Abstract
Fibroblast growth factor 2 (FGF2) is a major regulator of developmental, pathological, and therapeutic angiogenesis. Its activity is partially mediated by binding to syndecan 4 (S4), a proteoglycan receptor. Angiogenesis requires polarized activation of the small guanosine triphosphatase Rac1, which involves localized dissociation from RhoGDI1 and association with the plasma membrane. Previous work has shown that genetic deletion of S4 or its adapter, synectin, leads to depolarized Rac activation, decreased endothelial migration, and other physiological defects. In this study, we show that Rac1 activation downstream of S4 is mediated by the RhoG activation pathway. RhoG is maintained in an inactive state by RhoGDI1, which is found in a ternary complex with synectin and S4. Binding of S4 to synectin increases the latter's binding to RhoGDI1, which in turn enhances RhoGDI1's affinity for RhoG. S4 clustering activates PKCα, which phosphorylates RhoGDI1 at Ser96. This phosphorylation triggers release of RhoG, leading to polarized activation of Rac1. Thus, FGF2-induced Rac1 activation depends on the suppression of RhoG by a previously uncharacterized ternary S4–synectin–RhoGDI1 protein complex and activation via PKCα.
Introduction
FGFs are among the most potent inducers of endothelial cell migration, which is a critical event in angiogenesis and numerous other biological processes. FGFs signal via four high-affinity tyrosine kinase receptors (FGFR1–4) and the low-affinity heparan sulfate proteoglycan, syndecan 4 (S4; Murakami et al., 2008). The ability of S4 to signal independently of FGF receptors is largely credited to its ability to activate PKCα (Horowitz et al., 1999; Partovian et al., 2008) and to assemble a signaling complex via its postsynaptic density disc large ZO-1 (PDZ)–binding domain. This domain mediates S4's association with synectin, a ubiquitous PDZ-containing 38-kD cytoplasmic protein (Gao et al., 2000).
Studies involving the deletions of S4 and synectin have demonstrated their respective roles in physiological events as diverse as wound healing (Alexopoulou et al., 2007), arterial development (Chittenden et al., 2006; Dedkov et al., 2007), endotoxic shock protection (Ishiguro et al., 2001), murine vibrissae growth (Iwabuchi and Goetinck, 2006), and neural crest development (Matthews et al., 2008). Although the molecular causes of these phenotypes remain largely undefined, S4–synectin signaling is known to target the small Rho family GTPase Rac1 (Tkachenko et al., 2006; Matthews et al., 2008), which orchestrates actin polymerization in migrating cells.
Rac1 can be activated via several parallel pathways, and its active form is typically found in highest concentrations at the plasma membrane of migrating cells' leading edges. One upstream activator of Rac1 is the highly homologous small Rho GTPase, RhoG. This protein has been specifically implicated in cell migration, activating Rac1 upon binding ELMO and Dock180 (Katoh and Negishi, 2003; Katoh et al., 2006). RhoG is ubiquitously expressed and is a principal mediator of two separate endocytic pathways: macropinocytosis and caveolar endocytosis (Ellerbroek et al., 2004; Prieto-Sanchez et al., 2006). RhoG-mediated endocytosis is also exploited during infection by Salmonella (Patel and Galan, 2006) and Shigella (Handa et al., 2007) and is required for endothelial apical cup formation during leukocyte extravasation (van Buul et al., 2007).
The activation of Rho GTPases is primarily regulated by the guanine exchange factor (GEF) class of proteins. GEFs catalyze the exchange of GDP for GTP on their targets, whereas GTPase-activating proteins (GAPs) accelerate the intrinsic GTPase activity of these proteins and facilitate their rapid inactivation. In this way, the trimeric complex of RhoG, ELMO, and Dock180 functions as a GEF in the activation of Rac1 (Katoh and Negishi, 2003). A third class of proteins, guanine dissociation inhibitors (GDIs), serves to sequester pools of inactive GTPases, shielding them from GEF and GAP interactions. Three Rho family GTPase-interacting GDIs have been identified (RhoGDI1–3) with some degree of overlap in their GTPase targets (Dovas and Couchman, 2005).
We and others have shown that the genetic knockout of either S4 or synectin results in a signaling defect whereby cells exhibit a constitutively high level of Rac1 activity (Saoncella et al., 2004; Chittenden et al., 2006; Tkachenko et al., 2006; Bass et al., 2007; Matthews et al., 2008). These cells migrate poorly as a result of the mislocalization and overabundance of active Rac1, and mice with the constitutive signaling imbalance display various physiological abnormalities (Pankov et al., 2005; Chittenden et al., 2006; Partovian et al., 2008). Correct spatial and temporal regulation of Rac1 activity is therefore an indispensable prerequisite for directional cell migration, angiogenesis, and normal cardiovascular function.
In this study, we sought to identify (a) the mechanism of basal GTPase suppression before stimulation and (b) how Rac1 becomes activated downstream of S4 during endothelial cell migration. We report that S4-mediated Rac1 activation proceeds via the RhoG–Dock180–ELMO pathway. Before activation, RhoGDI1 keeps RhoG inactive and sequestered as part of a previously uncharacterized protein complex with S4 and synectin. Upon FGF2 treatment, S4 oligomerization leads to PKCα activation, resulting in the phosphorylation of RhoGDI1 at Ser96. Phosphorylation initiates the release of RhoG from the S4–synectin–RhoGDI protein complex and permits its activation, which in turn induces the polarized activation of Rac1.
Results
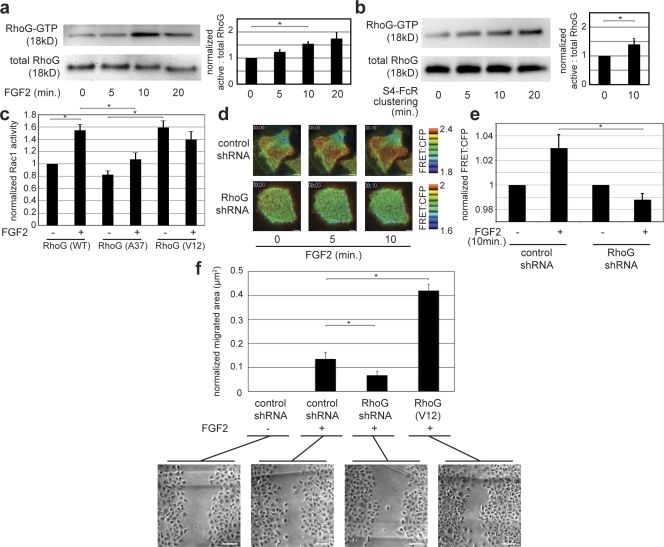
Rac1, a critical component of endothelial cell migration, can be activated by RhoG through the DOCK180–ELMO complex (Katoh and Negishi, 2003). Because RhoG plays a prominent role in endothelial cells (van Buul et al., 2007), we examined the involvement of RhoG in Rac1 activation when endothelial cells respond to FGF2. RhoG activity increased when rat fat pad endothelial cells (RFPECs) were stimulated with FGF2, peaking at ∼10 min (Fig. 1 a). These kinetics also mirrored those of FGF2-induced Rac1 activation (Fig. S1 a). Because FGF2 can signal via its high-affinity tyrosine kinase receptor or via S4, we examined whether S4 clustering alone is sufficient to activate RhoG. The activity of RhoG was measured upon antibody-induced clustering of an S4 chimera, which contains human Fc receptor (FcR) 1a (CD64) in place of the extracellular domain (Tkachenko and Simons, 2002). S4-FcR clustering led to RhoG activation (Fig. 1 b) with kinetics comparable with those of Rac1 activation by S4-FcR clustering (Tkachenko et al., 2006).
Figure 1.
FGF2 and S4 clustering activate Rac1 via RhoG. (a) RFPECs were cultured in 0.5% FBS/DME for 24 h before RhoG activity assay. Cells were treated with 50 ng/ml FGF2 for the indicated times before lysis and subsequent RhoG activity assay. Peak RhoG activity is observed at 10 min. (right) Quantification from three experiments is shown. *, P = 0.018. (b) RFPECs stably expressing the S4-FcR chimera were likewise cultured for 24 h in 0.5% FBS/DME before RhoG activity assay. The S4-FcR chimera was clustered for the indicated times. (right) Quantification from three experiments is shown. *, P = 0.042. (c) RFPECs were transfected with the indicated RhoG constructs for 48 h before assay. 24 h after transfection, cells were cultured in 0.5% FBS/DME. Each condition represents no stimulation (−) or 10 min of FGF2 stimulation at a concentration of 50 ng/ml (+). Rac1 activity assays were performed three times and quantified using a modified ELISA technique (see Materials and methods). * (left to right), P = 0.0008, 0.014, and 0.002. (d) RFPECs were plated on glass coverslips and transfected with Raichu-Rac1 along with the indicated shRNA constructs in a 1:4 molar ratio. Cells were serum starved with 0.5% FBS in 1:1 F12/DME for 12 h before imaging. FGF2 was added at the indicated time points, and the cells were imaged once per minute. The images show the pseudocolored FRET ratios calculated as YFP fluorescence/CFP fluorescence after background subtraction at each pixel. Higher ratio values (red) correlate with higher Rac1 activity. Bars, 10 µm. (e) Quantification of whole cell FRET ratios across six cells were performed in each condition shown in d. FGF2 stimulation (+) is for 10 min. *, P = 0.037. (f) RFPECs were transfected with the indicated constructs and plated on fibronectin-coated plastic dishes. The cells were grown to a confluent monolayer, at which time they were serum starved, and a scratch was introduced to disrupt the monolayer. The images and quantification represent the area migrated, averaged over three experiments with 24 frames measured per condition, 24 h after monolayer disruption. All results were normalized to the migration of nonstimulated control cells (leftmost condition). Constitutively active RhoG (V12) was used as a positive control for migration. * (bottom to top), P = 0.048 and 0.008. Bars, 75 µm. P-values in all experiments were calculated using a two-sample equal variance t test. Error bars represent SEM.
We next tested the requirement for RhoG in FGF2-induced Rac activation using two approaches for RhoG inhibition. First, RFPECs were transfected with wild-type (WT) RhoG, A37 RhoG (which does not bind its effector, ELMO), or V12 RhoG (constitutively active). Measurement of Rac activity in cell lysates 10 min after FGF2 stimulation demonstrated a 1.5-fold increase in cells expressing WT RhoG (Fig. 1 c), whereas A37 RhoG significantly blunted Rac1 activation. Conversely, the constitutively active V12 RhoG mutant induced high Rac1 activity that did not change after FGF2 treatment. Second, we examined the effect of knocking down RhoG expression, in this case measuring Rac1 activity in live cells using an intramolecular fluorescence resonance energy transfer (FRET) probe (Raichu-Rac1). This probe measures the local balance of GEF and GAP activities but is insensitive to regulation by RhoGDI (Itoh et al., 2002). In control cells, FGF2 increased Rac1 activity between 5 and 10 min (Fig. 1 d, top), whereas in cells expressing RhoG short hairpin RNA (shRNA), Rac1 baseline activity was reduced (Fig. S1 b), and stimulation by FGF2 was inhibited (Fig. 1, d [bottom] and e). These results confirm the effects of the RhoG dominant negative and indicate that changes in GEF or GAP activity, rather than RhoGDI, must mediate the effect.
We next investigated whether RhoG is required for FGF2-induced cell migration. Knockdown of RhoG diminished endothelial cell migration in response to FGF2, whereas expression of V12 RhoG potentiated cell migration (Fig. 1 f). Collectively, these data demonstrate that FGF2 activates RhoG via S4, that RhoG is required for Rac1 activation, and that RhoG mediates FGF2-induced cell migration.
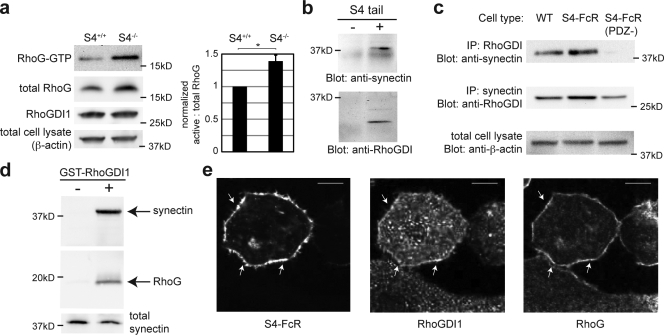
S4 is required to maintain low baseline Rac1 activity (Saoncella et al., 2004; Tkachenko et al., 2006; Bass et al., 2007), although the mechanism of this effect is unknown. Given our finding that both baseline Rac1 activity and its stimulation by FGF2 depend on RhoG, we investigated whether S4 likewise affects Rac1 baseline activity via RhoG. To begin, measurement of baseline RhoG activity in pulmonary microvascular endothelial cells from S4+/+ and S4−/− mice showed that loss of S4 significantly increased baseline RhoG activity (Fig. 2 a). The S4 cytoplasmic tail contains a PDZ-binding sequence that interacts with synectin, a ubiquitously expressed protein that also affects Rac1 activity (Gao et al., 2000; Chittenden et al., 2006). A yeast two-hybrid screen with synectin as bait revealed S4 and RhoGDI1 as binding partners (unpublished data). RhoGDI1 regulates the activity of Rho family GTPases by preventing interactions with GEFs. Therefore, we explored whether S4 influences RhoG activity through synectin and RhoGDI1. A biotinylated, synthetic peptide corresponding to the transmembrane and cytoplasmic domains of S4 bound not only synectin as expected (Fig. 2 b, top) but also pulled down RhoGDI (Fig. 2 b, bottom) from RFPEC lysates.
Figure 2.
S4 mediates baseline RhoG activity and associates with synectin, RhoGDI1, and RhoG. (a) Murine pulmonary microvascular endothelial cells from WT and S4 knockout mice were cultured in 0.5% FBS/DME for 24 h before assay of RhoG activity. β-Actin and RhoGDI were used as loading controls. (right) Quantification from three experiments is shown. *, P = 0.009. Error bar indicates SEM. (b) A synthetic biotinylated peptide corresponding to the transmembrane domain and cytoplasmic tail of S4 was used to pull down proteins after conjugation to streptavidin beads. Pull-downs were immunoblotted and probed for synectin (top) and RhoGDI (bottom). − indicates unconjugated streptavidin beads. In both cases, the target proteins were pulled down only when incubated with the S4 tail peptide. (c) Coimmunoprecipitations were performed with antibodies against RhoGDI and synectin in three cell lines: WT RFPECs (left), RFPECs expressing the S4-FcR chimera (middle), and RFPECs expressing the S4-FcR (PDZ−) chimera (right). β-Actin was used as a loading control. IP, immunoprecipitation. (d) Glutathione beads conjugated with recombinant RhoGDI1 (right) or without the recombinant protein (left) were incubated with lysates of RFPECs for 12 h at 4°C. After washing, proteins were denatured by boiling and subjected to SDS-PAGE. The proteins were transferred to a membrane and probed for RhoG and synectin. (e) RFPECs were transfected with the S4-FcR chimera. Cells were incubated with FITC-conjugated human IgG to stain the chimera (left), washed twice with PBS, fixed, permeabilized, and stained with antibodies against RhoGDI1 and RhoG (middle and right, respectively). Arrows indicate regions of colocalization. Bars, 10 µm.
Next, we performed coimmunoprecipitations using antibodies against each protein. RhoGDI immunoprecipitates contained synectin in WT RFPECs and in RFPECs overexpressing the S4-FcR chimera but not in RFPEC overexpressing the S4 chimera containing a nonfunctional PDZ-binding domain (Fig. 2 c, top). Synectin immunoprecipitation also brought down less RhoGDI in these cells compared with S4-overexpressing or WT S4 (Fig. 2 c, middle). Because the PDZ-binding domain of S4 is required to bind synectin, these data demonstrate the importance of S4–synectin interaction in synectin's binding to RhoGDI1.
Whether RhoG associates with this S4–synectin–RhoGDI1 complex was studied next. Published data are inconsistent on whether RhoG binds RhoGDI1 (Fauré and Dagher, 2001; Brunet et al., 2002). We found that glutathione beads conjugated with recombinant GST-RhoGDI1 bound both synectin and RhoG from RFPEC lysates, whereas control beads showed no binding (Fig. 2 d). The use of purified RhoGDI1 in combination with a specific anti-RhoG antibody (Meller et al., 2008) in these experiments provides strong evidence for this interaction. The cellular distribution of RhoG in living cells also changes in a RhoGDI1-dependent manner, further demonstrating the interaction of these two proteins (Fig. S1 c). We finally sought to confirm the existence of this protein complex within intact cells. The S4-FcR chimera was expressed and stained in RFPECs (Fig. 2 e). Despite the largely cytoplasmic distribution of RhoGDI1, certain membrane regions demonstrated colocalization of the S4 construct (Fig. 2 e, left), RhoGDI1 (Fig. 2 e, middle), and RhoG (Fig. 2 e, right). Validation of the RhoG monoclonal antibody for immunofluorescence is presented in Fig. S1 (d–f).
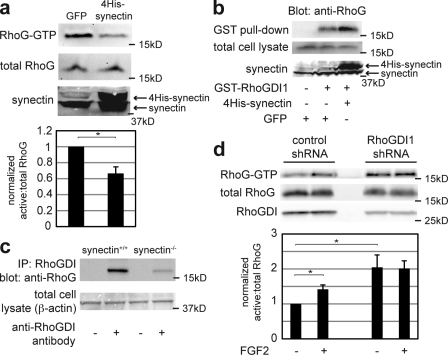
We hypothesized that the interaction between RhoG and RhoGDI1 is regulated, which may account for the contradictory reports in different studies (Fauré and Dagher, 2001; Brunet et al., 2002). To test whether synectin enhances RhoGDI's affinity for RhoG, we measured the effect of synectin overexpression in RFPECs on RhoG activity (Fig. 3 a). Consistent with our hypothesis, synectin overexpression decreased RhoG activity (Fig. 3 a), and endothelial cells from synectin knockout mice were also found to have high basal levels of RhoG activity (Fig. S1 g). To test whether synectin suppresses RhoG activity by increasing RhoGDI's affinity for RhoG, we used two complementary approaches. First, the effect of overexpressed synectin on binding of RhoG to recombinant GST-tagged RhoGDI1 was examined. RFPECs were transfected with His-tagged synectin or GFP as a control. Expression of synectin substantially increased binding of endogenous RhoG to RhoGDI1 beads (Fig. 3 b, compare left with middle), whereas no binding was observed to glutathione beads alone (Fig. 3 b, right). Interestingly, synectin overexpression also enhanced RhoGDI–Rac1 binding (Fig. S1 h). Second, we examined the effect of synectin knockout on the ability of RhoGDI1 to bind RhoG. In murine lung endothelial cells, immunoprecipitation of RhoGDI1 brought down less RhoG in synectin−/− cells than in WT cells (Fig. 3 c). Collectively, these data demonstrate that synectin enhances both the binding of RhoG by RhoGDI and the suppression of RhoG activity.
Figure 3.
Synectin and RhoGDI1 are required for RhoG suppression at baseline. (a) WT murine pulmonary microvascular endothelial cells were transfected with either GFP- or 4His-tagged synectin 48 h before lysis and RhoG activity assay. Cells were serum starved in 0.5% FBS/DME for 24 h before lysis. (bottom) Quantification from three experiments is shown. *, P = 0.052. (b) Mock-transfected RFPECs (left and middle) and those transfected with 4His-tagged synectin (right) were lysed 48 h after transfection. Pull-downs were performed using glutathione beads conjugated with GST-RhoGDI1 (middle and right) or glutathione beads alone (right). (c) Lysates from WT and synectin knockout murine pulmonary microvascular endothelial cells were normalized to equal protein concentrations and incubated with monoclonal anti-RhoGDI antibodies and protein A/G beads (+) or beads alone (−). After washing and protein elution, the samples were analyzed by SDS-PAGE and probed with an anti-RhoG antibody. IP, immunoprecipitation. (d) RFPECs were transfected with a control shRNA sequence or shRNA specific for RhoGDI1. 24 h after transfection, cells were placed in 0.5% FBS/DME for an additional 24 h, at which time they were lysed and assayed for RhoG activity. The quantifications show the mean of three experiments and reveal a more than twofold increase in baseline RhoG activity in cells treated with RhoGDI1 shRNA. * (bottom to top), P = 0.034 and 0.008. Error bars indicate SEM.
To test whether RhoGDI is required for suppression of RhoG by S4 and synectin, we measured the effect of RhoGDI1 knockdown on RhoG activity in RFPECs (Fig. 3 d). In control shRNA-treated cells, FGF2 treatment elevated RhoG activity by ∼1.5-fold after 10 min (Fig. 3 d, left). In cells treated with shRNA against RhoGDI1, baseline RhoG activity was approximately twofold higher than in control cells, and FGF2 triggered no further increase (Fig. 3 d, right). Related experiments involving RhoGDI2 (Ly-GDI or GDI-D4) have shown the ability of this GDI to affect the activity states of other Rho family GTPases (Ota et al., 2004). Our data demonstrate that maintenance of low RhoG activity in the absence of FGF2 requires RhoGDI1.
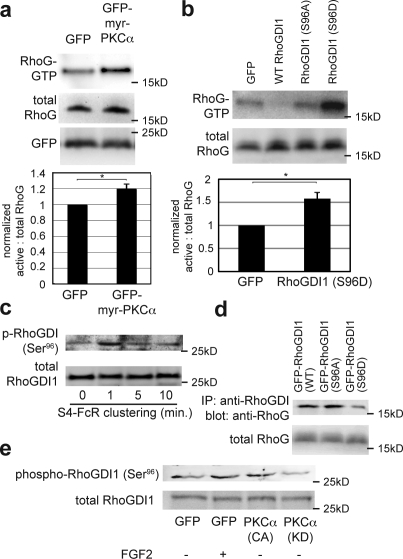
We next investigated the mechanism by which RhoG suppression is reversed by FGF2/S4 signaling. Previous studies indicate that S4 oligomerization leads to the activation of PKCα (Oh et al., 1997; Horowitz et al., 2002). To explore the role of this kinase in RhoG activation, we examined the effect of constitutively active (myristoylated) PKCα on baseline RhoG activity. RFPECs transfected with myristoylated PKCα showed increased RhoG activation at baseline (Fig. 4 a). Conversely, a dominant-negative PKCα construct blocked FGF2-induced RhoG activation (Fig. S1 i). RhoGDI1 is among the targets of PKCα (Price et al., 2003; Knezevic et al., 2007); therefore, we hypothesized that PKCα-dependent phosphorylation of RhoGDI1 might cause release of RhoG followed by its activation. To determine which RhoGDI1 phosphorylation site affects RhoG binding, RhoGDI1 constructs with mutated phosphorylation sites were screened (unpublished data). The Ser96 residue, when mutated to alanine (S96A), resulted in baseline levels of RhoG activity that were comparable with GFP controls, whereas a phosphomimetic mutation (S96D) led to strong activation of RhoG (Fig. 4 b). The S96A mutant did not suppress RhoG activity as efficiently as WT RhoGDI, mirroring this mutation's effect on RhoA (Knezevic et al., 2007) and implying that alanine substitution at this site also moderately weakens RhoGDI–RhoG binding. This result also indicates that in previously reported instances of simultaneous RhoG and RhoA activation (van Buul et al., 2007), the Ser96 residue of RhoGDI1 is a common site of regulation.
Figure 4.
PKCα phosphorylation of RhoGDI1 at Ser96 induces RhoG activation. (a) RFPECs were transduced with GFP or GFP-myristoylated PKCα adenoviral constructs for 48 h before RhoG activity assay. The cells were serum starved in 0.5% FBS/DME 24 h after transfection, at which time lysates were collected. (bottom) Quantification for three experiments is shown. *, P = 0.048 (two-sample equal variance t test). (b) RFPECs were transfected with the indicated constructs for 24 h and serum starved in 0.5% FBS/DME for an additional 24 h before RhoG activity assay. Expression of phosphomimetic RhoGDI1 (S96D) led to RhoG activation. Quantification represents the mean of three experiments. *, P = 0.038. (c) RFPECs with stable expression of the S4-FcR chimera were used to cluster the transmembrane and cytosolic domains of S4. These cells were treated with 2 µg/ml nonimmune human IgG and washed twice with PBS. Clustering was initiated with anti–human IgG F(ab′)2 fragments at 3 µg/ml for the indicated times. Cells were lysed and analyzed by immunoblotting. A rabbit polyclonal antibody specific for the phosphorylation of Ser96 was used for the top panel. (d) HeLa cells overexpressing the indicated GFP-tagged RhoGDI1 mutants were lysed 72 h after transfection and subjected to immunoprecipitation (IP) with anti-GFP antibodies conjugated to protein A/G beads. The immunoblot was probed for RhoG. RhoGDI1 (S96D) leads to decreased RhoG–RhoGDI1 interaction. (e) RFPECs were transfected with the indicated constructs and serum starved. FGF2 treatment was performed for 1 min. Lysates were subsequently analyzed by Western blotting using anti–phospho-RhoGDI (Ser96) antibodies. Both FGF2 and (myristoylated) constitutively active (CA) PKCα resulted in RhoGDI1 phosphorylation Ser96, whereas kinase-dead (KD) PKCα resulted in a lower baseline level of RhoGDI phosphorylation at this residue. Error bars represent SEM.
We next studied RhoGDI1 phosphorylation more directly with a phosphorylation site–specific antibody (validation shown in Fig. S1, j and k). RhoGDI1 Ser96 phosphorylation was detected within 1 min after clustering the S4-FcR chimera and then returned to a lower plateau level (Fig. 4 c). Thus, RhoGDI1 phosphorylation precedes RhoG and Rac1 activation. To establish a functional role for RhoGDI1 Ser96 phosphorylation, we expressed GFP-tagged RhoGDI1 mutants in HeLa cells (on account of their high transfection efficiency) and performed immunoprecipitations using antibodies against GFP. The S96D mutation resulted in decreased association between RhoGDI and RhoG (Fig. 4 d). We confirmed a published report that the Ser96 site does not affect Rac1 activity (Fig. S1 l; Knezevic et al., 2007). Thus, despite their homology, interactions of RhoGDI with RhoG and Rac1 are regulated differently, and the data are consistent with a direct effect on RhoG followed by an indirect effect on Rac1. Finally, we demonstrated that both FGF2 treatment and transfection with constitutively active PKCα result in phosphorylated Ser96, whereas transfection with dominant-negative PKCα diminishes this phosphorylation (Fig. 4 e). Collectively, these results support a model in which activation of PKCα by S4 triggers phosphorylation of RhoGDI and release of RhoG, which can then be activated by GEFs that reside near the plasma membrane (Fig. 5).
Figure 5.
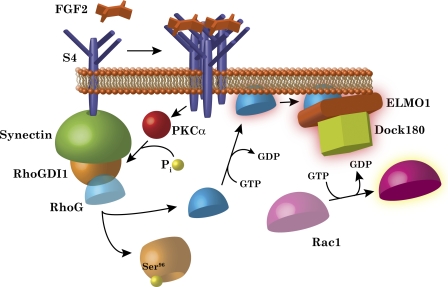
RhoG activation is regulated by a S4–synectin–RhoGDI1 complex. RhoG activity is suppressed at baseline by a complex consisting of S4, synectin, and RhoGDI1. Within this complex, S4 enhances synectin–RhoGDI1 binding, and synectin increases the affinity of RhoGDI1 for RhoG. Upon FGF2 stimulation and subsequent S4 oligomerization, PKCα becomes activated, which phosphorylates RhoGDI at its Ser96 residue. This induces the dissociation of RhoG from RhoGDI1, after which RhoG becomes activated. Active RhoG associates with ELMO1 and Dock180 to form a functional GEF complex, which is required for Rac1 activation in this pathway.
Discussion
Rho family GTPase activation is generally a fast and transient event effected by local shifts in the balance between GEFs and GAPs. RhoGDI1 is also important in GTPase regulation. Indeed, knockdown of this protein results in a more than twofold increase in baseline RhoG activity (Fig. 3 d). This result demonstrates that when RhoG is not sequestered by RhoGDI1, the GEF/GAP balance favors RhoG activation. We therefore sought to characterize the mechanism of RhoGDI-dependent GTPase suppression between activation cycles.
Studying the RhoGDI–RhoG interaction, we identified a novel multiprotein complex of S4–synectin–RhoGDI1 as the central regulatory component of RhoG activity. The contributions of each protein were then characterized beginning with S4. A single amino acid deletion in the PDZ-binding domain of S4 diminished the efficiency of ternary complex formation (Fig. 2 c). This finding explains previous results that a mutated PDZ-binding domain results in elevated Rac1 levels (Tkachenko et al., 2006) and demonstrates the importance of S4 in stabilizing the complex.
Synectin was also found to be an indispensable component of the ternary RhoG regulation complex. Our data are the first to describe a protein that enhances the affinity of RhoGDI1 for a GTPase (Fig. 3, a–c), although studies have characterized proteins that perform the opposite role: the ezrin–radixin–moesin (ERM)-CD44 system, involved in actin reorganization, is a direct binding partner of RhoGDI that decreases the affinity of the GDI for Rho GTPases (Hirao et al., 1996; Takahashi et al., 1997). Furthermore, the neurotrophin receptor p75NTR and nonreceptor tyrosine kinase Etk also decrease RhoGDI1–RhoA association, leading to RhoA activation (Kim et al., 2002; Yamashita and Tohyama, 2003). Although functionally opposed to synectin, ezrin similarly binds directly to both RhoGDI (Hirao et al., 1996) and syndecans (Granés et al., 2000); these observations present another interesting dimension to the antagonism between ezrin and synectin, one that is likely to be illuminated upon discovery of their specific RhoGDI-binding sites.
RhoGDI1 inhibits RhoG activation by GEFs and serves as the physical link between S4–synectin and RhoG (Fig. 1, d and e; Fig. 3, b and c; and Fig. S1 b). However, conflicting studies describe RhoG's ability to bind RhoGDI1 (Fauré and Dagher, 2001; Brunet et al., 2002). In this study, the use of purified RhoGDI1 with a specific anti-RhoG antibody enabled us to definitively determine that this interaction exists (Fig. 1, d and e; Fig 3, b and c; and Fig. S1 b) and is functionally significant in RhoG activity regulation (Fig. 3 d). S4 and synectin increased this interaction, which may furthermore explain the failure to detect binding in previous studies. Although synectin was shown to enhance the RhoGDI–GTPase interaction for both RhoG (Fig. 3 b) and Rac1 (Fig. S1 h), whether this effect encompasses additional Rho family GTPases remains to be determined.
Published studies involving RhoGDI2 (Ly-GDI or GDI-D4) have shown the ability of this GDI to affect the activity states and localization of Rho GTPases (Ota et al., 2004), and we investigated whether RhoGDI1 likewise determines the localization and activity of RhoG. Our finding that the Ser96 site is phosphorylated to release RhoG from RhoGDI is supported by related published findings. First, RhoGDI1 is phosphorylated by p21-activated kinase on two sites, causing Rac1 activation (DerMardirossian et al., 2004). RhoGDI1 Ser96 also becomes phosphorylated by PKCα and is involved in RhoA activation (Knezevic et al., 2007). Given that RhoA and RhoG are often activated simultaneously (van Buul et al., 2007), it is likely that Ser96 phosphorylation determines the activity of both GTPases similarly.
Finally, our confirmation of the observation that Ser96 phosphorylation does not affect Rac1 activity (Knezevic et al., 2007) indicates an important point of departure between RhoG and Rac1; these two proteins have high sequence homology, are part of the same signaling pathway, are activated with similar kinetics, and both bind to RhoGDI1. However, we demonstrate that activation via Ser96 phosphorylation affects only RhoG. Although critical in FGF2 signaling, RhoG is not common to all signaling cascades that converge upon Rac1. For example, syndecans and integrins contribute synergistically but distinctly to GTPase activation (Bass et al., 2007), and integrin-mediated Rac1 activation does not require RhoG (Meller et al., 2008). In the case of FGF2 stimulation, the finding that A37 RhoG blocks Rac1 activation (Fig. 1 c) strongly suggests ELMO1–Dock180 involvement (Katoh and Negishi, 2003), although other parallel RhoG-dependent modes of Rac1 activation could also contribute.
Previous studies indicated that S4 orchestrates the polarization of active Rac1 in the presence of chemotactic signals such as FGF2 and that S4 induces Rac-dependent cell migration in a manner that requires both its PDZ-binding domain and PKCα (Tkachenko et al., 2006; Bass et al., 2007). This study unifies these observations with the discovery of the S4–synectin–RhoGDI1 protein complex. Examining this ternary complex revealed a mechanism to explain these earlier reports that the S4 PDZ-binding domain is crucial for GTPase regulation: the PDZ-binding domain mediates the S4–synectin interaction and enhances the latter's affinity for RhoGDI1 (Fig. 2 c). Additionally, our observations substantiate the involvement of PKCα by showing that this kinase phosphorylates RhoGDI1, releasing RhoG to activate Rac1 (Fig. 4).
More importantly, we found that the S4–synectin–RhoGDI1 complex mediates the baseline suppression of RhoG. How such GTPases are constitutively maintained in their inactive states has been poorly understood, and our data reveal the mechanism by which S4 and synectin are required for this process. Disrupting any part of the S4–synectin–RhoGDI1 complex (by gene silencing or mutagenesis) creates a persistently dysregulated state of active RhoG by decreasing its binding to RhoGDI1 (Fig. 2 a; and Fig. 3, a and d). Although this invariably leads to a high baseline level of RhoG and Rac1 activity, FGF2 stimulation is still able to further activate these GTPases in cells lacking S4 or synectin (Chittenden et al., 2006). This result implies that the S4–synectin–RhoGDI1 complex mediates the sequestration and release of inactive RhoG but is dispensable for actual activation event, which is presumably catalyzed by a yet undetermined GEF. In the case of FGF2 signaling, it is likely that activation of this GEF proceeds downstream of FGF tyrosine kinase receptor (FGFR1–4) signaling.
In animal models, genetic deletions of S4, synectin, or RhoGDI1 result in pronounced vascular phenotypes (both developmental and homeostatic) that include arterial branching defects, endotoxic shock susceptibility, elevated blood pressure, and enhanced pulmonary vessel permeability (Ishiguro et al., 2001; Chittenden et al., 2006; Gorovoy et al., 2007; Partovian et al., 2008). Neural crest formation in Xenopus laevis and zebrafish are also dependent on S4/Rac1 signaling (Matthews et al., 2008), and other neural implications of S4–synectin–RhoGDI1 disruption are likely to parallel those discovered in the vascular system.
Given the breadth of molecular and physiological influence exerted by the S4–synectin–RhoGDI1 complex, we believe it represents a significant mechanism of GTPase regulation. Our data identify novel functions of both S4 and synectin in GTPase signaling, characterize the pathway of S4-mediated Rac1 activation during endothelial migration, and address the longstanding question of how Rho GTPases are maintained in a minimally activated state before growth factor stimulation.
Materials and methods
Cell culture, transfection, and transduction
RFPECs and HeLa cells were cultured in DME (Cambrex) containing 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin (Mediatech). RFPECs with stable expression of the S4-FcR and S4-FcR (PDZ−) chimeras (Tkachenko and Simons, 2002) were used for S4 clustering experiments. The S4-FcR (PDZ−) mutant includes a single amino acid truncation at the cytoplasmic terminus (Horowitz et al., 2002). Primary pulmonary murine endothelial cells from WT and S4 knockout mice were isolated by harvesting murine lungs and subjecting them to fine mincing and digestion in 25 ml collagenase 0.2% (wt/vol) at 37°C for 45 min. The crude cell preparation was pelleted and resuspended in Dulbecco's PBS. The cell suspension was incubated with PECAM-1–coated beads (IgG Dynal beads; Invitrogen) at room temperature for 10 min with end over end rotation. Using a magnetic separator, the bead-bound cells were recovered, washed with DME containing 20% FBS, suspended in 12 ml complete culture medium (DME containing 20% fetal calf serum supplemented with 100 ug/ml heparin, 100 ug/ml endothelial cell growth factor growth supplement [Biomedical Technologies], and nonessential amino acids, sodium pyruvate, L-glutamine, and antibiotics at standard concentrations), and plated in fibronectin-coated 75-cm2 tissue culture flasks. Transfection of RFPECs was performed using Fugene6 (Roche) or Amaxa (Amaxa, Inc.), and transfection of HeLa cells was performed using 293Fectin (Invitrogen) according to the manufacturer's protocols. Adenoviral transduction was performed using 100 MOI.
cDNA constructs
GFP- and myc-tagged RhoG and GST-ELMO1 constructs were provided by H. Katoh and M. Negishi (Kyoto University, Kyoto, Japan). The S4-FcR chimera was previously generated (Tkachenko et al., 2006). Synectin constructs were provided by A. Horowitz (Dartmouth Medical School, Hanover, NH). The RhoG shRNA construct targets nucleotides 348–367 (5′-CGTCTTCGTCATCTGTTTC-3′). GFP-tagged RhoGDI1 constructs were provided by D. Mehta (University of Illinois, Chicago, IL). Myc-tagged RhoGDI1, the Vav2 expression construct, and Raichu-Rac1 FRET probes were validated previously (Aoki et al., 2005; Moissoglu et al., 2006; Tkachenko et al., 2006). The adenoviral dominant-negative PKCα construct was cloned with a PKCϵ epitope tag (Horowitz et al., 1999).
Antibodies and reagents
Rabbit polyclonal antisynectin antibodies and mouse monoclonal anti-RhoG antibodies were generated previously (Chittenden et al., 2006; Meller et al., 2008). Phospho-specific RhoGDI1 Ser96 antibodies were generated by and in consultation with 21st Century Biochemicals, Inc. to the following sequence, which is conserved in mouse, rat, and human: LDLTGDLE[pS]FKKQSFV. Mouse monoclonal anti-GFP antibodies and rabbit polyclonal anti-RhoGDI1 antibodies were purchased from Santa Cruz Biotechnology, Inc. Mouse monoclonal anti–β actin antibodies were obtained from BD. Mouse monoclonal anti-RhoG antibodies and GST-tagged RhoGDI1 were purchased from Cytoskeleton, Inc. Biotinylated S4 tail peptide (biotin-MKKKDEGSYDLGKKPIYKKAPTNEFYA) corresponding to the C-terminal protein sequence was synthesized by Syngene and verified by mass spectrometry. Nonimmune human IgG and anti–human F(ab′)2 fragments were purchased from Jackson ImmunoResearch Laboratories. FGF2 was obtained from Novartis.
Rac1 and RhoG activity assays
ELISA-based Rac1 activity quantification was performed using the G-LISA kit (Cytoskeleton, Inc.) according the manufacturer's protocol. RhoG pull-down assays were performed by first purifying GST-ELMO1 and conjugating it to agarose beads using a GST Purification kit (Thermo Fisher Scientific). Rac1 pull-down assays were performed using p21-activated kinase–conjugated agarose beads (Cytoskeleton, Inc.). With the exception of these baits, both Rac1 and RhoG pull-downs were performed identically as follows: cells were serum starved in 0.5% FBS/DME for 24 h before assay. Upon stimulation for the indicated times, the cells were lysed on ice with M-PER (Thermo Fisher Scientific) supplemented with Complete Protease Inhibitor Cocktail (Roche). GST-ELMO1–conjugated beads were incubated with cell lysates at 4°C for 30 min before being washed four times and boiled in SDS-PAGE sample buffer (Thermo Fisher Scientific). Eluted proteins were subjected to immunoblot analysis and probed with either anti-RhoG or anti-Rac1 antibodies. The RhoG pull-down assay was validated using constitutively active (V12) RhoG and effector nonbinding (A37) RhoG as controls.
Immunoprecipitations, GST-tagged protein pull-downs, and Western blots
Cells were lysed using either M-PER or RIPA buffers (Thermo Fisher Scientific) containing Complete Mini Protease Inhibitor Cocktail (Roche) for immunoprecipitations, pull-downs, and Western blots. For immunoprecipitations, protein A/G sepharose beads (GE Healthcare) were conjugated with the corresponding antibodies and incubated with cell lysates overnight at 4°C. They were washed six times with lysis buffer, immersed in sample buffer (Thermo Fisher Scientific), boiled for 5 min, and analyzed by SDS-PAGE. GST-tagged RhoGDI pull-downs were performed similarly with glutathione agarose beads (Thermo Fisher Scientific) used instead of sepharose and purified GST-RhoGDI1 (Cytoskeleton, Inc.) used as bait. Biotinylated S4 tail pull-downs were performed using streptavidin-conjugated beads (Thermo Fisher Scientific). 10% polyacrylamide gels (Bio-Rad Laboratories) were used for all Western blots.
Microscopy
Live cell imaging of FRET probes was performed by excitation of CFP and measurement of both CFP and YFP emission in cells transfected with the indicated probes (Aoki et al., 2005). These experiments were performed using an environment-controlled (set to 37°C) widefield microscope (IX-81; Olympus) using an oil immersion 60× NA 1.4 objective (Olympus). MetaMorph software was used for acquisition. The filters used for the dual-emission imaging were obtained from Omega Optical: an excitation filter (XF1071), a dichroic mirror (XF2034), and two emission filters (XF3075 for CFP and XF3079 for FRET). The imaging medium was phenol red–free DME/F12 (1:1 ratio) supplemented with 1% BSA and covered by mineral oil (Sigma-Aldrich) to prevent evaporation. The camera used for these experiments was a CoolSNAP HQ model (Roper Scientific). Cell migration was performed by introducing scratch wounds to confluent monolayers of RFPECs and measuring migration after 24 h using a 10× NA 0.3 objective (Olympus).
Fixed sections were prepared by transfer of cells growing on fibronectin-coated glass-bottom dishes to ice, washing once with ice-cold PBS, and fixation with 4% paraformaldehyde/PBS at room temperature for 10 min. Samples were washed three times with PBS and incubated with 0.1% Triton X-700 for 10 min for permeabilization where indicated. They were blocked with 1% BSA/PBS for 30 min at room temperature before antibody incubation. Confocal imaging of fixed cells was performed at room temperature with PBS as imaging medium using an FV1000 system (Olympus) equipped with an oil immersion 60× NA 1.35 objective (Olympus) with FluoView software (Olympus) for acquisition. All figures were assembled using Photoshop and Illustrator software (Adobe).
Quantitative analyses
Cellular distributions of GFP-RhoG were quantified by creating 20 line scans of equal length (five cells with four line scans per condition) with their midpoints at the cell membrane. The intensity at each pixel was measured using ImageJ software (National Institutes of Health) and was averaged among corresponding pixels in all line scans of each condition. Each intensity reading was normalized to the highest observed intensity across all conditions. Western blots were scanned using either the G:Box (Syngene) or the Odyssey (LI-COR Biosciences) and quantified using GelEval software (FrogDance). FRET ratio analyses were performed using MetaMorph software. Cell migration was quantified using ImageJ software.
Online supplemental material
Fig. S1 shows Rac1 activation upon FGF2 treatment, RhoG knockdown and basal Rac1 activity, RhoGDI1 expression and the cellular distribution of RhoG, RhoG antibody validation for immunofluorescence applications, RhoG activity at baseline in synectin knockout endothelial cells, the effect of synectin overexpression on Rac1 binding to RhoGDI1, dominant-negative PKCα and FGF2-induced RhoG activation, the phospho-RhoGDI1 (Ser96) antibody specificity in Western blot analyses, and phosphomimetic and nonphosphorylatable mutations of RhoGDI1 (Ser96) and baseline Rac1 activity. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200810179/DC1.
Acknowledgments
We would like to thank Dr. Hironori Katoh and Dr. Manabu Negishi for their insightful discussions and invaluable suggestions. We also thank them and Nao Yamaki (Kyoto University, Kyoto, Japan) for providing tagged RhoG constructs and purified GST-fused ELMO1. We would like to thank Dr. Dolly Mehta for the gift of the GFP-RhoGDI constructs used for these experiments. Finally, we would also like to thank the members of the Simons and Matsuda laboratories for experimental guidance and expertise.
A. Elfenbein was supported by an American Heart Association Predoctoral Fellowship (grant 0615689T) and the Global Center of Excellence Postdoctoral Fellowship Center for Frontier Medicine (Japanese Ministry of Education, Culture, Sports, Science and Technology). This work was also supported by the National Heart, Lung, and Blood Institute (grant HL62289 to M. Simons).
Footnotes
Abbreviations used in this paper: FcR, Fc receptor; FRET, fluorescence resonance energy transfer; GAP, GTPase-activating protein; GDI, guanine dissociation inhibitor; GEF, guanine exchange factor; PDZ, postsynaptic density disc large ZO-1; RFPEC, rat fat pad endothelial cell; S4, syndecan 4; shRNA, short hairpin RNA; WT, wild type.
References
- Alexopoulou A.N., Multhaupt H.A., Couchman J.R. 2007. Syndecans in wound healing, inflammation and vascular biology.Int. J. Biochem. Cell Biol. 39:505–528 [DOI] [PubMed] [Google Scholar]
- Aoki K., Nakamura T., Fujikawa K., Matsuda M. 2005. Local phosphatidylinositol 3,4,5-trisphosphate accumulation recruits Vav2 and Vav3 to activate Rac1/Cdc42 and initiate neurite outgrowth in nerve growth factor-stimulated PC12 cells.Mol. Biol. Cell. 16:2207–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass M.D., Roach K.A., Morgan M.R., Mostafavi-Pour Z., Schoen T., Muramatsu T., Mayer U., Ballestrem C., Spatz J.P., Humphries M.J. 2007. Syndecan-4–dependent Rac1 regulation determines directional migration in response to the extracellular matrix.J. Cell Biol. 177:527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet N., Morin A., Olofsson B. 2002. RhoGDI-3 regulates RhoG and targets this protein to the Golgi complex through its unique N-terminal domain.Traffic. 3:342–357 [DOI] [PubMed] [Google Scholar]
- Chittenden T.W., Claes F., Lanahan A.A., Autiero M., Palac R.T., Tkachenko E.V., Elfenbein A., Ruiz de Almodovar C., Dedkov E., Tomanek R., et al. 2006. Selective regulation of arterial branching morphogenesis by synectin.Dev. Cell. 10:783–795 [DOI] [PubMed] [Google Scholar]
- Dedkov E.I., Thomas M.T., Sonka M., Yang F., Chittenden T.W., Rhodes J.M., Simons M., Ritman E.L., Tomanek R.J. 2007. Synectin/syndecan-4 regulate coronary arteriolar growth during development.Dev. Dyn. 236:2004–2010 [DOI] [PubMed] [Google Scholar]
- DerMardirossian C., Schnelzer A., Bokoch G.M. 2004. Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase.Mol. Cell. 15:117–127 [DOI] [PubMed] [Google Scholar]
- Dovas A., Couchman J.R. 2005. RhoGDI: multiple functions in the regulation of Rho family GTPase activities.Biochem. J. 390:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerbroek S.M., Wennerberg K., Arthur W.T., Dunty J.M., Bowman D.R., DeMali K.A., Der C., Burridge K. 2004. SGEF, a RhoG guanine nucleotide exchange factor that stimulates macropinocytosis.Mol. Biol. Cell. 15:3309–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauré J., Dagher M.C. 2001. Interactions between Rho GTPases and Rho GDP dissociation inhibitor (Rho-GDI).Biochimie. 83:409–414 [DOI] [PubMed] [Google Scholar]
- Gao Y., Li M., Chen W., Simons M. 2000. Synectin, syndecan-4 cytoplasmic domain binding PDZ protein, inhibits cell migration.J. Cell. Physiol. 184:373–379 [DOI] [PubMed] [Google Scholar]
- Gorovoy M., Neamu R., Niu J., Vogel S., Predescu D., Miyoshi J., Takai Y., Kini V., Mehta D., Malik A.B., Voyno-Yasenetskaya T. 2007. RhoGDI-1 modulation of the activity of monomeric RhoGTPase RhoA regulates endothelial barrier function in mouse lungs.Circ. Res. 101:50–58 [DOI] [PubMed] [Google Scholar]
- Granés F., Urena J.M., Rocamora N., Vilaró S. 2000. Ezrin links syndecan-2 to the cytoskeleton.J. Cell Sci. 113:1267–1276 [DOI] [PubMed] [Google Scholar]
- Handa Y., Suzuki M., Ohya K., Iwai H., Ishijima N., Koleske A.J., Fukui Y., Sasakawa C. 2007. Shigella IpgB1 promotes bacterial entry through the ELMO-Dock180 machinery.Nat. Cell Biol. 9:121–128 [DOI] [PubMed] [Google Scholar]
- Hirao M., Sato N., Kondo T., Yonemura S., Monden M., Sasaki T., Takai Y., Tsukita S. 1996. Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway.J. Cell Biol. 135:37–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz A., Murakami M., Gao Y., Simons M. 1999. Phosphatidylinositol-4,5-bisphosphate mediates the interaction of syndecan-4 with protein kinase C.Biochemistry. 38:15871–15877 [DOI] [PubMed] [Google Scholar]
- Horowitz A., Tkachenko E., Simons M. 2002. Fibroblast growth factor–specific modulation of cellular response by syndecan-4.J. Cell Biol. 157:715–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro K., Kadomatsu K., Kojima T., Muramatsu H., Iwase M., Yoshikai Y., Yanada M., Yamamoto K., Matsushita T., Nishimura M., et al. 2001. Syndecan-4 deficiency leads to high mortality of lipopolysaccharide-injected mice.J. Biol. Chem. 276:47483–47488 [DOI] [PubMed] [Google Scholar]
- Itoh R.E., Kurokawa K., Ohba Y., Yoshizaki H., Mochizuki N., Matsuda M. 2002. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells.Mol. Cell. Biol. 22:6582–6591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi T., Goetinck P.F. 2006. Syndecan-4 dependent FGF stimulation of mouse vibrissae growth.Mech. Dev. 123:831–841 [DOI] [PubMed] [Google Scholar]
- Katoh H., Negishi M. 2003. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo.Nature. 424:461–464 [DOI] [PubMed] [Google Scholar]
- Katoh H., Hiramoto K., Negishi M. 2006. Activation of Rac1 by RhoG regulates cell migration.J. Cell Sci. 119:56–65 [DOI] [PubMed] [Google Scholar]
- Kim O., Yang J., Qiu Y. 2002. Selective activation of small GTPase RhoA by tyrosine kinase Etk through its pleckstrin homology domain.J. Biol. Chem. 277:30066–30071 [DOI] [PubMed] [Google Scholar]
- Knezevic N., Roy A., Timblin B., Konstantoulaki M., Sharma T., Malik A.B., Mehta D. 2007. GDI-1 phosphorylation switch at serine 96 induces RhoA activation and increased endothelial permeability.Mol. Cell. Biol. 27:6323–6333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H.K., Marchant L., Carmona-Fontaine C., Kuriyama S., Larraín J., Holt M.R., Parsons M., Mayor R. 2008. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA.Development. 135:1771–1780 [DOI] [PubMed] [Google Scholar]
- Meller J., Vidali L., Schwartz M.A. 2008. Endogenous RhoG is dispensable for integrin-mediated cell spreading but contributes to Rac-independent migration.J. Cell Sci. 121:1981–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moissoglu K., Slepchenko B.M., Meller N., Horwitz A.F., Schwartz M.A. 2006. In vivo dynamics of Rac-membrane interactions.Mol. Biol. Cell. 17:2770–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Elfenbein A., Simons M. 2008. Non-canonical fibroblast growth factor signalling in angiogenesis.Cardiovasc. Res. 78:223–231 [DOI] [PubMed] [Google Scholar]
- Oh E.S., Woods A., Couchman J.R. 1997. Multimerization of the cytoplasmic domain of syndecan-4 is required for its ability to activate protein kinase C.J. Biol. Chem. 272:11805–11811 [DOI] [PubMed] [Google Scholar]
- Ota T., Maeda M., Suto S., Tatsuka M. 2004. LyGDI functions in cancer metastasis by anchoring Rho proteins to the cell membrane.Mol. Carcinog. 39:206–220 [DOI] [PubMed] [Google Scholar]
- Pankov R., Endo Y., Even-Ram S., Araki M., Clark K., Cukierman E., Matsumoto K., Yamada K.M. 2005. A Rac switch regulates random versus directionally persistent cell migration.J. Cell Biol. 170:793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partovian C., Ju R., Zhuang Z.W., Martin K.A., Simons M. 2008. Syndecan-4 regulates subcellular localization of mTOR Complex2 and Akt activation in a PKCalpha-dependent manner in endothelial cells.Mol. Cell. 32:140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J.C., Galan J.E. 2006. Differential activation and function of Rho GTPases during Salmonella–host cell interactions.J. Cell Biol. 175:453–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price L.S., Langeslag M., ten Klooster J.P., Hordijk P.L., Jalink K., Collard J.G. 2003. Calcium signaling regulates translocation and activation of Rac.J. Biol. Chem. 278:39413–39421 [DOI] [PubMed] [Google Scholar]
- Prieto-Sanchez R.M., Berenjeno I.M., Bustelo X.R. 2006. Involvement of the Rho/Rac family member RhoG in caveolar endocytosis.Oncogene. 25:2961–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saoncella S., Calautti E., Neveu W., Goetinck P.F. 2004. Syndecan-4 regulates ATF-2 transcriptional activity in a Rac1-dependent manner.J. Biol. Chem. 279:47172–47176 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Sasaki T., Mammoto A., Takaishi K., Kameyama T., Tsukita S., Takai Y. 1997. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein.J. Biol. Chem. 272:23371–23375 [DOI] [PubMed] [Google Scholar]
- Tkachenko E., Simons M. 2002. Clustering induces redistribution of syndecan-4 core protein into raft membrane domains.J. Biol. Chem. 277:19946–19951 [DOI] [PubMed] [Google Scholar]
- Tkachenko E., Elfenbein A., Tirziu D., Simons M. 2006. Syndecan-4 clustering induces cell migration in a PDZ-dependent manner.Circ. Res. 98:1398–1404 [DOI] [PubMed] [Google Scholar]
- van Buul J.D., Allingham M.J., Samson T., Meller J., Boulter E., García-Mata R., Burridge K. 2007. RhoG regulates endothelial apical cup assembly downstream from ICAM1 engagement and is involved in leukocyte trans-endothelial migration.J. Cell Biol. 178:1279–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Tohyama M. 2003. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI.Nat. Neurosci. 6:461–467 [DOI] [PubMed] [Google Scholar]