Abstract
We have been developing Aβ derivative vaccines with the objective to improve the safety of Aβ targeting immunotherapy. Our Aβ homologs are designed to have less direct toxicity and to produce a modified immune response compared to Aβ. In extensive mouse studies, all our vaccines have improved cognition in transgenic mice while eliciting different immune responses and reducing brain amyloid burden to a variable degree. While we are continuing to characterize these vaccines in mice, in preparation for studies in old primates and for human trials we assessed their effect in young lemur primates (n = 25) that with age develop Aβ plaques and tau aggregates as seen in Alzheimer’s disease.
In the primates, all the peptides administered with alum adjuvant elicited a moderate to robust anti-Aβ IgM response. Aβ1-42, K6Aβ1-30 and K6Aβ1-30[E18E19] resulted in a high anti-Aβ IgG response, whereas Aβ1-30[E18E19] produced a weaker more variable IgG titer. Notably, 22 weeks after the 3rd immunization, IgM and IgG levels in derivative-vaccinated primates were similar to preimmune values whereas Aβ1-42 treated primates maintained a moderate IgG titer. The increase in antibodies that recognized Aβ1-40 often correlated with increase in Aβ1-40 in plasma, which suggests that the antibodies were binding to Aβ in vivo. Interestingly, significant transient weight gain was observed (K6Aβ1-30-, Aβ1-30[E18E19]- and Aβ1-42-treated) or a trend in the same direction (K6Aβ1-30[E18E19]-treated, adjuvant controls) following the injections. Based on these findings, we have chosen K6Aβ1-30 for immunizations in old primates as the antibody response to this vaccine was less variable compared to other Aβ derivatives. Our present findings indicate that most of our Aβ derivatives elicit a substantial antibody response in primates, and importantly this effect is reversible which enhances the safety profile of our approach.
Keywords: Amyloid-β, Microcebus murinus, Lemur, Primate, Immunization, Alum
1. Introduction
Prior to the occurrence of brain inflammation in the Elan/Wyeth AN-1792 trial, we raised concerns about administering full-length Aβ1-42 self-antigen in humans, and we advocated the use of both adjuvants and immunogens that favored a Th2 response promoting antibody production instead of a Th1 response which mediates a cytotoxic T-cell response [1]. The primary objective in designing our Aβ derivatives was to maintain antibody epitopes while reducing their β-sheet content compared to Aβ1-42, in order to eliminate direct toxicity and amyloid seeding potential. These modifications also altered or removed potential T-cell epitopes as determined by computer algorithms [2,3]. Most of the potential T-cell epitopes in Aβ1-42 are located in the C-terminus of the peptide (which we removed) or in the hydrophobic region in the middle of the peptide (that we modified). Interestingly, recent findings in the prion field also indicate that immune responses to α-helical structures appear to preferentially involve the Th2 pathway whereas a β-sheet conformation favors Th1 activation [4].
Our initial report was on K6Aβ1-30 which has 6 lysines on the N-terminus to increase immunogenicity and enhance solubility. This modification in addition to removal of the C-terminal amino acids of Aβ1-42 also reduced its propensity to form β-sheets. The main antibody epitopes are located within the first 30 amino acids of the peptide and this peptide as expected elicited a similar albeit somewhat less antibody response as Aβ1-42 in mice which resulted in a comparable therapeutic efficacy [1]. Concurrently, we have been determining the therapeutic potential of other Aβ derivatives with internal substitutions to further diminish T-cell response [5]. It appears that a robust immune response towards Aβ is not needed to improve cognition since some of our Aβ derivatives elicit only very modest anti-Aβ antibody titers, while producing cognitive improvements [5–7]. Interestingly, one of our Aβ derived immunogen, K6Aβ1-30[E18E19], primarily produced a T-cell independent IgM response, and the anti-Aβ IgM titer correlated inversely with brain plaque burden [5]. IgM does not cross the blood brain barrier because of its size [8,9]; hence our observations indicate that it may be sufficient to clear Aβ peripherally to reduce amyloid burden in the brain, as we and others have previously suggested [1,10]. One of the advantages of this approach is that the potential of antibody-mediated CNS inflammation is avoided. Also, because of its T-cell independence, the IgM response is reversible and it subsides within a few weeks of vaccination. This reversibility is important if any adverse reactions occur in the course of the vaccination. In other words, this type of response resembles passive immunization but avoids the possibility of an anti-idiotypic response and subsequent side effects of multiple antibody injections as we have discussed previously [11,12].
The different immunogenicity profiles of these Aβ derivatives in transgenic models are intriguing but prior to clinical trials it is necessary to assess potential AD immunotherapy in other models. Ideally, those models should have an immune system that is closer to humans than the mouse equivalent; and endogenous Aβ levels that are comparable to humans. Non-human primates are the obvious choice and many of the subspecies develop similar plaque pathology as seen in AD. Two preliminary findings in four Vervet-[13] and two Rhesus primates [14] show that these animals like humans develop an immune response to Aβ1-40/42 that may have resulted in amyloid clearance [13]. Furthermore, immunization of aged canines with fibrillar Aβ1-42 reduces Aβ1-40/42 levels as well as their diffuse Aβ plaques, which is associated with stabilization of prefrontal-dependent learning ability [15]. The advantage of the Microcebus murinus lemur primate, which we have chosen to use, is that these animals develop tau pathology in addition to amyloid deposition, and are therefore better models of AD than many other primates [16–18]. They can, therefore, also be used to assess experimental therapy targeting pathological tau such as we and others have recently described in mouse models [19–21]. Also, their small size reduces housing cost and facilitates experimental work. This allows larger number of animals to be included in each experimental group, increasing the reliability of the data.
We report that immunization with Aβ derivatives in M. murinus can result in different antibody response than in mouse models. Importantly, the reversibility of this effect renders it a safer approach for future human trials.
2. Materials and methods
2.1. Peptides
Synthesized by the solid-phase technique (Keck, Yale Univ.), as we have described previously [1,5,7].
2.2. Primates
M. murinus (mouse lemur primate) develops Aβ plaques and hyperphosphorylated tau aggregates with age [16–18]. The advantages of these primates over simians are: (1) Smaller size (70–150 g) and shorter lifespan (10–13 years [16]), (2) Easier reproduction, (3) Both Aβ and tau pathology develop with age whereas simians only develop Aβ pathology. The animals were randomly obtained from a colony of 250 primates kept in our zoological facility in Montpellier, France. The primates employed were disease-free and had never been subjected to any experimental treatment. Twenty-five animals were enrolled in the study (five per group: Table 1).
Table 1.
Depicts the gender, age as well as the type and number of immunizations received by each of the primates.
| Animal | Sex | Age (years) | Group | Number of immunizations |
|---|---|---|---|---|
| 376 | Female | 1.8 | Control | 6 |
| 377 | Male | 1.8 | 3a | |
| 402 | Male | 1.3 | 6 | |
| 403 | Male | 1.2 | 6 | |
| 404 | Male | 1.2 | 3b | |
| 375 | Male | 1.9 | K6Aβ1-30 | 6 |
| 391 | Female | 1.4 | 6 | |
| 397 | Female | 1.4 | 6 | |
| 399 | Male | 1.2 | 6 | |
| 408 | Male | 1.2 | 6 | |
| 371 | Female | 2.0 | K6Aβ1-30[E18 E19] | 6 |
| 379 | Female | 1.9 | 6 | |
| 392 | Female | 1.4 | 6 | |
| 401 | Male | 1.3 | 6 | |
| 405 | Female | 1.2 | 6 | |
| 373 | Female | 1.9 | Aβ1-30[E18 E19] | 6 |
| 398 | Female | 1.2 | 6 | |
| 400 | Female | 1.2 | 6 | |
| 407 | Male | 1.2 | 6 | |
| 419 | Male | 0.7 | 2c | |
| 372 | Male | 2.0 | Aβ1-42 | 6 |
| 374 | Female | 1.9 | 6 | |
| 394 | Female | 1.3 | 6 | |
| 406 | Male | 1.2 | 6 | |
| 412 | Male | 0.7 | 6 |
Euthanized 21 days after the 4th bleed (T3) because of substantial weight loss. The date of death was 5 months and 23 days after the third adjuvant injection.
Discontinued in the study after the 4th bleed because it was required for breeding.
Euthanized 15 days after the 2nd bleed (T1) because of substantial weight loss. The date of death was 22 days after the 2nd immunization.
2.3. Injections and bleeds
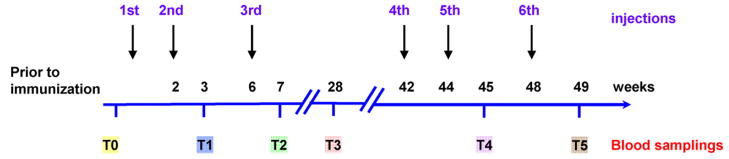
The time line for injections and bleeds is depicted in Fig. 1. Five animals per group received a subcutaneous injection with Aβ1-42 or its derivatives in alum adjuvants (Brenntag Biosector, Denmark). The peptides were mixed with alum adjuvant at a concentration of 1 mg/mL and the solution was rotated overnight at 4 °C prior to administration to allow the peptide to adsorb onto the aluminium particles. Controls received alum adjuvant alone (n = 5 per group). The primates received the second and third injections at 2 and 6 weeks. The fourth, fifth and sixth injections were at 42, 44 and 48 weeks, respectively. The primates were bled prior to the first immunization (T0), 1 week following the second (T1, 3 weeks) and third injection (T2, 7 weeks). T3 was at 28 weeks (22 weeks following the third injection) to assess the reversibility of the immune response. T4 and T5 were performed at 45 and 49 weeks, respectively (1 week following the fifth and sixth injection).
Fig. 1.
Schedule for immunizations and bleeds. A schematic diagram depicting the timeline of the immunizations and bleeds for measurements of antibody response and Aβ levels.
2.4. Antibody levels
Determined at 1:200 dilution of plasma using an ELISA assay as we have described previously [1,5], where the immunogen or the full-length Aβ1-40/42 peptide was coated onto microtiter wells (Immulon 2 HB, ThermoScientific, Waltham, MA). Antibody levels were detected by an anti-primate IgG and IgM linked to a horseradish peroxidase (Alpha Diagnostics, San Antonio, TX).
2.5. Aβ1-40 levels in plasma
For measurements of free Aβ1-40 in plasma, 10% dilution of untreated plasma were used, and the detection was performed by an ELISA kit (Biosource, Camarillo, CA) as described by the manufacturer. Under these conditions, Aβ1-42 levels in plasma were below the limit of detection.
2.6. Staining of AD brain tissue
Paraffin embedded brain sections (10 μm) through the superior frontal/cingulate gyrus from a familial AD case were stained with plasma dilutions (1:100) from several primates at different bleeds with varying Aβ antibody levels using standard protocol as described previously in more detail [22]. The donor met the NIA Reagen/CERAD criteria for definitive AD, with a Braak and Braak stage VI/VI, and the tissue was obtained from the NYU ADCC Neuropathology Core brain bank (grant AG 08051). The sections were deparaffinized and hydrated through xylene and ethanol gradient and were then washed in water followed by overnight incubation at 4 °C in plasma (1:100) or monoclonal antibody dilutions (6E10/4G8 (courtesy of Richard Kascsak, IBRDD, Staten Island), 1:1000). The plasma samples were diluted in a blocking diluent as described previously [23] that contained 2% bovine serum albumin and 10% goat serum to prevent non-specific staining. MOM diluent (Vector Labs, Burlingame, VT) was used for the 6E10/4G8 dilution. After three washes in PBS containing 0.1% Triton X-100 (PBS-T) for 10 min each, sections were incubated for 1 h in biotinylated goat anti-monkey IgG (1:200; Alpha Diagnostics) and biotinylated horse anti-mouse IgG (1:200; MOM kit). Subsequently, sections were washed again three times in PBS-T followed by 1 h incubation in avidin-peroxidase complex (1:200 dilution; Vector Labs). The sections were then washed in 0.2 M sodium acetate buffer and reacted with diaminobenzidine tetrahydrochloride (0.35 mg/ml; Sigma) with nickel intensification (nickel ammonium sulfate 25 mg/ml; Fisher Scientific, Fair Lawn, NJ) in the same buffer containing 0.3% hydrogen peroxide (Sigma, St. Louis, MO).
2.7. Statistical analysis
All the data were analyzed with GraphPad Prism 4.3 (San Diego, CA) or Statistica 6 (Statsoft Inc. Tulsa, OK). Correlation between plasma Aβ1-40 levels and anti-Aβ1-40 IgG or IgM response was analyzed by Pearson r correlation or Spearman rank correlation if the data failed at least two of three normality tests (Kolmogorov–Smirnov, D’Agostino and Pearson omnibus, and Shapiro–Wilk normality tests). The weight of the primates at different time points within each group was analyzed by one-way ANOVA repeated measures and Dunnett’s post hoc test in which the preimmunization weight was compared to the weight of the animals at subsequent intervals.
3. Results
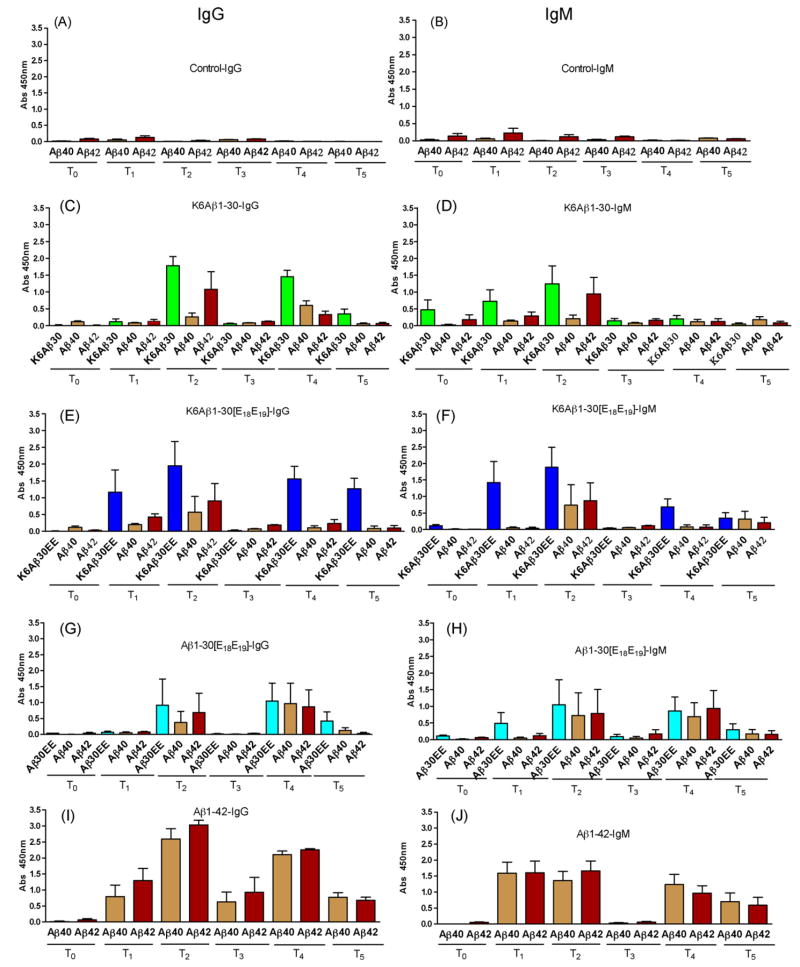
Very low levels of IgG and IgM antibodies that recognized Aβ1-40 or Aβ1-42 were observed in control primates that received adjuvant alone (Fig. 2A and B). We have previously observed similar degree of autoantibodies against Aβ in mouse models [1,5,7]. Of the three Aβ derivatives, the most consistent antibody response was observed with K6Aβ1-30 (Fig. 2C and D) but a similar degree of response was observed with K6Aβ1-30[E18E19] albeit more variable (Fig. 2E and F). Aβ1-30[E18E19] elicited the lowest antibody response which was as expected because it does not contain the 6 lysines on the N-terminus that are known to increase immunogenicity (Fig. 2G and H). Importantly, for all the Aβ derivatives the IgG antibody levels went back to baseline once immunization was discontinued as measured 22 weeks (T3) following the third immunization. This finding enhances the safety of this approach, because should any antibody-related side effects occur, these should subside with discontinuation of therapy. Aβ1-42 elicited the most robust antibody response, with the IgG levels not going back to baseline once treatment was halted (Fig. 2I and J).
Fig. 2.
Antibody response in lemur primates. The primates (n = 5 per group) were bled prior to immunization (T0). Subsequent vaccinations were at 0, 2, 6, 42, 44 and 48 weeks. Subsequent bleeds were at 3 (T1), 7 (T2), 28 (T3) 45 (T4) and 49 weeks (T5). (A, B) Very low levels of autoantibodies are observed in control animals (1:200 dilution). (C–H) All the Aβ derivatives have a moderate to high antibody response (IgG and IgM) but it is transient because 22 weeks after the 3rd injection (T3), antibody levels are similar to controls. However, antibodies are regenerated when the primates are reimmunized. (I, J) Aβ1-42 elicits an overall stronger antibody response that is maintained at moderate levels (IgG) 22 weeks (T3) after the 3rd injection. Also, more antibodies are generated when the primates are reimmunized. Treatment and antibody type measured (IgG or IgM) is indicated in each graphs’ title. The x-axis depicts which peptide (coated on ELISA plate) the antibodies are recognizing. The y-axis depicts the absorbance at 450 nm.
Regarding IgM levels, those are known to peak 1–2 weeks following immunization and then the antibodies are cleared. Our findings confirmed this well known fact as IgM levels in all the groups decreased to preimmunization levels at the T3 bleed. Overall, these results indicate that most of our Aβ derivatives elicit a substantial antibody response in primates, and importantly this effect is reversible (see T3) which enhances the safety profile of this type of immunization. It should be considered optimal to have this reversibility feature designed into any vaccine that targets self-antigens and has the potential to induce autoimmune toxicity. The primates elicited a similar antibody response towards Aβ as we have observed in mouse models with the exception of K6Aβ1-30[E18E19] that in BL6/SJL Tg2576 mice elicited very low IgG but high IgM response whereas in the primates both IgG and IgM levels were moderate to high.
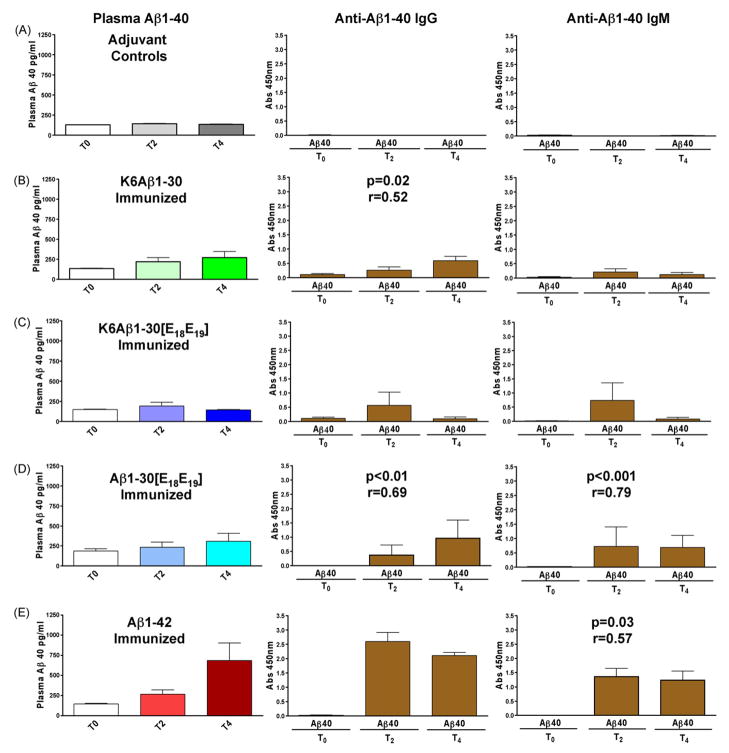
These young primates should not have any Aβ deposits within the brain but we sought to determine if their antibody response altered their plasma Aβ1-40 levels as an indicator of the physiological relevance of the immunization. Aβ1-40 levels in plasma were measured at T0, T2 and T4 (Fig. 3; for comparison the levels of anti-Aβ1-40 antibodies at the same intervals is depicted in the right panel). Aβ1-40 plasma levels correlated well with both IgG (Aβ1-30[E18E19], p < 0.01; K6Aβ1-30, p = 0.02) and IgM (Aβ1-30[E18E19], p < 0.001; Aβ1-42, p < 0.03) against Aβ1-40. These findings indicate that the antibodies that are generated in these primates in response to the immunization are having a biological effect on Aβ in vivo.
Fig. 3.
Plasma Aβ1-40 levels versus antibody response. A good correlation was often observed between plasma Aβ1-40 levels (left panel) and levels of anti-Aβ1-40 IgG (middle panel) or IgM (right panel) in the young lemurs. Aβ1-40 plasma levels correlated well with both IgG (Aβ1-30[E18 E19 ], p < 0.01; K6Aβ1-30, p = 0.02) and IgM (Aβ1-30[E18 E19 ], p < 0.001; Aβ1-42, p = 0.03) against Aβ1-40.
To assess if the antibodies generated in response to the vaccine would recognize AD plaques or congophilic angiopathy, AD brain sections were stained with plasma from animals in all treatment groups. Staining of parenchymal and vascular Aβ deposits was observed with plasma from all the immunized groups and as expected was more prominent in bleeds with higher Aβ antibody levels (Fig. 4).
Fig. 4.
Staining of parenchymal and vascular Aβ deposits with plasma from immunized primates. Representative examples of AD brain sections stained with plasma (T2; 1:100 dilution) from lemur primates immunized with (A) Aβ1-30[E18 E19 ] and (B) Aβ1-42. Adjacent section (C) was stained with a mixture of 6E10/4G8 monoclonal antibodies (1:1000) to reveal all Aβ deposits in this particular AD case. As expected, more plaques were observed in the Aβ1-42 immunized primate as it had higher Aβ antibody levels than the animal immunized with Aβ1-30[E18 E19 ] (Aβ1-42 treated primate: anti-Aβ1-40 = 3.3; anti-Aβ1-42 = 3.3. Aβ1-30[E18 E19 ]-treated primate: anti-Aβ1-40 = 1.5; anti-Aβ1-42 = 2.6. Values are arbitrary ELISA signals at 1:200 dilution at 450 nm). Similar titer-dependent staining was observed in the K6Aβ1-30- and K6Aβ1-30[E18 E19 ]-treated groups (data not shown). Original magnification: 50×.
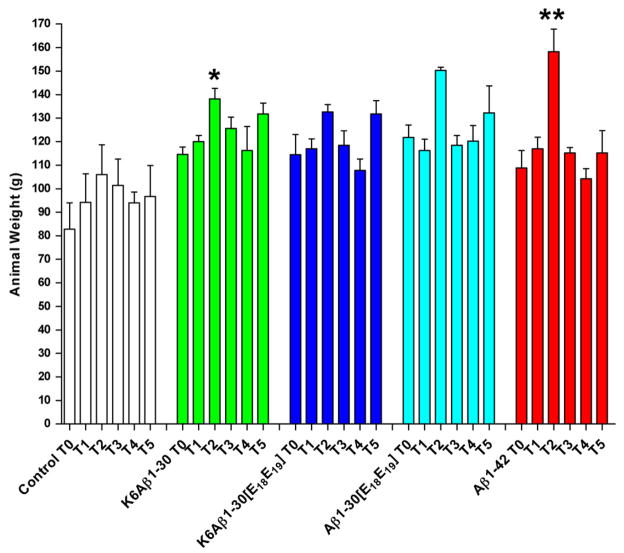
As one measure of general health, the animals were weighted the same day they were bled (T0–T5; Fig. 5). None of the groups lost weight during this period but two animals were euthanized during the study because of substantial weight loss: (1) One adjuvant control 6 weeks after the fourth bleed (T3) and (2) One Aβ1-30[E18E19]-treated primate, 2 weeks after the second bleed (T1). While the cause of the weight loss is unclear there is no indication that it was related to the adjuvant or the vaccine although that possibility cannot be completely ruled out. Interestingly, significant weight increase was observed in all the immunized groups, K6Aβ1-30 (p = 0.02), K6Aβ1-30[E18E19] (p = 0.01), Aβ1-30[E18E19] (p = 0.03), and Aβ1-42 (p = 0.0001), but not in the controls (p = 0.14). Post hoc analysis revealed significant weight gain at T2 compared to T0 in K6Aβ1-30- (p = 0.01), and Aβ1-42-treated primates (p = 0.0001), and a strong trend for weight gain in K6Aβ1-30[E18E19]-treated (p = 0.07) and Aβ1-30[E18E19]-treated primates (p = 0.10). The weight of those animals went back to preimmune (T0) values at subsequent intervals. Furthermore, weekly observations by a veterinarian indicated as well that the animals were in good health.
Fig. 5.
Weight of primates. A significant weight increase was observed in all the immunized groups, K6Aβ1-30 (p = 0.02), K6Aβ1-30[E18 E19 ] (p = 0.01), Aβ1-30[E18 E19 ] (p = 0.03), and Aβ1-42 (p = 0.0001), but not in the controls (p = 0.14). Post hoc analysis revealed significant weight gain at T2 compared to T0 in K6Aβ1-30- (p = 0.01), and Aβ1-42-treated primates (p = 0.0001), and a strong trend for weight gain in K6Aβ1-30[E18 E19 ]- (p = 0.07) and Aβ1-30[E18 E19 ]-treated primates (p = 0.10). The weight of those animals went back to preimmune (T0) values at subsequent intervals.
4. Discussion
Most of the immunization approaches targeting Aβ have been performed in transgenic models that overexpress mutated human amyloid precursor protein which results in high levels of human Aβ. The mice have normal endogenous levels of mouse Aβ as well. These models are ideal for initial screening of potential therapy but prior to clinical trials it is imperative to perform studies in animals such as primates that express normal levels of Aβ which has the same sequence as human Aβ. One of these models is M. murinus, a lemur primate that develops Alzheimer’s like brain pathology with age [16–18].
Our present findings in a relatively small group of young primates indicate that immunization with Aβ derivatives appears to be safe as a prophylactic measure. Transient weight gain was observed which indicates that the vaccines do not appear to have detrimental effects on the general health of these young animals. Importantly, anti-Aβ antibody levels went back to baseline when therapy was discontinued in the Aβ homolog treated primates but not in the Aβ1-42 treated animals. These observations show that active immunization with Aβ derivatives is analogous to passive immunization with regard to its reversibility in plasma antibody levels when immunization is discontinued. Furthermore, it will be less likely to induce an anti-idiotypic response as the antibodies are formed endogenously. As should be expected, not all mammals react in the same way. Head et al. observed that in young beagles immunized with Aβ1-42 in Titermax adjuvant, antibody titer was back to preimmune values by 12 weeks [24]. However their experimental design was different from ours with regard to dose, adjuvant and numbers of injections.
The increase of Aβ1-40 antibodies in the primates often correlated with increased Aβ1-40 in plasma which suggests that the antibodies generated in response to the vaccines were binding to Aβ in vivo. The spike in plasma levels of Aβ can be explained by increased half-life of bound Aβ in plasma and/or by antibody-mediated sequestration of Aβ from organs to blood.
Aβ T cell epitopes have not been described in lemur primates but as mid-region Aβ T cell epitopes have been detected in mice and humans, this type of response is more likely to occur following administration of K6Aβ1-30 than with the modified Aβ derivatives K6Aβ1-30[E18E19] and Aβ1-30[E18E19]. However, T-helper epitopes are necessary for antibody response and those often overlap with cytotoxic T cell epitopes depending on the haplotype of the individual which complicates vaccine design. With appropriate choice of adjuvant, for example alum (Th2) over QS-21 (Th1), cytotoxic T cell response can likely be contained.
Low ELISA signal in preimmune plasma or controls was occasionally detected in the antibody assays. As depicted in Figs. 2 and 3, the ELISA IgG and IgM signal at T0 or in the controls at all bleeds is either barely measurable or much lower than post-immunization. As several of the animals had a value of 0, it is more likely that the signal is specific for Aβ autoantibodies rather than representing a non-specific binding of the peroxidase-linked secondary antibody. We are not aware of studies that have measured Aβ autoantibodies in young humans but those are frequently observed in middle-aged and older individuals [25–27]. Furthermore, we have noticed an age-related increase in Aβ40 autoantibodies in older human controls (age range 56–81) as well as in the Aβ plaque mouse model Tg2576 (unpublished results).
It is unclear why transient weight gain occurred in the immunized primates but it is interesting that they were heaviest at the T2 bleed, the time point of their highest levels of Aβ antibodies. This observation may suggest that Aβ has a role in weight regulation. Several gastrointestinal hormones and neuropeptides are known to have complex effects on appetite and energy homeostasis [28]. As Aβ is found in all biological fluids, it likely has both peripheral and central effects that can be altered by immunotherapy although compensatory mechanisms may only lead to transient changes.
We are not aware of other investigators using modified Aβ derivatives (Aβ1-30[E18E19], K6Aβ1-30[E18E19]) as potential AD vaccines, but studies on unaltered Aβ fragments as vaccine components have recently been described by others [29–32]. Also, mice have been vaccinated with a phage displaying amino acids 3–6 of the Aβ peptide which resulted in a modest antibody response, promoted clearance of amyloid deposits [33], and improved cognition [34], supporting our previous findings that a relatively low antibody titer is sufficient to enhance memory and learning [5]. A different promising approach combines Aβ1-15, its immunodominant B-cell epitope, with a promiscuous T-cell epitope that in wild-type mice elicits high anti-Aβ antibody titers without T-cell reactivity towards Aβ [35]. A similar approach has recently been shown to reduce amyloid burden and learning deficits in AD mouse model in the absence of Aβ specific cellular immune response [36]. Together, the use of Aβ derivatives for active immunization is likely to have a better safety profile for humans than administering full-length Aβ. To selectively target highly toxic forms of Aβ should also be beneficial although the types of antibodies generated are likely to vary between individuals. For example, the use of oligomeric Aβ as immunogen has been shown to reduce Aβ burden in transgenic mice with less microglial response than other Aβ peptides [37]. Potential toxicity associated with use of oligomeric Aβ can be avoided by using monoclonal antibodies that selectively target these Aβ aggregates. At least three clinical trials by Elan/Wyeth (ACC-001), Cytos/Novartis (CAD106) and Merck (V950) are ongoing on this type of active immunotherapy targeting Aβ. Dosing in the ACC-001 trial was recently suspended temporarily, because one patient in the Phase II trial developed skin lesions [38]. The ACC-001 vaccine is Aβ1-7 linked to an immunostimulatory molecule, a mutated diphtheria toxin CRM-197, and is delivered with or without QS-21, a strong Th1-type adjuvant. It is unclear what may have caused this reaction in the patient who is currently recovering.
The main purpose of this present immunization study in young primates was twofold: (1) to assess the safety of this approach as a prophylactic measure using an animal model that is closer to humans than transgenic mice, and (2) to select one Aβ derivative for studies in old lemur primates. One control primate that received alum adjuvant alone and one Aβ1-30[E18E19]-treated primate were euthanized during the study because of a substantial weight loss. It is unlikely that this condition was related to the immunization as one of the animals was a control that only received adjuvant that is approved for human use, and should therefore be considered safe. Unexplained weight loss in a few animals has occasionally been observed over the years in this colony of about 250 primates.
While we continue to monitor the young primates for any adverse reactions, we have now initiated studies in older lemurs employing K6Aβ1-30 because it showed the most consistent antibody response in the young animals. As those older animals may have Alzheimer’s-like neuropathology, this approach should provide invaluable information for future clinical trials on this type of Aβ immunotherapy that embodies the advantages of active (endogenous response) and passive (reversibility) immunization approaches.
Acknowledgments
Supported by NIH grants AG20197, AG20245, AG05891, the Alzheimer’s Association (USA), France Alzheimer Association, and Intellect Neurosciences.
We thank Hameetha Banu Rajamohamedsait for technical assistance with staining the AD brain sections.
References
- 1.Sigurdsson EM, Scholtzova H, Mehta PD, Frangione B, Wisniewski T. Immunization with a non-toxic/non-fibrillar amyloid-β homologous peptide reduces Alzheimer’s disease associated pathology in transgenic mice. Am J Pathol. 2001;159(2):439–47. doi: 10.1016/s0002-9440(10)61715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh H, Raghava GP. ProPred: prediction of HLA-DR binding sites. Bioinformatics. 2001;17(12):1236–7. doi: 10.1093/bioinformatics/17.12.1236. [DOI] [PubMed] [Google Scholar]
- 3.Singh H, Raghava GP. ProPred1: prediction of promiscuous MHC Class-I binding sites. Bioinformatics. 2003;19(8):1009–14. doi: 10.1093/bioinformatics/btg108. [DOI] [PubMed] [Google Scholar]
- 4.Khalili-Shirazi A, Quaratino S, Londei M, et al. Protein conformation significantly influences immune responses to prion protein. J Immunol. 2005;174(6):3256–63. doi: 10.4049/jimmunol.174.6.3256. [DOI] [PubMed] [Google Scholar]
- 5.Sigurdsson EM, Knudsen E, Asuni A, et al. An attenuated immune response is sufficient to enhance cognition in an Alzheimer’s disease mouse model immunized with amyloid-beta derivatives. J Neurosci. 2004;24(28):6277–82. doi: 10.1523/JNEUROSCI.1344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scholtzova H, Wisniewski T, Ahlawat S, et al. Safety of potential vaccines for Alzheimer’s disease [Abstract] Soc Neurosci Abstr. 2002;227:1. [Google Scholar]
- 7.Asuni AA, Boutajangout A, Scholtzova H, et al. Vaccination of Alzheimer’s model mice with Abeta derivative in alum adjuvant reduces Abeta burden without microhemorrhages. Eur J Neurosci. 2006;24(9):2530–42. doi: 10.1111/j.1460-9568.2006.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vermes LM. Cerebrospinal fluid proteins: III. Normal values of immunoglobulins G, A and M (variations related to race, sex and age) Arq Neuropsiquiatr. 1983;41(1):25–49. doi: 10.1590/s0004-282x1983000100003. [DOI] [PubMed] [Google Scholar]
- 9.Nerenberg ST, Prasad R. Radioimmunoassays for Ig classes G, A, M, D, and E in spinal fluids: normal values of different age groups. J Lab Clin Med. 1975;86(5):887–98. [PubMed] [Google Scholar]
- 10.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-Abeta antibody alters CNS and plasma Abeta clearance and decreases brain Abeta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98:8850–5. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sigurdsson EM. Immunotherapy for conformational diseases. Curr Pharm Des. 2006;12(20):2569–85. doi: 10.2174/138161206777698837. [DOI] [PubMed] [Google Scholar]
- 12.Wisniewski T, Sigurdsson EM. Therapeutic approaches for prion and Alzheimer’s diseases 2. FEBS J. 2007;274(15):3784–98. doi: 10.1111/j.1742-4658.2007.05919.x. [DOI] [PubMed] [Google Scholar]
- 13.Lemere CA, Beierschmitt A, Iglesias M, et al. Alzheimer’s disease abeta vaccine reduces central nervous system abeta levels in a non-human primate, the Caribbean vervet. Am J Pathol. 2004;165(1):283–97. doi: 10.1016/s0002-9440(10)63296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandy S, DeMattos RB, Lemere CA, et al. Alzheimer A beta vaccination of rhesus monkeys (Macaca mulatta) Alzheimer Dis Assoc Disord. 2004;18(1):44–6. doi: 10.1097/00002093-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Head E, Pop V, Vasilevko V, et al. A two-year study with fibrillar beta-amyloid (A beta) immunization in aged canines: effects on cognitive function and brain A beta 1. J Neurosci. 2008;28(14):3555–66. doi: 10.1523/JNEUROSCI.0208-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bons N, Mestre N, Petter A. Senile plaques and neurofibrillary changes in the brain of an aged lemurian primate Microcebus murinus. Neurobiol Aging. 1992;13(1):99–105. doi: 10.1016/0197-4580(92)90016-q. [DOI] [PubMed] [Google Scholar]
- 17.Mestre-Frances N, Keller E, Calenda A, Barelli H, Checler F, Bons N. Immunohistochemical analysis of cerebral cortical and vascular lesions in the primate Microcebus murinus reveal distinct amyloid beta1-42 and beta1-40 immunoreactivity profiles. Neurobiol Dis. 2000;7(1):1–8. doi: 10.1006/nbdi.1999.0270. [DOI] [PubMed] [Google Scholar]
- 18.Giannakopoulos P, Silhol S, Jallageas V, et al. Quantitative analysis of tau protein-immunoreactive accumulations and beta amyloid protein deposits in the cerebral cortex of the mouse lemur Microcebus murinus. Acta Neuropathol (Berl) 1997;94(2):131–9. doi: 10.1007/s004010050684. [DOI] [PubMed] [Google Scholar]
- 19.Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27(34):9115–29. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sigurdsson EM, Quartermain D, Boutajangout A. Tau immunotherapy prevents cognitive decline and clears pathological tau in a tangle mouse model [Abstract] Alzheimer’s Demen. 2008;4(4):T191–2. [Google Scholar]
- 21.Roder HM, Hutton ML. Microtubule-associated protein tau as a therapeutic target in neurodegenerative disease. Expert Opin Ther Targets. 2007;11(4):435–42. doi: 10.1517/14728222.11.4.435. [DOI] [PubMed] [Google Scholar]
- 22.Sigurdsson EM. Histological staining of amyloid-beta in mouse brains. Methods Mol Biol. 2005;299:299–308. doi: 10.1385/1-59259-874-9:299. [DOI] [PubMed] [Google Scholar]
- 23.Sigurdsson EM, Lorens SA, Hejna MJ, Dong XW, Lee JM. Local and distant histopathological effects of unilateral amyloid-beta 25-35 injections into the amygdala of young F344 rats. Neurobiol Aging. 1996;17(6):893–901. doi: 10.1016/s0197-4580(96)00169-8. [DOI] [PubMed] [Google Scholar]
- 24.Head E, Barrett EG, Murphy MP, et al. Immunization with fibrillar A beta(1-42) in young and aged canines: antibody generation and characteristics, and effects on CSF and brain A beta 6. Vaccine. 2006;24(15):2824–34. doi: 10.1016/j.vaccine.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 25.Hyman BT, Smith C, Buldyrev I, et al. Autoantibodies to amyloid-beta and Alzheimer’s disease. Ann Neurol. 2001;49(6):808–10. doi: 10.1002/ana.1061. [DOI] [PubMed] [Google Scholar]
- 26.Szabo P, Relkin N, Weksler ME. Natural human antibodies to amyloid beta peptide 3. Autoimmun Rev. 2008;7(6):415–20. doi: 10.1016/j.autrev.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Neff F, Wei X, Nolker C, Bacher M, Du YS, Dodel R. Immunotherapy and naturally occurring autoantibodies in neurodegenerative disorders 2. Autoimmun Rev. 2008;7(6):501–7. doi: 10.1016/j.autrev.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Vincent RP, Ashrafian H, le Roux CW. Mechanisms of disease: the role of gastrointestinal hormones in appetite and obesity 2. Nat Clin Practice Gastroenterol Hepatol. 2008;5(5):268–77. doi: 10.1038/ncpgasthep1118. [DOI] [PubMed] [Google Scholar]
- 29.Leverone JF, Spooner ET, Lehman HK, Clements JD, Lemere CA. Abeta1-15 is less immunogenic than Abeta1-40/42 for intranasal immunization of wild-type mice but may be effective for “boosting”. Vaccine. 2003;21(17–18):2197–206. doi: 10.1016/s0264-410x(02)00754-5. [DOI] [PubMed] [Google Scholar]
- 30.Hara H, Monsonego A, Yuasa K, et al. Development of a safe oral Abeta vaccine using recombinant adeno-associated virus vector for Alzheimer’s disease. J Alzheimers Dis. 2004;6(5):483–8. doi: 10.3233/jad-2004-6504. [DOI] [PubMed] [Google Scholar]
- 31.Seabrook TJ, Thomas K, Jiang L, et al. Dendrimeric Abeta1-15 is an effective immunogen in wildtype and APP-tg mice. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Seabrook TJ, Jiang L, Thomas KE, Lemere CA. Boosting with intranasal dendrimeric Abeta1-15 but not Abeta1-15 peptide leads to an effective immune response following a single injection of Abeta1-40/42 in APP-tg mice. J Neuroinflammation. 2006;3(1):14. doi: 10.1186/1742-2094-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frenkel D, Dewachter I, Van Leuven F, Solomon B. Reduction of beta-amyloid plaques in brain of transgenic mouse model of Alzheimer’s disease by EFRH-phage immunization. Vaccine. 2003;21(11–12):1060–5. doi: 10.1016/s0264-410x(02)00609-6. [DOI] [PubMed] [Google Scholar]
- 34.Lavie V, Becker M, Cohen-Kupiec R, et al. EFRH-phage immunization of Alzheimer’s disease animal model improves behavioral performance in Morris water maze trials. J Mol Neurosci. 2004;24(1):105–13. doi: 10.1385/JMN:24:1:105. [DOI] [PubMed] [Google Scholar]
- 35.Agadjanyan MG, Ghochikyan A, Petrushina I, et al. Prototype Alzheimer’s disease vaccine using the immunodominant B cell epitope from beta-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J Immunol. 2005;174(3):1580–6. doi: 10.4049/jimmunol.174.3.1580. [DOI] [PubMed] [Google Scholar]
- 36.Maier M, Seabrook TJ, Lazo ND, et al. Short amyloid-beta (A beta) immunogens reduce cerebral A beta load and learning deficits in an Alzheimer’s disease mouse model in the absence of an A beta-specific cellular immune response. J Neurosci. 2006;26(18):4717–28. doi: 10.1523/JNEUROSCI.0381-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou J, Fonseca MI, Kayed R, et al. Novel Abeta peptide immunogens modulate plaque pathology and inflammation in a murine model of Alzheimer’s disease. J Neuroinflammation. 2005;2:28. doi: 10.1186/1742-2094-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fagan T. Trial Troika—Immunotherapy Interrupted, Lipitor Lags, Dimebon Delivers. [accessed April 28, 2008];Alzheimer Research Forum. 2008 April 25; http://www.alzforum.org/new/detail.asp?id=1807.