Abstract
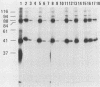
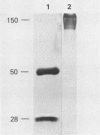
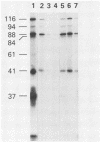
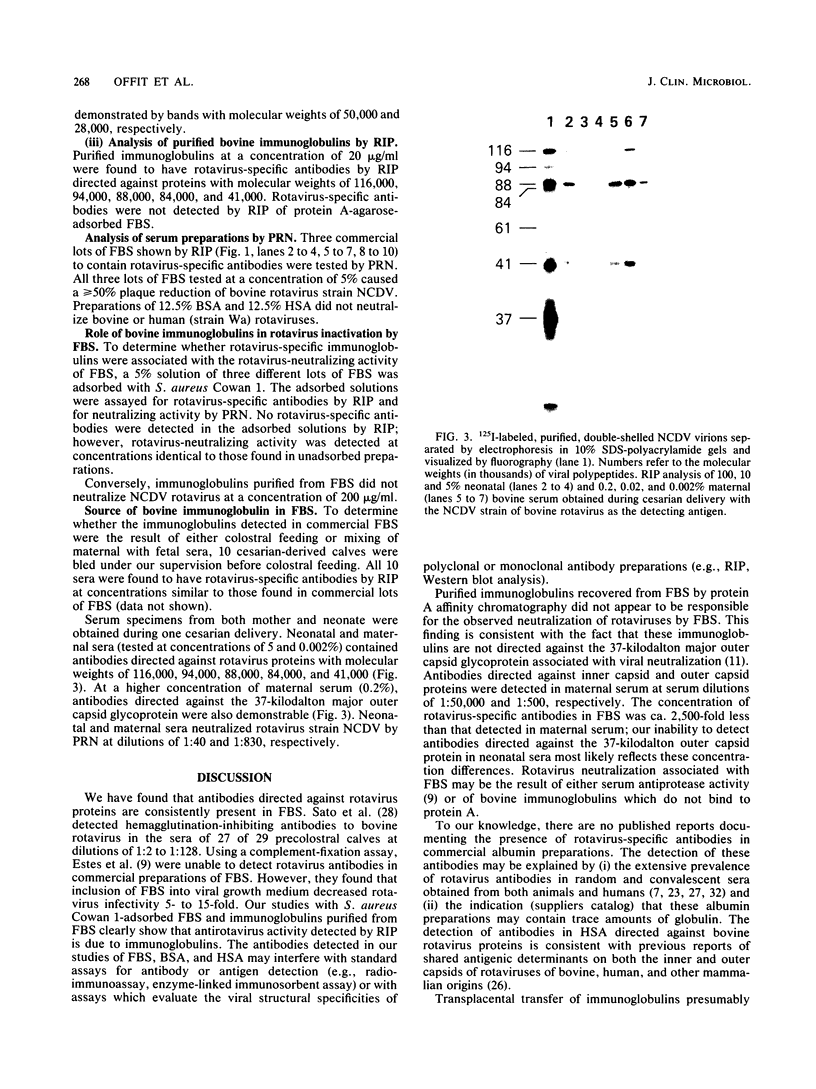
Rotavirus-specific antibodies were detected in fetal bovine serum, bovine serum albumin, and human serum albumin by radioimmunoprecipitation with the NCDV strain of bovine rotavirus as the detecting antigen. Fetal bovine sera neutralized bovine rotavirus in a plaque reduction neutralization test to titers of 1:20 or greater. Immunoglobulins purified from fetal bovine serum by protein A-agarose affinity chromatography precipitated rotavirus antigens but did not neutralize bovine rotavirus. Rotavirus antibodies in fetal bovine serum and in purified serum albumin preparations may interfere with diagnostic assays for the detection of rotavirus antigens or antibodies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER J. A., YORK C. J., GILLEPSIE J. H., MITCHELL G. B. Virus diarrhea in cattle. Am J Vet Res. 1954 Oct;15(57):525–531. [PubMed] [Google Scholar]
- Boone C. W., Mantel N., Caruso T. D., Jr, Kazam E., Stevenson R. E. Quality control studies on fetal bovine serum used in tissue culture. In Vitro. 1971 Nov-Dec;7(3):174–189. doi: 10.1007/BF02617963. [DOI] [PubMed] [Google Scholar]
- Bowne J. G., Luedke A. J., Jochim M. M., Metcalf H. E. Bluetongue disease in cattle. J Am Vet Med Assoc. 1968 Sep 15;153(6):662–668. [PubMed] [Google Scholar]
- CHOW T. L., MOLELLO J. A., OWEN N. V. ABORTION EXPERIMENTALLY INDUCED IN CATTLE BY INFECTIOUS BOVINE RHINOTRACHEITIS VIRUS. J Am Vet Med Assoc. 1964 May 1;144:1005–1007. [PubMed] [Google Scholar]
- Elias M. M. Distribution and titres of rotavirus antibodies in different age groups. J Hyg (Lond) 1977 Dec;79(3):365–372. doi: 10.1017/s0022172400053201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis W. A., Logan E. F., O'Brien J. J. Serum immunoglobulins in aborted and non-aborted bovine foetuses. Clin Exp Immunol. 1978 Jul;33(1):136–141. [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Gerba C. P., Smith E. M. Simian rotavirus SA11 replication in cell cultures. J Virol. 1979 Sep;31(3):810–815. doi: 10.1128/jvi.31.3.810-815.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband A. J., Brandon M. R., Lascelles A. K. Absorption and endogenous production of immunoglobulins in calves. Aust J Exp Biol Med Sci. 1972 Aug;50(4):491–498. doi: 10.1038/icb.1972.41. [DOI] [PubMed] [Google Scholar]
- Ivanoff M. R. Weak calf syndrome: serum immunoglobulin concentrations in precolostral calves. Am J Vet Res. 1975 Aug;36(08):1129–1131. [PubMed] [Google Scholar]
- Jalnapurkar B. V., Ajinkya S. M., Sardeshpande P. D. Immunoglobulin G in the sera of new born buffalo (Bos bubalus bubalis) calves. Vet Rec. 1976 Aug 21;99(8):146–146. doi: 10.1136/vr.99.8.146. [DOI] [PubMed] [Google Scholar]
- Kendrick J. W. Bovine viral diarrhea-mucosal disease virus infection in pregnant cows. Am J Vet Res. 1971 Apr;32(4):533–544. [PubMed] [Google Scholar]
- Kirkbride C. A., Martinovich D., Woodhouse D. A. Immunoglobulins and lesions in aborted bovine foetuses. N Z Vet J. 1977 Jul;25(7):180-2, 187. doi: 10.1080/00480169.1977.34399. [DOI] [PubMed] [Google Scholar]
- Klaus G. G., Bennett A., Jones E. W. A quantitative study of the transfer of colostral immunoglobulins to the newborn calf. Immunology. 1969 Mar;16(3):293–299. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Luedke A. J., Jochim M. M., Bowne J. G., Jones R. H. Observations on latent bluetongue virus infection in cattle. J Am Vet Med Assoc. 1970 Jun 15;156(12):1871–1879. [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Inouye S., Kono R. Plaque assay of neonatal calf diarrhea virus and the neutralizing antibody in human sera. J Clin Microbiol. 1977 Jan;5(1):1–4. doi: 10.1128/jcm.5.1.1-4.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKercher D. G., Saito J. K., Singh K. V. Serologic evidence of an etiologic role for bluetongue virus in hydranencephaly of calves. J Am Vet Med Assoc. 1970 Apr 15;156(8):1044–1047. [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Plotkin S. A. Response of mice to rotaviruses of bovine or primate origin assessed by radioimmunoassay, radioimmunoprecipitation, and plaque reduction neutralization. Infect Immun. 1983 Oct;42(1):293–300. doi: 10.1128/iai.42.1.293-300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Inaba Y., Tokuhisa S., Miura Y., Akashi H., Tanaka Y. Antibodies against several viruses in sera from normal bovine fetuses and precolostral calves. Natl Inst Anim Health Q (Tokyo) 1980 Summer;20(2):77–78. [PubMed] [Google Scholar]
- Sawyer M., Osburn B. I., Knight H. D., Kendrick J. W. A quantitative serologic assay for diagnosing congenital infections of cattle. Am J Vet Res. 1973 Oct;34(10):1281–1284. [PubMed] [Google Scholar]
- Schultz R. D., Dunne H. W., Heist C. E. Ontogeny of the bovine immune response. Infect Immun. 1973 Jun;7(6):981–991. doi: 10.1128/iai.7.6.981-991.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouless M. E., Bryden A. S., Flewett T. H., Woode G. N., Bridger J. C., Snodgrass D. R., Herring J. A. Serological relationships between rotaviruses from different species as studied by complement fixation and neutralization. Arch Virol. 1977;53(4):287–294. doi: 10.1007/BF01315627. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., Kapikian A. Z., Mebus C. A. Induction of cross-reactive serum neutralizing antibody to human rotavirus in calves after in utero administration of bovine rotavirus. J Clin Microbiol. 1983 Sep;18(3):505–508. doi: 10.1128/jcm.18.3.505-508.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]