Abstract
Repeated administration of many addictive drugs leads to a progressive increase in their locomotor effects. This increase in locomotor activity often develops concomitantly with increases in their positive-reinforcing effects, which are believed to contribute to the etiology of substance use disorders. The purpose of this study was to examine changes in sensitivity to the locomotor effects of opioids after their repeated administration and to determine the role of μ and κ receptors in mediating these effects. Separate groups of rats were treated with opioid receptor agonists and antagonists every other day for 10 days, and changes in locomotor activity were measured. Repeated administration of the μ agonists, morphine and buprenorphine, produced a progressive increase in locomotor activity during the treatment period, and this effect was blocked by coadministration of the opioid antagonist naltrexone. The κ agonist spiradoline decreased locomotor activity when administered alone and blocked the progressive increase in locomotor activity produced by morphine. The ability of spiradoline to block morphine-induced increases in locomotor activity was itself blocked by pretreatment with the κ antagonist nor-binaltorphimine. Repeated administration of high doses, but not low or moderate doses, of the mixed μ/κ agonists butorphanol, nalbuphine, and nalorphine produced a progressive increase in locomotor activity during the treatment period. Doses of butorphanol, nalbuphine, and nalorphine that failed to produce a progressive increase in locomotor activity when administered alone did so when subjects were pretreated with nor-binaltorphimine. These findings suggest that μ and κ receptors have functionally opposing effects on opioid-mediated locomotor activity and sensitization-related processes.
Locomotor activity after psychotropic drug administration has long been of interest to behavioral pharmacologists in general and substance abuse researchers in particular. The reasons for this interest can be traced to the fact that the anatomical structures and neurotransmitter systems mediating locomotor activity overlap those that mediate positive reinforcement and reward (for review, see Wise, 1987; Tzschentke, 2001). Because of this overlap, a careful examination of locomotor activity after drug administration can shed light on the neuropharmacological basis of substance abuse and other addictive behaviors.
Opioid analgesics produce a stereotypical pattern of locomotor activity that has been well characterized. After systemic administration, μ-opioid agonists initially produce a transient decrease in locomotor activity that gradually dissipates over the course of 60 to 120 min, which is then followed by an increase in locomotor activity lasting several hours (Babbini and Davis, 1972; Buxbaum et al., 1973). Sensitivity to both the initial decrease and the subsequent increase in locomotor activity changes after the repeated administration of opioids, such that the initial decrease becomes gradually smaller, and the subsequent increase becomes progressively larger (Vasko and Domino, 1978; Brady and Holtzman, 1981). These changes in sensitivity to the locomotor effects of opioids have been termed behavioral sensitization, as opposed to tolerance, because the initial decrease in locomotor activity often disappears entirely and is replaced by an increase in locomotor activity that becomes progressively greater with continued treatment (Vanderschuren et al., 1999b). Similar changes in sensitivity are observed after treatment with other drugs possessing significant abuse and dependence liability (McCreary et al., 1999; Sabeti et al., 2003), and cross-sensitization is often observed between pharmacological classes (Leri et al., 2003; McDaid et al., 2005). Studies examining behavioral sensitization and other sensitization-related processes are particularly important in substance abuse research because sensitization to the positive-reinforcing and incentive-motivational effects of drugs is believed to contribute to the etiology of substance use disorders (for review, see Robinson and Berridge, 2000; Morgan and Roberts, 2004).
There is evidence that μ- and κ-opioid receptors may play functionally opposing roles in the development of behavioral sensitization. For example, repeated administration of the μ opioid morphine produces sensitization to its locomotor effects (Powell and Holtzman, 2001) and cross-sensitization to the locomotor effects of cocaine (Cunningham et al., 1997; Vanderschuren et al., 1999a). In contrast, repeated administration of the κ opioid U69593 does not produce sensitization to its effects but blocks the development of sensitization to the effects of cocaine (Heidbreder et al., 1993; Shippenberg et al., 1996). The full extent of these functionally opposing actions on sensitization-related processes is unknown because only a few studies have coadministered μ and κ agonists using a protocol that would be expected to produce a systematic change in their behavioral effects. Complicating matters further, very few studies have examined changes in sensitivity to the behavioral effects of opioids possessing agonist activity at both μ and κ receptors, and few have made use of receptor-selective antagonists to tease apart the potential role of these opioid receptor subtypes.
The purpose of the present study was to examine changes in sensitivity to the locomotor effects of opioids after their repeated administration and to determine the relative contribution of μ and κ receptors in these effects. To this end, separate groups of rats were treated with opioid receptor agonists and antagonists every other day for 10 days, and changes in locomotor activity were measured. All locomotor activity data were collected during discrete trials conducted 15 min after drug administration. This period of time was chosen because changes in locomotor activity are readily apparent (Székely et al., 1980; Brady and Holtzman, 1981), plasma drug concentrations are approaching their peak (Berkowitz et al., 1975; Melzacka et al., 1985), and drug-drug interactions are easily observed and quantified (Porreca et al., 1981; Morgan et al., 1999).
Materials and Methods
Animals. Adult male Long-Evans rats, weighing approximately 250 g, were obtained from Charles River Laboratories (Raleigh, NC) and housed individually in polycarbonate cages, with food and drinking water freely available. All rats were housed in a temperature- and humidity-controlled colony room and maintained on a 12-h light/dark cycle (lights on, 7:00 AM). All subjects were maintained in accordance with the guidelines of the Davidson College Animal Care and Use Committee.
A total of 222 rats were divided among 32 groups: saline (three determinations; n = 18), 10 mg/kg cocaine (n = 6), 0.3 mg/kg naltrexone (n = 6), 3.0 mg/kg naltrexone (n = 6), 10 mg/kg nor-binaltorphimine (two determinations; n = 12), 3.0 mg/kg spiradoline (n = 6), 10 mg/kg spiradoline (n = 6), 3.0 mg/kg morphine (n = 6), 10 mg/kg morphine (two determinations; n = 12), 10 mg/kg morphine + 0.3 mg/kg naltrexone (n = 6), 0.3 mg/kg buprenorphine (n = 6), 1.0 mg/kg buprenorphine (n = 6), 1.0 mg/kg buprenorphine + 0.3 mg/kg naltrexone (n = 6), 10 mg/kg morphine + 10 mg/kg spiradoline (n = 6), 10 mg/kg morphine + 10 mg/kg spiradoline + 10 mg/kg nor-binaltorphimine (n = 6), 1.0 mg/kg buprenorphine + 10 mg/kg spiradoline (two determinations; n = 12), 1.0 mg/kg buprenorphine + 10 mg/kg spiradoline + 10 mg/kg nor-binaltorphimine (n = 6), 3.0 mg/kg butorphanol (n = 6), 10 mg/kg butorphanol (n = 6), 30 mg/kg butorphanol (n = 6), 10 mg/kg butorphanol + 10 mg/kg nor-binaltorphimine (n = 6), 30 mg/kg butorphanol + 10 mg/kg nor-binaltorphimine (n = 6), 3.0 mg/kg nalbuphine (n = 6), 10 mg/kg nalbuphine (n = 6), 30 mg/kg nalbuphine (n = 6), 10 mg/kg nalbuphine + 10 mg/kg nor-binaltorphimine (n = 6), 30 mg/kg nalbuphine + 10 mg/kg nor-binaltorphimine (n = 6), 3.0 mg/kg nalorphine (n = 6), 10 mg/kg nalorphine (n = 6), 30 mg/kg nalorphine (n = 6), 10 mg/kg nalorphine + 10 mg/kg nor-binaltorphimine (n = 6), and 30 mg/kg nalorphine + 10 mg/kg nor-binaltorphimine (n = 6).
The effects of 10 mg/kg morphine, 10 mg/kg nor-binaltorphimine, and 10 mg/kg spiradoline + 10 mg/kg buprenorphine were determined twice in separate groups of rats tested approximately 12 months apart. In all cases, the effects of these drugs and drug combinations did not differ between the two determinations. As a consequence, data from the two groups were combined for all statistical analyses. The effects of saline were determined in three separate groups of rats tested approximately 18 months apart. Similar to that observed with the other drugs and drug combinations, the effects of saline did not differ across the three determinations, and data from the three groups were combined for all statistical analyses. The entire study was completed over the course of 48 months.
Apparatus. All behavioral tests were conducted in a single, open-field, locomotor activity chamber (interior dimensions, 43 × 43 × 30 cm) obtained from MED Associates (St. Albans, VT). The chamber consisted of a polyvinyl chloride floor and acrylic sidewalls with aluminum corner supports. Two circuit boards were located on opposite sidewalls 2.5 cm above the floor of the chamber. One board contained 16 infrared photocells spaced 2.5 cm apart; the opposite board contained 16 infrared detectors with identical spacing. All photocells and detectors were interfaced through a computer running a Microsoft Windows operating system and MED Associates software.
Testing Procedure. Before behavioral testing, each rat was habituated to the apparatus and testing procedure by being placed into the activity chamber for 5 min a day for 3 consecutive days (Table 1). On the 3rd and final day of habituation, a saline control session was conducted in which each rat received an injection of saline (1.0 mg/kg i.p.) 15 min before being placed in the chamber.
TABLE 1.
Schedule of testing and events for all groups
| Day of Study | Manipulation | Location |
|---|---|---|
| 1 | Habituation | Locomotor chamber |
| 2 | Habituation | Locomotor chamber |
| 3 | Saline | Locomotor chamber |
| 4 | Test drug | Locomotor chamber |
| 5 | Colony room | |
| 6 | Test drug | Locomotor chamber |
| 7 | Colony Room | |
| 8 | Test drug | Locomotor chamber |
| 9 | Colony room | |
| 10 | Test drug | Locomotor chamber |
| 11 | Colony room | |
| 12 | Test drug | Locomotor chamber |
During drug treatment and locomotor activity testing, all groups were administered a test drug (or test drugs) every other day for 10 days. On days in which testing was conducted, each rat was brought to the laboratory, administered an intraperitoneal injection of the test drug, and then returned to its home cage. After 15 min, the rat was placed in the activity chamber for 5 min, and photo beam interruptions were recorded. After the testing period, the rat was removed from the chamber and returned to its home cage. For groups in which multiple drugs were administered, separate injections were administered on opposite sides of the peritoneal cavity. Because of its extremely long duration of action, nor-binaltorphimine was administered only once immediately after the saline control session on the 3rd day of habituation. Rats receiving nor-binaltorphimine were then administered saline every other day for the next 10 days, 15 min before being placed into the activity chamber. On days in which drugs were not administered, rats remained in the colony room and were left undisturbed.
All rats in this study were tested for cross-sensitization to cocaine 7 days after the end of drug treatment and locomotor testing. Data from those tests are described in Smith et al. (2009), and only data from the 10 days of drug treatment are described below.
Drugs. Naltrexone hydrochloride, spiradoline mesylate, butorphanol tartrate, nalbuphine hydrochloride, and nalorphine hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO). Cocaine hydrochloride, nor-binaltorphimine dihydrochloride, morphine sulfate, and buprenorphine hydrochloride were generously supplied by the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC). All compounds were dissolved in saline and injected in a volume of 1.0 ml/kg b.wt.
Data Analysis. Locomotor activity data were computed as distance traveled (centimeters) by software from MED Associates. Data from the 5 days of drug treatment were analyzed via repeated-measures analysis of variance. Post hoc tests, corrected for multiple pair-wise comparisons, were conducted where appropriate. Locomotor activity data from the first and last days of treatment were also expressed as differences from saline control values by subtracting the distance traveled during the saline control session from the distance traveled during the first and last test sessions. Planned comparisons between the first and last test sessions were then conducted used paired Student's t tests. An α level of 0.05 was used for all statistical tests.
Results
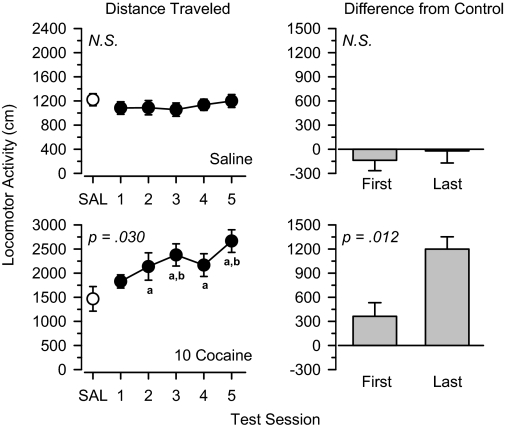
Saline and Cocaine. Saline and cocaine served as negative and positive controls of the study, respectively. As expected, locomotor activity was stable during repeated administration of 1.0 ml/kg saline, and no significant differences were observed across the five test sessions (Fig. 1). In contrast, locomotor activity systematically increased during repeated treatment with 10 mg/kg cocaine [F(4,20) = 3.326, p = 0.030]. Locomotor activity was increased relative to saline control values on each day of treatment, and a planned comparison between the first and last test sessions revealed that locomotor activity was significantly greater on the last day of treatment than the first day [t(5) = 3.846, p = 0.012].
Fig. 1.
Locomotor effects of 1.0 ml/kg saline (top) and 10 mg/kg cocaine (bottom) over 5 days of treatment. Left, locomotor activity expressed as distance traveled (centimeters) after saline administration (SAL) and 5 days of drug treatment. Right, locomotor activity on days 1 and 5 expressed as difference from saline control values (centimeters). Significant differences are indicated as follows: a, significantly different from saline (p < 0.05); and b, significantly different from day 1 (p < 0.05).
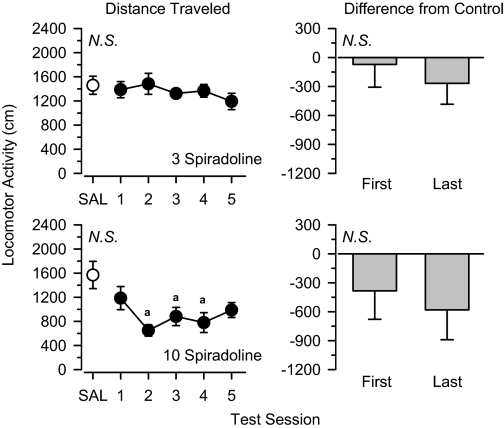
Spiradoline. Locomotor activity was stable across the five test sessions in animals treated with 3.0 mg/kg of the κ agonist spiradoline and approximated that observed after saline administration (Fig. 2). A dose of 10 mg/kg spiradoline reduced locomotor activity relative to saline control values, and this effect was apparent throughout the treatment period. Planned comparisons revealed no significant differences between the first and last days of treatment with either dose of spiradoline.
Fig. 2.
Locomotor effects of 3.0 mg/kg spiradoline (top) and 10 mg/kg spiradoline (bottom) over 5 days of treatment. Left, locomotor activity expressed as distance traveled (centimeters) after saline administration (SAL) and 5 days of drug treatment. Right, locomotor activity on days 1 and 5 expressed as difference from saline control values (centimeters). Significant differences are indicated as follows: a, significantly different from saline (p < 0.05).
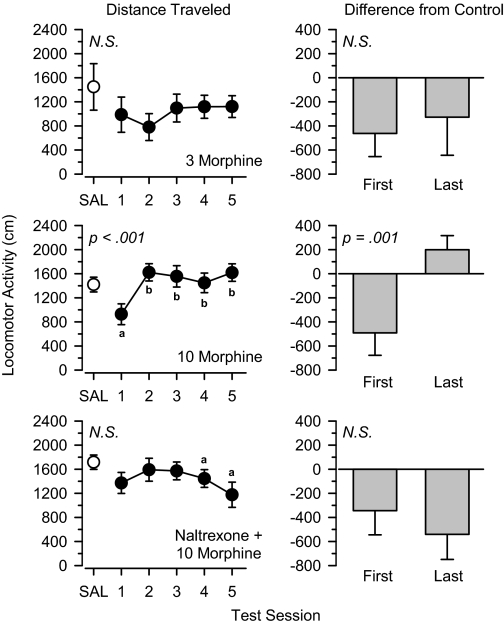
Morphine and Buprenorphine. A 3.0 mg/kg dose of the μ agonist morphine nonsignificantly decreased locomotor activity relative to saline control values on each day of treatment, but no systematic increase or decrease in activity was observed (Fig. 3). In contrast, locomotor activity increased significantly over the five test sessions during treatment with 10 mg/kg morphine [F(4,44) = 10.255, p < 0.001]. In this group, locomotor activity was lower than saline control values in the first test session but greater than saline control values in the last session, a difference that was statistically significant [t(11) = 4.517, p = 0.001]. Coadministration of a low dose (0.3 mg/kg) of the opioid antagonist naltrexone blocked this effect, such that locomotor activity remained lower than control values over the five test sessions, and no significant differences were observed between the first and last days of treatment. Naltrexone (0.3 and 3.0 mg/kg) did not alter locomotor activity over five test sessions when administered alone (Table 2).
Fig. 3.
Locomotor effects of 3.0 mg/kg morphine (top), 10 mg/kg morphine (middle), and 0.3 mg/kg naltrexone + 10 mg/kg morphine (bottom) over 5 days of treatment. Left, locomotor activity expressed as distance traveled (centimeters) after saline administration (SAL) and 5 days of drug treatment. Right, locomotor activity on days 1 and 5 expressed as difference from saline control values (centimeters). Significant differences are indicated as follows: a, significantly different from saline (p < 0.05); and b, significantly different from day 1 (p < 0.05).
TABLE 2.
Locomotor activity expressed as mean (S.E.) distance traveled in centimeters
| Day of Treatment | 0.3 Naltrexone | 3.0 Naltrexone | 10 Nor-Binaltorphimine |
|---|---|---|---|
| cm | |||
| Saline | 1601.53 (93.63) | 1167.22 (99.17) | 1472.47 (105.13) |
| Day 1 | 1468.18 (157.71) | 1100.32 (192.02) | 1438.88 (127.35) |
| Day 2 | 1414.09 (146.26) | 1472.49 (165.44) | 1406.21 (117.35) |
| Day 3 | 1438.80 (131.45) | 983.03 (181.93) | 1588.71 (124.88) |
| Day 4 | 1623.23 (119.25) | 1041.93 (187.74) | 1270.59 (129.02) |
| Day 5 | 1788.76 (77.57) | 1359.29 (139.48) | 1436.58 (92.73) |
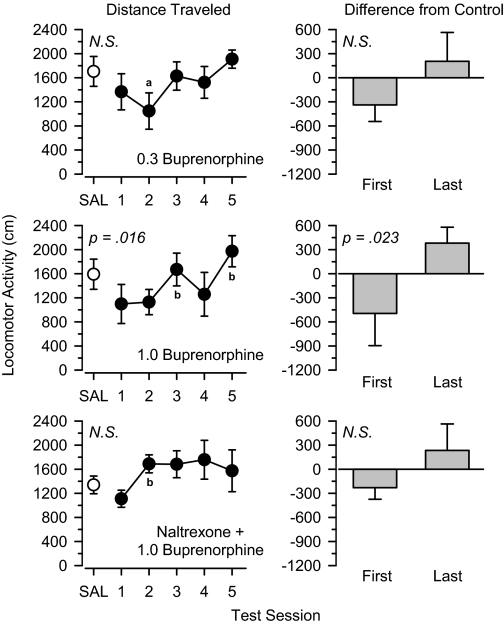
Locomotor activity increased slightly over the treatment period in rats treated with 0.3 mg/kg of the μ agonist buprenorphine, but this effect was not statistically significant (Fig. 4). Locomotor activity increased significantly during treatment with 1.0 mg/kg buprenorphine [F(4,20) = 3.957, p = 0.016]. In this group, locomotor activity was lower than saline control values on the first day of treatment and greater than saline control values on the last day of treatment, and this difference was statistically significant [t(5) = 3.220, p = 0.023]. Coadministration of naltrexone greatly attenuated the increase in locomotor activity produced by buprenorphine. Although locomotor activity increased on the 2nd day of treatment, no further increases were observed, and no significant differences were observed between the first and last test sessions.
Fig. 4.
Locomotor effects of 0.3 mg/kg buprenorphine (top), 1.0 mg/kg buprenorphine (middle), and 0.3 mg/kg naltrexone + 1.0 mg/kg buprenorphine (bottom) over 5 days of treatment. Left, locomotor activity expressed as distance traveled (centimeters) after saline administration (SAL) and 5 days of drug treatment. Right, locomotor activity on days 1 and 5 expressed as difference from saline control values (centimeters). Significant differences are indicated as follows: a, significantly different from saline (p < 0.05); and b, significantly different from day 1 (p < 0.05).
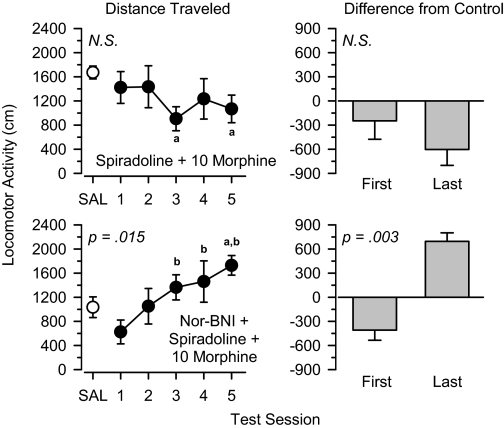
Spiradoline in Combination with Morphine and Buprenorphine. Coadministration of 10 mg/kg spiradoline blocked the increase in locomotor activity produced by 10 mg/kg morphine (Fig. 5). In this group, locomotor activity was lower than saline control values on both the first and last days of treatment. Pretreatment with the κ antagonist nor-binaltorphimine prevented spiradoline from blocking morphine-induced increases in locomotor activity. In this group, locomotor activity increased significantly over each of the five test sessions [F(4,20) = 4.009, p = 0.015]. Similar to that observed when 10 mg/kg morphine was administered alone, locomotor activity was lower than saline control values on the first day of treatment but greater than saline control values on the last day of treatment, and this difference was statistically significant [t(5) = 5.416, p = 0.003]. Nor-binaltorphimine (10 mg/kg) did not alter locomotor activity when administered alone (Table 2).
Fig. 5.
Locomotor effects of 10 mg/kg spiradoline + 10 mg/kg morphine (top) and 10 mg/kg nor-binaltorphimine + 10 mg/kg spiradoline + 10 mg/kg morphine (bottom) over 5 days of treatment. Left, locomotor activity expressed as distance traveled (centimeters) after saline administration (SAL) and 5 days of drug treatment. Right, locomotor activity on Days 1 and 5 expressed as difference from saline control values (centimeters). Significant differences are indicated as follows: a, significantly different from saline (p < 0.05); and b, significantly different from day 1 (p < 0.05).
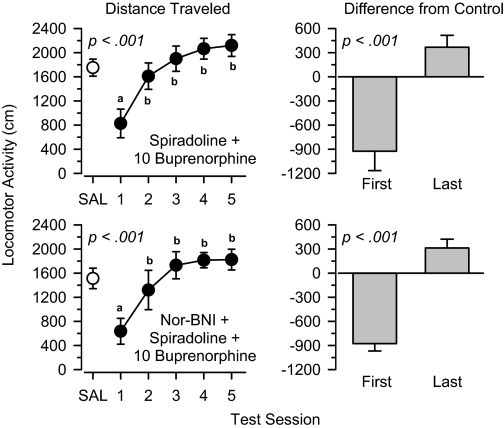
Coadministration of spiradoline failed to block the increase in locomotor activity produced by 1.0 mg/kg buprenorphine (Fig. 6). In this group, locomotor activity increased significantly across the five test sessions [F(4,44) = 14.170, p < 0.001], and locomotor activity was significantly greater on the last day of treatment than the first day [t(11) = 5.145, p < 0.001]. Pretreatment with nor-binaltorphimine did not alter the locomotor effects produced by spiradoline and buprenorphine. In this group, locomotor activity increased significantly over the course of treatment [F(4,20) = 8.159, p < 0.001], and locomotor activity on the last day of treatment was significantly greater than on the first day [t(5) = 8.439, p < 0.001].
Fig. 6.
Locomotor effects of 10 mg/kg spiradoline + 10 mg/kg buprenorphine (top) and 10 mg/kg nor-binaltorphimine + 10 mg/kg spiradoline + 10 mg/kg buprenorphine (bottom) over 5 days of treatment. Left, locomotor activity expressed as distance traveled (centimeters) after saline administration (SAL) and 5 days of drug treatment. Right, locomotor activity on days 1 and 5 expressed as difference from saline control values (centimeters). Significant differences are indicated as follows: a, significantly different from saline (p < 0.05); and b, significantly different from day1(p < 0.05).
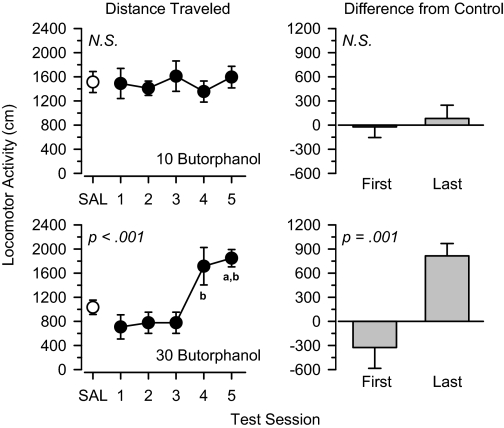
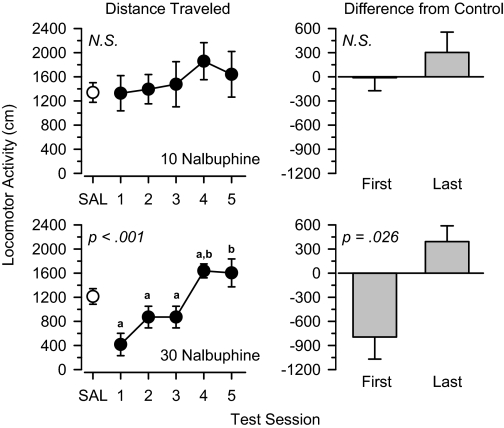
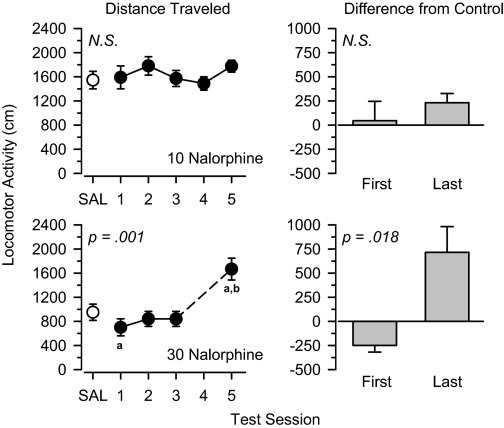
Butorphanol, Nalbuphine, and Nalorphine. The mixed μ/κ agonists butorphanol (Fig. 7), nalbuphine (Fig. 8), and nalorphine (Fig. 9) produced a similar pattern of results to one another. Locomotor activity was not altered by low (3.0 mg/kg; data not shown) and moderate (10 mg/kg; Figs. 7, 8, 9) doses of these drugs. In all cases, locomotor activity throughout the treatment period was similar to that observed under saline control conditions, and no significant differences were observed between the first and last days of treatment. In contrast, locomotor activity increased significantly across the five test sessions in rats treated with 30 mg/kg butorphanol [F(4,20) = 9.953, p < 0.001], 30 mg/kg nalbuphine [F(4,20) = 9.353, p < 0.001], and 30 mg/kg nalorphine [F(3,15) = 9.052, p = 0.001]. Under these conditions, the locomotor effects of butorphanol [t(5) = 7.103, p = 0.001], nalbuphine [t(5) = 3.128, p = 0.026], and nalorphine [t(5) = 3.472, p = 0.018] were significantly greater on the last day of treatment than the first day.
Fig. 7.
Locomotor effects of 10 mg/kg butorphanol (top) and 30 mg/kg butorphanol (bottom) over 5 days of treatment. Left, locomotor activity expressed as distance traveled (centimeters) after saline administration (SAL) and 5 days of drug treatment. Right, locomotor activity on days 1 and 5 expressed as difference from saline control values (centimeters). Significant differences are indicated as follows: a, significantly different from saline (p < 0.05); and b, significantly different from day 1 (p < 0.05).
Fig. 8.
Locomotor effects of 10 mg/kg nalbuphine (top) and 30 mg/kg nalbuphine (bottom) over 5 days of treatment. Left, locomotor activity expressed as distance traveled (centimeters) after saline administration (SAL) and 5 days of drug treatment. Right, locomotor activity on Days 1 and 5 expressed as difference from saline control values (centimeters). Significant differences are indicated as follows: a, significantly different from saline (p < 0.05); and b, significantly different from day 1 (p < 0.05).
Fig. 9.
Locomotor effects of 10 mg/kg nalorphine (top) and 30 mg/kg nalorphine (bottom) over 5 days of treatment. Left, locomotor activity expressed as distance traveled (centimeters) after saline administration (SAL) and 5 days of drug treatment. Right, locomotor activity on days 1 and 5 expressed as difference from saline control values (centimeters). Significant differences are indicated as follows: a, significantly different from saline (p < 0.05); and b, significantly different from day 1 (p < 0.05). Note: Data were lost from day 4 of 30 mg/kg nalorphine treatment because of computer error.
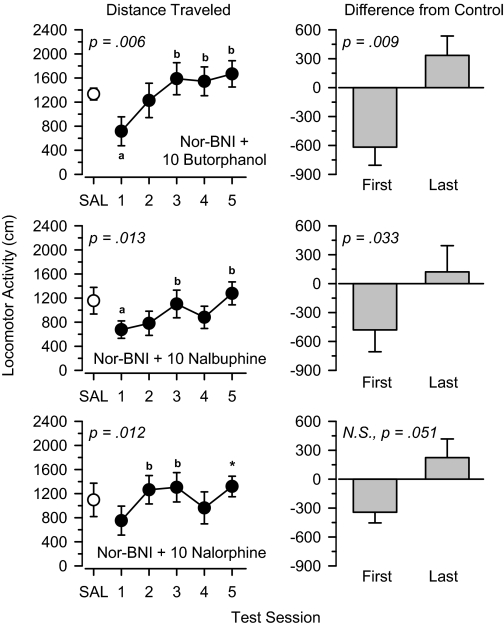
To determine whether the failure to see an increase in locomotor activity during treatment with 10 mg/kg butorphanol, nalbuphine, and nalorphine was due to their κ component of action, three additional groups of rats were pretreated with 10 mg/kg nor-binaltorphimine before treatment with the mixed μ/κ agonists (Fig. 10). Under these conditions, locomotor activity increased significantly during treatment with butorphanol [F(4,20) = 4.889, p = 0.006], nalbuphine [F(4,20) = 4.188, p = 0.013], and nalorphine [F(4,20) = 4.256, p = 0.012]. Locomotor activity increased significantly from the first test session to the last test session during treatment with butorphanol [t(5) = 4.120, p = 0.009] and nalbuphine [t(5) = 2.931, p = 0.033]. Activity also increased from the first to the last session in rats treated with nalorphine, but this effect failed to reach statistical significance [t(5) = 2.550, p = 0.051]. Pretreatment with nor-binaltorphimine did not alter the locomotor effects of 30 mg/kg butorphanol, 30 mg/kg nalbuphine, and 30 mg/kg nalorphine (data not shown). In all cases, locomotor activity increased significantly over the five test sessions, and significant differences were observed between the first and last days of treatment (p < 0.05 for all comparisons).
Fig. 10.
Locomotor effects of 10 mg/kg nor-binaltorphimine + 10 mg/kg butorphanol (top), 10 mg/kg nor-binaltorphimine + 10 mg/kg nalbuphine (middle), and 10 mg/kg nor-binaltorphimine + 10 mg/kg nalorphine (bottom) over 5 days of treatment. Left, locomotor activity expressed as distance traveled (centimeters) after saline administration (SAL) and 5 days of drug treatment. Right, locomotor activity on days 1 and 5 expressed as difference from saline control values (centimeters). Significant differences are indicated as follows: a, significantly different from saline (p < 0.05); and b, significantly different from day 1 (p < 0.05). *, nonsignificant difference from day 1 (p = 0.051).
Discussion
The major findings of this study are that: 1) agonist activity at μ receptors facilitates a progressive increase in locomotor activity that is consistent with characterizations of behavioral sensitization, and 2) agonist activity at κ receptors functionally opposes these effects. As novel findings, this study provided evidence that nor-binaltorphimine can prevent a κ agonist from blocking morphine-induced sensitization and provided evidence that the development of sensitization to the locomotor effects of mixed μ/κ agonists is limited by their κ component of action. It is important to note that the changes in sensitivity reported in this study cannot be attributed to nonpharmacological factors (e.g., maturation, habituation) because locomotor activity remained stable in three groups of control animals treated with saline. As a positive control, one group of rats in the present study was treated with cocaine according to the same dosing regimen and testing schedule as the 30 groups treated with opioids. In this group, cocaine increased locomotor activity on the first day of treatment, and this effect progressively increased over subsequent days of testing. These findings are consistent with a large number of studies examining the locomotor effects of cocaine after acute (Waddell and Holtzman, 1998; Badanich et al., 2008) and repeated (Laviola et al., 1995; Sabeti et al., 2003) administration.
Effects of μ Opioids. Consistent with previous studies reporting decreases in locomotor activity during the first 30 to 60 min of μ agonist administration in naive animals (e.g., Babbini and Davis, 1972; Buxbaum et al., 1973), morphine and buprenorphine decreased locomotor activity on the first day of treatment. Locomotor activity gradually increased over subsequent days of treatment, such that the amount of activity on the final day of treatment exceeded the amount of activity under saline control conditions. This increase in activity was observed at only higher doses of morphine and buprenorphine because locomotor activity did not differ significantly across the treatment period when lower doses were tested. These data are consistent with earlier studies reporting qualitative changes in the locomotor effects of μ agonists during the development of behavioral sensitization (Babbini and Davis, 1972; Vanderschuren et al., 1999b) and previous reports that the magnitude of sensitization is directly related to the dose administered (Brady and Holtzman, 1981; Shippenberg et al., 1998).
A low (0.3 mg/kg) dose of the opioid receptor antagonist naltrexone blocked the progressive increase in locomotor activity observed after repeated treatment with high doses of morphine and buprenorphine. In these groups, the locomotor effects observed on the first and last days of treatment were similar to those observed when lower doses of these drugs were administered alone. These data indicate that naltrexone lowered the functional dose of these drugs and support the hypothesis that agonist activity at μ receptors was responsible for the progressive increase in locomotor activity observed during the treatment period. Naltrexone, even at doses 10-fold higher than those used in the antagonism tests, did not alter locomotor activity when administered alone, suggesting that the ability of naltrexone to block the effects of morphine and buprenorphine was due to pharmacological antagonism and not due to any acute locomotor effects of naltrexone masking the effects of the other drugs.
Effects of κ Opioids. The κ agonist spiradoline decreased locomotor activity in a dose-dependent manner. Unlike that seen with morphine and buprenorphine, this effect was consistent across the 5 days of testing, and no progressive increase in locomotor activity was observed. Previous studies have revealed that κ agonists prevent the development of sensitization to the behavioral effects of cocaine (Heidbreder et al., 1993; Shippenberg et al., 1996; Puig-Ramos et al., 2008); however, their effects on the development of sensitization to morphine and other μ opioids are less clear. For instance, although an early study reported that the κ agonists U50488 and U69593 blocked the development of sensitization to morphine (Spanagel, 1995), a later study reported that U69593 did not block the development of sensitization to morphine or the development cross-sensitization to cocaine in morphine-treated animals (Shippenberg et al., 1998). In the present study, spiradoline blocked the progressive increase in locomotor activity produced by the high dose of morphine. It is unlikely that this effect was due to a simple masking of morphine-induced hyperactivity by spiradoline-induced hypoactivity because the effect of the drug combination on the first day of treatment (i.e., a 20% decrease in locomotor activity) was actually less than that seen when morphine was administered alone (i.e., a 35% decrease in locomotor activity). The ability of spiradoline to block the increase in locomotor activity produced by morphine was itself blocked by the κ-selective antagonist nor-binaltorphimine, indicating that this effect was mediated by κ receptors and suggesting that κ agonists functionally oppose the progressive increase in locomotor activity produced by morphine.
It is interesting that spiradoline did not block the progressive increase in locomotor activity produced by buprenorphine. Although these findings seemingly conflict with those obtained with morphine, it must be noted that buprenorphine differs from morphine in that it possesses antagonist activity at κ receptors. Indeed, buprenorphine binds to κ receptors with nanomolar affinity (Huang et al., 2001) and blocks the effects of the κ agonists bremazocine (Leander, 1987) and U50488 (Negus et al., 1989). κ Antagonist activity would explain the asymmetry between morphine and buprenorphine after spiradoline administration and would account for the ability of nor-binaltorphimine to eliminate these differences by blocking the effects of spiradoline in morphine-treated animals.
Effects of Mixed μ/κ Agonists. Butorphanol, nalbuphine, and nalorphine bind to both μ and κ receptors with nanomolar affinity (Emmerson et al., 1996; Remmers et al., 1999) and demonstrate both μ (Morgan and Picker, 1998) and κ (Smith and Picker, 1995) agonist activity across an extensive dose range. In the present study, low (3.0 mg/kg) and moderate (10 mg/kg) doses of these drugs did not alter locomotor activity on the first day of testing, and repeated administration of these doses did not lead to any consistent increase or decrease in locomotor activity over the treatment period. In contrast, a high dose (30 mg/kg) of these drugs produced small decreases in locomotor activity on the first day of treatment, which was followed by significant increases in locomotor activity over the remaining days of treatment. For all three drugs, locomotor activity was markedly greater on the last day of treatment than that observed under saline control conditions. These findings are similar to those obtained with morphine and buprenorphine, suggesting a role of μ-opioid receptors in these effects.
Given that butorphanol, nalbuphine, and nalorphine possess significant κ-agonist activity, we wanted to determine whether their κ component of action was functionally opposing their μ component of action and preventing a progressive increase in locomotor activity at moderate doses. To this end, additional groups of rats were pretreated with nor-binaltorphimine before repeated treatment with 10 mg/kg butorphanol, nalbuphine, and nalorphine. In these groups, each of the mixed μ/κ agonists produced a progressive increase in locomotor activity across the five test sessions, similar to that produced by morphine and buprenorphine. These data provide additional support for the hypothesis that agonist activity at κ receptors functionally opposes the progressive increase in locomotor activity mediated by μ receptors.
Implications for Substance Abuse and Addictive Behavior. Changes in sensitivity to the locomotor effects of addictive drugs are believed to develop concomitantly with changes in sensitivity to their positive-reinforcing and incentive-motivational effects (Spanagel, 1995; Vezina, 2004), and sensitization to these latter effects is believed to be involved in the etiology of substance use disorders (Robinson and Berridge, 2000; Morgan and Roberts, 2004). One goal of preclinical research is to identify effective analgesics that have lower abuse potential than traditional μ opioids and that are less likely to produce sensitization after repeated administration. κ Agonists meet these criteria, but dysphoria and hallucinations limit their clinical utility in human populations (Wadenberg, 2003; Dortch-Carnes and Potter, 2005). Mixed μ/κ agonists are effective analgesics and have a long history of being well tolerated in clinical pain patients (Schmidt et al., 1985; Vogelsang and Hayes, 1991). Furthermore, these drugs have less abuse liability than traditional μ agonists (Peachey, 1987; Hoskin and Hanks, 1991), and the present findings suggest they are less likely to produce sensitization after repeated administration. Despite such findings, clinicians remain concerned about the analgesic efficacy of these drugs and their potential to produce dysphoric reactions in some individuals. Until these issues are sufficiently resolved, mixed μ/κ opioids will have only limited utility in the treatment of pain disorders in vulnerable populations.
One additional goal of substance abuse research involves identifying behavioral and pharmacological interventions that prevent or attenuate the development of sensitization to the behavioral effects of addictive drugs. κ Opioids have previously been shown to be effective at blocking the development of sensitization to cocaine (Heidbreder et al., 1993; Shippenberg et al., 1996; Puig-Ramos et al., 2008), and the present findings suggest that κ opioids may also be effective at preventing the development of sensitization to morphine and other μ agonists. As noted above, dysphoria and hallucinations limit the clinical utility of κ opioids in human populations, and the clinical utility of mixed μ/κ opioids is still a matter of debate. Regardless, the present findings suggest that activation of κ receptors may serve to attenuate the development of sensitization produced by activation of μ receptors. If this is the case, then the endogenous μ and κ opioid receptor systems may represent future targets in the development of novel medications to prevent the escalation of drug use and addictive behavior in substance-abusing populations.
Acknowledgments
We thank Amy Becton for expert animal care and technical assistance and the National Institute on Drug Abuse for supplying the study drugs.
This work was supported in part by the National Institutes of Health [Grant DA14255]; the Howard Hughes Medical Institute [Grant 52006292]; the National Science Foundation; the Duke Endowment; and Davidson College.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.150011.
ABBREVIATIONS: U69593, (+)-(5α,7α,8β)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]benzeneacetamide; U50488, trans-(±)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl]benzeneacetamide.
References
- Badanich KA, Maldonado AM, and Kirstein CL (2008) Early adolescents show enhanced acute cocaine-induced locomotor activity in comparison to late adolescent and adult rats. Dev Psychobiol 50 127-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbini M and Davis WM (1972) Time-dose relationships for locomotor activity effects of morphine after acute or repeated treatment. Br J Pharmacol 46 213-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz BA, Ngai SH, Hempstead J, and Spector S (1975) Disposition of naloxone: use of a new radioimmunoassay. J Pharmacol Exp Ther 195 499-504. [PubMed] [Google Scholar]
- Brady LS and Holtzman SG (1981) Effects of intraventricular morphine and enkephalins on locomotor activity in nondependent, morphine-dependent and post-dependent rats. J Pharmacol Exp Ther 218 613-620. [PubMed] [Google Scholar]
- Buxbaum DM, Yarbrough GG, and Carter ME (1973) Biogenic amines and narcotic effects. I. Modification of morphine-induced analgesia and motor activity after alteration of cerebral amine levels. J Pharmacol Exp Ther 185 317-327. [PubMed] [Google Scholar]
- Cunningham ST, Finn M, and Kelley AE (1997) Sensitization of the locomotor response to psychostimulants after repeated opiate exposure: role of the nucleus accumbens. Neuropsychopharmacology 16 147-155. [DOI] [PubMed] [Google Scholar]
- Dortch-Carnes J and Potter DE (2005) Bremazocine: a kappa-opioid agonist with potent analgesic and other pharmacologic properties. CNS Drug Rev 11 195-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson PJ, Clark MJ, Mansour A, Akil H, Woods JH, and Medzihradsky F (1996) Characterization of opioid agonist efficacy in a C6 glioma cell line expressing the mu opioid receptor. J Pharmacol Exp Ther 278 1121-1127. [PubMed] [Google Scholar]
- Heidbreder CA, Goldberg SR, and Shippenberg TS (1993) The kappa-opioid receptor agonist U-69593 attenuates cocaine-induced behavioral sensitization in the rat. Brain Res 616 335-338. [DOI] [PubMed] [Google Scholar]
- Hoskin PJ and Hanks GW (1991) Opioid agonist-antagonist drugs in acute and chronic pain states. Drugs 41 326-344. [DOI] [PubMed] [Google Scholar]
- Huang P, Kehner GB, Cowan A, and Liu-Chen LY (2001) Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther 297 688-695. [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn C, Francis R, and Spear LP (1995) Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exp Ther 275 345-357. [PubMed] [Google Scholar]
- Leander JD (1987) Buprenorphine has potent kappa opioid receptor antagonist activity. Neuropharmacology 26 1445-1447. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rajabi H, and Stewart J (2003) Effects of cocaine in rats exposed to heroin. Neuropsychopharmacology 28 2102-2116. [DOI] [PubMed] [Google Scholar]
- McCreary AC, Bankson MG, and Cunningham KA (1999) Pharmacological studies of the acute and chronic effects of (+)-3, 4-methylenedioxymethamphetamine on locomotor activity: role of 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B/1D) receptors. J Pharmacol Exp Ther 290 965-973. [PubMed] [Google Scholar]
- McDaid J, Dallimore JE, Mackie AR, Mickiewicz AL, and Napier TC (2005) Cross-sensitization to morphine in cocaine-sensitized rats: behavioral assessments correlate with enhanced responding of ventral pallidal neurons to morphine and glutamate, with diminished effects of GABA. J Pharmacol Exp Ther 313 1182-1193. [DOI] [PubMed] [Google Scholar]
- Melzacka M, Nesselhut T, Havemann U, Vetulani J, and Kuschinsky K (1985) Pharmacokinetics of morphine in striatum and nucleus accumbens: relationship to pharmacological actions. Pharmacol Biochem Behav 23 295-301. [DOI] [PubMed] [Google Scholar]
- Morgan D, Cook CD, Smith MA, and Picker MJ (1999) An examination of the interactions between the antinociceptive effects of morphine and various mu-opioids: the role of intrinsic efficacy and stimulus intensity. Anesth Analg 88 407-413. [DOI] [PubMed] [Google Scholar]
- Morgan D and Picker MJ (1998) The mu opioid irreversible antagonist beta-funaltrexamine differentiates the discriminative stimulus effects of opioids with high and low efficacy at the mu opioid receptor. Psychopharmacology 140 20-28. [DOI] [PubMed] [Google Scholar]
- Morgan D and Roberts DC (2004) Sensitization to the reinforcing effects of cocaine following binge-abstinent self-administration. Neurosci Biobehav Rev 27 803-812. [DOI] [PubMed] [Google Scholar]
- Negus SS, Picker MJ, and Dykstra LA (1989) Kappa antagonist properties of buprenorphine in non-tolerant and morphine-tolerant rats. Psychopharmacology 98 141-143. [DOI] [PubMed] [Google Scholar]
- Peachey JE (1987) Clinical observations of agonist-antagonist analgesic dependence. Drug Alcohol Depend 20 347-365. [DOI] [PubMed] [Google Scholar]
- Porreca F, Cowan A, and Tallarida RJ (1981) Time course of antagonism of morphine antinociception by intracerebroventricularly administered naloxone in the rat. Eur J Pharmacol 76 55-59. [DOI] [PubMed] [Google Scholar]
- Powell KR and Holtzman SG (2001) Parametric evaluation of the development of sensitization to the effects of morphine on locomotor activity. Drug Alcohol Depend 62 83-90. [DOI] [PubMed] [Google Scholar]
- Puig-Ramos A, Santiago GS, and Segarra AC (2008) U-69593, a kappa opioid receptor agonist, decreases cocaine-induced behavioral sensitization in female rats. Behav Neurosci 122 151-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers AE, Clark MJ, Mansour A, Akil H, Woods JH, and Medzihradsky F (1999) Opioid efficacy in a C6 glioma cell line stably expressing the human kappa opioid receptor. J Pharmacol Exp Ther 288 827-833. [PubMed] [Google Scholar]
- Robinson TE and Berridge KC (2000) The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 95 S91-S117. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, and Zahniser NR (2003) Individual differences in cocaine-induced locomotor sensitization in low and high cocaine locomotor-responding rats are associated with differential inhibition of dopamine clearance in nucleus accumbens. J Pharmacol Exp Ther 305 180-190. [DOI] [PubMed] [Google Scholar]
- Schmidt WK, Tam SW, Shotzberger GS, Smith DH Jr, Clark R, and Vernier VG (1985) Nalbuphine. Drug Alcohol Depend 14 339-362. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, LeFevour A, and Heidbreder C (1996) kappa-Opioid receptor agonists prevent sensitization to the conditioned rewarding effects of cocaine. J Pharmacol Exp Ther 276 545-554. [PubMed] [Google Scholar]
- Shippenberg TS, LeFevour A, and Thompson AC (1998) Sensitization to the conditioned rewarding effects of morphine and cocaine: differential effects of the kappa-opioid receptor agonist U69593. Eur J Pharmacol 345 27-34. [DOI] [PubMed] [Google Scholar]
- Smith MA, Green-Naples JL, Felder JN, Iordanou JC, Lyte MA, and Walker KL (2009) The effects of repeated opioid administration on locomotor activity: II. unidirectional cross-sensitization to cocaine. J Pharmacol Exp Ther 330 476-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA and Picker MJ (1995) Examination of the kappa agonist and antagonist properties of opioids in the rat drug discrimination procedure: influence of training dose and intrinsic efficacy. Behav Pharmacol 6 703-717. [PubMed] [Google Scholar]
- Spanagel R (1995) Modulation of drug-induced sensitization processes by endogenous opioid systems. Behav Brain Res 70 37-49. [DOI] [PubMed] [Google Scholar]
- Székely JI, Miglécz E, and Rónai AZ (1980) Biphasic effects of a potent enkephalin analogue (D-Met2,Pro5)-enkephalinamide and morphine on locomotor activity in mice. Psychopharmacology 71 299-301. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM (2001) Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog Neurobiol 63 241-320. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Schoffelmeer AN, Mulder AH, and De Vries TJ (1999a) Dopaminergic mechanisms mediating the long-term expression of locomotor sensitization following pre-exposure to morphine or amphetamine. Psychopharmacology 143 244-253. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Schoffelmeer AN, Mulder AH, and De Vries TJ (1999b) Lack of cross-sensitization of the locomotor effects of morphine in amphetamine-treated rats. Neuropsychopharmacology 21 550-559. [DOI] [PubMed] [Google Scholar]
- Vasko MR and Domino EF (1978) Tolerance development to the biphasic effects of morphine on locomotor activity and brain acetylcholine in the rat. J Pharmacol Exp Ther 207 848-858. [PubMed] [Google Scholar]
- Vezina P (2004) Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev 27 827-839. [DOI] [PubMed] [Google Scholar]
- Vogelsang J and Hayes SR (1991) Butorphanol tartrate (Stadol): a review. J Post Anesth Nurs 6 129-135. [PubMed] [Google Scholar]
- Waddell AB and Holtzman SG (1998) Modulation of cocaine-induced motor activity in the rat by opioid receptor agonists. Behav Pharmacol 9 397-407. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML (2003) A review of the properties of spiradoline: a potent and selective kappa-opioid receptor agonist. CNS Drug Rev 9 187-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (1987). The role of reward pathways in the development of drug dependence. Pharmacol Ther 35 227-263. [DOI] [PubMed] [Google Scholar]