Abstract
Epstein-Barr virus (EBV)-associated gastric carcinoma (GC) is a distinct subtype with characteristic clinicopathological features. To better characterize its cellular characteristics, 43 cases of EBV-associated GC, 68 cases of EBV-negative GC, and non-neoplastic gastric mucosa in adults and fetuses were examined immunohistochemically. We quantified the expression of the major tight-junction protein claudin (CLDN) -1, -3, -4, -7, and -18 together with gastric mucins (MUC5AC and MUC6), intestinal mucin (MUC2), and CD10. EBV-associated GC showed a high frequency of CLDN18 expression (84%) and a low frequency of CLDN3 expression (5%). This expression profile corresponded to that of normal gastric epithelium in adults and fetuses. Almost half of the EBV-associated GC cases demonstrated gastric mucin expression, whereas the other half lacked mucin or CD10 expression. In contrast, as demonstrated by the expression profiles of CLDN3 and CLDN18, EBV-negative GC comprised a heterogeneous group of four different CLDN phenotypes: gastric, intestinal, mixed, and an undifferentiated type with variable expression patterns of mucins. These results indicate that EBV-associated GC is considerably homogenous with regard to cellular differentiation and that it preserves well the nature of the cells of origin. EBV-associated GC may undergo distinct carcinogenic processes, which differ from those of EBV-negative GC. (J Histochem Cytochem 57:775–785, 2009)
Keywords: Epstein-Barr virus, gastric carcinoma, immunohistochemistry, claudin, mucin
Epstein-Barr virus (EBV)-associated gastric carcinoma (GC) is a distinct subtype of GC and comprises 8–11% of all gastric carcinomas (Fukayama et al. 2008; Sousa et al. 2008; Uozaki and Fukayama 2008). Monoclonal EBV is present in nearly all neoplastic cells, thus strongly suggesting its causal role in gastric carcinogenesis. EBV-associated GC displays characteristic clinicopathological features as compared with EBV-negative GC, including male predominance, proximal localization within the stomach, and a relatively better prognosis. Two histological patterns that are characteristic of EBV-associated GC include a lymphoepithelioma-like pattern in which the tumor shows invasive growth accompanied by dense lymphocytic infiltrate and a “lacy” pattern within the mucosal layer, where neoplastic cells form irregularly anastomosing cords with glandular structures. In addition to these two patterns, the histological spectrum ranges from tubular adenocarcinomas to poorly differentiated carcinomas with variable degrees of lymphocytic infiltration.
Despite its histological diversity, EBV-associated GC shows relatively consistent cellular characteristics of mucin expression (Barua et al. 2006). EBV-associated GC is classified into either null or gastric (MUC5AC and MUC6) phenotypes, whereas EBV-negative GC consists of heterogeneous groups. More than half of all EBV-negative GC cases show an intestinal or mixed-type expression pattern of intestinal mucin (MUC2) and a brush border–related molecule, CD10. Phenotypic analysis based on the expression patterns of these molecules has recently been reappraised with regard to GC pathology (Reis et al. 2000; Pinto-de-Sousa et al. 2002). In the advanced stages of GC, certain phenotypes, rather than histological types, have been reported to be correlated with the invasiveness and recurrence of the tumor and the prognosis of the patient (Utsunomiya et al. 1998; Wakatsuki et al. 2008). Studies have shown that certain mucin phenotypes are closely associated with specific genetic changes in GC, which occur early in carcinogenesis. However, other studies assume that the gain or loss of mucin expression, particularly phenotypic changes from the gastric to intestinal or mixed type, occur gradually as the GC develops (Kawachi et al. 2003). Such conflicting observations may be partly due to the inconsistency between mucin phenotypes and the cell lineage, or to the histological diversity of GCs.
Claudins (CLDNs), the major tight-junction proteins, are expressed at the apical membrane of epithelial cells and play a critical role in controlling paracellular permeability and the maintenance of epithelial polarity (Tsukita and Furuse 2000). The CLDN family consists of 24 molecules and can be classified into two types: one that is ubiquitously expressed (CLDN1, -2, -3, -4, and -7), and the other that is highly specific to certain tissues and their constituent cells (CLDN5, -17, and -18) (Hewitt et al. 2006). Increasing numbers of studies that have investigated the different profiles of CLDN expression in various cancers (including GC) have been published recently. However, the effects of CLDN expression on histology and the biological/clinical behavior of GC, and the expression patterns of different CLDNs within normal gastric tissues remain controversial topics (Johnson et al. 2005; Lee et al. 2005; Resnick et al. 2005; Sanada et al. 2006; Matsuda et al. 2007; Park et al. 2007).
In the present study, we comparatively evaluated the expression patterns of CLDNs and mucins to characterize the non-neoplastic cells of fetal and adult gastric mucosa. We then examined expression of CLDNs in EBV-associated GC to clarify their cellular characteristics. Furthermore, we classified those cases with or without EBV infection into four phenotypes (i.e., gastric, intestinal, mixed, and undifferentiated CLDN) in an analogy of the expression profile of their normal counterparts. Finally, we investigated associations between CLDN phenotypes and mucin expression to determine their clinicopathological significance.
Materials and Methods
Patients and Samples
All GC specimens that were analyzed in the present study were retrieved from the Department of Pathology, Tokyo University Hospital. All aspects of the study were approved by the University of Tokyo Ethics Committee. Forty-three cases of EBV-associated GC from 38 patients were retrieved from files dated between 1996 and 2007. One patient had triple carcinomas, and three patients had double carcinomas, all of which were EBV-associated GC. As a control group, 68 samples from 68 patients with EBV-negative GC were randomly selected; 63 samples were from surgically resected stomachs and 5 samples were derived from endoscopic submucosal resection. Additionally, 10 non-neoplastic gastric tissue samples were retrieved; 5 were from patients with EBV-associated GC, and 5 were from those with EBV-negative GC. Of these 10 specimens, 4 were from cardia, 3 were from fundus, and 3 were from pylorus. Stomach, esophagus, and small and large intestine samples were obtained from autopsy cases of aborted or stillborn fetuses at the 9th (2 cases), 13th (1 case), 20th (1 case), 23rd (2 cases), and 35th (2 cases) week of gestation. All samples were fixed with 10% formalin and embedded in paraffin. Representative 4-μm-thick sections of each sample were routinely stained with hematoxylin and eosin.
All carcinoma tissues were histologically evaluated according to the Japanese Classification of Gastric Carcinoma and Lauren's classification (Lauren 1965; Japanese Gastric Cancer Association 1998). Predominant histological types were assessed at the invasive area of each lesion. We adopted the tumor node metastasis classification of the International Union Against Cancer to assess the depth of invasion (T grade), nodal involvement (N grade), and tumor stage (Sobin and Wittekind 2002). The presence of EBV in each tumor cell was confirmed by in situ hybridization with an EBER1 oligonucleotide probe as previously described (Fukayama et al. 1994).
Immunohistochemical Analysis
Antibodies used in this study were as follows: CLDN1 (polyclonal, 1:50), CLDN3 (polyclonal, 1:100), CLDN4 (clone 3E2C1, 1:400), CLDN7 (clone 5D10F3, 1:200), CLDN18 (polyclonal, 1:1000), MUC2 (clone Ccp58, 1:20), MUC5AC (clone CLH2, 1:100), MUC6 (clone CLH5, 1:100), and CD10 (clone 56C6, 1:30). All CLDN antibodies were purchased from Zymed (San Francisco, CA), and all MUC antibodies and CD10 were purchased from Novocastra (Newcastle, UK). All of these antibodies were used in the previous studies (Barua et al. 2006; Sanada et al. 2006; Soini et al. 2006; Hornsby et al. 2007).
All specimens were deparaffinized and rehydrated, and underwent an antigen retrieval procedure. Immunohistochemical staining with the antibodies listed above was performed using the Ventana BenchMark automated immunostainer (Tucson, AZ) with the labeled streptavidin-biotin peroxidase method and visualized with DAB. The positive controls were as follows: normal colonic mucosa for CLDN3, -4, and -7; normal duodenal mucosa for MUC2 and CD10; colon cancer for CLDN1; and normal gastric mucosa for CLDN18, MUC5AC, and MUC6. The immunohistochemical results were blinded and independently evaluated by two pathologists (A.S. and T.U.). Membranous staining for each CLDN was semiquantitatively assessed based on the intensity and distribution. The intensity of CLDN staining was scored as either 0, none; 1, weak; or 2, strong. The distribution of CLDN staining was scored according to the proportion of positively stained tumor cells: 0, none (<5%); 1, focal (5–50%); 2, regional (50–90%); and 3, diffuse (>90%). The tumor was considered immunohistochemically positive for CLDN1, -3, -4, and -7 when the sum of these two scores was 3 or higher. Because normal gastric mucosa strongly expresses CLDN18, the tumor was considered to retain its expression for CLDN18 when the sum of these scores was 4 or 5. The tumor was defined as positive for mucin core proteins or CD10 when more than 10% of the tumor cells were stained.
Statistical Analyses
Associations between clinicopathological parameters and the presence of EBV or CLDN phenotypes were analyzed mainly using Fisher's exact test and the χ2 test. They were analyzed with Student's t-test or ANOVA in the analyses of patients' ages and the size of carcinomas, and with Mann-Whitney U-test or Kruskal-Wallis test in the case of T and N grades and stages of the carcinomas. Differences were considered statistically significant at p<0.05.
Results
CLDN and Mucin Expression in Non-neoplastic Gastric Mucosa of Adults and Fetuses
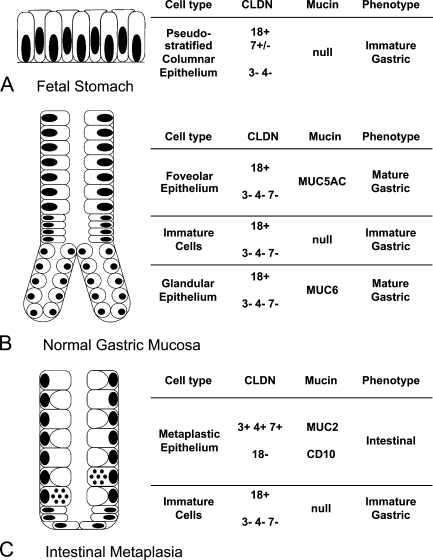
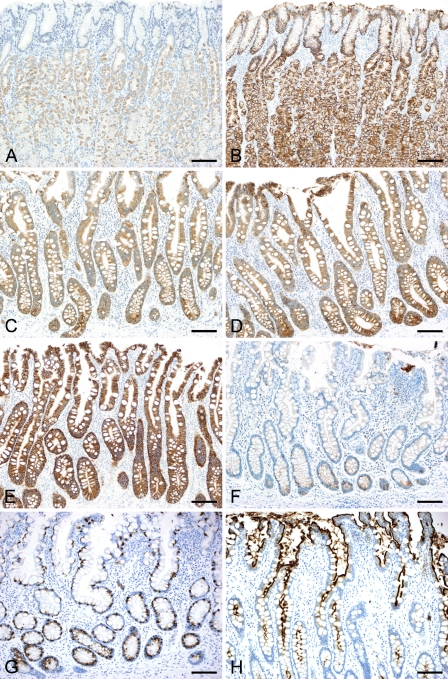
Expression profiles of CLDNs and mucins in non-neoplastic mucosa are summarized in Figure 1. In normal adult gastric mucosa, strong membranous positive staining for CLDN18 was observed in the apical and lateral membranes of the foveolar epithelia, glandular epithelia of cardiac, fundic and pyloric glands, and a small number of morphologically immature cells in the neck zone, whereas CLDN1, - 3, -4, and -7 were not expressed in these epithelia (Figures 2A and 2B). In contrast, intestinalized glands, both in complete type (with all the features of goblet cell metaplasia, the presence of Paneth cells and brush borders) and incomplete type, showed positive membranous immunoreactivity for CLDN3, -4, and -7 (Figures 2C–2E). CLDN18 expression decreased or was diminished in intestinal metaplasia, and only a few immature cells at the base of the pits preserved CLDN18 expression (Figure 2F). CLDN1 expression was negative in the metaplastic mucosa. MUC5AC and MUC6 expression was observed in the foveolar and glandular epithelia, respectively. A few morphologically immature cells in the neck zone were devoid of gastric mucin expression. Metaplastic epithelia expressed intestinal markers, MUC2 in goblet cells and CD10 in brush borders (Figures 2G and 2H).
Figure 1.
Claudin (CLDN) and mucin expression profiles in non-neoplastic gastric mucosa. Schematic illustration of normal gastric mucosa in the fetus (A), adult (B), and gastric mucosa with intestinal metaplasia (C). The expression patterns of each CLDN and mucin and their respective phenotypes are shown by different cell types. In the fetal stomach, CLDN7 expression was observed at the 9th week of gestational age, but diminished by the 23rd week.
Figure 2.
Immunoreactivity for CLDN3 and CLDN18 in non-neoplastic gastric mucosa. In normal gastric mucosa, the expression of CLDN3 (A) is negative, whereas that of CLDN18 (B) is positive in both the foveolar and glandular epithelia. Gastric mucosa with intestinal metaplasia is positive for CLDN3 (C), -4 (D), and -7 (E), and the expression of CLDN18 (F) is diminished, with only scattered positive cells remaining at the base. MUC2 expression (G) and CD10 expression (H) are also observed. The fetal stomach at the 9th week of gestational age is lined by pseudostratified columnar epithelia (I), which are positive for CLDN18 (J) and CLDN7 (K). (L–P) Fetal stomach at the 23rd week of gestational age. CLDN18 expression remains (M), whereas CLDN7 expression is diminished (N). Surface epithelium is positive for MUC5AC (O), and proper gland epithelium is positive for MUC6 (P). Bar = 100 μm.
In the fetal stomach, gastric epithelia appeared as immature, pseudostratified columnar cells without pit or gland formation at the 9th gestational week, and the proper glands appeared around the 13th to 20th week. Membranous staining for CLDN18 was observed as early as the 9th week (Figures 2I and 2J), and was maintained throughout the fetal period, on the surface foveolar epithelia and the epithelia of proper glands. CLDN7 expression was observed in the immature gastric epithelia at the 9th gestational week (Figure 2K). It decreased thereafter and was nearly absent by the 23rd week (Figures 2L–2N). The expression of CLDN1, -3, and -4 was not observed in the gastric epithelia at any developmental stage. Gastric mucin expression appeared in parallel with decreases in CLDN7 expression (Figures 2O and 2P). Positive staining for MUC5AC was observed in the foveolar epithelia at the 13th week, followed by MUC6 expression in the glandular epithelia at the 20th week.
No other alimentary tract expressed CLDN18 during fetal development. In contrast, CLDN7 expression was widely observed. CLDN3 and CLDN4 expression was detected in the small and large intestines near the second trimester. CLDN1 expression was rarely observed, with the exception of mature squamous epithelia of the esophagus in the last trimester.
Clinicopathological Features of EBV-associated GC and EBV-negative GC
The clinicopathological features of EBV-associated and EBV-negative GC are shown in Table 1. Nearly half of all EBV-associated GC cases arose in the upper (proximal) part of the stomach, whereas more than half of all EBV-negative GC cases were located at the middle or lower (distal) part (p=0.0005). No statistically significant difference was observed between the two groups in other parameters, including mean age, sex, size, T and N grade, and tumor stage. For EBV-associated GC, poorly differentiated, solid-type adenocarcinoma (por1) and moderately differentiated tubular adenocarcinoma (tub2) were the two main histological types observed. However, no significant differences in histology were detected by Lauren's classification.
Table 1.
Clinicopathological features of EBV-associated GC and EBV-negative GC
| EBV-associated GC | EBV-negative GC | |
|---|---|---|
| No. of cases (no. of patients) | 43 (38) | 68 (68) |
| Age, year (mean ± SD) | 66.1 ± 8.9 | 62.6 ± 11.8 |
| Sex (M/F) | 30/8 | 46/22 |
| Locationa (U/M/L/R) | 22/15/4/2 | 13/32/21/2 |
| Size, cm (mean ± SD) | 4.5 ± 3.2 | 5.5 ± 3.8 |
| T gradeb (T1/T2/T3/T4) | 23/12/6/2 | 32/17/17/2 |
| N gradeb (N0/N1/N2/N3) | 27/7/2/2 | 46/13/5/4 |
| Stageb (IA+IB/II/IIIA+IIIB/IV) | 27/4/3/4 | 41/12/7/8 |
| Histological typec (pap/tub1/tub2/muc/por1/por2/sig) | 0/4/17/0/17/3/2 | 1/15/15/1/2/19/15 |
| Lauren's classification (intestinal/diffuse) | 21/22 | 32/36 |
| Lymphatic invasion (positive/negative) | 32/11 | 42/26 |
| Venous invasion (positive/negative) | 26/17 | 31/37 |
The difference in tumor location between EBV-associated GC and EBV-negative GC was statistically significant (p=0.0005, χ2 test).
T and N grade and tumor stage were determined according to the tumor node metastasis (TNM) classification of the International Union Against Cancer (UICC).
The difference in histological type between EBV-associated GC and EBV-negative GC was statistically significant (p=0.0009, χ2 test).
EBV, Epstein-Barr virus; GC, gastric carcinoma; M, male; F, female; U, upper; M, middle; L, lower; R, residual stomach (state after partial gastrectomy); pap, papillary carcinoma; tub1, well-differentiated tubular carcinoma; tub2, moderately differentiated tubular carcinoma; muc, mucinous carcinoma; por1, poorly differentiated adenocarcinoma, solid type; por2, poorly differentiated adenocarcinoma, non–solid type; sig, signet ring cell carcinoma.
CLDN Expression in EBV-associated GC
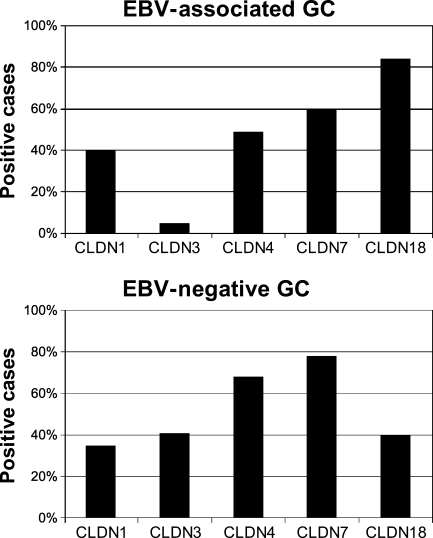
EBV-associated GC (n=43) showed positive immunoreactivity for CLDN1, -3, -4, -7, and -18 in 40%, 5%, 49%, 60%, and 84% of the cases, respectively. EBV-negative GC (n=68) showed positive immunoreactivity for CLDN1, -3, -4, -7, and -18 in 35%, 41%, 68%, 78%, and 40% of the cases, respectively (Figures 3 and 4). EBV-associated GC showed a higher incidence of CLDN18 expression (p<0.0001) and a lower incidence of CLDN3 expression (p<0.0001) as compared with EBV-negative GC. These results indicate that the CLDN3-negative and CLDN18-positive phenotype was predominant in EBV-associated GC (36/43, 84%), and that EBV-associated GC was considerably homogeneous with regard to cellular characteristics, in contrast to EBV-negative GC.
Figure 3.
CLDN expression in Epstein-Barr virus (EBV)-associated gastric carcinoma (GC) and EBV-negative GC. The percentage of positive cases for each CLDN in EBV-associated GC and EBV-negative GC is shown. The expression of CLDN18 is significantly higher in EBV-associated GC compared with EBV-negative GC (p<0.0001, Fisher's exact test), whereas CLDN3 expression is significantly higher in EBV-negative GC compared with EBV-associated GC (p<0.0001, Fisher's exact test).
Figure 4.
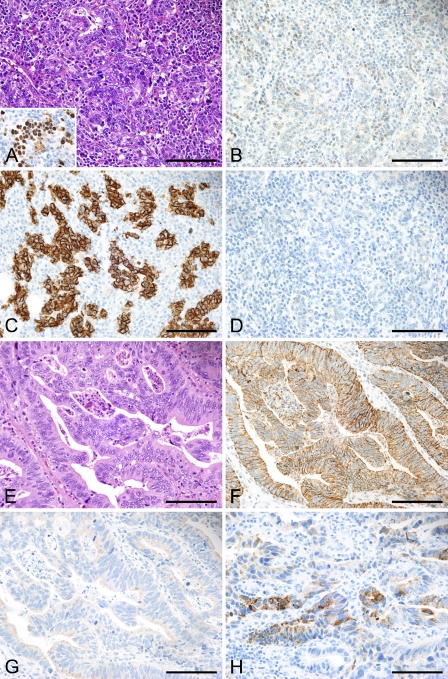
Immunoreactivity for CLDN and mucin in gastric carcinoma EBV-associated GC (A–D). Dense lymphocytic infiltrate within the tumor represents lymphoepithelioma-like histology (A, inset: EBER1 in situ hybridization). This case is negative for CLDN3 (B). Strong membranous staining for CLDN18 highlights tumor cells (C). The tumor is negative for gastric mucin (MUC6) (D). (E–H) EBV-negative GC. This case shows moderately differentiated tubular structures (E), which are positive for CLDN3 (F), and negative for CLDN18 (G). An intestinal mucin marker, MUC2 (H) is positive in tumor cells. Bar = 100 μm.
Table 2 outlines the association between CLDN expression and clinicopathological parameters in EBV-associated GC. CLDN7-positive cases were associated with old age (p<0.0001), large tumor size (p=0.0169), advanced T grade (p=0.0136) and stage (p=0.0072), and the presence of venous invasion (p=0.0261), compared with CLDN7-negative cases. Similarly, CLDN4-positive cases were associated with advanced T grade (p=0.0228) and stage (p=0.0322) compared with CLDN4-negative cases. None of the other CLDNs (i.e., CLDN1, -3, and -18) were associated with any of the above parameters.
Table 2.
Association between CLDN expression and clinicopathological parameters in EBV-associated GC
| CLDN1 | CLDN3 | CLDN4 | CLDN7 | CLDN18 | |
|---|---|---|---|---|---|
| No. of cases (no. of patients) | |||||
| Positive | 17 (17) | 2 (2) | 21 (19) | 26 (24) | 36 (32) |
| Negative | 26 (21) | 41 (36) | 22 (19) | 17 (14) | 7 (6) |
| Age, year (mean ± SD) Student's t-test | |||||
| Positive | 66.8 ± 8.3 | 61.5 ± 3.5 | 68.5 ± 8.1 | 70.0 ± 7.5 | 66.6 ± 9.4 |
| Negative | 65.3 ± 9.4 | 66.3 ± 9.0 | 63.5 ± 9.1 | 59.2 ± 6.6 | 62.8 ± 4.8 |
| p | ns | ns | ns | <0.0001 | ns |
| Sex (M/F) Fisher's exact test | |||||
| Positive | 14/3 | 2/0 | 13/6 | 19/5 | 24/8 |
| Negative | 16/5 | 28/8 | 17/2 | 11/3 | 6/0 |
| p | ns | ns | ns | ns | ns |
| Location (U/M/L/R) χ2 test | |||||
| Positive | 8/6/3/0 | 1/1/0/0 | 8/8/3/2 | 13/10/2/1 | 19/12/3/2 |
| Negative | 14/9/1/2 | 21/14/4/2 | 14/7/1/0 | 9/5/2/1 | 3/3/1/0 |
| p | ns | ns | ns | ns | ns |
| Size, cm (mean ± SD) Student's t-test | |||||
| Positive | 4.4 ± 4.0 | 5.7 ± 1.7 | 4.9 ± 3.5 | 5.4 ± 3.6 | 4.7 ± 3.3 |
| Negative | 4.5 ± 2.6 | 4.4 ± 3.2 | 4.0 ± 2.8 | 3.0 ± 1.8 | 3.2 ± 2.0 |
| p | ns | ns | ns | 0.0169 | ns |
| T gradea (T1/T2/T3/T4) Mann-Whitney U-test | |||||
| Positive | 9/3/4/1 | 0/1/1/0 | 8/6/6/1 | 10/9/5/2 | 19/10/5/2 |
| Negative | 14/9/2/1 | 23/11/5/2 | 15/6/0/1 | 13/3/1/0 | 4/2/1/0 |
| p | ns | ns | 0.0228 | 0.0136 | ns |
| N gradea (N0/N1/N2/N3) Mann-Whitney U-test | |||||
| Positive | 13/2/0/2 | 1/1/0/0 | 12/4/1/2 | 15/6/1/2 | 22/6/2/2 |
| Negative | 14/5/2/0 | 26/6/2/2 | 15/3/1/0 | 12/1/1/0 | 5/1/0/0 |
| p | ns | ns | ns | ns | ns |
| Stagea (IA/IB/II/IIIA/IIIB/IV) Mann-Whitney U-test | |||||
| Positive | 9/3/1/1/0/3 | 0/1/0/0/0/1 | 6/5/2/2/1/3 | 7/8/3/2/0/4 | 15/7/4/2/1/3 |
| Negative | 9/6/3/1/1/1 | 18/8/4/2/1/3 | 12/4/2/0/0/1 | 11/1/1/0/1/0 | 3/2/0/0/0/1 |
| p | ns | ns | 0.0322 | 0.0072 | ns |
| Histological type (pap/tub1/tub2/ muc/por1/por2/sig) χ2 test | |||||
| Positive | 0/1/8/0/7/1/0 | 0/0/1/0/1/0/0 | 0/3/9/0/8/1/0 | 0/3/9/0/13/1/0 | 0/4/15/0/14/2/1 |
| Negative | 0/3/9/0/10/2/2 | 0/4/16/0/16/3/2 | 0/1/8/0/9/2/2 | 0/1/8/0/4/2/2 | 0/0/2/0/3/1/1 |
| p | ns | ns | ns | ns | ns |
| Lauren's classification (intestinal/diffuse) Fisher's exact test | |||||
| Positive | 9/8 | 1/1 | 12/9 | 12/14 | 19/17 |
| Negative | 12/14 | 20/21 | 9/13 | 9/8 | 2/5 |
| p | ns | ns | ns | ns | ns |
| Lymphatic invasion (positive/negative) Fisher's exact test | |||||
| Positive | 14/3 | 2/0 | 14/7 | 17/9 | 25/11 |
| Negative | 18/8 | 30/11 | 18/4 | 15/2 | 7/0 |
| p | ns | ns | ns | ns | ns |
| Venous invasion (positive/negative) Fisher's exact test | |||||
| Positive | 9/8 | 1/1 | 10/11 | 12/14 | 21/15 |
| Negative | 17/9 | 25/16 | 16/6 | 14/3 | 5/2 |
| p | ns | ns | ns | 0.0261 | ns |
T and N grade and tumor stage were determined according to the TNM classification of the UICC. The clinicopathological differences between CLDN-positive and CLDN-negative groups were statistically analyzed. A p value of less than 0.05 is considered statistically significant.
CLDN, claudin; ns, not significant.
CLDN Phenotypes of GC With or Without EBV Infection
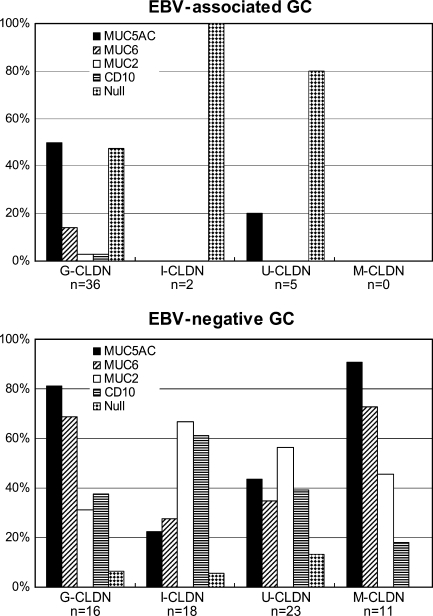
CLDN3 and CLDN18 expression patterns best described the homogeneous cellular characteristics of EBV-associated GC. Therefore, we further classified EBV-negative GCs into four distinct CLDN phenotypes according to the expression of these two CLDNs based on comparisons with normal tissues in the stomach: G (gastric)-CLDN; CLDN3-negative and CLDN18-positive, I (intestinal)-CLDN; CLDN3-positive and CLDN18-negative, M (mixed)-CLDN; both CLDN3- and 18-positive, U (undifferentiated)-CLDN; neither CLDN3- nor -18-positive. Among the 43 cases of EBV-associated GC, 36 cases (84%) were classified as G-CLDN, 2 cases (5%) as I-CLDN, and 5 cases (12%) as U-CLDN; none of them were classified as M-CLDN. In contrast, EBV-negative GC consisted of 16 cases (24%) of G-CLDN, 18 cases (27%) of I-CLDN, 11 cases (16%) of M-CLDN, and 23 cases (34%) of U-CLDN.
Association Between CLDN Phenotypes and Mucin Expression
Figure 5 shows the association between the CLDN phenotypes and mucin expression. In EBV-associated GC, MUC5AC was the most frequently expressed mucin (positive in 50% of G-CLDN and 20% of U-CLDN cases). In addition, cases that did not express any of the four mucin markers (i.e., null type) comprised 47% of G-CLDN, 100% of I-CLDN, and 80% of U-CLDN cases. In contrast, mucin expression was more widely observed in EBV-negative GC. Although not statistically significant, G-CLDN showed a high prevalence of MUC5AC and MUC6 expression, whereas in I-CLDN cases, the expression of MUC2 and CD10 was higher.
Figure 5.
Associations between CLDN phenotype and mucin expression. Percentages of positive cases for mucin markers and CD10 in each CLDN phenotype are shown. Cases without expression of any of these markers were defined as the null type; the percentage of each CLDN phenotype is given. G-CLDN, gastric CLDN; I-CLDN, intestinal CLDN; U-CLDN, undifferentiated CLDN; M-CLDN, mixed CLDN.
Association Between CLDN Phenotypes and Clinicopathological Parameters
Table 3 shows the clinicopathological characteristics of the G-CLDN phenotype of EBV-associated GC and each CLDN phenotype of EBV-negative GC. Associations between the clinicopathological parameters and CLDN phenotypes were analyzed. In EBV-negative GC, the G-CLDN phenotype was associated with a younger age, a larger tumor size, advanced T grade, diffuse-type histology (Lauren's classification), and frequent venous invasion. In contrast, in EBV-negative GC, the U-CLDN phenotype was associated with female predominance, a smaller tumor size, less-advanced T grade, and infrequent venous invasion. Clinicopathological differences between the four phenotypes in EBV-negative GC were statistically significant. When compared with the G-CLDN phenotype of EBV-associated GC, the G-CLDN phenotype of EBV-negative GC was associated with a younger age, a larger tumor size, a more-advanced T grade and tumor stage, and a more-frequent venous invasion. These differences were also statistically significant.
Table 3.
Association between CLDN phenotypes and clinicopathological parameters
| EBV-associated GC
|
EBV-negative GC
|
||||
|---|---|---|---|---|---|
| G-CLDN | G-CLDN | I-CLDN | M-CLDN | U-CLDN | |
| No. of cases (no. of patients) | 36 (32) | 16 (16) | 18 (18) | 11 (11) | 23 (23) |
| Age, year (mean ± SD) ANOVA | 66.6 ± 9.4 | 57.4 ± 12.5 | 69.3 ± 6.9 | 63.9 ± 10.4 | 60.2 ± 12.9 |
| p=0.0063 | p=0.0153 | ||||
| Sex (M/F) χ2 test | 24/8 | 13/3 | 14/4 | 8/3 | 11/12 |
| ns | ns | ||||
| Location (U/M/L/R) χ2 test | 19/12/3/2 | 3/6/7/0 | 6/9/3/0 | 1/5/3/2 | 3/13/7/0 |
| p=0.0105 | ns | ||||
| Size, cm (mean ± SD) ANOVA | 4.7 ± 3.3 | 7.6 ± 4.4 | 5.8 ± 3.5 | 6.2 ± 3.4 | 3.3 ± 2.4 |
| p=0.0132 | p=0.0031 | ||||
| T gradea (T1/T2/T3/T4) Kruskal-Wallis test | 19/10/5/2 | 2/6/7/1 | 9/5/3/1 | 5/4/2/0 | 16/2/5/0 |
| p=0.0053 | p=0.0106 | ||||
| N gradea (N0/N1/N2/N3) Kruskal-Wallis test | 22/6/2/2 | 8/6/2/0 | 12/2/2/2 | 8/2/0/1 | 18/3/1/1 |
| ns | ns | ||||
| Stagea (IA+IB/II/IIIA+IIIB/IV) Kruskal-Wallis test | 22/4/3/3 | 4/8/3/1 | 12/1/3/2 | 8/1/0/2 | 17/2/1/3 |
| p=0.0229 | ns | ||||
| Histological type (pap/tub1/tub2/muc/por1/por2/sig) χ2 test | 0/4/15/0/14/2/1 | 0/1/3/0/1/6/5 | 1/3/6/0/1/4/3 | 0/5/4/0/0/1/1 | 0/6/2/1/0/8/6 |
| p=0.0001 | ns | ||||
| Lauren's classification (intestinal/diffuse) χ2 test | 19/17 | 4/12 | 10/8 | 9/2 | 9/14 |
| ns | p=0.0238 | ||||
| Lymphatic invasion (positive/negative) χ2 test | 25/11 | 7/9 | 10/8 | 8/3 | 17/6 |
| ns | ns | ||||
| Venous invasion (positive/negative) χ2 test | 21/15 | 3/13 | 7/11 | 4/7 | 17/6 |
| p=0.0146 | p=0.0046 | ||||
T grade, N grade, and tumor stage were determined according to the TNM classification of the UICC.
The clinicopathological differences between the G-CLDN phenotype of EBV-associated GC and the G-CLDN phenotype of EBV-negative GC were statistically analyzed, and the p value is shown in the column of EBV-associated GC. Differences among four different CLDN phenotypes in EBV-negative GC were also assessed and the p value is shown in the column of EBV-negative GC. A p value of less than 0.05 is considered statistically significant.
Discussion
In this study, we demonstrated that EBV-associated GC displays a characteristic CLDN expression pattern, which is clearly distinguishable from that of EBV-negative GC. EBV-associated GC is correlated with well-preserved gastric CLDN (i.e., CLDN18) and less-frequent aberrant expression of CLDN3. Mucin expression in EBV-associated GC showed a similar trend. The association between mucin expression and tumor differentiation in GC has been well investigated in the previous reports. Reis et al. (2000) and Pinto-de-Sousa et al. (2002) demonstrated that the expression of MUC2 and MUC5AC is associated with mucinous-type GC and diffuse-type GC, respectively. In our 43 cases of EBV-associated GC, we observed a higher frequency of gastric or null-type mucin expression and rare intestinal mucin expression. These results are in agreement with our previous report, and indicate the lack of differentiation potential toward the intestinal phenotype (Barua et al. 2006).
When discussing the impact of CLDN expression on gastric carcinomas, it is necessary to elucidate the significance of CLDN expression in the stomach. In this study, we investigated the expression patterns of different CLDNs in the non-neoplastic gastric mucosa of adults and fetuses and found that CLDNs differed from each other in both their distribution patterns and possible physiological functions. The fact that CLDN18 expression is specific to the adult stomach has been well demonstrated using the SAGE (serial analysis of gene expression) database, RT-PCR of normal tissues, and immunohistochemistry, as in the present study (Hewitt et al. 2006; Sanada et al. 2006). By examining the fetal stomach, we found that CLDN18 is strongly expressed within the gastric epithelium before it differentiates into the pits and glands (and subsequently expresses gastric mucin). Furthermore, CLDN18 is expressed exclusively in the stomach; it is not found in other alimentary tracts, including the esophagus and small and large intestines. These findings strongly suggest that CLDN18 is involved in the differentiation into gastric epithelium, and this is also supported by the fact that CLDN18 expression is lost in gastric mucosa with intestinal metaplasia and in some gastric carcinomas. Additionally, morphologically immature cells present in the neck zone of the gastric pits or at the base of the metaplastic epithelium lack gastric mucin expression, but retain CLDN18 expression. From these results, we can conclude that CLDN18 expression is a more fundamental and sensitive gastric marker.
Note that EBV-associated GC possesses homogenous cellular characteristics of CLDN expression—well-preserved CLDN18 expression and a lack of aberrant CLDN3 expression. Taken together with the gastric or null-type mucin expression, EBV-associated GC demonstrates traits that are identical to those of mature or immature gastric epithelium. From these results, we hypothesized that EBV-associated GC would arise directly from immature proliferating cells and retain characteristics of the cells of origin throughout the carcinogenic process. Other CLDNs, which are prevalent in intestinal metaplasia, showed significantly lower expression levels. Therefore, EBV-associated GC may arise through a different process, not as a consequence of chronic gastritis and intestinal metaplasia. In contrast, when classified according to expression patterns of CLDN3 and CLDN18, EBV-negative GC cases were equally distributed among the four CLDN phenotypes. These results imply that EBV-negative GC consists of heterogeneous groups with variable differentiation potential and diverse genetic changes in the course of tumor progression. Further studies investigating the association between CLDNs and other tumor differentiation markers with biomolecular techniques are necessary to clarify the significance of CLDNs in carcinogenesis and tumor differentiation.
In addition to exploring the role of CLDNs as differentiation markers, these studies sought to investigate the significance of CLDN in the stomach. Normal gastric mucosa lacks expression of CLDN3, -4, -7, and -1; the former three are found in metaplastic mucosa, and all of them are found in some gastric carcinomas. Therefore, it is reasonable to consider the aberrant expression of these CLDNs as a “gain” of novel function in the metaplastic or carcinogenic process. In EBV-associated GC, for example, the expression of CLDN4 and CLDN7 was correlated with parameters indicating tumor invasiveness. In EBV-negative GC, the G-CLDN phenotype was associated with tumor aggressiveness compared with the other CLDN phenotypes; however, the G-CLDN phenotype of EBV-associated GC was not as aggressive as the G-CLDN phenotype of EBV-negative GC. This inconsistency may have been due to the effects of EBV infection. Further studies are needed to elucidate the impact of CLDN expression on GC and its association with EBV infection.
With regard to the regulation of CLDN expression, studies have cited the involvement of epigenetic modification of the CLDN promoter. Honda et al. (2006) demonstrated that promoter methylation and histone deacetylation contributed to CLDN4 silencing in ovarian cancer cell lines. Given that EBV-associated GC shows global CpG island methylation in the promoter region of various cancer-related genes, some CLDNs that are less frequently expressed in EBV-associated GC may be associated with altered methylation status within the promoter region (Chong et al. 2003; Chang et al. 2006).
In conclusion, by CLDN expression profiling, we demonstrated that EBV-associated GC is considerably homogeneous with regard to cellular differentiation, which is characterized by well-preserved gastric CLDN (CLDN18) expression and the lack of aberrant expression of intestinal CLDN (CLDN3). It also displays characteristic gastric or null type mucin expression. These expression profiles closely resemble those of immature proliferating epithelium of the stomach. In contrast, EBV-negative GC comprises a heterogeneous group with variable expression patterns of CLDNs and mucins. EBV-associated GC may undergo distinct carcinogenic processes, which differ from those of EBV-negative GC.
Acknowledgments
This work was supported by a grant-in aid for scientific research on priority areas (20249022) from the Ministry of Education, Culture, Science, Sports, and Technology of Japan.
References
- Barua RR, Uozaki H, Chong JM, Ushiku T, Hino R, Chang MS, Nagai H, et al. (2006) Phenotype analysis by MUC2, MUC5AC, MUC6, and CD10 expression in Epstein-Barr virus-associated gastric carcinoma. J Gastroenterol 41:733–739 [DOI] [PubMed] [Google Scholar]
- Chang MS, Uozaki H, Chong JM, Ushiku T, Sakuma K, Ishikawa S, Hino R, et al. (2006) CpG island methylation status in gastric carcinoma with and without infection of Epstein-Barr virus. Clin Cancer Res 12:2995–3002 [DOI] [PubMed] [Google Scholar]
- Chong JM, Sakuma K, Sudo M, Ushiku T, Uozaki H, Shibahara J, Nagai H, et al. (2003) Global and non-random CpG-island methylation in gastric carcinoma associated with Epstein-Barr virus. Cancer Sci 94:76–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukayama M, Hayashi Y, Iwasaki Y, Chong J, Ooba T, Takizawa T, Koike M, et al. (1994) Epstein-Barr virus-associated gastric carcinoma and Epstein-Barr virus infection of the stomach. Lab Invest 71:73–81 [PubMed] [Google Scholar]
- Fukayama M, Hino R, Uozaki H (2008) Epstein-Barr virus and gastric carcinoma: virus-host interactions leading to carcinoma. Cancer Sci 99:1726–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt KJ, Agarwal R, Morin PJ (2006) The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer 6:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda H, Pazin MJ, Ji H, Wernyj RP, Morin PJ (2006) Crucial roles of Sp1 and epigenetic modifications in the regulation of the CLDN4 promoter in ovarian cancer cells. J Biol Chem 281:21433–21444 [DOI] [PubMed] [Google Scholar]
- Hornsby CD, Cohen C, Amin MB, Picken MM, Lawson D, Yin-Goen Q, Young AN (2007) Claudin-7 immunohistochemistry in renal tumors: a candidate marker for chromophobe renal cell carcinoma identified by gene expression profiling. Arch Pathol Lab Med 131:1541–1546 [DOI] [PubMed] [Google Scholar]
- Japanese Gastric Cancer Association (1998) Japanese classification of gastric carcinoma: 2nd English edition. Gastric Cancer 1:10–24 [DOI] [PubMed]
- Johnson AH, Frierson HF, Zaika A, Powell SM, Roche J, Crowe S, Moskaluk CA, et al. (2005) Expression of tight-junction protein claudin-7 is an early event in gastric tumorigenesis. Am J Pathol 167:577–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi H, Takizawa T, Eishi Y, Shimizu S, Kumagai J, Funata N, Koike M (2003) Absence of either gastric or intestinal phenotype in microscopic differentiated gastric carcinomas. J Pathol 199:436–446 [DOI] [PubMed] [Google Scholar]
- Lauren P (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 64:31–49 [DOI] [PubMed] [Google Scholar]
- Lee SK, Moon J, Park SW, Song SY, Chung JB, Kang JK (2005) Loss of the tight junction protein claudin 4 correlates with histological growth-pattern and differentiation in advanced gastric adenocarcinoma. Oncol Rep 13:193–199 [PubMed] [Google Scholar]
- Matsuda Y, Semba S, Ueda J, Fuku T, Hasuo T, Chiba H, Sawada N, et al. (2007) Gastric and intestinal claudin expression at the invasive front of gastric carcinoma. Cancer Sci 98:1014–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Park KH, Oh TY, Hong SP, Jeon TJ, Kim CH, Park SW, et al. (2007) Up-regulated claudin 7 expression in intestinal-type gastric carcinoma. Oncol Rep 18:377–382 [PubMed] [Google Scholar]
- Pinto-de-Sousa J, David L, Reis CA, Gomes R, Silva L, Pimenta A (2002) Mucins MUC1, MUC2, MUC5AC and MUC6 expression in the evaluation of differentiation and clinico-biological behaviour of gastric carcinoma. Virchows Arch 440:304–310 [DOI] [PubMed] [Google Scholar]
- Reis CA, David L, Carvalho F, Mandel U, de Bolos C, Mirgorodskaya E, Clausen H, et al. (2000) Immunohistochemical study of the expression of MUC6 mucin and co-expression of other secreted mucins (MUC5AC and MUC2) in human gastric carcinomas. J Histochem Cytochem 48:377–388 [DOI] [PubMed] [Google Scholar]
- Resnick MB, Gavilanez M, Newton E, Konkin T, Bhattacharya B, Britt DE, Sabo E, et al. (2005) Claudin expression in gastric adenocarcinomas: a tissue microarray study with prognostic correlation. Hum Pathol 36:886–892 [DOI] [PubMed] [Google Scholar]
- Sanada Y, Oue N, Mitani Y, Yoshida K, Nakayama H, Yasui W (2006) Down-regulation of the claudin-18 gene, identified through serial analysis of gene expression data analysis, in gastric cancer with an intestinal phenotype. J Pathol 208:633–642 [DOI] [PubMed] [Google Scholar]
- Sobin L, Wittekind C (2002) TNM Classification of Malignant Tumours. 6th ed. Hoboken, NJ, John Wiley & Sons
- Soini Y, Tommola S, Helin H, Martikainen P (2006) Claudins 1, 3, 4 and 5 in gastric carcinoma, loss of claudin expression associates with the diffuse subtype. Virchows Arch 448:52–58 [DOI] [PubMed] [Google Scholar]
- Sousa H, Pinto-Correia AL, Medeiros R, Dinis-Ribeiro M (2008) Epstein-Barr virus is associated with gastric carcinoma: the question is what is the significance? World J Gastroenterol 14:4347–4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Furuse M (2000) Pores in the wall: claudins constitute tight junction strands containing aqueous pores. J Cell Biol 149:13–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozaki H, Fukayama M (2008) Epstein-Barr virus and gastric carcinoma: viral carcinogenesis through epigenetic mechanisms. Int J Clin Exp Pathol 1:198–216 [PMC free article] [PubMed] [Google Scholar]
- Utsunomiya T, Yonezawa S, Sakamoto H, Kitamura H, Hokita S, Aiko T, Tanaka S, et al. (1998) Expression of MUC1 and MUC2 mucins in gastric carcinomas: its relationship with the prognosis of the patients. Clin Cancer Res 4:2605–2614 [PubMed] [Google Scholar]
- Wakatsuki K, Yamada Y, Narikiyo M, Ueno M, Takayama T, Tamaki H, Miki K, et al. (2008) Clinicopathological and prognostic significance of mucin phenotype in gastric cancer. J Surg Oncol 98:124–129 [DOI] [PubMed] [Google Scholar]