Abstract
Interleukin 7 (IL-7) and T cell receptor (TCR) signals have been proposed to be the primary drivers of homeostatic T cell proliferation. However, it is not known why CD4+ T cells undergo less efficient homeostatic proliferation than CD8+ T cells. Here we showed that systemic IL-7 concentrations rise during lymphopenia due to diminished IL-7 utilization, but that IL-7 signaling on IL-7Rα+ dendritic cells (DCs) in lymphopenic settings paradoxically diminishes CD4+ T cell homeostatic proliferation. This effect is mediated, at least in part, by IL-7-mediated downregulation of MHC class II expression on IL-7Rα+ DCs. These results implicate IL-7Rα+ DCs as regulators of the peripheral CD4+ T cell niche, and indicate that IL-7 signals in DCs prevent uncontrolled CD4+ T cell expansion in vivo.
INTRODUCTION
Regeneration of T cells in lymphopenic hosts can occur via thymopoiesis or thymic-independent homeostatic proliferation of peripheral T cells. Thymic output diminishes early in life and, in clinical settings associated with lymphocyte depletion, is further limited by thymotoxic insults such as cytotoxic chemotherapy, radiotherapy, HIV infection, and graft versus host disease (GVHD)1-3. As a result, humans experiencing T cell depletion frequently rely upon homeostatic proliferation to restore peripheral T cell populations. Homeostatic proliferation efficiently regenerates peripheral CD8+ lymphocyte pools, but inefficiently regenerates CD4+ T cell populations4-6. Current models of T cell homeostasis do not explain the molecular and/or cellular basis for this difference in the ability to regenerate CD4+ and CD8+ T cell pools.
Previous murine studies concluded that homeostatic proliferation of naïve CD8+ and CD4+ T cells requires T cell receptor (TCR) signals delivered by peptides presented by class I and class II MHC molecules, respectively7-9. In addition, naïve CD4+ and CD8+ T cells fail to proliferate, and disappear when transferred into recipients lacking endogenous interleukin 7 (IL-7)10,11 and short-term administration of pharmacologic doses of IL-7 increases homeostatic proliferation of CD4+ and CD8+ lymphocytes12-14. These findings support a model in which TCR signaling and IL-7 act as primary drivers of naïve T cell homeostatic proliferation. At the same time, the biological impact of IL-7, at least with regard to proliferation, is greater on CD8+ compared to CD4+ T cells14,15, and elevated IL-7 concentrations in lymphopenic humans show stronger inverse correlations with CD4+ than with CD8+ T cell numbers16,17. The latter findings raise the possibility that chronic stimulation with excessive IL-7 could negatively impact CD4+ T cell homeostatic proliferation and potentially contribute to the differences observed between regeneration of CD4+ vs. CD8+ T cells. Here we sought to identify factors limiting homeostatic proliferation of naïve CD4+ T cells during lymphopenia, with a primary focus on the potential role of IL-7. We demonstrate that IL-7 concentrations become elevated in lymphopenic hosts due to diminished utilization. Chronically elevated systemic IL-7 levels diminish the capacity for IL-7Rα+ DCs to support CD4 homeostatic expansion, at least in part, via IL-7 mediated downregulation of MHC II expression. Thus, IL-7 signaling in DCs regulates the size of the peripheral naïve CD4+ niche.
RESULTS
IL-7 concentrations rise in lymphopenic mice
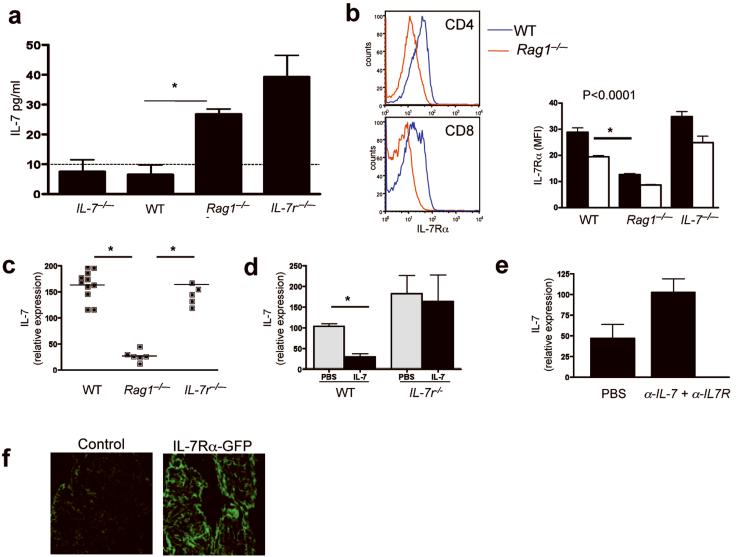
In humans16-18 and other primates19, lymphopenia is associated with increased concentrations of IL-7 in the circulation and in tissues. However, whether a similar link between lymphopenia and IL-7 exists in mice is not known. Using commercial ELISA kits that can measure IL-7 in quantities as low as 30 pg/ml, we detected approximately 40 pg/ml IL-7 in the serum of Il7r-/- mice, but we did not detect IL-7 in the serum of Rag1-/- or wild-type mice (data not shown). Using a bioassay based upon proliferation of an IL-7- dependent cell line that could reliably measure IL-7 concentrations as low as 10 pg/ml (Supplementary Fig. 1a, online), we detected 10 pg/ml, 25 pg/ml and 40 pg/ml IL-7 in the serum of wild-type, Rag1-/- and Il7r-/- mice, respectively (Fig. 1a). A second bioassay based upon IL-7-mediated downregulation of IL-7Rα (http://www.signaling-gateway.org/molecule/query?afcsid=A001267)20 demonstrated that congenic lymphocytes transferred into Rag1-/- or Il7r-/- recipients (Fig. 1b and data not shown) expressed significantly lower amounts of surface IL-7Rα compared to lymphocytes transferred into wild-type or Il7-/- recipients. This was not due to proliferation-associated downregulation of IL-7Rα in response to lymphopenia, as CFSE staining showed no proliferation at the 24 h time point used for these studies (data not shown); this IL-7Rα downregulation was also not due to IL-7-mediated blockade of anti-IL-7Rα binding to IL-7Rα (Supplementary Fig. 1b, online). Reduced IL-7Rα protein expression was accompanied by diminished expression of IL-7Rα mRNA, as reported previously (data not shown)20. These data confirm that lymphopenic mice, like lymphopenic primates16-19, exhibit elevated IL-7 concentrations. In addition these findings reveal that IL-7 quantities are higher in Il7r-/- mice than in similarly lymphopenic Rag1-/- mice, raising the prospect that IL-7Rα contributes to the regulation of IL-7 production.
Figure 1.
IL-7Rα signaling regulates stromal IL-7 production in vivo. (a) IL-7 concentrations in serum (mean pg/ml of blood for triplicate wells) from indicated mice measured by 2E8 cell proliferation. Dashed line denotes limit of detection. *, P < 0.005 Blood was pooled from 4 mice, two independent experiments. (b) Left, IL-7Rα expression on congenic T cells 24 h after transfer into wild-type (WT) or Rag1-/- mice. Right, IL-7Rα MFI on congenic CD4 (black bars) and CD8 (white bars) T cells transferred into indicated recipients. *, P < 0.0001. Results are expressed as mean IL-7Rα (MFI), (n=4-5/group, 3 independent experiments). (c) IL-7 mRNA expression in splenic stroma of indicated mice, as measure by RT-PCR. Horizontal line indicates mean, and each dot represents an individual mouse. *, P < 0.0001. (d) Wild-type and Il7r-/- mice were treated with PBS or rhIL-7 (10μg/day) for 3 d (n=4/group), and IL-7 mRNA expression by the stroma was measured by RT-PCR. Data show mean ±s.e. of 4 mice/group, two independent experiments. *, P<0.0005. (e) Rag1-/- mice were treated with PBS or anti-IL-7 (M25) plus anti-IL-7Rα (A7R34) (1mg/day) for 3 d, and IL-7 mRNA expression by the stroma was measured by RT-PCR. Data show mean ±s.e. for 3 mice/group. (f) Fluorescence microscopy of the spleen capsule of wild-type mice (control) and mice expressing GFP downstream of the IL-7Rα promoter. Results are representative of two independent experiments. Two GFP transgenic mice and one WT control were analyzed in two independent experiments.
IL-7Rα-mediated IL-7 feedback loop
The different IL-7 concentrations observed in wild-type, Rag1-/- and Il7r-/- mice raised the possibility that IL-7 production is regulated by lymphopenia and/or IL-7Rα. Notably however, quantitative RT-PCR demonstrated that splenic stromal cells from Rag1-/- mice expressed significantly less IL-7 mRNA than WT splenic stromal cells (Fig. 1c). Similar results were obtained using spleen or LN homogenates from SCID versus wild-type mice (data not shown). Il7r-/- splenic stroma expressed similar amounts of IL-7 mRNA as wild-type splenic stroma (Fig. 1c), consistent with the finding that Il7r-/- mice contain more serum IL-7 than Rag1-/- mice (Fig. 1a) and suggesting that IL-7 signaling via IL-7Rα on lymphoid stroma modulates IL-7 production. To test this hypothesis, we measured IL-7 transcripts following administration of recombinant human IL-7 (rhIL-7) to wild-type and Il7r-/- mice. rhIL-7 significantly reduced IL-7 mRNA expression in wild-type, but not Il7r-/- mice (Fig. 1d). Similarly, inhibition of IL-7 signaling using blocking IL-7- and IL-7Rα- specific monoclonal antibodies enhanced IL-7 mRNA expression in Rag1-/- mice (Fig. 1e). These results implicate IL-7 signaling on lymphoid stromal populations in the regulation of IL-7 production in vivo.
As stromal IL-7Rα expression has only previously been described on human marrow stromal populations21,22, we examined IL-7Rα expression in the capsule of the spleen, an area rich in splenic stromal cells. To this end we used mice engineered to express GFP under the regulation of the IL-7Rα promoter. These mice showed substantial GFP expression in the splenic capsule (Fig. 1f), confirming IL-7Rα transcription in this compartment. These findings demonstrate the existence of an IL-7Rα-mediated feedback loop that regulates IL-7 production. In addition, these data indicate that elevated IL-7 protein expression in lymphopenic hosts results from an accumulation, rather than increased production, of IL-7. Paradoxically, IL-7 mRNA production diminished during lymphopenia.
High IL-7 promotes CD8+ but not CD4+ T cell proliferation
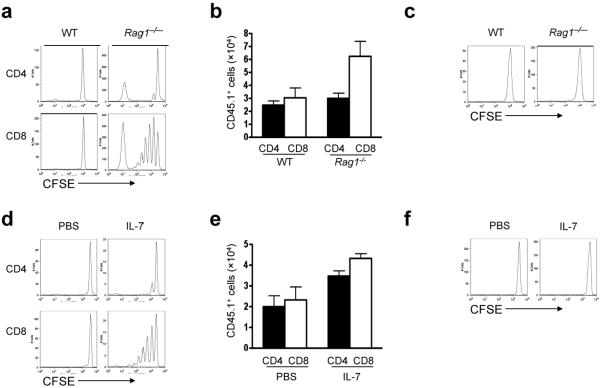
To compare the capacity for lymphopenia to drive homeostatic proliferation of CD4+ versus CD8+ T cell populations, lymph node (LN)-derived T cells were labeled with CFSE and transferred into Rag1-/- recipients. Within seven days, most CD8+ T cells proliferated, but the majority of CD4+ T cells did not divide (Fig. 2a,b). As reported previously, proliferation of polyclonal CD4+ populations in Rag1-/- mice was limited to a subset of rapidly dividing cells, which have been described as IL-7-independent, cross-reactive with environmental antigens and likely derived from memory CD4+ T cells23,24. Consistent with this hypothesis, we detected minimal proliferation of polyclonal CD4+ T cell populations depleted of CD44hi cells in Rag1-/- mice (Supplementary Fig. 2, online). Similarly, an exclusively naïve CD4+ population comprised of T cells from TCR transgenic `Marilyn' mice, which bear a single TCR restricted for MHCII in association with Dby, an antigen contained in the male HY complex, did not proliferate in female Rag1-/- mice; this finding agrees with previous findings using CD4+ T cells bearing other TCR transgenes25,26 (Fig. 2c). Therefore, lymphopenia efficiently supports homeostatic proliferation of bulk CD8+ but not CD4+ T cells, and induces essentially no proliferation of naïve CD4+ cells.
Figure 2.
Elevated systemic IL-7 preferentially expands CD8+ but not CD4+ T cells. (a,b) CD45.1+ LN T cells were labeled with CFSE and transferred into CD45.2+ wild-type (WT) or Rag1-/- recipients. (a) CFSE dilution was analyzed 7 d later. Representative CFSE profile. (b) Numbers of CD8+CD45.1+ and CD4+CD45.1+ T cells recovered from the spleens of recipients 7 d post-transfer (n=6-8 mice per group). (c) As in (a), but using CFSE-labeled Marilyn CD4+ cells. Results are representative of three independent experiments. (d,e) Polyclonal CD45.1+ LN T cells were labeled with CFSE and transferred into CD45.2+ wild-type recipients treated with PBS or IL-7 (10μg/day). (d) CFSE dilution was analyzed 7 d post-transfer. Representative CFSE profile. (e) Numbers of CD45.1+CD8+ and CD45.1+CD4+ T cells recovered from the spleens of recipient mice (n=3-6 mice/group) (f) As in (d), but using CFSE-labeled Marilyn CD4+ cells. These results are representative of four independent experiments.
Current models hold that IL-7 signaling is regulated by cytokine availability in vivo20. Therefore one potential explanation for these findings is a competitive advantage of CD8+ T cells compared to CD4+ T cells for limiting amounts of systemic IL-7, perhaps as a result of differential IL-7Rα expression. We could find no evidence to support this conclusion, as CD4+ T cell homeostatic proliferation did not increase after depletion of CD8+ T cells (data not shown). Nor did we observe differential IL-7Rα or common γ-chain (γc) expression on CD8+ versus CD4+ cells (data not shown), or differential STAT5 phosphorylation in CD4+ versus CD8+ T cells exposed to limiting concentrations of IL-7 ex vivo (Supplementary Fig. 3, online). Furthermore, transfer of LN T cells to mice receiving pharmacologic doses of rhIL-7, which generate serum human IL-7 levels one log greater than IL-7 concentrations found in lymphopenic mice27, resulted in efficient proliferation of CD8+ but not polyclonal or Marilyn CD4+ T cells (Fig. 2d-f). Notably, despite minimal proliferation of CD4+ T cells, we observed substantial accumulation of polyclonal (Fig. 2e) and Marilyn CD4+ T cells (data not shown) in IL-7 treated mice; these findings suggest that IL-7 enhances CD4+ T cell survival and thus may hold therapeutic utility despite limited proliferative effects. Similar results were observed when IL-7 was administered to Rag1-/- recipients (data not shown). Therefore, we conclude that exposure to elevated systemic IL-7, as occurs in lymphopenic mice or following pharmacologic IL-7 administration, preferentially expands CD8+ T cells.
In contrast, when antigen-presenting cell (APC) numbers were increased by treatment with rhFLT3 ligand, CD4+ T cells underwent substantial proliferation (Supplementary Fig. 4, online). This effect was markedly reduced in CD11c-DTR BM chimeric animals, in which diphtheria toxin (DT) induced depletion of CD11chi cells28. Therefore, accessibility to APCs, rather than availability of systemic IL-7, appears to be a primary factor limiting homeostatic proliferation of CD4+ T cells during lymphopenia.
Role of IL-7 signaling in BM-derived cells
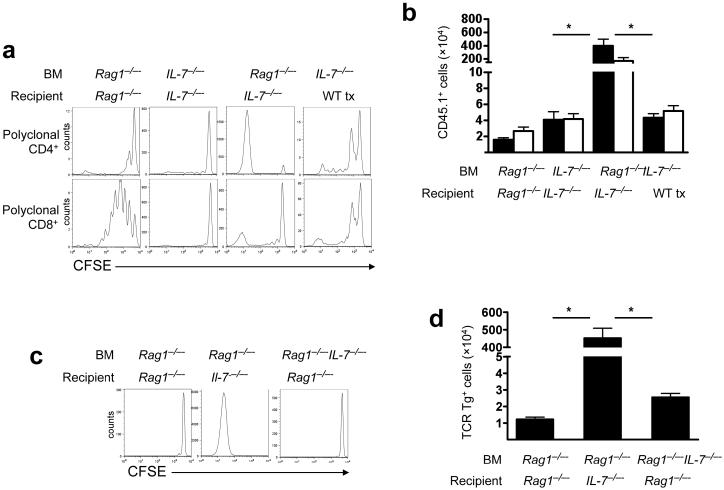
These results and previous studies29-33 demonstrating that modulation of APC numbers induces CD4+ T cell homeostatic proliferation emphasize the influence of MHCII+ DCs on the peripheral CD4+ T cell niche. However, several studies have also implicated IL-7 as necessary for CD4+ T cell homeostatic proliferation10,34,35. To investigate whether IL-7 produced by DCs is necessary or sufficient to support CD4+ T cell homeostatic proliferation, we transferred CFSE-labeled T cells into chimeric mice engineered such that IL-7 production is limited to radioresistant stroma or radiosensitive BM-derived cells (Supplementary Fig. 5, online). Despite lymphopenia in all groups, chimeras lacking IL-7 entirely (Il7-/- BM into Il7-/- recipients) failed to support CD4+ or CD8+ T cell homeostatic proliferation. IL-7 production by both stroma and BM-derived cells (Rag1-/- BM into Rag1-/- recipients) supported substantial CD8+ but limited CD4+ T cell homeostatic proliferation. Suprisingly, absence of stromal cell-derived IL-7 in the presence of BM-derived IL-7 (Rag1-/- BM into Il7-/- recipients) resulted in massive CD4+ T cell homeostatic proliferation, whereas chimeras producing IL-7 exclusively from radioresistant stromal cells (Il7-/- BM into thymectomized wild-type recipients) failed to support CD4+ T cell homeostatic proliferation (Fig. 3b,c). To confirm that the CD4+ T cell proliferation observed was indeed homeostatic, and not antigen-driven, proliferation24, we repeated this experiment using Marilyn CD4+ cells. The results confirmed that naïve CD4+ T cells do not undergo homeostatic proliferation when IL-7 is produced by both stroma and BM-derived cells or exclusively by the stroma; however, proliferation is supported when IL-7 production is limited to the BM-derived compartment (Fig. 3d,e). Therefore, BM-derived IL-7 is sufficient to support, whereas stromal cell-derived IL-7 paradoxically diminishes, CD4+ T cell homeostatic proliferation during lymphopenia. Settings where IL-7 production is limited to BM-derived cells facilitate maximal homeostatic proliferation of CD4+ T cells.
Figure 3.
BM-derived IL-7 supports, whereas stromal cell-derived IL-7 inhibits, CD4+ T cell homeostatic proliferation in lymphopenic hosts. (a,b) CFSE-labeled CD45.1+ polyclonal wild-type T cells were transferred into the indicated BM chimeras. Tx, thymectomized. (a) Representative CFSE profile measured 7 d post-transfer. (b) Numbers of CD8+CD45.1+ and CD4+CD45.1+ T cells recovered from the spleens of recipients 7 d post-transfer. CD4 (black bars) and CD8 (white bars) Data represent mean±s.e. for each group (n=6-10 mice/group) *, P<0.0001. (c,d) As in (a,b), but using CFSE-labeled Marilyn CD4+ cells (n=6-8 mice/group). *, P<0.0001. These results are representative of two independent experiments.
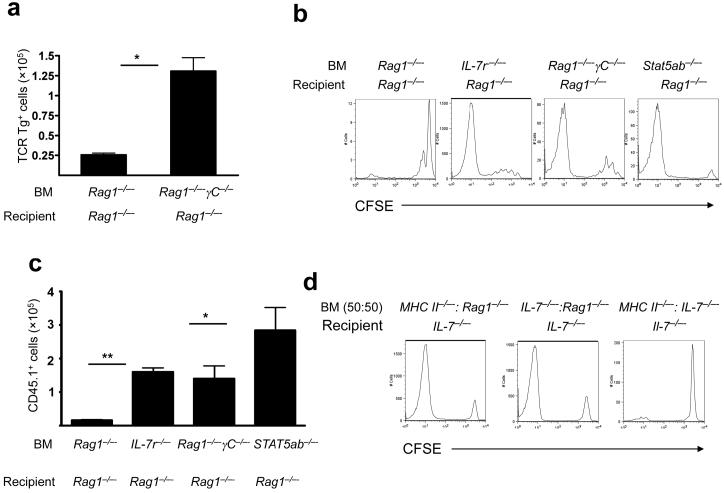
As hosts with elevated levels of IL-7 derived from stroma showed limited CD4+ T cell homeostatic proliferation (Fig. 3), we hypothesized that IL-7 signaling through IL-7Rα+ on hematopoietic cells could regulate CD4+ T cell homeostatic proliferation in vivo. To test this, we measured CD4+ T cell homeostatic proliferation in BM chimeras engineered such that BM-derived cells could not receive IL-7 signals (Il7r-/- BM into Rag1-/- recipients and Rag1-/- Il2rγ-/- BM into Rag1-/- recipients). Notably, Il7r-/- and Il2rγ-/- BM supported marked homeostatic proliferation of Marilyn (Fig. 4a) or polyclonal CD4+ T cells (Fig. 4b,c). Similarly, chimeras wherein downstream mediators of IL-7 signaling were lacking in BM-derived cells (Stat5a-/-Stat5b-/- BM into Rag1-/- recipients) also supported robust homeostatic proliferation of polyclonal CD4+ T cell populations (Fig. 4b,c). These results demonstrated that IL-7 signaling via IL-7Rα and STAT5 in BM-derived cells inhibits CD4+ T cell homeostatic proliferation. Notably, the robust CD4+ T cell homeostatic proliferation observed in the absence of γc signaling on APCs rules out the possibility that thymic stromal lymphopoietin (TSLP), mediates this inhibitory effect.
Figure 4.
IL-7 signaling on BM-derived cells inhibits CD4+ T cell homeostatic proliferation during lymphopenia. (a) Marilyn T cells were transferred into the indicated BM chimeras. On day +7 after lymphocyte transfer, Marilyn T cells recovered from the spleen were enumerated. Data show mean±s.e. (n=6 mice/group), results representative of three independent experiments. *, P<0.001. (b) CFSE-labeled wild-type CD4+CD45.1+ cells were transferred into indicated BM chimeras. Seven days post-transfer CFSE dilution was assessed (n=6-8 mice/group), results representative of two independent experiments. (c) Wild-type CD4+CD45.1+ cells were transferred into indicated BM chimeras. Seven days post-transfer donor cells recovered from the spleen were enumerated. Data show mean±s.e. (n=3-6 mice/group). *, P<0.05 and **, P<0.0001). (d) Lethally irradiated Il7-/- mice received a mixture of the indicated BM. Five weeks later, BM chimeras received CFSE-labeled wild-type CD45.1+ T cells and, 7 days later, CFSE dilution was analyzed, (n=6-10 mice/group, results representative of three independent experiments).
Previous studies concluded that stroma-derived IL-7 is necessary and sufficient to support CD8+ T cell homeostatic proliferation10; however, but the results presented here clearly demonstrate that BM-derived IL-7 is sufficient to support CD4+ T cell homeostatic proliferation. IL-7 is not produced by T cells, B cells or natural killer (NK) cells, but is produced by DCs and macrophages, albeit in lower amounts than by lymphoid stroma36-38. To determine whether BM-derived IL-7 could be delivered in trans with MHCII signals to CD4+ T cells, or whether IL-7 production and MHCII must be present on the same BM-derived cell in order to facilitate CD4+ T cell homeostatic proliferation, we compared CD4+ T cell homeostatic proliferation in lethally irradiated Il7-/- mice reconstituted with a 1:1 mixture of BM from MHC II-/- and Il7--/- mice, or a 1:1 mixture of BM from Rag1-/- and either MHCII-/- or Il7-/- mice. Mice expressing IL-7 and MHC in cis, or in the same cell (MHCII-/- and Rag1-/- BM into Il7-/- recipients or Il7-/- and Rag1-/- BM into Il7-/- recipients) exhibited robust CD4+ T cell homeostatic proliferation, whereas those expressing MHCII and IL-7 exclusively in trans (MHCII-/- and Il7-/- BM into Il7-/- recipients) showed minimal CD4+ T cell homeostatic proliferation (Fig. 4d). These results confirmed the critical role for MHCII in supporting CD4+ T cell homeostatic proliferation, support a model wherein access to a cells producing IL-7 and expressing MHCII is required for CD4+ T cell homeostatic proliferation, and demonstrate that the presence of stromal cell-derived IL-7 impairs CD4+ T cell homeostatic proliferation.
IL-7Rα signaling regulates DC MHCII expression
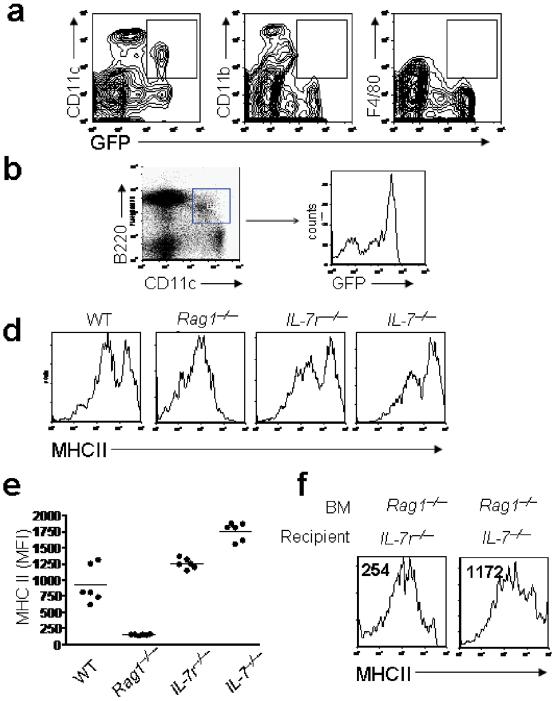
Given findings implicating MHCII in CD4+ T cell homeostatic proliferation, and the evidence presented above demonstrating that IL-7 signaling on BM-derived cells regulates CD4+ T cell homeostatic proliferation, we sought to evaluate IL7Rα expression on DC subsets by enumerating IL-7Rα mRNA transcripts in electronically sorted splenocyte subpopulations. DCs expressed less IL-7Rα mRNA than T cells, but among the various DC subsets, CD11b- CD11c+ DCs expressed the most abundant IL-7Rα transcripts (Supplementary Fig. 5, online). Using splenocytes from mice expressing GFP under control of the IL-7Rα promoter, we confirmed that a sizable fraction of CD11b-CD11c+ DCs express IL-7Rα (Fig. 5a), and demonstrated that the majority of cells bearing the phenotype ascribed to plasmacytoid DCs (B220+CD11b-CD11c+ MHCII+) also express IL-7Rα (Fig. 5b). We next evaluated the effects of IL-7 signaling on MHCII expression in vivo on APCs. We observed diminished MHCII expression on CD11b+CD11c-, CD11b+CD11c+ and CD11b-CD11c+ cells in Rag1-/- compared to wild-type mice (Fig. 5c), with CD11b-CD11c+B220+ cells expressing the lowest amounts of MHCII. Modulation of MHC Class II expression was mediated by IL-7 signaling, as APCs from Il7r-/- and Il7-/- mice, which are as lymphopenic as Rag1-/- mice, expressed wild-type amounts of MHCII (Fig 5d,e). Furthermore, MHCII expression was restored on Rag1-/- DCs transplanted into Il7-/- but not Il7r-/- recipients (Fig. 5f).
Figure 5.
IL-7 Signaling diminishes MHCII expression on APCs during lymphopenia. (a,b) APCs from mice expressing GFP under the control of the IL-7Rα promoter were analyzed by flow cytometry. *, P< 0.05. (c) Left, CD11b+CD11c-, CD11b+CD11c+ and CD11b-CD11c+ splenocytes in indicated mice were enumerated. Data show mean±s.e. Right, MHCII expression was measured on the designated splenocyte subsets from representative Il7r-/- (red shaded), and Rag-/- (blue line) mice. (d,e) MHCII expression on plasmacytoid DCs from indicated mice. (d) Representative histograms gated on CD11b-CD11c+B220+ cells. (e) MFI of MHCII expression. (n=6 mice/group) (f) MHCII expression on plasmacytoid DCs from indicated BM chimeras. These results are representative of three independent experiments.
Thus, IL-7Rα+ DCs primarily consist of CD11b-CD11c+B220+ ClassII+ cells, and high systemic IL-7 concentrations diminish MHCII expression on IL-7Rα+ DCs. Given the critical role for MHCII-CD4 interactions in CD4+ T cell homeostatic proliferation, these results implicate IL-7-mediated downregulation of MHCII expression as a likely mechanism through which IL-7Rα+ DCs regulate CD4+ T cell homeostatic proliferation in vivo.
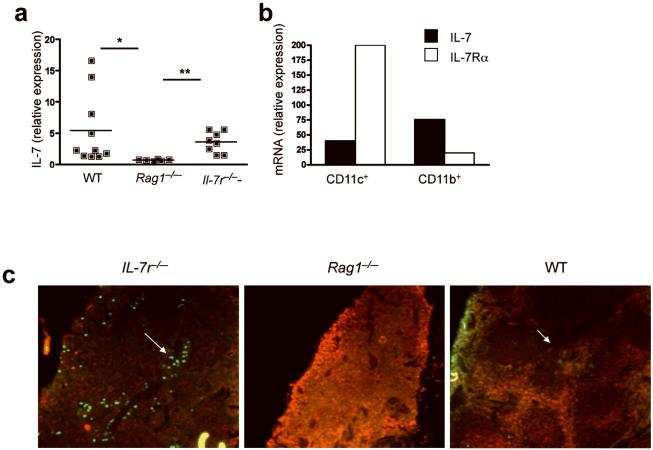
Feedback loop regulates DC IL-7 production
In addition to the effects of IL-7 signaling on MHCII expression, it remains possible that other effects of IL-7Rα signaling on DCs contribute to the diminished CD4+ T cell homeostatic proliferation observed in the presence of high levels of stromal cell-derived IL-7. Given that IL-7 production exclusively by BM-derived cells is necessary and sufficient to sustain CD4+ T cell homeostatic proliferation in Il7-/- recipient mice, we postulated that IL-7 production by DCs themselves may be physiologically relevant in vivo. Therefore, we sought to determine whether IL-7 signaling on DCs regulates IL-7 production by DCs as it does on stromal cells. Similar to results obtained with stromal cell preparations, IL-7 mRNA was diminished in splenocytes from Rag1-/- mice (Fig. 6a) compared to splenocytes from wild-type or Il7r-/- mice, demonstrating that IL-7Rα-mediated feedback also regulates non-stromal cell derived IL-7 production. Among IL-7-producing DC subsets, we noted an inverse relationship between IL-7Rα expression and IL-7 production, such that IL-7 production was lower in the IL-7Rα+CD11b-CD11c+ subset than in the IL-7Rα-/loCD11b+CD11c+ subset (Fig. 6b and Supplementary Fig. 6b, online). We noted higher numbers of cells producing more abundant IL-7 within the DC region of the spleen of Il7r-/- compared with Rag1-/- or wild-type mice (Fig. 6c). Thus, IL-7Rα signaling facilitates downregulation of MHCII expression and IL-7 production in DCs; these feedback loops could explain the detrimental effects of high systemic IL-7 quantities on CD4+ T cell homeostatic proliferation.
Figure 6.
Stromal cell-derived IL-7 regulates DC IL-7 production within the lymphoid microenvironment. (a) IL-7 mRNA expression in splenocytes from indicated mice was measured by RT-PCR. Horizontal lines indicate mean, and each dot represents an individual mouse. 6 to 10 individual mice were analyzed and the experiment was done twice *, P<0.05 and **, P<0.005. (b) IL-7 and IL-7Rα mRNA expression in electronically sorted CD11b-CD11c+ and CD11b+CD11c- cells from pooled wild-type mice. Results are representative of 2 independent experiments. (c) IL-7 (green) and CD45 (red) expression in spleens of indicated mice, as measured by immunofluorescence. Results are representative of 2 independent experiments.
IL-7Rα signals in T cells regulate proliferation
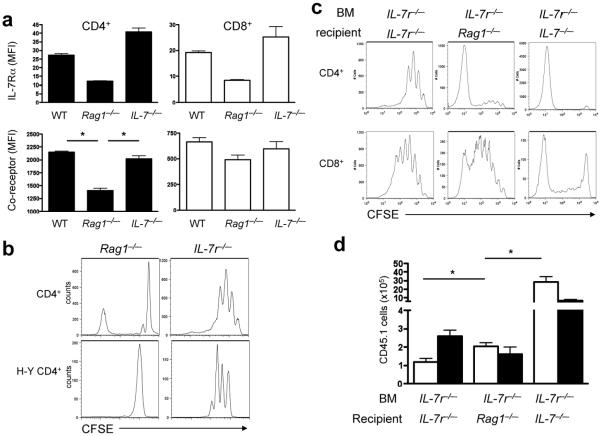
In addition to changes in APC function, we reasoned that high concentrations of systemic IL-7 could also act directly on T cells to suppress their capacity to undergo homeostatic proliferation. IL-7 signaling diminishes IL-7Rα expression20 and IL-7Rα downmodulation on proliferating CD4+ cells in settings of high IL-7 concentrations could directly constrain IL-7-dependent homeostatic proliferation. Indeed, CD4+ and CD8+ T cells transferred into Rag1-/- mice show diminished IL-7Rα expression compared to those transferred into Il7-/- mice (Fig. 1b, 7a). The degree of IL-7Rα downregulation was similar in CD4+ and CD8+ cells, suggesting that this effect is not responsible for the differential homeostatic proliferation of CD4+ versus CD8+ cells (Fig. 2). However, we also observed downregulation of CD4, but not CD8, co-receptor expression on T cells transferred into Rag1-/-, but not Il7-/- mice (Fig. 7a). Therefore, diminished IL-7Rα and/or CD4 co-receptor expression could also contribute to inefficient homeostatic proliferation of CD4+ T cells in lymphopenic hosts with high systemic IL-7 concentrations. The effects of reduced IL-7Rα and/or CD4 expression would be expected to synergize with the influence of diminished MHCII expression in lymphopenic mice.
Figure 7.
IL-7 acts directly on CD4+ T cells. (a) Polyclonal CD45.1+ T cells were transferred into indicated recipient mice. At day +7, lL-7Rα, CD4 and CD8 expression on CD45.1+ T cells was measured by flow cytometry. Data show mean±s.e. (n=3 mice/group), and the results are representative of two independent experiments. *, P<0.001. (b) CFSE-labeled polyclonal and naïve H-Y TCR transgenic CD4+ T cells were transferred into the indicated recipient mice. CFSE dilution was measured at day +7 ater adoptive transfers. transfer. (c,d) CFSE-labeled CD45.1+ T cells were transferred into the indicated BM chimeras. Seven days later, CFSE dilution was measured (c) and CD45.1+ T cells in the spleen were enumerated (d). Data in (d) show mean±s.e. (n=6-12/group). *, P<0.01 and **, P<0.001. These results are representative of 3 independent experiments.
To assess the impact of IL-7 signaling on T cells versus APCs, we evaluated homeostatic proliferation of wild-type CD4+ T cells transferred into Il7r-/- mice, wherein IL-7 signaling on APCs cannot be invoked to explain constrained CD4+ T cell homeostatic proliferation. Both polyclonal CD4+ and Marilyn CD4+ T cells underwent substantially increased homeostatic proliferation, compared to that seen in Rag1-/- mice (Fig. 7b). Next we compared the effect of varying amounts of stromal cell-derived IL-7 on the homeostatic proliferation of wild-type CD4+ T cells transferred into BM chimeras created such that APCs lack IL-7Rα (and therefore are maximally supportive of CD4+ T cell homeostatic proliferation). Even when APCs lack IL-7Rα, high systemic IL-7 concentrations (in Il7r-/- recipients) suppressed CD4+ T cell homeostatic proliferation; proliferation was increased and maximal in Rag1-/- and Il7-/- recipients, respectively (Fig. 7c,d). Therefore, in addition to APCs, T cells are influenced by high concentrations of systemic IL-7.
To compare the relative dominance of IL-7 effects on APCs versus T cells, we utilized Il7r-/- recipients (which contain maximal amounts of stromal cell-derived IL-7) and compared expansion of wild-type CD4+ cells in the presence or absence of IL-7 signaling in APCs. If T cell-centric effects of high systemic IL-7 concentrations were the predominant force inhibiting CD4+ T cell homeostatic proliferation, minimal proliferation would occur in this system, regardless of whether APCs had the capacity to transmit IL-7 signals. However, wild-type CD4+ T cell proliferation was substantially higher in recipients of Il7r-/- compared to Rag1-/- BM (Supplementary Fig. 7, online). We conclude therefore that IL-7 signaling on APCs is the primary regulator of naïve CD4+ T cell homeostatic proliferation during lymphopenia.
DISCUSSION
Multiple clinical studies have demonstrated that lymphopenic insults are followed by relatively rapid normalization of CD8+ T cells via thymic-independent homeostatic proliferation, whereas CD4+ T cells remain chronically depleted, unless thymic function is present1,2,6,39,40. Moreover, although IL-7 has been implicated as a driver of homeostatic proliferation, clinical studies show inverse relationships between systemic IL-7 concentrations and CD4+ T cell counts, raising the possibility that high quantities of IL-7 negatively regulate CD4+ T cell homeostasis. Such an inhibitory effect would make sense, as IL-7 is continually available within lymphoid tissues and is measured in substantial amounts in the serum17, the IL-7 receptor complex is expressed on most T cells and IL-7 signals induce potent co-stimulation. Some system to prevent uncontrolled IL-7-mediated T cell proliferation in vivo must be in place. Previous studies postulated that IL-7 effects are regulated by competition for limiting amounts of IL-7 and dynamic regulation of IL-7Rα expression on T cells20. This report identifies two novel points through which IL-7 regulates CD4+ T cell homeostasis. First, we demonstrated that IL-7 production in vivo is tightly regulated by a simple feedback loop. When tissue IL-7 concentrations rise (e.g. due to diminished utilization during lymphopenia, or as a result of injection of exogenous IL-7), IL-7 production diminishes in both stromal cells and APCs. Second, we demonstrated that IL-7 signaling on APCs and, to a lesser extent on T cells, controls homeostatic expansion of CD4+ cells in vivo.
The effects of IL-7 on IL-7Rα+ APCs are likely manifold, but we observed substantially diminished MHCII expression on IL-7Rα+ DCs in the presence of elevated IL-7 quantities in vivo, and enhanced MHCII expression on IL-7Rα+ DCs when IL-7 was absent. The transcription factor CIITA is required for MHCII expression, and p38 MAPK signaling pathway is a negative regulator of CIITA gene expression42. IL-7 activates p3843, and DCs grown in the presence of IL-7 express lower amounts of MHCII44. Hence we postulate IL-7-mediated downmodulation of MHCII expression on IL-7Rα+ DCs directly diminishes support for CD4+ T cell homeostatic proliferation. Consistent with this, we observed that expansion of APCs via Flt3 ligand potently augments CD4+ T cell homeostatic proliferation, further implicating availability of MHCII+ APCs is a primary factor regulating the size of the peripheral CD4+ T cell niche. A major impact of MHCII expression is consistent with models invoking a critical influence of TCR stimulation on CD4+ T cell homeostatic proliferation.25
In addition to MHCII expression, IL-7 signaling may influence other attributes of IL-7Rα+ DCs. Several reports have demonstrated that APCs produce IL-736-38,45, but the biological relevance of this observation has not been explored. Our studies in Il7-/- hosts demonstrate that co-expression of IL-7 and MHCII by the same APC is necessary to support CD4+ T cell homeostatic proliferation. This may relate to the relatively infrequent number of IL-7Rα+ DCs and/or the relatively low amount of IL-7 released by DCs, which would make it unlikely for naïve CD4+ T cells to receive both signals simultaneously by different cells. Whether this requirement holds true during lymphopenia, when systemic IL-7 quantities increase, remains unknown. Indeed, it seems paradoxical to invoke limiting amounts of IL-7 produced by DCs as regulating CD4+ homeostatic proliferation if tissue quantities of IL-7 are elevated. However, such an effect could occur if tissue IL-7 is not as physiologically accessible to CD4+ T cells as it is to CD8+ T cells. Future studies are necessary to clarify the physiological role of APC-derived IL-7, and other potential downstream effects of IL-7 signaling on IL-7Rα+ DCs that might influence CD4+ T cell homeostasis.
The full physiological picture of the dynamic regulation of homeostatic expansion in vivo must also consider IL-7-mediated modulation of IL-7Rα expression and co-receptor expression on T cells. Modulation of IL-7Rα expression on T cells contributes to constraint of T cell homeostatic proliferation in vivo, an effect that may be compounded by downmodulation of CD4 expression. Previous studies have demonstrated similar T cell-intrinsic forces that limit CD4+ T cell homeostatic proliferation, including IL-7—induced Fas expression that predisposes T cells to activation-induced cell death46. Despite these findings, our results demonstrate that IL-7Rα signaling on APCs is the dominant axis through which IL-7 regulates CD4+ proliferation during lymphopenia.
Our findings have several clinical implications. First, they implicate plasmacytoid DCs, which comprise the majority of IL-7Rα+ DCs, as a central regulator of the peripheral CD4+ T cell niche. We did not observe IL-7Rα mediated differences in APC numbers in the lymphopenic models presented in this report, but pDCs have been reported to be reduced in clinical settings associated with CD4+ T cell depletion such as HIV infection and following BM transplantation47-49, raising the possibility that IL-7Rα+ DC depletion could contribute to chronic CD4+ T cell depletion in humans. Second, these results predict that therapies that expand IL-7Rα+ DC populations and/or modulate IL-7 signaling in APCs could improve immune reconstitution and potentially improve the effectiveness of adoptive cell therapy. Finally, it is possible that IL-7Rα signaling on APCs could control autoaggressive T cells in vivo in settings where CD4+ populations mediate autoimmune pathology.
METHODS
Animals, BM chimeras, and cytokines
All experiments were approved by the NCI Animal Care and Use Committee. Mice were C57BL/6Ncr females (CD45.1+) or, where indicated, C57BL/6Ly5.2 (CD45.2+) and were purchased from the Animal Production Unit of the NCI. B6;129S7-Rag1tm1Mom/J and B6.129-H2dlAb1-Ea/J were purchased from Jackson and C57BL/6RAG-/-γC-/- mice were purchased from Taconic. C57BL/6 Il7-/-, C57BL/6 Il7r-/-, C57BL/6 CD11c-DTR, C57BL/6 Stat5a-/-Stat5b-/- and Rag-/- Marilyn mice were bred at NIH facilities. Rag-/-Il7-/- mice were kindly provided by S. Durum (NCI, Frederick, MD). For chimera generation, BM cells were obtained from both the tibias and femurs of donor mice. Recipient mice were irradiated with 1300 Rads (2 × 650) from a 137Cs source and injected i.v. with 107 BM cells. Five weeks later, chimeric mice were injected with congenic lymphocytes as specified. For depletion of CD11chi cells during Flt3 ligand (FL) treatment, chimeras were utilized since repeated dosing of diphtheria toxin (DT) has been previously reported to be lethal in CD11c-DTR mice26 whereas BM chimeric mice are physically tolerant to repeated DT administration allowing continued depletion of CD11chi cell population. CD45.1+ C57BL/6 CD11c-DTR donor BM cells were transplanted into lethally irradiated CD45.1+ C57BL/6 recipients. Five weeks following BM transplantation, 106 enriched LN T cells (CD45.2+) were adoptively transferred via the tail vein to the BM chimeas. Recombinant human FL or PBS was administered as a daily i.p. injection (10 μg) for 14 days. Among the FL-treated mice, some received DT treatment to ablate CD11chi cells. DT treatment (4ng/kg/body weight) was performed at day 0, +4, +8 and +12. Mice were sacrificed at day +14 and transferred lymphocytes analyzed. RhIL-7 was supplied by Cytheris Inc and administered i.p. (10 μg/d) for 12 consecutive days.
Measurement of IL-7
Serum was isolated via the retroorbital vein from anesthetized mice. Serum from 4 mice was pooled and incubated with the IL-7-dependent cell line, 2E8 (ATCC). Briefly, 2E8 cells were plated at 105 cells/well into a 96-well flat-bottomed microtiter plate with rmIL-7 serially diluted as a standard, or with unknown serum samples plated in triplicate; each well contained a final volume of 200μl. Plates were incubated for 5 days at 37°C, and proliferation was measured by adding 1 μCi of 3H-thymidine/well 18h before DNA harvesting. Thymidine incorporation was measured using a Beckman Packwood plate counter. Concentrations of unknown samples were extrapolated based on c.p.m induced by known IL-7 concentrations.
CFSE staining and adoptive transfer of lymphocytes
LNs from wild-type mice were homogenized and lymphocytes were negatively enriched using the mouse Pan T Cell Isolation Kit (Miltenyi Biotec). For enrichment of the naïve lymphocyte fraction, enriched T cells were labeled with anti-CD44 PE (Pharmigen), then anti-PE microbeads were used for removal of CD44+ cells (Miltenyi Biotec). Enriched T cells suspended at 107 cells per ml in PBS were incubated at 37°C with 1μl of 5 μM CFSE (Molecular Probes) for 15 min. Cells were washed twice in PBS and resuspended at of 2×106 cells/ml. Recipient mice received 1×106 CFSE-labeled polyclonal T cells and where indicated 0.5×106 Marilyn T cells. After 7 days, mice were sacrificed, and CFSE content was analyzed using a dual laser FACSCalibur (Becton Dickinson). All analyses were performed using FlowJo software (Tree Star, Inc.). Calculation of the absolute number of cells recovered per spleen used a gate that included lymphoid and myeloid cells according to the FSC and SSC profile.
Flow cytometry
Splenocytes were resuspended at 10×106 cells/ml in FACS buffer (bovine serum albumin (BSA) (0.2%) and NaN3 (0.1%)) and incubated with diluted monoclonal antibodies for 30 min on ice. Cells were then washed twice with FACS buffer and resuspended in 1% formalin in PBS. For GFP analysis, cells were resuspended in FACS buffer for immediate analysis. The following monoclonal antibodies were used: Vβ6-PE (clone RR4-7), CD3-FITC (clone 145-2C11) IgG1,k (clone A19-3), CD4-PE and CD4-PerCP-Cy5.5 (clone RM4-5), CD4-PE, CD8-FITC (clone Ly-2), CD8-PerCP-Cy5.5(clone Ly-2), CD11b-APC (clone M1/70), CD11b-PE (clone M1/70), CD11b-PerCP-Cy5.5 (clone M1/70), CD11c-PE (clone HL3) CD44-PE (cloneIM7), CD45.1-APC (clone A20), CD45.2-PerCP-Cy5.5 (Clone 104), CD62L-APC (MEL-14), I-A-I/I-E-PE (clone M5/114.15.2), B220-FITC and B220-PE (Clone RA3 6B2), STAT5-PE (clone 47) Nonspecific binding was determined using the following isotype controls: Rat IgG2a-FITC, PE, PerCP-Cy5.5 and APC (clone R35-95), Rat IgG2b-PE, PerCP-Cy5.5 and APC, (clone A95-1), Mouse IgG1, (clone 107.3), Armenian Hamster IgG1-FITC and PE (clone A19-3). all antibodies were purchased from Pharmingen. CD127-APC (clone A7R34) was purchased from e-Bioscience. Immunofluorescence was determined by automated multi-parameter flow cytometry analyzing at least 104 cells for purity and up to 107 for CFSE analysis. For cell sorting, 107 cells were labeled with the following antibodies: B220-FITC, I-A/I-E-PE, CD11b PerCP-Cy5.5, CD11c-APC. Cell sorting was performed using a FACSAria (Beckton Dickinson). For p-STAT5 detection, 106 cells were first labeled with antibodies directed at cell surface antigens, then washed using PBS supplemented with 2% BSA. Cells were then incubated with IL-7 for 30 minutes at 37°C, then immediately fixed and permeabilized (Cytofix/Cytoperm, BD Pharmigen), then stained using anti-p-STAT5 (clone 47, Pharmingen).
Real time PCR
For analysis of stroma versus splenocyte RNA, whole spleens were sliced longitudinally into 2 sections using sharp blades, then opened and washed using cold PBS washing splenocytes into a flask, and leaving the capsule clear of cell populations. The washed capsule was used as the stromal population, then minced with scissors prior to RNA isolation. Recovered splenocytes were treated with red cell lysis buffer (Lonza), washed, passed through nylon mesh then used as the splenocyte fraction. Total RNA was extracted from the fractions using FastRNA Pro Green Kit (Q-Bio Gene), then re-suspended in 100ul of DEPC water. RNA concentration was determined using a spectrophotometer. 1μg of RNA was converted to cDNA using a 1st Strand cDNA Synthesis Kit for RT-PCR (Roche). Reverse transcription was carried out in a total volume of 20μl using random primers according to the instruction of the manufacturer. RT-PCR was carried out using an Abi-Prism 7000 Sequencing Detection System with the following cycle profile: 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 sec and 60°C 1 min. PCR amplifications were performed in a total volume of 50μl containing 1μl of template DNA, 25μl of TaqMan Universal PCR Master Mix (Roche), 2.5μl of specific probes (see below), and 21.5μl of DEPC water. Results were quantified using a standard of double stranded plasmid DNA containing the gene of interest. Standard curves were created using 10 fold dilutions from 100pg to 100ag. Samples were run in duplicate and the mean crossing point was compared to a set of standards run on the same plate. The probes used in this study were purchased from TaqMan Gene Expression Assays (Applied Biosystems) (Supplementary table 1, online).
Immunostaining
Frozen tissues were sectioned at 5 μm intervals and fixed using acetone. After blocking for 30 min in PBS supplemented with 5% goat serum, sections were incubated with primary antibodies. Mouse monoclonal anti-IL-7 (clone M25) isotype IgG2b was generously provided by Immunex and used at 20μg/ml, and rat anti-murine CD45 (clone 30-F11) isotype IgG2b (BD Bioscience) used at 1:20 dilution; antibodies were added for 30 min at room temperature. Goat anti-rat IgG AlexaFluor 594 and goat anti-mouse IgG AlexaFuor 488 secondary antibodies (at 1:400 dilution) were applied to sections for 30 min in the dark at room temperature, and mounted with VectaShield containing DAPI nuclear stain (Vector Laboratories). Sections were viewed using a Zeiss AxioObserver Z1 microscope (Zeiss Inc.) using a 20× differential interference contrast objective and appropriate filter sets. Images were captured with a Zeiss AxioCam MRm monochrome digital camera and analyzed using AxioVision 4.6 software. (Carl Zeiss MicroImaging, Inc.,Thornwood NY)
Statistical analysis
Tests were performed with Prism 4.0 (GraphPad Software). Statistics were two-tailed, unpaired Student's t-test with 95% confidence bounds unless otherwise indicated. Bar graph error bars are ± 1 s.e.m.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank V. Kapoor and N. Voong flow cytometric expertise and Dr. M.A Caligiuri that kindly provided FL. We would also like to thank S. Durum, A. Singer and R. Gress for their careful reviews of the manuscript. This work was supported by the Intramural Program of the National Cancer Institute.
REFERENCES
- 1.Mackall CL, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–9. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 2.Heitger A, et al. Essential role of the thymus to reconstitute naive (CD45RA+) T-helper cells after human allogeneic bone marrow transplantation. Blood. 1997;90:850–7. [PubMed] [Google Scholar]
- 3.Dumont-Girard F, et al. Reconstitution of the T-cell compartment after bone marrow transplantation: restoration of the repertoire by thymic emigrants. Blood. 1998;92:4464–71. [PubMed] [Google Scholar]
- 4.Hakim FT, et al. Constraints on CD4 recovery postchemotherapy in adults: thymic insufficiency and apoptotic decline of expanded peripheral CD4 cells. Blood. 1997;90:3789–98. [PubMed] [Google Scholar]
- 5.Douek DC, et al. Evidence for increased T cell turnover and decreased thymic output in HIV infection. J Immunol. 2001;167:6663–8. doi: 10.4049/jimmunol.167.11.6663. [DOI] [PubMed] [Google Scholar]
- 6.Hakim FT, et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest. 2005;115:930–9. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–90. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Surh CD, Sprent J. Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? J Exp Med. 2000;192:F9. doi: 10.1084/jem.192.4.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seddon B, Zamoyska R. TCR signals mediated by Src family kinases are essential for the survival of naive T cells. J Immunol. 2002;169:2997–3005. doi: 10.4049/jimmunol.169.6.2997. [DOI] [PubMed] [Google Scholar]
- 10.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 11.Vivien L, Benoist C, Mathis D. T lymphocytes need IL-7 but not IL-4 or IL-6 to survive in vivo. Int Immunol. 2001;13:763. doi: 10.1093/intimm/13.6.763. [DOI] [PubMed] [Google Scholar]
- 12.Mackall CL, et al. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood. 2001;97:1491–7. doi: 10.1182/blood.v97.5.1491. [DOI] [PubMed] [Google Scholar]
- 13.Fry TJ, et al. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood. 2003;101:2294–9. doi: 10.1182/blood-2002-07-2297. [DOI] [PubMed] [Google Scholar]
- 14.Sportes C, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008 doi: 10.1084/jem.20071681. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geiselhart LA, et al. IL-7 administration alters the CD4:CD8 ratio, increases T cell numbers, and increases T cell function in the absence of activation. J Immunol. 2001;166:3019–27. doi: 10.4049/jimmunol.166.5.3019. [DOI] [PubMed] [Google Scholar]
- 16.Napolitano LA, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:73–9. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 17.Fry TJ, et al. A potential role for interleukin-7 in T-cell homeostasis. Blood. 2001;97:2983–90. doi: 10.1182/blood.v97.10.2983. [DOI] [PubMed] [Google Scholar]
- 18.Bolotin E, Annett G, Parkman R, Weinberg K. Serum levels of IL-7 in bone marrow transplant recipients: relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplant. 1999;23:783–8. doi: 10.1038/sj.bmt.1701655. [DOI] [PubMed] [Google Scholar]
- 19.Muthukumar A, et al. Elevated interleukin-7 levels not sufficient to maintain T-cell homeostasis during simian immunodeficiency virus-induced disease progression. Blood. 2004;103:973–9. doi: 10.1182/blood-2003-03-0874. [DOI] [PubMed] [Google Scholar]
- 20.Park JH, et al. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Iwata M, Graf L, Awaya N, Torok-Storb B. Functional interleukin-7 receptors (IL-7Rs) are expressed by marrow stromal cells: binding of IL-7 increases levels of IL-6 mRNA and secreted protein. Blood. 2002;100:1318–25. doi: 10.1182/blood-2002-01-0062. [DOI] [PubMed] [Google Scholar]
- 22.Pillai M, Torok-Storb B, Iwata M. Expression and function of IL-7 receptors in marrow stromal cells. Leuk Lymphoma. 2004;45:2403–8. doi: 10.1080/10428190412331283189. [DOI] [PubMed] [Google Scholar]
- 23.Min B, Foucras G, Meier-Schellersheim M, Paul WE. Spontaneous proliferation, a response of naive CD4 T cells determined by the diversity of the memory cell repertoire. Proc Natl Acad Sci U S A. 2004;101:3874–9. doi: 10.1073/pnas.0400606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kieper WC, et al. Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J Immunol. 2005;174:3158–63. doi: 10.4049/jimmunol.174.6.3158. [DOI] [PubMed] [Google Scholar]
- 25.Kassiotis G, Zamoyska R, Stockinger B. Involvement of avidity for major histocompatibility complex in homeostasis of naive and memory T cells. J Exp Med. 2003;197:1007–16. doi: 10.1084/jem.20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourgeois C, Kassiotis G, Stockinger B. A major role for memory CD4 T cells in the control of lymphopenia-induced proliferation of naive CD4 T cells. J Immunol. 2005;174:5316–23. doi: 10.4049/jimmunol.174.9.5316. [DOI] [PubMed] [Google Scholar]
- 27.Melchionda F, et al. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005;115:1177–87. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung S, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–20. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda S, Rodewald HR, Arakawa H, Bluethmann H, Shimizu T. MHC class II molecules are not required for survival of newly generated CD4+ T cells, but affect their long-term life span. Immunity. 1996;5:217–28. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- 30.Boursalian TE, Bottomly K. Survival of naive CD4 T cells: roles of restricting versus selecting MHC class II and cytokine milieu. J Immunol. 1999;162:3795–801. [PubMed] [Google Scholar]
- 31.Kieper WC, Burghardt JT, Surh CD. A role for TCR affinity in regulating naive T cell homeostasis. J Immunol. 2004;172:40–4. doi: 10.4049/jimmunol.172.1.40. [DOI] [PubMed] [Google Scholar]
- 32.Labrecque N, et al. How much TCR does a T cell need? Immunity. 2001;15:71–82. doi: 10.1016/s1074-7613(01)00170-4. [DOI] [PubMed] [Google Scholar]
- 33.Fry TJ, et al. Flt3 ligand enhances thymic-dependent and thymic-independent immune reconstitution. Blood. 2004;104:2794–800. doi: 10.1182/blood-2003-11-3789. [DOI] [PubMed] [Google Scholar]
- 34.Tan JT, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–7. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moniuszko M, et al. Recombinant interleukin-7 induces proliferation of naive macaque CD4+ and CD8+ T cells in vivo. J Virol. 2004;78:9740–9. doi: 10.1128/JVI.78.18.9740-9749.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Saint-Vis B, et al. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol. 1998;160:1666–76. [PubMed] [Google Scholar]
- 37.Sorg RV, McLellan AD, Hock BD, Fearnley DB, Hart DN. Human dendritic cells express functional interleukin-7. Immunobiology. 1998;198:514–26. doi: 10.1016/S0171-2985(98)80075-2. [DOI] [PubMed] [Google Scholar]
- 38.Harnaha J, et al. Interleukin-7 is a survival factor for CD4+ CD25+ T-cells and is expressed by diabetes-suppressive dendritic cells. Diabetes. 2006;55:158–70. [PubMed] [Google Scholar]
- 39.Mackall CL, et al. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood. 1997;89:3700–7. [PubMed] [Google Scholar]
- 40.Dion ML, et al. Slow disease progression and robust therapy-mediated CD4+ T-cell recovery are associated with efficient thymopoiesis during HIV-1 infection. Blood. 2007;109:2912–20. doi: 10.1182/blood-2006-09-047308. [DOI] [PubMed] [Google Scholar]
- 41.Jaleco S, et al. Homeostasis of naive and memory CD4+ T cells: IL-2 and IL-7 differentially regulate the balance between proliferation and Fas-mediated apoptosis. J Immunol. 2003;171:61–8. doi: 10.4049/jimmunol.171.1.61. [DOI] [PubMed] [Google Scholar]
- 42.Yao Y, et al. ERK and p38 MAPK signaling pathways negatively regulate CIITA gene expression in dendritic cells and macrophages. J Immunol. 2006;177:70–6. doi: 10.4049/jimmunol.177.1.70. [DOI] [PubMed] [Google Scholar]
- 43.Crawley JB, et al. T cell proliferation in response to interleukins 2 and 7 requires p38MAP kinase activation. J Biol Chem. 1997;272:15023–7. doi: 10.1074/jbc.272.23.15023. [DOI] [PubMed] [Google Scholar]
- 44.Moschella F, et al. Transcript profiling of human dendritic cells maturation-induced under defined culture conditions: comparison of the effects of tumour necrosis factor alpha, soluble CD40 ligand trimer and interferon gamma. Br J Haematol. 2001;114:444–57. doi: 10.1046/j.1365-2141.2001.02953.x. [DOI] [PubMed] [Google Scholar]
- 45.Vasir B, et al. Dendritic cells induce MUC1 expression and polarization on human T cells by an IL-7-dependent mechanism. J Immunol. 2005;174:2376–86. doi: 10.4049/jimmunol.174.4.2376. [DOI] [PubMed] [Google Scholar]
- 46.Fluur C, et al. Potential role for IL-7 in Fas-mediated T cell apoptosis during HIV infection. J Immunol. 2007;178:5340–50. doi: 10.4049/jimmunol.178.8.5340. [DOI] [PubMed] [Google Scholar]
- 47.Killian MS, Fujimura SH, Hecht FM, Levy JA. Similar changes in plasmacytoid dendritic cell and CD4 T-cell counts during primary HIV-1 infection and treatment. Aids. 2006;20:1247–52. doi: 10.1097/01.aids.0000232231.34253.bd. [DOI] [PubMed] [Google Scholar]
- 48.Giraud S, et al. Plasmacytoid dendritic cell reconstitution following bone marrow transplantation: subnormal recovery and functional deficit of IFN-alpha/beta production in response to herpes simplex virus. J Interferon Cytokine Res. 2005;25:135–43. doi: 10.1089/jir.2005.25.135. [DOI] [PubMed] [Google Scholar]
- 49.Mohty M, et al. Impact of plasmacytoid dendritic cells on outcome after reduced-intensity conditioning allogeneic stem cell transplantation. Leukemia. 2005;19:1–6. doi: 10.1038/sj.leu.2403558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.