Abstract
Analogs of vitamin E (tocols) are under development as radioprophylactic agents because of their high efficacy and lack of toxicity. Gamma-tocotrienol (GT3) is of particular interest because, in addition to being an antioxidant, it also inhibits 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase and accumulates to greater extent in endothelial cells than other tocols. We addressed in vivo whether HMG-CoA reductase inhibition contributes to the radioprotection conferred by GT3. Groups of mice were treated with vehicle, mevalonate (the product of the reaction catalyzed by HMG-CoA reductase), GT3 alone or GT3 in combination with mevalonate. Lethality and standard parameters of injury to the hematopoietic, intestinal and vascular/endothelial systems were assessed after exposure to total-body irradiation. GT3 improved post-irradiation survival and decreased radiation-induced vascular oxidative stress, an effect that was reversible by mevalonate. GT3 also enhanced hematopoietic recovery, reduced intestinal radiation injury, and accelerated the recovery of soluble markers of endothelial function. These parameters were not reversed by mevalonate co-administration. Our data confirm GT3’s radioprophylactic properties against hematopoietic injury and, for the first time, demonstrate benefits in terms of protection against gastrointestinal and vascular injury. The radioprotective efficacy of GT3 against vascular injury is related to its properties as an HMG-CoA reductase inhibitor.
INTRODUCTION
There is significant interest in developing vitamin E analogs (collectively referred to as tocols) as radioprophylactic agents because of their potent antioxidant properties, lack of performance-degrading toxicity, and presumed clinical benefit in chronic radiation fibrosis in some organ systems. The naturally occurring tocols comprise α-, β-, δ-and γ-tocopherol and α-, β-, δ- and γ-tocotrienol (1, 2). Most studies have been conducted with α-tocopherol, the most commonly used vitamin E supplement and the most abundant vitamin E isoform in human and animal tissues (3–9). However, other tocols, notably γ-tocotrienol (GT3), have been shown to be superior to α-tocopherol in reducing lethality secondary to hematopoietic/immune system injury after total-body irradiation (TBI) in mice (10).
Tocols exert their biological effects not only by virtue of their antioxidant properties but also by inhibiting the enzyme hydroxy-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, i.e., an effect similar to that of the drug class statins. GT3 exerts substantially stronger inhibitory effects on HMG-CoA reductase compared to other tocols and also accumulates in endothelial cells at much higher levels (11, 12). These properties suggest that GT3 may be particularly useful in protecting against vascular radiation injury and against tissue injury in organs where vascular injury plays a significant role.
This study addressed the extent to which HMG-CoA reductase inhibition is involved in the mechanisms of radio-protection by GT3 in vivo. The protective effects of GT3 were examined in three organ systems that play critical roles after exposure to ionizing radiation, the hematopoietic system, the intestine and the vascular system. We demonstrate here that, in addition to protecting against hematopoietic radiation toxicity, GT3 also ameliorates intestinal radiation injury, enhances recovery of the intestine after TBI, and reduces vascular oxidative stress in an HMG-CoA reductase-dependent manner. These findings may have significant implications for the future development of tocols as radioprophylactic agents and pertain particularly to tissue injury in organs where vascular damage is presumed to play a mechanistic role. Our data also suggest that combination therapies with tocols and statins should be explored to take advantage of possible synergistic or additive effects.
MATERIALS AND METHODS
Chemicals
GT3 was obtained from Yasoo Health Inc. (Johnson City, TN). Shortly before administration, GT3 was dispersed in a mixture of polyethylene glycol (PEG-400) (Sigma, St. Louis, MO) and a proprietary blend of fat-soluble emulsifiers with 20% ethyl alcohol and 1% benzyl alcohol added as preservatives (SPA, Stuart Products, Bedford, TX). PEG-400 with emulsifying agent (but without GT3) was used as vehicle control.
Unless otherwise specified, all other chemicals were obtained from Sigma Chemical Company (St. Louis, MO).
Animals
The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committees (IACUC) of the University of Arkansas for Medical Sciences and the Central Arkansas Veterans Health-care Systems (CAVHS).
Male CD2F1 mice (Harlan Sprague Dawley, Indianapolis, IN) with an initial body weight of 22–25 g were used in this study. Animals were housed in conventional cages under standardized conditions with controlled temperature and humidity and a 12–12-h day-night light cycle. Animals had free access to water and chow (Harlan Teklad laboratory diet 7012, Purina Mills, St. Louis, MO).
A total of 600 mice was used for these experiments, with four to eight mice per group. Mice were randomly assigned to one of the four following treatment groups: vehicle control, mevalonate alone, GT3 alone, and GT3 with co-administration of mevalonate. Twenty-four hours before irradiation, mice received a single dose of GT3 (400 mg/kg) or the excipient alone by subcutaneous (s.c.) injection. In addition, mice received either 25 mg/kg mevalonate in saline or saline alone by once-daily intra-peritoneal (i.p.) injections from 1 day before irradiation until 14 days after irradiation. This dose and administration schedule of mevalonate have been shown to be effective in other applications (13).
To study the effect of the different treatments on postirradiation survival, mice were exposed to 7–15 Gy TBI and observed for up to 30 days. To determine the effect of GT3 on radiation-induced tissue injury, mice received a single dose of TBI (8.5 Gy, unless otherwise specified) and were subsequently killed at set times after irradiation (0 h, 4 h, 1 day, 3.5 days, 7 days, 12 days, 14 days and 21 days). Previous experiments in CD2F1 mice have shown that 8.5 Gy TBI induces pronounced intestinal and hematopoietic injury, but with sufficient survival at 21 days.
Irradiation
Irradiation was performed with a J. L. Shepherd Mark I, model 25 137Cs irradiator (J. L. Shepherd & Associates, San Fernando, CA). Un-anesthetized mice were placed in cylindrical, well-ventilated Plexiglas chambers (J. L. Shepherd & Associates) divided into four 90° “pie slice” compartments by vertical dividers made of T-6061 aluminum (machinable grade) with a gold anodized coating. Two chambers were stacked on top of each other and placed on a turntable rotating at 5 rpm in the position furthest away from the radiation source, allowing eight mice to be irradiated at a time. The average dose rate was 1.35 Gy per minute, and the dose rate was corrected for decay each day mice were irradiated.
Dose uniformity was assessed by thermoluminescence dosimetry (TLD). Tissue-equivalent mouse phantoms were placed into each of the compartments of the same Plexiglas chambers used for irradiation. Two Harshaw TLD-100 lithium fluoride chips were placed into the center of each phantom and exposed to radiation for either 3 min or 8 min with the turntable rotating. The irradiated TLD chips and unirradiated control chips were subsequently analyzed by an independent company (K&S Associates, Nashville, TN). The measured coefficients of variation were 4.4% and 2.7%, respectively, for the two radiation exposures.
Survival Studies
For studies of postirradiation survival, mice were exposed to graded doses of TBI between 7 and 15 Gy. The mice were monitored up to 30 days after TBI, and the number of dead/moribund mice was recorded twice daily. Kaplan-Meier survival curves, median survival times, and lethality at 10 days and 30 days were recorded.
Assessment of Intestinal Radiation Injury
Mucosal surface area
Intestinal mucosal surface area is a well-validated, sensitive parameter of intestinal radiation injury. Mucosal surface area was measured in vertical H&E-stained sections of the jejunum using a projection/cycloid method as described by Baddeley et al. (14). The method has been validated by us previously specifically for surface area determination of the intestinal mucosa after irradiation (15).
Intestinal crypt colony assay
Microcolony crypt survival was performed as described by Withers and Elkind (16). Exactly 3.5 days after TBI (0, 8.5, 11, 13 and 15 Gy), mice were killed, and segments of proximal jejunum were obtained, fixed, embedded so that four transverse sections were obtained per specimen, cut at 3–5 μm, and stained with H&E. Surviving crypts, defined as crypts containing 10 or more adjacent, chromophilic non-Paneth cells, were counted. Four circumferences were scored per mouse, and microcolony survival was expressed as the average number of surviving crypts per circumference, with the average from each mouse considered as a single value for statistical purposes.
Plasma citrulline levels
The plasma level of citrulline is a well-validated biomarker for functional enterocyte mass (17). There is excellent correlation between plasma citrulline levels and more conventional markers of intestinal radiation injury, including mucosal surface area and the crypt colony survival assay (18, 19). Because citrulline levels can be determined in as little as 5 μl plasma, the citrulline assay may be used as an attractive minimally invasive, longitudinal marker of radiation-induced bowel injury. At 0, 3.5 and 7 days after 8.5 Gy TBI, whole blood was collected into EDTA-coated tubes (Fisher Scientific, Pittsburgh, PA.). Plasma was obtained by centrifugation (12,000 rpm, 5 min, 4°C) and stored at −80°C until analyzed. Citrulline concentrations were determined using a reverse-phase HPLC-fluorimetry method with precolumn OPA/ME derivatization, as described previously by Pérez-Neri et al. (20).
Bacterial translocation
Radiation-induced bacterial translocation starts around day 7 and peaks around 2 weeks after TBI (21, 22). In the present study, analysis of bacterial translocation was performed on day 10 after exposure to 9 Gy TBI. Livers were removed aseptically and homogenized immediately. Bacterial translocation was quantified by real-time PCR as described by van Minnen et al. (23). Briefly, DNA was isolated from sterile livers using a DNA purification kit (Promega, Madison, WI), and real-time PCR was performed using Power SYBR green PCR master mix (Applied Biosystems, Foster City, CA) and 16S rRNA gene-targeted primers, forward (5′-AAC GCG AAG AAC CTT AC-3′) and reverse (5′-CGG TGT GTA CAA GAC CC-3′). Serially diluted bacterial genomic DNA was used to generate the standard curve. PCR-derived bacterial counts were expressed as nanogram bacterial DNA per gram mouse liver tissue.
Assessment of Hematopoietic Injury and Recovery
Blood cell counts
At 0, 3.5, 7, 14 and 21 days after TBI (8.5 Gy), whole blood was collected into EDTA-coated tubes. Peripheral blood cell counts were obtained using a veterinary hemocytometer (Hematrue System, Heska Corporation, Loveland, CO) according to the manufacturer’s instructions.
Spleen colonies
Endogenous spleen colonies are a result of the proliferation of single hematopoietic stem cells and spleen colony counts are a marker of postirradiation hematopoietic recovery. At 12 days after TBI (8.5 Gy), spleens were collected in Bouin’s solution. After fixation, spleen colonies were clearly visible as yellowish nodules against a dark, smooth background. Spleen colonies were counted by two independent observers.
Assessment of Vascular Oxidative Stress and Endothelial Function
Vascular peroxynitrite production
Pre- and postirradiation peroxynitrite measurements provide information about the magnitude of radiation-induced oxidative/nitrosative stress. Peroxynitritrite readily oxidizes dihydrorhodamine 123 (DHR123). While the reaction is not entirely specific for peroxinitrite in that both HOCl and H2O2, provided the reaction is catalyzed by heme-containing peroxidases, are also capable of oxidizing DHR123, this assay is frequently used as a marker for peroxynitrite production. In the present study, abdominal aortas were removed by dissection at 0, 4 and 84 h after TBI. After collection, the aortas were incubated in 10 μM DHR123 (Axxora, San Diego, CA) in EGM-2 medium (Lonza, Walkersville, MA) for 90 min at 37°C in the dark. Subsequently, aortas were washed twice with PBS and homogenized in a buffer (PBS, 0.15% Tween-20, 0.15% SDS) using a Polytron PT 6100 homogenizer (Kinematica Inc., Bohemia, NY). Samples were centrifuged for 5 min at 2000 rpm and supernatant was collected to determine fluorescence (485/515) using a Synergy HT multiplate reader (BioTek Instruments, Winooski, VT). The protein concentration in the supernatant was measured using a modified Bradford reaction (Coomassie Plus Protein Assay, Thermo Scientific, Rockford, IL). Fluorescence was expressed per mg protein.
Soluble markers of endothelial function
Plasma levels of the endothelial cell adhesion molecules VCAM-1, ICAM-1 and E-selectin, the serine protease inhibitor PAI-1, and the gelatinase MMP-9 are commonly used to monitor changes in endothelial function. Whole blood was collected into EDTA-coated tubes (Fisher Scientific, Pittsburgh, PA). Plasma was generated by centrifugation (12000 rpm, 5 min, 4°C) and stored at −80°C until analysis. Soluble VCAM-1, ICAM-1, E-selectin, PAI-1 and MMP-9 were measured by multiplexing using a Bioplex system (Bio-Rad Laboratories, Hercules, CA) and the Lincoplex mouse CVD1 panel (Millipore, Billerica, MA).
Statistical Methods
Statistical analyses were performed using NCSS 2004 for Windows (NCSS, Kaysville, UT). Data are presented as means ± SEM, except for duration of survival, which is presented as median ± interquartile range (IQR). Two-sided tests were used throughout, and differences were considered statistically significant when the P value was less than 0.05. Survival curves were constructed using the Kaplan-Meier method and were compared using the log-rank test. Survival curves for the crypt colony assay were compared using regression analysis with radiation dose and treatment group as independent variables. Pairwise (univariate) comparisons were performed with the Student’s t test or Mann-Whitney U test. Comparisons among several treatment groups and/or times were performed with analysis of variance (ANOVA) with post hoc testing of group differences with Bonferroni’s or Dunnet’s test as appropriate.
RESULTS
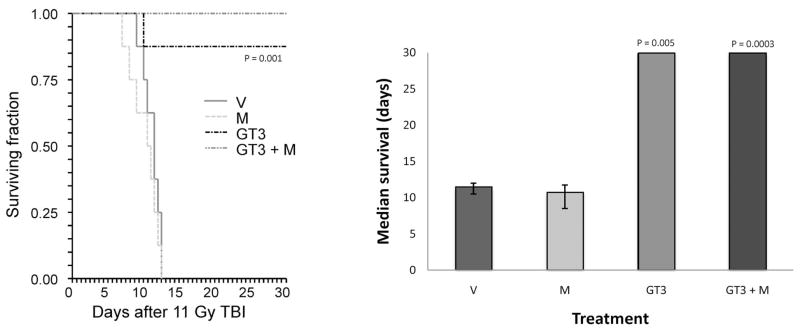
GT3, administered as a single s.c. dose 24 h before irradiation, had a pronounced effect on postirradiation survival. For example, after 11 Gy TBI, GT3 increased survival from 0% to 88% (Fig. 1A) and median survival time from 11.5 to 30 days (P = 0.001) (Fig. 1B). Co-administration of mevalonate did not reverse the effect of GT3 on overall survival.
FIG. 1.
Effect of GT3 and/or mevalonate administration on overall survival. Panel A: Kaplan-Meier survival curve from mice exposed to 11 Gy TBI. GT3 significantly prolonged survival and reduced lethality (P = 0.001). This effect was not reversed by mevalonate. Panel B: Improvement of the median duration of postirradiation survival by GT3 in mice exposed to 11 Gy TBI (median survival time ± IQR). V: vehicle; M: mevalonate; GT3: γ-tocotrienol; GT3 + M: γ-tocotrienol + mevalonate.
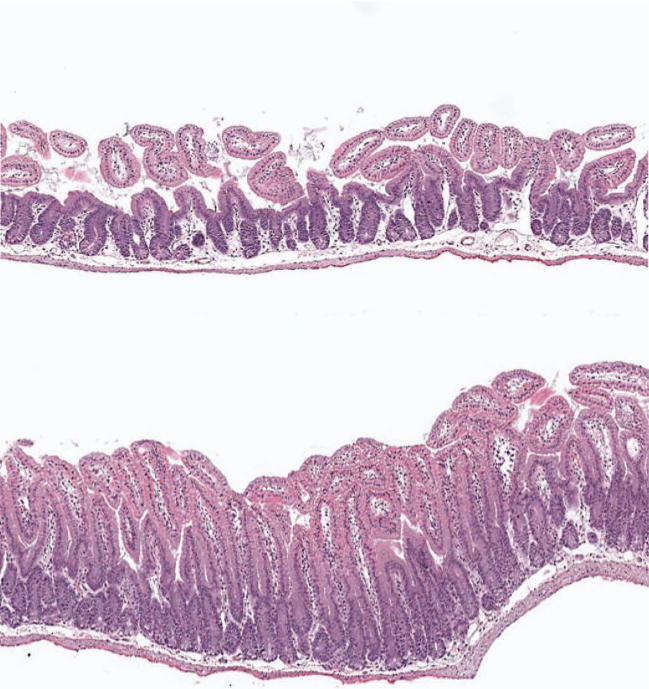
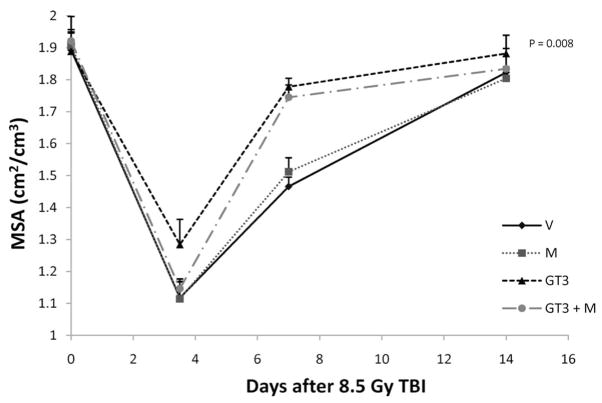
Each of the three assays used to assess intestinal radiation injury, mucosal surface area, the intestinal crypt microcolony assay, and plasma citrulline levels, showed a highly statistically significant effect of GT3 on the intestinal radiation response. Intestines from GT3-treated animals consistently exhibited less structural injury than intestines from vehicle-treated mice (Fig. 2). At 3.5 days after 8.5 Gy TBI, the mucosal surface area was decreased in all treatment groups (P < 10−6). From day 7 on, animals treated with GT3 and GT3 combined with mevalonate showed increased recovery of the mucosal surface area compared to the vehicle and mevalonate groups (P = 0.008) (Fig. 3).
FIG. 2.
Effect of GT3 on structural injury of the intestine. Representative images of intestines from a vehicle-treated (top) and a GT3-treated mouse on day 3.5 after 8.5 Gy TBI. Original magnification of both images 20×.
FIG. 3.
Effect of GT3 and/or mevalonate administration on mucosal surface area. TBI induced a reduction in mucosal surface area in all treatment groups (abbreviations as in Fig. 1). Administration of a single dose of GT3 24 h before irradiation significantly improved recovery of the intestinal mucosal surface area as measured from 7 days after irradiation on (P = 0.008). This effect was not reversed by mevalonate. The measures of variability (SE) are plotted only in the upward direction to minimize the number of overlapping lines.
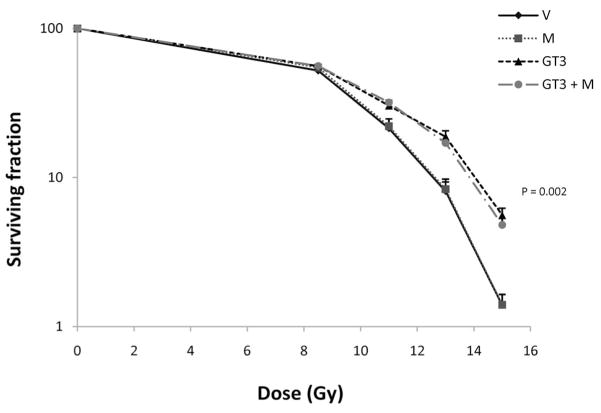
GT3 was also associated with a significant improvement in crypt survival measured at 84 h after TBI (P = 0.002). This effect was not reversed by co-administration of mevalonate (Fig. 4).
FIG. 4.
Effect of GT3 and/or mevalonate on postirradiation crypt survival. Pretreatment with a single dose of GT3 significantly improved intestinal crypt survival (P = 0.002) (abbreviations as in Fig. 1). This effect was not reversed by co-administration of mevalonate. The measures of variability (SE) are plotted only in the upward direction to minimize the number of overlapping lines.
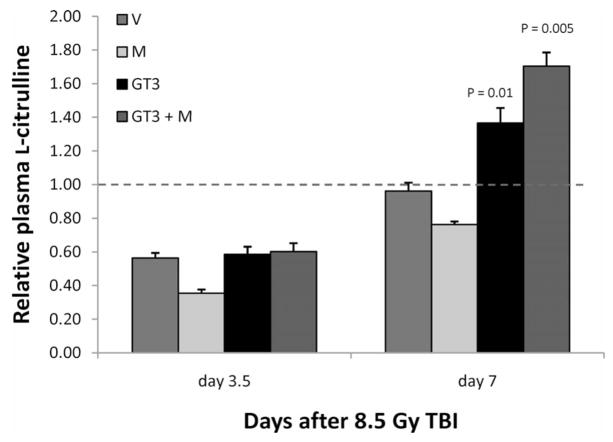
Mice from all treatment groups showed a decrease in plasma citrulline 3.5 days after irradiation, indicating decreased intestinal epithelial cell mass. By day 7, plasma citrulline levels had recovered. Interestingly, on day 7, GT3-treated animals and animals treated with GT3 and mevalonate exhibited citrulline levels that were significantly higher than in unirradiated mice (P = 0.01) (Fig. 5).
FIG. 5.
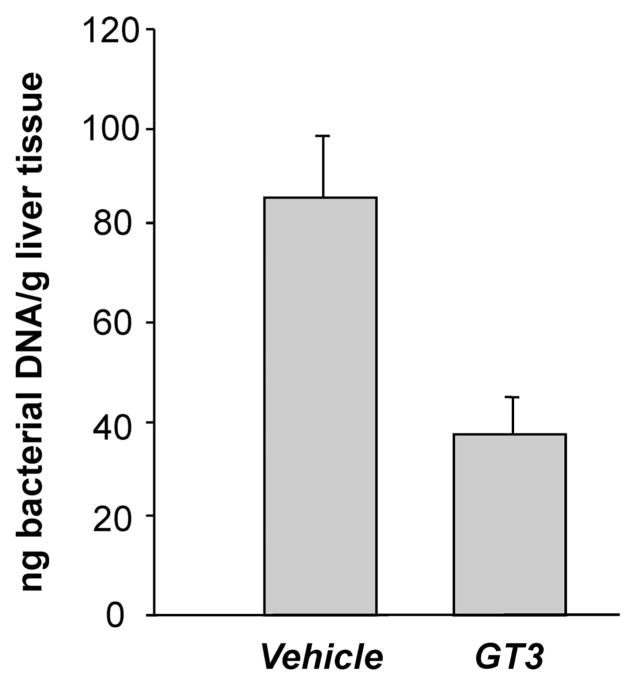
Effect of GT3 on postirradiation bacterial translocation. Ten days after exposure to 9 Gy TBI, significant amounts of bacterial DNA were observed in the livers of vehicle-treated mice. There was a highly statistically significant reduction in bacterial translocation in GT3-treated mice (P = 0.002).
GT3 administration was associated with a highly statistically significant reduction in bacterial translocation as assessed by the presence of bacterial DNA in the livers from irradiated mice (P = 0.002) (Fig. 6). No bacterial DNA was detected in the livers of unirradiated mice.
FIG. 6.
Relative plasma citrulline levels 3.5 days and 7 days after irradiation. There was a highly significant reduction in plasma L-citrulline levels at 3.5 days, but this effect was not attenuated by GT3 administration (abbreviations as in Fig. 1). At 7 days after irradiation, L-citrulline levels were back to baseline values in the vehicle- and mevalonate-treated animals, whereas in GT3-treated animals, L-citrulline levels exceeded baseline values (GT3 alone: P = 0.01; GT3 + mevalonate: P = 0.005).
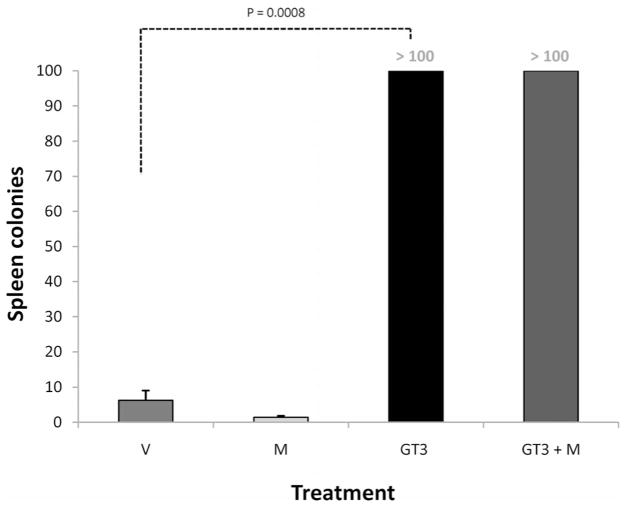
To confirm the effect of GT3 on radiation-induced hematopoietic injury, peripheral blood cell counts and spleen colony counts were obtained. As expected, the number of circulating blood cells decreased dramatically after irradiation. White blood cell counts started decreasing as early as 4 h after irradiation. In contrast to vehicle-treated animals, GT3-treated animals exhibited increased leukocyte counts (P = 0.01) and recovery of platelet counts (P = 0.01) and erythrocyte counts (P = 0.01) by day 14 after TBI (Table 1). Spleen colonies were counted 12 days after irradiation. Compared to vehicle-treated mice, GT3-treated animals exhibited enlarged spleens and increased numbers of spleen colonies (P = 0.0008) (Fig. 7). The positive effect of GT3 on hematopoietic recovery was not reversed by mevalonate.
TABLE 1.
The Effect of GT3 on Blood Cell Recovery after 8.5 Gy TBI
| Days after TBI | Vehicle | Mevalonate | GT3 | GT3 + mevalonate | |
|---|---|---|---|---|---|
| Erythrocytes (1012/liter) | 0 | 9.7 ± 0.1 | 9.6 ± 0.2 | 9.2 ± 0.3 | 9.3 ± 0.2 |
| 3.5 | 8.9 ± 0.1 | 8.8 ± 0.3 | 9.0 ± 0.3 | 8.8 ± 0.2 | |
| 7 | 7.5 ± 0.2 | 7.4 ± 0.1 | 7.4 ± 0.1 | 7.7 ± 0.1 | |
| 14 | 6.0 ± 0.3 | 6.5 ± 0.3 | 9.0 ± 0.1** | 8.9 ± 0.1** | |
| 21 | 9.2 ± 0.4 | 8.4 ± 0.5 | 10.0 ± 0.2 | 10.0 ± 0.2 | |
| Platelets (109/liter) | 0 | 456 ± 23 | 427 ± 10 | 452 ± 12 | 400 ± 12 |
| 3.5 | 390 ± 6 | 384 ± 19 | 437 ± 12 | 475 ± 9 | |
| 7 | 83 ± 4 | 89 ± 3 | 85 ± 5 | 88 ± 6 | |
| 14 | 88 ± 11 | 118 ± 22 | 462 ± 31** | 410 ± 26** | |
| 21 | 264 ± 22 | 224 ± 28 | 371 ± 18** | 365 ± 29* | |
| Leukocytes (109/liter) | 0 | 3.6 ± 0.3 | 4.6 ± 0.5 | 5.1 ± 0.5 | 4.5 ± 0.4 |
| 3.5 | 0.5 ± 0.2 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | |
| 7 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.6 ± 0.1 | |
| 14 | 0.5 ± 0.1 | 0.4 ± 0.1 | 2.1 ± 0.4** | 1.6 ± 0.2** | |
| 21 | 1.8 ± 0.1 | 1.2 ± 0.1 | 3.1 ± 0.3** | 2.6 ± 0.1** |
Notes. Values are means ± SEM;
P < 0.05;
P < 0.005.
At 14 days after irradiation, erythrocyte, platelet and leukocyte counts had recovered significantly in the GT3 group (P = 7 × 10−6; P < 10−6; P < 0.002, respectively) as well as in the GT3 + mevalonate group compared to the vehicle and mevalonate alone groups.
FIG. 7.
Effect of GT3 and/or mevalonate on spleen colony counts. Animals treated with GT3 and GT3 ± mevalonate showed increased numbers of spleen colonies compared to vehicle- and mevalonate-treated animals at 12 days after 8.5 Gy TBI (P = 0.0008; abbreviations as in Fig. 1). All spleens from GT3-treated animals exhibited countless (≫100) partly confluent colonies, and the number is thus arbitrarily set to 100.
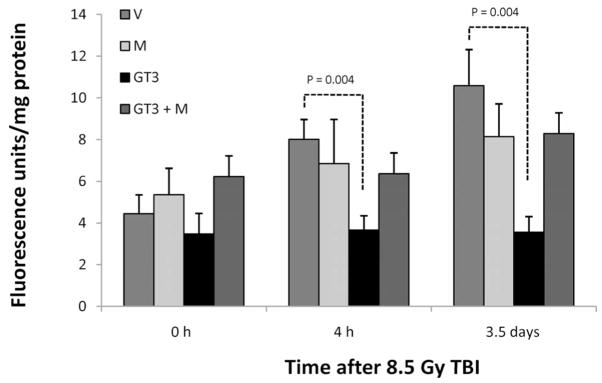
To study the effect of GT3 on the vascular and endothelial radiation response, we measured aortal peroxynitrite production along with circulating markers of endothelial function. Vascular peroxynitrite production was significantly increased during the first 3.5 days after radiation exposure. GT3 conferred a highly statistically significant degree of protection against radiation-induced vascular peroxynitrite formation at both 4 h (P = 0.004) and 3.5 days (P = 0.004) after irradiation. This effect of GT3 was prominently reversed by mevalonate administration, thus demonstrating dependence on inhibition of HMG-CoA reductase (Fig. 8).
FIG. 8.
Effect of GT3 and/or mevalonate on postirradiation vascular peroxynitrite formation. GT3 reduced radiation-induced peroxynitrite production in the abdominal aorta at 4 h (P = 0.004) and 3.5 days (P = 0.004) after TBI (abbreviations as in Fig. 1). This effect was reversed by co-treatment with mevalonate, indicating that it is dependent on HMG-CoA reductase inhibition.
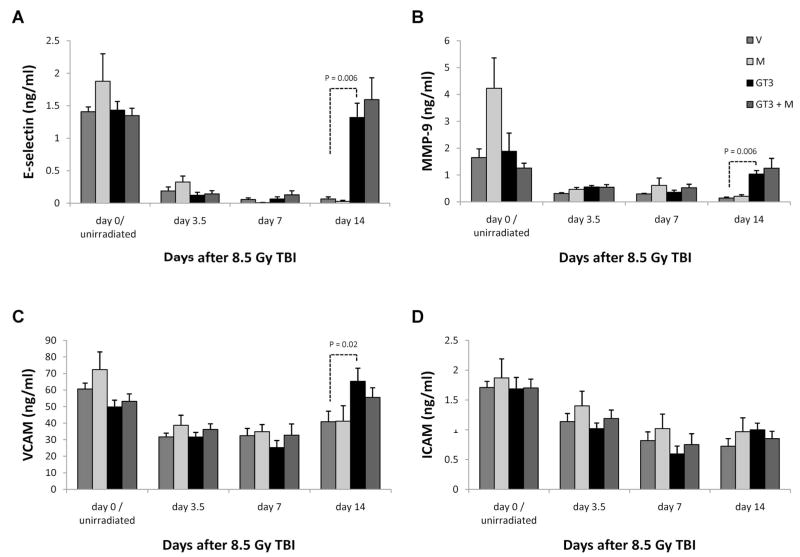
Plasma levels of four of the five measured endothelial membrane proteins, E-selectin, MMP-9, ICAM-1 and VCAM-1, showed a significant reduction at 3.5 and 7 days after irradiation. At 14 days, the plasma concentrations of E-selectin, MMP9 and VCAM-1 were approaching baseline values in the groups treated with GT3 and with GT3 and mevalonate, whereas the levels in vehicle-treated animals were still significantly, or in the case of MMP9 borderline significantly, abnormal (P = 0.006, P = 0.06, and P = 0.02, respectively) (Fig. 9). Circulating levels of soluble ICAM-1 remained reduced in all groups at 14 days after irradiation. No significant changes in soluble PAI-1 were observed in this experiment.
FIG. 9.
Effect of GT3 and/or mevalonate on circulating markers of endothelial dysfunction 8.5 Gy TBI induced a significant decrease in circulating E-selectin (panel A), MMP9 (panel B), VCAM (panel C) and ICAM (panel D) levels. At 14 days, E-selectin (P = 0.006), MMP9 (P = 0.006) and VCAM (P = 0.02) levels approached baseline values in GT3-treated animals while they were still severely reduced in mice treated with vehicle and mevalonate alone. There was no difference in ICAM levels among the treatment groups.
DISCUSSION
Development of effective radioprophylactic interventions continues to be a significant capability shortfall and an unmet need. At present, only amifostine is approved for this indication, but its use is hampered by a narrow window of administration relative to the time of irradiation as well as by serious performance-degrading side effects. Therefore, amifostine is generally considered unsuitable as a practical countermeasure (24). Considerable efforts are currently being directed at developing other compounds, including vitamin E analogs, as less toxic alternatives for radioprophylactic use.
Most studies investigating vitamin E analogs as radio-protective agents have focused on the use of γ-tocopherol. However, because of critical structural and functional differences among the various tocols, other isoforms may actually exhibit superior radioprotective properties. The eight different vitamin E isoforms, α-, β-, δ- and γ-tocopherol and α-, β-, δ- and γ-tocotrienol, differ in the number and position of the methyl groups on the chroman ring. Moreover, tocopherols and tocotrienols differ in terms of the side chain attached to the chroman ring: Tocopherols have a saturated phytyl side chain, while tocotrienols have an unsaturated isoprenoid chain. Consistent with the notion that GT3 may be a more potent radioprotector than α-tocopherol, a direct comparison in a mouse TBI model revealed a 40% survival benefit with GT3 compared to α-tocopherol (10). These data are also consistent with reports from research fields other than radiation biology that in general show that tocotrienols exhibit more potent health-promoting effects than α-tocopherol. For example, compared to tocopherols, tocotrienols are more effective as neuroprotective compounds, as anti-cancer agents, in reducing hyper-cholesterolemia, and in improving endothelial cell function (11, 12, 25–29). However, the exact molecular underpinnings of these functional differences between tocols and tocotrienols have not been elucidated.
It is commonly believed that differences among tocols are due to increased membrane mobility, distribution and/or cellular uptake of tocotrienols caused by their unsaturated isoprenoid side chain (11, 30–32). However, another property that applies specifically to GT3 and δ-tocotrienol is the inhibition of HMG-CoA reductase, i.e., an effect that is similar to the drug class statins. HMG-CoA reductase catalyzes the rate-limiting step in cholesterol synthesis and statins are the mainstay in the treatment of hyperlipidemia disorders. However, in addition to the hypercholesterolemic effects, the benefits of HMG-CoA reductase inhibition also include a plethora of cholesterol-independent effects. These effects are generally vasculoprotective and include decreased oxidative stress, improvement of endothelial cell function, and anti-inflammatory properties (33). There is substantial preclinical and clinical evidence supporting the notion that inhibition of HMG-CoA reductase ameliorates radiation injury in normal tissues. Hence statins have been shown to attenuate pulmonary injury and intestinal injury after localized irradiation (34–36). Moreover, clinical studies have shown statin use to be associated with reduced rectal toxicity after pelvic radiation therapy (37). GT3 is 30-fold more potent as an HMG-CoA reductase inhibitor compared to α-tocopherol (38), and it accumulates in endothelial cells to levels that are 25–95-fold greater (26). It is thus likely that some of the radioprotective properties of GT3 after TBI may relate to its properties as an inhibitor of HMG-CoA reductase. This may be particularly relevant to injury of normal tissues where endothelial dysfunction and/or vascular injury is presumed to play a role.
In contrast to statins, which directly inhibit the activity of HMG-CoA reductase, GT3 enhances the degradation of the enzyme (39, 40). While both δ-tocotrienol and GT3 stimulate ubiquitination of HMG-CoA reductase by Insig proteins and subsequent degradation by 26S proteasomes, GT3 appears to be more selective in this respect. In vivo experiments have confirmed that GT3 supplementation does indeed reduce HMG-CoA reductase activity in animals (41). Because of the difference between GT3 and statins in terms of the mechanism by which they inhibit HMG-CoA reductase, there may be opportunities for synergy or at least additive benefits of combining the two compounds for radioprotection.
The present study confirmed previous reports that prophylactic administration of GT3 before TBI ameliorates hematopoietic radiation toxicity and reduces lethality.4 In the present study, mice treated with a single dose of GT3 administered 24 h before TBI exhibited accelerated hematopoietic recovery. These results are consistent with the notion that part of the radioprotective effect of GT3 is mediated by the induction of G-CSF and other cytokines and chemokines that stimulate proliferation and differentiation of hematopoietic progenitor cells (7).
In addition, crypt survival and mucosal surface area were also significantly improved in GT3-treated mice. Hence our data demonstrate that GT3, in addition to reducing the consequences of hematopoietic/immune suppression, also ameliorates intestinal radiation toxicity. While plasma citrulline was unchanged at 3.5 days after TBI, the citrulline levels at 7 days after TBI exceeded baseline values in GT3-treated animals, suggesting that GT3 administration promotes recovery of the intestinal mucosa.
Whereas mevalonate at the dose and schedule given in the present study did not reverse the beneficial effect of GT3 on acute radiation-induced hematopoietic and intestinal injury, mevalonate clearly reversed the protective effect of GT3 on radiation-induced vascular peroxynitrite formation. This demonstrates that this effect of GT3 is mediated by inhibition of HMG-CoA reductase. Radiation-induced vascular damage plays a critical role in the mechanism of early delayed radiation responses in many different organ systems. In the gut, for example, endothelial apoptosis is thought to promote acute epithelial injury (42, 43) and to play an important role in the subsequent development of delayed radiation enteropathy (44). Similarly, endothelial dysfunction, and more specifically peroxynitrite production, has been shown to play a prominent role in pulmonary radiation toxicity (45–47).
The protective effect of GT3 on the hematopoietic niche may also be due in part to its properties as an HMG-CoA reductase inhibitor. The pleiotropic effects of HMG-CoA reductase inhibitors are largely mediated by increased activity of endothelial nitric oxide synthase (eNOS), which is critical for mobilization and proliferation of stem cells and thus ameliorates the consequences of myelotoxic insults (48). Hence compounds like GT3 that protect vascular endothelium against the effects of radiation by inhibiting HMG-CoA reductase may improve recovery from radiation injury in a variety of organ systems. Studies to determine the extent to which eNOS is required for the protective hematopoietic effect of GT3 are ongoing.
Although a reversal of the beneficial effect of GT3 by co-administration of mevalonate was observed only for vascular injury in the present study, it is conceivable that inhibition of HMG-CoA reductase by GT3 nevertheless contributes to the protection against intestinal and hematopoietic radiation injury. First, reversal of the effects of GT3 by mevalonate administration in vivo may be insufficient in some organ systems because of differences and/or different pharmacokinetics in tissue and cellular distribution of the two compounds. Second, it is possible that a higher mevalonate concentration is required in certain organs or that higher doses or more frequent or continuous administration (such as by miniosmotic pumps) is required for some effects to become apparent. Third, it is conceivable that the rate and/or duration of HMG-CoA reductase inhibition caused by single dose of GT3, as used in the present study (as opposed to its antioxidant effect), is not sufficient to have a pronounced effect on intestinal and hematopoietic injury. Finally, it is possible that inhibition of HMG-CoA reductase is more important in reducing the late sequelae after irradiation than the acute radiation responses, as shown for the effects of statins after localized, fractionated irradiation (36). Further research is clearly needed to explore the effects of administration of repeated doses of GT3 and to address the potential benefits of combining compounds that inhibit HMG-CoA reductase activity by different mechanisms.
In conclusion, a single prophylactic dose of GT3 greatly reduces lethality after TBI. In addition to attenuating hematopoietic and immune system dysfunction, GT3 also reduces intestinal and vascular oxidative stress. Inhibition of HMG-CoA reductase may play a significant role in the vascular radioprotection by GT3.
Acknowledgments
Assistance with tissue processing by Jennifer D. James of the Experimental Pathology Core Laboratory, Winthrop P. Rockefeller Cancer Institute, performance of citrulline assays by Ivan Spasojević of the Clinical Research PK/PD Laboratory, and performance of Luminex assays by Jeffrey C. Hale and Gregory D. Sempowski of the Immune Monitoring Core, both at Duke University Medical Center; as well as preparation of tissue-equivalent mouse phantoms for dosimetry by Miriam Hauer-Jensen are gratefully acknowledged. Dr. Berbée is enrolled in the Ph.D. program at the Department of Radiation Oncology, University of Maastricht. This work was supported by the Defense Threat Reduction Agency (grant HDTRA1-07-C-0028 to MH-J and H.10027-07-AR-R to KSK) and by NIH/NIAID (grant AI67798 to MH-J).
Footnotes
S. P. Ghosh, K. Hieber, T. C. Kao, M. Hauer-Jensen and K. S. Kumar, Radiation protection profile of gamma-tocotrienol, a superior tocol anti-oxidant. Presented at the Fifty-fourth Annual Meeting of the Radiation Research Society, Boston, MA, 2008.
References
- 1.Sen CK, Khanna S, Roy S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006;78:2088–2098. doi: 10.1016/j.lfs.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghosh SP, Hauer-Jensen M, Kumar KS. Chemistry of tocotrienols. In: Watson RR, Preedy VR, editors. Tocotrienols: Vitamin E beyond Tocopherols. CRC Press; Boca Raton, FL: 2009. pp. 85–96. [Google Scholar]
- 3.Bichay TJ, Roy RM. Modification of survival and hematopoiesis in mice by tocopherol injection following irradiation. Strahlenther Onkol. 1986;162:391–399. [PubMed] [Google Scholar]
- 4.Felemovicius I, Bonsack ME, Baptista ML, Delaney JP. Intestinal radioprotection by vitamin E (alpha-tocopherol) Ann Surg. 1995;222:504–508. doi: 10.1097/00000658-199522240-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srinivasan V, Weiss JF. Radioprotection by vitamin E: injectable vitamin E administered alone or with WR-3689 enhances survival of irradiated mice. Int J Radiat Oncol Biol Phys. 1992;23:841–845. doi: 10.1016/0360-3016(92)90657-4. [DOI] [PubMed] [Google Scholar]
- 6.Odagiri Y, Karube T, Katayama H, Takemoto K. Modification of the clastogenic activity of X-ray and 6-mercaptopurine in mice by prefeeding with vitamins C and E. J Nutr. 1992;122:1553–1558. doi: 10.1093/jn/122.7.1553. [DOI] [PubMed] [Google Scholar]
- 7.Singh VK, Shafran WE, Jackson WE, Seed TM, Kumar KS. Induction of cytokines by radioprotective tocopherol analogs. Exp Mol Pathol. 2006;81:51–61. doi: 10.1016/j.yexmp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Ramos FM, Pontual ML, de Almeida SM, Boscolo FN, Tabchoury CP, Novaes PD. Evaluation of radioprotective effect of vitamin E in salivary dysfunction in irradiated rats. Arch Oral Biol. 2006;51:96–101. doi: 10.1016/j.archoralbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Boerma M, Roberto KA, Hauer-Jensen M. Prevention and treatment of functional and structural radiation injury in the rat heart by pentoxifylline and alpha-tocopherol. Int J Radiat Oncol Biol Phys. 2008;72:170–177. doi: 10.1016/j.ijrobp.2008.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar KS, Ghosh SP, Hauer-Jensen M. Gamma-tocotrienol: potential as a countermeasure against radiological threat. In: Watson RR, Preedy VR, editors. Tocotrienols: Vitamin E beyond Tocopherols. CRC Press; Boca Raton, FL: 2009. pp. 379–398. [Google Scholar]
- 11.Noguchi N, Hanyu R, Nonaka A, Okimoto Y, Kodama T. Inhibition of THP-1 cell adhesion to endothelial cells by alpha-tocopherol and alpha-tocotrienol is dependent on intracellular concentration of the antioxidants. Free Radic Biol Med. 2003;34:1614–1620. doi: 10.1016/s0891-5849(03)00216-8. [DOI] [PubMed] [Google Scholar]
- 12.Naito Y, Shimozawa M, Kuroda M, Nakabe N, Manabe H, Katada K, Kokura S, Ichikawa H, Yoshida N, Yoshikawa T. Tocotrienols reduce 25-hydroxycholesterol-induced monocyte-endothelial cell interaction by inhibiting the surface expression of adhesion molecules. Atherosclerosis. 2005;180:19–25. doi: 10.1016/j.atherosclerosis.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Stalker TJ, Lefer AM, Scalia R. A new HMG-CoA reductase inhibitor, rosuvastatin, exerts anti-inflammatory effects on the micro-vascular endothelium: the role of mevalonic acid. Br J Pharmacol. 2001;133:406–412. doi: 10.1038/sj.bjp.0704070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142:259–276. doi: 10.1111/j.1365-2818.1986.tb04282.x. [DOI] [PubMed] [Google Scholar]
- 15.Langberg CW, Sauer T, Reitan JB, Hauer-Jensen M. Relationship between intestinal fibrosis and histopathologic and morphometric changes in consequential and late radiation enteropathy. Acta Oncol. 1996;35:81–87. doi: 10.3109/02841869609098484. [DOI] [PubMed] [Google Scholar]
- 16.Withers HR, Elkind MM. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17:261–267. doi: 10.1080/09553007014550291. [DOI] [PubMed] [Google Scholar]
- 17.Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr. 2008;27:328–339. doi: 10.1016/j.clnu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Lutgens L, Lambin P. Biomarkers for radiation-induced small bowel epithelial damage: an emerging role for plasma citrulline. World J Gastroenterol. 2007;13:3033–3042. doi: 10.3748/wjg.v13.i22.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lutgens LC, Deutz NE, Gueulette J, Cleutjens JP, Berger MP, Wouters BG, von Meyenfeldt MF, Lambin P. Citrulline: a physiologic marker enabling quantitation and monitoring of epithelial radiation-induced small bowel damage. Int J Radiat Oncol Biol Phys. 2003;57:1067–1074. doi: 10.1016/s0360-3016(03)00781-8. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Neri I, Montes S, Boll MC, Ramirez-Bermudez J, Rios C. Liquid chromatographic-fluorimetric method for the estimation of nitric oxide biosynthesis in the central nervous system. J Chromatogr B Anal Technol Biomed Life Sci. 2004;806:133–139. doi: 10.1016/j.jchromb.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 21.Brook I, MacVittie TJ, Walker RI. Recovery of aerobic and anaerobic bacteria from irradiated mice. Infect Immun. 1984;46:270–271. doi: 10.1128/iai.46.1.270-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi T, Ohmori T, Yanai M, Kawanishi G, Mitsuyama M, Nomoto K. The analysis of the defense mechanism against indigenous bacterial translocation in X-irradiated mice. Microbiol Immunol. 1991;35:315–324. doi: 10.1111/j.1348-0421.1991.tb01560.x. [DOI] [PubMed] [Google Scholar]
- 23.van Minnen LP, Timmerman HM, Lutgendorff F, Verheem A, Harmsen W, Konstantinov SR, Smidt H, Visser MR, Rijkers GT, Akkermans LM. Modification of intestinal flora with multispecies probiotics reduces bacterial translocation and improves clinical course in a rat model of acute pancreatitis. Surgery. 2007;141:470–480. doi: 10.1016/j.surg.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Hauer-Jensen M, Wang J, Denham JW. Bowel injury: current and evolving management strategies. Semin Radiat Oncol. 2003;13:357–371. doi: 10.1016/s1053-4296(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 25.McIntyre BS, Briski KP, Gapor A, Sylvester PW. Antiproliferative and apoptotic effects of tocopherols and tocotrienols on preneoplastic and neoplastic mouse mammary epithelial cells. Proc Soc Exp Biol Med. 2000;224:292–301. doi: 10.1046/j.1525-1373.2000.22434.x. [DOI] [PubMed] [Google Scholar]
- 26.Theriault A, Chao JT, Gapor A. Tocotrienol is the most effective vitamin E for reducing endothelial expression of adhesion molecules and adhesion to monocytes. Atherosclerosis. 2002;160:21–30. doi: 10.1016/s0021-9150(01)00540-8. [DOI] [PubMed] [Google Scholar]
- 27.Numakawa Y, Numakawa T, Matsumoto T, Yagasaki Y, Kumamaru E, Kunugi H, Taguchi T, Niki E. Vitamin E protected cultured cortical neurons from oxidative stress-induced cell death through the activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. J Neurochem. 2006;97:1191–1202. doi: 10.1111/j.1471-4159.2006.03827.x. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa K, Shibata A, Yamashita S, Tsuzuki T, Kariya J, Oikawa S, Miyazawa T. In vivo angiogenesis is suppressed by unsaturated vitamin E, tocotrienol. J Nutr. 2007;137:1938–1943. doi: 10.1093/jn/137.8.1938. [DOI] [PubMed] [Google Scholar]
- 29.Das S, Lekli I, Das M, Szabo G, Varadi J, Juhasz B, Bak I, Nesaretam K, Tosaki A, Das DK. Cardioprotection with palm oil tocotrienols: comparison of different isomers. Am J Physiol Heart Circ Physiol. 2008;294:H970–H978. doi: 10.1152/ajpheart.01200.2007. [DOI] [PubMed] [Google Scholar]
- 30.Sontag TJ, Parker RS. Influence of major structural features of tocopherols and tocotrienols on their omega-oxidation by tocopherol-omega-hydroxylase. J Lipid Res. 2007;48:1090–1098. doi: 10.1194/jlr.M600514-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida Y, Saito Y, Jones LS, Shigeri Y. Chemical reactivities and physical effects in comparison between tocopherols and tocotrienols: physiological significance and prospects as antioxidants. J Biosci Bioeng. 2007;104:439–445. doi: 10.1263/jbb.104.439. [DOI] [PubMed] [Google Scholar]
- 32.Atkinson J, Epand RF, Epand RM. Tocopherols and tocotrienols in membranes: a critical review. Free Radic Biol Med. 2008;44:739–764. doi: 10.1016/j.freeradbiomed.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams JP, Hernady E, Johnston CJ, Reed CM, Fenton B, Okunieff P, Finkelstein JN. Effect of administration of lova-statin on the development of late pulmonary effects after whole-lung irradiation in a murine model. Radiat Res. 2004;161:560–567. doi: 10.1667/rr3168. [DOI] [PubMed] [Google Scholar]
- 35.Haydont V, Bourgier C, Pocard M, Lusinchi A, Aigueperse J, Mathe D, Bourhis J, Vozenin-Brotons MC. Pravastatin inhibits the Rho/CCN2/extracellular matrix cascade in human fibrosis explants and improves radiation-induced intestinal fibrosis in rats. Clin Cancer Res. 2007;13:5331–5340. doi: 10.1158/1078-0432.CCR-07-0625. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Boerma M, Fu Q, Kulkarni A, Fink LM, Hauer-Jensen M. Simvastatin ameliorates radiation enteropathy development after localized, fractionated irradiation by a protein C-independent mechanism. Int J Radiat Oncol Biol Phys. 2007;68:1483–1490. doi: 10.1016/j.ijrobp.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irwin BC, Gupta R, Kim K, Han S, Ben-Josef E, Axelrod B, Tobi M. Calcium channel blockers may radiosensitize patients to radiation proctitis while statins, NSAIDs may radioprotect: a case–control study. Gastroenterology. 2006;130(S2):A460. [abstract] [Google Scholar]
- 38.Pearce BC, Parker RA, Deason ME, Qureshi AA, Wright JJ. Hypocholesterolemic activity of synthetic and natural tocotrienols. J Med Chem. 1992;35:3595–3606. doi: 10.1021/jm00098a002. [DOI] [PubMed] [Google Scholar]
- 39.Parker RA, Pearce BC, Clark RW, Gordon DA, Wright JJ. Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglu-taryl-coenzyme A reductase. J Biol Chem. 1993;268:11230–11238. [PubMed] [Google Scholar]
- 40.Song BL, DeBose-Boyd RA. Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme A reductase stimulated by delta- and gamma-tocotrienols. J Biol Chem. 2006;281:25054–25061. doi: 10.1074/jbc.M605575200. [DOI] [PubMed] [Google Scholar]
- 41.Qureshi AA, Peterson DM, Hasler-Rapacz JO, Rapacz J. Novel tocotrienols of rice bran suppress cholesterogenesis in hereditary hypercholesterolemic swine. J Nutr. 2001;131:223–230. doi: 10.1093/jn/131.2.223. [DOI] [PubMed] [Google Scholar]
- 42.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 43.Rotolo JA, Maj JG, Feldman R, Ren D, Haimovitz-Friedman A, Cordon-Cardo C, Cheng EH, Kolesnick R, Fuks Z. Bax and Bak do not exhibit functional redundancy in mediating radiation-induced endothelial apoptosis in the intestinal mucosa. Int J Radiat Oncol Biol Phys. 2008;70:804–815. doi: 10.1016/j.ijrobp.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Boerma M, Fu Q, Hauer-Jensen M. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J Gastroenterol. 2007;13:3047–3055. doi: 10.3748/wjg.v13.i22.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hallahan DE, Virudachalam S. Ionizing radiation mediates expression of cell adhesion molecules in distinct histological patterns within the lung. Cancer Res. 1997;57:2096–2099. [PubMed] [Google Scholar]
- 46.Hallahan DE, Virudachalam S. Intercellular adhesion molecule 1 knockout abrogates radiation induced pulmonary inflammation. Proc Natl Acad Sci USA. 1997;94:6432–6437. doi: 10.1073/pnas.94.12.6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giaid A, Lehnert SM, Chehayeb B, Chehayeb D, Kaplan I, Shenouda G. Inducible nitric oxide synthase and nitrotyrosine in mice with radiation-induced lung damage. Am J Clin Oncol. 2003;26:e67–e72. doi: 10.1097/01.COC.0000077940.05196.86. [DOI] [PubMed] [Google Scholar]
- 48.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]