | 44 | 0.53 |
| Medical history | |||||
| History of colorectal cancer in 1° relative (%) | 17 | 30 | 17 | 13 | 0.60 |
| Take NSAID*** regularly§ (%) | 22 | 13 | 9 | 22 | 0.60 |
| Take aspirin regularly (%) | 22 | 52 | 30 | 56 | 0.05 |
| If woman (n = 28), taking estrogens (%) | 4 | 9 | 4 | 4 | 1.00 |
| Habits | |||||
| Current smoker (%) | 9 | 4 | 0 | 0 | 0.61 |
| Take multivitamin (%) | 30 | 30 | 26 | 39 | 0.86 |
| Mean dietary intakes | |||||
| Total energy intake, kcal/d | 1,596 (528) | 1,788 (691) | 1,848 (821) | 1,845 (752) | 0.59 |
| Total calcium§§, mg/d | 618 (308) | 746 (335) | 843 (526) | 824 (714) | 0.41 |
| Total vitamin D§§, IU/d | 277 (230) | 336 (202) | 360 (317) | 415 (316) | 0.40 |
| Total fat, gm/d | 67 (32) | 72 (35) | 70 (32) | 74 (28) | 0.59 |
| Dietary fiber, gm/d | 15 (7) | 17 (9) | 18 (9) | 17 (11) | 0.97 |
| Alcohol, gm/d | 9 (14) | 11 (15) | 14 (18) | 10 (20) | 0.84 |
| Anthropometrics | |||||
| Body mass index (BMI), kg/m2 | 30.6 (7.2) | 29.4 (5.5) | 28.9 (5.56) | 31.6 (6.0) | 0.44 |
| Waist-to-hip ratio | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | 1.0 (0.1) | 0.17 |
| VDR BsmI genotype (%) | |||||
| bb | 35 | 39 | 48 | 30 | 0.25 |
| Bb | 35 | 57 | 43 | 52 | |
| BB | 30 | 4 | 9 | 17 | |
| Serum vitamin D | |||||
| 25-OH-vitamin D, ng/mL | 20.4 (7.6) | 25.7 (7.6) | 21.0 (8.3) | 20.9 (9.7) | 0.12 |
| 1,25-(OH)2-vitamin D, pg/mL | 39.2 (12.2) | 45.4 (35.3) | 44.5 (22.6) | 37.9 (12.5) | 0.60 |
Data are given as means (SD) unless otherwise specified.
By Fisher's exact χ2 test for categorical variables, and by ANOVA for continuous variables.
Nonsteroidal anti-inflammatory drug.
At least once a week.
Diet plus supplements.
Adherence to visit attendance averaged 92% and did not differ significantly among the four treatment groups. On average, at least 80% of pills were taken by 93% of participants at the first follow-up visit and 84% at the final follow-up visit. There were no adverse events attributed to study procedures or treatments. Seven participants (8%) were lost to follow-up due to perceived drug intolerance (n=2), unwillingness to continue participation (n=3), physician's advice (n=1), and death (n=1). Dropouts included one person from the vitamin D supplementation group, and two persons from each of other three groups.
At baseline, there were no significant differences between the four study groups in serum 25-OH- or 1,25-(OH)2-vitamin D levels (Table 2). By study end, serum 25-OH-vitamin D levels had statistically significantly increased in the vitamin D and calcium plus vitamin D groups, and appeared to have slightly, non-significantly decreased in the placebo and calcium groups (Table 2). As expected, serum levels of 1,25-(OH)2-vitamin D at the end of follow-up did not differ significantly between treatment groups (Table 2).
Table 2. Serum 25-OH-vitamin D and 1, 25-(OH)2-vitamin D, Bax and Bcl-2 Expression in Colorectal Crypts at Baseline and 6-months Follow-Up Shown as Batch-Standardized¶ Optical Density of Staining from the Immunohistochemically-detected Biomarkers.
| Baseline | 6-Months Follow-up | Absolute Difference** | Relative Effect § | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | p-value* | N | Mean | SD | p-value* | N | Mean | SD | p-value* | ||
| Serum Vitamin D | |||||||||||||
| 25-OH-vitamin D¥ | |||||||||||||
| Calcium + vitamin D | 23 | 20.93 | 9.65 | 0.84 | 21 | 28.51 | 7.94 | <0.0001 | 21 | 7.59 | 6.19 | <0.0001 | 1.56 |
| Vitamin D | 23 | 21.04 | 8.33 | 0.81 | 22 | 29.48 | 7.23 | <0.0001 | 22 | 8.44 | 6.05 | <0.0001 | 1.60 |
| Calcium | 23 | 25.67 | 7.59 | 0.05 | 21 | 23.20 | 8.88 | 0.03 | 21 | -2.46 | 4.45 | 0.88 | 1.03 |
| Placebo | 23 | 20.44 | 7.55 | N/A | 21 | 17.89 | 6.93 | N/A | 21 | -2.55 | 6.00 | N/A | 1.00 |
| 1,25-(OH)2-vitamin D¥ | |||||||||||||
| Calcium + vitamin D | 23 | 37.89 | 12.54 | 0.85 | 21 | 36.71 | 28.84 | 0.95 | 21 | -1.18 | 32.81 | 0.97 | 1.02 |
| Vitamin D | 23 | 44.48 | 22.58 | 0.44 | 22 | 47.40 | 27.97 | 0.13 | 22 | 2.92 | 35.74 | 0.57 | 1.12 |
| Calcium | 23 | 45.37 | 35.31 | 0.36 | 21 | 31.66 | 12.44 | 0.42 | 21 | -13.71 | 32.49 | 0.21 | 0.74 |
| Placebo | 23 | 39.17 | 12.19 | N/A | 21 | 37.13 | 10.70 | N/A | 21 | -2.04 | 9.76 | N/A | 1.00 |
| Biomarker Expression in Colorectal Crypts | |||||||||||||
| A. Entire crypts | |||||||||||||
| Bax | |||||||||||||
| Calcium + vitamin D | 23 | 0.97 | 0.50 | 0.59 | 21 | 1.01 | 0.31 | 0.43 | 21 | 0.01 | 0.60 | 0.36 | 1.33 |
| Vitamin D | 23 | 0.82 | 0.44 | 0.05 | 22 | 1.00 | 0.28 | 0.56 | 22 | 0.16 | 0.54 | 0.02 | 1.56 |
| Calcium | 23 | 1.00 | 0.36 | 0.70 | 21 | 1.04 | 0.42 | 0.43 | 21 | 0.03 | 0.59 | 0.31 | 1.33 |
| Placebo | 23 | 1.21 | 0.87 | N/A | 21 | 0.94 | 0.32 | N/A | 21 | -0.30 | 0.83 | N/A | 1.00 |
| Bcl-2 | |||||||||||||
| Calcium + vitamin D | 23 | 1.15 | 0.56 | 0.19 | 21 | 1.03 | 0.43 | 0.62 | 21 | -0.12 | 0.66 | 0.57 | 0.84 |
| Vitamin D | 23 | 0.87 | 0.47 | 0.58 | 22 | 1.06 | 0.40 | 0.57 | 22 | 0.17 | 0.58 | 0.25 | 1.14 |
| Calcium | 23 | 1.06 | 0.62 | 0.66 | 21 | 0.91 | 0.50 | 0.44 | 21 | -0.17 | 0.86 | 0.80 | 0.80 |
| Placebo | 23 | 0.93 | 0.46 | N/A | 21 | 0.99 | 0.40 | N/A | 21 | 0.07 | 0.60 | N/A | 1.00 |
| Bax/Bcl-2 ratio | |||||||||||||
| Calcium + vitamin D | 23 | 0.89 | 0.37 | 0.01 | 21 | 1.06 | 0.30 | 0.62 | 21 | 0.15 | 0.49 | 0.08 | 1.71 |
| Vitamin D | 23 | 1.03 | 0.46 | 0.05 | 22 | 1.06 | 0.43 | 0.91 | 22 | 0.02 | 0.43 | 0.37 | 1.47 |
| Calcium | 23 | 1.37 | 1.23 | 0.38 | 21 | 1.55 | 1.55 | 0.43 | 21 | 0.15 | 2.09 | 0.52 | 1.62 |
| Placebo | 23 | 1.54 | 1.55 | N/A | 21 | 1.08 | 0.50 | N/A | 21 | -0.51 | 1.63 | N/A | 1.00 |
| B. Upper 40% of crypts | |||||||||||||
| Bax | |||||||||||||
| Calcium + vitamin D | 21 | 0.014 | 0.01 | 0.29 | 21 | 0.018 | 0.01 | 0.40 | 21 | 0.004 | 0.01 | 0.13 | 1.52 |
| Vitamin D | 22 | 0.011 | 0.01 | 0.03 | 22 | 0.017 | 0.00 | 0.27 | 22 | 0.006 | 0.01 | 0.01 | 1.80 |
| Calcium | 21 | 0.016 | 0.01 | 0.63 | 21 | 0.017 | 0.01 | 0.52 | 21 | 0.002 | 0.01 | 0.60 | 1.25 |
| Placebo | 21 | 0.018 | 0.01 | N/A | 21 | 0.016 | 0.01 | N/A | 21 | -0.003 | 0.01 | N/A | 1.00 |
| Bcl-2 | |||||||||||||
| Calcium + vitamin D | 21 | 0.001 | 0.00 | 0.07 | 21 | 0.002 | 0.00 | 0.78 | 21 | 0.001 | 0.00 | 0.27 | 0.52 |
| Vitamin D | 22 | 0.001 | 0.00 | 0.76 | 22 | 0.002 | 0.00 | 0.90 | 22 | 0.001 | 0.00 | 0.66 | 0.71 |
| Calcium | 21 | 0.001 | 0.00 | 1.00 | 21 | 0.002 | 0.00 | 0.38 | 21 | 0.001 | 0.00 | 0.50 | 0.84 |
| Placebo | 21 | 0.001 | 0.00 | N/A | 21 | 0.002 | 0.00 | N/A | 21 | 0.002 | 0.00 | N/A | 1.00 |
| Bax/Bcl-2 ratio | |||||||||||||
| Calcium + vitamin D | 21 | 24.17 | 37.46 | 0.03 | 21 | 15.37 | 10.64 | 0.40 | 21 | -10.43 | 33.49 | 0.11 | 0.97 |
| Vitamin D | 22 | 22.33 | 17.83 | 0.17 | 22 | 23.17 | 30.69 | 0.46 | 22 | 0.17 | 23.40 | 0.14 | 1.59 |
| Calcium | 21 | 36.67 | 32.32 | 0.85 | 21 | 29.01 | 45.97 | 0.33 | 21 | -7.60 | 56.13 | 0.88 | 1.21 |
| Placebo | 21 | 45.61 | 57.42 | N/A | 21 | 29.80 | 66.10 | N/A | 21 | -16.78 | 94.47 | N/A | 1.00 |
| C. Ratio of upper 40% to lower 60% of crypts | |||||||||||||
| Calcium + vitamin D | 21 | 0.70 | 0.27 | 0.27 | 21 | 0.95 | 0.19 | 0.15 | 21 | 0.24 | 0.36 | 0.25 | 1.19 |
| Vitamin D | 22 | 0.62 | 0.32 | 0.07 | 22 | 0.89 | 0.16 | 0.97 | 22 | 0.27 | 0.34 | 0.06 | 1.26 |
| Calcium | 21 | 0.75 | 0.36 | 0.85 | 21 | 0.85 | 0.22 | 0.53 | 21 | 0.12 | 0.43 | 0.80 | 1.00 |
| Placebo | 21 | 0.77 | 0.25 | N/A | 21 | 0.87 | 0.14 | N/A | 21 | 0.09 | 0.33 | N/A | 1.00 |
| Bcl-2 | |||||||||||||
| Calcium + vitamin D | 21 | 0.05 | 0.03 | 0.23 | 21 | 0.07 | 0.05 | 0.86 | 21 | 0.02 | 0.05 | 0.69 | 0.70 |
| Vitamin D | 22 | 0.06 | 0.13 | 0.95 | 22 | 0.06 | 0.04 | 0.67 | 22 | 0.00 | 0.12 | 0.61 | 0.47 |
| Calcium | 21 | 0.03 | 0.03 | 0.54 | 21 | 0.08 | 0.09 | 0.62 | 21 | 0.05 | 0.09 | 0.96 | 1.09 |
| Placebo | 21 | 0.04 | 0.03 | N/A | 21 | 0.08 | 0.06 | N/A | 21 | 0.04 | 0.07 | N/A | 1.00 |
| Bax/Bcl-2 ratio | |||||||||||||
| Calcium + vitamin D | 21 | 32.68 | 53.91 | 0.08 | 21 | 20.04 | 13.26 | 0.47 | 21 | -14.29 | 47.55 | 0.28 | 0.91 |
| Vitamin D | 22 | 28.45 | 22.26 | 0.45 | 22 | 31.73 | 44.18 | 0.64 | 22 | 2.57 | 36.41 | 0.19 | 1.66 |
| Calcium | 21 | 41.29 | 35.05 | 0.97 | 21 | 19.66 | 11.34 | 0.73 | 21 | -20.97 | 33.31 | 0.90 | 0.71 |
| Placebo | 21 | 41.23 | 46.16 | N/A | 21 | 27.75 | 43.67 | N/A | 21 | -14.28 | 68.02 | N/A | 1.00 |
| D. Ratio of upper 20% to lower 20% of crypts | |||||||||||||
| Calcium + vitamin D | 21 | 0.55 | 0.32 | 0.21 | 21 | 0.84 | 0.27 | 0.20 | 21 | 0.28 | 0.42 | 0.22 | 1.31 |
| Vitamin D | 22 | 0.52 | 0.40 | 0.08 | 22 | 0.77 | 0.18 | 0.78 | 22 | 0.24 | 0.44 | 0.14 | 1.27 |
| Calcium | 21 | 0.63 | 0.40 | 0.97 | 21 | 0.74 | 0.29 | 0.57 | 21 | 0.11 | 0.50 | 0.92 | 0.99 |
| Placebo | 21 | 0.63 | 0.28 | N/A | 21 | 0.74 | 0.19 | N/A | 21 | 0.09 | 0.39 | N/A | 1.00 |
| Bcl-2 | |||||||||||||
| Calcium + vitamin D | 21 | 0.01 | 0.01 | 0.15 | 21 | 0.02 | 0.02 | 0.71 | 21 | 0.01 | 0.03 | 0.49 | 0.56 |
| Vitamin D | 22 | 0.01 | 0.03 | 0.60 | 22 | 0.02 | 0.02 | 0.43 | 22 | 0.001 | 0.02 | 0.12 | 0.32 |
| Calcium | 21 | 0.01 | 0.01 | 0.70 | 21 | 0.03 | 0.04 | 0.90 | 21 | 0.02 | 0.04 | 0.78 | 0.92 |
| Placebo | 21 | 0.01 | 0.01 | N/A | 21 | 0.02 | 0.02 | N/A | 21 | 0.02 | 0.03 | N/A | 1.00 |
| Bax/Bcl-2 ratio | |||||||||||||
| Calcium + vitamin D | 21 | 174.48 | 310.09 | 0.08 | 21 | 101.47 | 108.63 | 0.57 | 21 | -86.77 | 247.09 | 0.19 | 1.56 |
| Vitamin D | 22 | 175.65 | 211.44 | 0.16 | 22 | 303.18 | 682.54 | 0.43 | 22 | 120.26 | 659.71 | 0.04 | 4.64 |
| Calcium | 21 | 212.88 | 235.39 | 0.54 | 21 | 65.10 | 44.07 | 1.00 | 21 | -157.37 | 233.21 | 0.81 | 0.82 |
| Placebo | 21 | 225.01 | 215.08 | N/A | 21 | 83.73 | 90.47 | N/A | 21 | -151.99 | 251.61 | N/A | 1.00 |
Batch standardization for each biomarker was performed by dividing each individual measurement by the staining batch's average density.
Exact two-sided non-parametric test p-value for difference between each active treatment group and placebo group.
Absolute Difference = [treatment group follow-up - treatment group baseline] or [placebo group follow-up - placebo group baseline].
Relative effect = [(treatment group follow-up/treatment group baseline)/(placebo follow-up/placebo baseline)]; interpretation as for odds ratio (e.g., a relative effect of 1.8 indicates a proportional increase of 80% in the treatment group relative to that in the placebo group).
Differences at visit 2 & 4 and follow-up minus baseline measurements (Absolute Difference) were tested by analysis of variance (ANOVA).
Graphical Assessment of Changes over Six Months in the Distributions of Bax and Bcl-2 Expression along Normal Colorectal Crypts
The distributions of Bax and Bcl-2 staining densities (“expression”) along the colorectal crypts at the baseline and 6-months follow-up visits are shown in Figures 2 and 3, respectively. Bax and Bcl-2 staining densities were batch-standardized and multiplied by 100 for graphical presentation. In the placebo group, Bax expression appeared to decrease, especially in the crypt bases, while Bcl-2 expression appeared unchanged from baseline to follow-up (Figures 2.A and 3.A). In the calcium group, Bax expression did not appear to change from baseline to follow-up, whereas Bcl-2 expression tended to be lower in the crypt bases (Figures 2.B and 3.B). In the vitamin D group, there were no apparent changes in Bcl-2 expression; however, Bax expression appeared to increase, especially in the crypt opening onto the colon lumen (“crypt mouth”) (Figures 2.C and 3.C). In the calcium plus vitamin D group, there appeared to be slight decreases in Bcl-2 and Bax expression in the crypt bases and a slight increase in Bax expression in the crypt mouth (Figures 2.D and 3.D).
Figure 2. Distribution of Bax Staining Densities along Normal Colorectal Crypts by Treatment Group at Baseline and Follow-Up. A, Placebo Group. B, Calcium Group. C, Vitamin D Group. D, Calcium + Vitamin D Group.
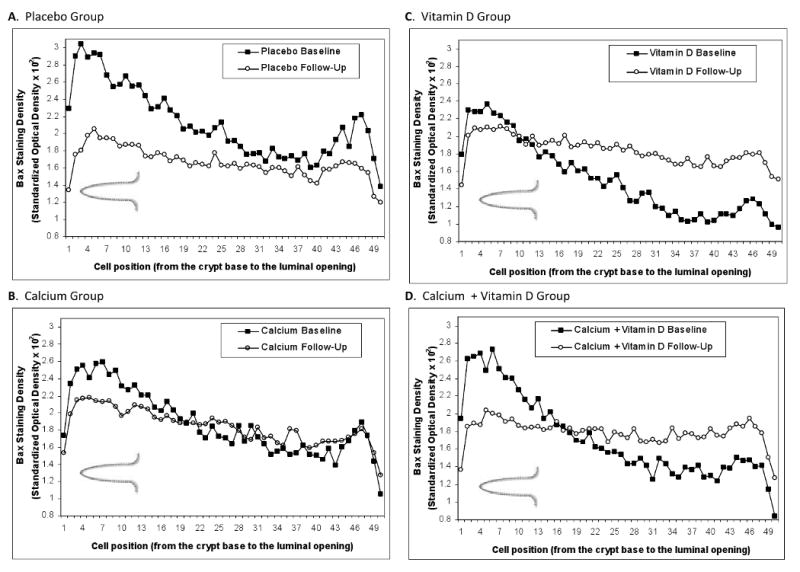
Figure 3. Distribution of Bcl-2 Staining Densities along Normal Colorectal Crypts by Treatment Group at Baseline and Follow-Up. A, Placebo Group. B, Calcium Group. C, Vitamin D Group. D, Calcium + Vitamin D Group.

Effects of Calcium and/or Vitamin D on the Separate and Relative Expressions of Bax and Bcl-2 in Normal Colorectal Crypts
After six months treatment, Bax expression along the full lengths of crypts increased proportionately by 56% (p=0.02) in the vitamin D group and 33% in both the calcium (p=0.31) and calcium plus vitamin D (p=0.36) groups relative to the placebo group (Table 2, A). The vitamin D treatment effect on Bax expression was more pronounced (80%, p=0.01) in the canonical differentiation zone, or upper 40%, of crypts (Table 2, B). Also, Bax expression in the upper 40% relative to the lower 60% of the crypts increased by 26% (p=0.06) in the vitamin D group relative to the placebo group (Table 2, C). There were no statistically significant treatment effects on Bcl-2 expression along the entire crypt, in the upper 40% of crypts, or in the ratios representing the biomarker's distribution in the crypts; however, there was a suggestion of some decrease in Bcl-2 expression in the calcium and calcium plus vitamin D groups (Table 2, A). The estimated relative treatment effects on the Bax/Bcl-2 ratio along the entire crypt in the calcium, vitamin D, and calcium plus vitamin D groups were increases of 62% (p=0.52), 47% (p=0.37), and 71% (p=0.08), respectively (Table 2, A). For the vitamin D group relative to the placebo group, the proportional increase in the Bax/Bcl-2 ratio in the upper 20% relative to the lower 20% of crypts was 464% (p=0.04).
Discussion
This study had intertwined missions of further clarification and/or development of calcium and vitamin D as chemopreventive agents against colorectal cancer in humans, understanding the mechanisms by which these agents reduce risk for the disease in humans, and the development of treatable biomarkers of risk for colorectal cancer. It addressed these missions by exploring the efficacy of two plausible and evidentially well-supported dietary agents, calcium and vitamin D3 (alone and in combination), on modulating a plausible set of molecular phenotypic biomarkers of risk for colorectal neoplasia. In this preliminary trial we found strong evidence for an increase in Bax expression in normal colon crypts of sporadic colorectal adenoma patients in response to six months of vitamin D supplementation, a finding that is consistent with the hypothesis that a higher intake of vitamin D may increase pro-apoptotic stimuli in the normal human colon mucosa and reduce risk for colorectal cancer. Our findings also indicated that the strongest treatment effect on Bax expression was in the upper sections of colorectal crypts, suggesting that vitamin D increases apoptosis in the parts of colorectal crypts most exposed to bowel lumen carcinogens. Although not statistically significant, our data also suggested that calcium and calcium plus vitamin D supplementation may reduce Bcl-2 expression, that treatment effects of calcium and/or vitamin D on Bax may be more pronounced relative to Bcl-2 expression (emphasizing the importance of evaluating both apoptotic markers together), and that there may be stronger treatment effects of vitamin D when combined with calcium than for vitamin D alone.
This trial emphasizes the importance of randomization to treatment assignment and a placebo-control group in cancer chemoprevention trials. In the placebo group we observed a decrease in Bax expression and in the Bax/Bcl-2 ratio after six months of follow-up. The cause(s) for the time-related influence(s) producing these decreases in the placebo group is unknown, but may have been due to the Hawthorne effect (participants in clinical trials change their health-related behaviors while under observation), some participants may have been developing recurrent polyps, laboratory drift, and/or chance. In clinical trials such extraneous temporal influences are presumed to occur equally across all treatment groups; therefore, change in the placebo group is “subtracted” from any change in an active treatment group to yield the true treatment effect. Thus, in this trial, without a placebo-control group the treatment effect on apoptotic markers would have been underestimated.
Although we hypothesized that the effects of calcium plus vitamin D on the apoptotic markers would be greater than from either agent alone, we found that Bax expression went up more in the vitamin D group than in the vitamin D plus calcium group. There are several possible explanations for this finding, including chance—especially considering the small sample size—and that the two agents may have attenuated the effects of either alone. In at least one 2×2 factorial experiment of calcium and vitamin D in rodents substantial treatment effects in proliferation markers were found for the individual agents but not for the combination (36). In a Women's Health Initiative randomized clinical trial there was no evidence for an overall treatment effect from the combination of supplemental calcium (1,000 mg daily) and vitamin D (400 IU daily) on colorectal cancer incidence (37); however, these overall results are difficult to interpret because of the low doses and high rates of intervention agent drop in and drop out. On the other hand, most animal studies that investigated the combination of calcium and vitamin D reported that supplemental vitamin D has stronger anti-neoplastic effects in animals given relatively high-calcium diets (38-40); in two large cohort studies (41, 42), there was clear evidence of a positive interaction between the two nutrients; and in the Calcium Polyp Prevention adenoma recurrence trial, there were strong indications that vitamin D enhanced the chemopreventive effect of calcium (43).
There is substantial evidence that markers of apoptosis, including Bax and Bcl-2 expression, are plausible candidates for treatable biomarkers of risk for colorectal neoplasms. Failure to delete cells with accumulated genetic and epigenetic changes via apoptosis is an important step in colon carcinogenesis that may lead to adenoma development, and thus colorectal cancer (44, 45). Inadequate rates of apoptosis may be a consequence of the separate and combined influences of multiple genetic, epigenetic, and environmental factors. Pro- or anti-apoptotic tendencies in the normal colon mucosa are reflected by the expression of Bax and Bcl-2 proteins, respectively. Thus, measuring both of these proteins in colon crypts provides a good indicator of apoptosis.
There is substantial evidence from in vitro (10-13) and animal studies (14, 15) that calcium and vitamin D enhance apoptosis in colonocytes. Possible mechanisms include direct effects on apoptotic proteins, mediated in part by the VDR and the CaSR (46, 47), induction of differentiation with subsequent promotion of apoptosis (10), indirect effects on apoptosis as a result of decreased inflammation (48), and others. Our data are consistent with the hypothesis that vitamin D promotes apoptosis in the normal human colon mucosa. We found an increase in the expression of pro-apoptotic Bax in the entire and upper 40% of colorectal crypts. We also observed statistically non-significant increases in Bax and decreases in Bcl-2 expression in the calcium and calcium plus vitamin D groups relative to the placebo group. The Bax/Bcl-2 ratio increased in the entire colorectal crypt after calcium and vitamin D supplementation, indicating a shift toward more apoptosis. The ratio of the upper 40% to the lower 60% of crypts represents the ratio of two functionally distinct zones, differentiation and proliferation. Vitamin D supplementation increased Bax and the balance of Bax to Bcl-2 in ratios of the upper 40% to the lower 60% of crypts, which can mostly be explained by an increase of Bax in the upper 40% of crypts. Another ratio that we constructed was of the upper 20% to the lower 20% of crypts, which represented the contrast between the areas of the crypts most proximal to or distant from damaging colon lumen contents. Again, in the ratio of the upper 20% to the lower 20% of crypts, we observed a significant increase in the balance of Bax to Bcl-2, some decrease in Bcl-2, and some increase in Bax in the vitamin D and calcium plus vitamin D groups. No effects of calcium supplementation on the apoptosis markers in the upper 20% relative to the lower 20% of crypts were observed.
There are few reported human studies of effects of supplemental calcium and vitamin D, including no large randomized trials of their combined effects, on apoptotic markers in the normal colon mucosa. One small, pilot cross-over study (n = 40) of calcium supplementation or low-fat dairy foods and biomarkers of apoptosis reported no change in epithelial cell apoptosis (measured by the terminal nucleotidyl transferase-mediated nick-end-labeling method (TUNEL)) or the expression of the pro-apoptotic gene product Bak in the normal-appearing colon mucosa (16). Another small study (n = 21) found no effects of calcium and vitamin D supplementation over six months on apoptosis by the TUNEL method or on Bcl-2 expression in either normal rectal mucosa or in situ polyps (17), but did find an increase in the frequency of Bak immunostained cells in the polyps (17). In a cross-sectional study weak, non-statistically significant direct associations between dietary calcium and an apoptosis score were found in patients both with and without adenomas (n = 498), and an inverse non-statistically significant association between serum vitamin D levels and an apoptosis score was found in adenoma patients (n = 92) (18).
This study has several limitations and strengths. Treatment effects could not be examined in parts of the colon other than the rectum; however, several studies suggest that patterns or levels of apoptosis across levels of the colon may be highly correlated (49, 50). Apoptotic markers are not proven biomarkers of risk for colon cancer; however, substantial basic science literature supports an important role for apoptosis in colon carcinogenesis. This study cannot prove that because vitamin D or calcium can increase apoptosis in normal colon tissue, they can reduce risk for colon cancer. On the other hand, this study is the only randomized, double-blind, placebo-controlled trial to have assessed the independent and combined effects of supplemental calcium and vitamin D on apoptosis markers in the normal colorectal epithelium, there was high protocol adherence by study participants, immunostaining was automated, and, via the use of novel quantitative image analysis procedures, biopsy analysis reliability was high.
Overall, these results from this pilot clinical trial suggest that 1) vitamin D and calcium, individually or together, may enhance apoptosis in the normal human colorectal epithelium; 2) that they do so via upregulating Bax expression alone or relative to Bcl-2 expression; 3) that the strongest treatment effects may be vitamin D related and in the upper sections of the colorectal crypts; and 4) that Bax expression alone or in combination with Bcl-2 expression, may be a treatable biomarker of risk for colorectal neoplasms.
Supplementary Material
Acknowledgments
Funding: National Cancer Institute, National Institutes of Health (R01 CA104637 to R. B.); Georgia Cancer Coalition Distinguished Scholar award (to R. B.). The National Cancer Institute and the Georgia Cancer Coalition had no influence on the design of the study; the collection, analysis, and interpretation of the data; the decision to submit the manuscript for publication; or the writing of the manuscript.
Note: We thank Jill Joelle Woodard and Bonita Feinstein for managing the study; Dr. Bruce W. Hollis for conducting blood vitamin D assays; Dr. Mark M. Bouzyk for conducting VDR genotyping analysis; Christopher Farino and Stuart Myerberg for development of the study database; Charles Reichley for development of the computer scoring software; the physicians of the Emory Clinic, GA for work on biopsy procurement; and all study participants for their time and dedication to the study.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Potter JD, Slattery ML, Bostick RM, Gapstur SM. Colon cancer: a review of the epidemiology. Epidemiol Rev. 1993;15(2):499–545. doi: 10.1093/oxfordjournals.epirev.a036132. [DOI] [PubMed] [Google Scholar]
- 3.Fraser G. Preventive Cardiology. New York, NY: Oxford University Press; 1986. [Google Scholar]
- 4.Newmark HL, Lipkin M. Calcium, vitamin D, and colon cancer. Cancer Res. 1992;52(7 Suppl):2067s–70s. [PubMed] [Google Scholar]
- 5.Newmark HL, Wargovich MJ, Bruce WR. Colon cancer and dietary fat, phosphate, and calcium: a hypothesis. J Natl Cancer Inst. 1984;72(6):1323–5. [PubMed] [Google Scholar]
- 6.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3(8):601–14. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarty S, Wang H, Canaff L, Hendy GN, Appelman H, Varani J. Calcium sensing receptor in human colon carcinoma: interaction with Ca(2+) and 1,25-dihydroxyvitamin D(3) Cancer Res. 2005;65(2):493–8. [PubMed] [Google Scholar]
- 8.Rodland KD. The role of the calcium-sensing receptor in cancer. Cell calcium. 2004;35(3):291–5. doi: 10.1016/j.ceca.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. American journal of physiology. 2005;289(1):F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 10.Diaz GD, Paraskeva C, Thomas MG, Binderup L, Hague A. Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: possible implications for prevention and therapy. Cancer Res. 2000;60(8):2304–12. [PubMed] [Google Scholar]
- 11.Vandewalle B, Wattez N, Lefebvre J. Effects of vitamin D3 derivatives on growth, differentiation and apoptosis in tumoral colonic HT 29 cells: possible implication of intracellular calcium. Cancer letters. 1995;97(1):99–106. doi: 10.1016/0304-3835(95)03958-y. [DOI] [PubMed] [Google Scholar]
- 12.Evans SR, Soldatenkov V, Shchepotin EB, Bogrash E, Shchepotin IB. Novel 19-nor-hexafluoride vitamin D3 analog (Ro 25-6760) inhibits human colon cancer in vitro via apoptosis. Int J Oncol. 1999;14(5):979–85. doi: 10.3892/ijo.14.5.979. [DOI] [PubMed] [Google Scholar]
- 13.Kumagai T, O'Kelly J, Said JW, Koeffler HP. Vitamin D2 analog 19-nor-1,25-dihydroxyvitamin D2: antitumor activity against leukemia, myeloma, and colon cancer cells. J Natl Cancer Inst. 2003;95(12):896–905. doi: 10.1093/jnci/95.12.896. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Tomotake H, Wan G, Watanabe H, Kato N. Combined effect of dietary calcium and iron on colonic aberrant crypt foci, cell proliferation and apoptosis, and fecal bile acids in 1,2-dimethylhydrazine-treated rats. Oncol Rep. 2001;8(4):893–7. doi: 10.3892/or.8.4.893. [DOI] [PubMed] [Google Scholar]
- 15.Harris DM, Go VL. Vitamin D and colon carcinogenesis. J Nutr. 2004;134(12 Suppl):3463S–71S. doi: 10.1093/jn/134.12.3463S. [DOI] [PubMed] [Google Scholar]
- 16.Holt PR, Wolper C, Moss SF, Yang K, Lipkin M. Comparison of calcium supplementation or low-fat dairy foods on epithelial cell proliferation and differentiation. Nutr Cancer. 2001;41(1-2):150–5. doi: 10.1080/01635581.2001.9680626. [DOI] [PubMed] [Google Scholar]
- 17.Holt PR, Bresalier RS, Ma CK, et al. Calcium plus vitamin D alters preneoplastic features of colorectal adenomas and rectal mucosa. Cancer. 2006;106(2):287–96. doi: 10.1002/cncr.21618. [DOI] [PubMed] [Google Scholar]
- 18.Miller EA, Keku TO, Satia JA, Martin CF, Galanko JA, Sandler RS. Calcium, vitamin D, and apoptosis in the rectal epithelium. Cancer Epidemiol Biomarkers Prev. 2005;14(2):525–8. doi: 10.1158/1055-9965.EPI-04-0466. [DOI] [PubMed] [Google Scholar]
- 19.Oh K, Willett WC, Wu K, Fuchs CS, Giovannucci EL. Calcium and vitamin D intakes in relation to risk of distal colorectal adenoma in women. American journal of epidemiology. 2007;165(10):1178–86. doi: 10.1093/aje/kwm026. [DOI] [PubMed] [Google Scholar]
- 20.Kesse E, Boutron-Ruault MC, Norat T, Riboli E, Clavel-Chapelon F. Dietary calcium, phosphorus, vitamin D, dairy products and the risk of colorectal adenoma and cancer among French women of the E3N-EPIC prospective study. Int J Cancer. 2005;117(1):137–44. doi: 10.1002/ijc.21148. [DOI] [PubMed] [Google Scholar]
- 21.Peters U, Chatterjee N, McGlynn KA, et al. Calcium intake and colorectal adenoma in a US colorectal cancer early detection program. The American journal of clinical nutrition. 2004;80(5):1358–65. doi: 10.1093/ajcn/80.5.1358. [DOI] [PubMed] [Google Scholar]
- 22.Baron JA, Beach M, Mandel JS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340:101–7. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 23.Weingarten MA, Zalmanovici A, Yaphe J. Dietary calcium supplementation for preventing colorectal cancer and adenomatous polyps. Cochrane Database Syst Rev. 2008;(1):CD003548. doi: 10.1002/14651858.CD003548.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez ME, Marshall JR, Sampliner R, Wilkinson J, Alberts DS. Calcium, vitamin D, and risk of adenoma recurrence (United States) Cancer Causes Control. 2002;13(3):213–20. doi: 10.1023/a:1015032215779. [DOI] [PubMed] [Google Scholar]
- 25.Peters U, Hayes RB, Chatterjee N, et al. Circulating vitamin D metabolites, polymorphism in vitamin D receptor, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2004;13:546–52. [PubMed] [Google Scholar]
- 26.Peters U, McGlynn KA, Chatterjee N, et al. Vitamin D, calcium, and vitamin D receptor polymorphism in colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2001;10:1267–74. [PubMed] [Google Scholar]
- 27.Bostick RM, Fosdick L, Lillemoe TJ, et al. Methodological findings and considerations in measuring colorectal epithelial cell proliferation in humans. Cancer Epidemiol Biomarkers Prev. 1997;6(11):931–42. [PubMed] [Google Scholar]
- 28.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. American journal of epidemiology. 1988;127(1):188–99. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 29.Baron JA, Beach M, Mandel JS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. The New England journal of medicine. 1999;340(2):101–7. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 30.Bostick RM, Fosdick L, Wood JR, et al. Calcium and colorectal epithelial cell proliferation in sporadic adenoma patients: a randomized, double-blinded, placebo-controlled clinical trial. Journal of the National Cancer Institute. 1995;87(17):1307–15. doi: 10.1093/jnci/87.17.1307. [DOI] [PubMed] [Google Scholar]
- 31.Lau KH, Baylink DJ. Vitamin D therapy of osteoporosis: plain vitamin D therapy versus active vitamin D analog (D-hormone) therapy. Calcified tissue international. 1999;65(4):295–306. doi: 10.1007/s002239900702. [DOI] [PubMed] [Google Scholar]
- 32.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. The American journal of clinical nutrition. 1999;69(5):842–56. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 33.Byrne PM, Freaney R, McKenna MJ. Vitamin D supplementation in the elderly: review of safety and effectiveness of different regimes. Calcified tissue international. 1995;56(6):518–20. doi: 10.1007/BF00298580. [DOI] [PubMed] [Google Scholar]
- 34.Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol. 1997;282:174–86. doi: 10.1016/s0076-6879(97)82106-4. [DOI] [PubMed] [Google Scholar]
- 35.Hollis BW, Kamerud JQ, Kurkowski A, Beaulieu J, Napoli JL. Quantification of circulating 1,25-dihydroxyvitamin D by radioimmunoassay with 125I-labeled tracer. Clin Chem. 1996;42(4):586–92. [PubMed] [Google Scholar]
- 36.Pence BC, Buddingh F. Inhibition of dietary fat-promoted colon carcinogenesis in rats by supplemental calcium or vitamin D3. Carcinogenesis. 1988;9(1):187–90. doi: 10.1093/carcin/9.1.187. [DOI] [PubMed] [Google Scholar]
- 37.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–96. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 38.Sitrin MD, Halline AG, Abrahams C, Brasitus TA. Dietary calcium and vitamin D modulate 1,2-dimethylhydrazine-induced colonic carcinogenesis in the rat. Cancer Res. 1991;51(20):5608–13. [PubMed] [Google Scholar]
- 39.Beaty MM, Lee EY, Glauert HP. Influence of dietary calcium and vitamin D on colon epithelial cell proliferation and 1,2-dimethylhydrazine-induced colon carcinogenesis in rats fed high fat diets. J Nutr. 1993;123(1):144–52. doi: 10.1093/jn/123.1.144. [DOI] [PubMed] [Google Scholar]
- 40.Kawaura A, Tanida N, Sawada K, Oda M, Shimoyama T. Supplemental administration of 1 alpha-hydroxyvitamin D3 inhibits promotion by intrarectal instillation of lithocholic acid in N-methyl-N-nitrosourea-induced colonic tumorigenesis in rats. Carcinogenesis. 1989;10(4):647–9. doi: 10.1093/carcin/10.4.647. [DOI] [PubMed] [Google Scholar]
- 41.Wu K, Willett WC, Fuchs CS, Colditz GA, Giovannucci EL. Calcium intake and risk of colon cancer in women and men. J Natl Cancer Inst. 2002;94(6):437–46. doi: 10.1093/jnci/94.6.437. [DOI] [PubMed] [Google Scholar]
- 42.Zheng W, Anderson KE, Kushi LH, et al. A prospective cohort study of intake of calcium, vitamin D, and other micronutrients in relation to incidence of rectal cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1998;7:221–5. [PubMed] [Google Scholar]
- 43.Grau MV, Baron JA, Sandler RS, et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. 2003;95:1765–71. doi: 10.1093/jnci/djg110. [DOI] [PubMed] [Google Scholar]
- 44.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. The Journal of cell biology. 1992;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hambly RJ, Saunders M, Rijken PJ, Rowland IR. Influence of dietary components associated with high or low risk of colon cancer on apoptosis in the rat colon. Food Chem Toxicol. 2002;40(6):801–8. doi: 10.1016/s0278-6915(02)00019-4. [DOI] [PubMed] [Google Scholar]
- 46.Lamprecht SA, Lipkin M. Cellular mechanisms of calcium and vitamin D in the inhibition of colorectal carcinogenesis. Ann N Y Acad Sci. 2001;952:73–87. doi: 10.1111/j.1749-6632.2001.tb02729.x. [DOI] [PubMed] [Google Scholar]
- 47.van den Bemd GJ, Pols HA, van Leeuwen JP. Anti-tumor effects of 1,25-dihydroxyvitamin D3 and vitamin D analogs. Current pharmaceutical design. 2000;6(7):717–32. doi: 10.2174/1381612003400498. [DOI] [PubMed] [Google Scholar]
- 48.Watson AJ. An overview of apoptosis and the prevention of colorectal cancer. Crit Rev Oncol Hematol. 2006;57(2):107–21. doi: 10.1016/j.critrevonc.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Liu LU, Holt PR, Krivosheyev V, Moss SF. Human right and left colon differ in epithelial cell apoptosis and in expression of Bak, a pro-apoptotic Bcl-2 homologue. Gut. 1999;45(1):45–50. doi: 10.1136/gut.45.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anti M, Armuzzi A, Morini S, et al. Severe imbalance of cell proliferation and apoptosis in the left colon and in the rectosigmoid tract in subjects with a history of large adenomas. Gut. 2001;48(2):238–46. doi: 10.1136/gut.48.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.