Abstract
Dsg1 (desmoglein 1) is a member of the cadherin family of Ca2+-dependent cell adhesion molecules that is first expressed in the epidermis as keratinocytes transit out of the basal layer and becomes concentrated in the uppermost cell layers of this stratified epithelium. In this study, we show that Dsg1 is not only required for maintaining epidermal tissue integrity in the superficial layers but also supports keratinocyte differentiation and suprabasal morphogenesis. Dsg1 lacking N-terminal ectodomain residues required for adhesion remained capable of promoting keratinocyte differentiation. Moreover, this capability did not depend on cytodomain interactions with the armadillo protein plakoglobin or coexpression of its companion suprabasal cadherin, Dsc1 (desmocollin 1). Instead, Dsg1 was required for suppression of epidermal growth factor receptor–Erk1/2 (extracellular signal-regulated kinase 1/2) signaling, thereby facilitating keratinocyte progression through a terminal differentiation program. In addition to serving as a rigid anchor between adjacent cells, this study implicates desmosomal cadherins as key components of a signaling axis governing epithelial morphogenesis.
Introduction
Desmogleins (Dsgs) and desmocollins (Dscs) are transmembrane glycoproteins that anchor adjacent epithelial cells to one another in desmosomes via their five extracellular domains (EC1–5; Green and Simpson, 2007; Garrod and Chidgey, 2008). The cytoplasmic domains of these desmosomal cadherins integrate the keratin-based cytoskeleton at stable sites of cell–cell contact by interacting with armadillo family members, including plakoglobin (PG) and the plakophilins, which, in turn, associate with the intermediate filament-binding protein desmoplakin. The resulting supracellular scaffolding gives tensile strength to tissues such as the heart and epidermis, which is required for effectively resisting the forces of mechanical stress.
The epidermis is a continually renewing stratified epithelium that forms the outer protective layer of the skin. During the epidermal life cycle, superficial cells lost through desquamation are replenished with a fresh population of keratinocytes entering the terminal differentiation program from the proliferating basal compartment. Desmosomal cadherin expression profiles are orchestrated with biochemical changes in growth factor signaling, cytoskeleton and cytoskeletal-associated proteins, and components required for establishing the epidermal barrier (Green and Simpson, 2007). Of the four Dsg and three Dsc subtypes, Dsg2/Dsc2 and Dsg3/Dsc3 are found in the basal layer. Conversely, Dsc1 is restricted to the postmitotic, upper cell layers, and Dsg4 is present only in the most terminally differentiated viable cell layers. Dsg1 exhibits a distinct expression pattern from other desmosomal cadherins in that it is first expressed at the interface between basal and suprabasal cells. Alterations in the normal expression pattern of desmosomal cadherins in mice disrupts epidermal structure and function, supporting the notion that these adhesion molecules also serve as key morphoregulators (Koch et al., 1998; Chidgey et al., 2001; Elias et al., 2001; Merritt et al., 2002; Kljuic et al., 2003; Hardman et al., 2005; Brennan et al., 2007; Chen et al., 2008). For example, the lack of Dsc1 or Dsg4 in the suprabasal layers impairs adhesion, proliferation, and differentiation of keratinocytes in interfollicular epidermis or hair follicles (Chidgey et al., 2001; Kljuic et al., 2003). The adhesive function of desmosomal cadherins is thought to be critical for this process, as the transgenic expression of an ectodomain-deleted Dsg3 interferes with desmosome formation and epidermal differentiation (Allen et al., 1996). A causal link between desmosomal adhesion and epithelial morphogenesis was further shown using inhibitory peptides that target amino acid residues present within EC1 of the adhesive cadherin ectodomain, which disrupt the positioning of luminal and myoepithelial cells in vitro (Runswick et al., 2001).
Desmosomal cadherins are part of a larger gene family that includes epithelial cadherin (E-cadherin), which is a critical orchestrator of intercellular junctional complexes and signaling pathways in epithelial tissues (Lien et al., 2006). Therefore, it has been suggested that, in addition to serving as adhesion receptors, desmosomal cadherins might also contribute to epidermal morphogenesis by coordinating intracellular signaling responses (Green and Simpson, 2007; Garrod and Chidgey, 2008). In support of this possibility, the forced expression of Dsc3 into the suprabasal layers not only led to epidermal proliferation and differentiation defects but also increased β-catenin signaling (Hardman et al., 2005). Similarly, introducing the basally restricted Dsg2 into the suprabasal layers enhanced epidermal proliferation and survival in a manner that relied, at least in part, on EGF receptor (EGFR) and nuclear factor κB signaling (Brennan et al., 2007). However, these studies did not directly test whether the alterations in signaling were related to the adhesive function of desmosomal cadherins.
To define the adhesive and signaling contributions of desmosomal cadherins in epithelial tissue morphogenesis, we focused on the role of Dsg1 in epidermal development because it is essential for adhesion in the uppermost cell layers and can be found at the interface between cells in the basal and suprabasal layers in vivo, where keratinocytes commit to a terminal differentiation program (Green and Simpson, 2007; Garrod and Chidgey, 2008). Providing further rationale for focusing on this particular desmosomal cadherin, the Grhl1 (grainyhead-like 1) transcription factor directly regulates DSG1 gene expression, and knockout of Grhl1 in mice leads to epidermal adhesive and differentiation defects (Wilanowski et al., 2008). Using a three-dimensional raft model of human epidermis combined with gain- and loss-of-function approaches, we demonstrate that Dsg1 regulates an early program of epidermal differentiation and morphogenesis that leads to an up-regulation of Dsc1 along with other suprabasal layer proteins. Interestingly, Dsg1 can promote keratinocyte differentiation in a manner that does not rely on its (a) suprabasal partner, Dsc1, (b) N-terminal ectodomain residues required for Dsg1-dependent adhesion, or (c) association with PG. Instead, our results demonstrate that Dsg1 is required for dampening EGFR–Erk1/2 signaling in the suprabasal layers, thus allowing for the progression of keratinocytes toward a more differentiated phenotype. Collectively, these experiments reveal that desmosomal cadherin functions transcend adhesion by networking with cell surface receptors that control epithelial morphogenesis.
Results
Dsg1 is required for suprabasal morphogenesis of organotypic human epidermal cultures
The analysis of Dsg1 function in vivo is complicated by the presence of two additional and highly homologous genes (dsg1-β and dsg1-γ) with overlapping expression patterns to dsg1-α in the epidermis of mice (Brennan et al., 2004). To determine the normal requirements for Dsg1 in stratified epithelial morphogenesis, we turned to an organotypic raft model of human epidermis to study the single human DSG1 gene purely in keratinocytes in a more morphologically relevant context than submerged cultures.
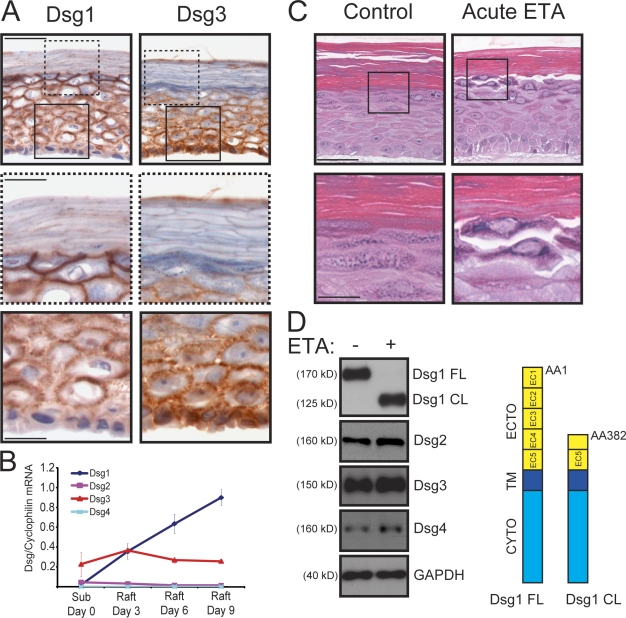
Dsg1 was present in rafts and exhibited a restricted expression pattern in differentiating keratinocytes (Fig. 1, A and B). As expected, Dsg1 was primarily found in the suprabasal layers that formed after 9 d at an air–liquid interface, whereas Dsg3 was immunolocalized between adjacent basal keratinocytes and was more abundant in the lower epidermal cell layers (Fig. 1 A). To test whether Dsg1 adhesive functions were conserved in these three-dimensional cultures, keratinocytes were matured on rafts for 9 d and then incubated for 24 h in the presence or absence of recombinant Staphylococcus aureus exfoliative toxin A (ETA; acute ETA treatment), a Ser protease which specifically cleaves after a Glu residue (381) located between EC3 and EC4 of Dsg1 but does not target other cadherins (Amagai et al., 2000; Hanakawa et al., 2004; Nagasaka et al., 2004). Similar to the phenotypic outcome in humans with ETA-induced bullous impetigo or Staphylococcal scalded skin syndrome, efficient removal of EC1–3 from Dsg1 in mature rafts resulted in prominent areas of dissociation between adjacent keratinocytes in the uppermost cell layers (Fig. 1, C and D). These regions corresponded to cell layers in which Dsg1 expression was most abundant and which further lacked Dsg3. Importantly, ETA had no effect on the protein levels or integrity of Dsg3 or the related Dsg2 and Dsg4, with the latter two found in lower abundance in raft cultures (Fig. 1, B and D). These observations helped validate the raft model for Dsg1 functional analysis.
Figure 1.
Differentiation-dependent Dsg1 maintains adhesion in the superficial layers of epidermal raft cultures. (A) IHC analysis of Dsg1 and Dsg3 in frozen sections prepared from 9-d-old rafts. Dsg1 was concentrated in the suprabasal layers (insets with dashed lines), whereas Dsg3 was prominent in the basal layer (insets with continuous lines). (B) Real-time PCR analysis of Dsg1–4 mRNA levels from keratinocytes maintained as submerged cultures (Sub day 0) or on rafts for 3, 6, or 9 d. The Dsg mRNA levels were normalized to cyclophilin 1 levels interpolated from a standard curve, and the mean of three independent experiments (±SEM) is represented in the graph. Dsg1 mRNA levels were tightly coordinated with raft maturation, whereas Dsg3 remained relatively constant. Maximal Dsg2 (0.0468 ± 0.0318) and Dsg4 (0.000874 ± 0.000101) mRNA transcript levels were detected in submerged and day 9 raft cultures, respectively. (C) H&E-stained sections of 9-d-old rafts incubated with or without 5 µg/ml recombinant ETA (acute ETA) for 24 h. Boxed insets show areas of cell–cell dissociation in the uppermost layers of ETA-treated rafts, whereas control cultures remained intact. (D) Western blot analysis of these raft cultures using a cytoplasmic domain (CYTO) antibody for Dsg1 demonstrated that the N-terminal portion (aa 1–381) of the adhesive ectodomain (ECTO) of the full-length protein (Dsg1 FL) was efficiently cleaved in the presence of ETA, resulting in a membrane-associated cytoplasmic fragment (Dsg1 CL; aa 382–1,049) that was retained in whole cell lysates. Dsg2–4 were not cleaved by ETA. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; TM, transmembrane domain. Bars: (A and C) 50 µm; (insets) 20 µm.
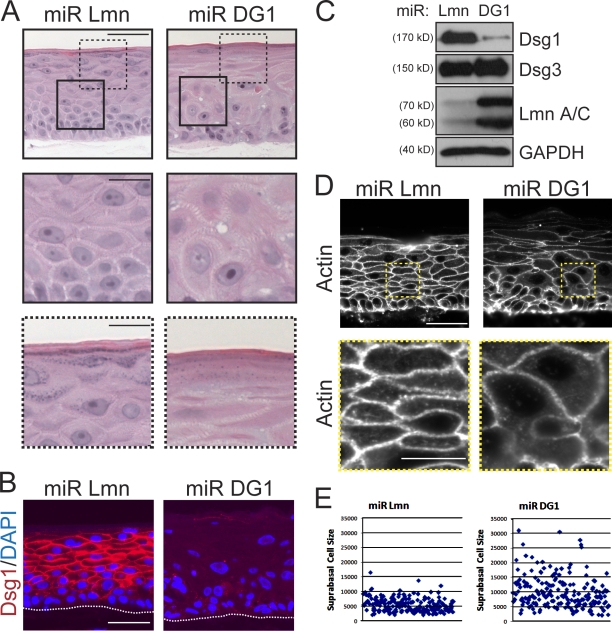
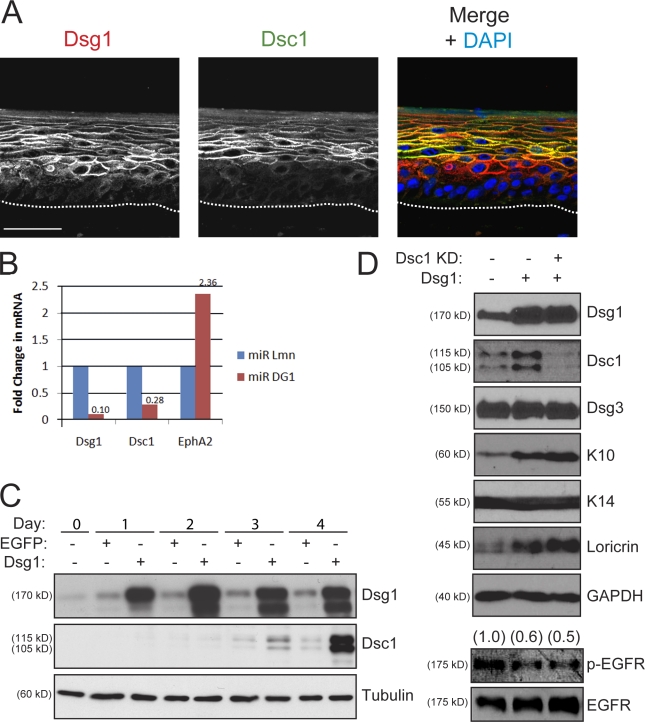
Dsg1 was commonly detected at the interface between basal and suprabasal keratinocytes (Fig. 1 A), leading us to posit that this desmosomal cadherin might play an early instructive role as epidermal cells commit to a differentiation program. To address this possibility, we silenced Dsg1 expression in raft cultures using retroviruses engineered to express microRNA (miRNA)-like sequences specific for this desmosomal cadherin (miR DG1) or lamin A/C (miR Lmn) as a control. Unlike lamin A/C–silenced rafts, there was a marked disorganization in the suprabasal layers that formed in Dsg1-deficient cultures (Fig. 2, A–C). In comparison with controls, we noted widened intercellular spaces (Fig. S3), irregular cell shapes in the intermediate layers (Fig. 2, D and E), and an immature granular layer suggestive of impaired differentiation (Fig. 2 A). Expression of a companion suprabasal cadherin, Dsc1, was markedly reduced; in contrast, the levels of classical and other desmosomal cadherins present in the lower layers were only marginally affected by the loss of Dsg1 and remained prominent at areas of cell–cell contact (Fig. S1).
Figure 2.
Dsg1 deficiency impairs morphogenesis of epidermal raft cultures. (A) H&E-stained sections of 6-d-old rafts expressing miR Lmn or miR DG1. Dsg1-deficient rafts exhibited abnormal suprabasal morphology (insets with continuous lines) and a poorly differentiated appearance (insets with dashed lines) compared with controls. (B) IHC analysis of Dsg1 in miR Lmn or miR DG1 rafts revealed a profound loss of Dsg1 at 6 d. The keratinocyte–collagen interface is highlighted by the dotted line. (C) Western blot analysis of Dsg1, Dsg3, lamin A/C (Lmn A/C), and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) from these raft cultures indicated that Dsg1 or lamin A/C levels were reduced by ∼90%. (D) An actin antibody was used to highlight the cellular cortex in Dsg1-deficient cultures and generate surface area measurements of individual suprabasal cells using indirect immunofluorescence staining. Although control cultures exhibited compact, uniformly shaped suprabasal cells, cultures lacking Dsg1 possessed suprabasal cells that displayed a highly irregular array of individual cell sizes. Boxed insets highlight suprabasal cell morphology between miR Lmn and miR DG1 rafts. (E) The surface area from individual suprabasal cells (n > 150) from miR Lmn or miR DG1 rafts was measured using MetaMorph software, and individual data points from a representative experiment are shown in the scatter plot. Bars: (A, B, and D) 50 µm; (insets) 20 µm.
The gross disturbances in cellular organization led us to more closely evaluate suprabasal keratinocyte morphology by imaging the cortical actin cytoskeleton, which outlines the cell periphery (Fig. 2 D). Dsg1-deficient keratinocytes were, on average, almost twice as large and more variable in size compared with those present in the suprabasal layers of miR Lmn rafts (Fig. 2 E). These data support the idea that an increase in Dsg1 expression is required for the normal adhesion and morphological transformation of keratinocytes transitioning into the suprabasal epidermis.
Dsg1 regulates epidermal differentiation independent of ectodomain residues required for adhesion
To determine whether the aberrant morphology of stratifying keratinocytes in Dsg1-deficient cultures was accompanied by alterations in the differentiation program, we investigated the expression of several well-characterized structural proteins that are induced upon keratinocyte differentiation. Consistent with the histological analysis, rafts lacking Dsg1 exhibited a marked reduction in both early (Dsc1 and keratin 10 [K10]) and late markers (loricrin and processed filaggrin) of epidermal differentiation (Fig. 3, B and C). In addition, proliferation, as assessed by Ki67 staining and BrdU incorporation (Fig. S2), and the expression of proteins more prominent in the basal layer such as K14 remained unchanged in Dsg1-deficient cultures.
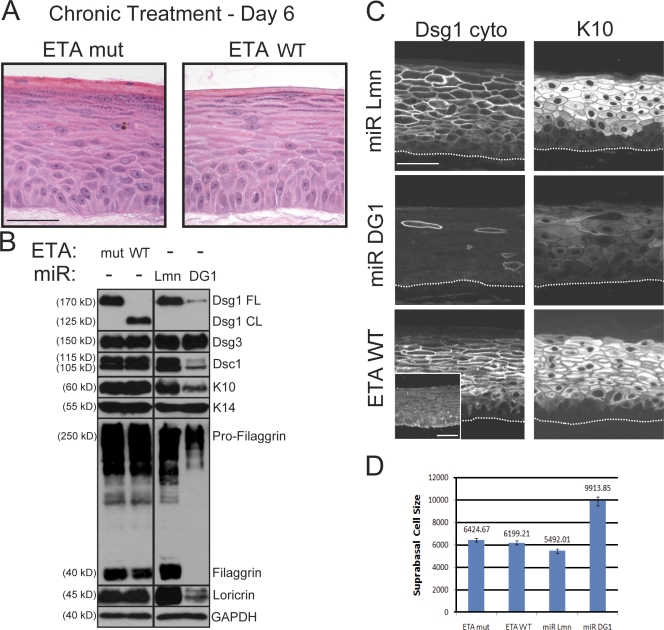
Figure 3.
N-terminal ectodomain residues of Dsg1 required for adhesion are not essential for epidermal raft development. (A) H&E analysis of raft cultures treated with WT ETA (ETA WT) or a protease-dead mutant (ETA mut) and maintained at an air–liquid interface for an additional 6 d. Chronic Dsg1 EC1–3 cleavage did not grossly alter suprabasal morphogenesis. (B) Western blot analysis revealed a reduction in Dsc1, K10, filaggrin, and loricrin in raft cultures deficient in Dsg1 (miR DG1) compared with miR Lmn controls. In contrast, there were no differences in the levels of these suprabasal proteins in rafts treated with ETA WT or mut. Levels of Dsg3 and the basal marker K14 were equivalent in all conditions. Black lines indicate that intervening lanes have been spliced out. CL, cleaved; FL, full length; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (C) IHC staining confirmed that both K10 and Dsc1 (Figs. S1 and S4) were reduced in Dsg1-silenced cultures (miR DG1), whereas control (miR Lmn) and ETA-treated (ETA WT) cultures robustly expressed these suprabasal markers. Also, note that ETA-treated cultures retained a cleaved fragment (aa 382–1,049) of Dsg1 at intercellular borders, as revealed by staining with an antibody against the cytoplasmic domain of Dsg1 (Dsg1 cyto), whereas the extracellular epitope (aa 1–381) was effectively removed by ETA (inset). The dotted lines indicate the boundary between keratinocytes and the collagen matrix. (D) The mean surface area (±SEM) of individual suprabasal cells from ETA-treated or Dsg1 knockdown rafts was determined using actin immunostaining to outline the cell cortex. Although control and ETA-treated cultures exhibited suprabasal cells of similar size, cultures lacking Dsg1 possessed suprabasal cells that were nearly twice as large. Bars, 50 µm.
To determine whether impaired morphogenesis and differentiation in knockdown rafts were solely caused by the loss of Dsg1-mediated adhesion, we chronically removed N-terminal ectodomain residues required for Dsg1-dependent adhesion (Getsios et al., 2004) by incubating rafts in wild-type (WT) or protease-deficient (mut) ETA as soon as these cultures were induced to differentiate and throughout their development (chronic ETA treatment). Consistent with previous studies in which truncated desmosomal cadherins were introduced into epithelial cells (Allen et al., 1996; Serpente et al., 2000), EM analysis revealed adhesive defects in these ETA-treated rafts with a reduction in suprabasal desmosome density similar to that observed upon Dsg1 silencing (Fig. S3). In stark contrast to miR DG1 rafts, chronic removal of the Dsg1 EC1–3 did not appreciably alter the cellular organization or size of suprabasal cells, which differentiated normally and remained grossly intact despite 6 d of constant exposure to ETA (Fig. 3, A–D). As in the case of acute ETA treatment (Fig. 1 D), the other desmosomal cadherins remained intact in raft cultures chronically exposed to ETA (Fig. S4). However, similar to immature embryonic and neonatal human skin (Wu et al., 2000), Dsg3 was more broadly distributed throughout all viable layers in the earlier 6-d-old raft cultures treated with ETA WT or ETA mut (Fig. S4) or silenced for Dsg1 expression (Fig. S1), perhaps contributing to the overall maintenance of tissue integrity (Figs. 2 A and 3 A) compared with acute ETA treatment at day 9, when the uppermost cell layers lack Dsg3 (Fig. 1 C). Collectively, these observations demonstrated that, in spite of comparable alterations in cell–cell adhesion in both Dsg1 cleavage and Dsg1 knockdown conditions, differentiation was impaired only in cells lacking Dsg1 altogether, indicating that the residual cleaved Dsg1 (aa 382–1,049) supported normal morphogenesis.
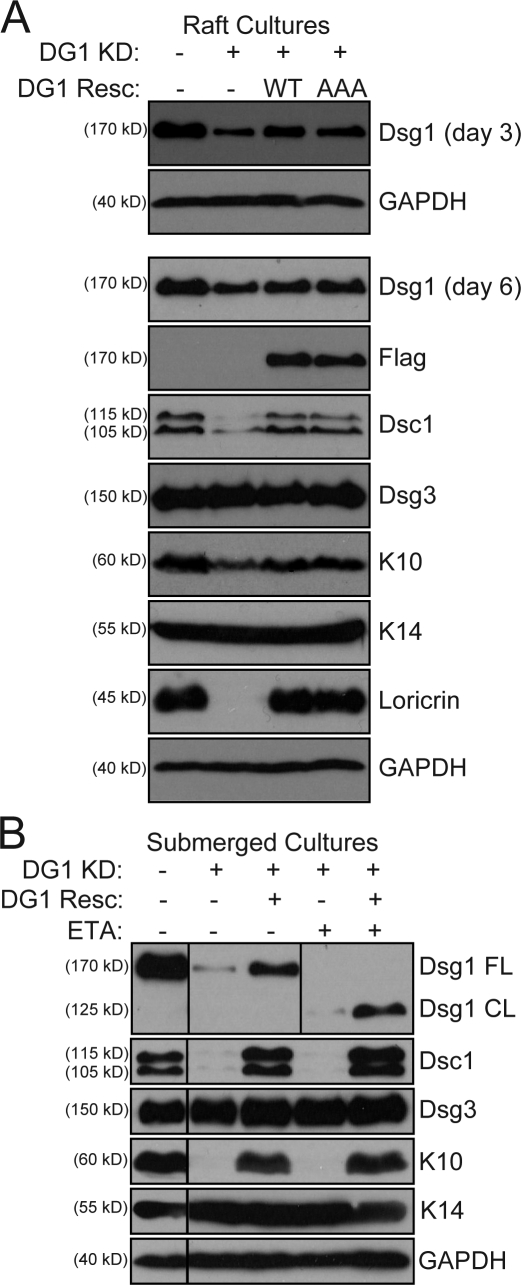
To establish that impaired progression through the differentiation program was a direct result of Dsg1 deficiency, a full-length Dsg1 cDNA with silent point mutations rendering it resistant to miR DG1 was simultaneously introduced along with retroviral miRNA into keratinocytes that were differentiated in rafts (Fig. 4 A) as well as submerged cultures (Fig. 4 B). The reconstitution of Dsg1 was capable of increasing the levels of Dsc1, K10, and loricrin in raft cultures (Fig. 4 A). Importantly, ETA-mediated cleavage of the EC1–3 domains of this Dsg1 rescue construct still allowed for restoration of these structural markers of keratinocyte differentiation (Fig. 4 B). These data provided direct evidence that Dsg1 can promote keratinocyte differentiation even when the N-terminal residues required for adhesion have been removed.
Figure 4.
Reconstitution of Dsg1 restores epidermal differentiation despite ectodomain cleavage. (A) Western blot analysis of keratinocytes cotransduced with miR Lmn or miR DG1 (DG1 KD) and either EGFP (DG1 Resc −) or silencing refractory WT or PG-binding mutant (AAA) Dsg1 constructs and then grown as raft cultures. Although Dsg1 was more efficiently silenced at day 3 (top), modest silencing was still evident at day 6 (bottom) with concomitant reduction of differentiation markers. Cotransduction with either miR-resistant Dsg1 WT or Dsg1 AAA was sufficient to increase Dsg1 levels and restore Dsc1/K10/loricrin levels to that of controls. (B) Similar rescue experiments were performed in submerged culture in which Dsg1 silencing was more efficient. Silencing of Dsg1 (DG1 KD) resulted in a decrease in Dsc1/K10 levels, and this deficiency was rescued by the silencing-resistant Dsg1 (DG1 Resc +; third lane) but not a susceptible WT Dsg1 construct (DG1 Resc −; second lane). Moreover, chronic treatment with ETA efficiently cleaved the N-terminal portion of the adhesive ectodomain of the Dsg1 rescue construct but did not affect its ability to rescue differentiation in Dsg1-silenced keratinocytes (fifth lane). Black lines indicate that intervening lanes have been spliced out. CL, cleaved; FL, full length; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Dsg1 promotes keratinocyte differentiation independent of Dsc1 levels, PG binding, or ectodomain residues required for adhesion
Consistent with previous work in human epidermis (Arnemann et al., 1993; Donetti et al., 2005; Bazzi et al., 2006), Dsc1 and Dsg4 exhibit a more restricted expression pattern than Dsg1 in the suprabasal layers of rafts (Fig. 5 A and not depicted). Dsg4-deficient mice exhibit major hair follicle defects, but the interfollicular epidermis, where this desmosomal cadherin is limited to the most differentiated viable cell layers, is relatively normal (Kljuic et al., 2003). Moreover, the low levels of Dsg4 protein in developing raft cultures did not change in response to Dsg1 silencing or cleavage (unpublished data). In addition, Dsg4 was limited to a single superficial cell layer in 6-d-old raft cultures, and this distribution did not change in ETA-treated or knockdown rafts (unpublished data). However, Dsg1 silencing led to a coordinate reduction in Dsc1, including at the mRNA level (Fig. 5 B), and ectopic Dsg1 expression was capable of increasing Dsc1 levels (Fig. 5 C; Getsios et al., 2004). As Dsc1 plays an important role in epidermal integrity and differentiation in mice (Chidgey et al., 2001), it was possible that Dsg1 initiated a cascade of events leading to the induction of Dsc1, which, in turn, was responsible for differentiation. We tested this possibility by silencing Dsc1 expression and asking whether this desmosomal cadherin was required for Dsg1-mediated differentiation. Ectopic expression of Dsg1 in submerged keratinocyte cultures was sufficient to increase markers of terminal differentiation, including Dsc1 (Fig. 5 D). Importantly, Dsg1 remained capable of promoting keratinocyte differentiation even when Dsc1 induction was blocked by siRNA treatment (Fig. 5 D).
Figure 5.
Dsg1 promotes keratinocyte differentiation in a manner that does not rely on its regulation of Dsc1 expression. (A) Dual-label IHC staining revealed that Dsg1 (red) is expressed in lower cellular layers of day 6 rafts compared with Dsc1 (green). A merged overlay image containing DAPI (blue) as a nuclear stain is mounted to the right. The dotted lines indicate the boundary between keratinocytes and the collagen matrix. (B) Real-time PCR analysis showed that Dsc1 mRNA levels are reduced in Dsg1 knockdown rafts. The mRNA levels of Dsg1, Dsc1, and a gene target of EGFR, EphA2, were normalized to 18-S ribosomal RNA using the comparative CT method. The data are representative of four independent experiments and are shown as fold change from miR Lmn controls. (C) Ectopic Dsg1-Flag in keratinocytes maintained in low (0.2 mM) Ca2+ to limit differentiation results in a time-dependent increase in Dsc1 expression compared with EGFP-transduced controls. Protein lysates from 0 to 4 d after retroviral transduction were analyzed using an antibody specific for Dsg1 (ectopic + endogenous), Dsc1, or tubulin as a loading control. (D) Dsg1-Flag was ectopically expressed in keratinocytes and additionally transfected with a pool of Dsc1 siRNA or control oligonucleotides (75 nM total) for 48 h in low Ca2+. Confluent cultures were switched into high (1.8 mM) Ca2+ to initiate differentiation and harvested after 24 h. Ectopic Dsg1 was capable of increasing the levels of differentiation-associated proteins, whereas the levels of p-EGFR were reduced. Dsc1 knockdown (KD) did not interfere with the Dsg1-dependent changes in K10, loricrin, or p-EGFR. Ratios of p-EGFR to total EGFR are indicated above the blots. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. Bar, 50 µm.
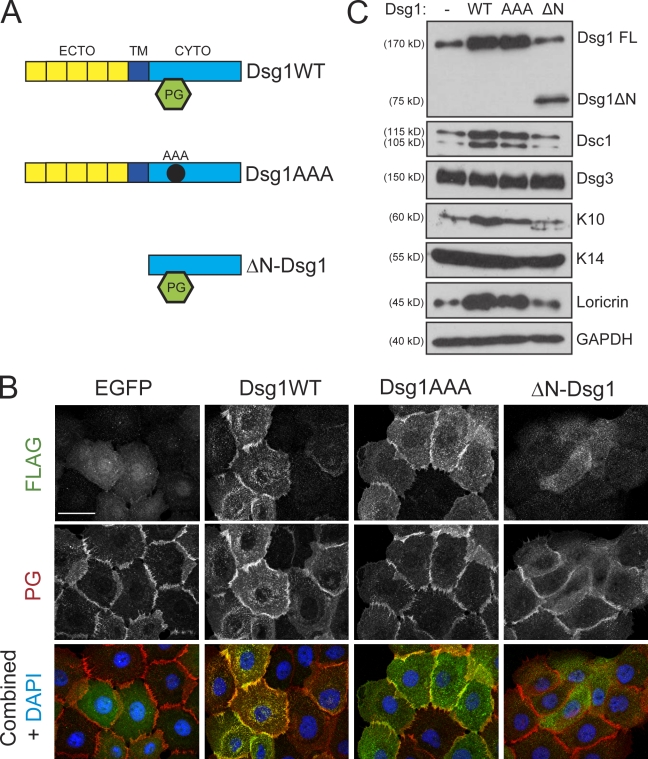
Previous experiments showed that the misexpression of Dsc3 in the suprabasal epidermis increased β-catenin stability and signaling (Hardman et al., 2005). However, Dsg1-deficient rafts exhibited a variable decrease in Dsc3 levels but no changes in β-catenin distribution or activation (Fig. S1). Moreover, ectopic expression of Dsg1 was capable of inducing differentiation without altering levels of Dsc3 or β-catenin (unpublished data). The desmosomal counterpart to β-catenin, PG, is a major binding partner for the Dsg1 cytoplasmic domain and has been shown to play a key role in nuclear signaling and keratinocyte differentiation (Chitaev et al., 1998; Teuliere et al., 2004; Williamson et al., 2006). Thus, this armadillo family member served as a logical candidate for mediating Dsg1-dependent differentiation. Therefore, we mutated three hydrophobic residues important for PG binding within the Dsg1 cytoplasmic domain to assess its contribution to Dsg1-mediated differentiation (Dsg1AAA; Fig. 6 A; Chitaev et al., 1998). Although Dsg1AAA still concentrated at areas of cell–cell contact (Fig. 6 B), its association with PG was severely limited as compared with the WT protein (Fig. S5). Despite this profound deficiency in PG binding, Dsg1AAA and WT Dsg1 were equally capable of restoring the levels of Dsc1, K10, and loricrin in Dsg1-deficient rafts (Fig. 4 A). Moreover, ectopic expression of either of these Dsg1 constructs increased differentiation markers (Fig. 6 C). These findings demonstrate that introduction of Dsg1 into keratinocytes is sufficient to accelerate a program of terminal differentiation and emphasize that recruitment of additional PG by this ectopic desmosomal cadherin is not a critical step in this cascade of differentiation-promoting events.
Figure 6.
Dsg1 promotes differentiation in the absence of robust PG binding. (A) To test which domains of Dsg1 would be sufficient to drive differentiation, we generated three Flag-tagged Dsg1 cDNA constructs: WT Dsg1 (Dsg1WT), a triple point mutant harboring three Ala substitutions (Dsg1AAA) within the predicted binding region for PG, or a truncation mutant lacking the ectodomain (ECTO) and transmembrane (TM) region (ΔN-Dsg1). CYTO, cytoplasmic domain. (B) The subcellular localization of Dsg1WT, Dsg1AAA, or ΔN-Dsg1 was determined in keratinocytes immunostained using a rabbit polyclonal antibody directed against Flag and a chicken polyclonal antibody against PG after exposing cells to high Ca2+ for 4 h to induce junction assembly. Both Dsg1WT and Dsg1AAA were efficiently recruited to areas of cell–cell contact; however, ΔN-Dsg1 was diffusely distributed throughout the cytoplasm. PG staining highlighted the intercellular borders; its localization at junctions was largely unaffected by any of the Dsg1 constructs. (C) Western blot analysis of keratinocytes transduced with these Dsg1 constructs and induced to differentiate for 2 d as submerged cultures. Although Dsg1WT and Dsg1AAA were sufficient to increase Dsc1/K10/loricrin, ΔN-Dsg1 did not affect these markers of differentiation compared with EGFP-transduced (Dsg1−) control cultures. FL, full length; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. Bar, 20 µm.
Because ETA-cleaved Dsg1 (Fig. 3 C) and the PG-binding mutant (Fig. 6 B) were both localized to intercellular junctions, we next asked whether the Dsg1 cytoplasmic domain itself was able to promote keratinocyte differentiation. A truncated mutant of this desmosomal cadherin lacking an extracellular and transmembrane domain (ΔN-Dsg1; Fig. 6 A) failed to localize at cell–cell junctions (Fig. 6 B) and exhibited increased solubility (Fig. S5 B) but retained the ability to bind PG, although not as efficiently as the full-length protein (Fig. S5 A). In contrast to WT Dsg1, which was capable of increasing Dsc1, K10, and loricrin expression even upon cleavage by ETA (Fig. 4 B), the levels of these differentiation markers were unchanged compared with controls upon ectopic ΔN-Dsg1 expression (Fig. 6 C). These findings suggested that the Dsg1 cytoplasmic tail may need to be anchored at the cell surface to trigger keratinocyte differentiation.
Dsg1 is required for the suppression of EGFR–Erk1/2 signaling during keratinocyte differentiation
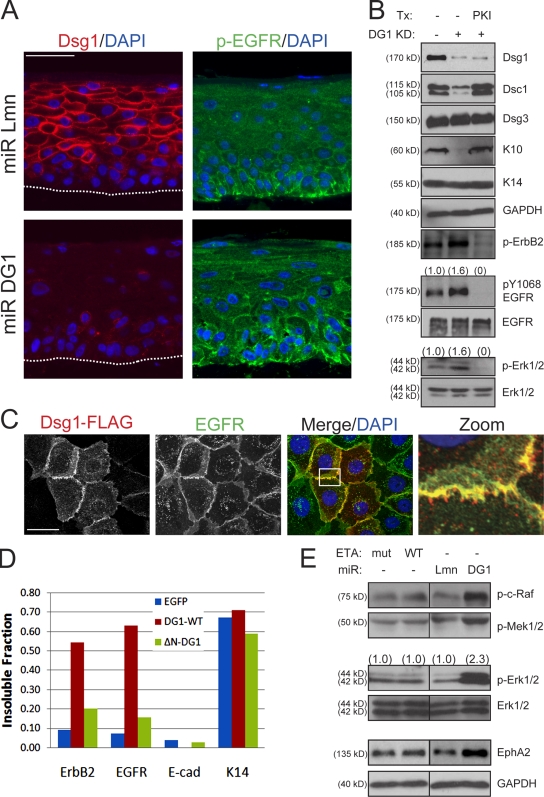
EGFR is concentrated and active in the basal layer where it maintains proliferating keratinocytes in an undifferentiated state (Stoll et al., 2001; Lacouture, 2006). Although this receptor Tyr kinase continues to be expressed in the suprabasal epidermis, which is coordinated with the induction of Dsg1, it is no longer active upon stratification, allowing these cells to adopt a more differentiated phenotype. The inability of a soluble Dsg1 tail to regulate keratinocyte differentiation suggests that localization is important. This observation led us to speculate that Dsg1 might be required to suppress EGFR signaling in the suprabasal layers and allow for normal progression through the epidermal differentiation program. In support of this possibility, phosphorylated EGFR was largely restricted to the basal layer of control rafts, but sustained activation and expansion of this activated receptor into the suprabasal layers were found after Dsg1 knockdown (Fig. 7, A and B). The reciprocal relationship between EGFR activity and Dsg1 was examined in more detail by ectopic expression of this desmosomal cadherin in submerged keratinocytes. These two transmembrane proteins did not overlap extensively in the cytoplasm but were colocalized within discrete regions of cell–cell contact (Fig. 7 C, zoom). Moreover, Dsg1 was capable of reducing the levels of activated EGFR in a manner that did not require Dsc1 (Fig. 5 D). Finally, the ectopic expression of Dsg1 but not the soluble Dsg1 tail was capable of recruiting EGFR and the related erbB2 receptor into a more insoluble pool (Fig. 7 D).
Figure 7.
Dsg1 is required for the suppression of EGFR–Erk1/2 signaling during epidermal differentiation. (A) IHC analysis of Dsg1 (left) and EGFR phosphorylated at Y1068 (p-EGFR; right) revealed a basally restricted p-EGFR staining pattern largely absent from Dsg1-positive suprabasal layers in control (miR Lmn) cultures, whereas Dsg1-deficient cultures (miR DG1) demonstrated a gross disorganization of p-EGFR, which was present throughout the basal and suprabasal layers. The dotted lines indicate the boundary between keratinocytes and the collagen matrix. (B) Western blot analysis of keratinocytes transduced with miR Lmn or miR DG1 and induced to undergo differentiation for 2 d as submerged cultures in the presence or absence of 10 µM of the EGFR–erbB2 inhibitor PKI166 (PKI). A significant increase in p-EGFR, p-erbB2, and p-Erk1/2 levels was detectable in keratinocytes upon Dsg1 silencing (Dsg1 KD) along with decreased K10/Dsc1. Blocking EGFR signaling using PKI166 suppressed p-EGFR, p-erbB2, and p-Erk levels in addition to restoring the capacity of Dsg1-deficient keratinocytes to differentiate. Ratios of p-EGFR to total EGFR or p-Erk1/2 to total Erk1/2 are indicated above the blots. Tx, treatment. (C) Dual-label indirect immunofluorescence and Apotome optical sectioning of Dsg1-Flag and EGFR after 4 h in high Ca2+ revealed areas of overlap in discrete regions of cell–cell contact (zoom) but not throughout the cell. The boxed area is magnified in the right panel. (D) Keratinocytes were transduced with EGFP, WT Dsg1-Flag (DG1-WT), or a soluble, truncated Dsg1 mutant (ΔN-DG1) and switched into high Ca2+ before being harvested in RIPA buffer. The insoluble pellet was resuspended in buffered SDS and run in parallel with equal protein amounts from the RIPA-soluble fraction. The ratio of insoluble/total erbB2, EGFR, E-cadherin (E-cad), and K14 is presented in the bar graph and is representative of three experiments. Full-length but not the truncated Dsg1 recruits EGFR–erbB2 into a more insoluble fraction where keratins are concentrated. Under these detergent conditions, the vast majority of E-cadherin remains in the soluble fraction. (E) Activation of EGFR-Ras-Raf-Erk signaling effectors as well as the EGFR–Erk1/2 gene target, EphA2, were elevated by Dsg1 knockdown but not chronic ETA treatment in rafts. Ratios of p-Erk to total Erk are indicated above the blots. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. Bars: (A) 50 µm; (C) 20 µm.
To determine whether the suppression of EGFR and erbB2 contributed to Dsg1-dependent differentiation, we inhibited these receptor Tyr kinases in keratinocytes lacking Dsg1. We reasoned that Dsg1-deficient cultures would be rescued by global inhibition of EGFR–erbB2 if their sustained activation was responsible for impaired differentiation. For this purpose, we incubated miR DG1–transduced keratinocytes with a dual specificity inhibitor (PKI166) for EGFR and erbB2 (Fig. 7 B; Traxler et al., 2001). In submerged cultures transduced with miR DG1, the inhibition of EGFR–erbB2 was capable of restoring the levels of early differentiation markers without inducing Dsg1 expression. However, chronic inactivation of the EGFR–erbB2 pathway by PKI166 failed to support raft culture maturation, which is consistent with its vital role in epidermal development in vivo (Miettinen et al., 1995; Murillas et al., 1995).
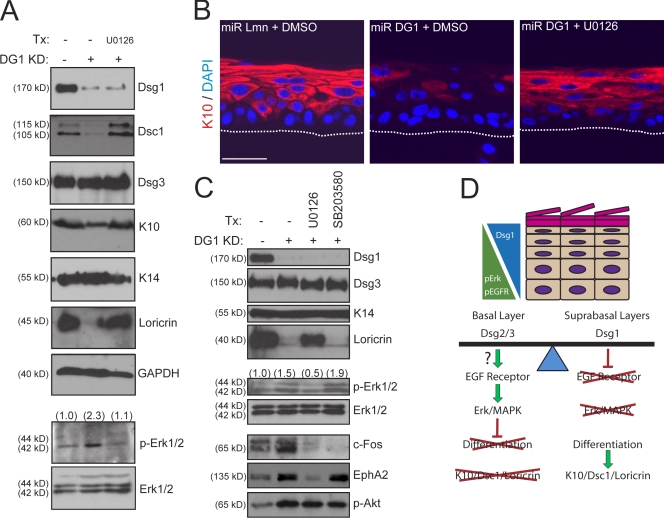
To identify downstream effectors of EGFR affected by Dsg1 deficiency, we examined the expression and activation status of the MAPK Erk1/2. Consistent with increased EGFR activity, the phosphorylation of Erk1/2 was elevated upon Dsg1 knockdown and was suppressed by PKI166 treatment (Fig. 7 B). In contrast to Dsg1-deficient cultures, the Ras–Raf–Mek (MAPK/extracellular signal-regulated kinase [Erk] kinase)–Erk1/2 pathway was unaltered in ETA-treated raft cultures (Fig. 7 E). To determine whether the Erk1/2 pathway, which had previously been implicated in keratinocyte differentiation (Gazel et al., 2008; Kolev et al., 2008), contributed to the defects upon Dsg1 knockdown, we used a pharmacological compound (U0126) with high specificity for the upstream kinase MEK1/2 (Davies et al., 2000). Similar to EGFR–erbB2 inhibition, the suppression of Erk1/2 restored differentiation in submerged cultures (Fig. 8 C) but also allowed for rescue of suprabasal differentiation in Dsg1 knockdown rafts (Fig. 8, A and B).
Figure 8.
Erk1/2 inhibition restores epidermal differentiation in Dsg1-deficient keratinocytes. (A) Western blot analysis of control or Dsg1 knockdown (KD) raft cultures incubated in the presence of vehicle control (DMSO) or 5 µM U0126. U0126 restored p-Erk1/2 to control levels and was able to increase Dsc1/K10/loricrin on the background of Dsg1 silencing. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (B) IHC analysis of K10 in 3-d-old control or Dsg1-deficient rafts treated with DMSO or U0126. Erk1/2 inhibition led to markedly improved suprabasal K10 expression. The dotted lines indicate the boundary between keratinocytes and the collagen matrix. (C) Treatment (Tx) of Dsg1-deficient keratinocytes with an inhibitor of Erk1/2 (5 µM U0126) or p38 MAPK (10 µM SB203580) signaling in submerged cultures induced to differentiate for an additional 2 d. Western blot analysis revealed a restoration of loricrin and K10/Dsc1 (not depicted) levels toward control keratinocytes upon suppression of p-Erk1/2 levels despite the lack of Dsg1. U0126 suppressed p-Erk1/2 and reduced downstream c-Fos and EphA2 expression but had no effect on p-AKT levels. Keratinocyte differentiation was not rescued using SB203580, which decreased c-Fos expression but not p-Erk1/2, p-AKT, or EphA2 levels. (A and C) Ratios of p-Erk to total Erk are indicated above the blots. (D) A model depicting the coordination of EGFR–Erk1/2 signaling and Dsg expression during epidermal differentiation. The expression of Dsg2 and Dsg3 is permissive for EGFR–Erk1/2 signaling in the basal layer, which suppresses terminal differentiation. In contrast, the induction of Dsg1 in the suprabasal layers dampens EGFR–Erk1/2 activity, allowing for the progression into a more differentiated state and expression of Dsc1, K10, and loricrin. Our findings predict that shifting the balance toward Dsg1 would cause premature differentiation, whereas the absence of Dsg1 in the suprabasal epidermis is expected to limit differentiation via the sustained activity of EGFR–Erk1/2. Consistent with animal models in which Dsg2 has been transgenically overexpressed (Brennan et al., 2007), increasing this desmosomal cadherin in the suprabasal layers can compete with Dsg1 function and promote persistent EGFR–Erk1/2 signaling and impaired differentiation. Bar, 50 µm.
Multiple signaling pathways beyond Erk1/2 are activated downstream of EGFR, including other MAPKs, PI3 kinase/AKT, and conventional PKCs. To determine their involvement in Dsg1-dependent differentiation, we inhibited these other kinases also implicated in keratinocyte differentiation (Denning et al., 1995; Calautti et al., 2005; Gazel et al., 2008). Although the activation of these downstream kinases were commonly elevated in Dsg1-deficient keratinocytes (shown for AKT; Fig. 8 C), the inhibition of these signaling pathways was not sufficient to restore differentiation as in the case of Erk1/2 suppression (not depicted). These findings suggest that Erk1/2 suppression is specifically required for Dsg1-mediated differentiation, which is consistent with a recent study on EGFR-mediated effects on keratinocytes (Kolev et al., 2008). In particular, inhibition of p38 MAPK did not rescue differentiation or alter the Erk1/2 pathway other than the expected down-regulation of the common MAPK target c-Fos (Fig. 8 C). In contrast, U0126 restored differentiation and was shown to decrease the levels of activated Erk1/2 and a specific target of the EGFR–Erk pathway, EphA2 (Larsen et al., 2007), without changing the activation of other EGFR effectors such as AKT. Consistent with the idea that the Dsg1 effects were mediated through elevated EGFR–Erk1/2 signaling, we found a marked increase in EphA2 mRNA (Fig. 5 B) and protein (Fig. 7 E) in Dsg1 knockdown but not ETA rafts. Because U0126 treatment also increased levels of Dsc1, we again ascertained that differentiation was rescued despite Dsc1 silencing (unpublished data). In summary, these findings place Dsg1 upstream in a signaling pathway leading to the suprabasal inhibition of EGFR–Erk1/2 signaling, which permits proper epidermal differentiation and morphogenesis.
Discussion
The targeting of Dsg1 by autoimmune antibodies and bacterial toxins causes blistering in the uppermost cell layers of the epidermis, highlighting its importance in adhesion (Green and Simpson, 2007; Garrod and Chidgey, 2008). However, because Dsg1 is first expressed as keratinocytes transit into the suprabasal layers, we considered an additional function for Dsg1 in regulating the differentiated state of epidermal cells. To define the role of Dsg1, we used gain- and loss-of-function approaches in a simplified in vitro model of human epidermis. These experiments revealed that Dsg1 is not only required for maintaining epidermal tissue integrity but also plays a key role in morphogenesis. Importantly, Dsg1 promotes epidermal differentiation in a manner that involves proper down-regulation of EGFR–Erk1/2 signaling but does not rely on ectodomain residues required for adhesion, the ability to bind PG, or coordinated Dsc1 expression. These experiments place Dsg1 in an early instructive pathway that directs the progression of keratinocytes through a terminal differentiation program.
Our data support a model in which Dsg1 acts as an important node in an epidermal adhesion and signaling network (Fig. 8 D). Keratinocytes held together by desmosomal cadherins in the basal layer are capable of responding to signals from EGFR to maintain steady-state proliferation in the epidermis. We propose that induction of Dsg1 serves to facilitate progression into a terminally differentiated state, at least in part, by preventing the sustained activation of the EGFR–Erk1/2 pathway in the suprabasal layers. However, basal layer desmosomal cadherins such as Dsg2 and Dsc3 might potentiate these growth promoting signals. In support of this possibility, transgenic misexpression of Dsg2 or Dsc3 in the suprabasal layers of mice results in elevated Erk1/2 signaling and impaired differentiation, respectively (Hardman et al., 2005; Brennan et al., 2007). Thus, basal and suprabasal desmosomal cadherins with opposing signaling potentials could serve to balance keratinocyte proliferation and differentiation to maintain epidermal tissue homeostasis.
Dsg4 exhibits a highly differentiation-dependent expression pattern in the human epidermis, being expressed in the uppermost viable cell layers above Dsg1, Dsc1, and K1/10 (Bazzi et al., 2006). Accordingly, the expression of these suprabasal proteins is unchanged in Dsg4 mutant mice that instead exhibit defects in hair follicle differentiation (Kljuic et al., 2003). In the present study, Dsg4 levels and distribution also remained unchanged in 6-d-old raft cultures in response to Dsg1 silencing or cleavage. Thus, Dsg4 likely plays a temporally and functionally distinct role from Dsg1, which is active in earlier epidermal differentiation events. However, coordination of desmosomal cadherin expression and growth factor signaling may be in play during hair follicle differentiation, where loss of Dsg4 or EGFR leads to premature differentiation of these keratinocytes with resultant hair defects (Hansen et al., 1997; Kljuic et al., 2003).
Directly interfering with desmosomal cadherin adhesion using small peptides that target EC1 of Dsg2 and Dsc2 disrupted epithelial positioning in an organotypic model of mammary alveolar morphogenesis (Runswick et al., 2001). The possibility that, in addition, desmosomal cadherins play supra-adhesive roles in development is supported by the observation that Dsg2- and Dsc3-null mutant mouse embryos die before desmosome formation (Eshkind et al., 2002; Den et al., 2006). In this study, we show that ETA-cleaved Dsg1 supports normal epidermal morphogenesis, demonstrating that this transmembrane protein can orchestrate a key signaling pathway in a manner that does not depend on ectodomain residues required for adhesion. E-cadherin, which is present in all viable epidermal cell layers (Hirai et al., 1989) has also been found to inhibit EGFR signaling, although downstream Erk1/2 phosphorylation was largely unaffected upon direct ligation of its adhesive ectodomain (Qian et al., 2004; Perrais et al., 2007). Moreover, classical cadherin depletion grossly disrupts epidermal integrity but does not result in MAPK-Erk1/2 activation or K10 loss (Tinkle et al., 2004, 2008). Therefore, the necessity of Dsg1 to suppress EGFR–Erk1/2 signaling and promote differentiation in a manner that does not require its adhesive ectodomain appears to be distinct from E-cadherin and represents a novel mechanism by which the activity of this receptor Tyr kinase can be regulated upon stratification.
The Dsg1 tail itself was unable to promote keratinocyte differentiation, although the cytoplasmic domain likely contributes to epidermal morphogenesis when anchored at the plasma membrane. Importantly, Dsg1's ability to recruit PG was not essential for triggering keratinocyte differentiation. This raises the possibility that the extended cytoplasmic tail of Dsg1, which harbors several additional regions outside of the catenin-binding site and not present in other cadherins, may recruit cytoplasmic proteins to the cell surface to regulate EGFR function. One such candidate could be the ERM (ezrin, radixin, moesin) family member, merlin, which has been shown to prevent EGFR signaling by sequestration of this receptor Tyr kinase into a more insoluble pool (Curto et al., 2007; Cole et al., 2008) in a manner similar to ectopic Dsg1 (Fig. 7 D).
In the absence of Dsg1, the sustained activation of EGFR–Erk1/2 in the suprabasal layers impaired differentiation without major changes in steady-state proliferation (Fig. S2). Because keratinocytes in different epidermal layers exhibit distinct responses to Ras-mediated Erk signaling (Bailleul et al., 1990; Brown et al., 1998), it is not too surprising that proliferation was unaltered by Dsg1 deficiency. However, our data support the notion that persistence of EGFR–Erk1/2 in the suprabasal layers prevents the induction of K10, which is not only a critical component of the intermediate filament network in differentiated keratinocytes but also limits the size of these cells in the upper layers (Reichelt and Magin, 2002). This could help explain why suprabasal keratinocytes lacking Dsg1 are larger and more variable in size compared with their WT counterparts and may contribute to persistent Erk1/2 signaling (Reichelt et al., 2004; Yano et al., 2004). Finally, Erk1/2 can activate CCAAT/enhancer-binding protein family members, which have been shown to regulate Dsc promoter activity (Hu et al., 2001; Smith et al., 2004). This might account for the defects in Dsc1 expression beyond posttranslational stabilization of this Dsg1-binding partner in the suprabasal layers. In support of this possibility, Dsc1 mRNA levels are markedly reduced upon Dsg1 silencing (Fig. 5 B). The ability of Dsg1 to induce Dsc1 expression is likely an important step in constructing robust adhesion complexes in the suprabasal epidermis because Dsg1-deficient rafts have desmosome defects (Fig. S3), but Dsc1 is not absolutely required for aspects of the Dsg1-mediated differentiation pathway reported in this study (Fig. 5 D).
Interestingly, skin blisters are not common in individuals with Dsg1 haploinsufficiency, who instead exhibit thickening in specific regions of the epidermis, which undergo extensive mechanical stress (Rickman et al., 1999; Wan et al., 2004). Alterations in the epithelial thickness were not apparent in Dsg1-deficient rafts, which might reflect differences in how keratinocytes respond to the lack of Dsg1 in situ because of signaling contributions from other cellular compartments such as the underlying dermis and immune system. Although reduced adhesion may account for some of these skin defects, our data raise the possibility of the EGFR–Erk1/2 signaling pathway as a novel therapeutic target for restoring epidermal architecture in patients with hyperkeratotic skin disease.
In summary, we have uncovered a novel role for desmosomal cadherins in epithelial differentiation by limiting the signaling potential of other cell surface receptors. In particular, Dsg1 is required for suppressing EGFR–Erk1/2 signaling to allow for normal epidermal differentiation and morphogenesis in a manner that does not rely on its adhesive capability but more likely on unique properties of the cytoplasmic tail or binding partners that are regulated by this extended region. Therefore, Dsg1 has multiple roles in the epidermis: instructing an early epidermal differentiation program upon stratification and maintaining robust adhesion in the uppermost layers.
Materials and methods
Generation of cDNA and miRNA constructs
The full-length human Dsg1 cDNA with a C-terminal Flag epitope in pTRE (p812) and pLZRS-Linker (p1007) along with pLZRS-pBMN-EGFP (p990) have been previously described (Getsios et al., 2004). An miRNA-resistant Dsg1 construct was generated from p812 by site-directed mutagenesis (Agilent Technologies) to incorporate two silent point mutations within the miR DG1 target region. Dsg1 deficient in PG binding was generated by incorporating three Ala substitutions (Leu751Ala; Phe755Ala; Leu758Ala) within the predicted binding region for PG (Dsg1AAA; Chitaev et al., 1998). The Dsg1 cytodomain-only construct (ΔN-Dsg1; aa 569–1,049) was generated by PCR amplification of a fragment of p812. All of these Flag-tagged Dsg1 cDNAs were subcloned into the BamHI site of pLZRS-Linker.
RNA interference of human lamin A/C (miR Lmn) or Dsg1 (miR DG1) was performed using miRNA 155–mimicking sequences cloned downstream of the 3′ untranslated region of EGFP. The EGFP cDNA was excised from pEGFP-N1 (Clontech Laboratories, Inc.) using EcoRI and NotI and subcloned into pME18S-f1 (a gift from K. Fujiwara, University of Rochester, Rochester, NY). The 3′ untranslated region (NotI–XbaI) was modified to contain two BstXI sites for insertion of oligonucleotide duplexes, and the resultant plasmid was termed pMIRAGE-G. Antisense and sense oligonucleotide duplexes for miR Lmn or miR DG1 were annealed as previously described (Brummelkamp et al., 2002), phosphorylated using T4 polynucleotide kinase, and ligated in tandem into the BstXI sites of pMIRAGE-G. The EGFP-miR EcoRI and XbaI fragments were then blunted with Klenow fragment and subcloned into the HindIII–XhoI site of pLZRS-Linker. Dsc1 silencing was performed using a pool of three Stealth siRNA oligonucleotides (25 nM each; Invitrogen) targeting this desmosomal cadherin or a GC-matched negative control, which were introduced into keratinocytes using the Dharmafectin1 transfection reagent (Thermo Fisher Scientific).
Constructs encoding WT and protease-dead (S195A) S. aureus ETA (Hanakawa et al., 2002) were provided by J. Stanley (University of Pennsylvania, Philadelphia, PA).
Cell culture and retroviral transduction
Epidermal keratinocytes were isolated from human foreskin as previously described (Halbert et al., 1992). The cells were propagated in medium 154 supplemented with human keratinocyte growth supplement, 1,000× gentamycin/amphotericin B solution (Invitrogen), and 0.07 or 0.2 mM CaCl2.
Keratinocytes were transduced with retroviral supernatants produced from Phoenix cells (provided by G. Nolan, Stanford University, Stanford, CA) as previously described (Getsios et al., 2004). For differentiation of submerged cultures, cells were grown to confluence and switched to E-medium containing 1.8 mM Ca2+ for 1–6 d (Meyers and Laimins, 1994). For raft cultures, transduced cells were expanded and grown at an air–medium interface according to published protocols (Meyers and Laimins, 1994). Organotypic cultures were grown for 3–10 d, at which time they were lysed for RNA/protein analysis, embedded in optimal cutting temperature compound for frozen sections, fixed in 10% neutral-buffered formalin, and embedded in paraffin for histology or fixed in 2% paraformaldehyde/2% glutaraldehyde in cacodylate buffer for EM analysis. For some experiments, cultures were treated with 2–5 µg/ml ETA, DMSO (Thermo Fisher Scientific), 10 µM PKI166 (Novartis), 5 µM U0126 (Cell Signaling Technology), or 10 µM SB203580 (EMD).
Antibodies and reagents
The following mouse monoclonal antibodies were used in this study: P124 (anti–Dsg1 extracellular domain; RDI); 4B2 (anti–Dsg1 cytodomain; Dusek et al., 2006); 27B2 (anti–Dsg1 cytodomain; Invitrogen); 6D8 and 18G8 (anti-Dsg2 and -Dsg4, respectively; gift from J. Wahl III, University of Nebraska Medical Center, Omaha, NE); AK15 (anti-Dsg3; gift from M. Amagai, Keio University, Minato-ku, Tokyo, Japan); U100 and U114 (anti-Dsc1a/b and -Dsc3a/b, respectively; RDI); 11E4 (anti-PG; gift from J. Wahl III); HECD1 (anti–E-cadherin; Takara); 8E7 (anti–activated β-catenin/ABC; Millipore); LH2 (anti-K10) and LL002 (anti-K14; both gifts from E.B. Lane, University of Dundee, Dundee, Scotland, UK); AKH1 (antifilaggrin; gift from R. Presland, University of Washington, Seattle, WA); C4 (antiactin; Thermo Fisher Scientific); anti-EGFR (Thermo Fisher Scientific); anti–EGFR phospho-Y1068 (p-Y1068; Cell Signaling Technology); D7 (anti-EphA2; Millipore); E7 (γ-tubulin) and anti-BrdU (Developmental Studies Hybridoma Bank); and M2 (anti-Flag; Sigma-Aldrich).
Polyclonal antibodies used included 1407 (chicken anti-PG; Aves Laboratories); c2206 (β-catenin; Sigma-Aldrich); rabbit antiloricrin (gift from D. Roop and M. Koster, Baylor College of Medicine, Houston, TX; Covance); rabbit anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase; Abcam); 982 (human anti–Dsg1 extracellular domain pemphigus foliaceus sera) and 1905 (rabbit anti-Dsg3; both gifts from J. Stanley); rabbit anti-Ki67 (Dako); rabbit anti-Flag and anti-erbB2 (Cell Signaling Technology); rabbit anti-EGFR, p-Ser473-AKT, p–c-Raf, and p-MEK1/2 (Cell Signaling Technology); c-Fos and lamin A/C (Santa Cruz Biotechnology, Inc.); and rabbit anti-Erk1/2 and anti–p-Erk1/2 (Promega).
Secondary antibodies used for Western blotting included goat anti–mouse, –rabbit, and –chicken peroxidase (Rockland; KPL). Secondary antibodies used for immunoperoxidase-DAB (Sigma-Aldrich) staining included biotinylated horse anti–mouse and –rabbit (Jackson ImmunoResearch Laboratories). Secondary antibodies used for immunofluorescence included goat anti–mouse, –rabbit, –chicken, and –human linked to fluorophores of 488 nm and 568 nm (Alexa Fluor; Invitrogen). DAPI (Sigma-Aldrich) was used for nuclear staining. BrdU was purchased from Sigma-Aldrich. Recombinant S. aureus WT or protease-dead (S195A) ETA was produced and purified as previously described (Hanakawa et al., 2002).
Real-time PCR
Total RNA was extracted in Tri-Reagent solution (Molecular Research Center, Inc.), DNase I (Invitrogen) treated, and reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad Laboratories) according to the manufacturer's instructions. Real-time PCR was performed on a sequence detector (SDS5700; Applied Biosystems) using the IQ SYBR green Supermix kit (Bio-Rad Laboratories) as directed by the manufacturer. Intron spanning primers were designed for human cyclophilin 1 and Dsg1–4 mRNA. For each experimental cDNA sample, the relative level of Dsg mRNA was interpolated from a standard curve for cyclophilin 1 that was generated from the analysis of serially diluted standards. Primer Bank (http://pga.mgh.harvard.edu/primerbank/) was used to select primers for the analysis of Dsg1, Dsc1, and EphA2 mRNA, which were normalized to 18-S ribosomal RNA using the comparative CT method (2-(Δ ΔCT)) after amplification using the Power SYBR green Master Mix (Applied Biosystems), and are represented as fold changes from control miR Lmn rafts.
Protein expression, solubility, and immunoprecipitation analysis
Protein was extracted from monolayer or raft cultures using urea sample buffer for whole cell lysates or radioimmunoprecipitation assay (RIPA) buffer containing 1% protease inhibitor cocktail (Sigma-Aldrich) and 1% phosphatase inhibitor cocktails II and IV (EMD) for phospho-specific antigens, immunoprecipitations, and detergent solubility experiments. Flag-tagged proteins were immunoprecipitated with anti-Flag M2-agarose (Sigma-Aldrich) and analyzed by SDS-PAGE and immunoblotting as described previously (Chen et al., 2002). RIPA-soluble and -insoluble fractions as well as immunoprecipitated complexes were denatured in SDS buffer and subjected to SDS-PAGE and Western blot analysis as previously described (Getsios et al., 2004). Relative band intensities for phosphorylated proteins were normalized to total protein levels calculated using ImageJ software (National Institutes of Health).
Histology, immunohistochemistry, immunocytochemistry, and EM analysis
Raft cultures were sectioned and processed for immunohistochemical (IHC) or hematoxylin and eosin (H&E) staining using conventional methods. Antigen retrieval was performed by heating to 95°C in 0.01 M of citrate buffer. Sections were blocked in 10% normal goat or horse serum (Jackson ImmunoResearch Laboratories) for 60 min at 37°C, incubated in primary antibody in 0.5% BSA overnight at 4°C, incubated in secondary antibody in 0.5% BSA for 60 min at 37°C, and mounted in polyvinyl alcohol. For frozen sections, optimal cutting temperature compound–embedded rafts were air dried, fixed in 4% paraformaldehyde, permeabilized in buffered 0.2% Triton X-100, and immunostained as described in the previous sentence. For immunocytochemistry of submerged cultures, cells were grown on glass coverslips, fixed in 3.7% formyl saline for 10 min at room temperature, permeabilized in acetone at −20°C for 2 min, and then processed for immunofluorescence as previously described (Getsios et al., 2004). Images were obtained with a 40× NA 1.0 Plan-Fluotar or a 63× NA 1.32 Plan-Apochromat objective on a microscope (DMR; Leica) using a charge-coupled device camera (Orca 100 model C4742-95; Hamamatsu Photonics) for fluorescence or a digital camera (DFC320; Leica) for color images and MetaMorph 6.1 software (MDS Analytical Technologies). For optical sectioning of tissue sections or colocalization experiments, images were acquired using a 20× NA 0.5 EC Plan-Neofluar or a 63× NA 1.4 Plan-Apochromat objective, respectively, on an epifluorescence microscope system (AxioVision Z1; Carl Zeiss, Inc.) fitted with an Apotome slide module and a digital camera (AxioCam MRm; Carl Zeiss, Inc.). For EM, raft cultures were fixed in 2% paraformaldehyde/2% glutaraldehyde and processed for conventional EM analysis as described previously (Green et al., 1991). Samples were viewed and photographed on an electron microscope (100CX; JEOL).
Raft cell size and proliferation analysis
Raft sections were processed for immunohistochemistry using antiactin (C4; Thermo Fisher Scientific) and DAPI. The size of suprabasal cells was quantitated by tracing the cortical actin outline of each cell and measuring the enclosed area using MetaMorph 6.1 software. Greater than 150 cells were measured for each condition; individual and mean cell sizes (±SEM) were graphed. To assess Ki67 expression and BrdU incorporation, rafts were pulsed with 10 µM BrdU for 1 h and then fixed after chasing with fresh media for 24 h and processed for IHC analysis. BrdU- or Ki67-positive cells were divided by the total number of DAPI-stained nuclei from 10 random fields; >500 cells were counted for each condition, and the percentage was graphed ±SD.
Online supplemental material
Fig. S1 shows that the expression and distribution of desmosomal and classical cadherins beyond Dsc1 remain unchanged in Dsg1-silenced raft cultures. Fig. S2 shows that Ki67 expression and BrdU incorporation are unaltered in Dsg1-deficient raft cultures. Fig. S3 shows adhesion defects by ultrastructure of chronic ETA-treated or Dsg1 knockdown cultures using EM. Fig. S4 shows that the expression and distribution of desmosomal and classical cadherins remain unchanged in chronic ETA-treated raft cultures. Fig. S5 shows the expression, detergent solubility, and PG-binding capability of the Dsg1 cDNA constructs used in this study. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200809044/DC1.
Acknowledgments
The authors would like to thank Drs. Andrew Kowalczyk, Robert Lavker, and Sergey Troyanovsky for comments on the paper, Dr. Laimonis Laimins for help in adopting the raft culture model of human epidermis, and Ms. Kerstyn Bryce and Mr. Todd Gocken for technical assistance. Gifts were provided by Novartis (PKI166) and Drs. John Stanley (ETA and Dsg1 and Dsg3 antibodies), Masayuki Amagai (Dsg3 antibody), Jim Wahl III (Dsg2, Dsg4, and PG antibodies), Birgitte Lane (K10 and -14 antibodies), Dennis Roop and Maranke Koster (loricrin antibody), Richard Presland (filaggrin antibody), and Keigi Fujiwara (pME18S-f1 plasmid).
This work was supported by grants from the National Institutes of Health (R01 AR041836 with partial support from AR43380 and CA122151) to K.J. Green and a research grant from the Dermatology Foundation to S. Getsios. Additional support was provided by the J.L. Mayberry Endowment to K.J. Green as well as a Career Development Award from the Dermatology Foundation and a Zell Foundation Award from the Robert H. Lurie Comprehensive Cancer Center to S. Getsios. C.L. Simpson is supported by a Kirschstein predoctoral fellowship from the National Institutes of Health National Institute of Environmental Health Sciences (F30 ES14990), a Malkin Scholarship from the Robert H. Lurie Comprehensive Cancer Center, and a Presidential Fellowship from Northwestern University. R. Harmon is supported by a National Institutes of Health T32 Training Grant (T32 GM08061). Histological analysis was conducted by the Mouse Phenotyping Core of the R.H. Lurie Comprehensive Cancer Center (National Institutes of Health grant P30 CA060553-159026).
Footnotes
Abbreviations used in this paper: Dsc, desmocollin; Dsg, desmoglein; E-cadherin, epithelial cadherin; EGFR, EGF receptor; Erk, extracellular signal-regulated kinase; ETA, exfoliative toxin A; IHC, immunohistochemical; miRNA, microRNA; PG, plakoglobin; RIPA, radioimmunoprecipitation assay; WT, wild type.
References
- Allen E., Yu Q.C., Fuchs E. 1996. Mice expressing a mutant desmosomal cadherin exhibit abnormalities in desmosomes, proliferation, and epidermal differentiation.J. Cell Biol. 133:1367–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amagai M., Matsuyoshi N., Wang Z.H., Andl C., Stanley J.R. 2000. Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1.Nat. Med. 6:1275–1277 [DOI] [PubMed] [Google Scholar]
- Arnemann J., Sullivan K.H., Magee A.I., King I.A., Buxton R.S. 1993. Stratification-related expression of isoforms of the desmosomal cadherins in human epidermis.J. Cell Sci. 104:741–750 [DOI] [PubMed] [Google Scholar]
- Bailleul B., Surani M.A., White S., Barton S.C., Brown K., Blessing M., Jorcano J., Balmain A. 1990. Skin hyperkeratosis and papilloma formation in transgenic mice expressing a ras oncogene from a suprabasal keratin promoter.Cell. 62:697–708 [DOI] [PubMed] [Google Scholar]
- Bazzi H., Getz A., Mahoney M.G., Ishida-Yamamoto A., Langbein L., Wahl J.K., III, Christiano A.M. 2006. Desmoglein 4 is expressed in highly differentiated keratinocytes and trichocytes in human epidermis and hair follicle.Differentiation. 74:129–140 [DOI] [PubMed] [Google Scholar]
- Brennan D., Hu Y., Kljuic A., Choi Y., Joubeh S., Bashkin M., Wahl J., Fertala A., Pulkkinen L., Uitto J., et al. 2004. Differential structural properties and expression patterns suggest functional significance for multiple mouse desmoglein 1 isoforms.Differentiation. 72:434–449 [DOI] [PubMed] [Google Scholar]
- Brennan D., Hu Y., Joubeh S., Choi Y.W., Whitaker-Menezes D., O'Brien T., Uitto J., Rodeck U., Mahoney M.G. 2007. Suprabasal Dsg2 expression in transgenic mouse skin confers a hyperproliferative and apoptosis-resistant phenotype to keratinocytes.J. Cell Sci. 120:758–771 [DOI] [PubMed] [Google Scholar]
- Brown K., Strathdee D., Bryson S., Lambie W., Balmain A. 1998. The malignant capacity of skin tumours induced by expression of a mutant H-ras transgene depends on the cell type targeted.Curr. Biol. 8:516–524 [DOI] [PubMed] [Google Scholar]
- Brummelkamp T.R., Bernards R., Agami R. 2002. A system for stable expression of short interfering RNAs in mammalian cells.Science. 296:550–553 [DOI] [PubMed] [Google Scholar]
- Calautti E., Li J., Saoncella S., Brissette J.L., Goetinck P.F. 2005. Phosphoinositide 3-kinase signaling to Akt promotes keratinocyte differentiation versus death.J. Biol. Chem. 280:32856–32865 [DOI] [PubMed] [Google Scholar]
- Chen J., Den Z., Koch P.J. 2008. Loss of desmocollin 3 in mice leads to epidermal blistering.J. Cell Sci. 121:2844–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Bonne S., Hatzfeld M., van Roy F., Green K.J. 2002. Protein binding and functional characterization of plakophilin 2. Evidence for its diverse roles in desmosomes and beta-catenin signaling.J. Biol. Chem. 277:10512–10522 [DOI] [PubMed] [Google Scholar]
- Chidgey M., Brakebusch C., Gustafsson E., Cruchley A., Hail C., Kirk S., Merritt A., North A., Tselepis C., Hewitt J., et al. 2001. Mice lacking desmocollin 1 show epidermal fragility accompanied by barrier defects and abnormal differentiation.J. Cell Biol. 155:821–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitaev N.A., Averbakh A.Z., Troyanovsky R.B., Troyanovsky S.M. 1998. Molecular organization of the desmoglein-plakoglobin complex.J. Cell Sci. 111:1941–1949 [DOI] [PubMed] [Google Scholar]
- Cole B.K., Curto M., Chan A.W., McClatchey A.I. 2008. Localization to the cortical cytoskeleton is necessary for Nf2/merlin-dependent epidermal growth factor receptor silencing.Mol. Cell. Biol. 28:1274–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curto M., Cole B.K., Lallemand D., Liu C.H., McClatchey A.I. 2007. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin.J. Cell Biol. 177:893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S.P., Reddy H., Caivano M., Cohen P. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors.Biochem. J. 351:95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Z., Cheng X., Merched-Sauvage M., Koch P.J. 2006. Desmocollin 3 is required for pre-implantation development of the mouse embryo.J. Cell Sci. 119:482–489 [DOI] [PubMed] [Google Scholar]
- Denning M.F., Dlugosz A.A., Williams E.K., Szallasi Z., Blumberg P.M., Yuspa S.H. 1995. Specific protein kinase C isozymes mediate the induction of keratinocyte differentiation markers by calcium.Cell Growth Differ. 6:149–157 [PubMed] [Google Scholar]
- Donetti E., Bedoni M., Boschini E., Dellavia C., Barajon I., Gagliano N. 2005. Desmocollin 1 and desmoglein 1 expression in human epidermis and keratinizing oral mucosa: a comparative immunohistochemical and molecular study.Arch. Dermatol. Res. 297:31–38 [DOI] [PubMed] [Google Scholar]
- Dusek R.L., Getsios S., Chen F., Park J.K., Amargo E.V., Cryns V.L., Green K.J. 2006. The differentiation-dependent desmosomal cadherin desmoglein 1 is a novel caspase-3 target that regulates apoptosis in keratinocytes.J. Biol. Chem. 281:3614–3624 [DOI] [PubMed] [Google Scholar]
- Elias P.M., Matsuyoshi N., Wu H., Lin C., Wang Z.H., Brown B.E., Stanley J.R. 2001. Desmoglein isoform distribution affects stratum corneum structure and function.J. Cell Biol. 153:243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshkind L., Tian Q., Schmidt A., Franke W.W., Windoffer R., Leube R.E. 2002. Loss of desmoglein 2 suggests essential functions for early embryonic development and proliferation of embryonal stem cells.Eur. J. Cell Biol. 81:592–598 [DOI] [PubMed] [Google Scholar]
- Garrod D., Chidgey M. 2008. Desmosome structure, composition and function.Biochim. Biophys. Acta. 1778:572–587 [DOI] [PubMed] [Google Scholar]
- Gazel A., Nijhawan R.I., Walsh R., Blumenberg M. 2008. Transcriptional profiling defines the roles of ERK and p38 kinases in epidermal keratinocytes.J. Cell. Physiol. 215:292–308 [DOI] [PubMed] [Google Scholar]
- Getsios S., Amargo E.V., Dusek R.L., Ishii K., Sheu L., Godsel L.M., Green K.J. 2004. Coordinated expression of desmoglein 1 and desmocollin 1 regulates intercellular adhesion.Differentiation. 72:419–433 [DOI] [PubMed] [Google Scholar]
- Green K.J., Simpson C.L. 2007. Desmosomes: new perspectives on a classic.J. Invest. Dermatol. 127:2499–2515 [DOI] [PubMed] [Google Scholar]
- Green K.J., Stappenbeck T.S., Noguchi S., Oyasu R., Nilles L.A. 1991. Desmoplakin expression and distribution in cultured rat bladder epithelial cells of varying tumorigenic potential.Exp. Cell Res. 193:134–143 [DOI] [PubMed] [Google Scholar]
- Halbert C.L., Demers C.W., Galloway D.A. 1992. The E6 and E7 genes of human papilloma virus type 6 have weak immortalizing activity in human epithelial cells.J. Virol. 66:2125–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa Y., Schechter N.M., Lin C., Garza L., Li H., Yamaguchi T., Fudaba Y., Nishifuji K., Sugai M., Amagai M., Stanley J.R. 2002. Molecular mechanisms of blister formation in bullous impetigo and staphylococcal scalded skin syndrome.J. Clin. Invest. 110:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa Y., Schechter N.M., Lin C., Nishifuji K., Amagai M., Stanley J.R. 2004. Enzymatic and molecular characteristics of the efficiency and specificity of exfoliative toxin cleavage of desmoglein 1.J. Biol. Chem. 279:5268–5277 [DOI] [PubMed] [Google Scholar]
- Hansen L.A., Alexander N., Hogan M.E., Sundberg J.P., Dlugosz A., Threadgill D.W., Magnuson T., Yuspa S.H. 1997. Genetically null mice reveal a central role for epidermal growth factor receptor in the differentiation of the hair follicle and normal hair development.Am. J. Pathol. 150:1959–1975 [PMC free article] [PubMed] [Google Scholar]
- Hardman M.J., Liu K., Avilion A.A., Merritt A., Brennan K., Garrod D.R., Byrne C. 2005. Desmosomal cadherin misexpression alters beta-catenin stability and epidermal differentiation.Mol. Cell. Biol. 25:969–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai Y., Nose A., Kobayashi S., Takeichi M. 1989. Expression and role of E- and P-cadherin adhesion molecules in embryonic histogenesis. II. Skin morphogenesis.Development. 105:271–277 [DOI] [PubMed] [Google Scholar]
- Hu J., Roy S.K., Shapiro P.S., Rodig S.R., Reddy S.P., Platanias L.C., Schreiber R.D., Kalvakolanu D.V. 2001. ERK1 and ERK2 activate CCAAAT/enhancer-binding protein-beta-dependent gene transcription in response to interferon-gamma.J. Biol. Chem. 276:287–297 [DOI] [PubMed] [Google Scholar]
- Kljuic A., Bazzi H., Sundberg J.P., Martinez-Mir A., O'Shaughnessy R., Mahoney M.G., Levy M., Montagutelli X., Ahmad W., Aita V.M., et al. 2003. Desmoglein 4 in hair follicle differentiation and epidermal adhesion: evidence from inherited hypotrichosis and acquired pemphigus vulgaris.Cell. 113:249–260 [DOI] [PubMed] [Google Scholar]
- Koch P.J., Mahoney M.G., Cotsarelis G., Rothenberger K., Lavker R.M., Stanley J.R. 1998. Desmoglein 3 anchors telogen hair in the follicle.J. Cell Sci. 111:2529–2537 [DOI] [PubMed] [Google Scholar]
- Kolev V., Mandinova A., Guinea-Viniegra J., Hu B., Lefort K., Lambertini C., Neel V., Dummer R., Wagner E.F., Dotto G.P. 2008. EGFR signalling as a negative regulator of Notch1 gene transcription and function in proliferating keratinocytes and cancer.Nat. Cell Biol. 10:902–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacouture M.E. 2006. Mechanisms of cutaneous toxicities to EGFR inhibitors.Nat. Rev. Cancer. 6:803–812 [DOI] [PubMed] [Google Scholar]
- Larsen A.B., Pedersen M.W., Stockhausen M.T., Grandal M.V., van Deurs B., Poulsen H.S. 2007. Activation of the EGFR gene target EphA2 inhibits epidermal growth factor-induced cancer cell motility.Mol. Cancer Res. 5:283–293 [DOI] [PubMed] [Google Scholar]
- Lien W.H., Klezovitch O., Vasioukhin V. 2006. Cadherin-catenin proteins in vertebrate development.Curr. Opin. Cell Biol. 18:499–506 [DOI] [PubMed] [Google Scholar]
- Merritt A.J., Berika M.Y., Zhai W., Kirk S.E., Ji B., Hardman M.J., Garrod D.R. 2002. Suprabasal desmoglein 3 expression in the epidermis of transgenic mice results in hyperproliferation and abnormal differentiation.Mol. Cell. Biol. 22:5846–5858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers C., Laimins L.A. 1994. In vitro systems for the study and propagation of human papillomaviruses.Curr. Top. Microbiol. Immunol. 186:199–215 [DOI] [PubMed] [Google Scholar]
- Miettinen P.J., Berger J.E., Meneses J., Phung Y., Pedersen R.A., Werb Z., Derynck R. 1995. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor.Nature. 376:337–341 [DOI] [PubMed] [Google Scholar]
- Murillas R., Larcher F., Conti C.J., Santos M., Ullrich A., Jorcano J.L. 1995. Expression of a dominant negative mutant of epidermal growth factor receptor in the epidermis of transgenic mice elicits striking alterations in hair follicle development and skin structure.EMBO J. 14:5216–5223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaka T., Nishifuji K., Ota T., Whittock N.V., Amagai M. 2004. Defining the pathogenic involvement of desmoglein 4 in pemphigus and staphylococcal scalded skin syndrome.J. Clin. Invest. 114:1484–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais M., Chen X., Perez-Moreno M., Gumbiner B.M. 2007. E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions.Mol. Biol. Cell. 18:2013–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Karpova T., Sheppard A.M., McNally J., Lowy D.R. 2004. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases.EMBO J. 23:1739–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt J., Magin T.M. 2002. Hyperproliferation, induction of c-Myc and 14-3-3sigma, but no cell fragility in keratin-10-null mice.J. Cell Sci. 115:2639–2650 [DOI] [PubMed] [Google Scholar]
- Reichelt J., Furstenberger G., Magin T.M. 2004. Loss of keratin 10 leads to mitogen-activated protein kinase (MAPK) activation, increased keratinocyte turnover, and decreased tumor formation in mice.J. Invest. Dermatol. 123:973–981 [DOI] [PubMed] [Google Scholar]
- Rickman L., Simrak D., Stevens H.P., Hunt D.M., King I.A., Bryant S.P., Eady R.A., Leigh I.M., Arnemann J., Magee A.I., et al. 1999. N-terminal deletion in a desmosomal cadherin causes the autosomal dominant skin disease striate palmoplantar keratoderma.Hum. Mol. Genet. 8:971–976 [DOI] [PubMed] [Google Scholar]
- Runswick S.K., O'Hare M.J., Jones L., Streuli C.H., Garrod D.R. 2001. Desmosomal adhesion regulates epithelial morphogenesis and cell positioning.Nat. Cell Biol. 3:823–830 [DOI] [PubMed] [Google Scholar]
- Serpente N., Marcozzi C., Roberts G.A., Bao Q., Angst B.D., Hirst E.M., Burdett I.D., Buxton R.S., Magee A.I. 2000. Extracellularly truncated desmoglein 1 compromises desmosomes in MDCK cells.Mol. Membr. Biol. 17:175–183 [DOI] [PubMed] [Google Scholar]
- Smith C., Zhu K., Merritt A., Picton R., Youngs D., Garrod D., Chidgey M. 2004. Regulation of desmocollin gene expression in the epidermis: CCAAT/enhancer-binding proteins modulate early and late events in keratinocyte differentiation.Biochem. J. 380:757–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll S.W., Kansra S., Peshick S., Fry D.W., Leopold W.R., Wiesen J.F., Sibilia M., Zhang T., Werb Z., Derynck R., et al. 2001. Differential utilization and localization of ErbB receptor tyrosine kinases in skin compared to normal and malignant keratinocytes.Neoplasia. 3:339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuliere J., Faraldo M.M., Shtutman M., Birchmeier W., Huelsken J., Thiery J.P., Glukhova M.A. 2004. beta-catenin-dependent and -independent effects of DeltaN-plakoglobin on epidermal growth and differentiation.Mol. Cell. Biol. 24:8649–8661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkle C.L., Lechler T., Pasolli H.A., Fuchs E. 2004. Conditional targeting of E-cadherin in skin: insights into hyperproliferative and degenerative responses.Proc. Natl. Acad. Sci. USA. 101:552–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkle C.L., Pasolli H.A., Stokes N., Fuchs E. 2008. New insights into cadherin function in epidermal sheet formation and maintenance of tissue integrity.Proc. Natl. Acad. Sci. USA. 105:15405–15410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traxler P., Bold G., Buchdunger E., Caravatti G., Furet P., Manley P., O'Reilly T., Wood J., Zimmermann J. 2001. Tyrosine kinase inhibitors: from rational design to clinical trials.Med. Res. Rev. 21:499–512 [DOI] [PubMed] [Google Scholar]
- Wan H., Dopping-Hepenstal P.J., Gratian M.J., Stone M.G., Zhu G., Purkis P.E., South A.P., Keane F., Armstrong D.K., Buxton R.S., et al. 2004. Striate palmoplantar keratoderma arising from desmoplakin and desmoglein 1 mutations is associated with contrasting perturbations of desmosomes and the keratin filament network.Br. J. Dermatol. 150:878–891 [DOI] [PubMed] [Google Scholar]
- Wilanowski T., Caddy J., Ting S.B., Hislop N.R., Cerruti L., Auden A., Zhao L.L., Asquith S., Ellis S., Sinclair R., et al. 2008. Perturbed desmosomal cadherin expression in grainy head-like 1-null mice.EMBO J. 27:886–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson L., Raess N.A., Caldelari R., Zakher A., de Bruin A., Posthaus H., Bolli R., Hunziker T., Suter M.M., Muller E.J. 2006. Pemphigus vulgaris identifies plakoglobin as key suppressor of c-Myc in the skin.EMBO J. 25:3298–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Wang Z.H., Yan A., Lyle S., Fakharzadeh S., Wahl J.K., Wheelock M.J., Ishikawa H., Uitto J., Amagai M., Stanley J.R. 2000. Protection against pemphigus foliaceus by desmoglein 3 in neonates.N. Engl. J. Med. 343:31–35 [DOI] [PubMed] [Google Scholar]
- Yano S., Komine M., Fujimoto M., Okochi H., Tamaki K. 2004. Mechanical stretching in vitro regulates signal transduction pathways and cellular proliferation in human epidermal keratinocytes.J. Invest. Dermatol. 122:783–790 [DOI] [PubMed] [Google Scholar]