Abstract
Cadherin-based adherens junctions (AJs) mediate cell adhesion and regulate cell shape change. The nectin–afadin complex also localizes to AJs and links to the cytoskeleton. Mammalian afadin has been suggested to be essential for adhesion and polarity establishment, but its mechanism of action is unclear. In contrast, Drosophila melanogaster’s afadin homologue Canoe (Cno) has suggested roles in signal transduction during morphogenesis. We completely removed Cno from embryos, testing these hypotheses. Surprisingly, Cno is not essential for AJ assembly or for AJ maintenance in many tissues. However, morphogenesis is impaired from the start. Apical constriction of mesodermal cells initiates but is not completed. The actomyosin cytoskeleton disconnects from AJs, uncoupling actomyosin constriction and cell shape change. Cno has multiple direct interactions with AJ proteins, but is not a core part of the cadherin–catenin complex. Instead, Cno localizes to AJs by a Rap1- and actin-dependent mechanism. These data suggest that Cno regulates linkage between AJs and the actin cytoskeleton during morphogenesis.
Introduction
Embryonic cells self-assemble tissues and organs. This morphogenesis process requires dynamic regulation of cell adhesion and cell shape change (Halbleib and Nelson, 2006), which are coordinated by cell–cell adherens junctions (AJs). AJs link neighboring cells to each other and to the apical actin cytoskeleton. Central to AJs are cadherins, which are transmembrane homophilic adhesion proteins. Their cytoplasmic tails bind β-catenin (fly Armadillo [Arm]), which binds α-catenin (αcat). αCat can directly bind actin filaments. Each of these proteins is essential for cell adhesion and epithelial integrity, with loss leading to very early defects in embryogenesis (Larue et al., 1994; Cox et al., 1996; Müller and Wieschaus, 1996; Kofron et al., 1997; Torres et al., 1997). It was assumed that AJs directly link to actin via the catenins. However, things are more complex. Although E-cadherin (Ecad) binds both catenins and αcat binds actin, these interactions are mutually exclusive, and thus, cadherin–catenin complexes cannot bind actin (Drees et al., 2005; Yamada et al., 2005). However, many morphogenetic events require intimate interactions between AJs and the cytoskeleton, prompting us to explore other proteins that may regulate adhesion and linkage to actin.
One interesting candidate is the nectin–afadin complex. Nectins are transmembrane immunoglobulin domain proteins colocalizing with Ecad at AJs (Takahashi et al., 1999) and mediating homophilic and heterophilic adhesion (Sakisaka et al., 2007). The four mouse nectins complicate loss of function analysis, but expression of soluble nectin extracellular domain diminishes cell adhesion in culture (Honda et al., 2003). These and other data (Tachibana et al., 2000; Fukuhara et al., 2002) led the authors to suggest that nectins are “necessary and sufficient for the recruitment of Ecad to the nectin-based cell–cell adhesion sites and [are] involved in the formation of Ecad-based cell–cell AJs” (Honda et al., 2003).
Nectins are thought to associate with actin via the filamentous actin (F-actin)–binding protein afadin (AF6), which binds via its PDZ (PSD-95/Dlg/zona occludens-1 [ZO-1] homology) domain to nectin C termini and localizes to AJs (Mandai et al., 1997). Afadin’s structure suggests a scaffolding role (Fig. 1 A). It has two Ras association (RA) domains, forkhead-associated and dilute domains, and a C-terminal actin-binding domain. Rap1 is thought to be the preferred binding partner for the RA domains (Linnemann et al., 1999), and afadin and Rap1 are functionally linked (Kooistra et al., 2007). Afadin provides a potential direct link between nectins and actin, and afadin also associates with other actin-binding proteins, including αcat (Tachibana et al., 2000; Pokutta et al., 2002).
Figure 1.
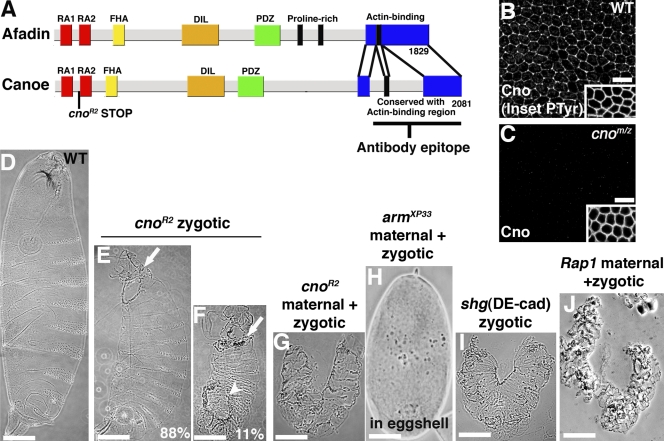
cno mutants have defects in morphogenesis. (A) Domain structures and cno mutant. (B and C) Stage 7 wild-type (WT) or cnoR2 MZ embryos stained for Cno and antiphosphotyrosine (PTyr; insets) to show cell borders imaged on same slide. (D–J) Cuticles, anterior up. Genotypes are indicated. (E and F) cnoR2 zygotic mutants are shown. Arrows, head involution defects; arrowhead, dorsal closure defects. (G) cnoR2 MZ is shown. Only dorsal cuticle remains. (H) armXP33 MZ mutant (in eggshell) is shown, cuticle fragmented. (I) shgR69 zygotic mutant retains only dorsal cuticle. (J) Rap1 MZ mutant retains only dorsal cuticle (see Results for Rap1 data). FHA, forkhead-associated domain; DIL, dilute domain. Bars: (B and C) 10 µm; (D–J) 100 µm.
This raised the possibility that afadin plays an important role in adhesion. Afadin knockdown in MDCK cells reduced Ecad at AJs after Ca2+ shift, although, surprisingly, total cell surface Ecad and catenin association were unchanged (Sato et al., 2006). Afadin-null embryoid bodies have many AJ and tight junction proteins mislocalized (Komura et al., 2008), suggesting that afadin is important in establishing polarity and cell adhesion. Afadin knockout in mice resulted in embryonic lethality, with defects during and after gastrulation. These authors concluded that afadin is “a key molecule essential for structural organization of cell–cell junctions of polarized epithelia during embryogenesis (Ikeda et al., 1999)” or that loss of afadin “disrupts epithelial cell–cell junctions and cell polarity during mouse development (Zhadanov et al., 1999).” However, afadin’s phenotype is much milder than those caused by loss of Ecad (Larue et al., 1994) or α-E-catenin (Torres et al., 1997), which disrupt the trophectoderm epithelium and block implantation.
Drosophila melanogaster has one afadin homologue, Canoe (Cno; Miyamoto et al., 1995), and at least one nectin, Echinoid (Ed), to which Cno binds (Wei et al., 2005). Cno also genetically interacts with and binds Rap1 (Boettner et al., 2003) and Polychaetoid (Pyd; fly ZO-1; Takahashi et al., 1998). Surprisingly, experiments with Cno suggested a different model in which it is a scaffold for signal transduction proteins. cno genetically interacts with receptor tyrosine kinase/Ras, JNK, Notch, and Wnt pathways (Miyamoto et al., 1995; Takahashi et al., 1998; Matsuo et al., 1999; Carmena et al., 2006), but mechanisms by which Cno influences signaling remain unclear. As in mice, Cno regulates morphogenesis. Zygotic mutants have defects in cell shape change during dorsal closure (Jürgens et al., 1984; Takahashi et al., 1998; Boettner et al., 2003) and in asymmetric divisions and cell fate choice in the nervous system and mesoderm (Carmena et al., 2006; Speicher et al., 2008). However, these studies left intact maternally contributed wild-type Cno.
These data provide several alternate hypotheses for Cno–afadin function: at one extreme, it may be essential in cell adhesion, whereas at the other, it may transduce signals regulating cell shape change. Drosophila provides powerful tools to distinguish between these mechanistic hypotheses. In this study, we examine the consequences of completely eliminating Cno function from the onset of embryogenesis. Our data suggest that Cno regulates links between AJs and actin during apical constriction, providing one possible solution to the dilemma posed by Drees et al. (2005) and Yamada et al. (2005).
Results
Complete loss of Cno leads to severe morphogenesis defects
Cno plays important roles in dorsal closure, mesoderm, and neural development (see Introduction), but these experiments only examined zygotic mutants. We hypothesized that maternal Cno masked earlier roles. To eliminate maternal and zygotic (MZ) Cno (cnoMZ mutants), we screened for new cno alleles on a FRT (flippase recombination target) chromosome (cno is very close to the flippase recombination target site), allowing us to remove Cno from the germline (Chou et al., 1993). cnoR2 has an early stop codon (K211Stop) after the first RA-binding domain (Fig. 1 A), suggesting that it is null. MZ cnoR2 mutants lost Cno immunoreactivity with a C-terminal antibody (Fig. 1, B vs. C; imaged on the same slide), confirming that there is not stop codon readthrough or reinitiation. Although it is possible that the remaining short protein fragment is produced, we think this is unlikely. First, nonsense-mediated mRNA decay usually efficiently degrades mRNAs with such early stop codons (Gatfield et al., 2003; Muhlemann et al., 2008). Second, we could not detect a stable product of cno2 with a much later stop codon (Q1310Stop; unpublished data). Finally, a second independent early truncation has a similar phenotype (see following paragraph).
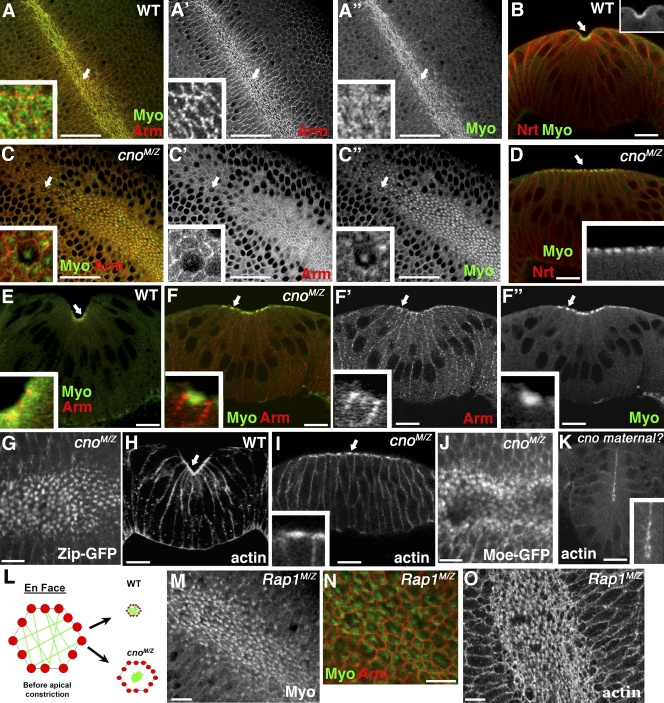
To assess how complete Cno loss affects morphogenesis, we examined cuticles secreted by epidermal cells (Fig. 1 D). Zygotic cno mutant embryos die; 88% have defects in head involution but close dorsally (Fig. 1 E), whereas 11% have defects in head involution and dorsal closure (Fig. 1 F). Loss of maternal Cno is not fully rescued by zygotic wild-type Cno; ∼30% of paternally rescued mutants die with defects in head involution (unpublished data). cnoMZ mutants (Fig. 1 G) are much more severe than zygotic mutants, which is consistent with strong maternal contribution. Most cnoMZ embryos (83%) entirely lack ventral cuticle, secreted by ventral neurogenic epidermis, but retain dorsal cuticle, secreted by nonneurogenic dorsal epidermis (Fig. 1 G). cnoR10 MZ mutants (a second putative null; Q140STOP) had similar phenotypes (unpublished data). The cnoMZ phenotype is not as severe as that of mutants completely lacking core AJ proteins DEcad (Tepass et al., 1996) or arm (arm–β-catenin; Cox et al., 1996; Müller and Wieschaus, 1996) in which only cuticle scraps are secreted (Fig. 1 H). This suggests that Cno is not essential for epithelial integrity. However, cnoMZ mutants mimic shotgun zygotic mutants; these mutants retain maternal DEcad but lack zygotic DEcad (Fig. 1 I; Tepass et al., 1996) and thus lose AJ function as maternal DEcad is depleted. This is consistent with Cno modulating adhesion during later morphogenesis.
Cno is not essential for AJ assembly and is only required for AJ maintenance in some tissues
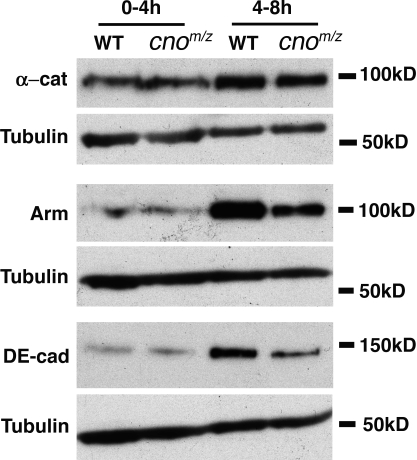
To further test Cno’s roles in AJs, we assessed AJ protein localization in cnoMZ mutants. We first examined AJ assembly. During cellularization, DEcad first localizes to basal junctions near the invaginating actomyosin front and then relocalizes to apical spot AJs; as the germband extends, these smooth out into belt AJs (Tepass and Hartenstein, 1994; Harris and Peifer, 2004). Initial AJ assembly in cnoMZ was indistinguishable from wild type (Arm and αcat also assembled correctly; Fig. 2, A vs. B and C vs. D; and Fig. S1, A–F; unpublished data), and AJ proteins became apically enriched (Fig. 2 F). Apical actin also appeared normal, colocalizing with DEcad (Fig. 2, A’ vs. B’ and C vs. D). This is in striking contrast to the loss of junctional DEcad and polarized F-actin in arm mutants (Fig. 2, E and E’; Cox et al., 1996). Maturation of spot AJs to belt AJs (Fig. S1, A–F) also proceeded normally. Finally, AJ protein levels were normal at these stages (Fig. 3, 0–4 h; Decad 102%, Arm 111%, and αcat 90% of wild type; mean of three experiments). Two Cno-binding proteins, Pyd and Ed, localize to AJs from the start, and both localize normally in cnoMZ mutants (Fig. S1, G–J). These data suggest that Cno is not essential for AJ assembly or initial maturation.
Figure 2.
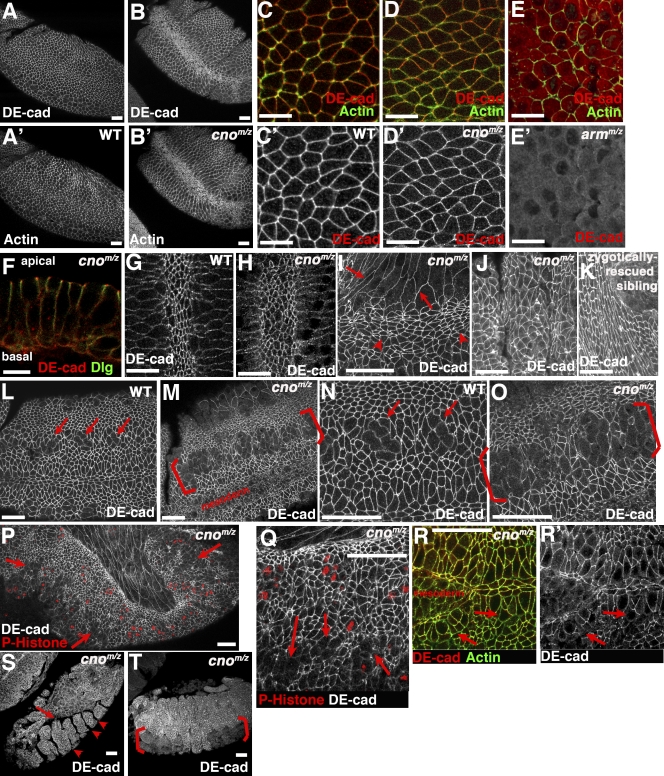
Cno is not essential for AJ assembly. Embryos, antigens, and genotypes are indicated. (A–F) Stage 8 is shown. (A–B’) Ventrolateral views, anterior top left. (C–C’) Close ups of A and A’ are shown (wild type [WT]). (D and D’) Close ups of B and B’ are shown (cnoMZ). (E and E’) armMZ is shown. Cortical DEcad lost. (F) Cross section, cnoMZ. DEcad remains apical. (G) Wild-type ventral furrow. (H) cnoMZ, DEcad maintained. (I) Stage 11, cnoMZ. AJ is normal in amnioserosa (arrows) and dorsal epidermis (arrowheads). (J and K) Dorsal epidermis, stage 13–14. (J) cnoMZ, AJs intact. (K) Paternally rescued sibling. (L–O) Lateral view, stage 9–10 is shown. (L and N) Close-up views of wild-type mitotic domains (arrows) are shown. (M and O) Close-up views of cnoMZ are shown. Some cells have reduced DEcad (brackets). (P and Q) Stage 12, cnoMZ. Arrows, fragmented AJs. (R and R’) Ventral midline, stage 11 cnoMZ. AJ fragmentation precedes loss of cortical actin (arrows). (S and T) Stage 13–14 cnoMZ. Amnioserosa detaches from epidermis (arrow), segmental groves never retract (arrowheads), and parts of ventral epidermis are missing (brackets). Bars: (A–B’ and K–T) 30 µm; (C–J) 10 µm.
Figure 3.
AJ protein levels in cnoMZ. Immunoblots, embryo extracts, and antigens are indicated. 0–4 h through mesoderm invagination and early germband extension. 4–8 h extended germband, stages 8–11. Tubulin is a loading control. WT, wild type.
In many embryonic cells, Cno is also not essential for AJ maintenance. In cnoMZ, AJs and cell shapes remain normal in amnioserosa (Fig. 2 I, arrows) and dorsal epidermal cells (Fig. 2, I [arrowheads] and J vs. K) through germband retraction. However, in a subset of ectoderm, AJs are not maintained normally. As the germband extends, ectodermal cells initiate mitosis; as they divide, they round up, and apical AJ protein accumulation is reduced (Fig. 2, L and N, arrows). As they exit mitosis, AJs reassemble, and cells become columnar again. In cnoMZ, although dorsal ectodermal cells retain columnar shape and normal AJs (Fig. 2 J), many ventral neurogenic ectodermal cells have reduced DEcad. It appears that after division they do not regain columnar shape with small apical ends (Fig. 2, M and O, brackets). To ensure that cells properly exited mitosis, we labeled embryos with the mitotic marker antiphospho–histone H3; large regions of ventral epidermis exited mitosis without properly reassembling AJs or regaining columnar shape (Fig. 2, P and Q, arrows). AJ fragmentation occurred before loss of cortical actin (Fig. 2 R, arrows). Arm and DEcad levels are also somewhat reduced at this stage (Fig. 3, 4–8 h; DEcad 87%, Arm 83%, and αcat 102% of wild type; mean of three experiments). Morphogenesis is compromised; the epidermis separates from the amnioserosa (Fig. 2 S, arrow), and segmental grooves never retract (Fig. 2 S, arrowheads). Ultimately, ventral cells are lost (Fig. 2 T, brackets), likely explaining the retention of dorsal but not ventral cuticle (Fig. 1 G). Thus, Cno is dispensable for AJ assembly and maintenance in many tissues but regulates AJ maintenance in some morphogenetically active cells.
Cno loss disrupts mesoderm invagination
Although AJs are established normally in Cno’s absence, morphogenesis is affected from the start. Gastrulation initiates after cellularization. The ventral-most cells form mesoderm and undergo coordinated apical constriction triggered by a pathway involving the ligand Fog, the G protein concertina, RhoGEF2, and Rho (Pilot and Lecuit, 2005). In response, mesodermal cells accumulate apical actin and myosin, apically constrict (Fig. 4, A and B), and internalize as a tube (Fig. 4 C). If AJs are disrupted, mesoderm invagination is compromised (Dawes-Hoang et al., 2005), and thus, coordinating AJs and actin is critical to couple actomyosin constriction to cell shape change.
Figure 4.
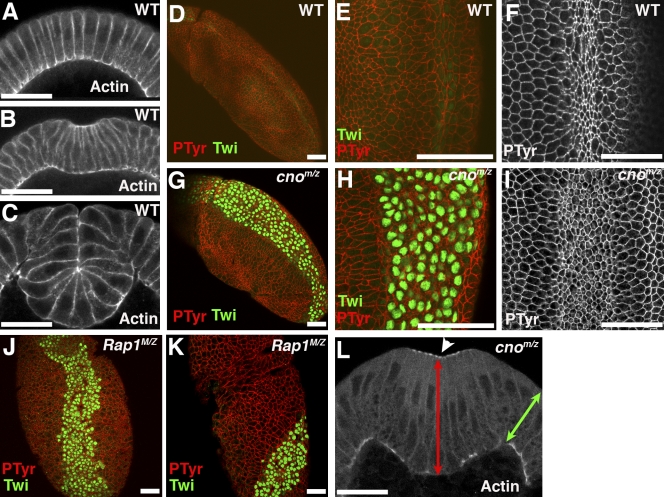
Cno is essential for mesoderm invagination. Embryos, antigens, and genotypes are indicated. (A–C) Cross sections of wild-type (WT) ventral furrow. Late cellularization (A), initial furrowing (B), and mesoderm internalized (C) are shown. (D–K) Ventral views, anterior up. (D and E) Wild type, mesoderm completely internalized. (F) Wild type during constriction. (G and H) cnoMZ, Twist (Twi)-positive cells not completely internalized. (I) cnoMZ mesoderm initiates constriction. (J and K) Rap1MZ phenocopies cnoMZ, but some exhibit twisted gastrulation. (L) cnoMZ mesodermal cells elongate along apical–basal axis (red arrow) relative to ectodermal neighbors (green arrow). Arrowhead, actin accumulating in balls. PTyr, phosphotyrosine. Bars, 30 µm.
cnoMZ morphogenetic defects begin at gastrulation. Wild-type mesoderm, marked by the transcription factor Twist, is completely internalized during gastrulation (Fig. 4, D and E). In contrast, cnoMZ mutants do not completely internalize mesoderm; many cells remain on the embryo surface and begin to divide in this aberrant location (Fig. 4, G and H). The degree of defect in mesoderm invagination varied from complete failure to defects only at the anterior and posterior ends (unpublished data).
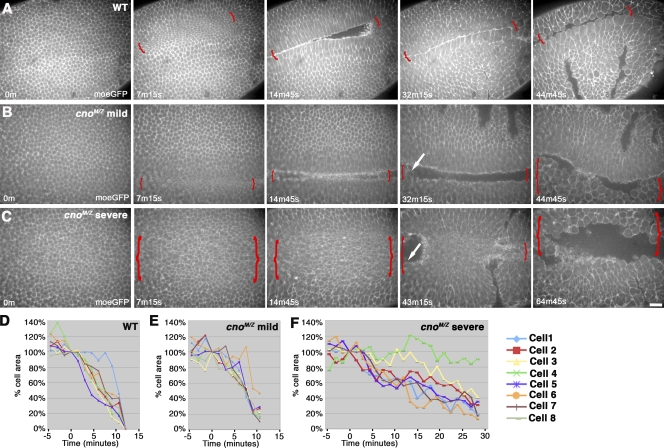
We next examined mechanisms by which this occurs. First, Cno, unlike Arm, is not essential for AJ assembly (Fig. 2, A–E’), even in invaginating mesoderm (Fig. 2, G vs. H). Second, Cno is not required for mesoderm specification, as cnoMZ mesoderm expresses Twist, the transcription factor conferring mesodermal fate (Fig. 4, G and H). A third hypothesis is that in Cno’s absence, mesodermal cells fail to initiate apical constriction, as do RhoGEF2 mutants (Barrett et al., 1997), or fail to constrict in a coordinated way, as do fog or concertina mutants (Sweeton et al., 1991). However, cnoMZ mutant cells initiate constriction and do so fairly synchronously (Fig. 4, F vs. I; occasional cells in both wild type and mutant constrict more slowly than their neighbors). However, cnoMZ cells arrest partway through apical constriction. Live analysis using moesin-GFP (moe-GFP) to highlight F-actin confirmed this. Wild-type mesodermal cells constrict rapidly and fairly synchronously (Fig. 5 A and Video 1). To quantify this, we measured change in cell cross-sectional areas of eight randomly chosen cells, confirming rapid, synchronous constriction in wild type, with occasional cells lagging behind (Fig. 5 D). cnoMZ mutants (distinguished from paternally rescued embryos using a marked balancer chromosome) initiated apical constriction in a timely manner but then had a variable phenotype (like the variability in mesoderm invagination). In less severe mutants, constriction went at the same rate as in wild type (Fig. 5, B and E; and Video 2) but halted prematurely; thus, as mesodermal cells initiated division (Fig. 5 B, arrow), they reemerged from the furrow. In more severe embryos (Fig. 5, C and F; and Video 3), constriction was slower than in wild type, and more cells lagged behind; this delay allowed mesodermal cells to divide before being internalized. These data suggest that Cno acts by a novel mechanism to ensure completion of apical constriction.
Figure 5.
Mesoderm invagination in cnoMZ. (A–C) Embryos, ventral views, anterior left, and genotypes are indicated. Moe-GFP reveals F-actin. Brackets, ventral furrow; arrows, mesoderm cells round up to divide and emerge from furrow. Still images from Videos 1 (A), 2 (B), and 3 (C) are shown. (D–F) Graphs show cell cross-sectional areas as ventral furrow invaginates. t = 0, defined as 100%. Wild-type (WT) cells constrict to essentially zero before invaginating, whereas mutant cells disappear in furrow before fully constricting. Bar, 30 µm.
To identify this mechanism, we looked in detail at cytoskeletal rearrangements. The first step is apical recruitment of actin and myosin (Fig. 6, B and H, arrows) in which they assemble into a contractile network (Fig. 6, A–A”; and not depicted); actin is also enriched in a ring at AJs (Fox and Peifer, 2007). In cnoMZ, actin and myosin are recruited to the apical cortex (Fig. 4 L, arrowhead; and Fig. 6 D, arrow). Wild-type constricting cells elongate along the apical–basal axis, and this occurs normally in cnoMZ mutants (Fig. 4 L).
Figure 6.
Cno regulates coupling of AJs to contractile network. Embryos, stage 6–8, antigens, and genotypes are indicated. (A–A’’, C–C’’, G, J, and M–O) Ventral views are shown. (B, D–F’, H, I, and K) Cross sections are shown. (A–A”, B, and E) Wild-type (WT) ventral furrow. Myosin (Myo) covers cell apices (arrows and insets). Constriction coupled to actomyosin contraction. (C–C’’, D, and F–F’’) cnoMZ is shown. Myosin condensed into balls that are not contiguous with AJs (arrows and insets). Cell shape change is not completed. (G) cnoMZ is shown. Myosin balls visualized live with zipper-GFP (Zip-GFP). (H) Wild type is shown. Actin accumulates evenly at the apical surface (arrow). (I) cnoMZ is shown. Actin condenses into balls that are not contiguous with actin at AJs. Constriction arrests (arrow and inset) are shown. (J) cnoMZ is shown. F-actin balls visualized live with moe-GFP. (K) Probable cno maternal mutant. Balls of actin (inset) observed even in embryos initiating invagination. (L) Model of alterations in actin, myosin and constriction in cnoMZ. (M–O) Rap1MZ is shown. (M and N) Similar balls of Myo form and separate from AJs. (O) Balls of actin. Bars: (A–A” and C–C”) 30 µm; (B and D–O) 10 µm.
In wild type, actomyosin constriction begins as soon as myosin arrives apically and is coupled to cell shape change, with AJs moving inward as constriction proceeds (Fig. 6, A–A”). One hypothesis is that Cno regulates the extent of actomyosin constriction, so it does not go to completion in cnoMZ mutants. However, this is not the case; instead, actomyosin constriction initiated correctly (Fig. 4 I) but became uncoupled from cell shape change. In wild type, actomyosin contraction is coupled to reduction in diameter of the cell’s apical end (Fig. 6, A–B, E, and H). In cnoMZ, myosin (Fig. 6, C–C’’, D, and F–F’’) and actin (Fig. 6 I) both coalesced into “balls” at the cell apex, which were not contiguous with AJs (Fig. 6, E vs. F–F’’). To explore dynamic cytoskeletal rearrangements, we used moe-GFP to visualize F-actin (Video 4) and zipper-GFP (myosin heavy chain) to visualize myosin (Video 6). In cnoMZ, balls of both F-actin (Fig. 6 J; Fig. S2, A vs. B; and Video 5) and myosin (Fig. 6 G; Fig. S2, C vs. D; and Video 7) coalesced as invagination proceeded. These data support a model (Fig. 6 L) in which cnoMZ cells apically constrict without fully effective linkage between AJs and the actomyosin network, the contractile network detaches from AJs before full cell constriction, and mesodermal cells are not efficiently internalized.
In contrast, other gastrulation events are more normal. Posterior midgut cells also apically constrict (Sweeton et al., 1991), leading to internalization (Fig. S1 K). cnoMZ mutants successfully internalize the gut (Fig. S1 L), although the midgut epithelium may be less organized (Fig. S1 M). Lateral ectodermal cells intercalate during germband elongation, narrowing the ectoderm in the dorsal–ventral axis and elongating it in the anterior–posterior axis. cnoMZ mutants extend their germbands, and intercalation proceeds normally (some cnoMZ mutants do not extend as far as wild type, but this may be a secondary consequence of ventral furrow failure; Fig. S1, N and O). Intercalation is thought to be driven by opposing planar polarization of myosin and AJ proteins (Fig. S1, P–P”; Bertet et al., 2004; Zallen and Wieschaus, 2004; Blankenship et al., 2006). Ectodermal cells in cnoMZ mutants planar polarize myosin and AJ proteins (Fig. S1, Q–Q”); in fact, planar polarization is even more pronounced than in wild type (Fig. S1, P–P” vs. Q–Q”), and mutants retain accentuated planar polarity through the end of germband extension (Fig. S1, R vs. S).
αCat localizes to actomyosin balls in cnoMZ
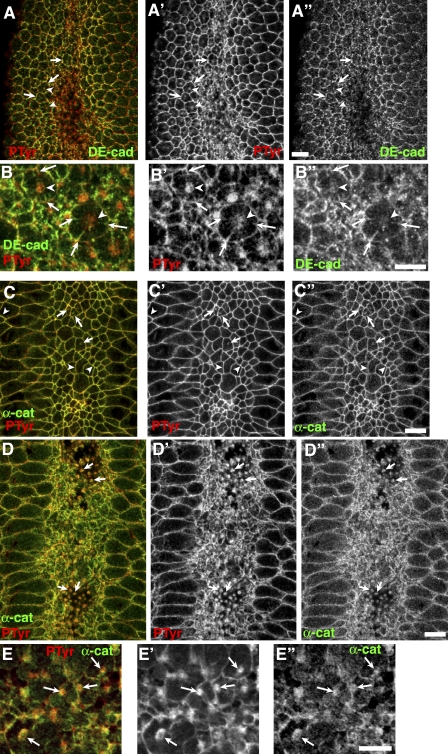
We next looked in detail at the apparent separation of AJs and the apical actomyosin web, examining whether AJ proteins accumulated in actomyosin balls in cnoMZ mutants. We first examined DEcad, a transmembrane protein. The actomyosin balls were apical to AJs (we visualized actomyosin balls with antiphosphotyrosine, as DEcad and phalloidin are not well preserved by the same fixation; Fig. 7, C–C” [sections of the same embryo at AJs] and D–D” [more apical]). DEcad was largely retained in AJs after detachment (Fig. 7, A–A”, arrows) and only weakly localized in actomyosin balls (Fig. 7, A–B”, arrowheads). We sometimes noted strands of DEcad joining balls to AJs (Fig. 7, B–B”, arrows); these were reminiscent of less dramatic deformations of the lateral membrane observed during normal apical constriction (Martin et al., 2009) and may represent points of remaining attachment between AJs and the balls. Ed also did not strongly accumulate in actomyosin balls (unpublished data). In contrast, αcat accumulated at easily detected levels in actomyosin balls (Fig. 7, C–E”, arrows) as well as remaining in AJs (Fig. 7, C–C”, arrowheads). This is consistent with the existence of two pools of αcat, one in AJs and one bound to actin (Drees et al., 2005).
Figure 7.
Pools of αcat at AJs and actomyosin balls. Ventral views, gastrulating cnoMZ, antigens are indicated. (A–B”) DEcad localizes to AJs (arrows) but is only very weakly found in actomyosin balls (arrowheads). Strands of DEcad connect AJs to balls. (C–E”) Apical (C–C”) and more basolateral (D–D”) views of the same embryo. E–E” show close-up views. Pools of αcat at AJs (C–C”, arrowheads) and actomyosin balls (C–E”, arrows). PTyr, phosphotyrosine. Bars, 10 µm.
Cno is enriched at tricellular AJs along with a subset of actin
Cno localizes to AJs in embryos and imaginal discs (Takahashi et al., 1998). However, apical junctions are already complex at their assembly. Bazooka (Baz; fly PAR-3) and DEcad localize apically from cellularization onset (Harris and Peifer, 2004), whereas aPKC, Par6, and Crumbs are recruited to an even more apical position during gastrulation (Tepass, 1996; Hutterer et al., 2004; Harris and Peifer, 2005). AJs initially assemble as spot AJs that do not precisely colocalize with actin and smooth out to form belt AJs during gastrulation.
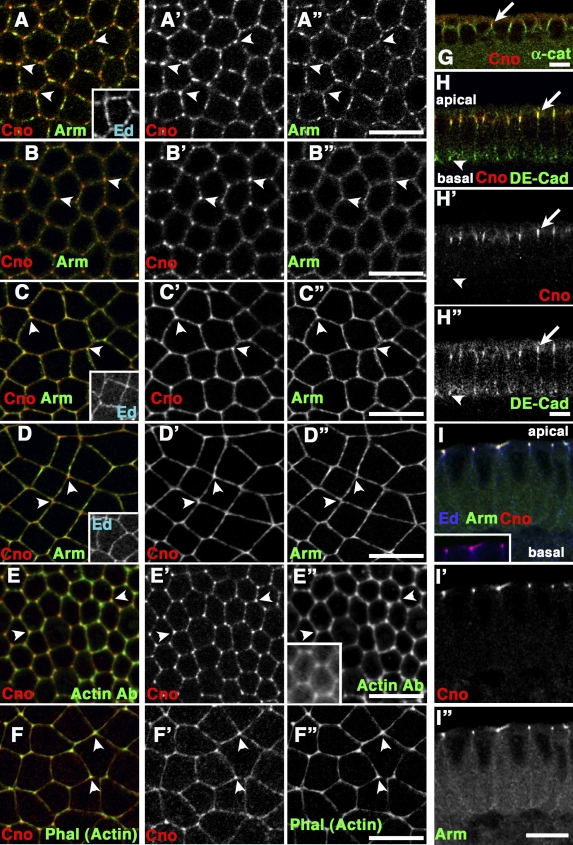
To place Cno in the apical junctional protein network, we examined its localization and explored how it localizes apically. Cno has similarities and differences in localization with AJ proteins. Apical junctions assemble as cells form from the syncytium. As actomyosin furrows ingress, DEcad localizes to basal junctions just behind invaginating actomyosin (Thomas and Williams, 1999; Hunter and Wieschaus, 2000) and also begins to localize to apical junctions, whereas Baz is apical throughout (Harris and Peifer, 2004). Cno also remains apical, colocalizing with DEcad at apical junctions (Fig. 8, H–H”, arrows) but not basal junctions (Fig. 8, H–H”, arrowheads). In fact, like AJ proteins and Baz (McCartney et al., 2001; Harris and Peifer, 2004), Cno is already cortical before cellularization, localizing at apical ends of syncytial furrows (Fig. 8 G, arrow). As embryos gastrulate, DEcad and Baz localize more tightly to apical AJs (Harris and Peifer, 2004), as does Cno (Fig. 8, I–I”). Thus, Cno is part of the apical junctional complex from the start.
Figure 8.
Cno is enriched at tricellular junctions with a subpool of actin. Wild type, antigens are indicated. (A–F”) Surface views are shown. (G–I”) Cross sections are shown. (A–B”) Cellularization is shown. (A–A”) Apically, Cno colocalizes with Arm and Ed (inset) in spot AJs, with enrichment at tricellular junctions (arrowheads). (B–B”) 2 µm more basal, Cno is strongly enriched at tricellular junctions relative to Arm (arrowheads). (C–D”) Mid (C–C”) to late (D–D”) gastrulation. Cno, Arm, and Ed (insets) form belt AJs. Cno remains enriched at tricellular junctions (arrowheads). (E–F”) Cno localizes with a subpool of actin at tricellular junctions (arrowheads) during cellularization (E–E”) and gastrulation (F–F”; E” [inset], actin visualized with moe-GFP). (G) Cno is already apical in the syncytial embryo (arrow). (H–H”) Cno colocalizes with DEcad in apical AJs (arrows) but not basal junctions (arrowheads). (I–I”) Gastrulation. Cno tightly localized at AJs with Arm and Ed. The inset shows Cno and Ed channels alone. Bars, 10 µm.
To get a detailed view of Cno localization, we looked at cells en face. AJs initially form as spot AJs around the apical cortex (Tepass and Hartenstein, 1994). Cno colocalizes at spot AJs apically, with some enrichment at tricellular junctions (Fig. 8, A–A”, arrowheads); however, when we imaged 2 µm more basally, Cno, unlike AJ proteins, is strikingly enriched at tricellular junctions (Fig. 8, B–B”, arrowheads). Intriguingly, a subset of actin is also enriched at tricellular junctions (visualized with antiactin antibody, this is also apparent using moe-GFP; Fig. 8, E [arrowheads] and E” [inset]). As gastrulation begins, spot AJs mature into less punctate belt AJs (Harris and Peifer, 2004). Like AJ proteins and Baz, Cno also becomes more evenly distributed but remains enriched at tricellular junctions, as does actin (actin visualized with phalloidin; Fig. 8, C–D” and F–F”, arrowheads). Thus, Cno is in apical junctions from the start but does not strictly colocalize with AJ proteins and localizes more closely with a subset of cortical actin.
Cno can bind DEcad but is not a core AJ component
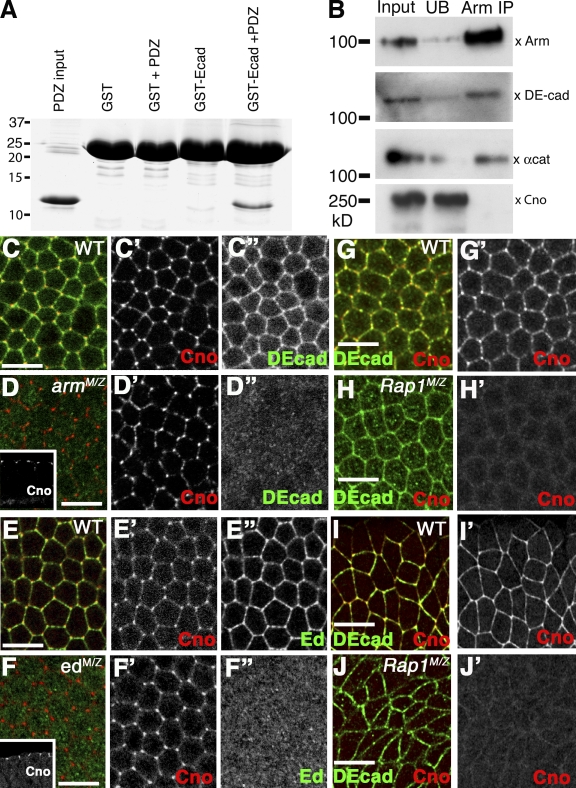
Cno–afadin has known direct interactions with AJ proteins, including nectins/Ed (Takahashi et al., 1999; Wei et al., 2005), αcat (Tachibana et al., 2000; Pokutta et al., 2002), and the tight/AJ protein ZO-1/Pyd (Takahashi et al., 1998; Yokoyama et al., 2001). This suggests that Cno may have multiple, partially redundant interactions with AJs. Cno–afadin interacts with nectins via its PDZ domain (Takahashi et al., 1999; Wei et al., 2005). Ed (ending in the sequence EIIV) and Nectin1 (ending in EWYV) have class II PDZ-binding sites. Interestingly, DEcad also has a putative C-terminal type II PDZ-binding site (ending in the sequence GWRI; matching the consensus XøXø, where ø is any hydrophobic amino acid; Hung and Sheng, 2002) that is strongly conserved in all Diptera, which diverged ∼250 million years ago (Zdobnov et al., 2002). Thus, we tested whether Cno’s PDZ domain can bind the DEcad tail. Purified Cno PDZ domain does not bind GST alone but does bind GST fused at its C terminus to the last seven amino acids of DEcad (Fig. 9 A). These data are consistent with DEcad as a Cno-binding partner. Given this and Cno’s localization to AJs, we explored whether Cno is a core component of the cadherin–catenin complex. DEcad, Arm, and αcat coimmunoprecipitate as a stable complex from embryonic extracts (Fig. 9 B). In contrast, Cno is not detected in these immunoprecipitations (Fig. 9 B), suggesting that it is not in the core complex.
Figure 9.
Rap1 but not AJs or Ed are required for apical Cno recruitment. (A) Purified Cno PDZ domain incubated with GST or GST fused to C-terminal seven amino acids of DEcad. Input, 1% of load; bound, 10% of bound fraction. (B) Embryonic extracts immunoprecipitated with anti-Arm. Input, unbound (UN), and immunoprecipitation (IP) fractions immunoblotted with the indicated antibodies. (C–J’) Antigens and genotypes are indicated. Apical surface is shown except for insets in D and F, which show cross sections. (C–H’) Late cellularization. (I–J’) Early gastrulation. (C–F”) Removing AJs (C–D”; arm043A01) or Ed (E–F”; edF72) does not affect Cno localization. (G–J’) Removing Rap1 reduces cortical Cno. WT, wild type. Bars, 10 µm.
Cno apical recruitment requires F-actin but not AJs or Ed
This raises questions about mechanisms by which Cno is recruited to and maintained at AJs. We first considered the hypothesis that cadherin–catenin complexes recruit Cno because Cno–afadin can bind both αcat (Pokutta et al., 2002) and DEcad (Fig. 9 A). To test this, we made armMZ mutants, in which both DEcad and αcat are lost from the cortex (Fig. 9, D–D”; Cox et al., 1996; Dawes-Hoang et al., 2005), disrupting AJs. Surprisingly, Cno localizes normally in armMZ mutants (Fig. 9, C’ vs. D’). This suggests that Cno has other means of reaching the cortex.
We next tested the hypothesis that Cno is recruited by Ed. Cno is mislocalized in ed mutant wing disc cells, suggesting that Ed helps localize Cno to AJs (Wei et al., 2005). Ed localizes to spot AJs and transitions to belt AJs (Fig. 8, A, C, D, and I, insets). Cno localized normally in edMZ mutants (Fig. 9, E’ vs. F’), which is consistent with the observation that edMZ mutants do not have morphogenetic defects until dorsal closure (Laplante and Nilson, 2006; Lin et al., 2007). Thus, although Cno binds Ed, Cno has other ways to localize to AJs in embryos.
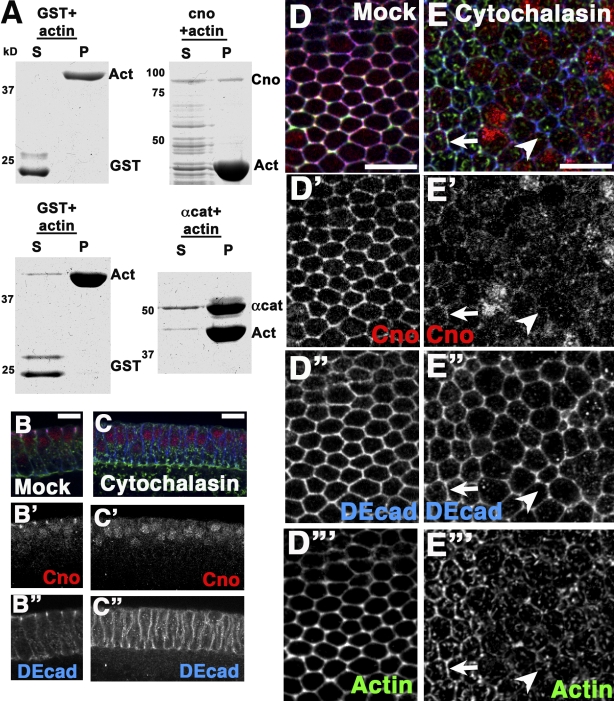
Baz, which also localizes to apical junctions independently of AJs, is positioned apically by cytoskeletal cues, including binding an apical actin-based scaffold (Harris and Peifer, 2004, 2005). Afadin is an F-actin–binding protein (Mandai et al., 1997). Thus, we examined whether Cno could directly bind F-actin like afadin. We fused GST to the C-terminal 491 aa of Cno, which shares sequence conservation with afadin’s F-actin–binding site, and performed actin sedimentation assays to determine whether Cno directly associates with F-actin. GST alone was a negative control, and GST-αcat (aa 671–906) was a positive control (Pokutta et al., 2002). Little GST pelleted with F-actin, as most remains in the supernatant (11% pelleted; mean of six experiments; Fig. 10 A). GST-αcat pelleted with F-actin (84% pelleted; mean of three experiments; Fig. 10 A). GST-Cno (aa 1,560–2,051) also pelleted with F-actin (41% pelleted; mean of four experiments; Fig. 10 A) to a degree similar to afadin (Lorger and Moelling, 2006), suggesting that Cno can directly bind F-actin.
Figure 10.
F-actin is required for Cno cortical localization. (A) Actin (Act) cosedimentation assays of GST-CnoCT, GST-αcat, and GST as a negative control are shown. S, supernatant; P, high speed pellet. (B–B” and D–D”’) DMSO-treated controls. (B–B”) DEcad at apical and basal junctions. Cno only at apical junctions. (C–C” and E–E”’) Cytochalasin treated. (C–C”) After depolymerization, DEcad all along lateral cortex. Cno cytoplasmic and nuclear. (D–D”’) Normal DEcad, Cno, and actin localization. (E–E”’) Actin depolymerized, some residual cortical actin in cells at left (arrows). DEcad remains cortical. Cno lost from cortex (arrowheads) except where residual cortical actin remains. Bars, 10 µm.
Cno’s ability to bind actin and its colocalization with a subpool of actin at tricellular junctions suggested the hypothesis that Cno is recruited apically by an actin-based scaffold. To test this, we examined Cno localization after depolymerizing actin with cytochalasin. When actin is depolymerized at the end of cellularization, DEcad remains cortical but distributes all along the apical–basal axis (Fig. 10, B” vs. C”; Harris and Peifer, 2005). Strikingly, Cno is lost from the cortex and accumulates in the cytoplasm or nucleus (residual cortical Cno was present in cells where some cortical actin remained; Fig. 10, C–C” and E–E’” [arrows]). We saw similar effects in extended germband embryos (Fig. S3). These data suggest that Cno is recruited/retained at the cortex at least in part by interacting with the cortical actin cytoskeleton.
Rap1 is essential for mesoderm invagination and Cno cortical recruitment
Both afadin and Cno bind the small GTPase Rap1, and this is thought to activate Cno during dorsal closure (Boettner et al., 2000, 2003). Thus, we examined whether Rap1 also works with Cno during mesoderm invagination by generating Rap1MZ mutants using the null allele Rap1CD3 (deleting the entire coding region; Asha et al., 1999). Previous work suggested that Rap1 plays a role in gastrulation, as midline cells, which meet at the ventral midline after gastrulation, did not do so in Rap1MZ (Asha et al., 1999). We extended this analysis. Loss of MZ Rap1 disrupts ventral cuticle (Fig. 1 J), and Twist-positive mesoderm remained on the surface after gastrulation (Fig. 4 J) as in cnoMZ. In some Rap1MZ mutants, the germband became twisted during gastrulation (Fig. 4 K), as is seen in mutants like fog that disrupt invagination of both mesoderm and the posterior midgut (Sweeton et al., 1991).
To further examine parallels between Rap1MZ and cnoMZ mutants, we examined localization of AJ and cytoskeletal proteins. Initial AJ assembly was normal in Rap1MZ (Fig. S4, A vs. B) as in cnoMZ (Fig. 2, A–D’). However, as in cnoMZ, coupling between actomyosin constriction and cell shape change was disrupted in Rap1MZ. Balls of actin (Fig. 6 O) and myosin (Fig. 6, M and N) appeared at the apical surface of mesodermal cells, and cell constriction halted prematurely, with myosin balls not contiguous with AJs (Fig. 6 N). These data are consistent with Cno and Rap1 acting together in this process.
Cno binds Rap1, and epistasis analysis suggests that Rap1 acts upstream of Cno in dorsal closure (Boettner et al., 2003). Thus, we explored whether Rap1 regulates Cno recruitment to AJs. We examined Cno localization during cellularization and early gastrulation in Rap1MZ mutants. Cno recruitment to the cortex was substantially reduced at cellularization and early gastrulation (Fig. 9, G–J’). This suggests that Rap1 binding plays an important role in Cno cortical recruitment.
We also explored Rap1 localization using GFP-Rap1 driven by its endogenous promoter (Knox and Brown, 2002) to see whether its localization was consistent with a role in recruiting Cno to AJs. During cellularization, GFP-Rap1 accumulated in the cytoplasm in a large structure just above nuclei (Fig. S4 C, arrowheads) and all along the lateral cell cortex from apical junctions (Fig. S4 C, arrows) to the basal end (Fig. S4 C, inset). GFP-Rap1 remained cortically enriched during gastrulation (Fig. S4, E and H). Interestingly, in apically constricting cells of the posterior midgut, although GFP-Rap1 is found all along lateral membranes (Fig. S4, G and G’, arrows), it accumulates at elevated levels in a region overlapping the AJs (Fig. S4, G and G’, arrowheads). We next examined whether Cno is required for GFP-Rap1 cortical localization. We saw no differences in GFP-Rap1 localization in wild type or cnoMZ (Fig. S4, D, F, and H–I’), which is consistent with Rap1 acting upstream of Cno in the pathway.
Discussion
AJs mediate cell adhesion and anchor and regulate the underlying actin cytoskeleton. We have a working model for how cadherin–catenin complexes regulate these events, but less is known about the parallel system of nectins and the linker Cno–afadin. Studies in mammalian cells and embryos largely focus on a model in which the nectin–afadin complex is critical for cell adhesion, working in parallel with cadherin–catenins (see Introduction). In contrast, studies of Drosophila Cno suggest that it is a scaffold for signal transduction (see Introduction). We completely removed MZ Cno, allowing us to assess the consequences of complete loss of function from the onset of embryogenesis and to explore Cno’s mechanism of action.
Cno is not essential for AJ assembly or maturation
Work in cultured mammalian cells using nectin misexpression or dominant-negative approaches led to the model that nectin–afadin complexes play a key role in cell adhesion, recruiting cadherins to nascent AJs (Tachibana et al., 2000; Honda et al., 2003). However, multiple nectins made genetic tests of this hypothesis problematic. Afadin knockout in mice resulted in defects at and after gastrulation and subsequent lethality (Ikeda et al., 1999; Zhadanov et al., 1999). However, defects occurred much later than those caused by loss of core AJ proteins (Larue et al., 1994; Torres et al., 1997). Thus, the mouse data suggested that loss of zygotic afadin does not disrupt adhesion to the same degree as loss of cadherin–catenin; however, as these embryos retained maternal afadin, an essential role for afadin in adhesion and epithelial integrity remained possible.
We tested whether Cno is essential for AJ assembly or maintenance by completely removing MZ Cno from the onset of fly embryogenesis. The results were striking. Initial assembly of cadherin–catenin-based AJs, establishment of epithelial cell polarity, and organization of apical actin were all normal in Cno-deficient embryos. Furthermore, the first step in AJ maturation, coalescence of spot AJs into belt AJs underlain by actin, was completed on schedule, unlike what was observed in afadin knockdown MDCK cells (Sato et al., 2006). These results are in strong contrast to loss of Arm, which disrupts all these events (Cox et al., 1996; Müller and Wieschaus, 1996). Thus, Cno is not essential for AJ assembly or initial maturation. Furthermore, many tissues maintained normal AJs and architecture through late embryogenesis, suggesting that Cno is not essential for AJ maintenance per se or essential to maintain actin–AJ connections in nonmorphogenetically active tissues, as these are essential for AJ integrity (Quinlan and Hyatt, 1999). Differences between our work and that in cultured mammalian cells could reflect differences in assembly and regulation of AJs in insects and mammals. However, they suggest further exploration of whether afadin is essential for AJ assembly in mammals is warranted; e.g., generating afadin-null epithelial cells or maternally mutant mice.
Loss of Cno does affect maintenance of tissue architecture in a subset of cells. Many cells in the neurogenic ectoderm lost columnar shape, and membrane DEcad was reduced. This coincided with two morphogenetic events: a series of cell divisions and invagination of a subset of cells to form the central nervous system. Both involve significant AJ remodeling, and thus, the ventral epidermis is particularly susceptible to reducing DEcad levels (Tepass et al., 1996; Uemura et al., 1996). The neuroepithelium is also the tissue most susceptible to afadin loss in mice (Ikeda et al., 1999; Zhadanov et al., 1999), perhaps because of similarly dynamic cell behavior. It will be interesting to explore Cno’s role in this morphogenetically active tissue in more detail, using genetic approaches to block cell division or neuroblast invagination; the latter alleviates effects of reducing DEcad (Tepass et al., 1996). It will also be interesting to explore mechanisms by which Cno acts; e.g., it may regulate cadherin trafficking as suggested in mammalian cells (Hoshino et al., 2005) or it may help cells reassume a columnar shape by regulating connections between cadherin–catenin and actin.
A role for Cno in regulating AJ–actin linkage
Cross talk between AJs and actin is critical in many contexts from maintaining stable adhesion to mediating morphogenesis (Gates and Peifer, 2005). The classical view of AJs postulated direct connection between cadherin–catenin complexes and actin mediated by αcat. However, recent work undermined this idea (Drees et al., 2005; Yamada et al., 2005), raising the question of how actin is connected to AJs and causing some to question whether such a connection even occurs. One morphogenetic event that compellingly suggests that AJs are connected to actin is apical constriction, during which constriction of the apical actomyosin web is coupled to shape change (Fig. S5 A, top). Disrupting AJs uncouples these events (Dawes-Hoang et al., 2005), supporting the need for a connection, but the nature of the link was unclear.
The phenotype of cno mutants is consistent with Cno playing a critical role in this connection. In its absence, AJs assemble normally, actin and myosin accumulate apically, and apical constriction initiates. However, cell constriction halts before completion, whereas cytoskeletal constriction continues, uncoupling these events (Fig. S5 A, bottom).
Our data are consistent with several models for Cno in this process. The first step in all is Cno recruitment to the cortex. To our surprise, this is not dependent on either the cadherin–catenin complex or the nectin Ed, although we cannot rule out a redundant role for them. Instead, the GTPase Rap1 is critical. One speculative possibility is that Rap1 binding the RA domains opens up a closed conformation, as is seen, for example, in formins (Fig. S5, B–D). Thus, Rap1 recruitment of Cno to the cortex could also activate it, allowing it to interact with other partners. At least one partner is F-actin. Consistent with this, Cno, like afadin, can bind F-actin, and the actin cytoskeleton plays a critical role in cortical Cno localization.
Once Cno is recruited apically by Rap1 and actin, it could then help stabilize links between actomyosin and AJs in several ways. It might be a direct link, binding actin and interacting by multiple redundant and low affinity interactions with several AJ proteins (Fig. S5 E). Cno–afadin has well-documented direct interactions with nectins, αcat, and ZO-1, and we documented a direct interaction of its PDZ domain with DEcad. Alternately, Cno may regulate interactions more indirectly. It is intriguing that αcat acts later during germband elongation in linking a stable population of F-actin at spot AJs with the larger cortical actin network (Cavey et al., 2008). Our observation that αcat is strongly enriched in actin balls that detach from AJs in Cno’s absence, while also remaining at AJs, is consistent with αcat acting on both sides of the linkage. Cno may regulate interactions between junctional and actin-bound pools of αcat either directly or acting as a scaffold to recruit another regulator (Fig. S5 F). It will be important to test these models; the new Drosophila αcat mutants (unpublished data; U. Tepass, personal communication) will help, as will two-color simultaneous imaging of F-actin and AJs. It will also be important to further analyze Cno’s actin-binding domain by site-directed mutagenesis. Other models for Cno function remain possible. Dictyostelium Rap1 regulates myosin disassembly during cell motility (Jeon et al., 2007), and activated myosin can activate Rap1 (Arora et al., 2008). For example, Cno–Rap1 might regulate actomyosin contractility, and in its absence, apical actomyosin might become hypercontractile. We did not observe any acceleration of cell constriction as might be expected from the simplest versions of this model (Fig. 5, D–F). However, Cno–Rap1 regulation of myosin remains an open possibility.
Regardless of the mechanism, Cno’s enrichment at tricellular junctions along with a subpopulation of actin suggests the possibility that these structures might have a special role in AJ–actin connections. Intriguingly, mouse tricellulin plays a special role at tricellular junctions in maintaining tight junctions (Ikenouchi et al., 2005). However, our analysis and that of Martin et al. (2009) suggest that all spot AJs maintain connection to the apical actin web during normal constriction and disconnection in cno mutants.
It will be interesting to explore how forces are generated in the apical cortex, how contractility is regulated, and how and where the contractile network is coupled to AJs. Constriction in the Drosophila ventral furrow is rhythmic, suggesting a racheting mechanism (Martin et al., 2009). This resembles what is seen in the one-cell Caenorhabditis elegans embryo (Munro et al., 2004). Another striking thing about the ventral furrow is that cells do not constrict isometrically but instead constrict more quickly in the dorsal–ventral dimension than in the anterior–posterior dimension (Fig. 4 F and Fig. 5 A). This bias seems less pronounced in cnoMZ mutants (Fig. 4 I and Fig. 5 C), perhaps suggesting a requirement for cortex–AJ connections to maintain asymmetric cell constriction.
Mammalian afadin plays a role in epithelial wound healing; in its absence, cells migrate into wounds more rapidly (Lorger and Moelling, 2006). Although afadin knockdown did not affect stable AJs, it reduced AJ association with the cytoskeleton after wounding, reducing adhesion and increasing directionality of cell migration. This function required afadin’s actin-binding domain, providing a second context in which Cno–afadin may help link AJs and actin.
However, Cno is not critical for all actin–AJ connections. Cadherin-based adhesion itself, which does not require Cno, involves actin–AJ interactions (Quinlan and Hyatt, 1999). Likewise, conversion of spot AJs to belt AJs, which involves connections to actin (Maddugoda et al., 2007; Cavey et al., 2008), does not require Cno. Loss of Cno also did not halt germband extension, which involves reciprocal planar polarization of myosin and AJs. However, Cno may play a restraining role in this process, as planar polarity is enhanced in cnoMZ mutants. This is interesting, as actin depolymerization also enhanced AJ planar polarity (Harris and Peifer, 2007), suggesting that AJ–actin connections restrain planar polarity. Perhaps in Cno’s absence, subtle uncoupling of AJs from actin occurs.
Thus, we hypothesize that Cno is one aspect of regulation of AJ–actin linkage. However, this linkage will be complex, with different proteins mediating interactions in different circumstances. The mammal-specific protein Eplin regulates maturation/remodeling of AJ–actin connections during AJ assembly (Abe and Takeichi, 2008). Likewise, αcat regulates lateral mobility of AJ complexes (Cavey et al., 2008) and myosin VI acting with vinculin, and Cno–afadin-binding partners in the ZO-1 family also regulate maturation of belt junctions (Ikenouchi et al., 2007; Maddugoda et al., 2007). Perhaps different proteins evolved to respond to distinct forces exerted on AJs, differing either in magnitude or acceleration. Our challenge is to identify all proteins regulating AJ–actin connections and to determine their mechanisms of action.
Materials and methods
Fly stocks
Mutations are described at http://flybase.org. Wild type was yellow white or Histone-GFP. All experiments were performed at 25°C unless noted otherwise. cnoR2 was generated by ethane methyl sulfonate on an isogenic FRT82B line. cnoR2 was sequenced by PCR amplifying fragments of the cno coding sequence and sequencing them at the University of North Carolina at Chapel Hill Genome Analysis Facility. Cuticle preparations were made as described previously in Wieschaus and Nüsslein-Volhard (1986). Unless noted otherwise, fly stocks were obtained from the Bloomington Stock Center. Sources of other stocks are provided in Table S1. cno germline clones were made by heat shocking 48–72-h-old hsFLP1; FRT82BcnoR2/FRT82BovoD1-18 larvae for 3 h at 37°C. arm043A01 and edF72 germline clones were generated similarly.
Immunofluorescence and image aquisition
The following fixations were used: myosin/Arm/Cno/Ed, heat methanol (Müller and Wieschaus, 1996), phalloidin/Dcad2 for 10 min, 10% formaldehyde, phalloidin for 5 min, and 37% formaldehyde. All others were fixed as described previously in Grevengoed et al. (2001). Embryos were methanol devitillinized or hand devitillinized for phalloidin. Embryo cross sections were performed as described previously (Dawes-Hoang et al., 2005). For drug treatments, dechorinated embryos were washed twice with 0.9% NaCl and incubated for 30 min in 1:1 octane/0.9% NaCl with 10 µg/ml cytochalasin D (dissolved in DMSO; Sigma-Aldrich). Control embryos were treated with DMSO carrier alone. Embryos were fixed immediately after drug treatment (Harris and Peifer, 2005). All embryos were blocked/stained in PBS/1% goat serum/0.1% Triton X-100 and mounted in Aqua-Polymount (Polysciences). Table S1 lists the antibodies and probes used. All images and videos were acquired at RT. Fixed samples were imaged with confocal microscopes (LSM510 or Pascal; Carl Zeiss, Inc.) using a 40× NA 1.3 Plan Neofluar oil immersion objective (Carl Zeiss, Inc.) and LSM software (Carl Zeiss, Inc.). Live imaging was performed using a spinning-disc confocal microscope (UltraVIEW; PerkinElmer), a digital camera (ORCA-ER; Hamamatsu Photonics), a 40× NA 1.3 Plan Fluor oil immersion objective (Nikon), and Metamorph software (MDS Analytical Technologies). Photoshop (CS2; Adobe) was used to adjust input levels, so the main range of signals spanned the entire output gray scale, and to adjust brightness and contrast.
Vector construction, protein expression, and protein purification
GST-αcat (aa 671–906) was provided by S. Pokutta and B. Weis (Stanford University, Stanford, CA; Pokutta et al., 2002). The Cno–C terminus (aa 1,560–2,051) fragment was amplified by PCR and cloned into pGEX (GE Healthcare). The Cno-PDZ (aa 833–929) fragment was amplified by PCR and cloned into pET28 (EMD). GST-Ecad (GST-DDQGWRI) was amplified by PCR and cloned into pET28. GST fusion constructs in the pGEX vector were expressed in Escherichia coli BL21-Gold (DE3) cells (Agilent Technologies). Bacteria were grown in LB+ media with 100 µg/ml ampicillin at 37°C to OD600 between 0.8 and 1.0, induced with 1 mM isopropyl-g-d-thiogalactopyranoside, and grown for 3 h at 37°C. Pelleted cells were resuspended in 20 mM Tris, pH 8.0, 200 mM NaCl, 1 mM EGTA, 1% Triton X-100, 0.1 mM PMSF, and a protease inhibitor cocktail (Roche) and lysed using a microfluidizer. The lysate was cleared by centrifugation and incubated with glutathione agarose (GE Healthcare) O/N at 4°C. GST fusion proteins were purified over 20-mL columns (Bio-Rad Laboratories) and were either kept on beads for subsequent manipulations or eluted with 20 mM Tris, pH 8.0, 200 mM NaCl, and 10 mM glutathione (Sigma-Aldrich). Constructs in the pET-28 vector (H6-CnoPDZ and H6-GST-Ecad) were expressed in E. coli BL21-Gold (DE3) cells (Agilent Technologies). Bacteria were grown in LB+ media with 20 µg/ml kanamycin at 37°C to OD600 between 0.8 and 1.0, induced with 1 mM isopropyl-g-d-thiogalactopyranoside, and grown for 3 h at 37°C. Pelleted cells were resuspended in 25 mM Tris, pH 8.0, 300 mM NaCl, 10 mM imidazole, 1% β-mercaptoethanol, and 0.1 mM PMSF and lysed using a microfluidizer. The lysate was cleared by centrifugation and incubated with Ni2+-NTA agarose (QIAGEN) for 3 h at 4°C. The columns were washed with 20-column volumes of lysis buffer and bound protein step eluted using 3-column volumes of lysis buffer supplemented with 285 mM imidazole.
Actin sedimentation assay
Rabbit skeletal muscle actin (Cytoskeleton, Inc.) was stored in 5 mM Tris, pH 8.0, 0.2 mM CaCl2, 0.5 mM DTT, and 0.2 mM ATP at 0.4 mg/ml. Either 1 or 5 µM actin was used. Aliquots of 156.25 µL were polymerized with 3.2 µL 50× polymerization buffer (2.5 M KCl, 100 mM MgCl2, 50 mM ATP, and protease inhibitor cocktail) for 1 h at RT. GST fusion proteins were precleared by centrifugation for 7 min at 436,000 g at 4°C (100 tubes; TLA-100 rotor; Beckman Coulter). Precleared GST fusion protein (final concentrations of 5 or 2 µM) was added to polymerized F-actin and incubated for 30 min at RT. Proteins bound to F-actin were separated from unbound protein by centrifugation for 7 min at 436,000 g at 4°C. Sample buffer was added to supernatant and pellet fractions, boiled, and loaded on a 10% polyacrylamide gel. Gels were stained with Coomassie brilliant blue.
GST pull-downs
50 µl glutathione beads were saturated with GST or GST-Ecad then washed using wash buffer (25 mM Tris, pH 8.0, 300 mM NaCl, and 0.1% β-mercaptoethanol). GST- and GST-Ecad–bound beads were incubated in batch with 1 ml purified Cno-PDZ, nutating at 4°C for 30 min. Resin was pelleted, and supernatant containing nonbound Cno-PDZ was removed. Beads were washed twice in batch using 1 ml wash buffer. Proteins were eluted from the beads using 100 µl of wash buffer supplemented with 50 mM glutathione. 10 µl of the eluate was loaded on a 20% polyacrylamide gel, as was 10 µl of the Cno-PDZ load. Gels were stained with Coomassie brilliant blue.
Protein preparation and immunoprecipitations
Protein samples were prepared by grinding dechorionated embryos on ice in Laemmli buffer with a plastic pestle and boiled for 5 min. Immunoprecipitations were performed as described previously in Harris and Peifer (2005). Samples were separated by 6% SDS-PAGE and immunoblotted (see Table S1 for antibody concentrations). Signal was detected using ECL Plus (GE Healthcare).
Online supplemental material
Fig. S1 shows that Cno is not required for the transition from spot to belt AJs, posterior midgut invagination, and that it is not essential for intercalation but restrains planar polarity during germband extension. Fig. S2 shows that the actomyosin cytoskeleton becomes uncoupled from cell shape change in cnoMZ mutants. Fig. S3 shows that actin is required to retain Cno at the cortex after gastrulation. Fig. S4 shows that GFP-Rap1 localization overlaps AJs and does not require Cno function. Fig. S5 shows models for Cno function. Video 1 shows wild-type ventral furrow formation, moe-GFP. Video 2 shows a mild cnoMZ mutant ventral furrow phenotype, moe-GFP. Video 3 shows a severe cnoMZ mutant ventral furrow phenotype, moe-GFP. Video 4 shows wild-type ventral furrow formation, moe-GFP. Video 5 shows a cnoMZ mutant ventral furrow phenotype highlighting the actin balls, moe-GFP. Video 6 shows wild-type ventral furrow formation, zipper-GFP. Video 7 shows a cnoMZ mutant ventral furrow phenotype highlighting the myosin balls, zipper-GFP. Table S1 provides genetic and antibody reagents used in this paper.
Acknowledgments
We thank the Bloomington Drosophila Stock Center, the Developmental Studies Hybridoma Bank, D. Kiehart, E. Wieschaus, L. Nilson, S. Roth, C. Field, G. Rogers, A. Fanning, S. Pokutta, and W. Weis for reagents, and we especially thank K. Takahashi for the generous gift of Cno antibody. We thank members of the Gaul laboratory, including J. Fak, P. Harjes, M. Heke, D. Leaman, and B. Boettner for help in generating FRT82 cnoR2, E. Jezuit and D. Meardon for technical assistance, A. Fanning for many informative discussions, and B. Goldstein, G. Shemer, D. Roberts, and J. Sawyer for critiques.
This work was supported by the National Institutes of Health (grant RO1GM47857 and grant 5 T32 HD046369 to J.K. Sawyer). J.K. Sawyer was supported by an American Heart Association predoctoral fellowship. The screen for cno alleles was supported by a grant (RPG-00-237-01-CSM to U. Gaul) from the American Cancer Society.
Footnotes
Abbreviations used in this paper: αcat, α-catenin; AJ, adherens junction; Arm, Armadillo; Baz, Bazooka; Cno, Canoe; Ecad, E-cadherin; Ed, Echinoid; moe-GFP, moesin-GFP; MZ, maternal and zygotic; Pyd, Polychaetoid; RA, Ras association; ZO-1, zona occludens-1.
References
- Abe K., Takeichi M. 2008. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt.Proc. Natl. Acad. Sci. USA. 105:13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora P.D., Conti M.A., Ravid S., Sacks D.B., Kapus A., Adelstein R.S., Bresnick A.R., McCulloch C.A. 2008. Rap1 activation in collagen phagocytosis is dependent on nonmuscle myosin II-A.Mol. Biol. Cell. 19:5032–5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asha H., de Ruiter N.D., Wang M.G., Hariharan I.K. 1999. The Rap1 GTPase functions as a regulator of morphogenesis in vivo.EMBO J. 18:605–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett K., Leptin M., Settleman J. 1997. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation.Cell. 91:905–915 [DOI] [PubMed] [Google Scholar]
- Bertet C., Sulak L., Lecuit T. 2004. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation.Nature. 429:667–671 [DOI] [PubMed] [Google Scholar]
- Blankenship J.T., Backovic S.T., Sanny J.S., Weitz O., Zallen J.A. 2006. Multicellular rosette formation links planar cell polarity to tissue morphogenesis.Dev. Cell. 11:459–470 [DOI] [PubMed] [Google Scholar]
- Boettner B., Govek E.E., Cross J., Van Aelst L. 2000. The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin.Proc. Natl. Acad. Sci. USA. 97:9064–9069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettner B., Harjes P., Ishimaru S., Heke M., Fan H.Q., Qin Y., Van Aelst L., Gaul U. 2003. The AF-6 homolog canoe acts as a Rap1 effector during dorsal closure of the Drosophila embryo.Genetics. 165:159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmena A., Speicher S., Baylies M. 2006. The PDZ protein Canoe/AF-6 links Ras-MAPK, Notch and Wingless/Wnt signaling pathways by directly interacting with Ras, Notch and Dishevelled.PLoS ONE. 1:e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavey M., Rauzi M., Lenne P.F., Lecuit T. 2008. A two-tiered mechanism for stabilization and immobilization of E-cadherin.Nature. 453:751–756 [DOI] [PubMed] [Google Scholar]
- Chou T.B., Noll E., Perrimon N. 1993. Autosomal P[ovoD1] dominant female-sterile insertions in Drosophila and their use in generating germ-line chimeras.Development. 119:1359–1369 [DOI] [PubMed] [Google Scholar]
- Cox R.T., Kirkpatrick C., Peifer M. 1996. Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophila embryogenesis.J. Cell Biol. 134:133–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes-Hoang R.E., Parmar K.M., Christiansen A.E., Phelps C.B., Brand A.H., Wieschaus E.F. 2005. folded gastrulation, cell shape change and the control of myosin localization.Development. 132:4165–4178 [DOI] [PubMed] [Google Scholar]
- Drees F., Pokutta S., Yamada S., Nelson W.J., Weis W.I. 2005. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly.Cell. 123:903–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox D.T., Peifer M. 2007. Abelson kinase (Abl) and RhoGEF2 regulate actin organization during cell constriction in Drosophila.Development. 134:567–578 [DOI] [PubMed] [Google Scholar]
- Fukuhara A., Irie K., Yamada A., Katata T., Honda T., Shimizu K., Nakanishi H., Takai Y. 2002. Role of nectin in organization of tight junctions in epithelial cells.Genes Cells. 7:1059–1072 [DOI] [PubMed] [Google Scholar]
- Gates J., Peifer M. 2005. Can 1000 reviews be wrong? Actin, alpha-Catenin, and adherens junctions.Cell. 123:769–772 [DOI] [PubMed] [Google Scholar]
- Gatfield D., Unterholzner L., Ciccarelli F.D., Bork P., Izaurralde E. 2003. Nonsense-mediated mRNA decay in Drosophila: at the intersection of the yeast and mammalian pathways.EMBO J. 22:3960–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevengoed E.E., Loureiro J.J., Jesse T.L., Peifer M. 2001. Abelson kinase regulates epithelial morphogenesis in Drosophila.J. Cell Biol. 155:1185–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbleib J.M., Nelson W.J. 2006. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis.Genes Dev. 20:3199–3214 [DOI] [PubMed] [Google Scholar]
- Harris T.J., Peifer M. 2004. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila.J. Cell Biol. 167:135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T.J., Peifer M. 2005. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila.J. Cell Biol. 170:813–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T.J., Peifer M. 2007. aPKC controls microtubule organization to balance adherens junction symmetry and planar polarity during development.Dev. Cell. 12:727–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Shimizu K., Kawakatsu T., Yasumi M., Shingai T., Fukuhara A., Ozaki-Kuroda K., Irie K., Nakanishi H., Takai Y. 2003. Antagonistic and agonistic effects of an extracellular fragment of nectin on formation of E-cadherin-based cell-cell adhesion.Genes Cells. 8:51–63 [DOI] [PubMed] [Google Scholar]
- Hoshino T., Sakisaka T., Baba T., Yamada T., Kimura T., Takai Y. 2005. Regulation of E-cadherin endocytosis by nectin through afadin, Rap1, and p120ctn.J. Biol. Chem. 280:24095–24103 [DOI] [PubMed] [Google Scholar]
- Hung A.Y., Sheng M. 2002. PDZ domains: structural modules for protein complex assembly.J. Biol. Chem. 277:5699–5702 [DOI] [PubMed] [Google Scholar]
- Hunter C., Wieschaus E. 2000. Regulated expression of nullo is required for the formation of distinct apical and basal adherens junctions in the Drosophila blastoderm.J. Cell Biol. 150:391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutterer A., Betschinger J., Petronczki M., Knoblich J.A. 2004. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis.Dev. Cell. 6:845–854 [DOI] [PubMed] [Google Scholar]
- Ikeda W., Nakanishi H., Miyoshi J., Mandai K., Ishizaki H., Tanaka M., Togawa A., Takahashi K., Nishioka H., Yoshida H., et al. 1999. Afadin: a key molecule essential for structural organization of cell–cell junctions of polarized epithelia during embryogenesis.J. Cell Biol. 146:1117–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenouchi J., Furuse M., Furuse K., Sasaki H., Tsukita S., Tsukita S. 2005. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells.J. Cell Biol. 171:939–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenouchi J., Umeda K., Tsukita S., Furuse M., Tsukita S. 2007. Requirement of ZO-1 for the formation of belt-like adherens junctions during epithelial cell polarization.J. Cell Biol. 176:779–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon T.J., Lee D.J., Merlot S., Weeks G., Firtel R.A. 2007. Rap1 controls cell adhesion and cell motility through the regulation of myosin II.J. Cell Biol. 176:1021–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens G., Wieschaus E., Nüsslein-Volhard C., Kluding H. 1984. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster.Rouxs Arch. Dev. Biol. 193:283–295 [DOI] [PubMed] [Google Scholar]
- Knox A.L., Brown N.H. 2002. Rap1 GTPase regulation of adherens junction positioning and cell adhesion.Science. 295:1285–1288 [DOI] [PubMed] [Google Scholar]
- Kofron M., Spagnuolo A., Klymkowsky M., Wylie C., Heasman J. 1997. The roles of maternal alpha-catenin and plakoglobin in the early Xenopus embryo.Development. 124:1553–1560 [DOI] [PubMed] [Google Scholar]
- Komura H., Ogita H., Ikeda W., Mizoguchi A., Miyoshi J., Takai Y. 2008. Establishment of cell polarity by afadin during the formation of embryoid bodies.Genes Cells. 13:79–90 [DOI] [PubMed] [Google Scholar]
- Kooistra M.R., Dube N., Bos J.L. 2007. Rap1: a key regulator in cell-cell junction formation.J. Cell Sci. 120:17–22 [DOI] [PubMed] [Google Scholar]
- Laplante C., Nilson L.A. 2006. Differential expression of the adhesion molecule Echinoid drives epithelial morphogenesis in Drosophila.Development. 133:3255–3264 [DOI] [PubMed] [Google Scholar]
- Larue L., Ohsugi M., Hirchenhain J., Kemler R. 1994. E-cadherin null mutant embryos fail to form a trophectoderm epithelium.Proc. Natl. Acad. Sci. USA. 91:8263–8267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.P., Chen H.M., Wei S.Y., Chen L.Y., Chang L.H., Sun Y.J., Huang S.Y., Hsu J.C. 2007. Cell adhesion molecule Echinoid associates with unconventional myosin VI/Jaguar motor to regulate cell morphology during dorsal closure in Drosophila.Dev. Biol. 311:423–433 [DOI] [PubMed] [Google Scholar]
- Linnemann T., Geyer M., Jaitner B.K., Block C., Kalbitzer H.R., Wittinghofer A., Herrmann C. 1999. Thermodynamic and kinetic characterization of the interaction between the Ras binding domain of AF6 and members of the Ras subfamily.J. Biol. Chem. 274:13556–13562 [DOI] [PubMed] [Google Scholar]
- Lorger M., Moelling K. 2006. Regulation of epithelial wound closure and intercellular adhesion by interaction of AF6 with actin cytoskeleton.J. Cell Sci. 119:3385–3398 [DOI] [PubMed] [Google Scholar]
- Maddugoda M.P., Crampton M.S., Shewan A.M., Yap A.S. 2007. Myosin VI and vinculin cooperate during the morphogenesis of cadherin cell–cell contacts in mammalian epithelial cells.J. Cell Biol. 178:529–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai K., Nakanishi H., Satoh A., Obaishi H., Wada M., Nishioka H., Itoh M., Mizoguchi A., Aoki T., Fujimoto T., et al. 1997. Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction.J. Cell Biol. 139:517–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A.C., Kaschube M., Wieschaus E.F. 2009. Pulsed contractions of an actin-myosin network drive apical constriction.Nature. 457:495–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T., Takahashi K., Suzuki E., Yamamoto D. 1999. The Canoe protein is necessary in adherens junctions for development of ommatidial architecture in the Drosophila compound eye.Cell Tissue Res. 298:397–404 [DOI] [PubMed] [Google Scholar]
- McCartney B.M., McEwen D.G., Grevengoed E., Maddox P., Bejsovec A., Peifer M. 2001. Drosophila APC2 and Armadillo participate in tethering mitotic spindles to cortical actin.Nat. Cell Biol. 3:933–938 [DOI] [PubMed] [Google Scholar]
- Miyamoto H., Nihonmatsu I., Kondo S., Ueda R., Togashi S., Hirata K., Ikegami Y., Yamamoto D. 1995. canoe encodes a novel protein containing a GLGF/DHR motif and functions with Notch and scabrous in common developmental pathways in Drosophila.Genes Dev. 9:612–625 [DOI] [PubMed] [Google Scholar]
- Muhlemann O., Eberle A.B., Stalder L., Zamudio Orozco R. 2008. Recognition and elimination of nonsense mRNA.Biochim. Biophys. Acta. 1779:538–549 [DOI] [PubMed] [Google Scholar]
- Müller H.-A.J., Wieschaus E. 1996. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J. Cell Biol. 134:149–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro E., Nance J., Priess J.R., Jr 2004. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo.Dev. Cell. 7:413–424 [DOI] [PubMed] [Google Scholar]
- Pilot F., Lecuit T. 2005. Compartmentalized morphogenesis in epithelia: from cell to tissue shape.Dev. Dyn. 232:685–694 [DOI] [PubMed] [Google Scholar]
- Pokutta S., Drees F., Takai Y., Nelson W.J., Weis W.I. 2002. Biochemical and structural definition of the l-afadin- and actin-binding sites of alpha-catenin.J. Biol. Chem. 277:18868–18874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M.P., Hyatt J.L. 1999. Establishment of the circumferential actin filament network is a prerequisite for localization of the cadherin-catenin complex in epithelial cells.Cell Growth Differ. 10:839–854 [PubMed] [Google Scholar]
- Sakisaka T., Ikeda W., Ogita H., Fujita N., Takai Y. 2007. The roles of nectins in cell adhesions: cooperation with other cell adhesion molecules and growth factor receptors.Curr. Opin. Cell Biol. 19:593–602 [DOI] [PubMed] [Google Scholar]
- Sato T., Fujita N., Yamada A., Ooshio T., Okamoto R., Irie K., Takai Y. 2006. Regulation of the assembly and adhesion activity of E-cadherin by nectin and afadin for the formation of adherens junctions in Madin-Darby canine kidney cells.J. Biol. Chem. 281:5288–5299 [DOI] [PubMed] [Google Scholar]
- Speicher S., Fischer A., Knoblich J., Carmena A. 2008. The PDZ protein Canoe regulates the asymmetric division of Drosophila neuroblasts and muscle progenitors.Curr. Biol. 18:831–837 [DOI] [PubMed] [Google Scholar]
- Sweeton D., Parks S., Costa M., Wieschaus E. 1991. Gastrulation in Drosophila: the formation of the ventral furrow and posterior midgut invaginations.Development. 112:775–789 [DOI] [PubMed] [Google Scholar]
- Tachibana K., Nakanishi H., Mandai K., Ozaki K., Ikeda W., Yamamoto Y., Nagafuchi A., Tsukita S., Takai Y. 2000. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain–associated proteins.J. Cell Biol. 150:1161–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Matsuo T., Katsube T., Ueda R., Yamamoto D. 1998. Direct binding between two PDZ domain proteins Canoe and ZO-1 and their roles in regulation of the jun N-terminal kinase pathway in Drosophila morphogenesis.Mech. Dev. 78:97–111 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Nakanishi H., Miyahara M., Mandai K., Satoh K., Satoh A., Nishioka H., Aoki J., Nomoto A., Mizoguchi A., Takai Y. 1999. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with Afadin, a PDZ domain-containing protein.J. Cell Biol. 145:539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U. 1996. Crumbs, a component of the apical membrane, is required for zonula adherens formation in primary epithelia of Drosophila.Dev. Biol. 177:217–225 [DOI] [PubMed] [Google Scholar]
- Tepass U., Hartenstein V. 1994. The development of cellular junctions in the Drosophila embryo.Dev. Biol. 161:563–596 [DOI] [PubMed] [Google Scholar]
- Tepass U., Gruszynski-DeFeo E., Haag T.A., Omatyar L., Torok T., Hartenstein V. 1996. shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia.Genes Dev. 10:672–685 [DOI] [PubMed] [Google Scholar]
- Thomas G.H., Williams J.A. 1999. Dynamic rearrangement of the spectrin membrane skeleton during the generation of epithelial polarity in Drosophila.J. Cell Sci. 112:2843–2852 [DOI] [PubMed] [Google Scholar]
- Torres M., Stoykova A., Huber O., Chowdhury K., Bonaldo P., Mansouri A., Butz S., Kemler R., Gruss P. 1997. An alpha-E-catenin gene trap mutation defines its function in preimplantation development.Proc. Natl. Acad. Sci. USA. 94:901–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Oda H., Kraut R., Hayashi S., Kotaoka Y., Takeichi M. 1996. Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo.Genes Dev. 10:659–671 [DOI] [PubMed] [Google Scholar]
- Wei S.Y., Escudero L.M., Yu F., Chang L.H., Chen L.Y., Ho Y.H., Lin C.M., Chou C.S., Chia W., Modolell J., Hsu J.C. 2005. Echinoid is a component of adherens junctions that cooperates with DEcadherin to mediate cell adhesion.Dev. Cell. 8:493–504 [DOI] [PubMed] [Google Scholar]
- Wieschaus E., Nüsslein-Volhard C. 1986. Looking at embryos. In Drosophila: A Practical Approach. Roberts D.B., editor IRL Press, Oxford, England: 199–228 [Google Scholar]
- Yamada S., Pokutta S., Drees F., Weis W.I., Nelson W.J. 2005. Deconstructing the cadherin-catenin-actin complex.Cell. 123:889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S., Tachibana K., Nakanishi H., Yamamoto Y., Irie K., Mandai K., Nagafuchi A., Monden M., Takai Y. 2001. alpha-catenin-independent recruitment of ZO-1 to nectin-based cell-cell adhesion sites through afadin.Mol. Biol. Cell. 12:1595–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen J.A., Wieschaus E. 2004. Patterned gene expression directs bipolar planar polarity in Drosophila.Dev. Cell. 6:343–355 [DOI] [PubMed] [Google Scholar]
- Zdobnov E.M., von Mering C., Letunic I., Torrents D., Suyama M., Copley R.R., Christophides G.K., Thomasova D., Holt R.A., Subramanian G.M., et al. 2002. Comparative genome and proteome analysis of Anopheles gambiae and Drosophila melanogaster.Science. 298:149–159 [DOI] [PubMed] [Google Scholar]
- Zhadanov A.B., Provance D.W., Jr., Speer C.A., Coffin J.D., Goss D., Blixt J.A., Reichert C.M., Mercer J.A. 1999. Absence of the tight junctional protein AF-6 disrupts epithelial cell-cell junctions and cell polarity during mouse development.Curr. Biol. 9:880–888 [DOI] [PubMed] [Google Scholar]