Abstract
Although natural antibodies (NAbs) are present from birth, little is known about what drives their selection, and whether they have housekeeping functions. The prototypic T15-NAb, first identified because of its protective role in infection, is representative of a special type of NAb response that specifically recognizes and forms complexes with apoptotic cells, and which promotes cell-corpse engulfment by phagocytes. We now show that this T15-NAb IgM-mediated clearance process is dependent on the recruitment of C1q and mannose-binding lectin (MBL), which have known immune modulatory activities that also provide “eat me” signals for enhancing phagocytosis. Further investigation revealed that the addition of T15-NAb significantly suppressed in vitro LPS-induced TNF-α and IL-6 secretion by the macrophage-like cell line, RAW264.7, as well as Toll-like receptor (TLR)-induced maturation and secretion of a range of pro-inflammatory cytokines and chemokines by bone-marrow derived conventional dendritic cells. Significantly, high doses of this B-1 cell produced NAb also suppressed in vivo TLR–induced pro-inflammatory responses. While infusions of apoptotic cells also suppressed such in vivo inflammatory responses and this effect was associated with the induction of high levels of IgM anti-apoptotic cell antibodies, apoptotic cell treatment was not effective at suppressing such TLR responses in B-cell deficient mice. Moreover, infusions of T15-NAb also efficiently inhibited both collagen-induced arthritis and anti-collagen II antibody-mediated arthritis. These studies identify and characterize a previously unknown regulatory circuit by which a NAb product of innate-like B cells aids homeostasis by control of fundamental inflammatory pathways.
To defend against infectious agents, yet also guard against autoimmune disease, complex activating and inhibitory pathways have evolved that interconnect the innate and adaptive immune systems and control their activation. The innate immune system senses for threats by recognizing microbe-associated molecular motifs using limited sets of cellular receptors, such as Toll-like receptors (TLRs), as well as soluble immune recognition opsonizing factors, such as complement, collagen-like lectins (i.e., collectins) and C-reactive protein. Some of these receptors also bind to stress-associated proteins and other self-ligands (reviewed in (1)). Professional phagocytic cells, macrophages (Mφ) and dendritic cells (DCs) thereby respond to environmental stimuli, microbial antigens and cytokines, which by facilitating or forbidding differentiation changes control the capacity of Mφ and DC for overall inflammatory responses as well as the immunogenicity of foreign and self antigens.
While the innate immune system is important or even essential for modulating lymphocyte responses, innate immune responses themselves are also reciprocally influenced by specialized tiers of the adaptive immune system, such as natural killer (NK), NKT, and γδ T cells, which can recruit DCs into pro-inflammatory responses (2). We have wondered how B lymphocytes might also affect innate responses, especially by B-1 cells, the primordial tier of the B-lymphocyte compartment that is the major source of the “non-immune” IgM NAbs constitutively produced throughout life, and which are also involved in responses to non-protein antigens (3). This distinct set of self-replenishing mature B lymphocyte have been described as innate-like as they express a restricted and recurrent antibody repertoire that arises by a programmed sequence during immune development (3,4). Indeed, certain B-1 cell clones appear to have regulatory roles through effects on innate immune cells even at remote sites in the body (5), although how this might occur is not known.
The prototypic T15 B-1 cell clonotype, defined by H-L puaired canonical antibody gene rearrangements without hypermutation, was first characterized 40 years ago (6) with later repeated independent isolations (e.g. S107 (7), HPCM2 (8), EO6 (9) and others). T15 clonotypic B cells spontaneously arise and become highly represented within the first week of life, even in mice raised under germ-free conditions (10), which suggests that microbial ligands are not primary mediators of clonal selection. It has long been known that T15-NAbs bind to phosphorylcholine (PC) determinants, and contribute to host defense to PC-containing pneumococci, and other microbes, and provide optimal protection from systemic infection (11). More recently, PC-determinants were also identified on oxidatively-modified low density lipoprotein (LDL) generated during atherogenesis (9). Significantly, pneumococcal immunization, which induced active B-cell responses that raised T15 antibody levels, greatly ameliorated the chronic inflammatory response in a murine model of hyperlipidemia and atherosclerosis (12). The mechanistic basis for these findings remains obscure, as the original hypothesis, that T15-NAb might enhance clearance of the pro-inflammatory oxidatively-modified LDL, has subsequently been ruled out (13). While more recent studies suggest the possibility that immunization may induce regulatory B-cells that serve as a source of inhibitory cytokines (14), we have suspected there are other antibody-mediated immune modulatory activities.
Other studies have previously shown that by immune recognition of the PC head group, T15-NAb can discriminate dead/dying cells from healthy cells (15–17). This is because the PC-head group, which is a ubiquitous component of cell membrane neutral phospholipids (e.g. phosphatidylcholine), is embedded within the lipid bilayer in healthy cells and therefore inaccessible to antibodies unless exposed by membrane changes that occur during apoptosis (15–17). Importantly, we have shown that T15-NAb is structurally and functionally representative of the PC-specific anti-apoptotic cell antibodies that are induced in vivo by apoptotic cell infusions (18).
Herein, we show that the prototypic T15-NAb can play a general role in modulating innate immune responses by inhibiting the activation of phagocytes, and thereby suppressing in vitro and in vivo inflammatory responses. We further show that these regulatory properties derive from the capacity to complex with apoptotic cells and recruit soluble innate immune recognition molecules, which together enhance uptake and clearance of apoptotic cells and inhibit TLR induced phagocyte activation and maturation.
Materials and Methods
Antibodies
T15-IgM (from the EO6 hybridoma)(9), and the IgM isotype control from the hybridoma, NC17-D8 (gift of L. Arnold, UNC, Chapel Hill NC), both express J-chain transcripts. Hybridomas were grown under serum-free conditions in hollow fiber (10,000 MWCO) bioreactors in Hybridoma Serum free media (Invitrogen, Carlsbad CA) to a cell density of ~5×108/ml) and then maintained for 30–45 days, by NCCC (Minneapolis, MN). Supernatants were purified with a 300 kDa tangential flow filtration (TFF) device, followed by a 10kDa TFF for further concentration, then dialyzed against PBS pH 7.2, with documented low endotoxin (< 0.5 EU/mg), then aliquots stored at −80°C. By native PAGE analysis and western blot, the predominant IgM populations were pentamers with <10% hexamers, without monomeric IgM or low molecular weight species.
Antibody assays
Standard sandwich ELISA were performed with precoats of goat anti-IgM, PC-albumin for control antigens, with detection with either biotinylated AB1-2 to detect T15-colonotypic antibodies (19), or anti-IgM or anti-IgG, as described (20). Assays were adapted to buffer usage required to detect MBL-binding, as described (21), with limits of detection of ~5 ng/ml. In these studies MBL binding by IgG could not be detected in sera either before or after thymocytes immunization. Array studies were performed as described (22).
Mice
Age and gender-matched adult C57BL/6, congenic B-cell deficient muMT, BALB/c and DBA/1 mice were provided by The Jackson Laboratory (Bar Harbor, ME) or bred under SPF-conditions as supervised by UCSD Animal Care Program. All animal protocols were approved by the UCSD IACUC.
In vitro complement deposition
Apoptotic thymocytes were incubated at 37°C with IgM at 20 ug/ml in Tris-buffered saline with 10 mM CaCl2, and/or Tris-buffered saline (TBS) with 20% Ig-deficient plasma for complement or TBS alone. After 40min, cells were washed and studied for apoptosis (7AAD and Annexin V), and with APC-labeled goat anti-IgM, and anti-C1q (goat, Cedarlane Labs), or human recombinant MBL (6 ug/ml) and biotinylated mouse anti-human MBL (clone 131-1), or biotinylated rat anti-murine MBL A (clone 2B4) anti-murine MBL C)(clone14D12)(21), in the presence of Fc block (23).
In vivo apoptotic clearance assays
Using a standard in vivo approach (24), B-cell deficient mice received thioglycollate treatment and 3d later received intravenous PBS or 1 mg of IgM. After 16 hr, 5 ×106 SNARF-1 labeled apoptotic or fresh thymocytes were instilled, then peritoneal cells recovered after 10 min. For immunofluorescence microscopic studies, cytospins were prepared and Mφ stained with FITC-anti-F4/80, with >800 Mφ counted per mouse and the proportion determined of recovered Mφ that had ingested (and not just surface bound) one or more labeled thymocytes. Although longer time periods were also examined, 10 min of in vivo exposure yielded the greatest differences between groups, as previously described (36). While dexamethasone-treated thymocytes yielded similar results, most studies used etoposide for apoptosis induction due to >95% Annexin V+ (i.e., apoptotic) thymocyte yields by flow cytometry. In other studies, to quantitate Mφ uptake, flow cytometric analyses were performed with 7AAD and Annexin V staining of apoptotic thymocytes that were tracked via CD3 (Becton-Dickinson), with peritoneal Mφ detected with FITC-conjugated F4/80 (Caltag).
RAW264.7 cell cultures
Cells were grown to 80% confluence with ~105 cells per well in 48 well plates in 48-well plates in RPMI and 10% FBS, glutamine and 0.01 M Hepes, then serum starved overnight. To some replicates were preincubated with T15 IgM or isotype control for 1 hr, followed by addition of LPS (E. coli 055:B5, List Biological Labs) at 0.1 ug/ml or polyinosinic-cystidic acid (poly I:C)(Amersham) at 3.3 ug/ml for overnight culture,
Bone-marrow derived dendritic cells
Bone marrow cells from C57BL/6 femurs/tibias were washed and cultured in RPMI containing 10% FBS 1% Pen-Strep-Glutamine, GM-CSF (10 ng/ml) and IL-4 (400 pg/ml), replenished on d3 (25). On day 6, DC were selected in the presence of Fc block with magnetic anti-CD11c beads using LS magnetic columns (Milltenyi) to >94% CD11c+ purity. For phagocytosis assays, DC were cultured with GM-CSF but without IL-4 and harvested on day 5 CD11c+ cells were purified, then cultured at 0.5 × 106/100 ul in 96 well plates overnight in a 1:1 ratio with CFSE labeled healthy or etoposide-induced apoptotic cells, as described (18). Some studies instead used STEMSPAN SF Expansion (Stem Cell Technologies) serum-free media. For heat inactivation, Ig-deficient sera were incubated for 30 min at 56°C.
For stimulation studies, DC were further cultured for 24–48 hrs without/with agonists for TLR3, (polyinosinic:polycytidylic acid, poly I:C) at 3.3 ug/ml; TLR4, LPS at 0.1 ug/ml; TLR7, imiquimod (Invivogen) at 1 ug/ml; or TLR9, phosphorothioate CpG oligo 1018 at 0.5 ug/ml. Replicate cultures included serial concentrations of T15-IgM or IgM-isotype control. Other cultures included blocking antibody to IL-10 or isotype control (R&D Systems) with Fc block, as per manufacturer’s directions. Cultures with T15-NAb blockade with AB1-2 anti-idiotype or isotype control also included Fc block. To assess DC maturation, cells were co-stained with PE- anti-mouse CD80 (clone 16-10A1) and for intra-cellular Alexa-Fluor 647 anti-IL-12 p40 (clone C17-8), as per manufacturer’s protocol (eBiosciences). QPCR was performed as previously described (20).
DC isolation
Splenic DC were isolated, as reported (25), then evaluated by flow cytometry for defined DC subsets and maturation/activation markers. Transcript analysis after 6 hr of in vitro stimulation of CD11c-enriched BM-derived DC were performed by Taqman (Applied Biosystems), using manufacturer’s directions.
In vivo challenge assays
Based on pilot studies with outcomes assessed after weekly treatments we selected a 2-wk treatment period, which is also the turnover period of most DC populations from stem cells (26). Hence, groups of adult C57BL/6 received 3 i.p. infusions (d0, 7 & 14) of 1.5mg of T15-IgM or isotype control. To assess the role of PC-binding specificity, some groups received 1.5mg of T15-IgM incubated with 2mg of PC-BSA for 30 min at RT prior to infusion. Other groups received intravenous 2.5×107 freshly isolated (healthy), apoptotic, or necrotic (by repeated freeze-thawing) thymocytes in PBS, with bleeds obtained on d16. To induce apoptosis, congenic murine thymocytes either received 600 Rads using a Cs137 emission source of radiation, or were treated with 10uM etoposide, then incubated ON in complete media at 37°C with 5% CO2, then washed three times in media before use. Alternatively, mice received T15-NAb with 2mg of PC-BSA (Biosearch) or BSA as a control. On d17, at 18 hr before sacrifice mice received saline or challenge with: poly I:C, 100ug; LPS, 30 ug; imiquimod, 100ug or PT CpG ODN1018, 200ug. As pilot studies did not demonstrate in vivo activation after imiquimod treatment, we instead used 300 ug of SM-360320 (27), due to 100-fold greater potency. Mice were bled at sacrifice, and suspensions of splenocytes and other lymphoid organs evaluated by flow cytometry using standard antibodies and methods (BD-Pharmingen)(17,20). Antibody immunoassays and inhibitions were performed with PC-BSA, ABA-BSA (Biosearch) or BSA (Sigma) using IgG (sub)class and T15 clonotype-specific antibodies, as previously described (20). Soluble factors in DC supernatants and sera were evaluated by Luminex assay or ELISA (Biosource-Invitrogen).
Inflammatory arthritis models
For CIA studies, 8-wk-old DBA/1 male mice were immunized with avian CII/CFA (Chondrex) at the tail base on d0 and i.p. boosted on d21 with CII/IFA. Anti-CII antibody levels were assayed, per manufacturer’s instructions (Chondrex). For histologic analyses, paws and knees of mice sacrificed on day 44 were decalcified, embedded and sectioned. H&E stained slides were scored for inflammatory infiltrates and joint erosions, and safranin O stained for cartilage damage (28). Collagen antibody-induced arthritis was induced in BALB/c mice with 2 mg of CII-specific monoclonal IgG cocktail injected intravenous on d0, and 72h later each animal received 50ug of LPS E.coli 011B4 i.p. (Arthogen-CIA kit, Chemicon Int.). Different groups received T15-IgM or control IgM at 2 mg, or buffer, given as a pre-treatment and every 7d thereafter. Clinical arthritis was scored visually from 0 to 4 per paw, with a maximum score of 16 (28).
Statistical analysis
Values are reported as mean+/−SEM unless otherwise stated. Significance was assigned for P<0.05 by two-tailed t test, with Welsh correction, or ANOVA, as appropriate (Instat, GraphPad).
Results
NAb enhances local deposition of C1q and MBL on apoptotic cells
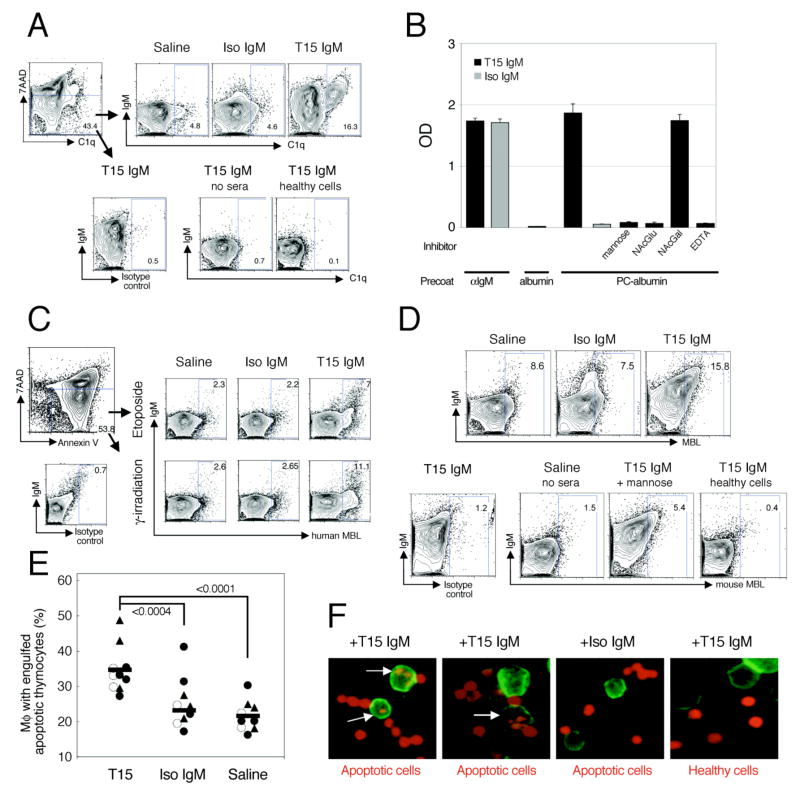
To understand the immune modulating properties of T15-NAb, we first characterized its antibody-effector capabilities, and then assessed how these may affect the innate immune system. In earlier studies, purified monoclonal T15 clonotypic antibodies were shown to recognize a subset of dying cells at both early (Annexin V+ 7AAD−) and late (Annexin V+ 7AAD+) stages of apoptosis in a PC-inhibitable fashion (17). As a physiologically relevant source of soluble opsonins, we used sera from B-cell deficient murine MuMT mice that are therefore deficient in immunoglobulins (Igs)(29). While incubation with Ig-deficient sera results in low level deposition of C1q on apoptotic cells (30), we found that the addition of T15-NAb of the IgM isotype increased the amount of C1q recruitment from Ig-deficient sera onto apoptotic cells (18). Notably, while neither T15-NAb nor C1q interacted with freshly isolated healthy thymocytes, T15-NAb was responsible for greater than 3-fold relative increases in C1q deposition on cells at early stages of apoptosis, based on gating on 7AAD− cells (Figure 1A).
Figure 1. T15-IgM NAb enhances deposition of C1q and MBL on apoptotic cells and increases their in vivo phagocytic clearance by peritoneal Mφ.
(A) To assess for C1q deposition, etoposide-treated apoptotic thymocytes were incubated in 50% muMT sera in saline or with monoclonal IgM (20 ug/ml), then washed and stained with 7AAD (to assess membrane integrity) and anti-murine C1q or isotype control, as indicated. While labeled Annexin V was used to document apoptosis, it was otherwise omitted to avoid interference with C1q-binding. By gating on early apoptotic cells (i.e., 7AAD−), which are indicated by the arrows, addition of T15 IgM was shown to increase more than 3-fold the level of C1q deposition from Ig-deficient sera, compared to saline or IgM isotype control. At bottom, control studies demonstrated no significant signal (left) on early apoptotic cells with the isotype control detection reagent, (middle) or with early apoptotic cells without MuMT sera but with T15 IgM (no sera), or (right) for C1q deposition onto freshly isolated live cells in the presence of T15-NAb. (B) ELISA studies show binding of IgM with a biotinylated detection reagent to different precoated antigens, which are listed at bottom. With a precoat of PC-albumin, T15-IgM (at 2 ug/ml) displays specific MBL- and antigenic-binding, but not to albumin alone. Specific MBL binding is blocked by mannose or N-acetylglucosamine (NAcGlu) but not by N-acetyl galactosamine (NAcGal) at 20 mM. Binding also requires CaCl2, and is absent in 10mM EDTA-containing buffer. (C) Etoposide-treated or γ-irradiated apoptotic thymocytes were incubated with human recombinant MBL in the absence or the presence of purified monoclonal IgM, then stained with labeled anti-human MBL. At top, binding is shown after gating on early (7AAD-Annexin V+) apoptotic cells. At bottom left, control studies demonstrated no significant signal on apoptotic cells with the biotinylated isotype-control detection reagent. (D) To assess for the capacity to recruit murine MBL from sera, etoposide-treated thymocytes were incubated with MuMT sera in the presence of saline or purified monoclonal IgM, as indicated. Cells were stained for IgM, and with labeled anti-mouse MBL A and MBL C. In control studies (bottom), only background reactivity is seen after incubation of apoptotic cells sera and T15-IgM, with staining with an isotype control reagent (left). Incubation without sera yielded background signal with anti-MBL A+C detection, while MBL deposition was greatly reduced by the addition of mannose (50 uM) to sera and T15-IgM. T15-IgM and sera incubation did not result in MBL deposition on healthy cells (right). (E) In vivo Mφ mediated apoptotic clearance was evaluated, in three independent experiments, as indicated (solid triangle, open circles and solid circles), with 2–4 B-cell deficient mice in each group. Mice received either T15-IgM, isotype control IgM or saline treatment before instillation of apoptotic thymocytes (total N=9–10/group). Values for each mouse represent the phagocytic index after 10 min, (i.e., proportion of recovered peritoneal Mφ that had engulfed labeled apoptotic thymocytes) (F) Cytospin preps show NAb enhanced phagocytic engulfment of apoptotic cells by peritoneal Mφ from B-cell deficient mice that received IgM or saline. After 16 hr, thymocytes labeled with SNARF-1 fluorochrome (red) were instilled i.p., with sacrifice 10 min later. Mφ were detected by F4/80 FITC (green). At top, treatment with specific IgM treatment is indicated, with either apoptotic or healthy freshly isolated thymocytes, as indicated below. Results are representative of three or more independent experiments.
Mannose binding lectin (MBL) is a multimeric collectin immune recognition protein that initiates the lectin pathway of complement activation, which play roles in immune defenses but can also interact with certain self-glycoproteins (31). Although not well known for contributions to antibody-effector functions, because of the reported roles of MBL in apoptotic-cell recognition (23) and modulation of inflammatory responses (reviewed in (31)), we also assessed the capacity of T15-NAb to recruit MBL. Indeed, solid phase immunoassays showed that both T15-NAb as well as the IgM-isotype control had dose-dependent binding to the labeled recombinant MBL used to detect binding (Figure 1B). However, only T15-NAb recognized the PC-albumin coated onto the wells and then also interacted with the labeled MBL reagent. MBL-binding to T15-NAb was inhibited by mannose or N-acetylglucosamine, but not by N-acetylgalactose, and was also calcium-dependent (Figure 1B), indicating that the carbohydrate recognition domain of MBL is responsible for these IgM-interactions presumably through Fcμ-associated N-glycans (32). In contrast to a single report that binding of a recombinant IgM-antibody to an experimental antigen disallows constant region interactions with MBL (32), we found that T15-NAb, but not the isotype control, was capable of concurrent binding interactions with both PC and MBL (Figure 1B). This indicated that binding interactions with T15-NAb could potentially amplify recruitment of MBL to immune complexes.
We therefore examined if T15-NAb could promote binding of human recombinant MBL to apoptotic thymocytes. As previously reported (23), incubation with MBL alone resulted in direct deposition of only low levels of this opsonin, predominantly on thymocytes at late stages of apoptosis and those undergoing secondary necrosis. By contrast, the addition of T15-NAb significantly enhanced MBL deposition with the greatest increases on thymocytes at early stages of apoptosis (Figure 1C and Supplemental Figure 1). In further analyses, murine MBL-specific antibodies (21) were used to directly detect mouse MBL deposited on apoptotic cells from Ig-deficient sera. Here, these specific detection antibodies showed that T15-IgM similarly induced the recruitment of both MBL A and C gene products from sera, either separately (not shown) or together, and the specificity was again confirmed as these interactions were inhibited by mannose (Figure 1D). Thus, a major function of T15-NAb is the recruitment of both C1q and MBL to primarily early, but also late, apoptotic cells.
NAb enhances in vivo macrophage clearance of apoptotic cells
To assess whether the T15-NAb can affect the phagocytic clearance of apoptotic cells, we used a standard sterile peritonitis model (24) with B-cell/Ig-deficient MuMT mice, which received pretreatment infusions of either T15-IgM, control isotype IgM or saline. Mice were then injected i.p. with labeled apoptotic thymocytes, and 10 minutes later peritoneal Mφ were recovered and examined for phagocytized thymocytes (24). We found that in saline or IgM isotype treated mice, a mean of ~23% of recovered Mφ had engulfed a labeled apoptotic cell or bleb, while after T15-IgM treatment the proportion of Mφ with ingested apoptotic thymocytes/fragments increased to ~36%. Hence, T15-IgM treatment resulted in a 50–60% increase in the level of apoptotic phagocytosis, compared to the isotype control (P<0.0004) or saline treatments (P<0.0001, Figure 1E). By contrast, we found that in T15-IgM treated mice, after injection of labeled healthy thymocytes <3% of recovered peritoneal Mφ had engulfed a labeled thymocyte (not shown). Notably, increases in the efficiency of apoptotic clearance in the same assay, akin to those mediated by T15-NAb, have also been documented when wild-type mice were compared with either C1q- or MBL-deficient mice (24,33). Indeed, we found that T15-NAb coated apoptotic thymocytes formed chains and clusters, which were engulfed by peritoneal Mφ (Figure 1F). Flow cytometric analysis of the recovered peritoneal cells demonstrated that T15-NAb enhanced the elimination of both early and late stage apoptotic cells (P<0.004, Supplemental Figure 1). Thus, T15-NAb significantly enhances the in vivo phagocytosis of apoptotic cells by peritoneal macrophages, with an influence akin to the individual contributions of MBL and C1q for phagocytic clearance.
NAb suppresses LPS-induced IL-6 secretion by RAW264.7 macrophage-like cell line
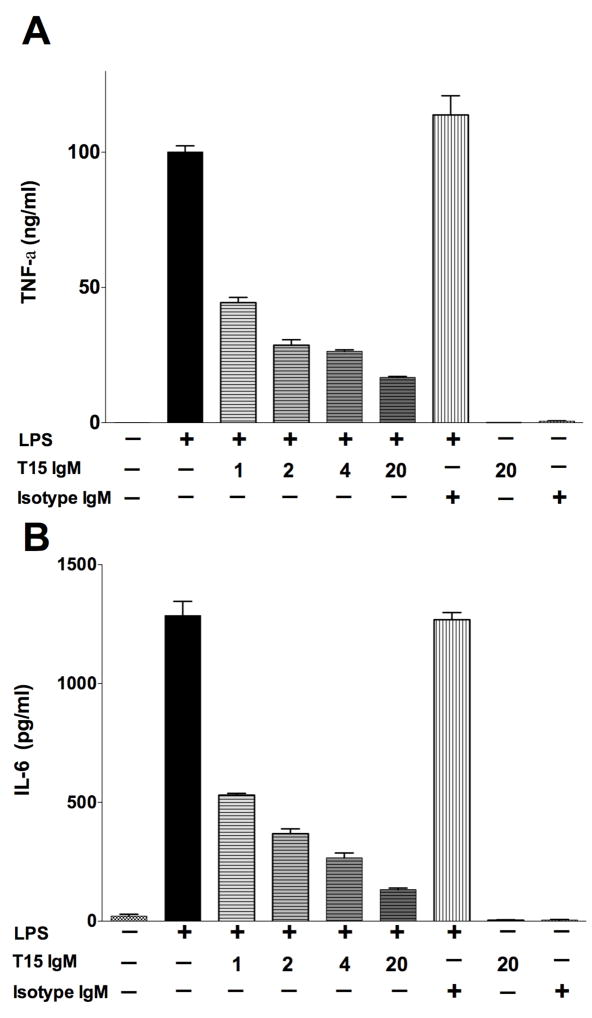
As interactions with apoptotic cells are reported to blunt inflammatory responses (34), and we found that T15-NAb enhances interactions of phagocytes with apoptotic cells, we also assessed whether this NAb can affect pro-inflammatory responses of the RAW264.7 macrophage-like cell line. In a representative study, T15 IgM displayed a significant dose-dependent inhibition of induced TNF-α secretion (P<0.0001), with 55% inhibition by 1 ug/ml, 74% at 4 ug/ml and 83% inhibition at 20ug/ml, while the IgM isotype control had no effect (Figure 2A). T15-NAb similarly inhibited TLR-induced IL-6 secretion (Figure 2B). However, TLR-mediated stimulation also caused a significant level of cell death (data not shown), so we could not control for a requirement for apoptotic cells for T15-mediated inhibition. Nonetheless, these findings document that the T15 NAb can induce a dose-dependent inhibition of TLR4-induced macrophage secretion of the key pro-inflammatory factors, TNF-α and IL-6.
Figure 2. T15-NAb inhibits LPS induced secretion of TNF-a and IL-6 by the RAW264.7 macrophage cell line.
RAW 264.7 murine macrophage cells were stimulated overnight without or with LPS (100 ng/ml), and supernatants were assayed for (A) TNF-α and (B) IL-6. Values represent mean +/− SEM or 3 or more replicate cultures. Representative of 4 experiments.
NAb associates with MBL and C1q to enhance DC phagocytosis of apoptotic cells
At early stages of differentiation, immature DC share many cell surface receptors as well as the phagocytic capacities of Mφ (35). We therefore used a standard culture system to generate CD11c+ immature DC (25), and studied the phagocytic capacity of bone marrow-derived conventional DCs (18,36–38). After incubation with labeled thymocytes, the purified CD11c+ immature DCs were discriminated in flow cytometry studies based on size (i.e., FSC), and/or staining for CD11c (Figure 3), and the proportion of immature DC sub-population that ingested CFSE-tagged apoptotic cells was quantified based on the associated shift in fluorescence (Figure 3A), using methods previously confirmed with side-by-side microscopic quantitation (18). Importantly, under serum-free conditions (i.e., devoid of Ig and opsonins), we have recently shown that DC displayed the same low frequency of phagocytosis of apoptotic thymocytes as with labeled healthy thymocytes ((18) and data not shown), which is consistent with the notion that efficient phagocytosis of apoptotic cells, compared to viable cells, is dependent on the availability of specific serum factors.
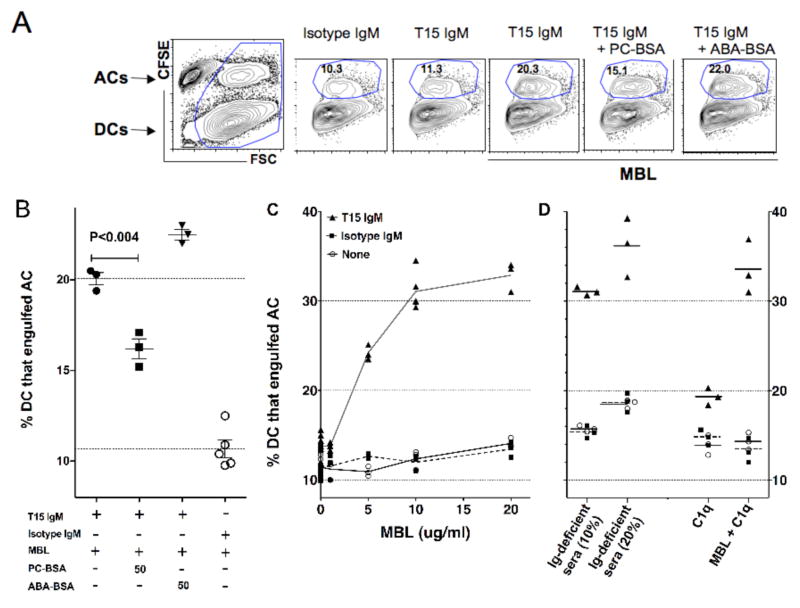
Figure 3. T15-NAb enhances in vitro phagocytosis of apoptotic thymocytes by conventional DC.
Bone marrow derived purified DC were cultured with equal numbers of CFSE-labeled apoptotic cells in serum-free media for 1 hr. Cultures were supplemented with IgM, purified human C1q, recombinant human MBL, or Ig-deficient murine sera as indicated. For analysis, cell fragments were removed based on forward scatter, which enabled the discrimination of the proportion of DCs that have engulfed apoptotic cells (ACs). (A) Cultures included limiting concentrations of T15 or isotype control IgM (5 ug/ml), with saturating amounts of MBL (40 ug/ml), as indicated. High levels of AC phagocytosis requires co-culture with both T15-NAb and MBL, while the level of phagocytosis is inhibited by preincubation with PC-BSA (50ug/ml) but not by control protein conjugate (ABA-BSA). (B) PC significantly inhibited T15-NAb mediated enhancement of AC phagocytosis, as shown in data compiled from replicate cultures. (C) MBL is responsible for large dose-dependent increases in AC engulfment increases in the presence of T15-IgM (at 20 ug/ml), with significantly less effects in cultures without IgM or with isotype control (20 ug/ml). (D) Replicate cultures demonstrate equivalent significant T15-IgM mediated increases with addition of Ig-deficient sera, while C1q also conveys significant but smaller increases in T15-NAb dependent AC phagocytosis, as recently reported (18).
As levels of the opsonins, C1q and MBL, are reported to directly correlate with the efficiency of apoptotic cell elimination (39), we also used this system to look for potential interactions of T15-NAb NAb in serum-free media with the addition of these opsonins. Here, we found that addition of recombinant MBL provided a significant dose-dependent increase in DC phagocytosis, with dramatic increases seen only when T15-NAb was present (Figure 3). Strikingly, in similar serum-free cultures with a fixed amount of T15-NAb, MBL conveyed much greater dose-dependent increases in the efficiency of phagocytosis than we have found was associated with supplementation with purified C1q (Figure 3C&D)(18). Furthermore, in cultures with T15-NAb and the highest level of MBL, the further addition of C1q resulted in only a minor additional increases in DC phagocytosis. In fact, the level of phagocytosis seen in cultures with MBL at 20 μg/ml were comparable to those instead supplemented with Ig-deficient sera (Figure 3C&D). Hence this NAb-dependent influence on innate immune function was limited by the availability of MBL and C1q, which were redundant in their capacity to enhance the influence of T15-NAb on DC phagocytosis. Unexpectedly, in this assay MBL appeared to be more potent than C1q, as MBL alone conveyed nearly the full level of phagocytosis associated with Ig-deficient sera. We also compared levels of phagocytosis with T15-IgM (at 20 ug/ml) without the addition of sera, or with supplementation with 10% Ig-deficient sera, or after heat inactivation, which was thereby shown to reduce by 83% the contribution of serum factors to T15-mediated enhancement of iDC phagocytosis of apoptotic cells (not shown).
To confirm the requirement of the antigen-binding specificity of the NAb, we performed studies with saturating amounts of MBL and a limiting concentration of T15 IgM. Here we found that with T15-NAb and MBL, the frequency of DC that engulfed apoptotic cells was still nearly twice as high as with serum-free conditions alone (Figure 3C&D). Importantly, preincubation with PC-BSA significantly reduced (>50%) the T15-IgM mediated increase in the phagocytic engulfment of apoptotic cells (P<0.004), while incubation with an irrelevant control BSA-conjugate instead increased phagocytosis by ~25% (p=0.0021)(Figure 3A). Hence, the capacity of T15-NAb to enhance apoptotic cell engulfment was also shown to be dependent on its PC-binding specificity.
NAb in association with MBL and C1q inhibits TLR-induced DC maturation
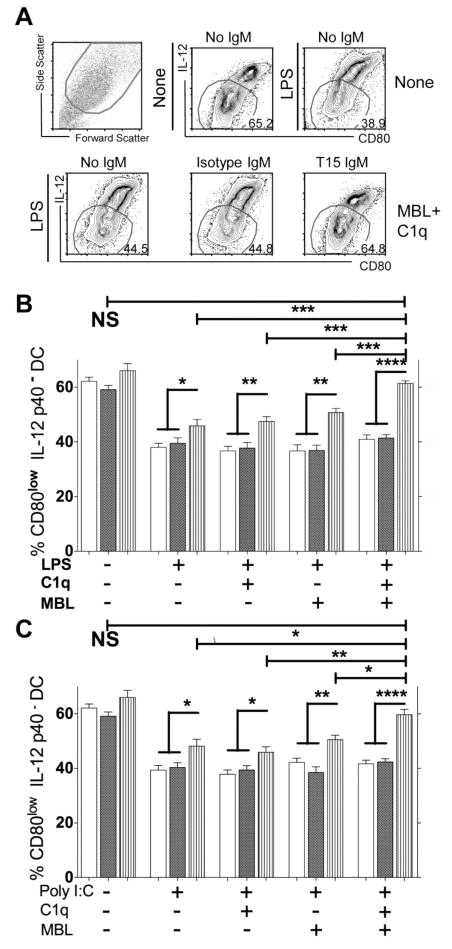
To assess the effects of NAb-apoptotic cell complexes on DC maturation, we studied how different culture conditions can affect co-expression of the membrane-associated co-stimulatory molecule, CD80, and intracellular IL-12 p40 expression, which can be upregulated following TLR stimulation. Notably, control studies demonstrated that even without TLR agonists, these primary CD11c+ DC display a range in their phenotype (Figure 4A), which in part reflects the persistent influence of GM-CSF and IL-4 (40) At first, we confirmed that either LPS or poly I:C induced the maturation of DC, based on evidence of decreased representation of less mature (i.e., CD80low IL-12p40−) DC, compared to culture without these stimuli (P<0.0001)(Figure 4A).
Figure 4. T15-NAb inhibits in vitro TLR induced maturation of conventional DC.
Bone marrow mononuclear cells were cultured with GM-CSF and IL-4 for 5 days, then purified with anti-CD11c beads prior to overnight culture in serum-free media. (A) LPS induces maturation of DC, based on increased CD80 and IL-12p40 expression, which is inhibited by T15 IgM (20 μg/ml) in cultures supplemented MBL (20 μg/ml) and C1q (80 μg/ml). DC are shown to be heterogeneous in their level of maturation, even in the absence of LPS or poly I:C. Based on scattergram analysis, these overnight cultures contained 10–18% dead cells and fragments. (B) T15-NAb, in the presence of MBL and C1q, inhibits LPS induced DC maturation. Values for CD80− IL-12p40low DC, as gated in panel A, is shown from four or more replicate cultures (mean+/− SEM). (C) T15-NAb, in the presence of MBL and C1q, inhibits poly I:C induced DC maturation. Data from four or more replicate cultures are shown. Open bars are without IgM, dark bars are with Isotype control, and vertical strips are with T15 IgM at 20 ug/ml. Significance as indicated: * P<0.05; ** ≤ 0.01; *** ≤0.0001; **** ≤0.0003. Representative of three independent experiments.
We next evaluated whether the addition of a large number of apoptotic cells alone can affect DC maturation. Notably, we uniformly found that in DC cultures stimulated with LPS or poly I:C, the addition of equal numbers of apoptotic thymocytes significantly inhibited the TLR-induced DC maturation. This result was a consistent finding whether or not antibody and opsonin was added, and these differences were highly significant when we compared paired cultures without or with added apoptotic cells (P<0.0001, paired t test, N=3 replicate cultures)(data not shown). Hence, as previously reported, apoptotic cells themselves, when present in substantial numbers, inhibit TLR-induced conventional DC maturation (41). Importantly, we found that after DC alone were in culture for 24 hr, 10–15% of recovered cells were Annexin V+ apoptotic cells and fragments (unpublished data)(Figure 4A). Predictably, when added to these DC cultures, T15-NAb coated these apoptotic DC (and their breakdown products) but not viable DC (Supplemental Figure 2). These T15-NAb-coated apoptotic DC were phagocytosed by viable immature DC (Supplemental Figure 2), while T15-NAb otherwise had no adverse effects on viability or proliferation. Thus, inherent to the biology of these primary cells, DC cultures contained apoptotic cells and debris that form into complexes with T15-NAb.
We therefore tested the hypothesis that T15-NAb can suppress TLR-induced DC maturation, in the presence of suitable opsonins, and only the limited number of the dead cells/fragments that are continuously generated in culture. We uniformly found that addition of T15-NAb increased the proportion of less mature DC, compared to cultures with isotype control or without IgM (Figure 4A–C). This was also found in T15-NAb containing cultures without supplemented opsonic factors, may reflect the potential carryover of serum factors on the apoptotic thymocytes, or the production by immature DC of small amounts of C1q and MBL (42,43) and possibly other factors. Nonetheless, T15-NAb significantly suppressed DC maturation in cultures stimulated with either poly I:C or LPS (P<0.05). There was also a trend toward less mature DC in cultures with T15-NAb but without TLR agonist (Figure 4B&C), presumably due to blunting of the residual influences of GM-CSF and IL-4.
There was a consistent hierarchy in the effects of C1q and MBL on T15-NAb suppression in these replicate LPS or poly I:C stimulated serum-free cultures. The least inhibition of DC maturation was found in the absence of additional supplements, with greater inhibition with the addition of C1q, and even greater suppression with MBL. The greatest inhibition was seen when T15-NAb was added to TLR-stimulated cultures that had been supplemented with both C1q and MBL, as this resulted in significantly more immature DC than other T15-NAb containing cultures, whether or not MBL or C1q were added (P<0.015). Indeed, T15-NAb, in the presence of both C1q and MBL, effectively blocked DC maturation induced by LPS (Figure 4B) or poly I:C (Figure 4C) to the level found in cultures without TLR agonist and T15-NAb.
NAb inhibits in vitro inflammatory responses of DC
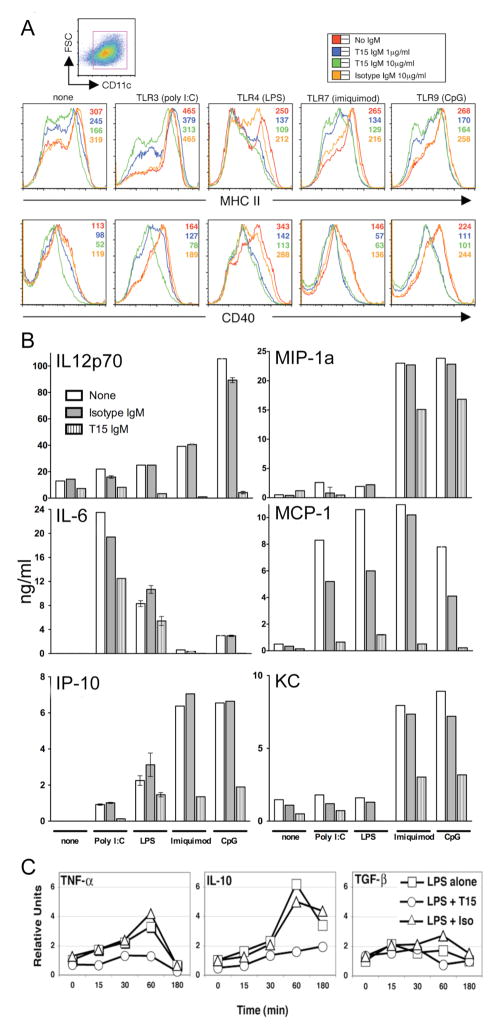
We next assessed whether T15-NAb, which binds apoptotic material in culture and enhances phagocytosis, can modulate other features of in vitro responses of DC in sera-containing media with a broad range of agonistic TLR ligands, including poly I:C, LPS, imiquimod, and CpG DNA. Indeed, inhibition was again documented for surface maturation/activation markers, MHC II, CD40, CD86, and CD80 (Figure 5A and unpublished data) and for secretion of pro-inflammatory cytokines (TNFα, IL-6, IL-12p70), CC chemokines (KC, MCP-1, MIP-1α), and CXC chemokine (IP-10) (Figure 5B and unpublished data). By real-time PCR analysis, T15-NAb also inhibited LPS induction of TNF-α, IL-1β, IL-6, and IL-12 transcripts (Figure 5C and unpublished data). By contrast, at even high concentrations, the B-1 cell derived IgM isotype control, which showed only minor binding to late-stage apoptotic cells, resulted in little or no inhibition. Further studies showed that T15-NAb-mediated inhibition of IL-6 production was >80% reduced by a T15-specific anti-idiotypic antibody that blocks the T15 PC-binding site (19). Hence, our findings support the hypothesis that the specific interactions of T15-NAb with dead and dying cells can inhibit DC maturation and suppress activation-associated expression of cytokine and chemokine factors.
Figure 5. T15-NAb treatment blunts in vitro DC responses to TLR agonists.
(A) CD11c+ selected myeloid DC were cultured in replicate with agonists for TLR3 (poly I:C), TLR4 (LPS), TLR7 (imiquimod), or TLR9 (PT CpG ODN1018), without or with T15-IgM or isotype control at indicated concentrations. (A) Histograms of MHC II and CD40 on DC after culture without or with stimulant (indicated above panel) are depicted. Mean fluorescence intensity is listed without and with IgM, at indicated concentrations. (B) Supernatants from these overnight cultures of conventional bone-marrow derived DC were assessed for levels of pro-inflammatory cytokines and chemokines, which were determined from standard curves (mean+/−SEM). Results are shown without (none) or with stimulants (poly I:C, pIC; imiquimod, imiq) without or with T15-IgM, isotype IgM control, at 10 ug/ml. The isotype control was associated with minor inhibition of MCP-1. (C) Transcript levels were determined by real time PCR for murine BM-derived CD11c+ DC, under indicated cultured conditions over time (min). DC were preincubated with T15-NAb or isotype control before time “0” sampling, then LPS was added. Amplification for TGFβ is β1 isoform-specific. Results are representative of three or more independent experiments.
To assess for potential pathways responsible for these T15-NAb-mediated inhibitory activities, we first examined expression of IL-10 and TGF-β1, which are both implicated in the inhibitory properties of regulatory DC responses. Neither, however, were induced, at either the transcript or protein level by T15 exposure and, in fact, T15-NAb inhibited the LPS-mediated induction of IL-10 (Figure 5C and unpublished data). The suppressive effects of T15-NAb were also unimpaired by IL-10 neutralizing antibodies or in DC from IL-10-deficient mice (unpublished data).
T15-NAb inhibits in vivo inflammatory responses
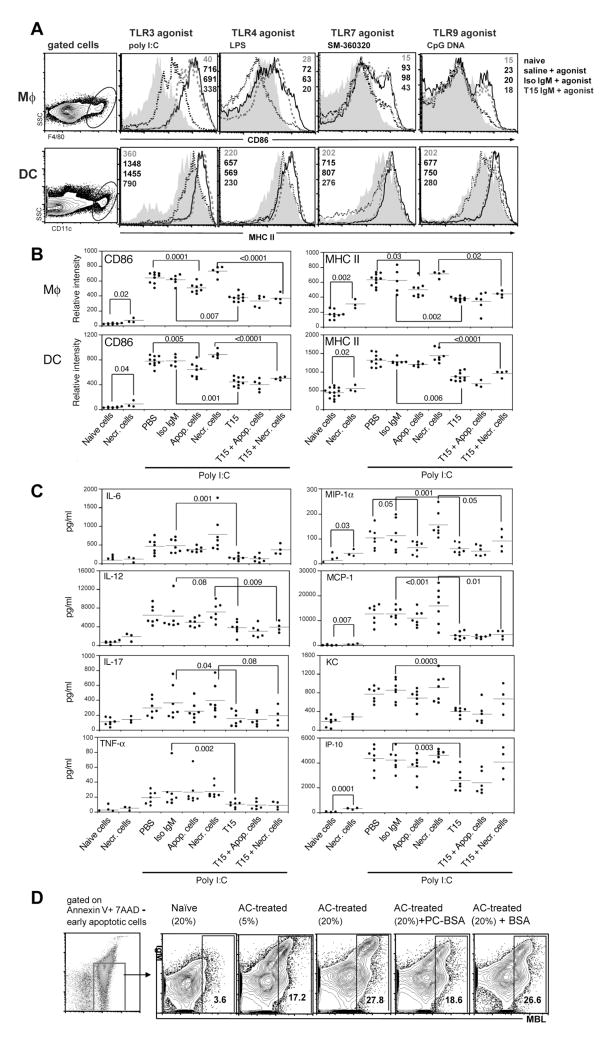
To determine whether T15-NAb can also inhibit in vivo inflammatory responses, we investigated the effects of infusions of purified T15-IgM on in vivo innate immune pro-inflammatory responses. Indeed, after two weeks of T15-NAb exposure, which corresponds to the approximate turnover period for DC populations (26), the T15-NAb group had 17–21% less splenic CD11chi DCs (P<0.02, N=7–8 per group) and significantly lower levels of surface-expressed MHC class II (P<0.02), which is consistent with evidence that T15-NAb can inhibit in vitro DC maturation. Importantly, responses to the TLR agonists, poly I:C (TLR3), LPS (TLR4) and CpG nucleotides (TLR9), were also inhibited by T15-NAb pretreatment, with impaired induction of activation and maturation markers, CD86 and MHC II, on splenic Mφ and CD11chi DC (Figure 6A&B). Furthermore, T15-NAb also significantly inhibited responses to the potent TLR7 agonist, SM-360320 (27)(Figure 6A), as well as poly I:C induction of other co-stimulatory molecules such as CD40, CD80 and B7-DC (unpublished data). T15-NAb treatment also blunted poly I:C-induced blood levels of pro-inflammatory cytokines (IL-6, IL-12, IL-17, TNFα) and chemokines (MIP1α, MCP-1, KC and IP-10)(Figure 6C). In addition, NAb treatment significantly reduced the production of IL-6 and IL-12 by peritoneal Mφ (unpublished data). Confirming the role of the PC-binding specificity, preincubation with an excess of PC-conjugate before T15-NAb infusion antagonized >78% of the in vivo inhibitory effects. Hence, elevated levels of T15-NAb drastically reduced the in vivo responsiveness of the innate immune system to a range of pro-inflammatory stimuli.
Figure 6. In vivo T15-IgM treatment blunts responses to TLR agonists.
(A) Groups of adult C57BL/6 mice received saline, IgM control or T15-IgM, then were challenged with agonists for TLR3 (poly I:C), TLR4 (LPS), TLR7 (SM-360320)(27), TLR9 (CpG ODN1018). After 18hr, splenic Mφ and CD11chi DC were evaluated, based on indicated gates (left panels). Representative histograms and mean fluorescence indices (MFI) are depicted for: top value from gray shaded area from naïve mouse; saline pretreatment then TLR agonist challenge dark solid line; isotype control followed by TLR agonist challenge from thick gray dashed line; bottom, T15-NAb followed by TLR agonist, black dashed line. Compared to naïve mice, challenge with TLR agonist induces high expression levels of MHC II and CD86, but was inhibited by T15-NAb. (B) T15-IgM or apoptotic-cell treatments inhibited poly I:C induced activation of splenic Mφ (F4/80+) and myeloid DC (CD11chi) from C57BL/6 mice. Groups received buffer alone (PBS), necrotic (Necr.) thymocytes, apoptotic (Apop.) thymocytes, isotype control (Iso IgM), T15 or the indicated combination. Results are shown for individual mice at 18 hr after challenge. Horizontal bars depict mean values for each group. There were no significant differences in the representation of splenic F4/80+ Mφ and CD11chi DC between different treatment groups. (C) T15-IgM or apoptotic-cell treatments inhibits poly I:C induction of serum levels of pro-inflammatory cytokines and chemokines in C57BL/6 mice. P values from two-tailed t test are shown. Results were pooled from three or more independent experiments. (D) Representative flow cytometric analyses after gating on early apoptotic thymocytes (i.e., Annexin V+ 7AAD−) of IgM and MBL from sera of naïve adult C57BL/6, or mice that received three weekly apoptotic cell (AC) treatments, with level of binding shown to be dose-dependent based on the % sera. PC-BSA incubation greatly reduced the level of MBL deposition. Representative of three independent studies.
Apoptotic cells induce anti-inflammatory NAbs with the properties of T15
We reasoned that apoptotic cells might be the main antigenic target for in vivo T15-related NAb responses. To examine the in vivo relationship between T15-NAb, apoptotic cells, and inflammation, we infused large numbers (25×106) of apoptotic thymocytes into naïve mice that had low but detectable natural T15 levels (Supplemental Figure 3, with data deposited in the GEO repository under accession number GSE14969 http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE14969). Notably, this treatment also blunted Mφ and DC activation responses (Figure 6B&C). Moreover, co-treatments of apoptotic cells plus T15-NAb trended toward greater suppression of in vivo poly I:C-induced activation responses, compared to either apoptotic cells alone, or apoptotic cells plus isotype control (Figure 6B&C). Inhibition was also observed for peripheral blood pro-inflammatory cytokines and chemokines (Figure 6C). Hence, in vivo apoptotic-cell treatment unexpectedly produced a similar inhibitory effect as demonstrated for T15-NAb.
While apoptotic cells were anti-inflammatory, primary necrotic thymocytes, which can exacerbate autoimmune disease (44), were not. In fact, infusion of necrotic cells alone significantly increased the expression of activation markers after poly I:C challenge (Figure 6B&C). Strikingly, T15-NAb, albeit to a lesser extent, still blunted the proinflammatory effect of necrotic-cell treatment (Figure 6B&C), suggesting that the raised T15-NAb levels resulted in formation of inhibitory complexes with host apoptotic cells.
Extending recent observations (18), we also tested whether infusions of apoptotic cells in these studies induced relevant T15-like antibody responses similar to pneumococcal immunization (12). Indeed, 10 days after a single infusion of such cells without adjuvant, 8-fold increases in circulating IgM anti-PC levels were detected. Although responses varied in individual mice, three weekly apoptotic-cell infusions generally raised circulating levels of T15-clonospecific (19) and IgM anti-PC antibodies more than 40-fold higher than in naïve mice (p<0.01)(Supplemental Figure 3A&B) (18). In contrast, infusions of healthy or necrotic-cells yielded only minor changes, which is consistent with findings that apoptotic-cells induced ~20-fold increased numbers of PC-specific splenic IgM-secreting cells (18). In fact, apoptotic-cell infusions induced IgM antibodies to PC-containing determinants to levels equivalent or higher than those that followed T15-NAb infusions (Supplemental Figure 3C), which required substantial doses due to the short half-life of IgM (45). Apoptotic cells also induced IgG anti-PC responses, but these levels were much lower (5.5±2.5 ug/ml) and overwhelmingly of the IgG3 subclass, indicating a mainly T-cell independent response (Supplemental Figure 3D, and unpublished data). Hence, despite evidence that apoptotic cells may suppress some innate immune functions, intravenous treatment nonetheless induced robust B-cell responses, even without the use of adjuvant.
To assess the relevance of these active immune responses to the above described T15-NAb studies, we have recently shown that incubation of sera from apoptotic cell treated mice resulted in IgM binding to apoptotic cells, and enhanced antibody-dependent C1q recruitment to apoptotic cells, both of which were inhibited by PC preincubation (18). We now used the solid phase MBL-recruitment anti-PC immunoassay, and found that apoptotic cell treatments induced high levels of MBL-binding anti-PC antibodies (384±89ug/ml, N=4), while negligible levels were found before treatment (< 2 ug/ml, P<0.0001). Akin to the properties of the monoclonal T15-NAb, MBL recruitment by post-immune anti-PC responses was also >90% inhibited by preincubation with mannose or N-acetyl glucosamine, or with EDTA in the media that demonstrated calcium dependence, while MBL binding was not inhibited by N-acetyl galactose (not shown). Compared to naïve sera, incubation in apoptotic-cell post-immune sera greatly increased levels of IgM binding to apoptotic cells, with similar increases in the recruitment of C1q and MBL. Notably, the greatest IgM-associated enhancement was seen on early apoptotic cells (i.e., 7AAD−)(18)(Figure 6D). Preincubation of T15-NAb with PC-BSA also greatly reduced the deposition of IgM and also MBL (Figure 6D) and C1q (18) on apoptotic cells. Furthermore, similar to purified T15-NAb (Figure 3A), sera obtained after apoptotic-cell treatment, which had markedly increased levels of IgM-antibodies to apoptotic cells, also suppressed in vitro TLR-mediated activation of cultured DC (unpublished data).
Suppression of inflammation by apoptotic cells requires B cells or T15-NAb
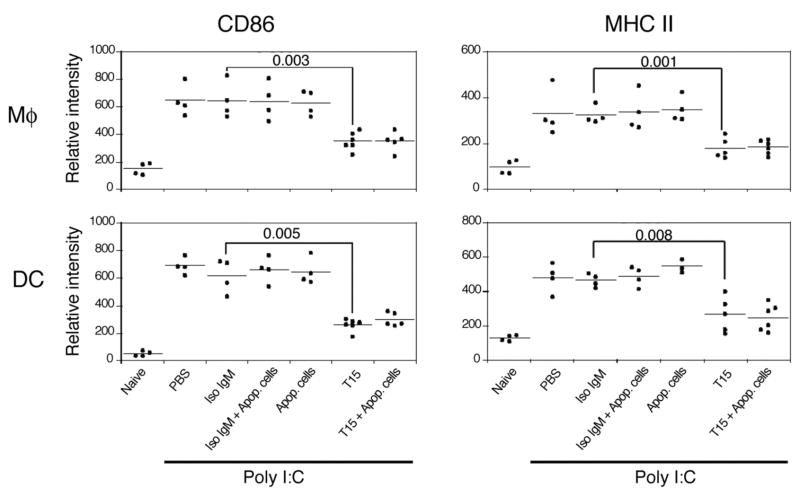
B-cell-deficient MuMT mice were next used to assess the requirement for IgM in the in vivo inhibitory properties of apoptotic-cells. Strikingly, infusions of apoptotic cells alone had little or no effect on poly I:C-induced cellular activation or cytokine/chemokine responses in B-cell deficient mice (Figure 7 and unpublished data). In contrast, T15-NAb, but not isotype control, treatment of B-cell deficient mice induced the same blunting of TLR-induced cell activation and cytokine/chemokine production as demonstrated in C57BL/6 mice. Overall, these findings indicate that the suppressive effect of apoptotic cell infusions in vivo is dependent on the induction of antibodies with certain specificities, which include anti-PC reactivity.
Figure 7. B-cell deficient mice are not protected by apoptotic-cell treatments.
T15-IgM but not apoptotic-cell treatments inhibits poly I:C-induced activation of splenic Mφ and myeloid DC in B-cell deficient (muMT) mice. Results are for MFI from flow cytometry studies, as shown in Figures 6A&B. Results are for individual mice are the pooled from two independent experiments.
NAb protects from inflammatory arthritis
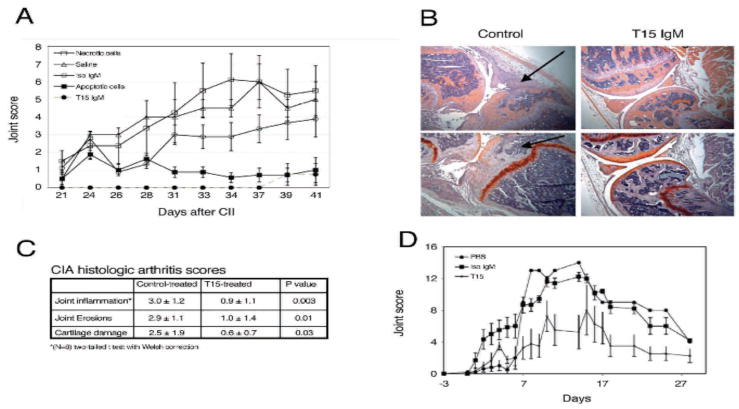
As inflammatory pathways involving Mφ, DC and TLR have been implicated in the pathogenesis of autoimmune arthritis (46), we studied collagen-induced arthritis (CIA) in DBA/1 mice (47) to test the hypothesis that high levels of T15-NAb might suppress the development of inflammatory disease (Figure 8 & Supplemental Figure 4). Significantly, pretreatment with the anti-PC NAb markedly reduced clinical disease activity, synovial leukocytic infiltrates, and bone and joint damage (Figure 8A–C). Notably, there were no differences in total IgG-, or IgG1- and IgG2a- subclass anti-CII levels induced by collagen immunization in the different treatment groups (unpublished data) suggesting that T15-NAb was primarily inhibiting the end organ inflammatory response. In other studies, infusions of apoptotic cells into DBA/1 mice yielded increased IgM anti-PC levels and protection from clinical arthritis, while infusions of primary necrotic cells did not (Figure 8A).
Figure 8. T15-NAb protects from inflammatory arthritis.
(A) DBA/1 mice were immunized with CII and boosted on day 20. T15-NAb at 2 mg/dose, isotype control, apoptotic thymocytes or necrotic cells (2.5 × 107) in saline, or saline alone were administered weekly. T15-NAb and apoptotic-cell treatments significantly reduced clinical arthritis joint scores compared to control treatments (isotype control, saline and necrotic cells) (P<0.001 by Bonferroni test). The isotype control group was not significantly different than saline-treated group. Data are pooled from two independent studies with separate treatment and control groups of four mice (total N=8). Depicted are mean values+/−SEM. (B) Protective T15-NAb reduced inflammatory cellular infiltrates in CIA. Compared to isotype control treatment at left, T15-IgM anti-PC NAb significantly reduced cartilage and bone destruction (arrowhead), and greatly reduced level of cellular infiltrates (arrow)(40Xmag). Below, knees from control-treated mice had progressive pathologic changes of compromised articular cartilage that is shown with safranin O (bright orange), while T15-IgM provided protection from cellular infiltrates, and cartilage and bone destruction. (C) Histologic arthritis scores are depicted for CIA treatment study, with values derived as previously described (28). (D) To induce autoantibody-mediated arthritis, BALB/c mice received a commercial cocktail of anti-CII antibodies, and data represent sequential measurements from two independent studies with separate treatment and control groups of four mice (total N=8). Weekly T15-NAb infusions significantly reduced arthritis based on clinical scores of joint scores, compared to saline or isotype control-treated mice, with P<0.0022 at the peak d14 response. Depicted are mean values +/− SD.
To further define the adaptive immune systems role in this process, we studied the effects of T15-NAb on passive transfer arthritis induced by anti-CII IgG, in which lymphocytes do not a play central role (48). Here, we again found that T15-NAb treatment significantly diminished joint swelling (Figure 8D). Together, these findings indicate that the regulatory properties of T15-NAb in these models of arthritis act through the blunting of pro-inflammatory effector mechanisms mediated by the recruitment of IgG-autoantibody immune complexes.
Discussion
In health, inflammatory responses are critical for combating infection and tissue injury, but of equal importance are control mechanisms that remove dying cells and prevent over-exuberant responses detrimental to the host. Here, we document several previously unrecognized and functionally important features of the prototypic anti-PC NAb, T15. First, by virtue of its capacities for PC-specific binding to apoptotic cells, in addition to C1q (18), T15-NAb also facilitates the deposition of MBL onto apoptotic cells, particularly early apoptotic cells that are generally less susceptible to direct opsonin recruitment. Second, T15-NAb, by recruiting the deposition of both C1q and MBL, enhances the phagocytosis of early and late apoptotic cells. Third, T15-NAb, by forming complexes with MBL and C1q on apoptotic cells, can effectively suppress TLR-induced maturation of conventional DCs. Fourth, T15-NAb inhibits Mφ and DC secretion of pro-inflammatory cytokines and chemokines in response to agonists for a broad range of TLR, and it is also capable of inhibition of in vivo phagocyte activation and suppression of potentially harmful inflammatory responses. Fifth, infusion of high doses of T15-NAb, or large numbers of apoptotic cells that induce IgM anti-PC Abs, can inhibit the development of autoimmune inflammatory arthritis. Taken together, these findings identify a hitherto unsuspected set of regulatory functions of this type of NAb.
Our findings therefore characterize the potential functional roles of T15-NAb, which is representative of a dominant antibody response induced by apoptotic cells. Multiplex autoantigen-microarray assays have shown that apoptotic-cell treatment of C57BL/6 mice induces a dominant IgM response directed toward PC-containing antigens; including PC-albumin, pneumococcal vaccine and capsular polysaccharide alone, or the self-antigen, oxidized LDL, with little or no reactivity to a large panel of other autoantigens (18)(Supplemental Figure 3)(17). In C57BL/6 mice, T15-related antibodies represent about half of all induced antibodies that recognize apoptotic cells (18). In fact, apoptotic cells induced relatively low levels of anti-DNA IgM (18)(Supplemental Figure 3), which contrasted with an earlier report (49). Taken together, apoptotic cells do not appear to induce non-specific polyclonal IgM responses, but instead provoke an antibody response with a strong bias toward immune recognition of PC-neo-determinants.
In contrast to the classical theory that a healthy immune system requires an absolute avoidance of self-reactive lymphocyte clones (i.e., “horror autotoxicus”)(50), the repertoires of innate-like B cells are known to incorporate some level of autoreactivity (3,51), and self-ligands are believed essential for their positive clonal selection and survival (52). Based on evidence that T15 B cells recognize an immunodominant self-antigen in apoptotic cells/debris, it is plausible that the apoptotic-cell turnover occurring during ontogenesis provides the self-ligands for the early selection of these B-1 cell clones. Moreover, as anti-apoptotic cell antibodies are part of the physiologic repertoire, our findings may explain why mice without circulating IgM spontaneously develop IgG-autoantibodies and lupus-like disease (53), as this may result in impaired clearance of apoptotic breakdown products, as well as the loss of an NAb regulatory influence that otherwise can suppress overexuberant responses of innate phagocytes (54).
Although the potential immunosuppressive roles of NAbs have been generally overlooked, there is nonetheless overwhelming evidence that C1q and MBL have immune modulating properties (31). Based on evidence that humans with a homozygotic deficiency in C1q have a high penetrance of lupus-like systemic autoimmunity (55), studies of murine models confirmed this also occurs on certain genetic backgrounds, and is associated with an accumulation of apoptotic bodies in the kidney and glomerulonephritis in 25% of animals (56). These data are consistent with the hypothesis that C1q deficiency, resulting in inefficient clearance of apoptotic cells, leads to the release of intracellular components, exposure of the immune system to self-Ags, and subsequent autoimmunity. Like C1q deficiency, MBL deficient mice also display defects in apoptotic clearance and are prone to inflammatory conditions (33), although it is controversial whether MBL deficiency also influences autoimmune predisposition or disease severity (57).
While MBL is well known for its contributions to the clearance of microbial pathogens and apoptotic cells, its potential role in IgM-antibody effector functions has been little explored. While these opsonins can be directly deposited at low levels onto apoptotic cells, this is primarily on late stage apoptotic and secondary necrotic cells (23). Our data show that MBL (and C1q) are recruited by T15-NAb to early stage apoptotic cells, and this is associated with both enhanced phagocytosis and reduced inflammation. Significantly in vivo apoptotic-cell immunization was shown to induce IgM-anti-PC responses that also recruited MBL- and C1q- binding, confirming the physiologic relevance of their essential yet redundant roles in NAb-effector functions. The effector function for MBL recruitment has also been associated with B-1 cell derived IgM antibodies with a different self-specificity (58).
Apoptotic-cell phagocytosis by immature DC enables constant sampling and presentation of self-antigens in a manner believed essential for the maintenance of tolerance (59). Yet, if apoptotic cells are not quickly cleared, cellular progression to necrosis can lead to the release of pro-inflammatory substances and autoantigens that can lead to breaches in self-tolerance (60). Hence the efficient elimination of the immense number of cell corpses generated each day is therefore indispensable for tissue homeostasis, resolution of inflammation, and prevention of autoimmune disease. We provide direct evidence that a monoclonal B-1 cell NAb can directly induce deposition of MBL and C1q, which can each directly enhance cell-corpse clearance, as well as inhibit immature DC activation and differentiation. The anti-inflammatory influences of T15-NAb are no doubt aided by the capacity to flag cells and fragments at earlier stages of apoptosis for clearance.
The association of MBL with an apoptotic cell alone has been reported to be inadequate for activation of the complement cascade (23), and host cells themselves express specific complement inhibitors that also provide protection from inappropriate complement activation (61). However, other recent reports have shown that polyclonal serum IgM can induce the deposition of C3 products, which enable recognition by Mac-1/CR3 on Mφ also implicated in the clearance of apoptotic cells (62,63). We therefore repeated our in vitro challenge studies with TLR agonists in serum-free cultures of C3-deficient DC, but found no reduced capacity of T15-NAb to enhance phagocytic clearance and inhibit inflammatory responses (18)(data not shown). Our pilot studies also indicate that the in vivo anti-inflammatory activities of T15-NAb are unimpaired in C3-deficient mice (unpublished data). Taken together, we believe these findings rigorously rule out an absolute requirement for downstream propagation of complement pathway activation in the immune modulatory properties of T15-NAb.
Our studies highlight the otherwise overlooked capacity for antigen-specific IgM antibodies to affect the fundamental process of apoptotic clearance and regulation of TLR responses. As discussed above, we postulate that T15-NAb, which recognizes apoptosis-specific determinants, by increasing the amount of C1q and MBL bound on apoptotic cells facilitates their phagocytosis and also enhances apoptotic cell-mediated inhibition of macrophage and DC activation. This process potentially involves receptors, such as CD36, CD93 αvβ3, calreticulin, and the low density lipoprotein receptor (LRP-1, CD91) that have been implicated in MBL- and C1q-enhanced apoptotic clearance (39,64,65). However the topic remains controversial, especially in light of the recent report, which also used etopside-induced apoptotic thymocytes, to demonstrate that LRP-1 was not essential for the enhancement of phagocytosis by murine macrophage (38). In addition, T15-NAb also appears to enhance immunomodulatory cellular interactions of complexed apoptotic cells and fragments, for which there are also a number of candidate receptors and at times conflicting literature (reviewed in (66)). Yet, even though T15-NAb enhanced the phagocytosis of cell corpses and also blunted TLR-induced DC maturation, the underlying pathways are not necessarily entirely identical, as these effects can be mediated by distinct residues in the intracellular domain of an apoptotic cell receptor (67). Our preliminary studies indicate that the suppressive effects of T15-NAb on DC involve inhibition of inflammatory signaling pathways (unpublished observations). However, although previous studies implicated IL-10 and TGF-β1 in the anti-inflammatory effects of apoptotic cells (reviewed in (66)), we were unable to find evidence to support this for T15-NAb, but this may in part reflect that such responses may primarily occur at later time points. Albeit, it remains possible that IL-10 and TGF-β might contribute to the in vivo effects of T15-NAb.
We first appreciated the pro-homeostatic properties of T15-NAb in the setting of the chronic inflammatory disease, atherosclerosis. Indeed, earlier in vitro studies initially suggested that T15-IgM might act by blocking apoptotic-cell binding to elicited Mφ (15). By contrast, we now document that these NAb-mediated properties are linked to the contributions of the opsonins, MBL and C1q, that were likely absent or in low amounts in the earlier study (15). Indeed, our current studies, which used more physiologically relevant assays, instead clearly showed that T15-NAb enhanced in vivo phagocytic clearance by Mφ and directly inhibited inflammatory responses. These mechanisms now appear to be a more likely explanation for previous evidence that immunization with a PC-vaccine suppressed atherosclerosis (12).
The current studies of autoimmune arthritis models also showed that treatment with T15-NAb significantly reduced inflammatory joint injury despite grossly unimpaired collagen-specific lymphocyte responses. Our studies were limited to prevention of the initiation phase of disease. However we believe these findings may still be clinically relevant, despite the fact that C1q and MBL deficiency do not specifically predispose to inflammatory arthritis, and such conditions are not known to result from defects in apoptotic clearance. Yet in vitro and in vivo studies in C57BL/6 did demonstrate a potent capacity to inhibit phagocyte production of TNF-α, IL-6, IL-17, IL-12 and key chemokines that recruit other cell types into inflammatory responses. Hence, we postulate that raising levels of such NAbs may provide benefits in conditions as diverse as rheumatoid arthritis, and perhaps also atherosclerosis (12), that are associated with innate immune cell recruitment and uncontrolled activation at sites of pathologic inflammation. Overall, these findings suggest that administration of T15-NAb, or induction of T15-NAb by apoptotic cells or microbial products, might be of general utility for the suppression of deleterious inflammatory conditions.
Earlier studies of T15 antibodies suggested that the driving force for the recurrent expression of these germline-encoded NAbs was linked to protection against microbial pathogens that bear PC molecular patterns, which is reminiscent of functions of receptors of innate immune cells. Our studies may now provide clues to unsuspected primary functional roles of the earliest B lymphocytes, the strong evolutionary pressure to maintain the anti-PC specificity, and why we are born with antibodies in our bloodstreams. Indeed, NAbs with the binding properties of murine T15, to PC and other phospholipid-derived determinants, are also highly conserved in humans from birth (5,68), with documented increases in healthy twins genetically predisposed but unaffected by SLE (22). Such antibodies may also be highly represented in the repertoire of malignant CD5+ chronic lymphocytic leukemia that are human B-1 cell equivalents (69). While there are many other known innate pathways for limiting inflammatory responses, we postulate that some T-cell independent immunoregulatory NAb circuit arose as a means to enhance the clearance of apoptotic cells, and to suppress the induction and hasten the resolution of inflammatory responses. These same primitive pathways may provide a novel therapeutic strategy for the treatment of chronic inflammatory diseases.
Supplementary Material
Acknowledgments
We appreciate helpful discussions with L.A. Herzenberg, M. Botto, T. DuClos and C. Mold. We acknowledge the support of the Digital Imaging Shared Resource of the UCSD Moores Cancer Center.
This work was supported by grants AI40305, AR47360, AR50659, and AI46637 from NIH, the Alliance for Lupus Research and Within Our Reach grant from REF (to GJS) and an Arthritis Foundation fellowship (to SK).
Nonstandard abbreviations used
- CII
Collagen type II
- MDA
Malondialdehyde
- MBL
Mannose Binding Lectin
- NAb
Natural antibody
- PC
Phosphoryl choline
- poly I
C, polyinosinic:polycytidylic acid
- ODN
oligonucleotide
References
- 1.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–35. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202:203–7. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herzenberg LA. Toward a layered immune system. Cell. 1989;59:953–954. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- 4.Perlmutter RM, Kearney JF, Chang SP, Hood LE. Developmentally controlled expression of immunoglobulin VH genes. Science. 1985;227:1597–601. doi: 10.1126/science.3975629. [DOI] [PubMed] [Google Scholar]
- 5.Binder CJ, Silverman GJ. Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin Immunopathol. 2005;26:385–404. doi: 10.1007/s00281-004-0185-z. [DOI] [PubMed] [Google Scholar]
- 6.Cohn M, Notani G, Rice SA. Characterization of the antibody to the C-carbohydrate produced by a transplantable mouse plasmacytoma. Immunochemistry. 1969;6:111–23. doi: 10.1016/0019-2791(69)90183-9. [DOI] [PubMed] [Google Scholar]
- 7.Cook WD, Scharff MD. Antigen-binding mutants of mouse myeloma cells. Proc Natl Acad Sci USA. 1977;74:5687–91. doi: 10.1073/pnas.74.12.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gearhart PJ, Johnson ND, Douglas R, Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981;291:29–34. doi: 10.1038/291029a0. [DOI] [PubMed] [Google Scholar]
- 9.Shaw PX, Horkko S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–40. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sigal NH, Gearhart PJ, Klinman NR. The frequency of phosphorylcholine-specific B cells in conventional and germfree BALB/C mice. J Immunol. 1975;114:1354–1358. [PubMed] [Google Scholar]
- 11.McDaniel LS, Benjamin WHJ, Forman C, Briles DE. Blood clearance by anti-phosphocholine antibodies as a mechanism of protection in experimental pneumococcal bacteremia. J Immunol. 1984;133:3308–3312. [PubMed] [Google Scholar]
- 12.Binder C, Hörkkö S, Dewan A, Chang MK, Kieu E, Goodyear CS, Shaw PX, Palinski W, Witztum J, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation.:Molecular mimicry between oxidized LDL and Streptococcus pneumoniae. Nature Medicine. 2003;9:736–43. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 13.Reardon CA, Miller ER, Blachowicz L, Lukens J, Binder CJ, Witztum JL, Getz GS. Autoantibodies to OxLDL fail to alter clearance of injected OxLDL in apolipoprotein E deficient mice. J Lipid Res. 2004;45:1374–54. doi: 10.1194/jlr.M400075-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Gray M, Miles K, Salter D, Gray D, Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc Natl Acad Sci USA. 2007;104:14080–5. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang MK, Bergmark C, Laurila A, Hörkkö S, Han KH, Friedman P, Dennis EA, Witztum JL. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci USA. 1999;96:6353–8. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SJ, Gershov D, Ma X, Brot N, Elkon KB. I-PLA(2) activation during apoptosis promotes the exposure of membrane lysophosphatidylcholine leading to binding by natural immunoglobulin M antibodies and complement activation. J Exp Med. 2002;196:655–65. doi: 10.1084/jem.20020542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw PX, Goodyear CS, Chang MK, Witztum J, Silverman GJ. The Autoreactivity of Anti-Phosphorylcholine Antibodies for Atherosclerosis-Associated Neo-antigens and Apoptotic Cells. J Immunol. 2003;170:6151–7. doi: 10.4049/jimmunol.170.12.6151. [DOI] [PubMed] [Google Scholar]
- 18.Chen YF, Park YB, Patel E, Silverman GJ. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol. 2008 doi: 10.4049/jimmunol.0804191. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kearney JF, Barletta R, Quan ZS, Quintans J. Monoclonal vs. heterogeneous anti-H-8 antibodies in the analysis of the anti-phosphorylcholine response in BALB/c mice. Eur J Immunol. 1981;11:877–883. doi: 10.1002/eji.1830111106. [DOI] [PubMed] [Google Scholar]
- 20.Silverman GJ, Cary SP, Dwyer DC, Luo L, Wagenknecht R, Curtiss VE. A B cell superantigen-induced persistent “Hole” in the B-1 repertoire. J Exp Med. 2000;192:87–98. doi: 10.1084/jem.192.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Jensen L, Hansen S, Petersen SV, Takahashi K, Ezekowitz AB, Hansen FD, Jensenius JC, Thiel S. Characterization and quantification of mouse mannan-binding lectins (MBL-A and MBL-C) and study of acute phase responses. Scand J Immunol. 2001;53:489–97. doi: 10.1046/j.1365-3083.2001.00908.x. [DOI] [PubMed] [Google Scholar]
- 22.Silverman GJ, Srikrishnan R, Germar K, Goodyear CS, Andrews KA, Ginzler EM, Tsao BP. Genetic imprinting of autoantibody repertoires in SLE patients. Clin Exp Immunol. 2008;153:102–116. doi: 10.1111/j.1365-2249.2008.03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nauta AJ, Raaschou-Jensen N, Roos A, Daha MR, Madsen HO, Borrias-Essers MC, Ryder LP, Koch C, Garred P. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur J Immunol. 2003;33:2853–63. doi: 10.1002/eji.200323888. [DOI] [PubMed] [Google Scholar]
- 24.Taylor PR, Carugati A, Fadok VA, Cook HT, Andrews M, Carroll MC, Savill JS, Henson PM, Botto M, Walport MJ. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–66. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inaba K, Turley S, Yamaide F, Iyoda T, Mahnke K, Inaba M, Pack M, Subklewe M, Sauter B, Sheff D, Albert M, Bhardwaj N, Mellman I, Steinman RM. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J Exp Med. 1998;188:2163–73. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100:1734–41. [PubMed] [Google Scholar]
- 27.Kurimoto A, Ogino T, Ichii S, Isobe Y, Tobe M, Ogita H, Takaku H, Sajiki H, Hirota K, Kawakami H. Synthesis and evaluation of 2-substituted 8-hydroxyadenines as potent interferon inducers with improved oral bioavailabilities. Bioorg Med Chem. 2004;12:1091–9. doi: 10.1016/j.bmc.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Simelyte E, Rosengren S, Boyle DL, Corr M, Green DR, Firestein GS. Regulation of arthritis by p53: critical role of adaptive immunity. Arthritis Rheum. 2005;52:1876–84. doi: 10.1002/art.21099. [DOI] [PubMed] [Google Scholar]
- 29.Kitamura D, Roes J, Kuhn R, Rajewsky KA. B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–6. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 30.Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J Immunol. 1997;158:4525–8. [PubMed] [Google Scholar]
- 31.Bohlson SS, Fraser DA, Tenner AJ. Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol Immunol. 2007;44:33–43. doi: 10.1016/j.molimm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Arnold JN, Wormald MR, Suter DM, Radcliffe CM, Harvey DJ, Dwek RA, Rudd PM, Sim RB. Human serum IgM glycosylation: identification of glycoforms that can bind to mannan-binding lectin. J Biol Chem. 2005;280:29080–7. doi: 10.1074/jbc.M504528200. [DOI] [PubMed] [Google Scholar]
- 33.Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174:3220–6. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- 34.Erwig LP, Henson PM. Immunological consequences of apoptotic cell phagocytosis. Am J Pathol. 2007;171:2–8. doi: 10.2353/ajpath.2007.070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nauta AJ, Castellano G, Xu W, Woltman AM, Borrias MC, Daha MR, van Kooten C, Roos A. Opsonization with C1q and mannose-binding lectin targets apoptotic cells to dendritic cells. J Immunol. 2004;173:3044–50. doi: 10.4049/jimmunol.173.5.3044. [DOI] [PubMed] [Google Scholar]
- 36.Hart SP, Dransfield I, Rossi AG. Phagocytosis of apoptotic cells. Methods. 2008;44:280–5. doi: 10.1016/j.ymeth.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi M, Kobayashi Y. Cytokine production in association with phagocytosis of apoptotic cells by immature dendritic cells. Cell Immunol. 2003;226(2):105–15. doi: 10.1016/j.cellimm.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Lillis AP, Greenlee MC, Mikhailenko I, Pizzo SV, Tenner AJ, Strickland DK, Bohlson SS. Murine low-density lipoprotein receptor-related protein 1 (LRP) is required for phagocytosis of targets bearing LRP ligands but is not required for C1q-triggered enhancement of phagocytosis. J Immunol. 2008;181:364–73. doi: 10.4049/jimmunol.181.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–95. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wells JW, Darling D, Farzaneh F, Galea-Lauri J. Influence of interleukin-4 on the phenotype and function of bone marrow-derived murine dendritic cells generated under serum-free conditions. Scand J Immunol. 2005;61:251–9. doi: 10.1111/j.1365-3083.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- 41.Sen P, Wallet MA, Yi Z, Huang Y, Henderson M, Mathews CE, Earp HS, Matsushima G, Baldwin AS, Jr, Tisch RM. Apoptotic cells induce Mer tyrosine kinase-dependent blockade of NF-kappaB activation in dendritic cells. Blood. 2007;109:653–60. doi: 10.1182/blood-2006-04-017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bensa JC, Reboul A, Colomb MG. Biosynthesis in vitro of complement subcomponents C1q, C1s and C1 inhibitor by resting and stimulated human monocytes. Biochem J. 1983;216:385–92. doi: 10.1042/bj2160385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reboul A, Prandini MH, Bensa JC, Colomb MG. Characterization of C1q, C1s and C-1 Inh synthesized by stimulated human monocytes in vitro. FEBS Lett. 1985;190:65–8. doi: 10.1016/0014-5793(85)80428-2. [DOI] [PubMed] [Google Scholar]
- 44.Ma L, Chan KW, Trendell-Smith NJ, Wu A, Tian L, Lam AC, Chan AK, Lo CK, Chik S, Ko KH, To CK, Kam SK, Li XS, Yang CH, Leung SY, Ng MH, Stott DI, MacPherson GG, Huang FP. Systemic autoimmune disease induced by dendritic cells that have captured necrotic but not apoptotic cells in susceptible mouse strains. Eur J Immunol. 2005;35:3364–75. doi: 10.1002/eji.200535192. [DOI] [PubMed] [Google Scholar]
- 45.Hughey CT, Brewer JW, Colosia AD, Rosse WF, Corley RB. Production of IgM hexamers by normal and autoimmune B cells: implications for the physiologic role of hexameric IgM. J Immunol. 1998;161:4091–7. [PubMed] [Google Scholar]
- 46.Cravens PD, Lipsky PE. Dendritic cells, chemokine receptors and autoimmune inflammatory diseases. Immunol Cell Biol. 2002;80:497–505. doi: 10.1046/j.1440-1711.2002.01118.x. [DOI] [PubMed] [Google Scholar]
- 47.Abdollahi-Roodsaz S, Joosten LA, Roelofs MF, Radstake TR, Matera G, Popa C, van der Meer JW, Netea MG, van den Berg WB. Inhibition of toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 2007;56:2957–67. doi: 10.1002/art.22848. [DOI] [PubMed] [Google Scholar]
- 48.Nandakumar KS, Svensson L, Holmdahl R. Collagen type II-specific monoclonal antibody-induced arthritis in mice: description of the disease and the influence of age, sex, and genes. Am J Pathol. 2003;163:1827–37. doi: 10.1016/S0002-9440(10)63542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mevorach D, Zhou JL, Song X, Elkon KB. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J Exp Med. 1998;188:387–92. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ehrlich P. On immunity with special reference to cell life. Proc R Soc Lond Biol. 1900;66:424–428. [Google Scholar]
- 51.Chen X, Martin F, Forbush KA, Perlmutter RM, Kearney JF. Evidence for selection of a population of multi-reactive B cells into the splenic marginal zone. Int Immunol. 1997;9:27–41. doi: 10.1093/intimm/9.1.27. [DOI] [PubMed] [Google Scholar]
- 52.Hayakawa K, Asano M, Shinton SA, Gui M, Allman D, Stewart CL, Silver J, Hardy RR. Positive selection of natural autoreactive B cells. Science. 1999;285:113–6. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 53.Boes M, Schmidt T, Linkemann K, Beaudette BC, Marshak-Rothstein A, Chen J. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci USA. 2000;97:1184–9. doi: 10.1073/pnas.97.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ehrenstein MR, Cook HT, Neuberger MS. Deficiency in serum immunoglobulin (Ig)M predisposes to development of IgG autoantibodies. J Exp Med. 2000;191:1253–8. doi: 10.1084/jem.191.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Botto M, Walport MJ. C1q, autoimmunity and apoptosis. Immunobiology. 2002;205:395–406. doi: 10.1078/0171-2985-00141. [DOI] [PubMed] [Google Scholar]
- 56.Botto M, Dell’Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, Walport MJ. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–9. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 57.Monticielo OA, Mucenic T, Xavier RM, Brenol JC, Chies JA. The role of mannose-binding lectin in systemic lupus erythematosus. Clin Rheumatol. 2008;27:413–9. doi: 10.1007/s10067-008-0838-8. [DOI] [PubMed] [Google Scholar]
- 58.Zhang M, Takahashi K, Alicot EM, Vorup-Jensen T, Kessler B, Thiel S, Jensenius JC, Ezekowitz RA, Moore FD, Carroll MC. Activation of the lectin pathway by natural IgM in a model of ischemia/reperfusion injury. J Immunol. 2006;177:4727–34. doi: 10.4049/jimmunol.177.7.4727. [DOI] [PubMed] [Google Scholar]
- 59.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 60.Mahoney JA, Rosen A. Apoptosis and autoimmunity. Curr Opin Immunol. 2005;17:583–8. doi: 10.1016/j.coi.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 61.Kavanagh D, Richards A, Atkinson J. Complement regulatory genes and hemolytic uremic syndromes. Annu Rev Med. 2008;59:293–309. doi: 10.1146/annurev.med.59.060106.185110. [DOI] [PubMed] [Google Scholar]
- 62.Quartier P, Potter PK, Ehrenstein MR, Walport MJ, Botto M. Predominant role of IgM-dependent activation of the classical pathway in the clearance of dying cells by murine bone marrow-derived macrophages in vitro. Eur J Immunol. 2005;35:252–60. doi: 10.1002/eji.200425497. [DOI] [PubMed] [Google Scholar]
- 63.Ogden CA, Kowalewski R, Peng Y, Montenegro V, Elkon KB. IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 2005;38:259–64. doi: 10.1080/08916930500124452. [DOI] [PubMed] [Google Scholar]
- 64.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–34. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 65.Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, Walport MJ, Fisher JH, Henson PM, Greene KE. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol. 2002;169:3978–86. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- 66.Bratton DL, Henson PM. Apoptotic cell recognition: will the real phosphatidylserine receptor(s) please stand up? Curr Biol. 2008;18:R76–9. doi: 10.1016/j.cub.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 67.Crotty S, Ahmed R. Immunological memory in humans. Semin Immunol. 2004;16:197–203. doi: 10.1016/j.smim.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 68.Merbl Y, Zucker-Toledano M, Quintana FJ, Cohen IR. Newborn humans manifest autoantibodies to defined self molecules detected by antigen microarray informatics. J Clin Invest. 2007;117:712–718. doi: 10.1172/JCI29943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Catera R, Silverman GJ, Hatzi K, Seiler T, Didier S, Zhang L, Hervé M, Meffre E, Oscier D, Vlassara H, Scofield RH, Chen Y, Allen SL, Kolitz J, Rai KR, Chu CC, Chiorazzi N. Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and chemical modifications. Molecular Medicine. 2008;14:665–674. doi: 10.2119/2008-00102.Catera. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.