Abstract
The association between ovarian cancer risk and reproductive factors has been well established, and two main theories, incessant ovulation and gonadotropin stimulation, have been proposed to explain the mechanism. Recent studies using animal models of ovarian tumorigenesis, and analysis of ovarian tissues from prophylactic oophorectomies, suggest that depletion of ovarian follicles might underlie the epidemiological findings linking reproductive history and ovarian cancer risk.
Introduction
The aetiology of ovarian cancer is complex and incompletely understood, although epidemiological data decisively link ovulation frequency and reproductive hormones to ovarian cancer risk.1–3 Increased parity and oral contraceptive use are the clearest examples of factors that decrease ovarian cancer risk, both of which limit ovulation. Two main theories, incessant ovulation4 and gonadotropin stimulation,5,6 have been proposed to explain the aetiology of ovulation in ovarian cancer risk,7,8 but neither completely nor satisfactorily explains the dramatic increase in ovarian cancer incidence that occurs in the immediate postmenopausal period and that continues to rise as the ovary ages after menopause. Most (90%) ovarian cancers are derived from the surface epithelium, and nearly 85–90% of ovarian cancer develops postmenopause. Therefore, understanding how the depletion of germ cells, loss of follicular structure that surrounds the oocyte or germ cell, and cessation of ovulation, all of which define menopause, influence the development of ovarian carcinomas is of particular importance. On the basis of studies with the germ-cell-deficient Wv mice (figure 1) and examination of human ovarian tissues from prophylactic oophorectomies, we suggest that depletion of germ cells and the loss of ovarian follicular function that follows might underlie the link between reproductive factors and ovarian cancer risk.
Figure 1. Ovarian morphological changes in germ-cell-deficient Wv mice.
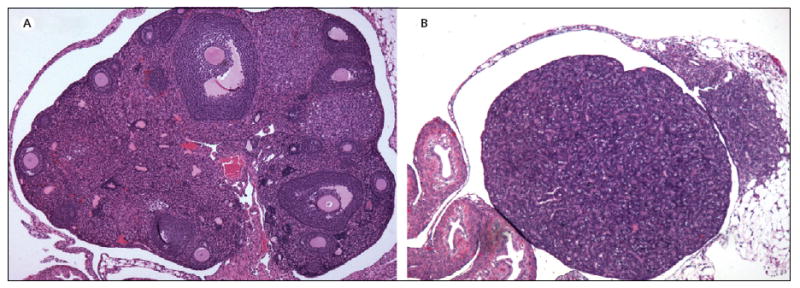
(A) Mature wildtype-mouse ovary contains many germ cells and follicles at various stages of development. (B) Wv ovary from a same-age mouse is depleted of germ cells and follicles and epithelial morphological changes, including tubular adenomas, permeate the ovary.
Aetiology of ovulation in ovarian cancer
Nearly 40 years ago, Fathalla proposed the theory of incessant ovulation to clarify the association between ovulation frequency and the risk of developing epithelial ovarian cancer.4 This hypothesis attributes the occurrence of ovarian cancer in modern day women (and domestic egg-laying hens), which is rare in other mammals, to ovulation that recurs monthly throughout the reproductive lifetime of women if not punctuated by anovulatory periods during pregnancy and breast-feeding.4 The repetitive wounding during the release of the ovum and the cell proliferation that occurs postovulation to repair the ovarian surface epithelium have been proposed to result in mutations accumulating in the epithelial cells and ultimately the formation of tumours.9 This central mechanism is supported experimentally in cell culture and is generally well accepted. Moreover, combined oestrogen–progesterone formulations of oral contraceptives that act by suppressing ovulation decrease the risk of ovarian cancer by about 40% after 3 years of use.10,11 Why pregnancy and oral contraceptives provide a long-term protection, however, might be more complex than by simply limiting ovulation, because more recent studies suggest that progesterone, which is increased during pregnancy and by oral contraceptives, might also affect the clearing of transformed cells from the ovarian surface-epithelial layers.12,13 Because the likelihood of cells carrying potentially transforming mutations increases with age, the age of either the last full-term pregnancy or the last regular use of progestin-containing oral contraceptives is a protective factor, and the benefit decreases after time.13
Gonadotropin stimulation in ovarian cancer risk
On the basis of the same epidemiological data, the gonadotropin stimulation theory, by contrast, postulates that surges of pituitary gonadotropins that initiate each ovulation and persist in high levels for many years after menopause also stimulate the ovarian surface-epithelial cells and induce cell transformation.5,6 During ovulation, gonadotropins stimulate an inflammatory-like process mediated by many cytokines and proteolytic enzymes, which leads to rupture of the ovarian surface-epithelial layer for the release of the ovum.14–16 After rupture of the follicle and the release of the ovum at the ovarian surface, the oestrogen-producing follicle is converted to a progesterone-producing corpus luteum, which then feeds back to inhibit gonadotropin levels. In menopause, which is caused by a total depletion in the number of germ cells present in the ovary and accompanied by the loss of the follicular structure that surrounds the germ cells, the feedback endocrine loop from the corpus luteum is absent, and serum gonadotropins and levels of proinflammatory cytokines are even higher in perimenopausal ovaries.17,18 Thus, increased gonadotropin levels in postmenopausal women might foster an inflammatory environment that cannot lead to ovulation, but might contribute to ovarian cancer risk,19 by causing either remodelling or morphological changes in the surface epithelium, which allow the transformation of genetically compromised cells and the development of cancerous lesions.20,21 This theory seems more fitting to explain the dramatic increase in ovarian cancer incidence in the perimenopausal and postmenopausal years.22–24 The average age of menopause, although it varies somewhat between women and cultures, is 51 years, which closely precedes the average age of ovarian cancer diagnosis, which is 54 years.
Ovarian ageing
The incidence of ovarian cancer continues to increase after menopause, and age—even more so than a family history of ovarian cancer—is the best prediction of ovarian cancer risk.1–3 Ovaries from older women have been noted to show more morphological changes than those from younger women.25 Presumably ovulation and subsequent repair cause these age-dependent changes, or so-called ovarian ageing,23,26 which might represent preneoplastic areas or lesions. Recently, prophylactic surgeries have allowed a closer and crucial examination of these morphological changes and potential preneoplastic lesions in healthy women.27–29 These morphological features include papillomatosis, deep-surface invaginations, inclusion cysts, and epithelial stratifi cations, which are noted most frequently in perimenopausal and immediate postmenopausal ovaries of both BRCA1/2 carriers and non-carriers,25 and might very well be the result of ovarian ageing.
Germ-cell-defi cient Wv mouse model
Recent laboratory studies of the germ-cell-deficient Wv mouse also provide intriguing ideas about the aetiology of ovarian cancer.30 The Wv mice harbour a point mutation in the c-Kit gene that greatly decreases the tyrosine-kinase activity of c-Kit, affecting the development of germ cells, pigment cells, and mast cells.31 Homozygous Wv-mutant mice have a similar lifespan as wildtype mice, but are essentially sterile due to the germ-cell defect.32 Wv/Wv females contain less than 1% of the normal number of oocytes at birth, and once reproductive age is reached, ovarian follicles are rapidly depleted.33 Subsequently, serum gonadotropins are increased and substantial epithelial morphological changes develop, including surface invaginations, inclusion cysts, papillomatosis, and benign ovarian tumours, known as tubular adenomas.30,34 These tumours are derived from ovarian surface-epithelial cells, resembling human ovarian changes that might result from ageing.30 In the absence of germ cells, as in the Wv mouse, suppression of gonadotropin release prevents the development of the ovarian tubular adenomas,35 yet gonadotropin administration alone (ie, germ cells are present in the ovaries) does not result in epithelial tumours.36,37 Moreover, transgenic mouse models targeting the gonadotropin pathway without depletion of oocytes do not develop epithelial tumours. Female mice overexpressing follicle-stimulating hormone (FSH) in levels far exceeding those in postmenopausal women develop haemorrhagic and cystic ovaries, but do not develop epithelial lesions.38 Additionally, genetic knockouts of the FSH receptor in the ovary, in which circulating serum FSH levels are high but non-functioning, do not develop epithelial cancers, but instead sex-cord tumours, which represent only a small subset of human ovarian cancers.39 Thus, the early depletion of germ cells and subsequent increase in gonadotropins in the Wv mice might constitute a relevant, albeit exaggerated model, mimicking the postmenopausal biology and ovarian morphological ageing in women.
Follicle depletion
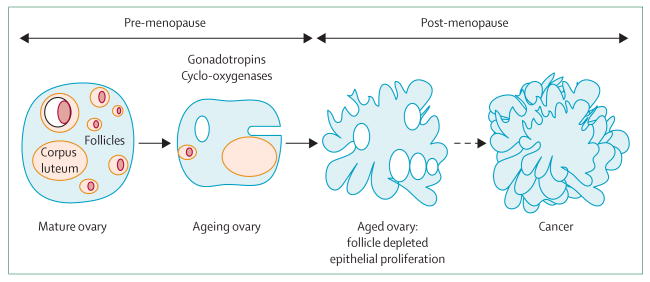
Studies of the Wv mice prompted us to postulate that depletion of ovarian germ cells and follicles might underlie the aetiology of ovarian cancer risk associated with reproductive factors and menopause status (figure 2), and might in fact unify incessant ovulation and gonadotropin stimulation as mechanisms.
Figure 2. Model depicting the hypothesis that germ-cell and follicle depletion underlies the aetiology of ovarian cancer risk associated with reproductive factors and menopause.
Follicle depletion explains the age-dependent risk of ovarian cancer: ovarian cancer generally develops in the immediate postmenopausal years, when ovarian follicles are depleted. The depletion of ovarian follicles obviously precedes and causes increased serum gonadotropins, which stimulate an inflammatory environment in the ovary that is permissive to transformation of surface epithelial cells and tumour development. Furthermore, both incessant ovulation and gonadotropin stimulation will accelerate the depletion of ovarian follicles. Additionally, protective factors, such as the use of birth-control pills,40 and cyclo-oxygenase inhibitors,41–43 might preserve follicles. Cyclo-oxygenases participate in follicle development and ovulation,44,45 and their inhibition might slow follicle maturation and extend follicle lifespan. Findings show that lifetime ovulation correlates with premenopausal, but not postmenopausal, ovarian cancer risk,46 and a decrease of pituitary gonadotropin release with hormone-replacement therapy does not decrease ovarian cancer risk.47 These findings also support the notion that the presence of ovarian follicles, rather than ovulation and gonadotropin stimulation, is a major determinant of ovarian cancer risk.
Conclusion
The follicle-depletion hypothesis predicts that follicle preservation and delaying reproductive ageing might prevent ovarian cancer or decrease the risk of developing this disease, and that menopause timing might correlate with ovarian cancer incidence, which can be verified by epidemiological studies designed to assess these factors.
Search strategy and selection criteria
Information for this Personal View was obtained by searches of PubMed using the search terms: “ovarian cancer”, “menopause”, “follicle depletion”, “gonadotropins”, “ovarian cancer risk”, and “Wv mice”. Only papers published in English between 1957 and 2007 were included.
Acknowledgments
We are grateful to colleagues in the Ovarian Cancer Program at Fox Chase Cancer Center (Philadelphia, PA, USA) for their suggestions for the development of our research and ideas. We especially thank laboratory members, Kathy Qi Cai, Wan-Lin Yang, and Jennifer Smedberg, for their work, on which the ideas in this paper are based. These studies were supported by grants R01 CA099471 from the US National Cancer Institute and the US National Institutes of Health, and W81XWH-06-1-0095 from the US Department of Defense (X-XX), and grants from the Marsha Rivkin Ovarian Cancer Research Foundation (Seattle, WA, USA), and the Department of Defense Ovarian Concept Award W81XWH-07-1-0303 (ERS). Additional funding from the Teal Ribbon Ovarian Cancer Research Foundation (Philadelphia, PA, USA) is greatly appreciated.
Footnotes
Conflicts of interest The authors declared no conflicts of interest.
Contributor Information
Elizabeth R Smith, Department of Medicine, Sylvester Comprehensive Cancer Center, University of Miami School of Medicine, Miami, FL, USA.
Xiang-Xi Xu, Department of Medicine, Sylvester Comprehensive Cancer Center ,Department of Obstetrics and Gynecology, University of Miami School of Medicine, Miami, FL, USA.
References
- 1.Tortolero-Luna G, Mitchell MF. The epidemiology of ovarian cancer. J Cell Biochem Suppl. 1995;23:200–07. doi: 10.1002/jcb.240590927. [DOI] [PubMed] [Google Scholar]
- 2.Yancik R. Ovarian cancer. Age contrasts in incidence, histology, disease stage at diagnosis, and mortality Cancer. 1993;71(2 suppl):517–23. doi: 10.1002/cncr.2820710205. [DOI] [PubMed] [Google Scholar]
- 3.Riman T, Persson I, Nilsson S. Hormonal aspects of epithelial ovarian cancer: review of epidemiological evidence. Clin Endocrinol. 1998;49:695–707. doi: 10.1046/j.1365-2265.1998.00577.x. [DOI] [PubMed] [Google Scholar]
- 4.Fathalla MF. Incessant ovulation—a factor in ovarian neoplasia? Lancet. 1971;2:163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- 5.Cramer DW, Welch WR. Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. J Natl Cancer Inst. 1983;71:717–21. [PubMed] [Google Scholar]
- 6.Mohle J, Whittemore A, Pike M, Darby S. Gonadotrophins and ovarian cancer risk. J Natl Cancer Inst. 1985;75:178–80. [PubMed] [Google Scholar]
- 7.Ozols RF, Bookman MA, Connolly DC, et al. Focus on epithelial ovarian cancer. Cancer Cell. 2004;5:19–24. doi: 10.1016/s1535-6108(04)00002-9. [DOI] [PubMed] [Google Scholar]
- 8.Lukanova A, Kaaks R. Endogenous hormones and ovarian cancer: epidemiology and current hypotheses. Cancer Epidemiol Biomarkers Prev. 2005;14:98–107. [PubMed] [Google Scholar]
- 9.Godwin AK, Testa JR, Handel LM, et al. Spontaneous transformation of rat ovarian surface epithelial cells: association with cytogenetic changes and implications of repeated ovulation in the etiology of ovarian cancer. J Natl Cancer Inst. 1992;84:592–601. doi: 10.1093/jnci/84.8.592. [DOI] [PubMed] [Google Scholar]
- 10.Barnes MN, Grizzle WE, Grubbs CJ, Partridge EE. Paradigms for primary prevention of ovarian carcinomas. CA Cancer J Clin. 2002;52:216–25. doi: 10.3322/canjclin.52.4.216. [DOI] [PubMed] [Google Scholar]
- 11.Hankinson SE, Colditz GA, Hunter DJ, Spencer TL, Rosner B, Stampfer MJ. A quantitative assessment of oral contraceptive use and risk of ovarian cancer. Obstret Gynecol. 1992;80:708–14. [PubMed] [Google Scholar]
- 12.Rodriguez GC, Walmer DK, Cline M, et al. Effect of progestin on the ovarian epithelium of macaques: cancer prevention through apoptosis? J Soc Gynecol Investig. 1998;5:271–76. doi: 10.1016/s1071-5576(98)00017-3. [DOI] [PubMed] [Google Scholar]
- 13.Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst. 1998;90:1774–86. doi: 10.1093/jnci/90.23.1774. [DOI] [PubMed] [Google Scholar]
- 14.Yang WL, Godwin AK, Xu XX. Tumor necrosis factor-alpha-induced matrix proteolytic enzyme production and basement membrane remodeling by human ovarian surface epithelial cells: molecular basis linking ovulation and cancer risk. Cancer Res. 2004;64:1534–40. doi: 10.1158/0008-5472.can-03-2928. [DOI] [PubMed] [Google Scholar]
- 15.Talbot P, Martin GG, Ashby H. Formation of the rupture site in preovulatory hamster and mouse follicles: loss of the surface epithelium. Gamete Res. 1987;17:287–302. doi: 10.1002/mrd.1120170403. [DOI] [PubMed] [Google Scholar]
- 16.Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the infl ammatory-like response. Annu Rev Physiol. 2002;64:69–92. doi: 10.1146/annurev.physiol.64.081501.131029. [DOI] [PubMed] [Google Scholar]
- 17.Eldridge JC, McPherson JC, 3rd, Mahesh VB. Maturation of the negative feedback control of gonadotropin secretion in the female rat. Endocrinology. 1974;94:1536–40. doi: 10.1210/endo-94-6-1536. [DOI] [PubMed] [Google Scholar]
- 18.Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proimflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 19.Smith ER, Xu XX. Etiology of epithelial ovarian cancer: a cellular mechanism for the role of gonadotropins. Gynecol Oncol. 2003;91:1–2. doi: 10.1016/s0090-8258(03)00463-3. [DOI] [PubMed] [Google Scholar]
- 20.Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459–67. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- 21.Smith ER, Daly MB, Xu XX. A mechanism for Cox-2 inhibitor anti-inflammatory activity in chemoprevention of epithelial cancers. Cancer Epidemiol Biomarkers Prev. 2004;13:144–45. doi: 10.1158/1055-9965.epi-461-2. [DOI] [PubMed] [Google Scholar]
- 22.Gosden RG. Biology of menopause: the causes and consequences of ovarian aging. Burlington, MA: Academic Press; 1985. [Google Scholar]
- 23.Finn CA. Reproductive ageing and the menopause. Int J Dev Biol. 2001;45:613–17. [PubMed] [Google Scholar]
- 24.Lobo RA, Kelsey J, Marcus R, editors. Menopause: biology and pathobiology. 1st. Burlington, MA: Academic Press; 2000. [Google Scholar]
- 25.Cai KQ, Klein-Szanto A, Karthik D, et al. Age-dependent morphological alterations of human ovaries from populations with and without BRCA mutations. Gynecol Oncol. 2006;103:719–28. doi: 10.1016/j.ygyno.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 26.Nicosia SV. The aging ovary. Med Clin North Am. 1987;71:1–9. [PubMed] [Google Scholar]
- 27.Salazar H, Godwin AK, Daly MB, et al. Microscopic benign and invasive malignant neoplasms and a cancer-prone phenotype in prophylactic oophorectomies. J Natl Cancer Inst. 1996;88:1810–20. doi: 10.1093/jnci/88.24.1810. [DOI] [PubMed] [Google Scholar]
- 28.Barakat RR, Federici MG, Saigo PE, Robson ME, Offit K, Boyd J. Absence of premalignant histologic, molecular, or cell biologic alterations in prophylactic oophorectomy specimens from BRCA1 heterozygotes. Cancer. 2000;89:383–90. doi: 10.1002/1097-0142(20000715)89:2<383::aid-cncr25>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 29.Roland IR, Yang WL, Yang DH, et al. Loss of surface and cyst epithelial basement membranes and pre-neoplastic morphological changes in prophylactic oophorectomies. Cancer. 2003;98:2607–23. doi: 10.1002/cncr.11847. [DOI] [PubMed] [Google Scholar]
- 30.Yang WL, Cai KQ, Smith ER, Klein-Szanto A, Hamilton TC, Xu XX. A reduction of Cox-2 gene dosage counters the menopausal ovarian morphological aging and tumor phenotype in Wv mice. Am J Pathol. 2007;170:1325–36. doi: 10.2353/ajpath.2007.060769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reith AD, Rottapel R, Giddens E, Brady C, Forrester L, Bernstein A. W mutant mice with mild or severe developmental defects contain distinct point mutations in the kinase domain of the c-kit receptor. Genes Dev. 1990;4:390–400. doi: 10.1101/gad.4.3.390. [DOI] [PubMed] [Google Scholar]
- 32.Mintz B. Embryological development of primordial germ-cells in the mouse: influence of a new mutation, Wj. J Embryol Exp Morphol. 1957;5:396–403. [Google Scholar]
- 33.Murphy ED. Hyperplastic and early neoplastic changes in the ovaries of mice after genic deletion of germ cells. J Natl Cancer Inst. 1972;48:1283–95. [PubMed] [Google Scholar]
- 34.Murphy ED, Beamer WG. Plasma gonadotropin levels during early stages of ovarian tumorigenesis in mice of the Wx-Wv genotype. Cancer Res. 1973;33:721–23. [PubMed] [Google Scholar]
- 35.Blaakaer J, Baeksted M, Micic S, Albrectsen P, Rygaard K, Bock J. Gonadotropin-releasing hormone agonist suppression of ovarian tumorigenesis in mice of the Wx/Wv genotype. Biol Reprod. 1995;53:775–79. doi: 10.1095/biolreprod53.4.775. [DOI] [PubMed] [Google Scholar]
- 36.Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–35. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 37.Vanderhyden BC. Loss of ovarian function and the risk of ovarian cancer. Cell Tissue Res. 2005;322:117–24. doi: 10.1007/s00441-005-1100-1. [DOI] [PubMed] [Google Scholar]
- 38.Kumar TR, Palapattu G, Wang P, et al. Transgenic models to study gonadotropin function: the role of follicle-stimulating hormone in gonadal growth and tumorigenesis. Mol Endocrinol. 1999;13:851–65. doi: 10.1210/mend.13.6.0297. [DOI] [PubMed] [Google Scholar]
- 39.Danilovich N, Roy I, Sairam RM. Ovarian pathology and high incidence of sex cord tumors in follitropin receptor knockout (FORKO) mice. Endocrinology. 2001;142:3673–84. doi: 10.1210/endo.142.8.8320. [DOI] [PubMed] [Google Scholar]
- 40.Walker GR, Schlesselman JJ, Ness RB. Family history of cancer, oral contraceptive use, and ovarian cancer risk. Am J Obstet Gynecol. 2002;186:8–14. doi: 10.1067/mob.2002.118657. [DOI] [PubMed] [Google Scholar]
- 41.Schildkraut JM, Moorman PG, Halabi S, Calingaert B, Marks JR, Berchuck A. Analgesic drug use and risk of ovarian cancer. Epidemiology. 2006;17:104–07. doi: 10.1097/01.ede.0000190538.55645.f8. [DOI] [PubMed] [Google Scholar]
- 42.Cramer DW, Harlow BL, Titus-Ernstoff L, Bohlke K, Welch WR, Greenberg ER. Over-the-counter analgesics and risk of ovarian cancer. Lancet. 1998;351:104–07. doi: 10.1016/S0140-6736(97)08064-1. [DOI] [PubMed] [Google Scholar]
- 43.Reese J, Zhao X, Ma WG, Brown N, Maziasz TJ, Dey SK. Comparative analysis of pharmacologic and/or genetic disruption of cyclooxygenase-1 and cyclooxygenase-2 function in female reproduction in mice. Endocrinology. 2001;142:3198–206. doi: 10.1210/endo.142.7.8307. [DOI] [PubMed] [Google Scholar]
- 44.Downs SM, Longo FJ. An ultrastructural study of preovulatory apical development in mouse ovarian follicles: effects of indomethacin. Anat Rec. 1983;205:159–68. doi: 10.1002/ar.1092050206. [DOI] [PubMed] [Google Scholar]
- 45.Downs SM, Longo FJ. Prostaglandins and preovulatory follicular maturation in mice. J Exp Zool. 1983;228:99–108. doi: 10.1002/jez.1402280111. [DOI] [PubMed] [Google Scholar]
- 46.Tung KH, Wilkens LR, Wu AH, et al. Effect of anovulation factors on pre- and postmenopausal ovarian cancer risk: revisiting the incessant ovulation hypothesis. Am J Epidemiol. 2005;161:321–29. doi: 10.1093/aje/kwi046. [DOI] [PubMed] [Google Scholar]
- 47.Chiaffarino F, Pelucchi C, Parazzini F, Negri E, Franceschi S, La Vecchia C. Reproductive and hormonal factors and ovarian cancer. Ann Oncol. 2001;12:337–41. doi: 10.1023/a:1011128408146. [DOI] [PubMed] [Google Scholar]