Abstract
Primary microcephaly, Seckel syndrome, and microcephalic osteodysplastic primordial dwarfism type II (MOPD II) are disorders exhibiting marked microcephaly, with small brain sizes reflecting reduced neuron production during fetal life. Although primary microcephaly can be caused by mutations in microcephalin (MCPH1), cells from patients with Seckel syndrome and MOPD II harbor mutations in ataxia telangiectasia and Rad3 related (ATR) or pericentrin (PCNT), leading to disturbed ATR signaling. In this study, we show that a lack of MCPH1 or PCNT results in a loss of Chk1 from centrosomes with subsequently deregulated activation of centrosomal cyclin B–Cdk1.
Introduction
Primary microcephaly is a neurodevelopmental disorder characterized by reduced brain size. The first causative gene identified encodes microcephalin (MCPH1), a protein implicated in DNA damage signaling via ataxia telangiectasia and Rad3 related (ATR; Jackson et al., 2002; Alderton et al., 2006). Accordingly, the clinical features of MCPH1 syndrome patients resemble those of Seckel syndrome, another microcephalic disorder that can be caused by mutations in ATR or defective ATR signaling (O'Driscoll et al., 2003; Alderton et al., 2004). Recently, it has been reported that mutations in pericentrin (PCNT), resulting in the loss of PCNT from the centrosome, also cause Seckel syndrome (Griffith et al., 2008). PCNT-Seckel cells, like ATR-Seckel cells, show defects in ATR-dependent G2/M checkpoint control. This finding is further supported by the recent description of PCNT mutations in microcephalic osteodysplastic primordial dwarfism type II (MOPD II), a microcephalic disorder in which growth restriction is thought to be the consequence of mitotic failure (Rauch et al., 2008).
Mechanistically, it has been shown that MCPH1 prevents premature entry into mitosis and speculated that loss of PCNT might prevent centrosomal recruitment of the checkpoint kinase Chk1, an ATR target that controls initial activation of cyclin B–Cdk1 at the centrosome (Krämer et al., 2004; Alderton et al., 2006; Löffler et al., 2006a; Griffith et al., 2008). Activation of cyclin B–Cdk1 is the key event required for the initiation of mitosis (Nurse, 1990). Cdk1 and cyclin B accumulate at the centrosome during interphase (Bailly et al., 1989, 1992), where initial activation of the cyclin B–Cdk1 complex occurs in late prophase (Jackman et al., 2003). The accumulation of active cyclin B–Cdk1 within the nucleus shortly thereafter irreversibly commits the cell to mitosis. Therefore, regulation of cyclin B–Cdk1 activity at the centrosome is critical for controlling both cytoplasmic and nuclear mitotic events. During unperturbed cell cycles, activation of cyclin B–Cdk1 at the centrosome is mediated by Cdc25B (De Souza et al., 2000; Krämer et al., 2004), which itself is positively regulated by phosphorylation at S353 by aurora A and inhibited by Chk1-mediated S230 phosphorylation at centrosomes (Dutertre et al., 2004; Löffler et al., 2006b; Schmitt et al., 2006).
To directly address whether loss of Chk1 from centrosomes constitutes a mechanism for premature entry into mitosis in microcephaly syndromes, we examined both primary patient-derived cells from individuals with MCPH1427insA (MCPH1 syndrome) and PCNT3109G>T (MOPD II) mutations as well as cells treated with MCPH1- and PCNT-specific siRNAs, respectively.
Results and discussion
Chk1 is lost from centrosomes in both MCPH1- and PCNT-deficient cells
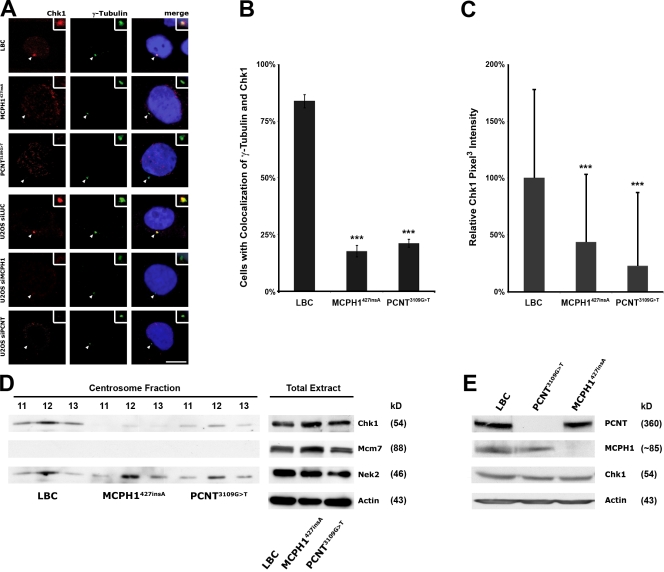
First, we analyzed whether deficiency of either MCPH1 or PCNT impacts on the abundance of Chk1 at centrosomes in human cells. Although immunofluorescence microscopy analysis with a monoclonal antibody against Chk1 revealed a pronounced centrosomal staining in both U2OS and control lymphoblastoid cells (LBCs), Chk1 levels were reduced at centrosomes in MCPH1 and PCNT siRNA–treated U2OS cells as well as in primary MCPH1427insA and PCNT3109G>T cells from patients with primary microcephaly or MOPD II, respectively (Fig. 1, A–C; and Fig. S1, A–C). This effect was independent from the cell cycle stage, as Chk1 levels were reduced to a similar extent in both cells containing a single centrosome, reflecting G0/G1 phase, and two centrosomes, reflecting S/G2 phase, respectively (Fig. S1 C). Virtually identical results were obtained when a rabbit polyclonal antibody against Chk1 was used (unpublished data).
Figure 1.
Chk1 levels are reduced at centrosomes in both MCPH1- and PCNT-deficient cells. (A) Normal, MCPH1427insA, and PCNT3109G>T LBCs as well as U2OS cells transfected with luciferase- (as control [siLUC]), MCPH1-, or PCNT-specific siRNA were costained with mouse anti-Chk1 (red) and rabbit anti–γ-tubulin (green) antibodies and analyzed by confocal microscopy. Bar, 10 µm. (B) The mean percentages of cells with centrosomal colocalization of γ-tubulin and Chk1 are indicated. Error bars represent the standard deviation after combining the results of three different experiments. Statistical significance versus control (LBC) by two-tailed Student's t test is as follows: ***, P = 9 × 10−6 (MCPH1427insA); P = 0.00003 (PCNT3109G>T). (C) Quantification of pixel intensity profiles constructed from optically sectioned (z axis) fluorescence images of control, MCPH1427insA, and PCNT3109G>T LBCs. Error bars represent standard deviations from the analysis of 100 cells. Statistical significance versus control (LBC) by two-tailed Student's t test is as follows: ***, P = 1.4 × 10−7 (MCPH1427insA); P = 9.8 × 10−12 (PCNT3109G>T). (D) Loss of Chk1 protein in isolated centrosome preparations. Immunoblots were performed on three sucrose gradient fractions of centrosome preparations (left) and whole cell lysates (right) as an input control from normal, MCPH1427insA, and PCNT3109G>T LBCs using antibodies against Chk1 and, for comparison, Nek2 (a centrosomal protein) and Mcm7 (a nuclear protein). For whole cell lysates, actin was included as a loading control. (E) Western blot analysis of PCNT, MCPH1, and Chk1 in whole cell lysates from control, MCPH1427insA, and PCNT3109G>T LBCs. Actin was included as a loading control. Arrowheads point to centrosomes, which are shown enlarged in insets.
Next, we examined the association of Chk1 with centrosomes using centrosome preparations from MCPH1427insA as well as PCNT3109G>T cells and normal LBCs. Although comparable protein amounts were loaded, the centrosome preparations from MCPH1427insA and PCNT3109G>T cells contained reduced levels of Chk1 as compared with centrosomes from control LBCs (Fig. 1 D). The loss of Chk1 from centrosomes in MCPH1- and PCNT-deficient cells was not attributable to a general shortage of cellular Chk1 in these cells, as whole cell lysates from control and MCPH1427insA or PCNT3109G>T LBCs contained almost identical Chk1 levels (Fig. 1 E). Likewise, we have described previously that Chk1 is lost from centrosomes during mitosis, although total cellular Chk1 levels are not reduced during this phase of the cell cycle (Krämer et al., 2004). On the basis of these combined in situ and biochemical analyses, we conclude that in MCPH1- and PCNT-deficient cells, Chk1 is lost from centrosomes.
MCPH1 targets Chk1 to the centrosome
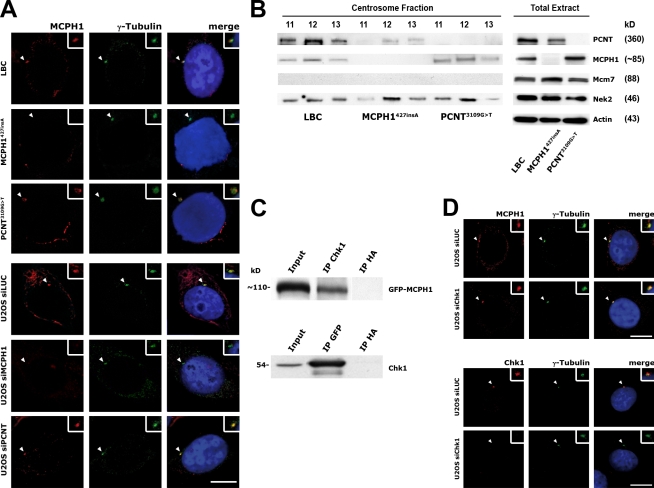
How Chk1 is recruited to interphase centrosomes during unperturbed cell cycles remains unknown. MCPH1 has been reported to localize to centrosomes in chicken DT40 cells and to directly interact with Chk1 in vitro (Alderton et al., 2006; Jeffers et al., 2008), thereby possibly contributing to the localization of Chk1 to centrosomes. To test for this possibility, we first confirmed that endogenous human MCPH1 localizes to interphase centrosomes in control but not MCPH1427insA LBCs or MCPH1 siRNA–treated U2OS cells by immunofluorescence microscopy using an antibody raised against an N-terminal fragment (1–267 aa) of MCPH1 (CCU.M1; see Materials and methods; Fig. 2 A and Fig. S1 D). Also, in contrast to MCPH1427insA LBCs, MCPH1 was detectable in centrosome preparations from control LBCs (Fig. 2 B). Therefore, we conclude that MCPH1 indeed localizes to centrosomes.
Figure 2.
MCPH1 interacts with and targets Chk1 to the centrosome. (A) Control, MCPH1427insA, and PCNT3109G>T LBCs as well as U2OS cells transfected with luciferase- (as control [siLUC]), MCPH1-, or PCNT-specific siRNA were costained with mouse anti-MCPH1 (red) and rabbit anti–γ-tubulin (green) antibodies and analyzed by confocal microscopy. (B) Reduced levels of PCNT in isolated centrosome preparations from MCPH1427insA LBCs. Immunoblots were performed on three sucrose gradient fractions of centrosome preparations (left) and whole cell lysates (right) as an input control from normal, MCPH1427insA, and PCNT3109G>T LBCs using antibodies against PCNT, MCPH1, and, for comparison, Nek2 as a loading control and Mcm7 to exclude nuclear contamination. For whole cell lysates, actin was included as a loading control. (C) Transiently expressed GFP-MCPH1 and Chk1 interact with each other in vivo. Chk1 was immunoprecipitated from U2OS cells 24 h after transient transfection with GFP-MCPH1. Reciprocally, GFP-MCPH1 was immunoprecipitated using an anti-GFP antibody. Immunoprecipitation with an anti-HA antibody served as a negative control. Input represents 10% of the amount used for immunoprecipitation. White lines indicate that intervening lanes have been spliced out. (D) Depletion of Chk1 does not impact on centrosomal MCPH1 levels. U2OS cells transfected with either luciferase- or Chk1-specific siRNA were costained with mouse anti-MCPH1 (red) and rabbit anti–γ-tubulin (green) antibodies (top) or mouse anti-Chk1 (red) and rabbit anti–γ-tubulin (green) antibodies (bottom) and analyzed by confocal microscopy. Arrowheads point to centrosomes, which are shown enlarged in insets. Bars, 10 µm.
To verify the interaction of MCPH1 with Chk1 in vivo, endogenous Chk1 was immunoprecipitated from U2OS cells transiently expressing MCPH1 fused to GFP (GFP-MCPH1), with GFP-MCPH1 being readily detectable in the immunoprecipitates (Fig. 2 C). Similarly, immunoprecipitation of GFP-MCPH1 using an anti-GFP antibody led to coimmunoprecipitation of endogenous Chk1. In contrast to the loss of centrosomal Chk1 in MCPH1-deficient cells, the centrosomal levels of MCPH1 remained virtually unchanged in U2OS cells transfected with Chk1 siRNA (Fig. 2 D), which is analogous to what has been reported for Chk1−/− DT40 cells (Jeffers et al., 2008). Thus, we conclude that MCPH1 and Chk1 interact with each other and that MCPH1 targets Chk1 to the centrosome.
MCPH1-dependent recruitment of Chk1 to centrosomes is mediated by PCNT
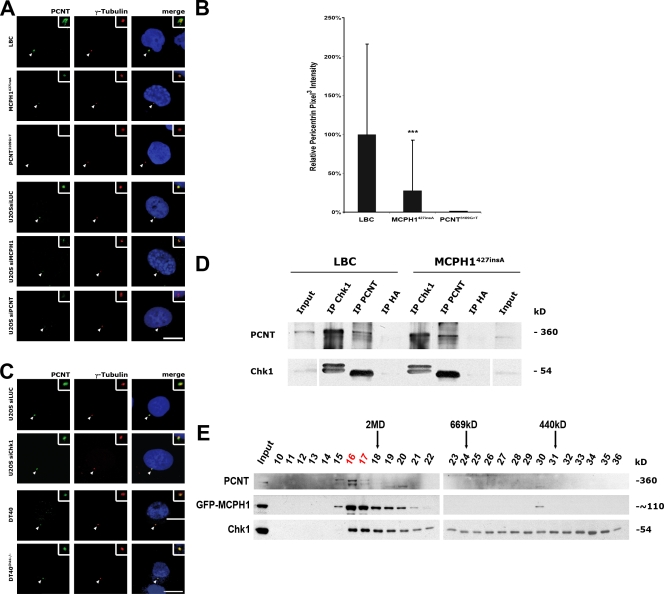
As PCNT has an established role in targeting regulatory proteins to the centrosome (Diviani et al., 2000; Chen et al., 2004), direct or indirect binding of Chk1 by PCNT might mediate the centrosomal localization of Chk1 (Griffith et al., 2008). To determine whether the loss of Chk1 from centrosomes in MCPH1-deficient cells is a consequence of a shortage of centrosomal PCNT, control and MCPH1427insA LBCs were immunostained for PCNT. In contrast to control LBCs, centrosomal PCNT signals were diminished in MCPH1427insA cells (Fig. 3, A and B; and Fig. S2 A). Immunostaining of MCPH1 siRNA– and mock siRNA–treated U2OS cells with antibodies to PCNT led to similar results. Consistently, centrosome preparations from MCPH1427insA cells contained less PCNT as compared with control LBCs (Fig. 2 B). Also, GFP-MCPH1 coimmunoprecipitated with endogenous PCNT when transiently expressed in U2OS cells (Fig. S2 B). Similarly, immunoprecipitation of GFP-MCPH1 led to coimmunoprecipitation of endogenous PCNT. Thus, MCPH1 and PCNT interact with each other, and in addition to a loss of centrosomal Chk1, PCNT is depleted from centrosomes in MCPH1-deficient cells. To the contrary, PCNT deficiency did not impact on the abundance of MCPH1 at centrosomes in PCNT3109G>T or PCNT siRNA–treated U2OS cells (Fig. 2 A). Also, the centrosomal levels of PCNT remained unchanged in both U2OS cells transfected with Chk1 siRNA and Chk1−/− DT40 cells (Fig. 3 C; Zachos et al., 2003).
Figure 3.
MCPH1 recruits Chk1 to the centrosome via PCNT. (A) Centrosomal PCNT levels are reduced in MCPH1427insA and PCNT3109G>T LBCs relative to control lymphoblasts. Normal, MCPH1427insA, and PCNT3109G>T LBCs as well as U2OS cells transfected with luciferase- (as control [siLUC]), MCPH1-, or PCNT-specific siRNA were costained with rabbit anti-PCNT (green) and mouse anti–γ-tubulin (red) antibodies and analyzed by confocal microscopy. (B) Quantification of pixel intensity profiles constructed from optically sectioned (z axis) fluorescence images of normal cells. Error bars represent standard deviations from the analysis of 100 cells. Statistical significance versus control (LBC) by two-tailed Student's t test is as follows: ***, P = 7.2 × 10−6 (MCPH1427insA). (C) Control and Chk1−/− chicken DT40 cells as well as U2OS cells transfected with luciferase- or Chk1-specific siRNA were costained with rabbit anti-PCNT (green) and mouse anti–γ-tubulin (red) and analyzed by confocal microscopy. (D) Endogenous PCNT and Chk1 interact with each other in vivo. Endogenous Chk1 was detected in immunoprecipitates using an anti-PCNT antibody in both control and MCPH1427insA LBCs. Reciprocally, endogenous PCNT was detectable after immunoprecipitation of Chk1 in both cell lines as well. Immunoprecipitation with an anti-HA antibody served as a negative control. Input represents 10% of the amount used for immunoprecipitation. White lines indicate that intervening lanes have been spliced out. (E) Cofractionation of PCNT, MCPH1, and Chk1 in U2OS whole cell lysates. Lysates were prepared from U2OS cells transiently transfected with GFP-MCPH1 24 h before lysis and size fractionated by fast protein liquid chromatography using a Superose 6 column. Proteins from consecutive fractions were analyzed by Western blotting using antibodies to PCNT, GFP, and Chk1. The size of marker proteins is shown on top. Numbers in red indicate the fractions that contain all three proteins (GFP-MCPH1, PCNT, and Chk1). Arrowheads point to centrosomes, which are shown enlarged in insets. Bars, 10 µm.
To examine the relationship between PCNT and Chk1 in more detail, endogenous PCNT was immunoprecipitated from control and MCPH1427insA LBCs, respectively. Endogenous Chk1 was detectable in immunoprecipitates from both cell types (Fig. 3 D). Similarly, immunoprecipitation of endogenous Chk1 led to coimmunoprecipitation of endogenous PCNT. Thus, PCNT and Chk1 interact with each other independent from the presence of MCPH1. Collectively, we conclude that MCPH1 contributes to the centrosomal localization of PCNT and that both MCPH1 and PCNT are required to recruit Chk1 to the centrosome.
To further confirm an interaction between Chk1, MCPH1, and PCNT, we size fractionated whole cell lysates from U2OS cells transiently transfected with GFP-MCPH1 by Superose 6 gel filtration chromatography (Fig. 3 E). As additional evidence for complex formation, all three proteins comigrated together in two fractions, which correspond to a molecular mass of >2 MD.
We next examined other components of the centrosome to establish whether they were affected by the absence of MCPH1 or PCNT (Fig. S2, C and D). Centrin, Nek2, and Cep170 were all localized normally. However, γ-tubulin was significantly reduced at interphase centrosomes in MCPH1427insA and PCNT3109G>T LBCs as compared with control LBCs, which is consistent with what has been described for mitotic PCNT-Seckel cells (Griffith et al., 2008).
MCPH1 or PCNT depletion–induced loss of Chk1 from centrosomes leads to premature activation of cyclin B–Cdk1
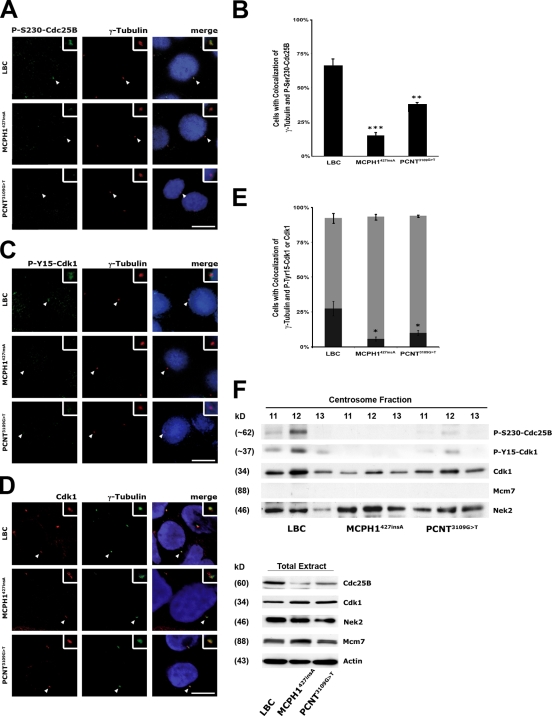
Centrosome-associated Chk1 prevents premature activation of cyclin B–Cdk1 and thereby mitotic entry by inhibitory phosphorylation of centrosomal Cdc25B at S230 with consecutive inhibition of P-Y15-Cdk1 dephosphorylation (Krämer et al., 2004; Schmitt et al., 2006). To determine whether down-regulation of MCPH1 or PCNT has an impact on the phosphorylation status of the centrosomal fractions of Cdc25B and Cdk1, primary MCPH1427insA and PCNT3109G>T LBCs as well as control LBCs were synchronized in G1/early S phase by a mimosine block and released for 8 h to reach G2 phase. Subsequently, cells were coimmunostained with antibodies to P-S230-Cdc25B or P-Y15-Cdk1 and γ-tubulin. Although centrosomes were decorated with anti–P-S230-Cdc25B in 65.7 ± 5.1% in control LBCs, only 14.0 ± 2.6% and 36.7 ± 1.5% of MCPH1427insA and PCNT3109G>T LBCs harbored centrosomes that stained positive for P-S230-Cdc25B (Fig. 4, A and B). Similarly, in contrast to control LBCs (27.3 ± 5.1%), only 5.7 ± 1.5% and 10.0 ± 1.7% of MCPH1427insA and PCNT3109G>T LBCs, respectively, harbored centrosomes with discernible P-Y15-Cdk1 decoration, despite almost all centrosomes stained positive for total Cdk1 (Fig. 4, C–E).
Figure 4.
Loss of Chk1 from centrosomes induces activation of centrosome-associated Cdc25B and Cdk1. (A, C, and D) Normal, MCPH1427insA, and PCNT3109G>T LBCs were synchronized in G1/early S phase by a mimosine block, released for 8 h to reach G2 phase, and subsequently costained with rabbit anti–P-S230-Cdc25B (green) and mouse anti–γ-tubulin (red) antibodies (A), rabbit anti–P-Y15-Cdk1 (green) and mouse anti–γ-tubulin (red) antibodies (C), or mouse anti-Cdk1 (red) and rabbit anti–γ-tubulin (green) antibodies (D) and analyzed by confocal microscopy. (B) Loss of Chk1 from centrosomes induces activation of centrosome-associated Cdc25B. The mean percentages of cells with centrosomal colocalization of γ-tubulin and P-S230-Cdc25B are indicated. Statistical significance versus control (LBC) by two-tailed Student's t test is as follows: ***, P = 0.0006 (MCPH1427insA); **, P = 0.006 (PCNT3109G>T). (E) Loss of Chk1 from centrosomes induces activation of centrosome-associated Cdk1. The mean percentages of cells with centrosomal colocalization of γ-tubulin and total Cdk1 (light gray bars) or P-Y15-Cdk1 (dark gray bars) are indicated. Statistical significance versus control (LBC) by two-tailed Student's t test is as follows: *, P = 0.013 (MCPH1427insA); P = 0.019 (PCNT3109G>T). (F) Reduced levels of P-S230-Cdc25B and P-Y15-Cdk1 in isolated centrosome preparations from MCPH1427insA and PCNT3109G>T LBCs. Immunoblots were performed on three sucrose centrifugation fractions of centrosome preparations (left) and whole cell lysates (right) as an input control from control (LBC), MCPH1427insA, and PCNT3109G>T LBCs using antibodies against P-S230-Cdc25B, P-Y15-Cdk1, Cdk1, and, for comparison, Nek2 as a loading control and Mcm7 to exclude nuclear contamination. For whole cell lysates, antibodies to Cdc25B, Cdk1, and actin were included. Arrowheads point to centrosomes, which are shown enlarged in insets. Error bars represent the standard deviation after combining the results of three different experiments. Bars, 10 µm.
Next, we examined the phosphorylation status of Cdc25B and Cdk1 using centrosome preparations from MCPH1427insA as well as PCNT3109G>T cells and normal LBCs. Although comparable protein amounts were loaded, the centrosome preparations from MCPH1427insA and PCNT3109G>T cells contained reduced levels of P-S230-Cdc25B or P-Y15-Cdk1 as compared with control LBCs (Fig. 4 F). Similar to immunofluorescence stainings, reduced levels of P-Y15-Cdk1 at centrosomes in MCPH1- and PCNT-deficient cells were not attributable to a general shortage of centrosomal Cdk1 in these cells, as centrosome preparations from control and MCPH1427insA or PCNT3109G>T LBCs contained almost identical Cdk1 levels (Fig. 4 F).
These findings suggest that MCPH1 or PCNT depletion–induced loss of Chk1 from centrosomes leads to reduced inhibitory phosphorylation of both centrosomal Cdc25B at S230 and Cdk1 at Y15 and therefore to premature entry into mitosis. In agreement with these findings, it has been reported recently that human and Drosophila melanogaster MCPH1-deficient cells have low levels of total P-Y15-Cdk1 in S and G2 phases, resulting in accelerated mitotic entry in MCPH1427insA LBCs and premature centrosome separation in MCPH1 mutant Drosophila embryos (Alderton et al., 2006; Brunk et al., 2007). To assay for the rates of cells in late G2 or mitosis, U2OS cells treated with mock-, MCPH1-, or PCNT-specific siRNAs were examined by flow cytometry measurement using P-S10–histone H3 as a mitotic marker. 72 h after MCPH1 or PCNT siRNA transfection, significantly more cells were in late G2 or mitosis (Fig. 5 A). Similar results were obtained when the cell cycle distribution of exponentially growing control, MCPH1427insA, and PCNT3109G>T LBCs was examined (Fig. 5 B and Fig. S3 A). In addition, transient transfection of MCPH1427insA LBCs with wild-type MCPH1-Flag led to a significant reduction of G2/mitotic cells from 1.28 ± 0.2% after mock transfection to 0.75 ± 0.1% 12 h after transfection with MCPH1-Flag (Fig. S3 B).
Figure 5.
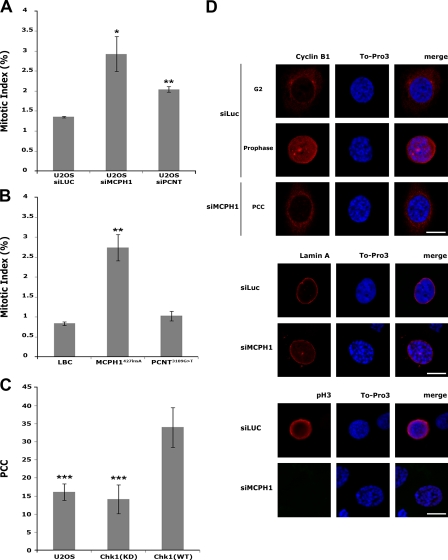
Down-regulation of MCPH1 induces premature entry into mitosis via depletion of centrosomal Chk1. (A and B) U2OS cells transfected with luciferase- (as control [siLUC]), MCPH1-, or PCNT-specific siRNA (A) as well as normal, MCPH1427insA, and PCNT3109G>T LBCs (B) were costained with propidium iodide and anti–P-S10–histone H3 and analyzed by fluorescence-activated cell sorting to quantify cells in mitosis. Statistical significance versus control by two-tailed Student's t test is as follows: (A) *, P = 0.02 (U2OS-siMCPH1); **, P = 0.001 (U2OS-siPCNT); (B) **, P = 0.009 (MCPH1427insA); P = 0.09 (PCNT3109G>T). (C) Parental U2OS cells or U2OS cells conditionally expressing wild-type (Chk1[WT]) or kinase-dead (Chk1[KD]) GFP-Chk1-PACT were transfected with MCPH1-specific siRNA and analyzed by fluorescence microscopy. The mean percentages of cells with PCC are indicated. Statistical significance versus Chk1(WT) by two-tailed Student's t test is as follows: ***, P = 0.0008 (U2OS); P = 0.0002 (Chk1[KD]). (D) Characterization of the PCC phenotype. U2OS cells were transfected with luciferase- or MCPH1-specific siRNA and immunostained with antibodies to cyclin B1 (top), lamin A (middle), and P-S10–histone H3 (pH3; bottom). DNA was counterstained with To-Pro 3. After transfection with MCPH1-specific siRNA, PCC cells have an intact nuclear membrane as judged by lamin A staining, are P-S10–histone H3 negative, and show no nuclear cyclin B1 accumulation, thereby demonstrating that they exhibit G2 rather than mitotic characteristics. Error bars represent the standard deviation after combining the results of three different experiments. Bars, 10 µm.
Time-lapse video microscopy of MCPH1 and PCNT siRNA–treated U2OS cells revealed that mitosis in these cells was prolonged as compared with U2OS cells treated with luciferase siRNA (Fig. S3, C–E). After PCNT siRNA treatment, the frequency of cells dying out of mitosis was increased as well.
Forced immobilization of Chk1 at centrosomes leads to accumulation of MCPH1-deficient cells with premature chromosome condensation (PCC) in G2 phase
MCPH1 deficiency leads to a striking elevation in G2 phase–like cells with PCC mediated by deregulated activity of nuclear condensin II (Trimborn et al., 2004, 2006; Alderton et al., 2006). If MCPH1 indeed functions to locally regulate activation of Cdk1 via centrosomal targeting of Chk1, then specific interference with centrosome-associated Chk1 should alter the impact of MCPH1 depletion on the G2/M transition. To address this issue, we took advantage of a U2OS clone conditionally expressing GFP-Chk1 fused to the PCNT-AKAP450 centrosomal-targeting (PACT) domain of AKAP450, an immobile protein of the pericentriolar matrix (Gillingham and Munro, 2000; Krämer et al., 2004). In these cells, forced immobilization of wild-type Chk1 to centrosomes impairs activation of centrosome-associated Cdk1 and thereby entry into mitosis (Krämer et al., 2004). When parental U2OS cells as well as wild-type and kinase-dead versions of GFP-Chk1-PACT–expressing cells were treated with an MCPH1-specific siRNA, 34.0 ± 5.5% of wild-type but only 14.2 ± 3.9% of kinase-dead GFP-Chk1-PACT–expressing cells and 16.2 ± 2.3% of parental U2OS cells showed a PCC phenotype 72 h after transfection (Fig. 5, C and D). These findings suggest that down-regulation of MCPH1 induces premature entry into mitosis via depletion of the centrosomal pool of Chk1. Forced immobilization of Chk1 at the centrosome prevents cell cycle progression into mitosis, leading to accumulation of cells with PCC in G2 phase.
In addition to MCPH1, three other primary microcephaly genes have been identified, ASPM, CENPJ, and CDK5RAP2 (Bond et al., 2002, 2005; Bond and Woods, 2006). Their protein products have a centrosomal localization in common and are required for centrosomal function. Therefore, a unifying theme for primary microcephaly of genes functioning in the process of cell division has been proposed. However, in contrast to mutations in ATR, MCPH1, and PCNT, which can reduce both body and brain size, effects of ASPM, CENPJ, and CDK5RAP2 mutations are restricted to the brain, presumably by acting specifically on neurogenic mitosis (Bond et al., 2005). Our data provide evidence that the recruitment of Chk1 to centrosomes depends on both MCPH1 and PCNT during unperturbed cell cycle progression. In MCPH1 syndrome and MOPD II, which result from mutations in MCPH1 and PCNT, respectively, loss of Chk1 from centrosomes leads to unscheduled entry into mitosis. Consequently, loss of regulatory restraints on the initial activation of cyclin B–Cdk1 at the centrosome might be a common final path in microcephalic disorders with reduced body size, thereby explaining for the first time why seemingly unrelated genetic defects result in a common human disease phenotype.
Materials and methods
Cell culture
U2OS were maintained in DME with 10% FCS. Control, MCPH1427insA, and PCNT3109G>T LBCs (Alderton et al., 2006; Rauch et al., 2008) were cultured in RPMI 1640 supplemented with 15% FCS. Derivatives of U2OS cells conditionally expressing wild-type or kinase-dead alleles of GFP-Chk1-PACT were generated as reported previously (Krämer et al., 2004). DT40 cells were maintained in RPMI 1640 with 10% chicken serum (Sigma-Aldrich).
Mimosine release
Cells were incubated overnight in 0.2 mM mimosine for synchronization at G1/early S phase. For release, cells were washed and seeded into complete medium for up to 12 h before further processing.
Microscopy
U2OS cells grown on glass coverslips or control, MCPH1427insA, and PCNT3109G>T LBCs cytocentrifuged on glass slides at 800 rpm for 4 min using a cytocentrifuge (Cytospin 3; Shandon) were fixed in −20°C methanol/acetone (1:1) for 7 min. After fixation, the cells were permeabilized in 1% Triton X-100 and 0.5% NP-40 for 10 min. Immunofluorescence microscopy was performed with the combinations of antibodies specified in Figs. 1–5, S1, and S2. Fluorescence images were captured and processed using a true confocal scanner laser-scanning microscope (SP2; Leica) equipped with confocal software (2.61; Leica) and a Plan Apo 63× 1.32 NA oil immersion objective (Leica). Images were cropped and processed using Photoshop (CS3; Adobe). Matching confocal planes were analyzed in all colocalization experiments. For the quantification of centrosomal Chk1, PCNT, and γ-tubulin signals, 3D datasets were collected using a confocal laser-scanning microscope (LSM 710; Carl Zeiss, Inc.) equipped with Axiovision software (4.7.2; Carl Zeiss, Inc.) and a Plan Apo 63× 1.4 NA oil immersion objective (Carl Zeiss, Inc.). A defined volume containing the centrosome was used to measure the total intensity of the signal. Signal intensities of centrosomes from 100 interphase cells per cell line were averaged, and relative intensities in comparison with control LBCs were calculated. 3D picture reconstruction was performed using ImageJ software (1.40g; provided by W. Rasband, National Institutes of Health, Bethesda, MD).
Plasmids
MCPH1 cDNA (GenBank/EMBL/DDBJ accession no. NM_024596) was cloned into pEGFP-C1 (Clontech Laboratories, Inc.) containing an N-terminal GFP tag as well as into pCMV-Tag4A (Agilent Technologies) containing a C-terminal Flag tag.
Antibodies
A phospho-specific rabbit antiserum to S230-Cdc25B (SE4394) was raised and characterized as described previously (Schmitt et al., 2006). Mouse monoclonal antibodies to Cdk1 and GFP (B-2), rabbit antiserum to actin (I-19), Chk1 (FL-476), and HA (Y11) as well as goat and donkey antisera to HRP were obtained from Santa Cruz Biotechnology, Inc.; a mouse monoclonal antibody to Chk1 (DCS-310) and a rabbit antiserum to γ-tubulin were obtained from Sigma-Aldrich; a mouse monoclonal antibody to γ-tubulin (TU-30) was obtained from Exbio; a mouse monoclonal antibody to Nek2 was obtained from BD; a goat antiserum to MCPH1 was obtained from R&D Systems; rabbit antiserum to PCNT, P-Y15-Cdk1, and P-S10–histone H3 were obtained from Abcam, EMD, and Millipore, respectively. Mouse monoclonal antibodies to centrin, Cep170, and lamin A were provided by J.L. Salisbury (Mayo Clinic, Rochester, MN), E.A. Nigg (University of Basel, Basel, Switzerland), and H. Herrmann-Lerdon (German Cancer Research Center, Heidelberg, Germany), respectively. Highly cross-absorbed secondary reagents Alexa Fluor 488 and 568 were obtained from Invitrogen.
RNA interference
For siRNA-mediated ablation of Chk1, MCPH1, and PCNT, U2OS cells were transfected twice (at 0 and 24 h) with the following oligonucleotide sequences: Chk1 siRNA, 5′-GGACUUCUCUCCAGUAAACdTdT-3′; MCPH1 siRNA, 5′-AGGAAGUUGGAAGGAUCCAdTdT-3′; PCNT siRNA, 5′-GCAGCUGAGCUGAAGGAGAdTdT-3′; and luciferase siRNA, 5′-UAAGGCUAUGAAGAGAUACdTdT-3′ by Oligofectamine reagent (Invitrogen). Cells were harvested 72 h after the first transfection.
Isolation and analysis of human centrosomes
Centrosomes were isolated from control, MCPH1427insA, and PCNT3109G>T LBCs as described previously (Moudjou and Bornens, 1994).
Gel filtration
Whole cell lysates were cleared by centrifugation and passaged through a 0.2-µm filter. 200 µl cleared lysate was applied to a Superose 6 gel filtration column (HR 10/30; GE Healthcare) and resolved on a fast protein liquid chromatography system (GE Healthcare) according to the manufacturer's instructions.
Immunoprecipitation
For immunoprecipitation, cells were lysed with RIPA buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 0.2 mM PMSF, 5 mM NaF, and 1 mM Na3VO4) containing a protease inhibitor cocktail (Roche). Subsequently, the cell lysates were centrifuged at 14,000 g for 20 min. Precipitating antibodies were preincubated with protein G/A agarose beads (50% slurry; Roche) for 1 h at room temperature followed by three washes for 5 min each in RIPA buffer and overnight incubation at 4°C after adding aliquots of the cell lysate supernatant. Negative controls were generated by immunoprecipitation with an antibody to HA (Clontech Laboratories, Inc.). After three washes for 5 min each in RIPA buffer, the proteins were analyzed by immunoblotting.
Transfection
U2OS cells were transiently transfected with 2 µg pEGFP-C1-MCPH1 using Fugene 6 (Roche) and MCPH1427insA LBCs with 1.5 µg pCMV-Tag4A-MCPH1 by electroporation using a MicroPorator (MP-100; Peqlab) according to the manufacturer's instructions.
Generation of an MCPH1-specific monoclonal antibody
A bacterially expressed and affinity-purified His-tagged MCPH1 fragment (MCPH1-1_267aa.HIS) was used to immunize mice following a modified standard immunization protocol (Köhler and Milstein, 1975). Fusions resulted in the generation of six specific monoclonal antibodies that were subsequently subcloned. Specificity of the antibody that was eventually used for further experiments (CCU.M1), which was typed as mouse IgM, was verified by using bacterially expressed recombinant proteins as well as control and MCPH1427insA LBCs for Western blotting and U2OS cells for immunofluorescence staining.
Live cell imaging
Live cell imaging of cells growing in CO2-independent Leibovitz medium (Invitrogen) on microdishes (Ibidi) was performed at 37°C in a humidified atmosphere containing 5% CO2 using the BioStation IM system (Nikon) equipped with a 63× 0.8 NA Plan Fluor objective (Nikon).
Flow cytometry
Cell cycle analysis by flow cytometry including the quantification of cells in mitosis by P-S10–histone H3 staining was performed as previously described (Rebacz et al., 2007).
Online supplemental material
Fig. S1 presents a time course of siRNA-mediated depletion of PCNT and MCPH1 as well as a characterization of a novel anti-MCPH1 antibody and illustrates that Chk1 levels are reduced at centrosomes in both MCPH1- and PCNT-deficient cells. Fig. S2 illustrates that centrosomal PCNT levels are reduced in MCPH1427insA and PCNT3109G>T LBCs irrespective of their cell cycle status, that transiently expressed GFP-MCPH1 and endogenous PCNT interact with each other in vivo, and that centrosomal levels of centrin, Nek2, and Cep170, but not γ-tubulin, are unchanged in MCPH1- and PCNT-deficient cells. Fig. S3 depicts the cell cycle distribution of MCPH1427insA and PCNT3109G>T LBCs, demonstrates that rescue of MCPH1427insA LBCs via transfection with wild-type MCPH1-Flag leads to a restored mitotic index, and outlines the mitotic fate of MCPH1- and PCNT-depleted U2OS cells. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200810159/DC1.
Acknowledgments
We thank D.A. Gillespie for providing Chk1−/− DT40 cells, J.L. Salisbury, E.A. Nigg, and H. Herrmann-Lerdon for providing antibodies to centrin, Cep170, and lamin A, respectively, S. Poppelreuther (Carl Zeiss, Inc., Heidelberg, Germany) for technical support, and B. Schreiter and S. Franz for excellent technical assistance.
We are grateful to the Deutsche Forschungsgemeinschaft and the European Commission (project Genome Instability in Cancer and Precancer) for financial support.
Footnotes
Abbreviations used in this paper: ATR, ataxia telangiectasia and Rad3 related; LBC, lymphoblastoid cell; MCPH1, microcephalin; MOPD II, microcephalic osteodysplastic primordial dwarfism type II; PACT, PCNT-AKAP450 centrosomal targeting; PCC, premature chromosome condensation; PCNT, pericentrin.
References
- Alderton G.K., Joenje H., Varon R., Borglum A.D., Jeggo P.A., O'Driscoll M. 2004. Seckel syndrome exhibits cellular features demonstrating defects in the ATR-signalling pathway.Hum. Mol. Genet. 13:3127–3138 [DOI] [PubMed] [Google Scholar]
- Alderton G.K., Galbiati L., Griffith E., Surinya K.H., Neitzel H., Jackson A.P., Jeggo P.A., O'Driscoll M. 2006. Regulation of mitotic entry by microcephalin and its overlap with ATR signalling.Nat. Cell Biol. 8:725–733 [DOI] [PubMed] [Google Scholar]
- Bailly E., Doree M., Nurse P., Bornens M. 1989. P34cdc2 is located in both the nucleus and cytoplasm; part is centrosomally associated at G2/M and enters vesicles at anaphase.EMBO J. 8:3985–3995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly E., Pines J., Hunter T., Bornens M. 1992. Cytoplasmic accumulation of cyclin B1 in human cells: association with a detergent-resistant compartment within the centrosome.J. Cell Sci. 101:529–545 [DOI] [PubMed] [Google Scholar]
- Bond J., Woods C.G. 2006. Cytoskeletal genes regulating brain size.Curr. Opin. Cell Biol. 18:95–101 [DOI] [PubMed] [Google Scholar]
- Bond J., Roberts E., Mochida G.H., Hampshire D.J., Scott S., Askham J.M., Springell K., Mahadevan M., Crow Y.J., Markham A.F., et al. 2002. ASPM is a major determinant of cerebral cortical size.Nat. Genet. 32:316–320 [DOI] [PubMed] [Google Scholar]
- Bond J., Roberts E., Springell K., Lizarraga S., Scott S., Higgins J., Hampshire D.J., Morrison E.E., Leal G.F., Silva E.O., et al. 2005. A centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size.Nat. Genet. 37:353–355 [DOI] [PubMed] [Google Scholar]
- Brunk K., Vernay B., Griffith E., Reynolds N.L., Strutt D., Ingham P.W., Jackson A.P. 2007. Microcephalin coordinates mitosis in the syncytial Drosophila embryo.J. Cell Sci. 120:3578–3588 [DOI] [PubMed] [Google Scholar]
- Chen D., Purohit A., Halilovic E., Doxsey S.J., Newton A.C. 2004. Centrosomal anchoring of protein kinase C betaII by pericentrin controls microtubule organization, spindle function, and cytokinesis.J. Biol. Chem. 279:4829–4839 [DOI] [PubMed] [Google Scholar]
- De Souza C.P., Ellem K.A., Gabrielli B.G. 2000. Centrosomal and cytoplasmic Cdc2/cyclin B1 activation precedes nuclear mitotic events.Exp. Cell Res. 257:11–21 [DOI] [PubMed] [Google Scholar]
- Diviani D., Langeberg L.K., Doxsey S.J., Scott J.D. 2000. Pericentrin anchors protein kinase A at the centrosome through a newly identified RII-binding domain.Curr. Biol. 10:417–420 [DOI] [PubMed] [Google Scholar]
- Dutertre S., Cazales M., Quaranta M., Froment C., Trabut V., Dozier C., Mirey G., Bouche J.P., Theis-Febvre N., Schmitt E., et al. 2004. Phosphorylation of CDC25B by aurora-A at the centrosome contributes to the G2-M transition.J. Cell Sci. 117:2523–2531 [DOI] [PubMed] [Google Scholar]
- Gillingham A.K., Munro S. 2000. The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin.EMBO Rep. 1:524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith E., Walker S., Martin C.-A., Vagnarelli P., Stiff T., Vernay B., Al Sanna N., Saggar A., Hamel B., Earnshaw W.C., et al. 2008. Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling.Nat. Genet. 40:232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman M., Lindon C., Nigg E.A., Pines J. 2003. Active cyclin B1-Cdk1 first appears on centrosomes in prophase.Nat. Cell Biol. 5:143–148 [DOI] [PubMed] [Google Scholar]
- Jackson A.P., Eastwood H., Bell S.M., Adu J., Toomes C., Carr I.M., Roberts E., Hampshire D.J., Crow Y.J., Mighell A.J., et al. 2002. Identification of microcephalin, a protein implicated in determining the size of the human brain.Am. J. Hum. Genet. 71:136–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers L.J., Coull B.J., Stack S.J., Morrison C.G. 2008. Distinct BRCT domains in Mcph1/Brit1 mediate ionizing radiation-induced focus formation and centrosomal localization.Oncogene. 27:139–144 [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity.Nature. 256:495–497 [DOI] [PubMed] [Google Scholar]
- Krämer A., Mailand N., Lukas C., Syljuasen R.G., Wilkinson C.J., Nigg E.A., Bartek J., Lukas J. 2004. Centrosome-associated Chk1 prevents premature activation of cyclin-B-Cdk1 kinase.Nat. Cell Biol. 6:884–891 [DOI] [PubMed] [Google Scholar]
- Löffler H., Lukas J., Bartek J., Krämer A. 2006a. Structure meets function – centrosomes, genome maintenance and the DNA damage response.Exp. Cell Res. 312:2633–2640 [DOI] [PubMed] [Google Scholar]
- Löffler H., Rebacz B., Ho A.D., Lukas J., Bartek J., Krämer A. 2006b. Chk1-dependent regulation of Cdc25B functions to coordinate mitotic events.Cell Cycle. 5:2543–2547 [DOI] [PubMed] [Google Scholar]
- Moudjou M., Bornens M. 1994. Isolation of centrosomes from cultured animal cells. Cell Biology: A Laboratory Handbook. Celis J.E., editor Academic Press, San Diego: 595–604 [Google Scholar]
- Nurse P. 1990. Universal control mechanism regulating onset of M-phase.Nature. 344:503–508 [DOI] [PubMed] [Google Scholar]
- O'Driscoll M., Ruiz-Perez V.L., Woods C.G., Jeggo P.A., Goodship J.A. 2003. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome.Nat. Genet. 33:497–501 [DOI] [PubMed] [Google Scholar]
- Rauch A., Thiel C.T., Schindler D., Wick U., Crow Y.J., Ekici A.B., van Essen A.J., Goecke T.O., Al-Gazali L., Chrzanowska K.H., et al. 2008. Mutations in the pericentrin (PCNT) gene cause primordial dwarfism.Science. 319:816–819 [DOI] [PubMed] [Google Scholar]
- Rebacz B., Larsen T.O., Clausen M.H., Ronnest M.H., Löffler H., Ho A.D., Krämer A. 2007. Identification of griseofulvin as an inhbitor of centrosomal clustering in a phenotype-based screen.Cancer Res. 67:6342–6350 [DOI] [PubMed] [Google Scholar]
- Schmitt E., Boutros R., Froment C., Monsarrat B., Ducommun B., Dozier C. 2006. CHK1 phosphorylates CDC25B during the cell cycle in the absence of DNA damage.J. Cell Sci. 119:4269–4275 [DOI] [PubMed] [Google Scholar]
- Trimborn M., Bell S.M., Felix C., Rashid Y., Jafri H., Griffiths P.D., Neumann L.M., Krebs A., Reis A., Sperling K., et al. 2004. Mutations in microcephalin cause aberrant regulation of chromosome condensation.Am. J. Hum. Genet. 75:261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimborn M., Schindler D., Neitzel H., Hirano T. 2006. Misregulated chromosome condensation in MCPH1 primary microcephaly is mediated by condensin II.Cell Cycle. 5:322–326 [DOI] [PubMed] [Google Scholar]
- Zachos G., Rainey M.D., Gillespie D.A. 2003. Chk1-deficient tumour cells are viable but exhibit multiple checkpoint and survival defects.EMBO J. 22:713–723 [DOI] [PMC free article] [PubMed] [Google Scholar]