Abstract
Yeast Saccharomyces cerevisiae has been a valuable model organism for the study of the endosomal system of eukaryotic cells. Morphological analyses, however, have been limited because of the lack of specific protein markers and of procedures that lead to a satisfactory ultrastructural resolution. We have recently developed an immunoelectron microscopy (IEM) protocol adapted from the Tokuyasu method to prepare cryosections from mildly fixed yeast. This novel approach allows excellent cell preservation and a unique resolution of the yeast morphology. Here, we present a protocol that combines this procedure with the specific labeling of the various endosomal compartments with positively charged Nanogold. In particular, we show that this new protocol generates excellent results when applied for the examination of early and late endosomes, and of mutants with an endosomal trafficking defect. Importantly, this method is compatible with immunogold labeling of protein markers, and it is consequently appropriate for localization studies of both resident and cargo proteins. This new IEM protocol will be a valuable tool for the large community of scientists using yeast as a model system to investigate the membrane transport and the biogenesis of the endosomal system. (J Histochem Cytochem 57:801–809, 2009)
Keywords: yeast, endocytosis, endosomes, vacuole, positively charged Nanogold, electron microscopy, Tokuyasu cryosectioning, immunogold labeling
The yeast Saccharomyces cerevisiae has been shown to internalize extracellular fluid, ligands bound to their receptors, and plasma membrane (PM) proteins (Shaw et al. 2001; Katzmann et al. 2002). Once internalized by endocytosis, most of the characterized molecules are delivered to the vacuole, where they are degraded. Transport to the vacuole passes through two morphologically and functionally different compartments, the early endosome (EE) and late endosome (LE) compartments (Prescianotto-Baschong and Riezman 1998,2002; Munn 2000; Katzmann et al. 2002). The latter organelles invaginate part of their limiting membrane, leading to the formation of multivesicular bodies (MVBs), which successively fuse with the vacuole, releasing their internal vesicles into the interior of this organelle (Hurley and Emr 2006). The EE and LE compartments are also the convergence points with the biosynthetic routes delivering endosomal and vacuolar resident proteins from the Golgi (Conibear and Stevens 1998; Bowers and Stevens 2005). The sorting of several of these resident proteins requires cycling receptors that together with specific soluble N-ethylmaleimide–sensitive fusion attachment receptors, are retrieved back to the Golgi from both the EE and LE compartments for reuse (Bowers and Stevens 2005).
Despite the numerous transport pathways connecting the various compartments, the yeast endosomal system is relatively less complex than that of mammals. In addition to the EE and LE compartments, high-eukaryotic cells possess recycling endosomes and tissue-specific lysosome-related organelles, which results in a more intricate network of endosomal transport routes (Luzio et al. 2007; van Meel and Klumperman 2008). Therefore, the yeast system represents an advantageous experimental setup. Importantly, genetic screens have led to the isolation of more than 50 vacuolar protein sorting (vps) mutants (Jones 1977; Robinson et al. 1988; Rothman et al. 1989), and most of their gene products are conserved among eukaryotes. The characterization of the Vps proteins has helped enormously in clarifying the transport events occurring in the eukaryotic endosomal system and has led to the identification of crucial players such as the endosomal sorting complex required for transport, the homotypic fusion and vacuole protein sorting, the class C core vacuole/endosome tethering, the retromer, and the Golgi-associated retrograde protein complexes (Bowers and Stevens 2005; Hurley and Emr 2006; Peplowska et al. 2007).
Biochemical and fluorescence microscopy–based approaches have been extensively used to study the yeast endosomal system in wild-type cells and in the vps mutants. Investigations at an ultrastructural level, in contrast, have only been minimal, mainly for two reasons. The first is the non-optimal morphological resolution of yeast electron microscopy preparations, and the second is the absence of protein markers for the yeast endocytic pathway that can be easily detected (Prescianotto-Baschong and Riezman 2002).
A successful approach employed to visualize the yeast endosomal compartments has been the internalization of positively charged Nanogold by yeast spheroplasts (Prescianotto-Baschong and Riezman 1998). These particles strongly bind to the negatively charged lipids present on the cell surface, and after endocytosis, they reach the vacuole, passing through the EE and LE compartments. We have recently developed a new immunoelectron microscopy (IEM) procedure adapted from the Tokuyasu method to prepare cryosections from mildly fixed yeast cells (Griffith et al. 2008). This novel approach allows immunogold labeling for performing localization studies in combination with an excellent resolution of the morphology. Here we present a protocol that integrates this procedure with the specific labeling of endosomal structures with positively charged Nanogold and show examples of how this new protocol can be applied.
Materials and Methods
Strains, Media, and Preparation of Spheroplasts
The S. cerevisiae strains used in this study are listed in Table 1. Cells were grown in a rich medium (yeast extract peptone dexthrose (YPD); 1% yeast extract, 2% peptone, 2% glucose) to exponential phase [optical density (OD)600 = 0.7–1.0]. Ten OD600-unit equivalents of cells were collected by centrifugation at 3000 rpm for 5 min and converted to spheroplasts as follows: First, they were resuspended in 5 ml of 100 mM PIPES (pH 9.6) and 10 mM dithiothreitol and incubated at 30C for 10 min. Then the cells were collected again by centrifugation and resuspended in 5 ml of YPD medium containing 1 M sorbitol and 5 mg of lytic enzyme MP Biomedicals; Illkirch, France) and incubated at 30C for 30 min. The suspension was finally centrifuged at 1500 rpm for 5 min, and spheroplasts were resuspended in 960 μl of ice-cold YPD medium containing 1 M sorbitol.
Table 1.
Yeast S. cerevisiae strains used in this study
| Name | Genotype | Reference |
|---|---|---|
| CWY40 | SEY6210 vam3Δ::TRP1 | (Wang et al. 2002) |
| MBY3 | SEY6210 vps4Δ::TRP1 | (Babst et al. 1997) |
| SEY6210 | MATαura3-52 leu2-3, 112 his3-Δ200 trp1-Δ901lys2-801 suc2-Δ9 mel GAL | (Robinson et al. 1988) |
Positively Charged Nanogold Labeling and Preparation of Cryosections
A stock solution of 0.1 nmol/μl was made by resuspending 30 nmol of positively charged Nanogold (1.4 nm mean diameter; Nanoprobes, Stony Brook, NY) in 300 μl of distilled water by extensive vortexing. Spheroplasts were gently mixed with 4 nmol of positively charged Nanogold (40 μl of the stock solution) and placed on ice for 15 min. Samples were then transferred to room temperature for the indicated times. Nanogold uptake by the spheroplasts was stopped by addition of 1 ml of double-strength fixative [4% (w/v) paraformaldehyde (PFA), 0.4% (v/v) glutaraldehyde (GA), 1 M sorbitol in 0.1 M PHEM buffer (20 mM PIPES, 50 mM HEPES, pH 6.9, 20 mM EGTA, and 4 mM MgCl2] at room temperature. Tubes were gently inverted several times during 30 min and then centrifuged twice at 6000 rpm for 25 sec. The fixative was then replaced by fresh standard-strength fixative with 1 M sorbitol for an additional 2 hr at room temperature on a slowly moving rotator. Samples were then processed for cryosectioning as described (Griffith et al. 2008) with the omission of the periodic acid treatment. Thin sections of ∼50 nm were cut and mounted on Formvar carbon-coated nickel grids.
Silver Enhancement
The positively charged Nanogold was enhanced with the HQ Silver kit (Nanoprobes) for 6 min at 24C as described by the manufacturer. Sections were then stained for 5 min on 2% uranyl oxalate acetate (pH 7) at room temperature (Tokuyasu 1978) and then passed over a drop of distilled water to a mixture of 1.8% methyl cellulose and 0.6% uranyl acetate (pH 4) on ice. After 5 min, the grids were looped out, the excess viscous solution was drained away, and the sections allowed to dry (Griffiths 1983). Where indicated, sections were immunogold labeled after enhancement.
Immunogold Labeling of Cryosections
Sections were immunolabeled using mouse anti-Pma1 (EnCor Biotechtologies; Gainesville, FL) and anti-Kar2 (a kind gift of Ineke Braakman) antibodies as described (Griffith et al. 2008). Protein A–gold conjugates were prepared according to the method described by Slot and Geuze (2007).
Image Acquisition
Sections were viewed in a JEOL 1010 or a JEOL 1200 electron microscope (JEOL; Tokyo, Japan), and images were recorded on Kodak 4489 sheet films (Kodak; Rochester, NY).
Results
Combination of Cryosectioning Preparations With the Nanogold Labeling
Positively charged Nanogold binds to biological membranes by associating to the negatively charged polar heads of phospholipids. This property has been exploited to label the yeast endosomal system (Prescianotto-Baschong and Riezman 1998). In particular, positively charged Nanogold associates with the PM and enters the endocytic transport route in association with lipids. When Nanogold is incubated with yeast spheroplasts at 4C for 15 min, these particles are exclusively localized to the PM because endocytosis is blocked under these conditions (Prescianotto-Baschong and Riezman 1998). Transfer of the cells to room temperature allows internalization to occur, and the Nanogold reaches the vacuole, passing through the compartments of the endosomal system in a time-course manner.
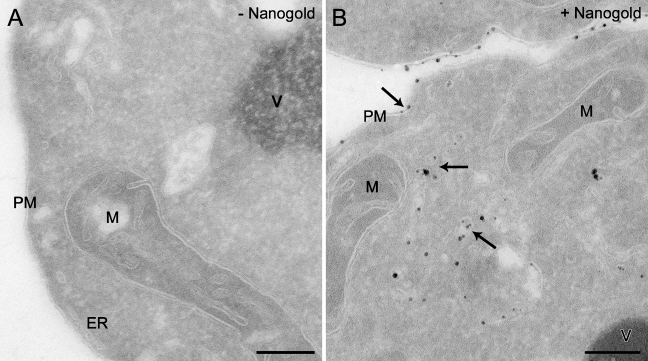
To explore whether this labeling procedure could be combined with the cryosectioning protocol adapted to yeast (Griffith et al. 2008), spheroplasts generated from wild-type cells were incubated at 4C for 15 min in the presence or absence of Nanogold and subsequently transferred to room temperature for 30 min before being fixed with 2% PFA/0.2% GA and processed for IEM as described in Materials and Methods. Nanogold particles are too small to be perceived by electron microscopy, and therefore a chemical reaction known as silver enhancement must be performed on the obtained cryosections to increase their size and render them visible. As shown in Figure 1, the processing of spheroplasts using the protocol developed for the intact yeast also allows an optimal preservation of the cell morphology, and the resolution of membranous structures is excellent. Importantly, the silver enhancement leads to the appearance of numerous particles disseminated along the PM and in the lumen of intracellular structures (Figure 1B, arrows). In contrast, these enhanced gold particles are not present in preparations obtained from mock-treated spheroplasts, confirming the specificity of the enhancement (Figure 1A). In other words, there is no nonspecific nucleation without Nanogold. The IEM protocol for the analysis of intact yeast includes a step in which the cell wall is permeabilized by treatment with periodic acid (Griffith et al. 2008). This reaction is omitted from the procedure employed with spheroplasts, because it is no longer necessary and we additionally found that it severely impairs the silver enhancement reaction (not shown). Another difference from the original approach is the addition of 1 M sorbitol to the initial fixation solution, which helps prevent osmotic damage to the cells (not shown).
Figure 1.
Ultrastructural analysis of Nanogold-labeled yeast using the Tokuyasu method to prepare cryosections. Spheroplasts obtained from the wild-type SEY6210 strain were incubated in the presence (A) or absence (B) of 4 nmol of positively charged Nanogold at 4C for 15 min before being transferred to room temperature for 30 min. After fixation, cells were processed following the Tokuyasu method adapted to yeast, and the silver enhancement reaction was performed on the obtained cryosections. Arrows indicate some of the membranes labeled with the Nanogold. ER, endoplasmic reticulum; M, mitochondria; PM, plasma membrane; V, vacuole. Bar = 200 μm.
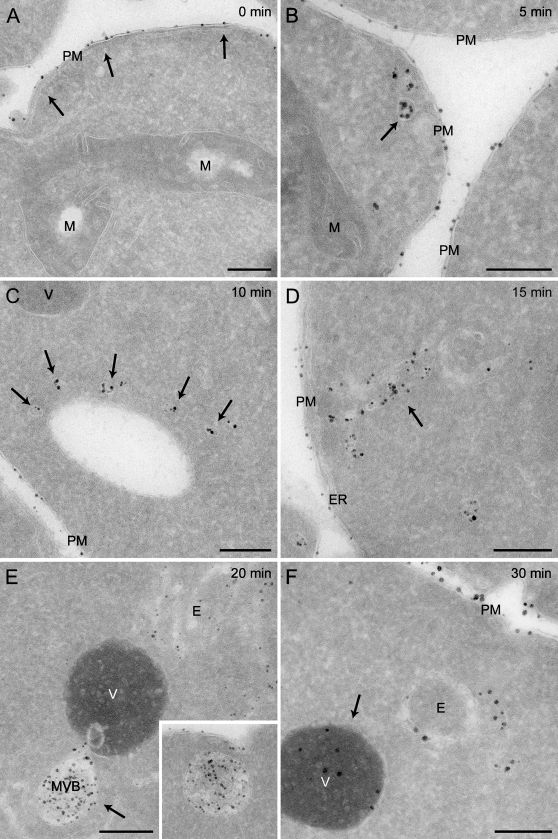
Using our optimized protocol, we performed a time-course experiment to explore the morphology of the compartments through which the endocytosed material passes en route to the vacuole. After incubation at 4C for 15 min in the presence of Nanogold, spheroplasts obtained from wild-type cells were transferred to room temperature and fixed after 0, 5, 10, 15, 20, and 30 min before being processed as described in Materials and Methods. As shown in Figure 2, the Nanogold was detected in various morphologically distinct compartments. As expected, these particles were exclusively found at the PM when spheroplasts were solely kept at 4C (Figure 2A, arrows). When endocytosis was allowed to occur for 5 min, the Nanogold was also detected in small vesicles very likely of endocytic origin (Figure 2B, arrow). After 10 and 15 min, clusters of vesicles and tubules became labeled as well, and those are the yeast EE (Figures 2C and 2D, arrows; Prescianotto-Baschong and Riezman 1998,2002). At these time points, the Nanogold was also occasionally observed in the LE/MVBs (not shown), but the labeling of these structures became much more prominent and uniform after 20 and 30 min (Figure 2E, arrow and inset, and not shown). Some vacuoles were finally labeled with the Nanogold after 30 min (Figure 2F, arrow). These data are consistent with previous observations (Prescianotto-Baschong and Riezman 1998,2002) and confirm that Nanogold can be used to label the compartments of the endocytic route. In addition, they underline the resolution power of the IEM protocol adapted to yeast.
Figure 2.
Time-course labeling of the yeast endocytic compartments with positively charged Nanogold. Spheroplasts obtained from the wild-type SEY6210 strain were incubated in the presence of 4 nmol of positively charged Nanogold at 4C for 15 min before being transferred to room temperature. Aliquots of the resuspension were collected after 0 (A), 5 (B), 10 (C), 15 (D), 20 (E), and 30 min (F) before being fixed and processed as described in Materials and Methods. Arrows point to the membranous structures that become labeled at the indicated times, e.g., PM (A), endocytic vesicles (B), cluster of early endosome (EE) vesicles (C), EE (D), MVB (E), and vacuole (F). (E) inset, MVB with clearly resolved internal vesicles. The Nanogold uptake times are indicated at the top of each panel. E, endosomes; M, mitochondria; MVB, multivesicular body; PM, plasma membrane; V, vacuole. Bar = 200 μm.
Analysis of Membranous Structures Accumulated in Various vps Mutants
The type of experiment just described allows monitoring the dynamic passage of endocytosed material through the different compartments of the yeast endosomal system. To verify whether our Nanogold labeling procedure is also effective in identifying particular endosomal structures persistently accumulated in specific mutant strains, we analyzed two deletions that block different transport events in the endosomal system. We selected two proteins essential for diverse steps of the endocytic route. Vps4 is a member of the AAATPase (ATPase associated with diverse cellular activities) protein family and participates in MVB biogenesis (Babst et al. 1997,1998; Odorizzi et al. 1998). In its absence, the process mediating the invagination of the LE-limiting membrane that leads to the MVB formation is blocked. As a consequence, a cluster of late endosomal compartments known as the class E compartment is accumulated in the proximity of the vacuole. Vam3 is a vacuolar tSNARE (target-soluble N-ethylmaleimide–sensitive factor-attachment protein receptor) essential for all the fusion events with the vacuole (Darsow et al. 1997; Wada et al. 1997). In vam3Δ cells, the endocytosed material is accumulated into MVBs because these organelles are unable to fuse with the vacuole.
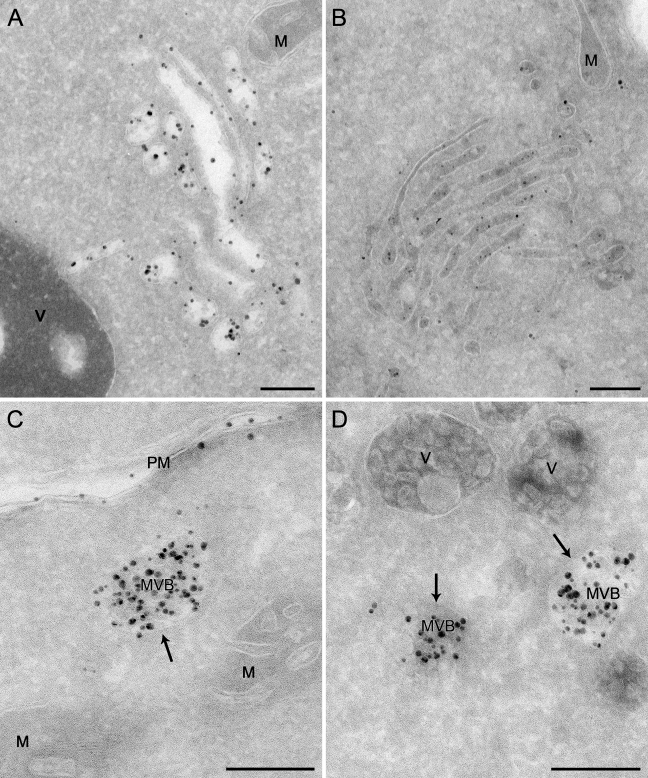
The vps4Δ and vam3Δ deletion strains were grown to an exponential phase before being converted into spheroplasts and incubated at 4C for 15 min in the presence of 4 nmol of positively charged Nanogold. Cells were then transferred to room temperature for 30 min before being processed for cryosectioning and silver enhancement. In addition to being seen on the PM, the Nanogold particles were detected in membranous compartments peculiar to each of two analyzed mutants. In the vps4Δ knockout, the labeling was principally concentrated in large clusters of vesicles and tubules adjacent to the vacuole, the class E compartment (Figures 3A and 3B; Babst et al. 1997,1998; Odorizzi et al. 1998). In most cases, the luminal content of these membranous rearrangements was lost, possibly because it is not easily fixed (Figure 3A); however, it was occasionally preserved (Figure 3B). According to the reported phenotype (Darsow et al. 1997; Wada et al. 1997), MVBs were easily detectable in the absence of Vam3, and they were loaded with the endocytosed Nanogold particles (Figures 3C and 3D, arrows).
Figure 3.
Labeling of the endosomal structures accumulated in various mutants with positively charged Nanogold. Spheroplasts obtained from the MBY3 (vps4Δ) and CWY40 (vam3Δ) strains were incubated in the presence of 4 nmol of positively charged Nanogold at 4C for 15 min before being transferred to room temperature for 30 min. Spheroplasts were then fixed and processed as described in Materials and Methods. (A,B) The class E compartment typical of the vps4Δ cells. (A) A preparation in which the lumen of the vesicles and tubules composing this compartment has been lost, probably because it was not optimally fixable. (B) An example in which the lumen of these organelles remained preserved. (C,D) The MVB accumulated in the vam3Δ strain (arrows). M, mitochondria; MVB, multivesicular body; PM, plasma membrane; V, vacuole. Bar = 200 nm.
On the basis of this series of observations, we concluded that our protocol is a valuable experimental tool for the identification and morphological characterization of endosomal structures aberrantly accumulated in specific mutants.
Immunolabeling of Nanogold-containing Cryosections
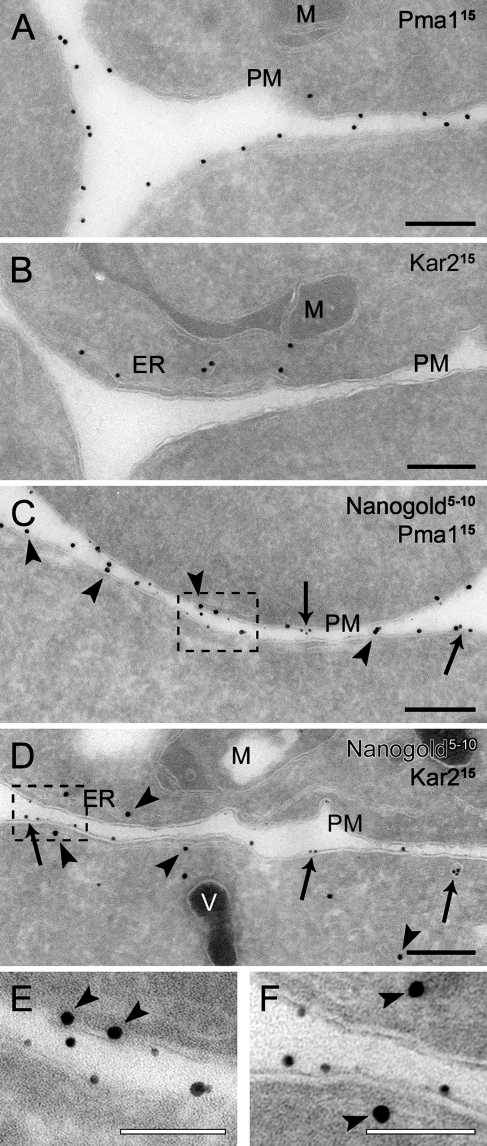
Another important application of this protocol would be to combine it with an immunogold labeling procedure. Our cryosectioning method adapted to yeast is compatible with immunolabeling with colloidal gold particles conjugated to protein A (protein A–gold; Griffith et al. 2008). To test whether we could distinguish between the particles generated by the silver enhancement reaction and the protein A–gold, we labeled preparations obtained from spheroplasts incubated with Nanogold exclusively at 4C for 15 min with antibodies against either the PM protein Pma1 or the endoplasmic reticulum (ER) chaperone Kar2 before and after the silver enhancement. These antibodies specifically decorated the PM and the ER adjacent to it when used on non-enhanced cryosections (Figures 4A and 4B; Griffith et al. 2008). The same labeling profiles were also obtained on the samples where the silver enhancement was performed before the immunological reaction (Figures 4C and 4D). Importantly, the enhanced Nanogold particles were lighter in density (Figures 4C–4F, arrows); consequently, they were easy to distinguish from the 15-nm protein A–gold (Figures 4C–4F, arrowheads). In addition, clear colocalization was observed between the Nanogold and Pma1 but not Kar2, revealing the resolution power of the protocol. It should be noted that a silver enhancement reaction that is too extended can lead to the formation of Nanogold particles of a size identical to that of the gold conjugated to the antibodies. Therefore, it is advisable to always make two controls, where cryosections are only either immunogold-labeled or silver-enhanced. The analysis of these two preparations will allow determination of whether the two types of particles are distinguishable. If they are not, the labeling experiment must be repeated and the time of the silver enhancement reaction must be reduced to generate smaller Nanogold. Silver enhancement after the labeling is also possible, but we observed that the protein A–gold particles were also enhanced at the expense of the Nanogold, which was not clearly visible (not shown). Taken together, these results demonstrate that our Nanogold protocol can be combined with immunogold labeling.
Figure 4.
Double labeling of cryosections with positively charged Nanogold particles and immunological reactions. Wild-type cells (SEY6210) grown to log phase were converted to spheroplasts and incubated with 4 nmol of positively charged Nanogold at 4C for 15 min before being immediately fixed. Cryosections with (C,D) or without (A,B) a silver enhancement reaction were incubated with anti-Pma1 (A,C) or anti-Kar2 (B,D) antibodies, followed by 15-nm protein A–gold particle conjugates. Arrows indicate Nanogold particles, whereas arrowheads indicate immunogold labeling. (E,F) Enlargements of defined labeled areas of C and D, respectively, to better illustrate the clear difference in size and density between the silver-enhanced Nanogold and gold conjugated to antibodies. The size of the particles is indicated at the top of each panel. ER, endoplasmic reticulum; M, mitochondria; PM, plasma membrane; V, vacuole. Bars: A–D = 200 nm; E,F = 100 nm.
Discussion
Investigations at an ultrastructural level are important for the analysis of protein localization and the morphology of carriers and organelles in both wild-type and mutant cells. This is particularly crucial when examining the yeast endosomal system, because most of the compartments appear as puncta when visualized by fluorescence microscopy. Consequently, the use of a probe entering the endocytic route in a time-dependent manner and detectable by electron microscopy is useful to mark specific compartments but also to assess the functionality of this transport process. Importantly, only a restricted number of antisera recognizing endosomal protein markers appear to work on IEM preparations in yeast (Lewis et al. 2000; Babst et al. 2002; Prescianotto-Baschong and Riezman 2002; Griffith et al. 2008). In certain cases, this problem has been circumvented by fusing proteins with specific epitope tags (Odorizzi et al. 1998; Babst et al. 2002; van Donselaar et al. 2007). Often, however, the cellular levels of a specific factor make it undetectable by IEM, and overexpression is not an option, because that can cause mislocalization. As a consequence, the labeling of the endocytic compartments with a specific probe provides a valid alternative, especially if it is known when the probe will be present in a particular compartment during a time-course experiment.
We have turned to positively charged Nanogold because this compound has been successfully used in the past for IEM analyses in yeast (Prescianotto-Baschong and Riezman 1998,2002; Seron et al. 1998). We have also explored whether yeast spheroplasts are able to endocytose BSA-gold as mammalian cells (Slot et al. 1988), or possibly protein A–gold, but in our hands, these labeling procedures are not working. As has already been shown (Prescianotto-Baschong and Riezman 1998), Nanogold is taken up by yeast spheroplasts through endocytosis because these particles are found on the PM or in the lumen of intracellular vesicles, and this incorporation event is blocked at 4C (Figures 1–4). The silver enhancement reaction required for the visualization of the Nanogold is compatible with our cryosectioning protocol when the periodic acid treatment required for the cell wall permeabilization is omitted (Figures 1–4). Importantly, the use of spheroplasts and the silver enhancement only marginally weaken the excellent cell preservation and contrast obtained by our IEM method. As a result, the resolution of the yeast morphology of our preparations remains higher than that of those obtained from cells embedded into a resin, highlighting the advantages of our protocol in ultrastructural analyses (Figures 1–4; Prescianotto-Baschong and Riezman 1998; Griffith et al. 2008). However, it should be pointed out that good-quality preparations can also be obtained with Epon-embedded cryo-fixed yeast (e.g., Osumi et al. 2006).
The procedure presented here is also a valuable tool for the ultrastructural examination of mutants with an endosomal trafficking defect (Figure 3). Moreover, the fact that the Nanogold passes through the EE and LE compartments in a time-dependent manner allows accurate marking of these organelles (Figure 2). Our procedure is not applicable exclusively to morphological analyses but can be combined with immunogold labeling, and is consequently appropriate for localization studies of both resident and cargo proteins (Figure 4). These investigations are particularly facilitated in yeast because if antibodies do not react on any chemically fixed preparation or are not available, the genetics offers an exceptional opportunity. Protein tags can be genomically inserted at either the 5′ or 3′ end of yeast genes using simple PCR-based integration methods (Longtine et al. 1998; Gauss et al. 2005), and this permits the creation of fusion proteins under the control of native promoters that can be detected using commercially available antibodies.
It is also possible to see additional applications of the protocol presented here. The FM 4-64 (Molecular Probes/Invitrogen; Eugene, OR) is a lipophilic fluorescent marker dye that associates to the PM, is endocytosed, and reaches the vacuole, passing through endosomes in a time-dependent manner (Vida and Emr 1995). FM 4-64 associates exclusively to the PM when cells are kept at 4C, because endocytosis is inhibited under these conditions. Then the transfer of FM 4-64–loaded cells to room temperature allows the endocytosis to occur, and the internalization of this dye can be followed in a time-course manner (Huckaba et al. 2004; Sipos et al. 2004). Because both the Nanogold and FM 4-64 enter cells in association with lipids (Vida and Emr 1995; Prescianotto-Baschong and Riezman 1998), their internalization kinetics are identical (Vida and Emr 1995; Prescianotto-Baschong and Riezman 1998; Huckaba et al. 2004; Sipos et al. 2004). Consequently, it would be possible to correlate IEM data of Nanogold uptake with fluorescence microscopy observations of FM 4-64.
Another important application of our method could potentially be the use of Nanogold particles to monitor receptor-mediated endocytosis. By using monomaleido and mono-sulfo-N-hydroxy-succinimido derivatives (Nanoprobes), it is possible to cross-link Nanogold to proteins. In particular, attachment to the a- or the α-factor would permit one to follow the endocytosis of these two pheromones through their specific receptors, Ste3 and Ste2, respectively (Hicke 1997,1999).
In summary, we have described here a new protocol that allows combining the efficient labeling of the yeast endocytic route using positively charged Nanogold with the high resolution of the Tokuyasu cryosectioning method. In addition, the possibility of performing immunogold labeling will provide researchers with a valuable new tool for the dynamic ultrastructural analysis of the S. cerevisiae endosomal system.
Acknowledgments
F.R. is supported by The Netherlands Organization for Health Research and Development (ZonMW-VIDI-917.76.329) and by a Utrecht University High Potential Grant.
The authors thank Muriel Mari and Ann de Mazière for critical reading of the manuscript, Daniel Klionsky, Scott Emr, and Ineke Braakman for reagents, and Marc van Peski and René Scriwanek for assistance with the preparation of the figures.
References
- Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD (2002) ESCRT-III: an endosome-associated heterooligomeric protein complex required for MVB sorting. Dev Cell 3:271–282 [DOI] [PubMed] [Google Scholar]
- Babst M, Sato TK, Banta LM, Emr SD (1997) Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO J 16:1820–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M, Wendland B, Estepa EJ, Emr SD (1998) The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J 17:2982–2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers K, Stevens TH (2005) Protein transport from the late Golgi to the vacuole in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta 1744:438–454 [DOI] [PubMed] [Google Scholar]
- Conibear E, Stevens TH (1998) Multiple sorting pathways between the late Golgi and the vacuole in yeast. Biochim Biophys Acta 1404:211–230 [DOI] [PubMed] [Google Scholar]
- Darsow T, Rieder SE, Emr SD (1997) A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J Cell Biol 138:517–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss R, Trautwein M, Sommer T, Spang A (2005) New modules for the repeated internal and N-terminal epitope tagging of genes in Saccharomyces cerevisiae. Yeast 22:1–12 [DOI] [PubMed] [Google Scholar]
- Griffith J, Mari M, De Maziere A, Reggiori F (2008) A cryosectioning procedure for the ultrastructural analysis and the immunogold labelling of yeast Saccharomyces cerevisiae. Traffic 9:1060–1072 [DOI] [PubMed] [Google Scholar]
- Griffiths GM (1983) Selective contrast for electron microscopy using thawed frozen sections and immunocytochemistry. In Revel JP, Barnard T, Haggis GH, eds. Science of Biological Specimen Preparation. AMF O'Hare, SEM, 153–159
- Hicke L (1997) Ubiquitin-dependent internalization and down-regulation of plasma membrane proteins. FASEB J 11:1215–1226 [DOI] [PubMed] [Google Scholar]
- Hicke L (1999) Gettin' down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends Cell Biol 9:107–112 [DOI] [PubMed] [Google Scholar]
- Huckaba TM, Gay AC, Pantalena LF, Yang HC, Pon LA (2004) Live cell imaging of the assembly, disassembly, and actin cable-dependent movement of endosomes and actin patches in the budding yeast, Saccharomyces cerevisiae. J Cell Biol 167:519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JH, Emr SD (2006) The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu Rev Biophys Biomol Struct 35:277–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EW (1977) Proteinase mutants of Saccharomyces cerevisiae. Genetics 85:23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann DJ, Odorizzi G, Emr SD (2002) Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol 3:893–905 [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Nichols BJ, Prescianotto-Baschong C, Riezman H, Pelham HR (2000) Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol Biol Cell 11:23–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A III, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, et al. (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953–961 [DOI] [PubMed] [Google Scholar]
- Luzio JP, Pryor PR, Bright NA (2007) Lysosomes: fusion and function. Nat Rev Mol Cell Biol 8:622–632 [DOI] [PubMed] [Google Scholar]
- Munn AL (2000) The yeast endocytic membrane transport system. Microsc Res Tech 51:547–562 [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Babst M, Emr SD (1998) Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell 95:847–858 [DOI] [PubMed] [Google Scholar]
- Osumi M, Konomi M, Sugawara T, Takagi T, Baba M (2006) High-pressure freezing is a powerful tool for visualization of Schizosaccharomyces pombe cells: ultra-low temperature and low-voltage scanning electron microscopy and immunoelectron microscopy. J Electron Microsc (Tokyo) 55:75–88 [DOI] [PubMed] [Google Scholar]
- Peplowska K, Markgraf DF, Ostrowicz CW, Bange G, Ungermann C (2007) The CORVET tethering complex interacts with the yeast Rab5 homolog Vps21 and is involved in endo-lysosomal biogenesis. Dev Cell 12:739–750 [DOI] [PubMed] [Google Scholar]
- Prescianotto-Baschong C, Riezman H (1998) Morphology of the yeast endocytic pathway. Mol Biol Cell 9:173–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescianotto-Baschong C, Riezman H (2002) Ordering of compartments in the yeast endocytic pathway. Traffic 3:37–49 [DOI] [PubMed] [Google Scholar]
- Robinson JS, Klionsky DJ, Banta LM, Emr SD (1988) Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol Cell Biol 8:4936–4948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JH, Howald I, Stevens TH (1989) Characterization of genes required for protein sorting and vacuolar function in the yeast Saccharomyces cerevisiae. EMBO J 8:2057–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seron K, Tieaho V, Prescianotto-Baschong C, Aust T, Blondel MO, Guillaud P, Devilliers G, et al. (1998) A yeast t-SNARE involved in endocytosis. Mol Biol Cell 9:2873–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JD, Cummings KB, Huyer G, Michaelis S, Wendland B (2001) Yeast as a model system for studying endocytosis. Exp Cell Res 271:1–9 [DOI] [PubMed] [Google Scholar]
- Sipos G, Brickner JH, Brace EJ, Chen L, Rambourg A, Kepes F, Fuller RS (2004) Soi3p/Rav1p functions at the early endosome to regulate endocytic trafficking to the vacuole and localization of trans-Golgi network transmembrane proteins. Mol Biol Cell 15:3196–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ (2007) Cryosectioning and immunolabeling. Nat Protocols 2:2480–2491 [DOI] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Weerkaamp AJ (1988) Localization of macromolecular components by application of the immunogold technique on cryosectioned bacteria. Methods Microbiol 20:211–236 [Google Scholar]
- Tokuyasu KT (1978) A study of positive staining of ultrathin frozen sections. J Ultrastruct Res 63:287–307 [DOI] [PubMed] [Google Scholar]
- van Donselaar E, Posthuma G, Zeuschner D, Humbel BM, Slot JW (2007) Immunogold labeling of cryosections from high-pressure frozen cells. Traffic 8:471–485 [DOI] [PubMed] [Google Scholar]
- van Meel E, Klumperman J (2008) Imaging and imagination: understanding the endo-lysosomal system. Histochem Cell Biol 129:253–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida TA, Emr SD (1995) A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol 128:779–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y, Nakamura N, Ohsumi Y, Hirata A (1997) Vam3p, a new member of syntaxin related protein, is required for vacuolar assembly in the yeast Saccharomyces cerevisiae. J Cell Sci 110:1299–1306 [DOI] [PubMed] [Google Scholar]
- Wang C-W, Stromhaug PE, Shima J, Klionsky DJ (2002) The Ccz1-Mon1 protein complex is required for the late step of multiple vacuole delivery pathways. J Biol Chem 277:47917–47927 [DOI] [PMC free article] [PubMed] [Google Scholar]