Abstract
The massive visual input from the eye to the brain requires selective processing of some visual information at the expense of other information, a process referred to as visual attention. Increases in the responses of visual neurons with attention have been extensively studied along the visual processing streams in monkey cerebral cortex, from primary visual areas to parietal and frontal cortex1–4. Here we show, by recording neurons in attending monkeys, that attention modulates visual signals before they even reach cortex by increasing responses of both parvocellular and magnocellular neurons in the first relay between retina and cortex, the lateral geniculate nucleus (LGN), at the same time it decreases neuronal responses in the adjacent thalamic reticular nucleus (TRN). Francis Crick5, argued for such modulation of the LGN by observing that it is inhibited by the TRN, and suggested that “if the thalamus is the gateway to the cortex, the reticular complex might be described as the guardian of the gateway”, a reciprocal relationship we now show to be more than just hypothesis. The reciprocal modulation in LGN and TRN appears only during the initial visual response, but the modulation of LGN reappears later in the response, suggesting separate early and late sources of attentional modulation in LGN.
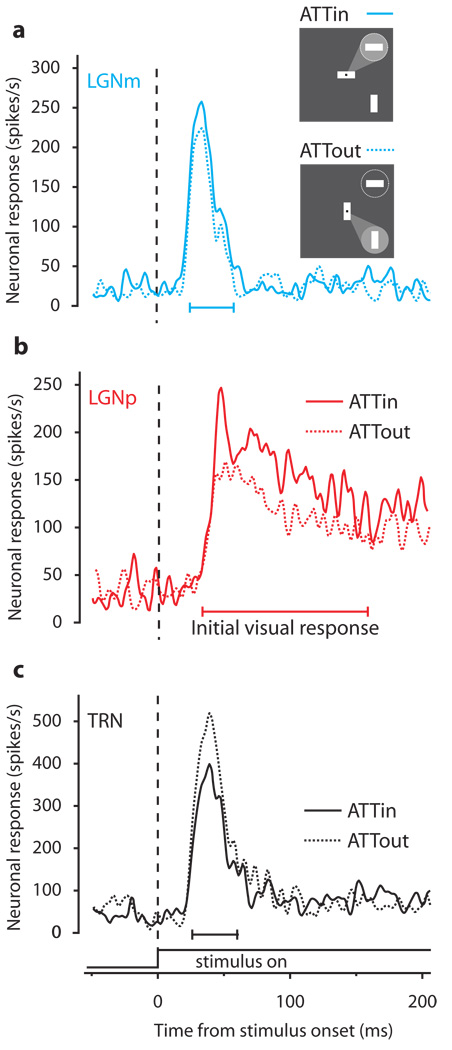
We recorded responses of LGN and TRN neurons in three awake behaving macaque monkeys (Macaca mulatta). Monkeys were directed by a central cue at the point of fixation to attend to one of two peripheral visual stimuli on randomly interleaved trials (inset in Figure 1a). One of these stimuli was in the receptive field (RF) of the recorded neuron. Figure 1a shows the responses of an example magnocellular LGN neuron (LGNm) to a light bar within the RF when attention was directed out of the RF (dashed curve, ATTout) or into the RF (solid curve, ATTin). Responses shown are from correct trials. The ATTin response falls above the ATTout response, indicating an increase in neuronal response with attention. The mean response to the same stimulus increased 12% with attention. Figure 1b shows the responses of a parvocellular LGN neuron (LGNp) that also increased (21%) when attention was directed into the RF.
Figure 1.
Sample responses to shifts of attention in LGN and TRN. Solid traces are spike density plots of the neuron’s ATTin response (as illustrated by the “spotlight” of attention in the inset cartoon directed to the circle representing the RF). Dashed traces are ATTout responses. Responses are aligned to stimulus onset (the dashed vertical line), and have been smoothed with a Gaussian window of 2 ms SD. a, Responses of sample magnocellular LGN neuron (LGNm). b, Responses of sample parvocellular LGN neuron (LGNp). c, Responses of sample TRN neuron.
If a similar increase in attention were to occur in TRN, however, Crick’s hypothesized interaction between TRN and LGN encounters a problem: TRN inhibits LGN. The visual sector of TRN receives excitatory inputs from LGN, but projects modulatory inhibitory input back to LGN6–13. Therefore, TRN responses should instead decrease with attention, reducing the inhibitory influence of the TRN on LGN, thereby causing the increase in the responses of LGN neurons that we observe. We did in fact find a decrease in the TRN visual response with attention (Figure 1c). When attention was directed into the RF of this TRN neuron, the mean response to the same visual stimulus was 13% less than when attention was directed out of the RF.
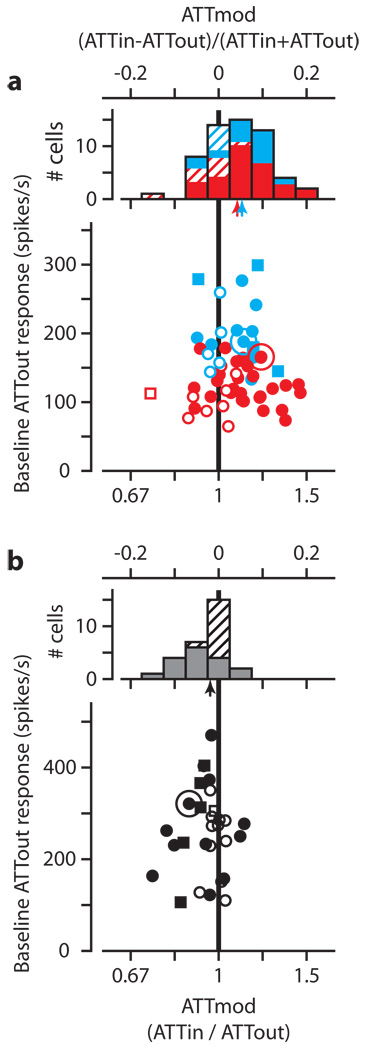
We have summarized the effect of attention on mean visual responses of 57 on-center LGN neurons (19 LGNm and 38 LGNp) in Figure 2a, and of 29 TRN neurons in Figure 2b. In each plot, the ordinate is the baseline ATTout response and the abscissa is the attentional modulation, ATTmod. ATTmod can be expressed either as the contrast measure (ATTin−ATTout)/(ATTin + ATTout), or the ratio of modulation (ATTin/ATTout). We have included both, with the bottom axes representing the ratio of modulation, and the top axes indicating the contrast measure of ATTmod. Figure 2a shows the bulk of points to the right of the vertical unity line indicating that the predominant effect of attention in LGN neurons was to increase mean responses to the visual stimulus. Distributions of ATTmod appear above the scatterplot, with small arrows indicating sample medians. In LGN, attention increased the median response 11 ± 2.6% in the magnocellular layers (p = 0.011), and 9 ± 1.1% in the parvocellular layers (p = 0.0007). All indications of variability are plus or minus one standard error of the median, and all p-values were determined using the Wilcoxon signed rank test for zero median, unless otherwise specified.
Figure 2.
Effect of attention on LGN and TRN. a, Scatterplot showing mean baseline ATTout response versus attentional modulation (ATTmod) for 19 LGNm neurons (blue) and 38 LGNp neurons (red). Solid symbols are significant response changes (Wilcoxon rank-sum test, p<0.01). Squares denote experiments in which performance did not guarantee attention (see full Methods section). Distributions of ATTmod appear above the scatterplots. Hatched areas of each bar denote changes that did not reach significance. Arrows show median ATTmod for LGNm (blue) and LGNp (red). b, Similar scatterplot and histogram for TRN. In all plots, the larger circles indicate the Figure 1 example neurons.
In contrast to LGN, values of attentional modulation in TRN (Figure 2b) tend to lie to the left of the unity line, showing a median decrease in neuronal response with attention of 4 ± 0.6% (p = 0.004). Over our sample of neurons, the reciprocal effect of attention holds; LGN responses increase with attention whereas TRN responses decrease.
If attention modulates neuronal responses, we would not necessarily expect such modulation during trials on which the monkeys made incorrect behavioral responses. For TRN neurons with more than five error trials, responses on those trials increased by 1.5 ± 1.5% (n=18, p = 0.62). Similarly, for LGNm and LGNp neurons, respectively, responses changed by 2.3 ± 3.6% (n=11, p = 0.58) and 1.3 ± 2.6% (n=22, p = 0.71). The lack of significant response modulation on incorrect trials provides further evidence that the factor enabling the monkeys to perform the task correctly was the same one modulating neuronal responses: visual attention.
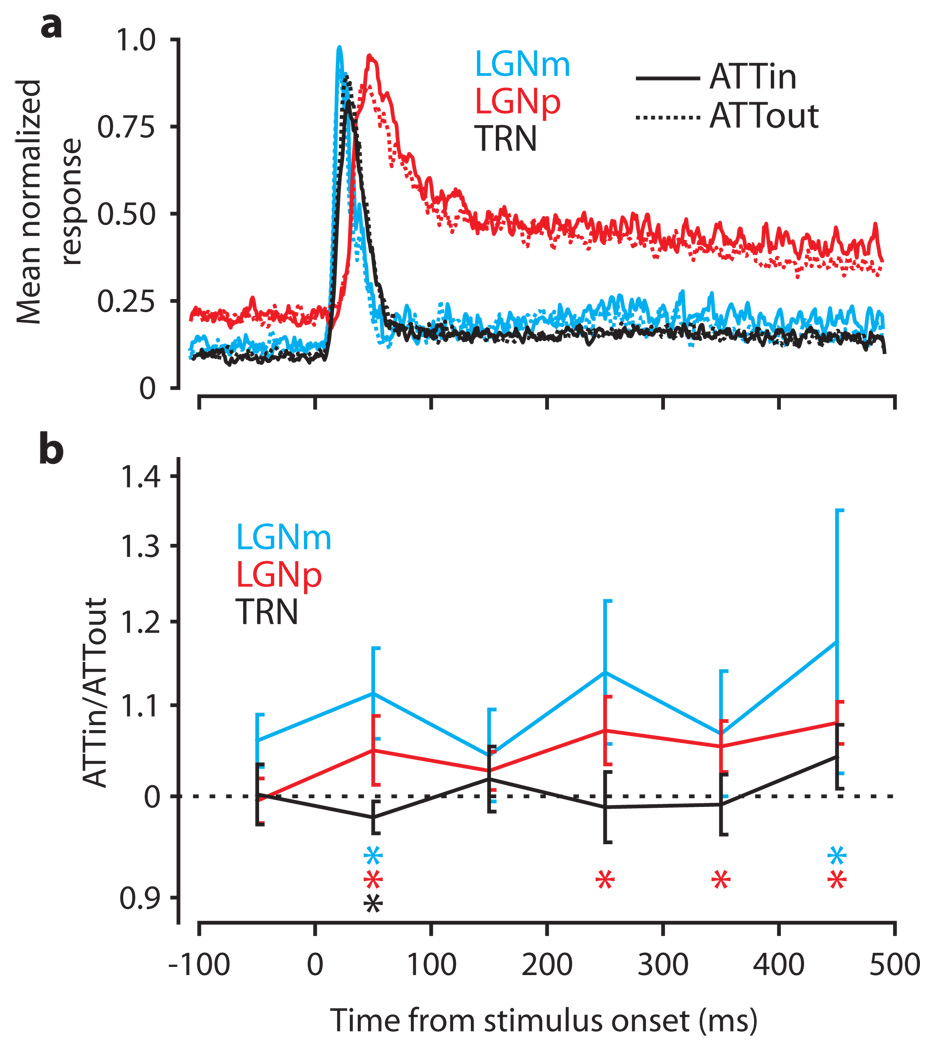
We found no significant modulation of the background activity preceding the initial visual response or in the latency or duration of the initial visual response in either LGN or TRN. To observe any residual effect of attention beyond the initial visual response, we examined mean neuronal responses from 100 ms before stimuli appeared to 500 ms after they appeared (the shortest presentation time common to all trials). For each neuron, we normalized the response to the neuron’s maximum firing rate. Figure 3a shows the mean normalized response for each area with solid curves for the ATTin condition and dashed curves for the ATTout condition. We calculated ATTmod for the six 100 ms time epochs in this time scale. Figure 3b shows ATTmod (as ATTin/ATTout) over time. Median changes for each area are connected across epochs with solid lines, and error bars denote ± 1 standard error of the median. Significant changes within an epoch are denoted by colored asterisks.
Figure 3.
Time courses of visual and attentional influences. a, Mean normalized ATTin (solid curves) and ATTout (dashed curves) responses for each area. Each curve is the mean normalized spike density plot over all neurons in an area. Mean responses have been smoothed with a Gaussian kernel of 2.8 ms SD. b, Median effect of attention on each area in 100 ms epochs. Each trace shows ATTmod over time. Error bars are ± 1 SE of the median. Significant changes in each epoch are denoted by colored asterisks coded to each area below the curves.
All areas demonstrate a significant response modulation in the initial 100 ms epoch after the stimuli appear. However, this modulation disappears in the next 100 ms epoch, but LGNm and LGNp show a second, later period of modulation that becomes significant in both divisions as time progresses. Also, both LGNm and LGNp showed significant attentional modulation just before the monkey needed to make a decision about the stimulus. Note that in contrast to LGN, TRN had no second period of attentional modulation.
Because only the initial visual response in TRN is modulated by attention, measuring over the whole 500 ms period would have yielded a much smaller modulation in TRN (−1.8%) that would not have been significant (p = 0.31). However, due to the second phase of modulation in LGN, we still would have measured attentional modulation of 13% in LGNm (p = 0.014) and 8.1% in LGNp (p < 0.0001), but the influence of TRN on the initial visual response would have gone undetected.
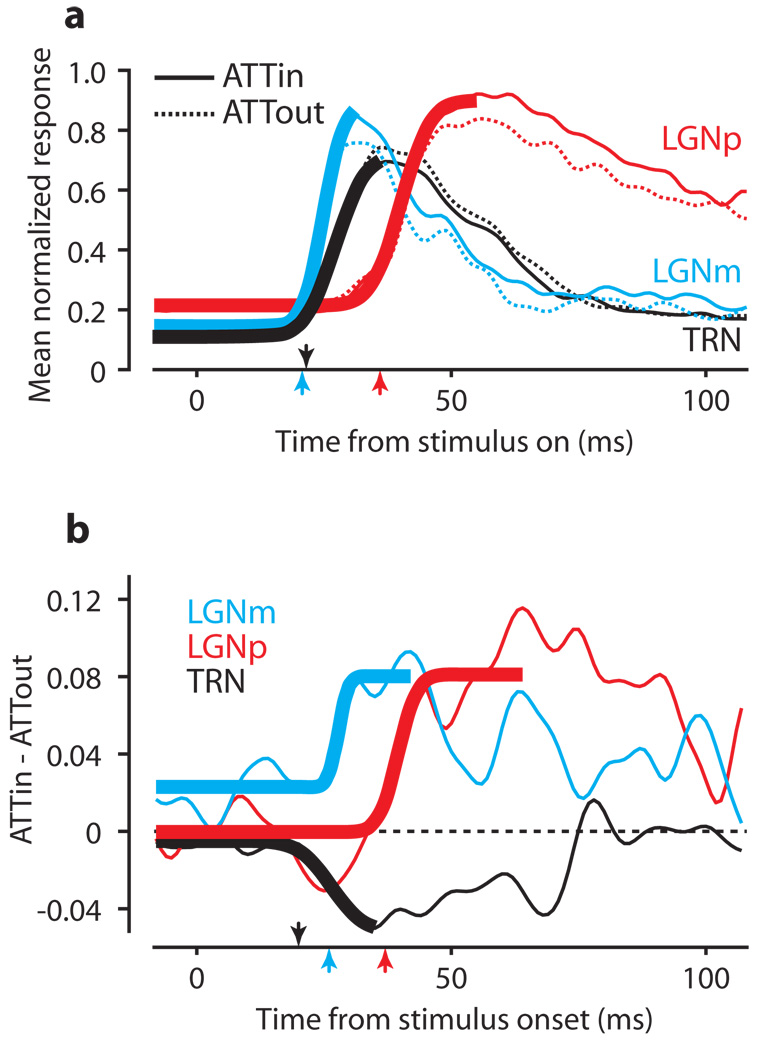
We see that careful consideration of responses over time is critical to detect the attentional effects in TRN, but analysis of the interactions between LGN and TRN requires an even more precise examination of response timing. To compare visual response latencies, Figure 4a shows the mean normalized initial responses for neurons in each area aligned on stimulus onset. To determine the significance of visual latency differences, we performed a bootstrap analysis (see Supplementary Notes) yielding estimates of the median visual latency in each area, and the significance of differences between areas using the Wilcoxon rank-sum test for equal medians. Although TRN responses (median latency 22 ± 0.92 ms) begin well before those in LGNp (p < 0.001, median latency 37 ± 1.43 ms), LGNm neurons (median latency 21 ± 1.25 ms) tend to respond before TRN neurons (p < 0.001).
Figure 4.
Latencies of visual and attentional influences. a, First 100 ms of the visual responses from Figure 3a. Thick lines are sample descriptive fits to ATTin responses from the bootstrap analysis. Arrows indicate median visual latencies. b, Latency of attentional effect in each area. Each trace shows the difference between the mean ATTin and ATTout responses. Thick lines show sample descriptive fits used to extract latency estimates in the bootstrap analysis. Arrows indicate the median latencies for the effect of attention.
To track the timing of attentional modulation in each area, we represented the effect of attention in Figure 4b as the difference between the mean ATTin and ATTout curves from Figure 4a. The latency of attentional modulation was obtained from a similar bootstrap analysis. Whereas the visual response appears first in LGNm, Figure 4b shows that attentional modulation occurs first in TRN (22 ± 0.37 ms), 4 ms before LGNm (26 ± 0.31 ms, p < 0.001). The attentional effect shows up significantly later in LGNp (37 ± 0.31 ms) than either TRN or LGNm (p < 0.001 for both). Therefore, even though LGNm visual responses precede those of TRN (consistent with LGNm driving the visual response in TRN), attention affects TRN responses first, consistent with attentional modulation in LGN coming from TRN.
In conclusion, we find that attention modulates thalamic visual responses in two phases: an initial modulation that enhances LGN responses and attenuates TRN responses, followed by a slowly building later enhancement limited to LGN. Until now, demonstration of attentional modulation of LGN neurons has been limited to preliminary experiments on monkey14 and fMRI studies in humans15. For the TRN, in addition to the recent growth in anatomical and cellular studies of monkey visual TRN6, 8, 9, 13, we recently found attentional modulation of neuronal activity in visual TRN during a visual/auditory attention task16. The differences between the visual/auditory attention task and the current task, along with a comparison of their results, are found in the Supplementary Discussion.
The initial LGN modulation might provide a substantial fraction of the modulation seen subsequently in cortical area V117–23. While it is difficult to compare across studies, the approximately 10% increase in responses we find in LGN is similar to the 6.9% median increase across V1 neurons17, and the 8.9% median increase in V1 simple cells18. The presence of the initial modulation in both TRN and LGN, their reciprocal increase and decrease, and the timing of their visual and attentional responses are consistent with TRN serving as the source of the initial LGN modulation as proposed by Crick.
The later attentional effects in LGN, and effects others have reported in higher cortical visual areas, might be more closely related to goal-directed attention which frequently also develops later in the visual response particularly in higher cortical areas2, 4, 24. This later modulation in LGN might in fact reflect feedback from cortex onto the LGN25, 26 via the established connections from V1 layer 625, 27, whereas the initial modulation in LGN by way of TRN may have its origins in subcortical structures, possibly including the superior colliculus28–30. While obviously separate in time course, the two phases of modulation may represent two distinct attentional influences, and may be early indicators for identifying and distinguishing feed-forward and feedback visual attentional mechanisms.
METHODS SUMMARY
Two monkeys performed a task in which a central cue directed them to attend to one of two peripheral stimuli (a horizontal and a vertical bar of light – see inset in Figure 1a). On each trial, while the monkey fixated on a central spot, a cue appeared at the fixation point matching one of the two upcoming stimuli. On any trial, the cue had an equal chance of matching either the vertical or horizontal stimulus. After 250 ms, the two peripheral stimuli appeared, one in the receptive field (RF) of the neuron, and the other some distance from the RF. After a period of 500 to 1000 ms, each peripheral stimulus independently had a 50% chance of transiently dimming about 40% in luminance. The monkey indicated if the stimulus matching the cue dimmed by making a saccade to it. If the matching stimulus did not dim, the correct response was to remain fixating. The correct response depended only on the stimulus matching the central cue. We compared neuronal responses when the cue matched the stimulus in the RF (ATTin) with responses when the cue matched the remote stimulus (ATTout). For each neuron studied, we always presented the same stimulus in the RF, and only the cue changed randomly between trials. This insured that neurons responded to the same stimulus regardless of which stimulus matched the central cue. Eye movements were monitored to make certain the monkey remained fixating during trials. The possible contribution of changes in the monkeys’ eye position on the attentional modulation is considered in Supplementary Discussion. Because the stimulus in the RF always remained the same, this also allowed the monkey to shift attention as soon as the cue came on as the location of each stimulus was consistent from trial to trial.
METHODS
Physiological methods
One recording chamber allowed access to both TRN and LGN. It was implanted stereotaxically 10mm anterior from the interaural line and 13mm lateral from the midline on three male rhesus monkeys (monkey B, O, and G – monkey G provided only TRN data). The surgical procedures, recording of single neurons and eye positions, and control of the monkeys’ behavior have been described previously16. All procedures were approved by the Institute Animal Care and Use Committee and complied with Public Health Service Policy on the humane care and use of laboratory animals.
We initially localized LGN using an MRI image after the recording cylinder was implanted. LGN recordings were verified by the nature of the visual response and the signature alternation of the ocularity of these responses as the electrode progressed through layers. TRN was located by its position in relation to LGN. The short latency of the visual response (about 22 ms) and its brief duration (~60 ms) identified the neurons as being from TRN as determined previously with histological verification16, rather than from the parvocellular layers in dorsal LGN.
For most units, RF center and extent were quantitatively determined. While the monkey fixated on a central spot 0.4° deg in diameter, a spot of light (0.8 to 1.0°) appeared sequentially in a series of locations centered on the estimated RF center, arranged in a 5 by 5 grid. Horizontal and vertical separation between grid points was equal to the diameter of the spot. Each trial lasted as long as 800 ms and the spot appeared in each randomly chosen location for either 200 ms (LGN) or 100 ms (TRN). Responses to the spot at each location were analyzed online and the center of the RF was adjusted accordingly. We subsequently determined RF diameter in a similar manner, this time by presenting a sequence of spots with varying diameters centered on the RF. Spots were 0.5 to 6° in diameter, and appeared in random sequence during each 800 ms trial, each spot remaining for 200 ms (LGN) or 100 ms (TRN). The quantitatively determined RF center and diameter were then used to place the stimuli for the attention task. Visual stimuli in all tasks were back projected onto a tangent screen 58 cm in front of the monkey by a liquid crystal display projector.
Attention task details
During a trial, the monkey was required to maintain fixation within 1.0 or 1.5° of the central fixation point (Supplementary Figure 1). If the monkey broke fixation early, the trial was aborted and excluded from analysis. The central cue was 1.5° × 0.6° or 2.0° × 0.8°, whichever was closest to the size of the peripheral stimuli. The peripheral stimuli were 1.5 to 2.0 deg long and 0.6 to 0.8 ° wide, depending on the eccentricity and size of the RF. One of the stimuli was placed in the RF of the LGN or TRN neuron. The other stimulus was placed some distance away, typically about 20°, but at the same eccentricity from the fixation point as the stimulus in the RF. Each peripheral bar of light independently had a 50% chance of dimming for 600 ms. Therefore on 25% of trials both stimuli dimmed (simultaneously), on 25% of trials neither stimulus dimmed, on 25% of trials only the horizontal stimulus dimmed, and on 25% of trials only the vertical stimulus dimmed.
We used a criterion of 75% correct responses to indicate that the monkey was attending to the cued target, although performance was typically better. To flag possible response strategies we first divided the trials into eight trial types according to which stimulus was cued (horizontal or vertical) and which stimuli dimmed (horizontal only, vertical only, both, or neither). If the monkey followed some response strategy rather than attending as directed by the cue, performance on one or more of these trial types would necessarily suffer. We flagged possible response strategies by requiring the monkey get each of these eight trial types correct at least 50% of the time. So when we refer to the monkey performing the attention task to criteria, the monkey is getting at least 75% of the trials correct overall and is getting at least 50% of each type of trial correct.
Analysis of results
We measured the activity of neurons as the mean neuronal response within several different epochs. We measured mean background activity in the 100 ms period before onset of the visual stimuli. Visual response latency was determined by fitting a normal cumulative density function (CDF) to the spike density plot obtained over at least 20 but usually more than 50 trials and smoothed with a 2.8 ms SD Gaussian kernel (Supplementary Figure 2A). The onset of the visual response was taken as the time at which the fit curve reached 10% of the neuron’s peak response. The latency of this response was the time between onset of the visual stimulus (determined by a photo cell attached to the screen) and this response onset time. The end of the initial response was taken as the point at which a Weibull probability density function (PDF) fit to the falling phase of the response declined 75% from the neuron’s peak response to the asymptote of the fit curve. The duration of the initial visual response was then the time between the visual response onset and the end of the initial visual response. Both the Gaussian CDF and the Weibull PDF were fit by minimizing the sum-squared error between the fit curve and the spike density plots. The mean response is the average response rate during this epoch.
Comparisons with other studies
The percent response modulation we report for other studies was calculated from available firing rates with baseline activity included. From the neuronal response rates with and without attention, we were able to extract a comparable percent change from the ratio of modulation by calculating 100 × ((ATTin / ATTout) − 1).
Supplementary Material
is linked to the online version of the paper at www.nature.com/nature.
Acknowledgements
This work was supported by the intramural research program of the National Eye Institute. We are grateful for the assistance of John McClurkin, Altah Nichols, Mitchell Smith, Tom Ruffner, and Ginger Tansey.
Footnotes
Reprints and permission information is available at npg.nature.com/reprintsandpermissions.
REFERENCES
- 1.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 2.Maunsell JH, Cook EP. The role of attention in visual processing. Philos Trans R Soc Lond B Biol Sci. 2002;357:1063–1072. doi: 10.1098/rstb.2002.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAdams CJ, Maunsell JH. Effects of attention on the reliability of individual neurons in monkey visual cortex. Neuron. 1999;23:765–773. doi: 10.1016/s0896-6273(01)80034-9. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- 5.Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci U S A. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conley M, Diamond IT. Organization of the Visual Sector of the Thalamic Reticular Nucleus in Galago. Eur J Neurosci. 1990;2:211–226. doi: 10.1111/j.1460-9568.1990.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 7.Crabtree JW, Killackey HP. The Topographic Organization and Axis of Projection within the Visual Sector of the Rabbit's Thalamic Reticular Nucleus. Eur J Neurosci. 1989;1:94–109. doi: 10.1111/j.1460-9568.1989.tb00777.x. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgibbon T, Szmajda BA, Martin PR. First order connections of the visual sector of the thalamic reticular nucleus in marmoset monkeys (Callithrix jacchus) Vis Neurosci. 2007;24:857–874. doi: 10.1017/S0952523807070770. [DOI] [PubMed] [Google Scholar]
- 9.Harting JK, Van Lieshout DP, Feig S. Connectional studies of the primate lateral geniculate nucleus: distribution of axons arising from the thalamic reticular nucleus of Galago crassicaudatus. J Comp Neurol. 1991;310:411–427. doi: 10.1002/cne.903100310. [DOI] [PubMed] [Google Scholar]
- 10.Jones EG. Some aspects of the organization of the thalamic reticular complex. J Comp Neurol. 1975;162:285–308. doi: 10.1002/cne.901620302. [DOI] [PubMed] [Google Scholar]
- 11.Kim U, Sanchez-Vives MV, McCormick DA. Functional dynamics of GABAergic inhibition in the thalamus. Science. 1997;278:130–134. doi: 10.1126/science.278.5335.130. [DOI] [PubMed] [Google Scholar]
- 12.Montero VM, Guillery RW, Woolsey CN. Retinotopic organization within the thalamic reticular nucleus demonstrated by a double label autoradiographic technique. Brain Res. 1977;138:407–421. doi: 10.1016/0006-8993(77)90681-3. [DOI] [PubMed] [Google Scholar]
- 13.Uhlrich DJ, Manning KA, Feig SL. Laminar and cellular targets of individual thalamic reticular nucleus axons in the lateral geniculate nucleus in the prosimian primate Galago. J Comp Neurol. 2003;458:128–143. doi: 10.1002/cne.10568. [DOI] [PubMed] [Google Scholar]
- 14.Casagrande VA, Sary G, Royal D, Ruiz O. On the impact of attention and motor planning on the lateral geniculate nucleus. Prog Brain Res. 2005;149:11–29. doi: 10.1016/S0079-6123(05)49002-0. [DOI] [PubMed] [Google Scholar]
- 15.O'Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci. 2002;5:1203–1209. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- 16.McAlonan K, Cavanaugh J, Wurtz RH. Attentional modulation of thalamic reticular neurons. J Neurosci. 2006;26:4444–4450. doi: 10.1523/JNEUROSCI.5602-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAdams CJ, Maunsell JH. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J Neurosci. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAdams CJ, Reid RC. Attention modulates the responses of simple cells in monkey primary visual cortex. J Neurosci. 2005;25:11023–11033. doi: 10.1523/JNEUROSCI.2904-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motter BC. Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J Neurophysiol. 1993;70:909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- 20.Roelfsema PR, Lamme VA, Spekreijse H. Object-based attention in the primary visual cortex of the macaque monkey. Nature. 1998;395:376–381. doi: 10.1038/26475. [DOI] [PubMed] [Google Scholar]
- 21.Ito M, Gilbert CD. Attention modulates contextual influences in the primary visual cortex of alert monkeys. Neuron. 1999;22:593–604. doi: 10.1016/s0896-6273(00)80713-8. [DOI] [PubMed] [Google Scholar]
- 22.Posner MI, Gilbert CD. Attention and primary visual cortex. Proc Natl Acad Sci U S A. 1999;96:2585–2587. doi: 10.1073/pnas.96.6.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts M, Delicato LS, Herrero J, Gieselmann MA, Thiele A. Attention alters spatial integration in macaque V1 in an eccentricity-dependent manner. Nat Neurosci. 2007;10:1483–1491. doi: 10.1038/nn1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Treue S. Neural correlates of attention in primate visual cortex. Trends Neurosci. 2001;24:295–300. doi: 10.1016/s0166-2236(00)01814-2. [DOI] [PubMed] [Google Scholar]
- 25.Briggs F, Usrey WM. A fast, reciprocal pathway between the lateral geniculate nucleus and visual cortex in the macaque monkey. J Neurosci. 2007;27:5431–5436. doi: 10.1523/JNEUROSCI.1035-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sillito AM, Cudeiro J, Jones HE. Always returning: feedback and sensory processing in visual cortex and thalamus. Trends Neurosci. 2006;29:307–316. doi: 10.1016/j.tins.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Lund JS, Lund RD, Hendrickson AE, Bunt AH, Fuchs AF. The origin of efferent pathways from the primary visual cortex, area 17, of the macaque monkey as shown by retrograde transport of horseradish peroxidase. J Comp Neurol. 1975;164:287–303. doi: 10.1002/cne.901640303. [DOI] [PubMed] [Google Scholar]
- 28.Jones EG. Cambridge, UK: Cambridge University Press; 2007. pp. 1270–1271. [Google Scholar]
- 29.Kolmac CI, Mitrofanis J. Patterns of brainstem projection to the thalamic reticular nucleus. J Comp Neurol. 1998;396:531–543. [PubMed] [Google Scholar]
- 30.Wilson JR, Hendrickson AE, Sherk H, Tigges J. Sources of subcortical afferents to the macaque's dorsal lateral geniculate nucleus. Anat Rec. 1995;242:566–574. doi: 10.1002/ar.1092420413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
is linked to the online version of the paper at www.nature.com/nature.