Abstract
Methamphetamine abuse results in lasting, partial depletions of striatal dopamine and cognitive dysfunction. However, the effect of partial dopamine depletions on the expression of an effector immediate early gene, Arc (activity regulated, cytoskeletal-associated protein), known to be involved in synaptic modifications underlying learning and memory has heretofore not been examined. Male Sprague-Dawley rats were pretreated with a neurotoxic regimen of methamphetamine or saline. Seven weeks later, rats were trained in a motor-response task on a T-maze for five days, and then underwent reversal training on day five. Rats were sacrificed 5 min after reaching criterion on the reversal task, and the brains removed and processed using double-label fluorescent in situ hybridization for Arc and preproenkephalin (PPE) mRNA expression in the dorsomedial striatum. Rats pretreated with methamphetamine had an average (±SEM) 54.4±7.9% loss of dopamine in dorsomedial striatum. Interestingly, there was no difference in reversal trials to criterion in methamphetamine- vs. saline-pretreated rats. However, the expression of Arc mRNA in dorsomedial striatum was attenuated in methamphetamine-pretreated animals, particularly in PPE-negative neurons. Furthermore, the correlation between Arc mRNA expression in dorsomedial striatum and learning was abolished in methamphetamine-pretreated animals. These data suggest that methamphetamine-induced partial monoamine loss is associated with disrupted induction of the effector immediate early gene Arc during a behavioral task, particularly in PPE-negative (presumed striatonigral) neurons, as well as with disruption of the relation between Arc mRNA expression in dorsomedial striatum and reversal learning.
Keywords: amphetamines, basal ganglia, in situ hybridization, monoamine, motor learning, striatum
Little is known about the effects of partial monoamine depletions on striatal efferent neuron function associated with basal ganglia-dependent learning. Methamphetamine (METH) exposure causes significant, yet partial, decreases in monoamine innervation of the striatum in rodents (Hotchkiss and Gibb, 1980; Ricaurte et al., 1980), as well as in humans (Wilson et al., 1996; Volkow et al., 2001; Wang et al., 2004). Previous work also has shown that such METH-induced monoamine depletions are associated with impaired cognitive function both in humans (Volkow et al., 2001) and rodents (Friedman et al., 1998; Chapman et al., 2001; Belcher et al., 2005; Daberkow et al., 2005; Belcher et al., 2008). However, the extent to which such depletions alter mechanisms underlying striatal neuroplasticity involved in learning and memory functions is unknown.
Spiny efferent neurons comprise ~95% of the neurons in the striatum (Kemp and Powell, 1971). Approximately half of these neurons, the striatonigral or “direct” pathway neurons, contain substance P and its preprotachykinin (PPT) precursor mRNA (Powell et al., 1973). The other half, the striatopallidal or “indirect” pathway neurons, contain met-enkephalin and its preproenkephalin (PPE) precursor mRNA (Aronin et al., 1984; Gerfen and Young, 1988; Gerfen et al., 1990). Therefore, the expression of PPE or PPT mRNA is used as a phenotypic marker of striatal efferent neurons.
The impact of partial dopamine loss on the function of striatonigral and striatopallidal efferent neurons is somewhat unclear. On the one hand, previous work from our lab and others suggests that striatonigral neuron function may be selectively affected by partial monoamine loss. First, the expression of PPT, but not PPE, mRNA is decreased by partial dopamine loss induced by 6-hydroxydopamine (Nisenbaum et al., 1996) or by a neurotoxic regimen of METH (Chapman et al., 2001; Johnson-Davis et al., 2002). Furthermore, cytochrome oxidase histochemical staining in the brains of rats with partial monoamine depletions is increased in the entopeduncular nucleus and substantia nigra pars reticulata—the primary terminal fields of the striatonigral neurons—but not in the globus pallidus or subthalamic nucleus—the primary terminal fields of the striatopallidal neurons (Chapman et al., 2001). On the other hand, studies from other groups suggest that partial dopamine loss is associated with selective effects on striatopallidal efferent neurons. That is, Ariano and colleagues have reported that partial dopamine loss induced by 6-OHDA is associated with activation of apoptotic cascades in striatopallidal neurons (Ariano et al., 2005). Cadet and colleagues also have reported that METH regimens that are toxic to monoamine systems result, 24 hr-three days later, in Fas Ligand and TUNEL staining in enkephalin-containing, as well as other phenotypically undefined, striatal neurons (Jayanthi et al., 2005; Thiriet et al., 2005). Thus, the extent to which partial monoamine depletions may differentially affect the function of striatal efferent neuron populations is currently unclear.
Arc/Arg3.1 is an effector immediate early gene, the protein product of which (Arc, activity regulated cytoskeleton associated protein) is thought to be critically involved in synaptic modifications subserving learning and memory (Steward et al., 1998; Guzowski et al., 2000; Guzowski et al., 2001; Steward and Worley, 2001). The expression of Arc mRNA is correlated with learning. For example, Arc mRNA expression in the hippocampus, but not the striatum, is correlated with behavioral performance on the spatial version of the Morris water maze (Guzowski et al., 2001). Likewise, we have previously reported (Daberkow et al., 2007) that the expression of Arc mRNA in both PPE+ and PPE− neurons of the dorsomedial (DM) striatum, but not Arc mRNA expression in the dorsolateral striatum or hippocampus, is significantly correlated with number of trials to criterion during reversal learning on a T-maze task—a task known to be dependent on the function of the DM striatum (Ragozzino et al., 2002). This correlation was shown to be associated with the new learning occuring during the reversal trials, as opposed to motor activity in general, as yoked control rats that ran the same number of trials on the T-maze but who did not learn a new motor response did not have a significant correlation between trials run and arc mRNA expression in DM striatum (Daberkow et al., 2007). These previous reports therefore establish that arc mRNA expression in a brain region known to be necessary for performance on a particular learning task is correlated with the behavioral indices of that learning.
Therefore, to further examine the impact of partial monoamine loss in striatum on the function of striatopallidal and striatonigral efferent neurons under behaviorally relevant conditions, we examined the expression of Arc mRNA induced in phenotypically identified efferent neurons of the DM striatum by reversal learning on a T-maze task in saline- and METH-pretreated rats.
METHODS
Animals
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA; 300–350g) were housed in tub cages in a room controlled for temperature and lighting (12:12 hr). Control rats (caged controls) were housed and fed over the same period of time as rats undergoing METH (or saline) pretreatment and motor-response training, but were not exposed to any behavioral training. All animal care and experimental procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Utah.
Methamphetamine pretreatment
Rats were exposed to a neurotoxic regimen of (±)-METH hydrochloride (NIDA, Rockville, MD) as previously described (Daberkow et al., 2005). Rats were given four injections of METH (10 mg/kg, s.c.; n=7) or saline (n=7) at 2-hr intervals. Two hours after the last injection, rats were returned to their home cages and given free access to food and water until trained and run in the behavioral experiment four to seven weeks later.
Behavioral training
One month after METH- or saline-pretreatment, rats were handled daily for two weeks and allowed to eat the food reward (Froot Loops cereal; Kellogg, Battle Creek, MI) for at least 10 min each day. Rats were then exposed to the T-maze with all arms open for 5 days, being allowed to explore the maze and consume ½ pieces of cereal in the food wells for 15 min each day. The T-maze consisted of a four-arm plus maze with one of the arms closed off on each trial by a removable door. Each arm was 55-cm long×10-cm wide×15-cm high. Removable start boxes with doors were at the end of each arm and contained a food well 2.5 cm in diameter and 1.5-cm deep.
The day prior to the first learning session, the turn bias of each rat was determined. Each rat then underwent acquisition training as previously described (Daberkow et al., 2007), except that rats in the current study repeated the acquisition training daily for five days, as opposed to three days as reported previously. Briefly, for five days the rats were rewarded to turn opposite their turn bias, when started from all four arms chosen in a pseudorandom order, until they reached criterion (90% correct responses over 10 trials). The intertrial interval was approximately 10 s, during which time the rat was moved to a new start arm and the new goal arm was loaded with a half a piece of Froot Loop cereal. On the fifth day, after the acquisition trials, the rats were then rewarded to turn in the opposite direction. These “reversal” trials were run until the rat reached criterion in the new direction.
Dopamine depletions & fluorescent in situ hybridization histochemistry (FISH)
All rats were sacrificed by exposure to CO2 and decapitated 5 min after reaching criterion on the reversal trials. The brains were rapidly removed and flash frozen in −80° C 2-methyl-butane. At the beginning and end of behavioral training, caged control rats were removed from their home cages and immediately sacrificed to determine the basal level of Arc mRNA expression.
Dopamine depletions were determined in tissue punches (~1mm3) taken from the DM striatum while sectioning the tissue for in situ hybridization histochemistry. Dopamine content in the tissue punches was analyzed with HPLC coupled to electrochemical detection as previously described (Daberkow et al., 2005).
Brain tissue from all groups to be directly compared was processed together and hybridized in parallel. Fresh-frozen sections (12 µm) were taken through striatum and mounted, fixed and stored as previously described (Chapman et al., 2001; Daberkow et al., 2007). Expression of Arc mRNA in identified subpopulations of striatal efferent neurons was accomplished by double-label FISH for Arc and PPE mRNAs, also as previously described (Daberkow et al., 2007). Ribonucleotide probes complementary to the mRNAs for Arc (Lyford et al., 1995) and PPE (Yoshikawa et al., 1984) were synthesized from cDNAs using digoxigenin-UTP (DIG-UTP; Arc) and fluorescein-UTP (FITC-UTP; PPE) with T7 (Arc) and SP6 (PPE) RNA polymerases and DIG and FITC RNA labeling kits (Roche Applied Science, Indianapolis, IN). Double labeling of striatal sections with dilutions of probes at 1:1000 (~350 ng/ml) and antibodies at 1:1000 was done as previously described (Guzowski et al., 1999; Daberkow et al., 2007). Probes then were visualized using tyramide signal amplification (Perkin/Elmer Life Sciences, Boston, MA) with cyanine-3 (cy-3; Arc) and cyanine-5 (cy-5; PPE) fluorophores as per the manufacturer’s instructions. Slides were washed in TNT buffer, counterstained with SYTOX Green (1:35,000; Molecular Probes), and mounted in anti-fade (Molecular Probes).
Imaging and Statistical Analyses
Images were collected using an Olympus FVX confocal microscopy system with an IX70 microscope, 488nm Argon laser line, and 60x/1.4 NA oil-immersion lens (plan APO). Photo-multiplier tube (PMT) assignments and pinhole size contrast values were kept constant across different confocal sessions. Areas of analysis were z-sectioned in 1-micron thick optical sections. A total of 10 z-sections were compressed for each field. A sample area of 0.7mm × 0.7mm from the DM striatum of each rat (at +1.0mm anterior to bregma) was imaged and digitized for subsequent analysis, as previously described (Daberkow et al., 2007).
Cells with cytoplasmic Arc mRNA expression were counted in each field by an experimenter blinded to the treatment groups. The number of cells positively stained for Arc mRNA and positive or negative for PPE mRNA were counted in each field, yielding numbers of Arc+/PPE+ and Arc+/PPE− neurons per field. In previous work we established that the field size analyzed contains approximately 1,000 cells and that approximately 7% of those cells are Arc mRNA positive in normal rats exposed to such a T-maze task (Daberkow et al., 2007). Previous work in our lab also has established that it is the cytoplasmic expression of Arc mRNA in striatal efferent neurons that correlates with behavioral performance during reversal learning on the T-maze (Daberkow et al., 2007). Therefore, for the present study, cells were considered to be positively labeled for Arc mRNA (Arc+) if they contained perinuclear/cytoplasmic labeling of Arc mRNA that did not overlap with the SYTOX-stained nucleus (Guzowski et al., 1999).During this cellular analysis, Arc+ cells containing the blue/cy-5 PPE mRNA signal in and around the nucleus were classified as PPE-positive (PPE+). Arc+ cells that did not also contain blue/cy-5 staining were classified as PPE-negative (PPE−).
The numbers of Arc+ neurons were compared across treatment groups and neuronal phenotype with a two-way analysis of variance (ANOVA; treatment × neuron phenotype) followed by post hoc t-tests. In addition, the numbers of Arc+/PPE+ and Arc+/PPE− cells in each field were correlated with the number of trials for each rat to reach criterion on the reversal-learning task. Statistical significance was set at p ≤ 0.05.
RESULTS
Dopamine depletions
The dopamine content (in ng/mg protein) in the DM striatum of saline- and METH-pretreated rats is shown in Figure 1. On average, METH administration decreased dopamine tissue content by 54.4 ±7.9% (mean ± SEM, n=7) in the DM striatum (range 36.1–83.9%).
Fig. 1.
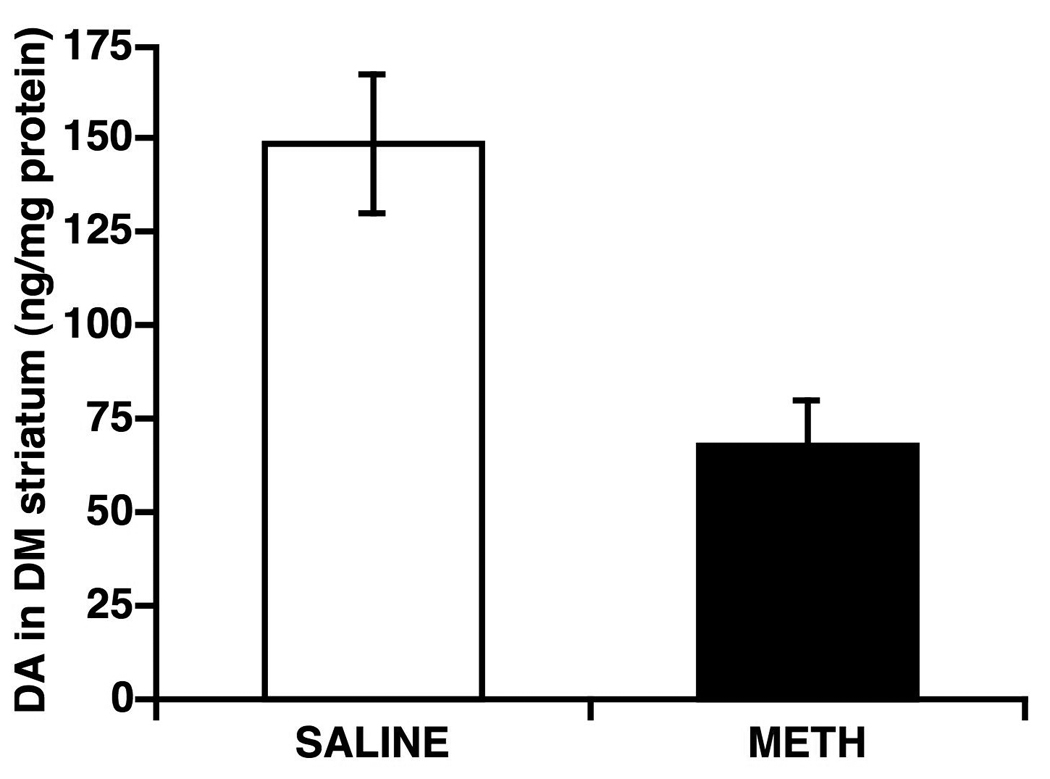
Dopamine (DA) tissue content (ng/mg protein; mean ± SEM) in the dorsomedial (DM) striata of rats pretreated with saline (4 × 1 mL at 2-hr intervals, n=7) or a neurotoxic regimen of methamphetamine (METH; 4 × 10 mg/kg, sc at 2-hr intervals, n=7) seven weeks prior to sacrifice.
Behavioral performance
There were no significant differences between the saline-pretreated and METH-pretreated rats in the number of trials required to reach criterion on either the first day of acquisition trials (Fig. 2; t=0.30, p=0.77) or on the reversal trials (Fig. 2; t=0.19, p=0.85).
Fig. 2.
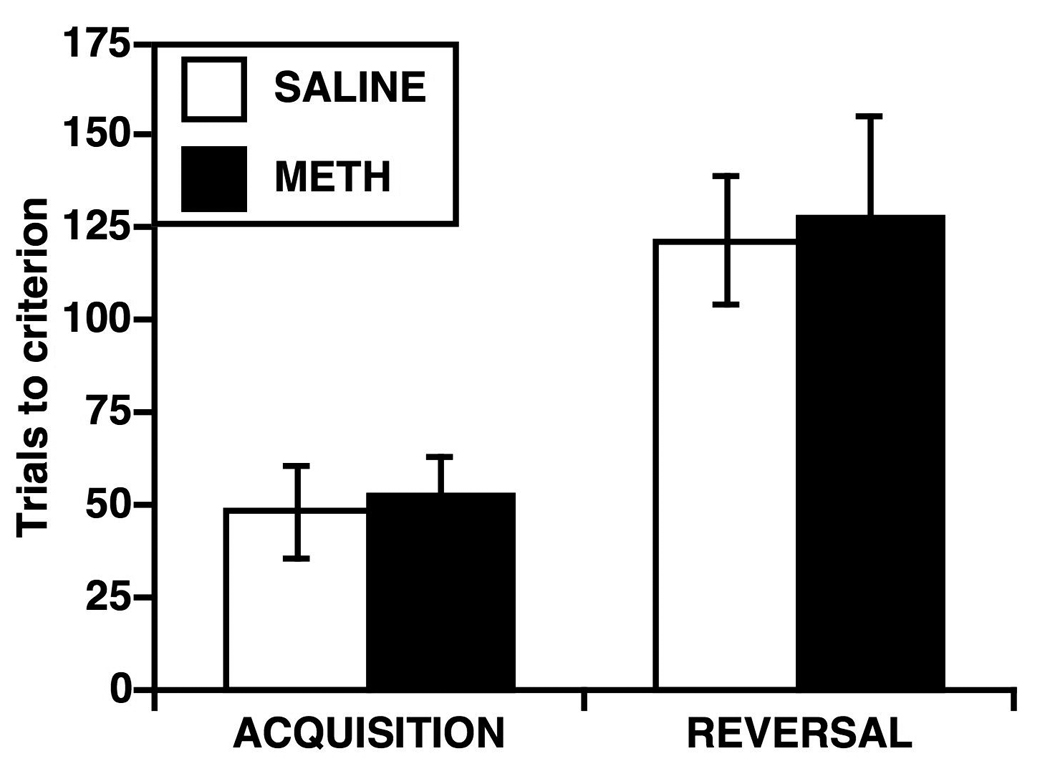
Number of trials required on a motor response learning task on a T-maze to reach criterion (90% correct responses over 10 trials) on the first day of acquisition training and on the reversal training on the final day of testing. Saline-pretreated rats (SALINE) received 4 injections of saline (1 mL) and methamphetamine (METH)-pretreated rats received 4 injections of METH (10 mg/kg, s.c.) at 2-hr intervals six weeks prior to the start of the motor response training. Data are mean ± SEM, n=7 / group.
METH-induced neurotoxicity is associated with impaired striatal Arc mRNA expression
Analysis of the numbers of PPE− and PPE+ neurons expressing Arc mRNA in the cytoplasm in the DM striata of saline- vs. METH-pretreated rats revealed a significant main effect of pretreatment (F2,15=4.85, p<0.05) and a significant pretreatment × neuronal phenotype interaction (Fig. 3C, F2,15=5.09, p<0.05). Post-hoc analysis revealed that there were significant increases in the numbers of Arc+/PPE− (t=−2.7, p<0.05) and Arc+/PPE+ (t=−3.2, p<0.05) neurons in the DM striatum of saline-pretreated rats engaged in reversal learning relative to caged controls (Fig. 3A, C). In the DM striatum of METH-pretreated rats, however, there were no significant increases in the numbers of Arc+/PPE− (t=−1.9, p>0.05) and Arc+/PPE+ (t=−1.9, p>0.05) neurons expressing Arc mRNA relative to caged controls (Fig. 3B, C). In addition, there was a strong trend towards a greater number of Arc+/PPE− neurons in saline- vs. METH-pretreated rats (Fig. 3C, t=2.0, p=0.06), which was not the case in the Arc+/PPE+ subpopulation of neurons (t=0.98, p=0.35). Likewise, there was a strong trend towards a greater number of Arc+/PPE− than Arc+/PPE+ neurons in the DM striatum of saline-pretreated (t=2.3, p=0.06), but not METH-pretreated (t=−1.2, p=0.28), rats (Fig. 3C).
Fig. 3.
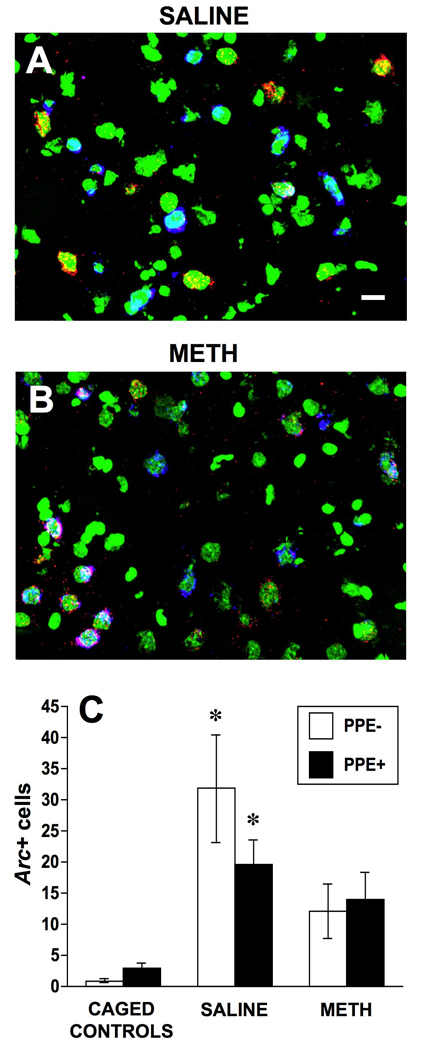
Confocal images showing Arc mRNA (cy-3/red), preproenkephalin mRNA (PPE, cy-5/blue) and nuclear (SYTOX Green) staining in the dorsomedial striatum of a saline- (4 × 1 mL/kg, s.c. at 2-hr intervals; A) and a methamphetamine- (METH; 4 × 10 mg/kg, s.c. at 2-hr intervals; B) pretreated rat. The average number (± SEM, n= 4 for caged controls and n=7 for both saline- and METH-pretreated groups) of PPE− and PPE+ neurons expressing Arc mRNA in the cytoplasm in a 0.7 mm × 0.7 mm field of the dorsomedial striatum (C). * Significantly different from respective phenotype in caged controls, p<0.05. Scale bar, 10 µm.
Arc mRNA expression in DM striatum is correlated with reversal learning in saline-, but not METH-, pretreated rats
In saline-pretreated rats, there was a significant inverse correlation between the number of Arc+/PPE− (Fig. 4A, r2=0.80, p<0.01) and Arc+/PPE+ (Fig. 4C, r2=0.81, p<0.01) neurons in DM striatum and the number of trials to reach criterion on the reversal-learning task, consistent with our previous observations (Daberkow et al., 2007). However, in DM striatum of METH-pretreated animals, the correlations between trials to criterion on the reversal trials and the numbers of Arc+/PPE− (Fig. 4B, r2=0.09, p>0.05) or Arc+/PPE+ (Fig. 4D, r2=0.09, p>0.05) neurons were not significant.
Fig. 4.
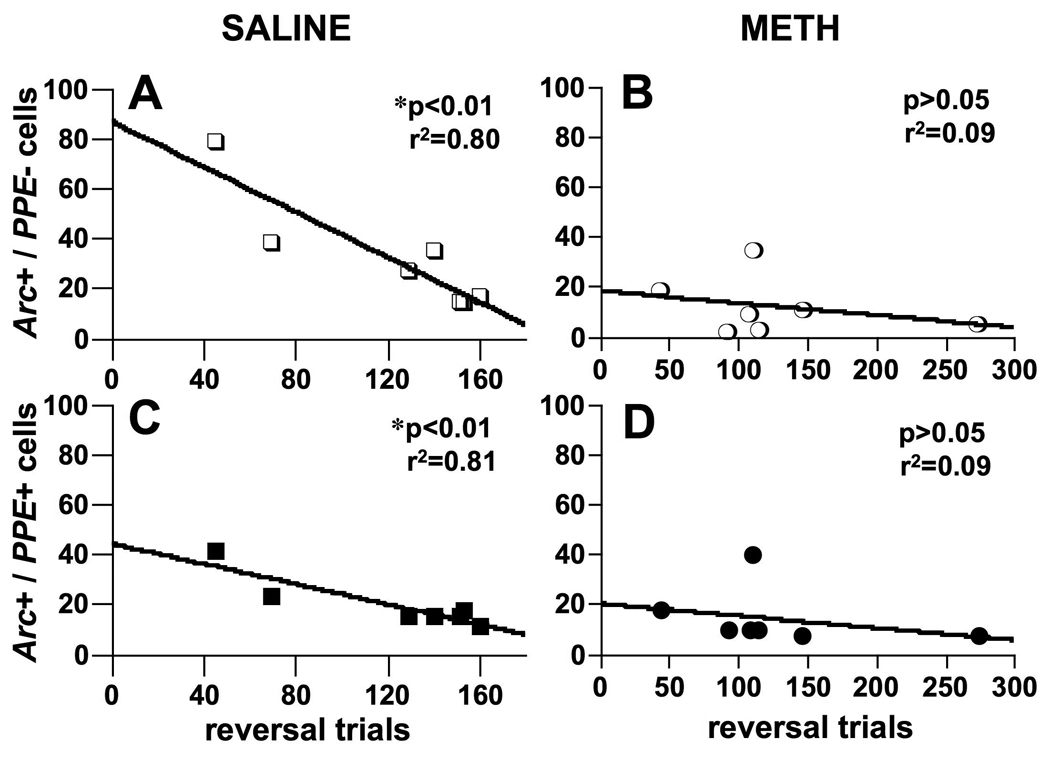
Correlations between the numbers of preproenkephalin-negative (PPE−; A, B; open symbols) and preproenkephalin-positive (PPE+; C, D; closed symbols) neurons expressing Arc mRNA (Arc+) in a 0.7 mm × 0.7 mm field of dorsomedial striatum and the number of trials to reach 9/10 correct responses during reversal learning in a motor response task on a T-maze. Values are from rats pretreated with saline (4 × 1 mL/kg; A, C, squares) or a neurotoxic regimen of methamphetamine (METH; 4 × 10 mg/kg, s.c. at 2-hr intervals; B, D, circles) approximately seven weeks prior to testing. * Significant correlation.
DISCUSSION
The present results show that prior exposure of rats to a neurotoxic regimen of METH that produces a partial loss of the dopamine innervation of the striatum is associated with impaired Arc mRNA expression in the DM striatum and disruption of the relation between the degree of Arc mRNA expression in this brain region and reversal learning on a T-maze task. Furthermore, the data suggest that although the expression of Arc mRNA is disrupted in both PPE+ and PPE− neurons in striatum, the expression in PPE− neurons may be more severely impaired. These data extend previous observations that METH-induced monoamine depletions are associated with impaired function of striatal efferent neurons by showing that such impairment extends to behaviorally induced expression of the effector immediate early gene Arc.
The present data show that animals with METH-induced neurotoxicity fail to express Arc mRNA in PPE+ and PPE− striatal efferent neurons to the same degree as intact animals in response to this behavioral task. Interestingly, this impairment in Arc mRNA expression was most notable in the PPE− neurons, suggesting a greater impact of the METH-induced neurotoxicity on the function of PPE− neurons. These PPE− neurons are presumed to be striatonigral (direct pathway) neurons by virtue of the fact that striatal efferent neurons comprise approximately 95% of the neurons in the striatum and roughly half of these neurons are striatopallidal (PPE+) and half are striatonigral (PPE−; Gerfen and Young, 1988). Thus, the majority, although not all, of the PPE− neurons will be striatonigral efferent neurons. Furthermore, recent work has shown that all striatal neurons expressing Arc mRNA in the context of spatial navigation also express GAD67 (Vazdarjanova et al., 2006), which is expressed predominately in striatal efferent neurons (Mercugliano et al., 1992). Thus, the present data suggest that the neurotoxicity induced by METH has a greater impact on the regulation of this learning-related molecule in striatonigral efferent neurons.
The extent to which dopamine, as opposed to serotonin, loss contributes to the observed deficits in Arc activation in the METH-pretreated animals is not known. Exposure to neurotoxic regimens of METH, such as that used in the present study, results in partial loss of both the dopamine and serotonin innervation of the striatum (Hotchkiss and Gibb, 1980; Ricaurte et al., 1980). In the present study, due to technical constraints, we only measured striatal concentrations of dopamine, not serotonin, to be certain that the neurotoxic regimen of METH had, in fact, induced toxicity to striatal monoamine systems. However, it is likely that serotonin also was significantly depleted in these animals (Johnson-Davis et al., 2002; Daberkow et al., 2005). Work by Nisenbaum and colleagues (Nisenbaum et al., 1996) and Ariano and colleagues (Ariano et al., 2005) show that selective dopamine loss induced by 6-OHDA produces effects on indices of striatal efferent neuron function similar to those observed subsequent to neurotoxic regimens of METH, suggesting that partial dopamine loss produced by METH may be sufficient to induce such altered function. However, the serotonin innervation of the striatum also regulates striatonigral neuron function (Campbell et al., 2001). Further work clearly is required to elaborate the potential for partial dopamine loss alone to alter striatal neuron function, as well as to determine the impact of partial serotonin loss per se on striatal efferent neuron function.
A potentially important aspect of Arc expression is that the correlation between Arc mRNA expression and behavior, rather than the total amount of Arc mRNA expression, may be a better indicator of the role of Arc and a particular brain region in a behavioral task (Guzowski et al., 2001). In the present study, there were significant correlations between the numbers of PPE− and PPE+ neurons with cytoplasmic Arc mRNA expression in the DM striatum of saline-pretreated rats and the number of trials to reach criterion on a reversal learning task on a T-maze. These data are consistent with our previously published observations (Daberkow et al., 2007) even though, on average, saline-pretreated rats in the present study tended to take more trials to reach the reversal criterion (121.0 ± 17.4) than did the rats in our previous work (87.1 ± 14.7; Daberkow et al., 2007), presumably due to the greater number of acquisition training days used in the present work. However, no such significant correlation was observed in the METH-pretreated rats, which performed as well as the saline-pretreated rats on the behavioral task (127.6 ± 27.1 trials to criterion) and who showed an even broader distribution of trials to criterion on the reversal task relative to the saline-pretreated rats. There are several possible interpretations of this loss of correlation between behavioral performance and striatal Arc mRNA expression in the METH-pretreated rats. First, it may be the case that Arc mRNA expression in the DM striatum, although correlated with behavioral performance under normal conditions, plays no functional role in the actual learning on this task. Thus, impaired activation of the gene in the context of METH-induced neurotoxicity has no consequence for behavioral performance. Second, because Arc mRNA expression was attenuated in the METH-pretreated rats, the smaller range of values of Arc mRNA expression may have simply made it more difficult to discern a relation between Arc expression and performance that, in fact, still exists. Finally, to the extent that the correlation between the degree of Arc mRNA expression in a particular brain region and the measure of learning reflects the involvement of that brain region in that particular learning process (Guzowski et al., 2001; Daberkow et al., 2007), the loss of correlation in the METH-pretreated animals may suggest that they are using a different brain area to solve this task, relative to normal controls. Similar results have been reported for patients early in the course of Parkinson’s disease in that their performance on a probabilistic classification task is similar to that of normal controls, but functional MRI scans reveal that the Parkinsonian patients show activation of the medial temporal lobe during this task whereas normal controls show activation of the caudate (Moody et al., 2004). Additional behavioral and molecular analyses will be necessary to delineate the significance of the correlation between Arc mRNA expression in DM striatum and behavioral performance, as well as the significance of the loss of the correlation in METH-pretreated rats.
In conclusion, the present study has shown that METH-induced monoamine depletions are associated with impaired expression, particularly in PPE- neurons, of Arc mRNA activated by a behavioral task known to be dependent on the function of the DM striatum. These findings therefore provide further evidence that the function of striatal efferent neurons is impaired in the context of partial monoamine loss induced by METH and that the impairment may be greater in striatonigral efferent neurons. Furthermore, the data show a loss of correlation between Arc mRNA expression in striatum and motor response learning in METH-pretreated animals despite apparently normal learning, raising the possibilities that Arc expression is not required for this learning or that METH-pretreated rats may be relying on a different behavioral strategy and brain regions to solve this task.
Acknowledgements
We would like to thank Dr. Paul Worley for the Arc cDNA and Dr. John Guzowski for helpful discussions regarding this work. This work was supported by PHS grants DA13367, NS41673, DA018479.
References
- Ariano MA, Grissell AE, Littlejohn FC, Buchanan TM, Elsworth JD, Collier TJ, Steece-Collier K. Partial dopamine loss enhances activated caspase-3 activity: differential outcomes in striatal projection systems. J Neurosci Res. 2005;82:387–396. doi: 10.1002/jnr.20644. [DOI] [PubMed] [Google Scholar]
- Aronin N, Difiglia M, Graveland GA, Schwartz WJ, Wu JY. Localization of immunoreactive enkephalins in GABA synthesizing neurons of the rat neostriatum. Brain Res. 1984;300:376–380. doi: 10.1016/0006-8993(84)90850-3. [DOI] [PubMed] [Google Scholar]
- Belcher AM, Feinstein EM, O'Dell SJ, Marshall JF. Methamphetamine influences on recognition memory: comparison of escalating and single-day dosing regimens. Neuropsychopharmacology. 2008;33:1453–1463. doi: 10.1038/sj.npp.1301510. [DOI] [PubMed] [Google Scholar]
- Belcher AM, O'Dell SJ, Marshall JF. Impaired object recognition memory following methamphetamine, but not p-chloroamphetamine- or d-amphetamine-induced neurotoxicity. Neuropsychopharmacology. 2005;30:2026–2034. doi: 10.1038/sj.npp.1300771. [DOI] [PubMed] [Google Scholar]
- Campbell BM, Gresch PJ, Walker PD. Neonatal dopamine depletion reveals a synergistic mechanism of mRNA regulation that is mediated by dopamine(D1) and serotonin(2) receptors and is targeted to tachykinin neurons of the dorsomedial striatum. Neuroscience. 2001;105:671–680. doi: 10.1016/s0306-4522(01)00218-4. [DOI] [PubMed] [Google Scholar]
- Chapman DE, Hanson GR, Kesner RP, Keefe KA. Long-term changes in basal ganglia function after a neurotoxic regimen of methamphetamine. J Pharmacol Exp Ther. 2001;296:520–527. [PubMed] [Google Scholar]
- Daberkow DP, Kesner RP, Keefe KA. Relation between methamphetamine-induced monoamine depletions in the striatum and sequential motor learning. Pharmacol Biochem Behav. 2005;81:198–204. doi: 10.1016/j.pbb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Daberkow DP, Riedy MD, Kesner RP, Keefe KA. Arc mRNA induction in striatal efferent neurons associated with response learning. Eur J Neurosci. 2007;26:228–241. doi: 10.1111/j.1460-9568.2007.05630.x. [DOI] [PubMed] [Google Scholar]
- Friedman SD, Castaneda E, Hodge GK. Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacol Biochem Behav. 1998;61:35–44. doi: 10.1016/s0091-3057(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Young WS., 3rd Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 1988;460:161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacol Exp Ther. 1980;214:257–262. [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Ladenheim B, McCoy MT, Cluster A, Cai NS, Cadet JL. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 2005;102:868–873. doi: 10.1073/pnas.0404990102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Davis KL, Hanson GR, Keefe KA. Long-term post-synaptic consequences of methamphetamine on preprotachykinin mRNA expression. J Neurochem. 2002;82:1472–1479. doi: 10.1046/j.1471-4159.2002.01095.x. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The structure of the caudate nucleus of the cat: light and electron microscopy. Philos Trans R Soc Lond B Biol Sci. 1971;262:383–401. doi: 10.1098/rstb.1971.0102. [DOI] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Mercugliano M, Soghomonian JJ, Qin Y, Nguyen HQ, Feldblum S, Erlander MG, Tobin AJ, Chesselet MF. Comparative distribution of messenger RNAs encoding glutamic acid decarboxylases (Mr 65,000 and Mr 67,000) in the basal ganglia of the rat. J Comp Neurol. 1992;318:245–254. doi: 10.1002/cne.903180302. [DOI] [PubMed] [Google Scholar]
- Moody TD, Bookheimer SY, Vanek Z, Knowlton BJ. An implicit learning task activates medial temporal lobe in patients with Parkinson's disease. Behav Neurosci. 2004;118:438–442. doi: 10.1037/0735-7044.118.2.438. [DOI] [PubMed] [Google Scholar]
- Nisenbaum LK, Crowley WR, Kitai ST. Partial striatal dopamine depletion differentially affects striatal substance P and enkephalin messenger RNA expression. Brain Res Mol Brain Res. 1996;37:209–216. doi: 10.1016/0169-328x(95)00317-l. [DOI] [PubMed] [Google Scholar]
- Powell D, Leeman S, Tregear GW, Niall HD, Potts JT., Jr Radioimmunoassay for substance P. Nat New Biol. 1973;241:252–254. doi: 10.1038/newbio241252a0. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Jih J, Tzavos A. Involvement of the dorsomedial striatum in behavioral flexibility: role of muscarinic cholinergic receptors. Brain Res. 2002;953:205–214. doi: 10.1016/s0006-8993(02)03287-0. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Thiriet N, Deng X, Solinas M, Ladenheim B, Curtis W, Goldberg SR, Palmiter RD, Cadet JL. Neuropeptide Y protects against methamphetamine-induced neuronal apoptosis in the mouse striatum. J Neurosci. 2005;25:5273–5279. doi: 10.1523/JNEUROSCI.4893-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, Ramirez-Amaya V, Insel N, Plummer TK, Rosi S, Chowdhury S, Mikhael D, Worley PF, Guzowski JF, Barnes CA. Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J Comp Neurol. 2006;498:317–329. doi: 10.1002/cne.21003. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler JS. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004;161:242–248. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K, Williams C, Sabol SL. Rat brain preproenkephalin mRNA. cDNA cloning, primary structure, and distribution in the central nervous system. J Biol Chem. 1984;259:14301–14308. [PubMed] [Google Scholar]


