Introduction
Circadian rhythms are endogenously generated rhythms with a period length of about 24-hrs. Evidence gathered over the past decade indicates that the circadian timing system develops prenatally and the suprachiasmatic nuclei, the site of a circadian clock, are present by mid-gestation in primates. Recent evidence also shows that the circadian system of primate infants is responsive to light at very premature stages and that low intensity lighting can regulate the developing clock. After birth, there is progressive maturation of the circadian system outputs, with pronounced rhythms in sleep-wake and hormone secretion generally developing after two months of age. Showing the importance of photic regulation of circadian phase in infants, exposure of premature infants to low-intensity cycled lighting results in the early establishment of rest-activity patterns that are in phase with the 24-hour light-dark cycle. With the continued elucidation of circadian system development and influences on human physiology and illness, it is anticipated that consideration of circadian biology will become an increasingly important component of neonatal care.
The Circadian Timing System
Circadian rhythms are endogenously driven rhythms with a period length of about 24-hrs1-3. Notable examples of circadian rhythms include the sleep-wake cycle and daily rhythms in hormone production. Circadian rhythms are also involved in the pathogenesis of illnesses, such as reactive airway disease and myocardial infarction3-7.
The system responsible for the generation and regulation of circadian rhythms is the circadian timing system. This neural system consists of a biological clock, input pathways, and output pathways1. The paired suprachiasmatic nuclei (SCN) in the anterior hypothalamus are the site of a biological clock. The SCN are located above the optic chiasm at the base of the third ventricle8. The SCN exhibit endogenous rhythmicity and have a period of oscillation close to 24-hrs. Peripheral clocks also play a role in circadian rhythm expression9-11.
Lesion studies in rodents provided the initial evidence that the SCN are the site of a circadian pacemaker8. In vivo and in vitro studies have since shown day-night rhythms in electrical activity, metabolic activity, and gene expression. Transplantation of fetal SCN cells into SCN-lesioned animals restores rhythmicity to the recipient further supporting that the SCN contain a biological clock8. Circadian oscillations have been seen in individual rodent SCN cells, and expressed rhythmicity reflects the collective oscillations of many SCN cells12, 13.
Because SCN oscillations are not exactly 24-hrs, it is necessary to reset the circadian pacemaker each day to prevent endogenous clock oscillations from drifting (or free-running) out of phase with the external light-dark cycle. Input pathways relay photic information from the retina to the SCN to synchronize (or entrain) the oscillations of the clock to the 24-hr light-dark cycle14, 15. A direct pathway from the retina to the SCN, the retinohypothalamic tract (RHT), has been shown to be both necessary and sufficient for photic entrainment14. The raphe nucleus also influences SCN function via serotinergic projections14.
Output pathways are responsible for the overt expression of circadian rhythms. Several discrete neural pathways projecting from the SCN to several hypothalamic and non hypothalamic sites have been defined16-18. Via these pathways, the circadian system acts to broadly influence neural physiology. Output pathways of the circadian system also regulate the rhythmic production of several hormones including melatonin and cortisol4, 5, 16-18.
The Primate Circadian System
Several lines of evidence support that the paired SCN are the site of a biological clock in primates. Similar to rodents, the primate SCN are located above the optic chiasm at the base of the third ventricle19. In contrast to rodents, human SCN cells are not densely clustered making the nuclei less visually apparent19-21. However, using probes for melatonin receptors and SCN peptides, the human SCN can be identified19-21. Using DG, day-night oscillations in SCN metabolic activity have been detected in squirrel monkeys and baboons 22-24.
Lesion studies performed in the early 1980s suggested the presence of a circadian pacemaker outside of the SCN in monkeys25. However, analysis of these reports revealed that either the completeness of the lesions was not verified, or monkeys were not studied in constant conditions26. Reexamination of this issue challenges the existence of primate circadian pacemakers outside the SCN. Squirrel monkeys with total SCN lesions show a complete absence of circadian rhythmicity when animals are monitored in constant conditions26. Supporting that the SCN are the site of a circadian pacemaker in humans, tumors and congenital lesions in the SCN region result in the loss of temperature rhythms and organized sleep-wake patterns27, 28.
The RHT has been anatomically characterized in prosimian (lemurs, shrews) and simian (squirrel monkeys, rhesus macaques, baboons, chimpanzees and apes) species19. This tract also has been identified in studies of postmortem human specimens using techniques that label degenerating retinal axons29, 30. Although it was suggested that cutaneous light exposure can influence circadian function31, there is little support for the notion that there is extraretinal photoreception in mammals32-34. Furthermore, other investigators have failed to reproduce phase shifting effects of cutaneous light exposure22.
Outputs of the primate circadian system have been widely characterized in human clinical studies. Many day-night rhythms have been documented4, 5, 33. Several of these rhythms have been shown to persist in constant conditions indicating that they are true endogenously generated circadian rhythms. Notable examples of circadian rhythms include the sleep-wake cycle, daily rhythms in body temperature, and day-night rhythms in cortisol and melatonin production4, 5, 33. Day-night differences in gonadotropin, testosterone, growth hormone and thyrotropin secretion are also present35, 36.
Development of the Primate SCN
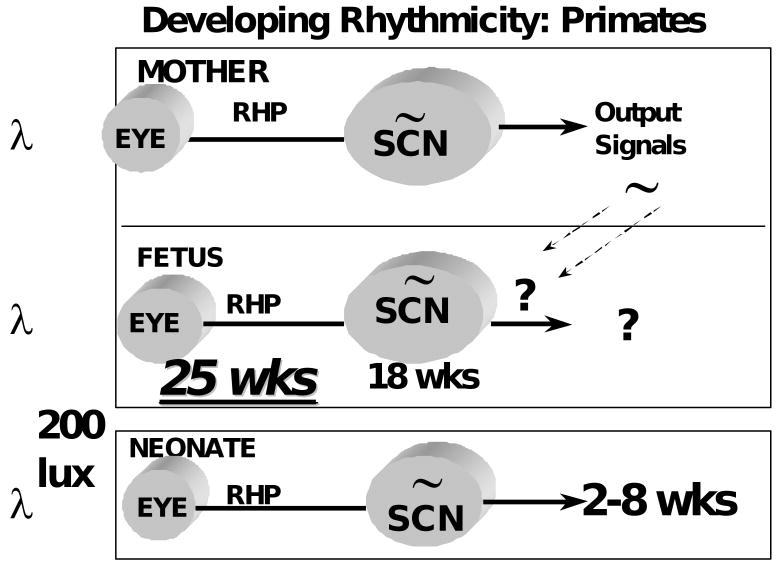
Although rodent studies have led to our understanding of developmental circadian physiology35-38, notable differences between rodents and primates have prevented the extension of rodent data to clinical care. In general, rodents are more immature at birth than humans. Differences in the sensitivity to light and other aspects of circadian physiology between humans and rodents also have been observed. However, based on evidence gathered over the past decade, it appears that the circadian clock in the SCN forms and begins oscillating in utero in primates.
In squirrel monkeys, SCN neurogenesis occurs early in gestation over days 27-4839. Because monkey and human embryonic development are very similar over the first 100 days of gestation40, it is therefore likely that the human SCN neurons form early in gestation.
It is not currently known when the primate SCN are first apparent morphologically. Yet, using [125I]melatonin and [125I]SKF38393 to label the nuclei, the human SCN have been detected at gestation week 18 41, 42 (Figure 1).
Figure 1.
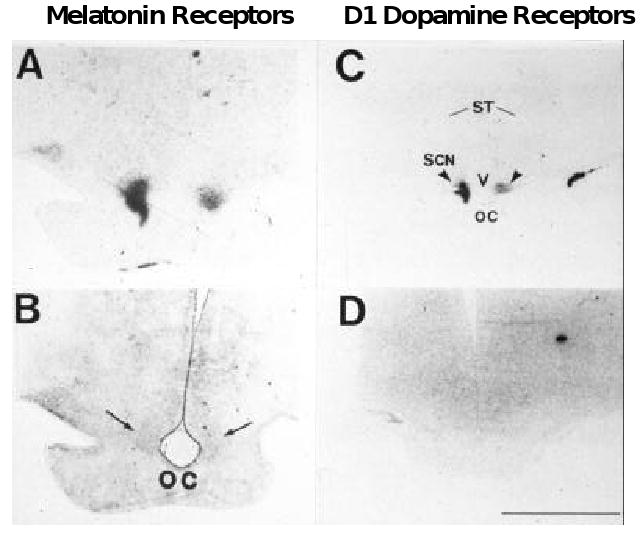
A. Localization of [125I]melatonin binding to the SCN of an 18-week gestation human fetus. Specific labeling is shown in black. B. The stained section used to generate the autoradiograph in A. Reproduced by permission from ref 33. C. Localization of [125I]SKF38393 binding to D1 dopamine receptors in the SCN of a 20-week post conceptual human infant. Specific labeling is shown in black. D. Non-specific labeling. Reproduced by permission from ref 34. OC, optic chiasm; ST, striatum. Arrows identify the SCN.
Functional studies suggest that the primate SCN oscillate prenatally. Studies of squirrel monkeys reveal day-night differences in SCN metabolic activity at the end of gestation43. It is not known if SCN oscillations are present at earlier ages. The physiologic processes influenced by the fetal clock have yet to be elucidated in primates.
Similar to rodents, the timing of the onset of labor and birth in humans is influenced by the circadian cycle with peak incidences between midnight and the early morning44. However, we do not know if the fetal clock plays a role in the circadian gating of birth in humans.
Immunocytochemistry studies show that SCN maturation continues after birth45. The SCN contain distinct populations of neurons that express arginine vasopressin or vasoactive intestinal polypeptide45. In term infants, the number of vasopressinergic neurons is 20% of the number present in adults45. It is not until one year of age that infants and adults have comparable vasopressin neuron numbers45. The number of vasoactive intestinal polypeptide containing SCN cells also increase after birth45.
Development of Primate Photic Entrainment
A critical issue in knowing if environmental cycles need to be considered in the care of infants is knowing when the primate circadian system becomes functionally responsive to light. The RHT has been identified in a 36 week gestation human newborn46. However, because of human study limitations, has not been possible to determine if the circadian clock of human infants is functionally responsive to light at birth.
Non-invasive methods used to examine regional changes in brain activity, such as function magnetic resonance (fMRI) imaging or positron emission tomography (PET), hold promise in being able to directly examine SCN function. In human adults, we have been able to observe acute increases in SCN metabolic activity after light exposure at night using 18F-DG in PET studies47. However, because of the small size of the SCN, consistent visualization of SCN activity is difficult to achieve and these methods have not been applied to infants.
Because of human study limitations, we have studied baboons, which are excellent models for human infants, to provide insights into the developing human clock. By monitoring changes in SCN metabolic activity and gene expression (Figure 2), light responsiveness can be demonstrated at birth in term baboon infants24. The presence of the RHT can also be demonstrated24.
Figure 2.
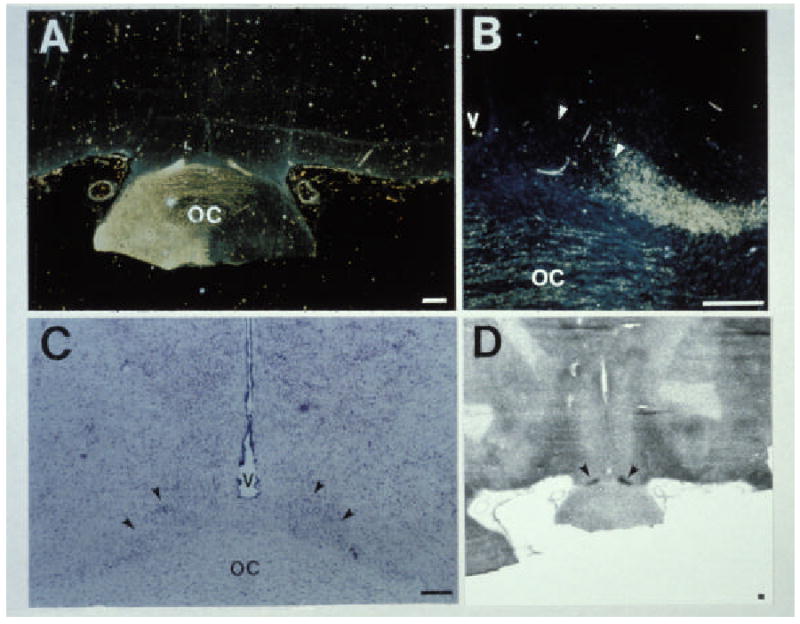
Innervation of the SCN by the retinohypothalamic tract (RHT) in a newborn baboon infant. A. Low-power image showing labeling of retinal fibers in the optic chiasm by horseradish peroxidase. B. Adjacent tissue section showing the location of the SCN. C. High power image showing projections of the RHT into the right SCN. D. Autoradiographic image generated from [14C]2-deoxyglocose uptake studies showing that light exposure at night induces increases in SCN metabolic activity. Areas of increased uptake are dark. Arrows identify the SCN. Scale bar = 5 mm. Reproduced by permission from ref 16.
By monitoring the effects of different lighting conditions on newborn baboon activity patterns, we have been able to show that newborn baboons are entrained by low intensity (200 lux) lighting24. These findings are similar to those seen in human adults showing that circadian phase can be regulated by low intensity (ca. 180 lux) lighting48, 49. Thus, it is likely that low intensity lighting, similar to that found indoors, can regulate the developing primate clock.
To determine when photic responsiveness first occurs in primates, we have studied premature baboon infants50. To our surprise, we find that the SCN are functionally innervated by the retina at stages equivalent to 25 wks post-conception human infants 50 (Figure 3).
Figure 3.
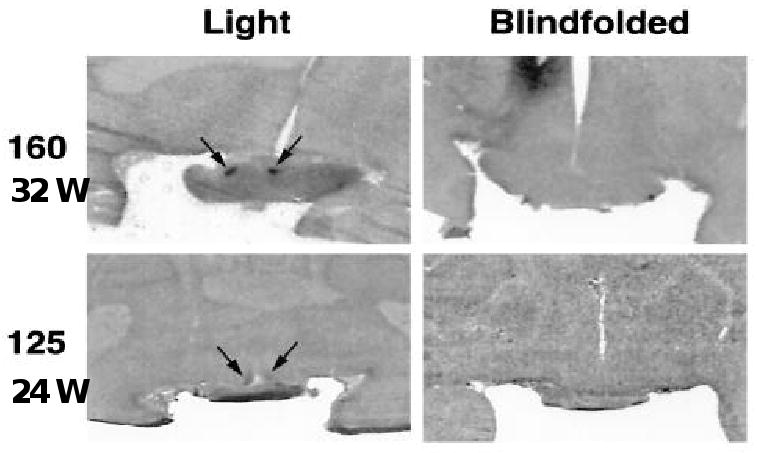
Autoradiograph images of preterm baboon brain sections showing SCN DG uptake after light exposure at night. Animals shown were PC 160 or 125 and were either blindfolded or directly exposed to light. The images are obtained from mid-SCN levels. Arrows identify the SCN image. Reproduced by permission from ref 42.
The primate circadian system is therefore sensitive to light in very premature infants when postnatal survival with intensive support becomes possible.
Development of Expressed Rhtymicity
The development of expressed rhythmicity has received attention in both human and nonhuman primates. During pregnancy, day-night rhythms are observed for a variety of hormones (esterone and progesterone) and physiological parameters (uterine contractility) in mothers51, 52. In human fetuses, day-night rhythms in heart rate, respiratory rate, and adrenal steroidogenesis have been detected51, 52. However, these rhythms appear to be driven by the mother.
When term human infants are examined, day-night rhythms are difficult to detect in the neonatal period47, 53-58. Consolidated periods of activity and rest are not generally observed until after the first or second month of life. Activity plots of human newborns reveal that sleep is generally distributed over the 24-hr day during the first few weeks of life (FIGURE 3). At 6 wks of age, infants are awake more during the daytime than at night. By 12 wks of age, daytime sleep duration decreases further and much more sleep occurs at night. Importantly, although consolidated periods of rest and activity are not apparent until more than one to two months after birth, day-night differences in activity can be detected as early as one week of age in some babies.
At the age when day-night differences in infant activity become clearly apparent, day-night rhythms in hormone production are observed. Day-night rhythms in melatonin production can be detected at 12 weeks of age59, 60. Circadian variation in cortisol levels appears between after 3-6 months of age61-63. With advancing age, circadian rhythms have been detected for a variety of other hormones and circulating factors64.
Because infant care influences activity patterns, it is possible that patterns of developing circadian rhythmicity in human infants reflects influences of caregivers rather than endogenous rhythmicity. Thus, to characterize the development of expressed rhythmicity in primates, we have examined the development of expressed rhythmicity in newborn baboons raised in constant conditions (continuous dim lighting, evenly spaced care)24. Similar to human infants, baboon infants do not manifest clear day-night differences in activity patterns in the early neonatal period (Figure 4). Yet, at one month of age, day-night differences in activity patterns are observed. Developing primate rhythmicity thus reflects maturation from a state of relative arrhythmicity to rhythmicity over the first few months of life.
Figure 4.
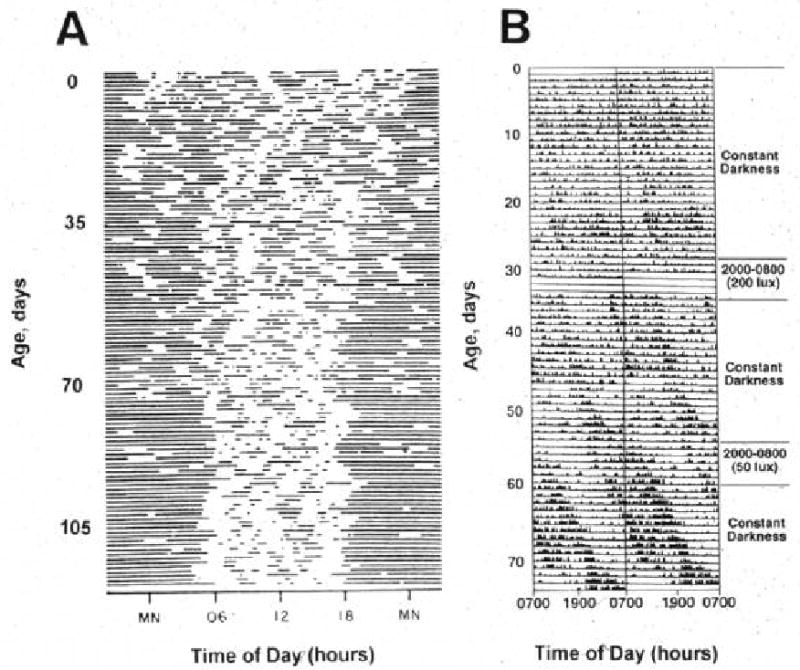
Rest-activity patterns of a newborn human (A) and a baboon (B) infant (right). Double-plotted actograms are shown. In A, dark bars represent sleep. In B, dark bars represent activity. Please note that the circadian phase of the baboons infant was shifted by exposure to a 200 lux reversed light-dark cycle at 30 days of age, and much less so by 50 lux of exposure at 55 days of age. Reproduced by permission from ref 16.
As in rodents, it appears that infant circadian phase is synchronous with that of the mother in baboon and human infants. However, in some humans and baboons24, 65, infant phase may be out of synchrony with that of the mother at birth. Thus, whereas there is maternal-infant synchrony of circadian phase in most primates, it may not be universal.
Rhtymicity in Premature Infants
The large number of premature infants hospitalized for extended periods has greatly facilitated studies of rhythmicity in preterm infants. Over the past decade, studies of patterns of infant activity, heart rate, temperature, and sleep state have not surprisingly flourished56-58, 66. Several of these studies have revealed the presence of ultradian rhythms (rhythms with period lengths of much less than 24 hrs). Endogenously driven circadian rhythms, however, are not clearly apparent.
When temperature and heart rate are studied beginning at a postconceptual (PC) age 24-29, circadian rhythmicity is generally not apparent even at 17 weeks after birth67. Studies of preterm infants at PC 32 weeks, have failed to detect day-night differences in sleep patterns whereas some differences are noted in term infants68. Analysis of temperature, heart rate, and activity patterns at PC 35 weeks have revealed ultradian rhythms, but no clear cut circadian rhythms68-70. Because feeding and physical contact influence infant temperature, heart rates and activity patterns, it is likely that infant care schedules drive the ultradian rhythms seen in preterm infants. These interventions may also mask the detection of circadian rhythms.
The Yale Neonatal Entrainment Study
Following the discovery that the primate circadian clock is responsive to light in very premature infants, we next assessed the effects of photic entrainment on premature infants 71. In these studies, the development of rest-activity patterns was examined in human preterm infants exposed to continuous dim lighting or low-intensity cycled lighting before discharge from hospital to home.
In general, day/night differences in rest and activity are not apparent in hospitalized control infants (Figure 5), whereas day/night differences in rest and activity are seen in experimental infants. Over the first ten days at home, distinct day/night differences in activity are not seen in controls, but experimental infants are more active during the day than at night. It was not until 21-30 days after discharge that day/night activity ratios in control infants match those seen in experimental infants shortly after discharge. Yet, even at this age, experimental infants are considerably more active during the day as compared to control infants. Despite the differences in rest-activity patterns among groups, no differences in weight gain or change in head circumference are seen.
Figure 5.
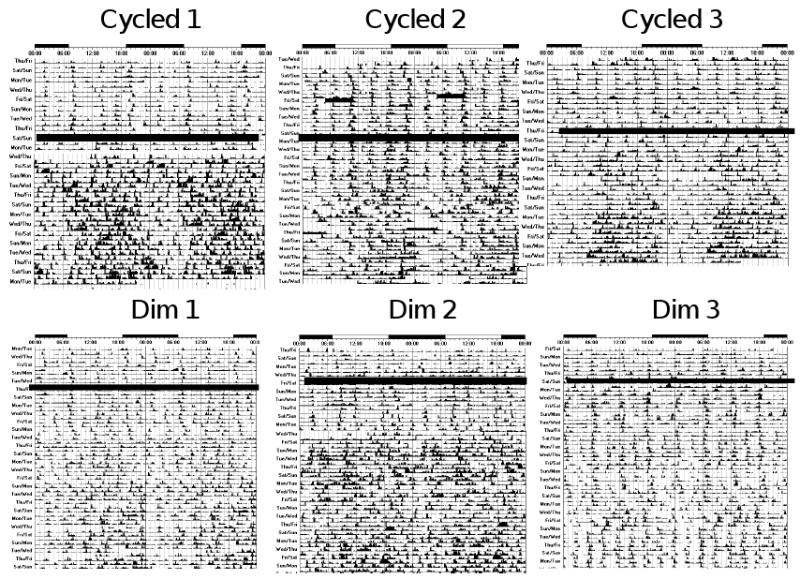
Actograms of rest-activity in representative infants exposed to cycled lighting in the (top panels) or constant dim light (bottom panels). Dark bars represent activity; the same activity scale is used in each plot. The time of day is shown on top. The thick dark line in middle of plots depicts the date of discharge. Note that distinct patterns of rest and activity in the infants are more apparent after discharge in infants exposed to cycled lighting than dim lighting before discharge from the hospital. In infants exposed to dim lighting, day-night differences in rest-activity patterns in synchrony with the light-dark cycle are generally not apparent until about 20 days after discharge from the hospital.
These observations show that exposure to low-intensity cycled lighting for 10 days before discharge induces distinct patterns of rest/activity in preterm infants that are in synchrony with the light-dark cycle that they will encounter at home. These effects are even more pronounced as soon as the child is discharged to home. In contrast, the appearance of rest/activity patterns in synchrony with the solar light-dark cycle is delayed in infants that have been exposed to continuous dim lighting in the hospital.
Other Studies of Lighting and Infants
Potential influences of cycled lighting on premature infants have been the subject of a few previous studies. In the Stanford Cycled Lighting Study, differences in circadian rhythms in temperature were not detected among infants exposed to either continuous dim lighting or cycled lighting before discharge 69, 70, 72. These infants were studied 1 and 3 months after discharge. Because we observe that infants in both groups manifest similar circadian phase by 30 days of age, treatment effects on the rhythm of core body temperature may no longer be distinct after one month of age.
Other investigators have suggested that exposing infants to light/dark cycles improves infant weight gain. Mann and co-workers found that exposure to light/dark cycles before discharge resulted in better weight gain and more sleep over the 24-hour day than did chaotic lighting patterns73. These effects were seen 6 weeks after discharge and not sooner73. Because of this lag period, it has been suggested that the observed effects were not a direct result of cycled lighting exposure on the infant72. More recently, it has been suggested that exposing infants to light/dark cycles improves the in-hospital growth of babies if exposure occurs before 36 weeks of age74. Yet, the infants in near-darkness group in this study appeared more ill than the other groups. Considering that it is difficult to detect circadian activity in premature infants47, 75, the potential mechanisms by which lighting could directly influence the growth of premature infants is not clear. By studying infants that were closely matched at enrollment, we failed to observe influences of lighting on growth either in-hospital or at home.
Previous studies have suggested that day/night rhythmicity is not apparent in prematurely born babies until nearly one month after term-birth age equivalency is reached (>42 weeks postmenstrual age)69, 70, 72, 76. These conclusions have been based on 24-48 hour assessments of rectal temperature and/or sleep patterns. However, using actigraphy to continuously monitor rest-activity patterns, we find that circadian phase can be detected in infants exposed to cycled lighting as early as a postmenstrual age of 34 weeks. In our previous studies of non-human primate infants reared in constant conditions, we also found that day/night differences in rest and activity were apparent shortly after term birth24. Most importantly, we find that day/night differences in activity could be detected several weeks before it was possible to detect circadian rhythms in core temperature using internal telemetry devices24. Thus, analysis of rest activity patterns may provide the earliest index of developing circadian rhythmicity in infants.
Nursery Lighinting Practices
The practice of nursery lighting has changed over the past several decades without a clear basis. Cycled lighting was often used in the hospital nurseries in the fifties and sixties. Yet, continuous bright lighting became favored when isolettes and neonatal intensive care units were introduced in the seventies. In reaction to continuous bright light, continuous dim light was introduced in the eighties and nineties, along with covering infant isolettes with blankets and quilts.
Although continuous dim lighting is the current practice in most nurseries in the United States, the scientific basis for this practice is not clear77. It has been suggested that ambient lighting may contribute to eye disease in premature infants78. Yet, rigorous clinical studies have failed to show adverse effects of low intensity lighting on premature infants79-81.
Investigators who propose a NIDCAP (Neonatal Individualized Developmental Care Assessment Program) have suggested that since the womb is dark, infants should be dark-reared82. This approach overlooks the fact that prenatally the infant is exposed to maternal time-of-day cues that synchronize the fetal clock with the external light/dark cycle37. Rearing premature infants in the dark thus deprives babies of the time-of-day information that they would have received during full gestation. Data also show that the NIDCAP approach does not improve developmental outcome or sleep of premature infants83, 84. Thus, a rational approach considering the importance of circadian rhythmicity and environmental lighting cycles is needed in the care of hospitalized infants.
Summary
Increasing evidence indicates that the circadian timing system is a fundamental homeostatic system that potently influences human behavior and physiology throughout development (Figure 6).
Figure 6.
Schematic representation of primate circadian system development based on studies of non-human primates. Estimated human ages are given.
After birth there is progressive maturation of the circadian system with day-night rhythms in activity and hormone secretion developing between one and three months of age. Recent evidence shows that the circadian system of primate infants is responsive to light at very premature stages and that low intensity lighting can regulate the developing clock. With the continued elucidation of circadian system development and influences on human physiology and illness, it is anticipated that consideration of circadian biology will become an increasingly important component of neonatal care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Panda S, Hogenesch JB, Kay SA. Circadian rhythms from flies to human. Nature. 2002;417(6886):329–35. doi: 10.1038/417329a. [DOI] [PubMed] [Google Scholar]
- 2.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(2):R271–7. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 3.Maywood ES, O'Neill J, Wong GK, Reddy AB, Hastings MH. Circadian timing in health and disease. Prog Brain Res. 2006;153:253–69. doi: 10.1016/S0079-6123(06)53015-8. [DOI] [PubMed] [Google Scholar]
- 4.Moore-Ede MC, Czeisler CA, Richardson GS. Circadian timekeeping in health and disease. Part 1. Basic properties of circadian pacemakers. NEnglJMed. 1983;309(8):469–76. doi: 10.1056/NEJM198308253090806. [DOI] [PubMed] [Google Scholar]
- 5.Moore-Ede MC, Czeisler CA, Richardson GS. Circadian timekeeping in health and disease. Part 2. Clinical implications of circadian rhythmicity. NEnglJMed. 1983;309(9):530–6. doi: 10.1056/NEJM198309013090905. [DOI] [PubMed] [Google Scholar]
- 6.Arendt J. Melatonin and human rhythms. Chronobiol Int. 2006;23(12):21–37. doi: 10.1080/07420520500464361. [DOI] [PubMed] [Google Scholar]
- 7.Tofler GH, Muller JE. Triggering of acute cardiovascular disease and potential preventive strategies. Circulation. 2006;114(17):1863–72. doi: 10.1161/CIRCULATIONAHA.105.596189. [DOI] [PubMed] [Google Scholar]
- 8.Weaver DR. The suprachiasmatic nucleus: a 25-year retrospective. J Biol Rhythms. 1998;13(2):100–12. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- 9.Ebisawa T. Circadian Rhythms in the CNS and Peripheral Clock Disorders: Human Sleep Disorders and Clock Genes. J Pharmacol Sci. 2007 doi: 10.1254/jphs.fmj06003x5. [DOI] [PubMed] [Google Scholar]
- 10.Maemura K, Takeda N, Nagai R. Circadian Rhythms in the CNS and Peripheral Clock Disorders: Role of the Biological Clock in Cardiovascular Diseases. J Pharmacol Sci. 2007 doi: 10.1254/jphs.fmj06003x2. [DOI] [PubMed] [Google Scholar]
- 11.Ohdo S. Circadian Rhythms in the CNS and Peripheral Clock Disorders: Chronopharmacological Findings on Antitumor Drugs. J Pharmacol Sci. 2007 doi: 10.1254/jphs.fmj06003x6. [DOI] [PubMed] [Google Scholar]
- 12.Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14(4):697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 13.Liu C, Weaver DR, Strogatz SH, Reppert SM. Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell. 1997;91(6):855–60. doi: 10.1016/s0092-8674(00)80473-0. [DOI] [PubMed] [Google Scholar]
- 14.Morin LP. The circadian visual system. Brain ResBrain ResRev. 1994;19(1):102–27. doi: 10.1016/0165-0173(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 15.Morin LP, Allen CN. The circadian visual system, 2005. Brain Res Brain Res Rev. 2006;51(1):1–60. doi: 10.1016/j.brainresrev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Kornhauser JM, Mayo KE, Takahashi JS. Light, immediate-early genes, and circadian rhythms. Behav Genet. 1996;26(3):221–40. doi: 10.1007/BF02359382. [DOI] [PubMed] [Google Scholar]
- 17.Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. JCompNeurol. 1987;258(2):204–29. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- 18.Watts AG, Swanson LW. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. JCompNeurol. 1987;258(2):230–52. doi: 10.1002/cne.902580205. [DOI] [PubMed] [Google Scholar]
- 19.Moore RY. Organization of the primate circadian system. JBiolRhythms. 1993;8(Suppl):S3–9. S3–9. [PubMed] [Google Scholar]
- 20.Lydic R, Schoene WC, Czeisler CA, Moore-Ede MC. Suprachiasmatic region of the human hypothalamus: homolog to the primate circadian pacemaker? Sleep. 1980;2(3):355–61. doi: 10.1093/sleep/2.3.355. [DOI] [PubMed] [Google Scholar]
- 21.Lydic R, Albers HE, Tepper B, Moore-Ede MC. Three-dimensional structure of the mammalian suprachiasmatic nuclei: a comparative study of five species. JCompNeurol. 1982;204(3):225–37. doi: 10.1002/cne.902040303. [DOI] [PubMed] [Google Scholar]
- 22.Lushington K, Galka R, Sassi LN, Kennaway DJ, Dawson D. Extraocular light exposure does not phase shift saliva melatonin rhythms in sleeping subjects. J Biol Rhythms. 2002;17(4):377–86. doi: 10.1177/074873002129002582. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz WJ, Reppert SM, Eagan SM, Moore-Ede MC. In vivo metabolic activity of the suprachiasmatic nuclei: a comparative study. Brain Res. 1983;274(1):184–7. doi: 10.1016/0006-8993(83)90538-3. [DOI] [PubMed] [Google Scholar]
- 24.Rivkees SA, Hofman PL, Fortman J. Newborn primate infants are entrained by low intensity lighting. ProcNatlAcadSciUSA. 1997;94(1):292–7. doi: 10.1073/pnas.94.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reppert SM, Perlow MJ, Ungerleider LG, et al. Effects of damage to the suprachiasmatic area of the anterior hypothalamus on the daily melatonin and cortisol rhythms in the rhesus monkey. J Neurosci. 1981;1(12):1414–25. doi: 10.1523/JNEUROSCI.01-12-01414.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J Neurosci. 1993;13(3):1065–79. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz WJ, Busis NA, Hedley-Whyte ET. A discrete lesion of ventral hypothalamus and optic chiasm that disturbed the daily temperature rhythm. JNeurol. 1986;233(1):1–4. doi: 10.1007/BF00313981. [DOI] [PubMed] [Google Scholar]
- 28.Rivkees S. Arrhythmicity in a child with septo-optic dysplasia and establishment of sleep-wake cyclicity with melatonin. J Pediatrics. 2001;139:463–5. doi: 10.1067/mpd.2001.117074. [DOI] [PubMed] [Google Scholar]
- 29.Friedman DI, Johnson JK, Chorsky RL, Stopa EG. Labeling of human retinohypothalamic tract with the carbocyanine dye, DiI. Brain Res. 1991;560(12):297–302. doi: 10.1016/0006-8993(91)91246-w. [DOI] [PubMed] [Google Scholar]
- 30.Sadun AA, Schaechter JD, Smith LE. A retinohypothalamic pathway in man: light mediation of circadian rhythms. Brain Res. 1984;302(2):371–7. doi: 10.1016/0006-8993(84)90252-x. [DOI] [PubMed] [Google Scholar]
- 31.Campbell SS, Murphy PJ. Extraocular circadian phototransduction in humans. Science. 1998;279(5349):396–9. doi: 10.1126/science.279.5349.396. [DOI] [PubMed] [Google Scholar]
- 32.Hebert M, Martin SK, Eastman CI. Nocturnal melatonin secretion is not suppressed by light exposure behind the knee in humans. Neurosci Lett. 1999;274(2):127–30. doi: 10.1016/s0304-3940(99)00685-0. [DOI] [PubMed] [Google Scholar]
- 33.Foster RG. Shedding light on the biological clock. Neuron. 1998;20(5):829–32. doi: 10.1016/s0896-6273(00)80464-x. [DOI] [PubMed] [Google Scholar]
- 34.Jean-Louis G, Kripke DF, Cole RJ, Elliot JA. No melatonin suppression by illumination of popliteal fossae or eyelids. J Biol Rhythms. 2000;15(3):265–9. doi: 10.1177/074873040001500307. [DOI] [PubMed] [Google Scholar]
- 35.Weitzman ED, Czeisler CA, Zimmerman JC, Moore-Ede MC. Biological rhythms in man: relationship of sleep-wake, cortisol, growth hormone, and temperature during temporal isolation. Adv Biochem Psychopharmacol. 1981;28:475–99. [PubMed] [Google Scholar]
- 36.Czeisler CA, Klerman EB. Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res. 1999;54:97–130. [PubMed] [Google Scholar]
- 37.Reppert SM, Weaver DR, Rivkees SA. Maternal communication of circadian phase to the developing mammal. Psychoneuroendocrinology. 1988;13(12):63–78. doi: 10.1016/0306-4530(88)90007-8. [DOI] [PubMed] [Google Scholar]
- 38.Reppert SM. Interaction between the circadian clocks of mother and fetus. CibaFoundSymp. 1995;183:198–207. doi: 10.1002/9780470514597.ch11. discuss:198-207; discussion - [DOI] [PubMed] [Google Scholar]
- 39.van Eerdenburg FJ, Rakic P. Early neurogenesis in the anterior hypothalamus of the rhesus monkey. Brain ResDevBrain Res. 1994;79(2):290–6. doi: 10.1016/0165-3806(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 40.Nowell-Morris L, Faherenbruch CE. Practical and evolutionary considerations for the use of nonhuman primate model in prenatal research. New York: Alan R. Liss; 1985. [Google Scholar]
- 41.Reppert SM, Weaver DR, Rivkees SA, Stopa EG. Putative melatonin receptors in a human biological clock. Science. 1988;242(4875):78–81. doi: 10.1126/science.2845576. [DOI] [PubMed] [Google Scholar]
- 42.Rivkees SA, Lachowicz JE. Functional D1 and D5 dopamine receptors are expressed in the suprachiasmatic, supraoptic, and paraventricular nuclei of primates. Synapse. 1997;26(1):1–10. doi: 10.1002/(SICI)1098-2396(199705)26:1<1::AID-SYN1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 43.Reppert SM, Schwartz WJ. Functional activity of the suprachiasmatic nuclei in the fetal primate. NeurosciLett. 1984;46(2):145–9. doi: 10.1016/0304-3940(84)90432-4. [DOI] [PubMed] [Google Scholar]
- 44.Jolly A. Hour of birth in primates and man. Folia Primatol. 1972;18(1):108–21. doi: 10.1159/000155472. [DOI] [PubMed] [Google Scholar]
- 45.Swaab DF. Development of the human hypothalamus. Neurochem Res. 1995;20(5):509–19. doi: 10.1007/BF01694533. [DOI] [PubMed] [Google Scholar]
- 46.Glotzbach SF, Sollars P, Pagano M. Development of the human retinohypothalamic tract. Soc Neurosci. 1992;18:857. [Google Scholar]
- 47.Rivkees SA, Hao H. Developing circadian rhythmicity. Semin Perinatol. 2000;24(4):232–42. doi: 10.1053/sper.2000.8598. [DOI] [PubMed] [Google Scholar]
- 48.Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Dose-response relationships for resetting of human circadian clock by light. Nature. 1996;379(6565):540–2. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- 49.Shanahan TL, Czeisler CA. Physiological effects of light on the human circadian pacemaker. Semin Perinatol. 2000;24(4):299–320. doi: 10.1053/sper.2000.9123. [DOI] [PubMed] [Google Scholar]
- 50.Hao H, Rivkees SA. The biological clock of very premature primate infants is responsive to light. Proc Natl Acad Sci U S A. 1999;96(5):2426–9. doi: 10.1073/pnas.96.5.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seron-Ferre M, Ducsay CA, Valenzuela GJ. Circadian rhythms during pregnancy. EndocrRev. 1993;14(5):594–609. doi: 10.1210/edrv-14-5-594. [DOI] [PubMed] [Google Scholar]
- 52.Seron-Ferre M, Torres-Farfan C, Forcelledo ML, Valenzuela GJ. The development of circadian rhythms in the fetus and neonate. Semin Perinatol. 2001;25(6):363–70. doi: 10.1053/sper.2001.29037. [DOI] [PubMed] [Google Scholar]
- 53.Parmelee AH., Jr Sleep cycles in infants. DevMedChild Neurol. 1969;11(6):794–5. doi: 10.1111/j.1469-8749.1969.tb01527.x. [DOI] [PubMed] [Google Scholar]
- 54.Meier-Koll A, Hall U, Hellwig U, Kott G, Meier-Koll V. A biological oscillator system and the development of sleep-waking behavior during early infancy. Chronobiologia. 1978;5(4):425–40. [PubMed] [Google Scholar]
- 55.Kleitman J, Engelman Sleep characteristics of infants. J Appl Physiolol. 1953;6:127–34. doi: 10.1152/jappl.1953.6.5.269. [DOI] [PubMed] [Google Scholar]
- 56.Darnall RA, Ariagno RL, Kinney HC. The late preterm infant and the control of breathing, sleep, and brainstem development: a review. Clin Perinatol. 2006;33(4):883–914. doi: 10.1016/j.clp.2006.10.004. abstract x. [DOI] [PubMed] [Google Scholar]
- 57.Jenni OG, Deboer T, Achermann P. Development of the 24-h rest-activity pattern in human infants. Infant Behav Dev. 2006;29(2):143–52. doi: 10.1016/j.infbeh.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 58.McKay SM, Angulo-Barroso RM. Longitudinal assessment of leg motor activity and sleep patterns in infants with and without Down syndrome. Infant Behav Dev. 2006;29(2):153–68. doi: 10.1016/j.infbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 59.Kennaway DJ, Stamp GE, Goble FC. Development of melatonin production in infants and the impact of prematurity. JClinEndocrinolMetab. 1992;75(2):367–9. doi: 10.1210/jcem.75.2.1639937. [DOI] [PubMed] [Google Scholar]
- 60.Kennaway DJ, Goble FC, Stamp GE. Factors influencing the development of melatonin rhythmicity in humans. JClinEndocrinolMetab. 1996;81(4):1525–32. doi: 10.1210/jcem.81.4.8636362. [DOI] [PubMed] [Google Scholar]
- 61.Beitins IZ, Kowarski A, Migeon CJ, Graham GG. Adrenal function in normal infants and in marasmus and kwashiorkor. Cortisol secretion, diurnal variation of plasma cortisol, and urinary excretion of 17-hydroxycorticoids, free corticoids, and cortisol. J Pediatr. 1975;86(2):302–8. doi: 10.1016/s0022-3476(75)80495-1. [DOI] [PubMed] [Google Scholar]
- 62.Onishi S, Miyazawa G, Nishimura Y, et al. Postnatal development of circadian rhythm in serum cortisol levels in children. Pediatrics. 1983;72(3):399–404. [PubMed] [Google Scholar]
- 63.Price DA, Close GC, Fielding BA. Age of appearance of circadian rhythm in salivary cortisol values in infancy. ArchDisChild. 1983;58(6):454–6. doi: 10.1136/adc.58.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hellbrugge T, Lange JE, Rutenfranz J. Circadian periodicity of physiological functions in different stages of infancy and childhood. Ann NY Acad Sci. 1964;117:361–73. doi: 10.1111/j.1749-6632.1964.tb48193.x. [DOI] [PubMed] [Google Scholar]
- 65.Parmelee AH, Jr, Wenner WH, Akiyama Y, Schultz M, Stern E. Sleep states in premature infants. DevMedChild Neurol. 1967;9(1):70–7. doi: 10.1111/j.1469-8749.1967.tb02212.x. [DOI] [PubMed] [Google Scholar]
- 66.Rivkees SA. Developing circadian rhythmicity. Basic and clinical aspects. PediatrClinNorth Am. 1997;44(2):467–87. doi: 10.1016/s0031-3955(05)70486-7. [DOI] [PubMed] [Google Scholar]
- 67.D'Souza SW, Tenreiro S, Minors D, Chiswick ML, Sims DG, Waterhouse J. Skin temperature and heart rate rhythms in infants of extreme prematurity. ArchDisChild. 1992;67(7):784–8. doi: 10.1136/adc.67.7_spec_no.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anders TF, Keener MA, Kraemer H. Sleep-wake state organization, neonatal assessment and development in premature infants during the first year of life. II. Sleep. 1985;8(3):193–206. doi: 10.1093/sleep/8.3.193. [DOI] [PubMed] [Google Scholar]
- 69.Glotzbach SF, Edgar DM, Boeddiker M, Ariagno RL. Biological rhythmicity in normal infants during the first 3 months of life. Pediatrics. 1994;94(4 Pt 1):482–8. [PubMed] [Google Scholar]
- 70.Glotzbach SF, Edgar DM, Ariagno RL. Biological rhythmicity in preterm infants prior to discharge from neonatal intensive care. Pediatrics. 1995;95(2):231–7. [PubMed] [Google Scholar]
- 71.Rivkees SA, Mayes L, Jacobs H, Gross I. Rest-activity patterns of premature infants are regulated by cycled lighting. Pediatrics. 2004;113 doi: 10.1542/peds.113.4.833. in press. [DOI] [PubMed] [Google Scholar]
- 72.Mirmiran M, Ariagno RL. Influence of light in the NICU on the development of circadian rhythms in preterm infants. Semin Perinatol. 2000;24(4):247–57. doi: 10.1053/sper.2000.8593. [DOI] [PubMed] [Google Scholar]
- 73.Mann NP, Haddow R, Stokes L, Goodley S, Rutter N. Effect of night and day on preterm infants in a newborn nursery: randomised trial. Br Med J (Clin Res Ed) 1986;293(6557):1265–7. doi: 10.1136/bmj.293.6557.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brandon DH, Holditch-Davis D, Belyea M. Preterm infants born at less than 31 weeks' gestation have improved growth in cycled light compared with continuous near darkness. J Pediatr. 2002;140(2):192–9. doi: 10.1067/mpd.2002.121932. [DOI] [PubMed] [Google Scholar]
- 75.Mirmiran M, Lunshof S. Perinatal development of human circadian rhythms. ProgBrain Res. 1996;111:217–26. 217–26. doi: 10.1016/s0079-6123(08)60410-0. [DOI] [PubMed] [Google Scholar]
- 76.McGraw K, Hoffmann R, Harker C, Herman JH. The development of circadian rhythms in a human infant. Sleep. 1999;22(3):303–10. doi: 10.1093/sleep/22.3.303. [DOI] [PubMed] [Google Scholar]
- 77.Ariagno RL, Mirmiran M. Shedding light on the very low birth weight infant. J Pediatr. 2001;139(4):476–7. doi: 10.1067/mpd.2001.118879. [DOI] [PubMed] [Google Scholar]
- 78.Glass P, Avery GB, Subramanian KN, Keys MP, Sostek AM, Friendly DS. Effect of bright light in the hospital nursery on the incidence of retinopathy of prematurity. NEnglJMed. 1985;313(7):401–4. doi: 10.1056/NEJM198508153130701. [DOI] [PubMed] [Google Scholar]
- 79.Fielder AR, Moseley MJ. Environmental light and the preterm infant. Semin Perinatol. 2000;24(4):291–8. doi: 10.1053/sper.2000.8597. [DOI] [PubMed] [Google Scholar]
- 80.Reynolds JD, Hardy RJ, Kennedy KA, Spencer R, van Heuven WA, Fielder AR. Lack of efficacy of light reduction in preventing retinopathy of prematurity. Light Reduction in Retinopathy of Prematurity (LIGHT- ROP) Cooperative Group. NEnglJMed. 1998;338(22):1572–6. doi: 10.1056/NEJM199805283382202. [DOI] [PubMed] [Google Scholar]
- 81.Kennedy KA, Fielder AR, Hardy RJ, Tung B, Gordon DC, Reynolds JD. Reduced lighting does not improve medical outcomes in very low birth weight infants. J Pediatr. 2001;139(4):527–31. doi: 10.1067/mpd.2001.117579. [DOI] [PubMed] [Google Scholar]
- 82.Als H, Lawhon G, Duffy FH, McAnulty GB, Gibes-Grossman R, Blickman JG. Individualized developmental care for the very low-birth-weight preterm infant. Medical and neurofunctional effects. Jama. 1994;272(11):853–8. [PubMed] [Google Scholar]
- 83.Ariagno RL, Thoman EB, Boeddiker MA, et al. Developmental care does not alter sleep and development of premature infants. Pediatrics. 1997;100(6):E9. doi: 10.1542/peds.100.6.e9. [DOI] [PubMed] [Google Scholar]
- 84.Symington A, Pinelli J. Developmental care for promoting development and preventing morbidity in preterm infants. Cochrane Database Syst Rev. 2006;(2):CD001814. doi: 10.1002/14651858.CD001814.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]