Abstract
Immunohistochemistry (IHC) is routinely used in diagnostic pathology to detect infectious agents, to immunophenotype neoplastic cells, and to prognosticate neoplastic diseases. Formalin fixation is considered a limiting factor for IHC because formalin can cross-link antigens and mask epitopes. Prolonged formalin fixation is presumed to result in decreased antigen detection; however, this effect has only been evaluated with a few antibodies. The goal of this study was to evaluate the effect of prolonged formalin fixation on the immunohistochemical detection of 61 different antigens. Approximately 5-mm-thick tissue slices were fixed in 10% neutral-buffered formalin. Tissue slices were removed from formalin, processed, and paraffin-embedded at 1-day, 3-day, and then at ∼1-week intervals. IHC was performed on all sections in tandem after all tissues were processed. Immunoreactions were evaluated by three pathologists according to a four-tier grading system. Immunoreactivity of cytokeratin 7, high-molecular-weight cytokeratin, and laminin was diminished by prolonged formalin fixation. However, immunohistochemical reactivity remained moderate to strong with up to 7 weeks of fixation for all other antibodies. These results suggest that prolonged formalin fixation has minimal effects on antigen detection for most commonly used antibodies. These results further validate the use of IHC in diagnostic pathology. (J Histochem Cytochem 57:753–761, 2009)
Keywords: immunohistochemistry, formalin, formalin fixation, diagnostic pathology
Immunohistochemistry is an essential tool for diagnostic pathology. Routine uses of diagnostic IHC include immunophenotyping and prognosticating neoplastic diseases, detecting or identifying pathogens associated with histologic lesions, and characterizing cellular infiltrates. The primary advantage of IHC over other protein detection techniques is that antigens can be detected in the context of tissue and cellular morphology. Therefore, antigen expression is not only detected in the diseased tissue, but associations between the presence of antigen and specific cell types or histologic lesions can be assessed with IHC.
Many tissues used in diagnostic histopathology and IHC are routinely fixed in 10% neutral-buffered formalin, which is a 4% formaldehyde solution buffered to a neutral pH. Formalin is a cross-linking fixative that forms hydroxymethyl groups on reactive amino acid side chains and subsequently cross-links peptides. Formalin can also react with nucleotides and some unsaturated fatty acids (Eltoum et al. 2001b; Ramos-Vara 2005). Formalin inhibits cellular processes, prevents tissue degradation, preserves tissue architecture, and kills pathogens within lesions (Eltoum et al. 2001b; Ramos-Vara 2005). The use of formalin-fixed paraffin-embedded tissues for IHC eliminates the need for fresh or fresh-frozen tissues, and allows the use of archival paraffin-embedded tissues in diagnostic cases and retrospective studies.
Formalin provides excellent preservation of tissue architecture; however, formalin fixation can mask epitopes and result in decreased immunoreactivity (Arnold et al. 1996; Werner et al. 2000; Ramos-Vara 2005). Formalin fixation is a time-dependent process in which increased fixation time results in continued formaldehyde group binding to proteins to a point of equilibrium (Fox et al. 1985). Studies have shown that formalin fixation, especially if prolonged, results in decreased antigenicity (Battifora and Kopinski 1986; Arnold et al. 1996; Shi et al. 1998), which limits the use of formalin-fixed tissues for diagnostic IHC (Arnold et al. 1996; Eltoum et al. 2001a; Ramos-Vara 2005). Enzymatic digestion and heat-induced epitope retrieval (HIER) protocols have improved antigen detection following prolonged fixation (Battifora and Kopinski 1986; Shi et al. 1998; Boenisch 2005); however, studies evaluating antigen detection after prolonged formalin fixation have been limited. Inclusive studies evaluating the effect of prolonged fixation on many antibodies, using a variety of antigen retrieval protocols, and including antigens with various cellular localizations, are lacking. The goal of this study was to evaluate the effect of prolonged formalin fixation on immunohistochemical antigen detection with 61 antibody–antigen combinations, and to characterize the influence of cellular antigen localization and antigen retrieval techniques on antigen detection after prolonged formalin fixation.
Materials and Methods
Tissues and Tissue Processing
Tissues were collected from animals or surgical biopsy specimens submitted to Purdue University Animal Disease Diagnostic Laboratory for necropsy or histologic examination, respectively. Tissues used for each IHC test were known to contain the antigen of interest. All tissues were canine except for a feline extramedullary plasmacytoma [for multiple myeloma oncogene-1 (MUM-1) evaluation], feline pancreas (for amylin evaluation), equine lymphoid tissue (for CD3, CD20, CD79a, and BLA.36 evaluation), and a bovine intestine infected with Mycobacterium avium paratuberculosis (for CD68 evaluation). Tissues, collected at necropsy or biopsy, were fixed in 10% neutral-buffered formalin for ∼24–48 hr. Following this initial fixation, ∼3–5-mm-thick tissue slices were individually placed in histologic cassettes and returned to formalin. One cassette was removed on day 1, day 3, and at 1-week intervals for at least 7 weeks and routinely processed into paraffin blocks. Exceptions include calcitonin, for which there was only sufficient tissue for days 1 and 3, and weeks 3, 4, and 5; CD1a, CD68, CD117, Oct-3/4, progesterone receptor, and tryptase, which were not processed on day 3; amylin and feline MUM-1, which were not processed on day 3 and week 1; and canine MUM-1, which was not processed on day 3 and week 5.
IHC
All paraffin blocks for a given antibody were sectioned on the same day. Tissues were manually deparaffinized with xylene, rehydrated in graded ethanol, and rinsed in distilled water. IHC was simultaneously performed for all time points for a given antibody using an autostainer (Dako; Carpinteria, CA) according to previously reported methods (Ramos-Vara and Beissenherz 2000). Antibodies are listed in Table 1 with information regarding the clone, source, antigen retrieval protocol, immunoperoxidase kit, and expected cellular antigen localization. Citrate buffer (pH 6.0) and EDTA (pH 9.0) HIER was performed in a decloaking chamber (Biocare Medical; Concord, CA) using concentrated solutions (Dako). Prior to incubation with primary antibodies, slides were incubated with a ready-to-use endogenous peroxidase blocking reagent (Dako) for 5 min. Nonspecific antibody binding was blocked by 5-min incubation with ready-to-use protein block reagent (Dako). All immunoreactions were detected with diaminobenzidine (DAB). Following IHC, slides were counterstained in hematoxylin, dehydrated in graded ethanol, cleared in xylene, and coverslipped.
Table 1.
Antigens, antibodies, and immunohistochemistry protocols
| Antigena | Antibody (clone) | Source | Localization | Antigen retrieval | Detection kit |
|---|---|---|---|---|---|
| Actin muscle | Mouse (HHF35) | Dako | Cytoplasm | Proteinase K | Envision+ |
| Actin sarcomeric | Mouse (α-Sr-1) | Dako | Cytoplasm | No treatment | Envision+ |
| Actin smooth muscle | Mouse (1A4) | Dako | Cytoplasm | No treatment | LSAB 2 |
| Amylin | Mouse (R10/99) | AbD Serotec | Extracellular | Citrate HIER | Envision+ |
| BLA.36 | Mouse (A27-42) | Biogenex | Membrane | Citrate HIER | LSAB+ |
| BLA.36 equine | Mouse (A27-42) | Biogenex | Membrane | Citrate HIER | LSAB+ |
| Calcitonin | Rabbit polyclonal | Dako | Cytoplasm | Citrate HIER | LSAB 2 |
| Calponin | Mouse (CALP) | Dako | Cytoplasm | Proteinase K | Envision+ |
| Calretinin | Rabbit polyclonal | Zymed | Cytoplasm | EDTA HIER | Envision+ |
| CD10 | Mouse (56C6) | Vector | Membrane | Citrate HIER | Envision+ |
| CD117 | Rabbit polyclonal | Dako | Membrane | Citrate HIER | Envision+ |
| CD11d | Mouse (CA18.3C6) | Dr. Moore UC Davis | Membrane | Citrate HIER | Envision+ |
| CD18 | Mouse (CA16.3C10) | Dr. Moore UC Davis | Membrane | Citrate HIER | Envision+ |
| CD1a | Mouse (O10) | Dako | Cytoplasm | Citrate HIER | Envision+ |
| CD20 | Rabbit polyclonal | LabVision | Membrane | No treatment | LSAB 2 |
| CD20 equine | Rabbit polyclonal | LabVision | Membrane | No treatment | LSAB 2 |
| CD3 | Rabbit polyclonal | Dako | Membrane | Citrate HIER | LSAB 2 |
| CD3 equine | Rabbit polyclonal | Dako | Membrane | Citrate HIER | LSAB 2 |
| CD31 | Mouse (JC/70A) | Dako | Membrane | Citrate HIER | LSAB+ |
| CD45 | Mouse (CA12.10C12) | Dr. Moore UC Davis | Membrane | Citrate HIER | LSAB+ |
| CD79a | Mouse (HM57) | Dako | Membrane | EDTA HIER | Envision+ |
| CD79a equine | Mouse (HM57) | Dako | Membrane | EDTA HIER | Envision+ |
| CD68 bovine | Mouse (EMB11) | Dako | Cytoplasm | Proteinase K | LSAB 2 |
| Chromogranin A+B | Rabbit polyclonal | Research Diagnostics | Cytoplasm | Proteinase K | Envision+ |
| Claudin | Rabbit polyclonal | Abcam | Membrane | Citrate HIER | Envision+ |
| Cytokeratins AE1-AE3 | Mouse (AE1 and AE3) | Dako | Cytoplasm | Citrate HIER | LSAB+ |
| Cytokeratin 5 | Mouse (XM26) | Vector | Cytoplasm | No treatment | Envision+ |
| Cytokeratin 7 | Mouse (OV-TL 12/30) | Dako | Cytoplasm | Proteinase K | Envision+ |
| Cytokeratins 8/18 | Mouse (5D3) | Novocastra | Cytoplasm | Proteinase K | Envision+ |
| Cytokeratins HMW | Mouse (34βE12) | Dako | Cytoplasm | Proteinase K | Envision+ |
| Cytokeratins pan | Mouse (MNF116) | Dako | Cytoplasm | Proteinase K | LSAB 2 |
| Desmin | Mouse (D33) | Dako | Cytoplasm | Citrate HIER | LSAB 2 |
| E-cadherin | Mouse (36) | BD transduction | Membrane | Citrate HIER | LSAB+ |
| Factor VIII-RA | Rabbit polyclonal | Dako | Cytoplasm | Proteinase K | LSAB 2 |
| GATA4 | Goat polyclonal | Santa Cruz | Nuclear | Citrate HIER | LSAB+ |
| Glial fibrillary acidic protein | Rabbit polyclonal | Dako | Cytoplasm | No treatment | LSAB 2 |
| Glucagon | Rabbit polyclonal | Dako | Cytoplasm | Citrate HIER | LSAB 2 |
| Glut-1 | Rabbit polyclonal | Dako | Cytoplasm | Citrate HIER | Envision+ |
| Inhibin-α | Mouse (R1) | Serotec | Cytoplasm | Citrate HIER | Envision+ |
| Insulin | Mouse (Z006) | Zymed | Cytoplasm | Citrate HIER | LSAB 2 |
| Ki67 | Mouse (7B11) | Zymed | Nuclear | Citrate HIER | LSAB 2 |
| Laminin | Rabbit polyclonal | Dako | Extracellular | Proteinase K | Envision+ |
| Lysozyme | Rabbit polyclonal | Dako | Cytoplasm | Proteinase K | Envision+ |
| MHC II | Mouse (TAL. 1B5) | Dako | Membrane | Citrate HIER | Envision+ |
| MUM1 | Mouse (MUM1p) | Dako | Nuclear | EDTA HIER | ImmPRESS |
| MUM1 feline | Mouse (MUM1p) | Dako | Nuclear | EDTA HIER | ImmPRESS |
| Myeloid/histiocytic antigen | Mouse (MAC 387) | Dako | Cytoplasm | Proteinase K | Envision+ |
| Myoglobulin | Rabbit polyclonal | Dako | Cytoplasm | No treatment | Envision+ |
| Myosin smooth muscle | Mouse (SMMS-1) | Dako | Cytoplasm | Citrate HIER | LSAB+ |
| Neurofilament | Mouse (SMI-31) | Covance | Cytoplasm | No treatment | Envision+ |
| Neuron-specific enolase | Mouse (BBS/NC/VI-H14) | Dako | Cytoplasm | Citrate HIER | Envision+ |
| Oct-3/4 | Mouse (C-10) | Santa Cruz | Nuclear | Citrate HIER | Envision+ |
| p63 | Mouse (4A4) | Santa Cruz | Nuclear | EDTA HIER | Envision+ |
| PGP 9.5 | Rabbit polyclonal | Dako | Pancellular | No treatment | Envision+ |
| Progesterone receptor | Rabbit mono (SP21) | LabVision | Nuclear | Citrate HIER | LSAB+ |
| Prox-1 | Rabbit polyclonal | AngioBio | Nuclear | Citrate HIER | LSAB+ |
| S-100 | Rabbit polyclonal | Dako | Pancellular | Citrate HIER | LSAB 2 |
| Somatostatin | Rabbit polyclonal | Dako | Cytoplasm | Citrate HIER | Envision+ |
| Synaptophysin | Rabbit mono (SP11) | LabVision | Cytoplasm | No Treatment | LSAB+ |
| Tryptase | Mouse (AA1) | Dako | Cytoplasm | Citrate HIER | Envision+ |
| Vimentin | Rabbit mono (SP20) | LabVision | Cytoplasm | Proteinase K | LSAB 2 |
All antigens were detected in canine tissues unless specifically noted.
HMW, high molecular weight; factor VIII-RA, factor VIII–related antigen; MCH II, major histocompatibility complex II; MUM1, multiple myeloma oncogene 1; PGP 9.5, protein gene product 9.5; mono, monoclonal; HIER, heat-induced epitope retrieval; LSAB, linked strepavidin-biotin.
Serotec, Raleigh, NC; Biogenex, San Ramon, CA; Zymed, San Francisco, CA; Vector, Burlingame, CA; LabVision, Fremont, CA; Research Diagnostics, Concord, MA; Abcam, Cambridge, MA; Novocastra, Bannockburn, IL; BD Transduction, San Jose, CA; Santa Cruz, Santa Cruz, CA; Covance, Madison, WI; AngioBio, Del Mar, CA.
IHC Evaluation
All slides were independently evaluated and graded by three pathologists. Slides were graded based on the intensity of immunoreactions using a four-tier grading system of 3+ (strong), 2+ (moderate), 1+ (faint), and 0 (none). Slides were initially evaluated to determine the range of immunoreactivity among the time points, then graded against the strongest time point, which was graded as 3+. In cases of disagreement, a consensus grade was determined by re-evaluation of slides.
Photomicrographs
All photomicrographs for each antibody were taken at the same settings and saved as tagged image file format images. Images were converted to 300 dots per inch on Adobe Photoshop (Adobe; San Jose, CA), cropped to ∼5 cm × 4.2 cm, and merged in a color plate. The merged color plate was sharpened with a sharpening tool in Adobe Photoshop.
Results
Thirty-two antigens were localized to the cytoplasm; 17 to the plasma membrane; 8 to the nucleus; 2 to the nucleus and the cytoplasm; and 2 were extracellular. Thirty-two antigens were retrieved with citrate buffer (pH 6.0) HIER; 6 were retrieved with EDTA (pH 9.0) HIER; 13 with proteinase K; and 10 received no antigen retrieval.
For the first 43 antibodies tested, each time point was run in duplicate to evaluate intrinsic slide-to-slide immunostaining variability. A total of 390 time points were evaluated in duplicate. Variation in immunostaining grades was noted in 50/390 (12.8%) time points by a single pathologist (JDW). Most discrepancies were between 2+ and 3+ staining reactions. Single slides were run at each time point for the remaining antibodies.
Consensus grades for each antibody at each time point are shown in Table 2. Data were not available for GATA-4 at weeks 4–7 because of failure of the immunoreaction. This was not interpreted as loss of immunoreactivity, because there was strong immunoreactivity at weeks 3 and 8. Data were not available for Oct-3/4 at week 1, because tissues were lost from the slide.
Table 2.
Consensus immunoreactivity grades for each antibody at each time point
| Day
|
Weekb
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibodya | 1 | 3 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Actin muscle | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
| Actin Sarcomeric | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
| Actin smooth muscle | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
| Amylin | 3 | n/a | n/a | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| BLA.36 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| BLA.36 equine | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a |
| Calcitonin | 3 | 3 | n/a | n/a | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Calponin | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| Calretinin | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| CD117 | 3 | n/a | 3 | 2 | 3 | 3 | 3 | 3 | 2 | 2 | 2 | 1 | n/a | n/a | n/a |
| CD10 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| CD11d | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| CD18 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| CD1a | 3 | n/a | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a |
| CD20 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
| CD20 equine | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a |
| CD3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| CD3 equine | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a |
| CD31 | 3 | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
| CD45 | 3 | 3 | 3 | 3 | 2 | 2 | 2 | 2 | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
| CD68 bovine | 3 | n/a | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a |
| CD79a | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| CD79a equine | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a |
| Chromogranin A+B | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
| Claudin | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| Cytokeratin 5 | 3 | 3 | 3 | 2 | 2 | 2 | 3 | 2 | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
| Cytokeratin 7 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
| Cytokeratins 8/18 | 3 | 2 | 2 | 2 | 3 | 2 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| Cytokeratin AE1/AE3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| Cytokeratins HMW | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
| Cytokeratins pan | 2 | 2 | 3 | 3 | 3 | 2 | 2 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| Desmin | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
| E-Cadherin | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| Factor VIII-RA | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| GATA4 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a |
| GFAP | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
| Glucagon | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| Glut-1 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| Inhibin | 3 | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a |
| Insulin | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| Ki67 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| Laminin | 3 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
| Lysozyme | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 2 | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
| MHC II | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| MUM1 | 3 | n/a | 3 | 3 | 3 | 3 | n/a | 3 | 3 | n/a | n/a | 3 | n/a | n/a | n/a |
| MUM1 feline | 3 | n/a | n/a | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 2 | 2 | n/a | n/a | n/a |
| Myel/histio antigen | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| Myoglobulin | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| Myosin smooth muscle | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
| Neurofilaments | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| Neuron-specific enolase | 2 | 3 | 2 | 3 | 3 | 3 | 2 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| OCT-3/4 | 3 | n/a | n/a | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | n/a | n/a | n/a |
| p63 | 2 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| PGP 9.5 | 3 | 3 | 3 | 3 | 2 | 3 | 2 | 2 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| Progesterone receptor | 3 | n/a | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a |
| Prox-1 | 3 | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| S-100 | 3 | 3 | 3 | 2 | 3 | 3 | 2 | 2 | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
| Somatostatin | 2 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
| Synaptophysin | 3 | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
| Tryptase | 2 | n/a | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | n/a |
| Vimentin | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
All antigens were detected in canine tissues unless otherwise noted.
Week 1 corresponds to 7 ± 3 days. All other weeks are at 7-day intervals following week 1, except for weeks 3, 7, and 8 for GATA4 and inhibin-α, which were processed 2 days early owing to holiday schedule. Equine lymphoid tissue was processed at 4 days instead of 3 days.
GFAP, glial fibrillary acidic protein; myelo/histio antigen, myeloid/histiocytic antigen; n/a, time point was not evaluated.
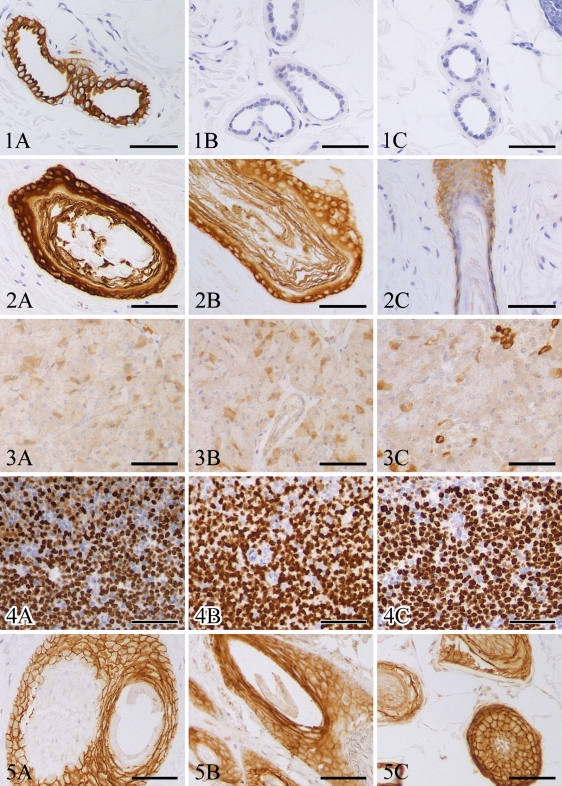
For most antibodies, immunostaining was moderate to strong at all points up to 7 weeks of fixation. Notable exceptions were cytokeratin 7, laminin, and high-molecular-weight cytokeratins, which had marked decreases in immunoreactivity with 3 days, 2 weeks, and 6 weeks of fixation, respectively (Figures 1 and 2). CD117 had strong immunoreactivity up to week 6, moderate immunoreactivity from weeks 7 to 9, and only faint immunoreactivity at week 10, which was the last time point evaluated. Some antigens, including glucagon, had increased immunoreactivity following 3-day fixation and continued moderate to strong immunoreactivity with up to 7 weeks of fixation (Figure 3). Many antibodies had mild fluctuations in immunoreactivity between time points; however, these variations were between +2 and +3 immunoreactivity, with little ambiguity as to the presence of antigen (Figures 4 and 5).
Figure 1.
Sections of canine haired skin; formalin-fixed for 1 day (A), 3 days (B), and 7 days (C); and immunohistochemically labeled with anti–cytokeratin 7 antibodies. DAB chromagen, hematoxylin counterstain. Bar = 50 μm.
Figure 2.
Sections of canine haired skin; formalin-fixed for 1 day (A), 7 days (B), and 42 days (C); and immunohistochemically labeled with anti–high-molecular-weight cytokeratin antibodies. DAB chromagen, hematoxylin counterstain. Bar = 50 μm.
Figure 3.
Sections of canine pancreas; formalin-fixed for 1 day (A), 3 days (B), and 49 days (C); and immunohistochemically labeled with anti-glucagon antibodies. DAB chromagen, hematoxylin counterstain. Bar = 50 μm.
Figure 4.
Sections of canine lymph node; formalin-fixed for 1 day (A), 21 days (B), and 49 days (C); and immunohistochemically labeled with anti-Ki67 antibodies. DAB chromagen, hematoxylin counterstain. Bar = 50 μm.
Figure 5.
Sections of canine haired skin; formalin-fixed for 1 day (A), 21 days (B), and 49 days (C); and immunohistochemically labeled with anti–E-cadherin antibodies. DAB chromagen, hematoxylin counterstain. Bar = 50 μm.
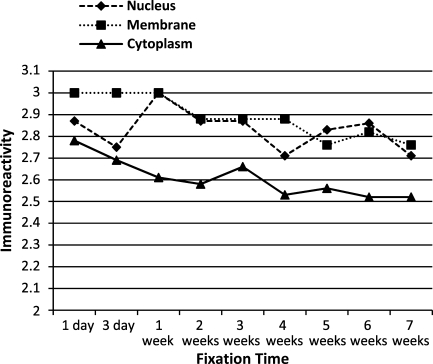
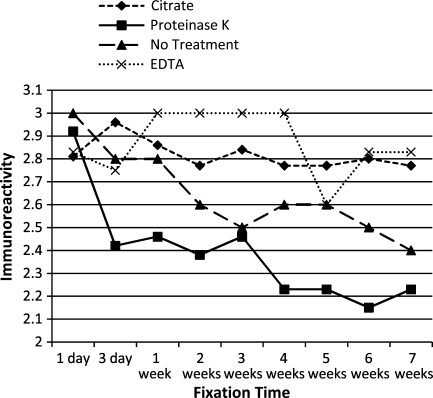
To evaluate the effects of antigen cellular localization and antigen retrieval, the mean immunoreactivity scores of antigens with similar cellular localizations or antigen retrieval protocols were determined and plotted across all time points. Antigens localized to the cytoplasm tended to have decreased immunoreactivity at all time points, compared with those localized to the nucleus or plasma membrane (Figure 6). Antigens that required proteinase K digestion and, to a lesser extent, antigens that did not receive antigen retrieval tended to have decreased immunoreactivity following prolonged fixation, compared with those receiving HIER (Figure 7). Despite these trends, however, the mean immunoreactivities for all antigen groups, based on cellular localization or antigen retrieval, were moderate to strong following up to 7 weeks of fixation.
Figure 6.
Mean immunoreactivity over time of antigens grouped by cellular localization.
Figure 7.
Mean immunoreactivity over time of antigens grouped by antigen retrieval protocol.
Discussion
In this study, most antibodies tested were able to detect target antigens with moderate to strong immunoreactivity following up to 7 weeks of formalin fixation. These results suggest that using standard procedures, prolonged formalin fixation is not a major limiting factor for most antibodies used in diagnostic IHC. However, some antibodies had moderate to marked decreases in their detection abilities following prolonged fixation. Therefore, it is likely that the effects of prolonged formalin fixation depend on the antibody, the antigen of interest, and the antigen retrieval protocol.
To the authors' knowledge, this is the largest and most comprehensive study evaluating the effects of prolonged formalin fixation. Previous studies have evaluated the effects of formalin fixation on single or a few cellular or infectious disease antigens. In one of the earliest studies of the adverse effects of prolonged fixation, Battifora and Kopinski (1986) showed that protease digestion can ameliorate the adverse effects of prolonged formalin fixation on cytokeratin detection; however, prolonged fixation required longer protease digestion, which risks tissue destruction. In subsequent studies, Shi et al. (1991,1998) showed that HIER permits antigen detection following up to 30 days of fixation; however, Shi et al. (1998) also demonstrated the importance of optimizing antigen retrieval protocols for each antibody used. Ramos-Vara et al. (2000,2002,2003) have shown persistence of immunoreactivity for uroplakin III, thyroid transcription factor-1, melan A, vimentin, and neuron-specific enolase following prolonged fixation; and Miller et al. (2005) were able to detect bovine viral diarrhea virus antigen following 36 days of fixation, but not at 176 days. A recent study evaluating the effects of prolonged formalin fixation on immunohistochemical detection of African horse sickness virus showed moderate to strong antigen detection following up to 365 days of fixation in 10 horses; however, the quality of staining was subjectively decreased (Clift 2008). Other studies have shown moderate to significant decreases in immunoreactivity following prolonged fixation. von Wasielewski et al. (1998) showed progressive decreases in estrogen and progesterone receptor immunoreactivity, which was notable at 8 and 16 days of fixation. Boenisch (2005) found consistent staining with 26 of 30 antibodies, including the BLA.36, CD1a, CD31, CD79α, cytokeratin AE1/AE3, and pancytokeratin clones used in this study, following 8 days of fixation; however, most antigens had moderate to marked decreases in antigenicity following 3 months of fixation. Interestingly, increasing antigen retrieval temperatures from 97C to 121C resulted in increased staining intensities for 5 of 7 antibodies tested after 3 months of fixation. Varma et al. (1999) were able to detect high-molecular-weight cytokeratin following 30 days of fixation if HIER was conducted with a hot plate, but results were variable if a microwave was used, and there was progressive loss of antigenicity after 1 week of fixation if antigens were retrieved with protease digestion. These studies demonstrate the importance of optimizing antigen retrieval protocols for each antibody used and evaluating the effects of prolonged fixation in antibody standardization and validation. Van Alstine et al. (2002) reported a significantly decreased ability to detect porcine reproductive and respiratory syndrome (PRRS) virus antigen at 5 days of fixation, and were unable to detect any antigen at later time points. Interestingly, using the same antibody, other investigators could detect PRRS virus antigen following 7 days of fixation (Rossow et al. 1996). These studies used different antigen retrieval protocols and immunodetection kits, which might have caused variations in their results. Although some studies have shown impaired antigen detection ability following prolonged fixation, results of most studies and this study suggest that with proper optimization and standardization of IHC protocols, most antigens can be successfully detected in tissues following up to 30 days or more of formalin fixation.
Effects of prolonged formalin fixation have been previously evaluated for cytokeratin 7, vimentin, neuron-specific enolase, and S100 in canine tissues (Ramos-Vara et al. 2000,2003). In this study, vimentin and neuron-specific enolase had moderate to strong staining at all time points; however, Ramos-Vara et al. (2000) found weak immunoreactivity at 1 day of fixation, followed by moderate to strong immunoreactivity at later time points. The most likely explanation for this discrepancy would be variations in tissue thickness during fixation. Increased tissue thickness could result in inadequate fixation at day 1 and subsequent weak or false-negative results. Ramos-Vara et al. (2000) also reported dramatically decreased immunoreactivity to S100 following 26 days of fixation, with almost no immunoreactivity at 41 days. In this study, however, although immunoreactivity was decreased at week 5, there was still moderate immunoreactivity at week 7. Additionally, cytokeratin 7 was not detected following 3 days of fixation in this study, but Ramos-Vara et al. (2003) previously reported cytokeratin 7 immunodetection with up to 28 days of fixation. The reason for the discrepancies in these studies is uncertain, considering that fixation and antigen retrieval protocols were similar. One possibility is that the variation in the tissues evaluated could have resulted in different protein–protein cross-links and differential effects of prolonged fixation. IHC protocols might need to be standardized, not only for the antibody and antigen, but also for the tissue in which the antigen is to be detected. The effect of tissue type should be independently evaluated.
Although there was minimal variation in immunoreactivity across multiple time points for most antibodies, many antibodies had variable immunoreactivity among different tissues on a single slide and within individual tissue sections. Variation within and among tissue sections was a major reason for discrepancies between pathologists. To avoid these discrepancies, tissues were graded in areas of the most-intense immunoreactivity. The pattern of variation tended to be either strong peripheral with weaker central immunoreactivity or a gradation of immunoreactivity throughout the section. Similar variations have also been noted in diagnostic samples. One likely cause of this variation is a difference in the degree of tissue fixation or in the time of tissue fixation throughout the sample. It takes ∼25 hr for formalin to penetrate a 1-cm3 sphere of tissue (Eltoum et al. 2001b). If tissues are processed before complete fixation, unfixed tissue will be ethanol-fixed during processing, which can produce altered IHC results (Fox et al. 1985; Yaziji and Barry 2006). Additionally, tissue deep in the specimen continues to autolyze until it is penetrated by formalin. Therefore, although tissues eventually become thoroughly fixed, the differing degrees of autolysis before fixation can cause variations in immunoreactivity. In many tissues, the strongest immunoreactivity tended to be at the periphery, where formalin exposure was likely to have occurred earliest and lasted longer than in more-central areas. This corroborates the concept that prolonged formalin fixation is not a major limiting factor for diagnostic IHC if autolysis can be avoided, particularly when HIER methods are used.
Formalin fixation times are rarely standardized in diagnostic pathology, especially when samples are submitted by outside hospitals (Shi et al. 1998). Owing to this variability, it is important to understand the effects of prolonged formalin fixation on each antibody used in the laboratory. Understanding this effect will facilitate interpretation of IHC results, especially when results are unexpectedly negative. The results of this study suggest that for most antibodies, moderate variations in fixation times up to 7 weeks should not significantly affect IHC results. However, the effects of prolonged formalin fixation are antibody dependent. Therefore, evaluating the effects of prolonged formalin fixation should be included in standardization and validation protocols for each marker used in diagnostic IHC. Whenever possible, protocols should be optimized to detect antigens following up to 4 weeks of formalin fixation (Ramos-Vara et al. 2008).
Although formalin fixation is a critical step in any IHC protocol, several other variables can affect IHC results, including under-fixation, delayed fixation, tissue processing, slide processing, antigen retrieval, and antibody detection (Werner et al. 2000; Miller et al. 2005; Yaziji and Barry 2006; Goldstein et al. 2007; Ramos-Vara et al. 2008). Whenever a new antibody is used in a laboratory, IHC protocols should be optimized and developed into standard operating procedures (Ramos-Vara et al. 2008). Standard operating procedures should be rigorously followed to avoid aberrant results, to allow for accurate interpretation of IHC results, and to compare results across multiple samples. In this study, IHC was performed according to the IHC protocols in our laboratory, and specimens from all time points for a given antibody were included in one run, to avoid intrinsic variations in IHC results and to accurately evaluate IHC results across time points.
In this study, we evaluated only the effects of prolonged formalin fixation; however, in some laboratories, under-fixation is a more common problem. During tissue processing, under-fixed tissues will be fixed in ethanol, which is a coagulative fixative (Fox et al. 1985; Yaziji and Barry 2006). Protocols standardized for formalin-fixed tissues might not work with other fixatives; therefore, under-fixation might be more likely to have detrimental effects on IHC results than over-fixation (Battifora and Kopinski 1986; Yaziji and Barry 2006). Based on the results of this study, it is probably better to fix tissues for an additional 24–48 hr than to process them at the first available opportunity and risk under-fixation. Additionally, if antibodies will be routinely used with tissues fixed in non-formalin fixatives, such as ethanol, B5, glutaraldehyde, or Bouin's solution, or if tissues will be further processed with decalcifying or bleaching solutions, IHC protocols should be standardized for use with these reagents.
The results of this study validate the use of IHC in diagnostic pathology in samples fixed for several weeks in formalin. Specifically, these results suggest that prolonged formalin fixation is not a major limiting factor in diagnostic IHC and most antibodies can detect antigens following up to 7-weeks fixation. However, these results also suggest that the effects of prolonged formalin fixation are antibody and antigen dependent. Therefore, evaluating the effects of prolonged fixation should be a routine component of antibody validation. Understanding these effects will allow for accurate interpretation of IHC results, especially in situations where fixation times are not controlled or standardized.
Acknowledgments
The authors acknowledge the pathology and histology sections of the Purdue University Animal Disease Diagnostic Laboratory.
References
- Arnold MM, Srivastava S, Fredenburgh J, Stockard CR, Myers RB, Grizzle WE (1996) Effects of fixation and tissue processing on immunohistochemical demonstration of specific antigens. Biotech Histochem 71:224–230 [DOI] [PubMed] [Google Scholar]
- Battifora H, Kopinski M (1986) The influence of protease digestion and duration of fixation on the immunostaining of keratins. A comparison of formalin and ethanol fixation. J Histochem Cytochem 34:1095–1100 [DOI] [PubMed] [Google Scholar]
- Boenisch T (2005) Effect of heat-induced antigen retrieval following inconsistent formalin fixation. Appl Immunohistochem Mol Morphol 13:283–286 [DOI] [PubMed] [Google Scholar]
- Clift SJ (2008) Standardization and Validation of an Immunoperoxidase Test for African Horse Sickness Virus. Thesis in Paraclinical Sciences. Pretoria, University of Pretoria, 150
- Eltoum I, Fredenburgh J, Grizzle WE (2001a) Advanced concepts in fixation. 1. Effects of fixation on immunohistochemistry, reversibility of fixation and recovery of proteins, nucleic acids, and other molecules from fixed and processed tissues. 2. Development methods of fixation. J Histotechnol 24:201–210 [Google Scholar]
- Eltoum I, Fredenburgh J, Myers RB, Grizzle WE (2001b) Introduction to the theory and practice of fixation of tissues. J Histotechnol 24:173–190 [Google Scholar]
- Fox CH, Johnson FB, Whiting J, Roller PP (1985) Formaldehyde fixation. J Histochem Cytochem 33:845–853 [DOI] [PubMed] [Google Scholar]
- Goldstein NS, Hewitt SM, Taylor CR, Yaziji H, Hicks DG (2007) Recommendations for improved standardization of immunohistochemistry. Appl Immunohistochem Mol Morphol 15:124–133 [DOI] [PubMed] [Google Scholar]
- Miller MA, Ramos-Vara JA, Kleiboeker SB, Larson RL (2005) Effects of delayed or prolonged fixation on immunohistochemical detection of bovine viral diarrhea virus type I in skin of two persistently infected calves. J Vet Diagn Invest 17:461–463 [DOI] [PubMed] [Google Scholar]
- Ramos-Vara JA (2005) Technical aspects of immunohistochemistry. Vet Pathol 42:405–426 [DOI] [PubMed] [Google Scholar]
- Ramos-Vara JA, Beissenherz ME (2000) Optimization of immunohistochemical methods using two different antigen retrieval methods on formalin-fixed paraffin-embedded tissues: experience with 63 markers. J Vet Diagn Invest 12:307–311 [DOI] [PubMed] [Google Scholar]
- Ramos-Vara JA, Beissenherz ME, Miller MA, Johnson GC, Pace LW, Fard A, Kottler SJ (2000) Retrospective study of 338 canine oral melanomas with clinical, histologic, and immunohistochemical review of 129 cases. Vet Pathol 37:597–608 [DOI] [PubMed] [Google Scholar]
- Ramos-Vara JA, Kiupel M, Baszler T, Bliven L, Brodersen B, Chelack B, Czub S, et al. (2008) Suggested guidelines for immunohistochemical techniques in veterinary diagnostic laboratories. J Vet Diagn Invest 20:393–413 [DOI] [PubMed] [Google Scholar]
- Ramos-Vara JA, Miller MA, Boucher M, Roudabush A, Johnson GC (2003) Immunohistochemical detection of uroplakin III, cytokeratin 7, and cytokeratin 20 in canine urothelial tumors. Vet Pathol 40:55–62 [DOI] [PubMed] [Google Scholar]
- Ramos-Vara JA, Miller MA, Johnson GC, Pace LW (2002) Immunohistochemical detection of thyroid transcription factor-1, thyroglobulin, and calcitonin in canine normal, hyperplastic, and neoplastic thyroid gland. Vet Pathol 39:480–487 [DOI] [PubMed] [Google Scholar]
- Rossow KD, Benfield DA, Goyal SM, Nelson EA, Christopher-Hennings J, Collins JE (1996) Chronological immunohistochemical detection and localization of porcine reproductive and respiratory syndrome virus in gnotobiotic pigs. Vet Pathol 33:551–556 [DOI] [PubMed] [Google Scholar]
- Shi SR, Cote RJ, Chaiwun B, Young LL, Shi Y, Hawes D, Chen T, et al. (1998) Standardization of immunohistochemistry based on antigen retrieval technique for routine formalin-fixed tissue sections. Appl Immunohistochem 6:89–96 [Google Scholar]
- Shi SR, Key ME, Kalra KL (1991) Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 39:741–748 [DOI] [PubMed] [Google Scholar]
- Van Alstine WG, Popielarczyk M, Albregts SR (2002) Effect of formalin fixation on the immunohistochemical detection of PRRS virus antigen in experimentally and naturally infected pigs. J Vet Diagn Invest 14:504–507 [DOI] [PubMed] [Google Scholar]
- Varma M, Linden MD, Amin MB (1999) Effect of formalin fixation and epitope retrieval techniques on antibody 34betaE12 immunostaining of prostatic tissues. Mod Pathol 12:472–478 [PubMed] [Google Scholar]
- von Wasielewski R, Mengel M, Notle M, Werner M (1998) Influences of fixation, antibody clones, and signal amplification on steroid receptor analysis. Breast J 4:33–40 [Google Scholar]
- Werner M, Chott A, Fabiano A, Battifora H (2000) Effect of formalin tissue fixation and processing on immunohistochemistry. Am J Surg Pathol 24:1016–1019 [DOI] [PubMed] [Google Scholar]
- Yaziji H, Barry T (2006) Diagnostic immunohistochemistry: what can go wrong? Adv Anat Pathol 13:238–246 [DOI] [PubMed] [Google Scholar]