Abstract
Sensitization refers to an increase in sensitivity to the effects of a drug and is believed to play a role in the etiology of substance use disorders. Cross-sensitization has been observed between drugs from different pharmacological classes and may play a role in the escalation of drug use in polydrug-abusing populations. The purpose of this study was to examine cross-sensitization between opioids and cocaine and to determine the extent to which cross-sensitization is mediated by an opioid's selectivity for μ, κ, and δ receptors. Separate groups of rats were treated with opioid receptor agonists and antagonists every other day for 10 days, and the locomotor effects of cocaine were tested 8 days later. The μ agonists, morphine and buprenorphine, and the δ agonist, BW373U86 [(±)-4-[(R*)-[(2S*,5R*)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-hydroxyphenyl)methyl]-N,N-diethylbenzamide hydrochloride], produced cross-sensitization to cocaine, such that repeated administration of these drugs over a 10-day period significantly enhanced cocaine's locomotor effects when tested later. Coadministration of the opioid antagonist naltrexone prevented morphine and buprenorphine from producing cross-sensitization. Coadministration of naltrexone, but not the δ antagonist naltrindole, also prevented BW373U86 from producing cross-sensitization. The κ agonist spiradoline failed to produce cross-sensitization, but coadministration of spiradoline prevented morphine and buprenorphine from producing cross-sensitization. The ability of spiradoline to block cross-sensitization was itself blocked by the κ antagonist nor-binaltorphimine. The mixed μ/κ opioids butorphanol, nalbuphine, and nalorphine did not produce cross-sensitization under any condition examined. These data indicate that agonist activity at μ receptors positively modulates cross-sensitization between opioids and cocaine, whereas agonist activity at κ receptors negatively modulates this effect.
Sensitization, an increase in sensitivity to a drug after its repeated administration, is frequently observed after the repeated intermittent administration of many drugs of abuse and is believed to contribute to the etiology of substance abuse and dependence (for review, see Robinson and Berridge, 2000; Morgan and Roberts, 2004). Increased sensitivity to the psychomotor effects of drugs can be determined and quantified easily in animal subjects and has been described in numerous studies examining the locomotor effects of both μ-opioid agonists and cocaine (Jackson and Nutt, 1993; Vanderschuren et al., 1999b; Sabeti et al., 2003). Both the acute (Vaccarino and Corrigall, 1987; Beyer and Steketee, 2001) and sensitized (Vezina et al., 1987; Crombag et al., 2002) locomotor effects of these drugs are due to increases in activity of mesolimbic and mesocortical dopamine systems that originate in the ventral tegmental area and terminate in the nucleus accumbens and prefrontal cortex, respectively. These same neuronal systems also contribute to their positive-reinforcing and incentive-motivational effects, which are believed to play a critical role in their high abuse and dependence liability (for review, see Maldonado, 2003; Robinson and Kolb, 2004). Consistent with this premise, treatment protocols that produce sensitization to a drug's locomotor effects also produce sensitization to a drug's positive-reinforcing effects, leading to greater rates of drug self-administration in sensitized animals (De Vries et al., 1998; Schenk and Partridge, 2000).
A number of preclinical studies have shown that cross-sensitization can develop between μ-opioid agonists and cocaine. For instance, cocaine-treated rats exhibit cross-sensitization to the locomotor (McDaid et al., 2005) and conditioned rewarding (Shippenberg et al., 1998) effects of morphine, and these effects can be observed after a single cocaine injection (Kim et al., 2004). Likewise, heroin-treated rats exhibit cross-sensitization to the locomotor effects of cocaine (Leri et al., 2003), and morphine-treated rats exhibit cross-sensitization to both the locomotor (Lett, 1989; Cunningham et al., 1997) and conditioned rewarding (Shippenberg et al., 1998) effects of cocaine. Similar effects may also be observed at the biochemical level because morphine-treated rats show an enhanced response to cocaine-induced increases in c-fos expression (Erdtmann-Vourliotis et al., 2000). The ability of opioids acting at other receptor sites or multiple receptor sites to influence sensitization-related processes is less clear. For instance, although several studies have reported that κ opioid agonists block the development of sensitization to cocaine itself (Heidbreder et al., 1993b; Spanagel, 1995), the κ agonist U69593 failed to block cross-sensitization to cocaine in rats treated with morphine (Shippenberg et al., 1998), and although sensitization has been observed in rats treated with the mixed μ/δ agonist d-Ala(2)-Met(5)-enkephalinamide (Kalivas et al., 1985), we know of no studies that have specifically examined the ability of δ receptor agonists or mixed μ/κ opioids to produce cross-sensitization to cocaine.
The purpose of the present study was to examine the role of an opioid's relative selectivity for μ, κ, and δ receptors in modulating cross-sensitization to cocaine. To this end, separate groups of rats were treated with various μ, κ, δ, and mixed μ/κ opioids every other day for 10 days. After a 7-day incubation period, the locomotor effects of cocaine were examined in all treatment groups using a cumulative dosing procedure. We have used this procedure previously to examine the acute interactions between opioids and cocaine (Smith et al., 2003), and the present study represents an extension of this research by modeling patterns of drug exposure and abstinence that may contribute to the etiology of substance use disorders.
Materials and Methods
Animals. Male, Long-Evans rats, weighing approximately 250 g upon arrival, were obtained from Charles River Laboratories (Raleigh, NC). Rats were housed individually in polycarbonate cages in a colony room maintained on a 12-h light/dark cycle (lights on, 7:00 AM). Food and drinking water were freely available in the home cage. All subjects were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of Davidson College.
A total of 258 rats were divided among 39 groups: saline (three determinations; n = 18), 10 mg/kg cocaine (n = 6), 2.0 mg/kg d-amphetamine (n = 6), 2.0 mg/kg methamphetamine (n = 6), 0.3 mg/kg naltrexone (n = 6), 3.0 mg/kg naltrexone (n = 6), 10 mg/kg nor-binaltorphimine (two determinations; n = 12), 1.0 mg/kg naltrindole (n = 6), 3.0 mg/kg spiradoline (n = 6), 10 mg/kg spiradoline (n = 6), 3.0 mg/kg morphine (n = 6), 10 mg/kg morphine (two determinations; n = 12), 10 mg/kg morphine + 0.3 mg/kg naltrexone (n = 6), 0.3 mg/kg buprenorphine (n = 6), 1.0 mg/kg buprenorphine (n = 6), 1.0 mg/kg buprenorphine + 0.3 mg/kg naltrexone (n = 6), 3.0 mg/kg BW373U86 (n = 6), 10 mg/kg BW373U86 (n = 6), 10 mg/kg BW373U86 + 1.0 mg/kg naltrindole (n = 6), 10 mg/kg BW373U86 + 0.3 mg/kg naltrexone (n = 6), 10 mg/kg morphine + 10 mg/kg spiradoline (n = 6), 10 mg/kg morphine + 10 mg/kg spiradoline + 10 mg/kg nor-binaltorphimine (n = 6), 1.0 mg/kg buprenorphine + 10 mg/kg spiradoline (n = 6), 1.0 mg/kg buprenorphine + 10 mg/kg spiradoline + 10 mg/kg nor-binaltorphimine (n = 6), 3.0 mg/kg butorphanol (n = 6), 10 mg/kg butorphanol (n = 6), 30 mg/kg butorphanol (n = 6), 10 mg/kg butorphanol + 10 mg/kg nor-binaltorphimine (n = 6), 30 mg/kg butorphanol + 10 mg/kg nor-binaltorphimine (n = 6), 3.0 mg/kg nalbuphine (n = 6), 10 mg/kg nalbuphine (n = 6), 30 mg/kg nalbuphine (n = 6), 10 mg/kg nalbuphine + 10 mg/kg nor-binaltorphimine (n = 6), 30 mg/kg nalbuphine + 10 mg/kg nor-binaltorphimine (n = 6), 3.0 mg/kg nalorphine (n = 6), 10 mg/kg nalorphine (n = 6), 30 mg/kg nalorphine (n = 6), 10 mg/kg nalorphine + 10 mg/kg nor-binaltorphimine (n = 6), and 30 mg/kg nalorphine + 10 mg/kg nor-binaltorphimine (n = 6). Any rat that missed a test session because of illness was removed from the study and not replaced.
Apparatus. All behavioral tests were conducted in a single, open-field, locomotor activity chamber with interior dimensions of 43 × 43 × 30 cm. The chamber consisted of a polyvinyl chloride base with clear acrylic sidewalls and aluminum corner supports. Two circuit boards were located on opposite sidewalls mounted 2.5 cm above the base of the apparatus. One board contained 16 infrared photocells spaced 2.5 cm apart, whereas the other contained 16 infrared detectors with the same spacing. All photocells and detectors were interfaced to a microprocessor that continuously recorded photo beam interruptions throughout a session using software supplied by MED Associates (St. Albans, VT).
Induction of Sensitization. Before behavioral testing, each rat was habituated to the behavioral procedure by being placed into the activity chamber for 5 min a day for 3 consecutive days (for timeline of events, see Table 1). On the final day of habituation (day 3), each rat received an intraperitoneal injection of 1.0 ml/kg saline 15 min before being placed into the chamber. On the following day (day 4), a sensitization procedure previously shown to induce cross-sensitization between morphine and cocaine was initiated (Cunningham et al., 1997).
TABLE 1.
Schedule of testing and events for all groups
| Day of Study | Manipulation | Location |
|---|---|---|
| 1 | Habituation | Locomotor chamber |
| 2 | Habituation | Locomotor chamber |
| 3 | Saline | Locomotor chamber |
| 4 | Test drug | Locomotor chamber |
| 5 | Colony room | |
| 6 | Test drug | Locomotor chamber |
| 7 | Colony room | |
| 8 | Test drug | Locomotor chamber |
| 9 | Colony room | |
| 10 | Test drug | Locomotor chamber |
| 11 | Colony room | |
| 12 | Test drug | Locomotor chamber |
| 13 | Colony room | |
| 14 | Colony room | |
| 15 | Colony room | |
| 16 | Colony room | |
| 17 | Colony room | |
| 18 | Saline control session | Locomotor chamber |
| 19 | Colony room | |
| 20 | Cocaine test session | Locomotor chamber |
During the induction period, rats were administered a test drug (or test drugs) on alternate days for 10 consecutive days (days 4, 6, 8, 10, and 12). On days in which drugs were administered, each rat was brought to the testing room, administered an intraperitoneal injection, and immediately returned to its home cage. After 15 min, the rat was placed into the activity chamber for 5 min, and the number of photo beam interruptions was recorded. After the testing interval elapsed, the rat was removed from the chamber and returned to its home cage. For groups in which multiple drugs were administered (e.g., morphine + spiradoline, buprenorphine + naltrexone), separate injections were administered on opposite sides of the peritoneal cavity. Because of its extremely long duration of action, nor-binaltorphimine was administered only once, immediately after the final habituation session and 24 h before the initiation of the induction period (day 3). Rats receiving nor-binaltorphimine were administered saline every other day during the induction period, 15 min before being placed in the activity chamber. On days in which drugs were not administered, rats remained in the colony room and were left undisturbed. After the induction period, an incubation period of 7 days was initiated. During this period, no drugs were administered, and except as noted below, the rats were left undisturbed in the colony room.
Behavioral Testing. Saline control sessions and cocaine test sessions were conducted according to procedures described previously (Smith et al., 2003). On the 6th day of the incubation period (day 18), a saline control session was conducted in which the effects of repeated saline administration were examined. During this session, each rat was administered saline (1.0 ml/kg i.p.) and immediately returned to its home cage. After 15 min, the rat was placed into the activity chamber, and locomotor activity was measured for 2 min. After this test, the rat was removed from the chamber, administered a second injection of saline, and immediately returned to its home cage. After another 15-min interval, the rat was again placed into the activity chamber, and locomotor activity was measured for 2 min. After this test, the cycle was repeated for a third and final component, after which the rats were returned to the colony room until the final session was conducted with cocaine.
Cocaine was examined on the 8th day of the incubation period and 2 days after the saline control session (day 20). Cocaine testing followed a protocol similar to that described for saline, with the exception that cumulative doses of cocaine were administered at the beginning of each component in lieu of saline. In all tests, rats were administered acute doses of 3.0, 7.0, and 20 mg/kg cocaine over the course of the test session, resulting in cumulative doses of 3.0, 10, and 30 mg/kg cocaine.
Schedule of Testing. The entire study was conducted over the course of 48 months, with no more than eight groups running concurrently with one another. Because rats treated with saline would serve as the comparison group for all other groups, the effects of saline were determined in three separate groups tested approximately 18 months apart. These groups were tested during the 1st, 2nd, and 4th years of the project. In addition, the effects of 10 mg/kg nor-binaltorphimine and 10 mg/kg morphine were each determined in two groups tested approximately 12 months apart.
Drugs. Cocaine hydrochloride, d-amphetamine sulfate, methamphetamine hydrochloride, naltrindole hydrochloride, nor-binaltorphimine dihydrochloride, morphine sulfate, and buprenorphine hydrochloride were generously supplied by the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC). BW373U86 was a gift from M. J. Picker (University of North Carolina, Chapel Hill, NC). Naltrexone hydrochloride, spiradoline mesylate, butorphanol tartrate, nalbuphine hydrochloride, and nalorphine hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO). All compounds were dissolved in saline and injected in a volume of 1.0 ml/kg b.wt.
Data Analysis. Locomotor activity data were computed as distance traveled (centimeters) by software supplied by MED Associates. Because of significant cohort differences in baseline measures of locomotor activity, all data were expressed as a percentage of saline control values according to methods described previously (Smith et al., 2003). These values were calculated individually for each rat by dividing the distance traveled during each component of the cocaine test session by the distance traveled during the relevant component of the saline control session and then multiplying by 100. Dose and group data were analyzed via repeated-measures ANOVA, with dose of cocaine serving as a within-subjects factor and group serving as a between-subjects factor. As a secondary analysis, area under the curve (AUC) estimates were obtained for each dose-effect curve using the Trapezoidal Rule. These AUC estimates were then analyzed via one-way ANOVA using group as a factor. Post hoc tests, corrected for multiple pairwise comparisons, were conducted where appropriate. An α level of 0.05 was used for all statistical tests.
Results
Baseline and Control Data. Three rats were removed from the study because of illness before testing with cocaine, with one rat removed from each of the following groups: saline (second determination), 10 mg/kg nor-binaltorphimine (first determination), and 0.3 mg/kg naltrexone. Thus, 255 rats of the original 258 rats completed the study. Data obtained during the induction period of the study (days 4–12) are described in Smith et al. (2009), and only data from the testing period of the study (days 18–20) are described below.
Because of significant cohort differences in baseline measures of locomotor activity, each group served as its own control, and data obtained in the cocaine test session were compared with those obtained in the saline control session. Locomotor activity decreased across the three components of the saline control session in all groups, and these effects were statistically significant in 22 of the 39 groups (Table 2). When collapsed across groups, the mean (S.E.) distance traveled was 604.01 (14.56), 535.43 (12.05), and 480.20 cm (13.74) in the first, second, and third components, respectively.
TABLE 2.
Mean (S.E.) distance traveled (centimeters) for each group during the saline control session
| Group | First Component | Second Component | Third Component |
|---|---|---|---|
| cm | |||
| Salinea | 582.08 (36.17) | 460.63 (33.67) | 375.92 (27.76) |
| 10 mg/kg cocainea | 594.49 (40.85) | 495.48 (59.39) | 412.09 (33.84) |
| 2.0 mg/kg d-Amphetamine | 379.35 (44.10) | 368.27 (54.32) | 325.45 (64.77) |
| 2.0 mg/kg Methamphetamine | 300.29 (38.95) | 295.22 (62.59) | 272.73 (56.76) |
| 0.3 mg/kg Naltrexone | 561.62 (56.99) | 545.87 (64.29) | 471.38 (67.49) |
| 3.0 mg/kg Naltrexone | 615.55 (50.80) | 557.37 (56.03) | 532.14 (66.45) |
| 10 mg/kg Nor-binaltorphimine | 571.64 (42.39) | 544.87 (42.18) | 485.25 (31.39) |
| 10 mg/kg Naltrindole | 615.29 (71.29) | 571.59 (63.76) | 457.02 (78.07) |
| 3.0 mg/kg Spiradolinea | 589.09 (19.26) | 572.60 (27.72) | 496.68 (20.65) |
| 10 mg/kg Spiradoline | 483.97 (47.77) | 456.94 (58.17) | 374.41 (28.32) |
| 3.0 mg/kg Morphinea | 622.84 (70.60) | 521.59 (59.52) | 431.45 (38.89) |
| 10 mg/kg Morphinea | 581.26 (36.96) | 466.19 (46.76) | 404.48 (30.12) |
| 10 mg/kg Morphine + 0.3 mg/kg naltrexonea | 671.73 (90.44) | 541.79 (68.82) | 464.05 (40.19) |
| 0.3 mg/kg Buprenorphinea | 630.98 (17.79) | 560.13 (50.84) | 474.90 (31.52) |
| 1.0 mg/kg Buprenorphinea | 616.65 (45.15) | 512.41 (86.67) | 446.44 (51.41) |
| 1.0 mg/kg Buprenorphine + 0.3 mg/kg naltrexone | 681.89 (75.63) | 599.16 (86.34) | 598.42 (75.93) |
| 3.0 mg/kg BW373U86a | 543.59 (43.37) | 453.39 (28.19) | 394.29 (47.31) |
| 10 mg/kg BW373U86a | 579.08 (42.02) | 433.46 (34.04) | 417.59 (86.72) |
| 10 mg/kg BW373U86 + 1.0 naltrindolea | 522.93 (49.06) | 474.64 (62.31) | 385.84 (52.34) |
| 10 mg/kg BW373U86 + 0.3 mg/kg naltrexonea | 628.30 (48.47) | 508.33 (69.02) | 453.19 (43.22) |
| 10 mg/kg Morphine + 10 mg/kg spiradolinea | 684.06 (41.01) | 556.84 (45.94) | 416.45 (40.50) |
| 10 mg/kg Morphine + 10 mg/kg spiradoline + 10 mg/kg nor-binaltorphiminea | 506.05 (68.29) | 481.14 (66.04) | 370.32 (62.64) |
| 1.0 mg/kg Buprenorphine + 10 mg/kg spiradoline | 668.10 (53.52) | 624.87 (69.03) | 613.50 (75.14) |
| 1.0 mg/kg Buprenorphine + 10 mg/kg spiradoline + 10 mg/kg nor-binaltorphiminea | 655.97 (35.59) | 555.50 (62.29) | 510.67 (62.48) |
| 3.0 mg/kg Butorphanola | 777.31 (36.73) | 659.74 (32.56) | 659.63 (49.15) |
| 10 mg/kg Butorphanola | 805.98 (49.02) | 616.88 (51.86) | 589.81 (43.06) |
| 30 mg/kg Butorphanola | 668.57 (29.02) | 660.01 (41.13) | 512.40 (44.82) |
| 10 mg/kg Butorphanol + 10 mg/kg nor-binaltorphimine | 597.22 (45.18) | 594.14 (47.38) | 579.34 (85.82) |
| 30 mg/kg Butorphanol + 10 mg/kg nor-binaltorphimine | 591.24 (54.85) | 513.29 (53.20) | 508.66 (55.41) |
| 3.0 mg/kg Nalbuphine | 620.37 (37.67) | 598.65 (37.62) | 560.35 (23.94) |
| 10 mg/kg Nalbuphinea | 627.60 (68.77) | 552.16 (80.81) | 455.37 (79.29) |
| 30 mg/kg Nalbuphinea | 614.53 (36.45) | 516.62 (41.95) | 530.46 (25.70) |
| 10 mg/kg Nalbuphine + 10 mg/kg nor-binaltorphiminea | 718.15 (41.24) | 588.03 (49.08) | 550.25 (45.85) |
| 30 mg/kg Nalbuphine + 10 mg/kg nor-binaltorphimine | 633.51 (66.63) | 580.30 (59.36) | 576.59 (75.02) |
| 3.0 mg/kg Nalorphine | 641.68 (50.18) | 588.61 (50.01) | 538.53 (53.28) |
| 10 mg/kg Nalorphinea | 593.78 (23.95) | 538.93 (38.07) | 461.33 (61.19) |
| 30 mg/kg Nalorphine | 630.84 (24.06) | 612.19 (61.08) | 572.81 (78.86) |
| 10 mg/kg Nalorphine + 10 mg/kg nor-binaltorphimine | 653.67 (42.14) | 619.25 (44.05) | 585.34 (68.57) |
| 30 mg/kg Nalorphine + 10 mg/kg nor-binaltorphimine | 495.44 (11.39) | 484.85 (70.42) | 462.36 (42.86) |
Significant main effect of component (p < 0.05)
Cocaine Test Data. When expressed as a percentage of saline control values, cocaine produced dose-dependent increases in locomotor activity in all groups of rats. In 36 of 39 groups, the effects of cocaine increased linearly across the three doses, with the greatest effect occurring at the highest dose tested (30 mg/kg). In those groups that did not exhibit a linear increase, the effects of cocaine peaked at the intermediate dose (10 mg/kg). Repeated-measures ANOVA using dose as a within-subjects variable revealed significant dose effects in all instances (p < 0.05). In the sections that follow, only group effects are discussed.
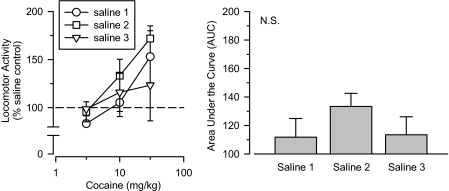
Saline. Rats treated with saline served as the comparison group with which all other groups were compared. As such, the effects of saline were determined in three separate groups tested approximately 18 months apart (Fig. 1). It is important that the three groups did not differ from one another in their sensitivity to cocaine, as determined by a dose-response analysis [F(2,14) = 0.619; N.S.] and by an AUC analysis [F(2,14) = 0.651; N.S.]. Given that no significant group differences were observed, the three groups were combined into a single comparison group for all subsequent analyses.
Fig. 1.
Effects of cumulative doses of cocaine in three groups of rats treated with saline and tested approximately 18 months apart (saline 1, saline 2, saline 3). Left, locomotor activity expressed as a percentage of saline control values. Right, AUC estimates for each group. Vertical lines, S.E.; where not indicated, the S.E. fell within the data point.
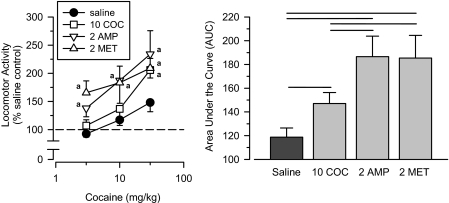
Cocaine, d-Amphetamine, and Methamphetamine. Rats treated with the indirect dopamine agonists cocaine, d-amphetamine, and methamphetamine served as positive control groups. All three groups were more sensitive to cocaine than the saline comparison group (Fig. 2), indicating that the sensitization protocol was sufficient for cocaine to induce sensitization to itself and for cross-sensitization to develop between other psychomotor stimulants and cocaine. Consistent with these observations, a repeated-measures ANOVA revealed a significant main effect of group [F(3,31) = 7.127; p = 0.001], and an AUC analysis revealed that all three groups differed from the saline comparison group (p < 0.05). Although the d-amphetamine and methamphetamine groups did not differ from one another, significantly greater degrees of sensitization were seen in these groups than in the cocaine group (p < 0.05).
Fig. 2.
Effects of cumulative doses of cocaine in separate groups of rats treated with saline (saline), 10 mg/kg cocaine (10 COC), 2.0 mg/kg d-amphetamine (2 AMP), and 2.0 mg/kg methamphetamine (2 MET). Left, locomotor activity expressed as a percentage of saline control values. Right, AUC estimates for each group. Horizontal lines, significant differences between groups (p < 0.05). Vertical lines, S.E.; where not indicated, the S.E. fell within the data point. Significant differences are indicated as follows: a, significantly different from saline control group (p < 0.05).
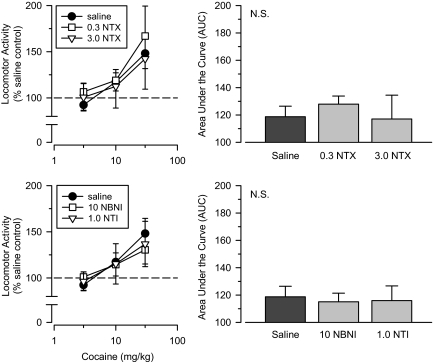
Opioid Receptor Antagonists. Low (0.3 mg/kg) and high (3.0 mg/kg) doses of the opioid receptor antagonist naltrexone were examined in separate groups of rats (Fig. 3). A repeated-measures ANOVA revealed no significant differences in sensitivity to cocaine between the two naltrexone groups and the saline comparison group. Furthermore, AUC estimates for both naltrexone groups were similar to those obtained in rats treated with saline.
Fig. 3.
Top, effects of cumulative doses of cocaine in separate groups of rats treated with saline (saline), 0.3 mg/kg naltrexone (0.3 NTX), and 3.0 mg/kg naltrexone (3.0 NTX). Bottom, effects of cumulative doses of cocaine in separate groups of rats treated with saline (saline), 10 mg/kg nor-binaltorphimine (10 NBNI), and 1.0 mg/kg naltrindole (1.0 NTI). Left, locomotor activity expressed as a percentage of saline control values. Right, AUC estimates for each group. Vertical lines, S.E.; where not indicated, the S.E. fell within the data point.
The effects of the κ receptor antagonist nor-binaltorphimine were examined in separate groups of rats tested approximately 12 months apart. Because the effects of nor-binaltorphimine did not differ between the two determinations, the two groups were combined into one group for all statistical analyses. The effects of the δ-selective antagonist naltrindole were examined in a separate group of rats on only one occasion. When tested with cocaine, neither the nor-binaltorphimine group nor the naltrindole group differed significantly from the saline comparison group. Similar to that seen with naltrexone, AUC estimates for both groups were comparable with those obtained in rats treated with saline (Fig. 3).
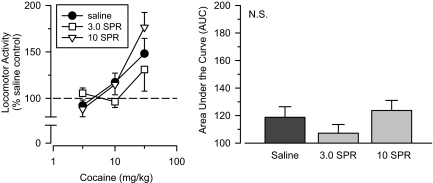
Spiradoline. Both low (3.0 mg/kg) and high (10 mg/kg) doses of the κ agonist spiradoline failed to produce cross-sensitization to cocaine (Fig. 4). Repeated-measures ANOVA revealed no significant group differences between the two spiradoline groups and the saline comparison group. Consistent with this finding, AUC estimates for the two groups were similar to each other and comparable with those obtained in saline-treated rats.
Fig. 4.
Effects of cumulative doses of cocaine in separate groups of rats treated with saline (saline), 3.0 mg/kg spiradoline (3.0 SPR), and 10 mg/kg spiradoline (10 SPR). Left, locomotor activity expressed as a percentage of saline control values. Right, AUC estimates for each group. Vertical lines represent the S.E.; where not indicated, the S.E. fell within the data point.
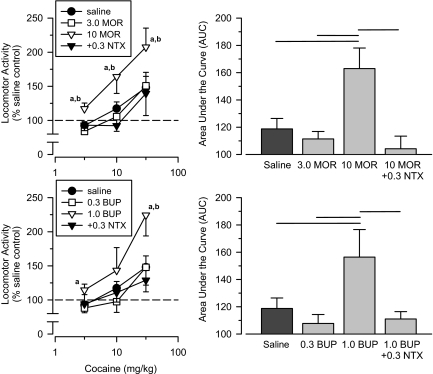
Morphine and Buprenorphine. Rats treated with the μ agonist morphine exhibited cross-sensitization to cocaine, but this effect depended on the dose of morphine (Fig. 5). Although rats treated with a low dose of morphine (3 mg/kg) did not exhibit cross-sensitization, rats treated with a high dose (10 mg/kg) exhibited significant cross-sensitization when tested with cocaine. It should be noted that the effects of 10 mg/kg morphine were examined in two groups of rats that were tested approximately 12 months apart. Both groups showed significant cross-sensitization to cocaine, but they did not differ significantly from one another. As such, the two groups were combined into one group for all statistical analyses. In another group of rats, coadministration of a low dose of naltrexone (0.3 mg/kg) blocked completely the ability of 10 mg/kg morphine to produce cross-sensitization to cocaine. A repeated-measures ANOVA revealed a significant main effect of group [F(3,37) = 4.393; p = 0.010], and an AUC analysis revealed that the 10 mg/kg morphine group differed significantly from all other groups (p < 0.05), including the saline comparison group and the morphine + naltrexone group.
Fig. 5.
Top, effects of cumulative doses of cocaine in separate groups of rats treated with saline (saline), 3.0 mg/kg morphine (3.0 MOR), 10 mg/kg morphine (10 MOR), and 10 mg/kg morphine + 0.3 mg/kg naltrexone (10 MOR + 0.3 NTX). Bottom, effects of cumulative doses of cocaine in separate groups of rats treated with saline (saline), 0.3 mg/kg buprenorphine (0.3 BUP), 1.0 mg/kg buprenorphine (1.0 BUP), and 1.0 mg/kg buprenorphine + 0.3 mg/kg naltrexone (1.0 BUP + 0.3 NTX). Left, locomotor activity expressed as a percentage of saline control values. Right, AUC estimates for each group. Horizontal lines, significant differences between groups (p < 0.05). Vertical lines, S.E.; where not indicated, the S.E. fell within the data point. Significant differences are indicated as follows: a, significantly different from saline control group (p < 0.05); and b, significantly different from naltrexone-treated group (p < 0.05).
Similar to that seen with morphine, rats treated with the μ agonist buprenorphine showed cross-sensitization to cocaine, and this effect was dose-dependent (Fig. 5). Rats treated with a high dose of buprenorphine (1.0 mg/kg) exhibited a significant degree of cross-sensitization that was similar in magnitude to that seen with 10 mg/kg morphine. Cross-sensitization was not observed in rats treated with the low dose of buprenorphine (0.3 mg/kg). In a separate group of rats, coadministration of 0.3 mg/kg naltrexone blocked completely the ability of 1.0 mg/kg buprenorphine to produce cross-sensitization. A repeated-measures ANOVA revealed a significant main effect of group [F(3,31) = 3.223; p = 0.036], and an AUC analysis revealed that the 1.0 mg/kg buprenorphine group differed significantly from all other groups (p < 0.05).
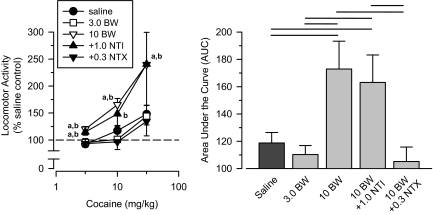
BW373U86. Rats treated with a high (10 mg/kg) but not a low (3 mg/kg) dose of the δ agonist BW373U86 exhibited significant cross-sensitization to cocaine (Fig. 6). Coadministration of a moderate dose of the δ-selective antagonist naltrindole (1.0 mg/kg) failed to block cross-sensitization in BW373U86-treated rats. In contrast, a low dose of naltrexone (0.3 mg/kg) blocked completely the ability of 10 mg/kg BW373U86 to produce cross-sensitization to cocaine. A repeated-measures ANOVA revealed a significant main effect of group [F(4,36) = 3.385; p = 0.019], and an AUC analysis revealed that the 10 mg/kg BW373U86 group differed significantly from the saline comparison group, the 3 mg/kg BW373U86 group, and the BW373U86 + naltrexone group (p < 0.05); however, this group did not differ significantly from the BW373U86 + naltrindole group.
Fig. 6.
Effects of cumulative doses of cocaine in separate groups of rats treated with saline (saline), 3.0 mg/kg BW373U86 (3.0 BW), 10 mg/kg BW373U86 (10 BW), 10 mg/kg BW373U86 + 1.0 mg/kg naltrindole (10 BW + 1.0 NTI), and 10 mg/kg BW373U86 + 0.3 mg/kg naltrexone (10 BW + 0.3 NTX). Left, locomotor activity expressed as a percentage of saline control values. Right, AUC estimates for each group. Horizontal lines, significant differences between groups (p < 0.05). Vertical lines, S.E.; where not indicated, the S.E. fell within the data point. Significant differences are indicated as follows: a, significantly different from saline control group (p < 0.05); and b, significantly different from naltrexone-treated group (p < 0.05).
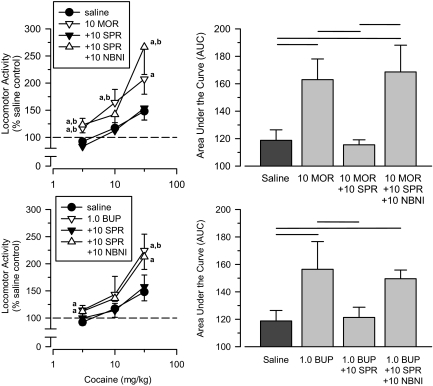
Spiradoline Reversal of Cross-Sensitization. To determine whether agonist activity at κ receptors could block cross-sensitization between μ opioids and cocaine, separate groups of rats were administered 10 mg/kg spiradoline in combination with doses of morphine (10 mg/kg) and buprenorphine (1.0 mg/kg) that produced cross-sensitization to cocaine in earlier tests. To demonstrate that the ability of spiradoline to block cross-sensitization was mediated by κ receptors, subsequent tests pretreated separate groups of rats with 10 mg/kg nor-binaltorphimine before the induction period. These groups were then compared with the groups tested with the μ agonist alone and the saline comparison group.
Spiradoline blocked completely the ability of morphine to produce cross-sensitization (Fig. 7). This effect of spiradoline was mediated by κ receptors because nor-binaltorphimine was able to prevent spiradoline from blocking morphine's ability to produce cross-sensitization. A repeated-measures ANOVA revealed a main effect of group [F(3,37) = 4.785; p = 0.006], and an AUC analysis revealed that the morphine + spiradoline group differed from both the morphine group and the morphine + spiradoline + nor-binaltorphimine group (p < 0.05).
Fig. 7.
Top, effects of cumulative doses of cocaine in separate groups of rats treated with saline (saline), 10 mg/kg morphine (10 MOR; redrawn from Fig. 5), 10 mg/kg morphine + 10 mg/kg spiradoline (10 MOR + 10 SPR), and 10 mg/kg morphine + 10 mg/kg spiradoline + 10 mg/kg nor-binaltorphimine (10 MOR + 10 SPR + 10 NBNI). Bottom, effects of cumulative doses of cocaine in separate groups of rats treated with saline (saline), 1.0 mg/kg buprenorphine (1.0 BUP; redrawn from Fig. 5), 1.0 mg/kg buprenorphine + 10 mg/kg spiradoline (1.0 BUP + 10 SPR), and 1.0 mg/kg buprenorphine + 10 mg/kg spiradoline + 10 mg/kg nor-binaltorphimine (1.0 BUP + 10 SPR + 10 NBNI). Left, locomotor activity expressed as a percentage of saline control values. Right, AUC estimates for each group. Horizontal lines, significant differences between groups (p < 0.05). Vertical lines, S.E.; where not indicated, the S.E. fell within the data point. Significant differences are indicated as follows: a, significantly different from saline control group (p < 0.05); and b, significantly different from spiradoline-treated group (p < 0.05).
Spiradoline also blocked completely the ability of buprenorphine to produce cross-sensitization to cocaine (Fig. 7). Similar to that seen with morphine, nor-binaltorphimine was able to prevent spiradoline from blocking the ability of buprenorphine to produce cross-sensitization to cocaine. A repeated-measures ANOVA revealed a significant main effect of group [F(3,31) = 3.138; p = 0.039], and an AUC analysis revealed that the group treated with buprenorphine + spiradoline differed significantly from the group treated with buprenorphine. The group treated with buprenorphine + spiradoline + nor-binaltorphimine did not differ significantly from the group treated with buprenorphine + spiradoline (p = 0.07); however, this group did differ from the saline comparison group (p < 0.05), indicating that significant cross-sensitization did occur in rats treated with nor-binaltorphimine.
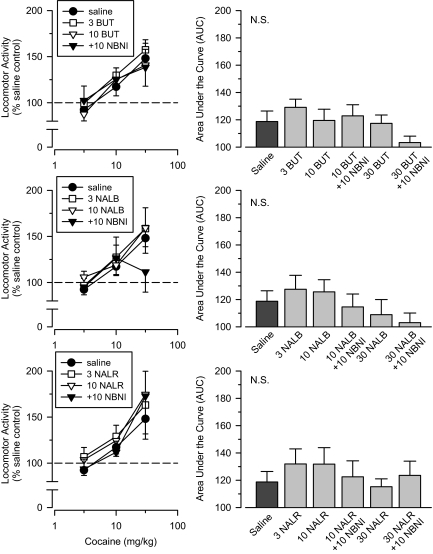
Butorphanol, Nalbuphine, and Nalorphine. All groups treated with the mixed μ/κ opioids, butorphanol, nalbuphine, and nalorphine, exhibited a similar pattern of effects to one another; as a consequence, data from these groups are discussed collectively. Initial tests with 3 and 10 mg/kg of each of butorphanol, nalbuphine, and nalorphine failed to produce cross-sensitization to cocaine (Fig. 8). To determine whether their κ component of action was preventing their μ component to produce cross-sensitization, separate groups were pretreated with 10 mg/kg nor-binaltorphimine before treatment with 10 mg/kg of each of the three test drugs. Even with their κ component of action blocked, none of the groups exhibited cross-sensitization to cocaine. To determine whether the inability of these drugs to produce cross-sensitization was due to a failure to test an adequate dose, additional groups were treated with 30 mg/kg of each of the test drugs alone. Similar to that observed with the lower doses, cross-sensitization was not apparent in any group. As a final test, three additional groups were treated with 30 mg/kg of each of the three test drugs after pretreatment with 10 mg/kg nor-binaltorphimine. Once again, no cross-sensitization was observed in any group. Repeated-measures ANOVA failed to reveal significant main effects of group for any of the three test drugs, and AUC analyses failed to reveal any significant differences among groups, including the saline comparison group (Fig. 8).
Fig. 8.
Top, effects of cumulative doses of cocaine in separate groups of rats treated with saline (saline), 3.0 mg/kg butorphanol (3.0 BUT), 10 mg/kg butorphanol (10 BUT), 10 mg/kg butorphanol + 10 mg/kg nor-binaltorphimine (10 BUT + 10 NBNI), 30 mg/kg butorphanol (30 BUT; right only), and 30 mg/kg butorphanol + 10 mg/kg nor-binaltorphimine (30 BUT + 10 NBNI; right only). Middle, effects of cumulative doses of cocaine in separate groups of rats treated with saline (saline), 3.0 mg/kg nalbuphine (3.0 NALB), 10 mg/kg nalbuphine (10 NALB), 10 mg/kg nalbuphine + 10 mg/kg nor-binaltorphimine (10 NALB + 10 NBNI), 30 mg/kg nalbuphine (30 NALB; right only), and 30 mg/kg nalbuphine + 10 mg/kg nor-binaltorphimine (30 NALB + 10 NBNI; right only). Bottom, effects of cumulative doses of cocaine in separate groups of rats treated with saline (saline), 3.0 mg/kg nalorphine (3.0 NALR), 10 mg/kg nalorphine (10 NALR), 10 mg/kg nalorphine + 10 mg/kg nor-binaltorphimine (10 NALR + 10 NBNI), 30 mg/kg nalorphine (30 NALR; right only), and 30 mg/kg nalorphine + 10 mg/kg nor-binaltorphimine (30 NALR + 10 NBNI; right only). Left, locomotor activity expressed as a percentage of saline control values. Right, AUC estimates for each group. Vertical lines, S.E.; where not indicated, the S.E. fell within the data point.
Discussion
The principal finding of this study is that agonist activity at μ receptors positively modulates cross-sensitization between opioids and cocaine, whereas agonist activity at κ receptors negatively modulates this effect. In all groups, the locomotor effects of cocaine were examined in a single test session using a cumulative dosing procedure. The advantage of this procedure is that it allows for the determination of an entire dose-effect curve within a single experimental session. This is important in studies examining cross-sensitization because it prevents cocaine from producing sensitization to itself when single doses are administered across test sessions (Jackson and Nutt, 1993). We have shown previously that cocaine produces dose- and time-dependent effects in this procedure that are identical to other methods of data collection (Smith et al., 2003). In the present study, cocaine produced dose-dependent increases in locomotor activity in all groups across a dose range equivalent to that used in previous studies.
Effects of Saline and Psychomotor Stimulants. Different groups of rats were tested in this study over a 4-year time period; thus, several different cohorts were obtained from the vender over the course of behavioral testing. Baseline differences in locomotor activity were observed both within and across cohorts; as a consequence, all locomotor activity was expressed as a percentage of the individual control values of each group. Three saline control groups derived from different cohorts were tested at approximately 18-month intervals. Cocaine produced dose-dependent increases in locomotor activity in all three groups, but the potency and efficacy of cocaine did not differ significantly between the groups. The data from the three groups were thus combined to form a single control group, which served as the negative comparison group for all other test groups.
The psychomotor stimulants and indirect dopamine agonists cocaine, d-amphetamine, and methamphetamine served as positive controls in the study, and sensitization was observed in all three groups. It is interesting that the degree of sensitization seen with d-amphetamine and methamphetamine was greater than that seen with cocaine. No attempt was made to match the doses of these drugs in terms of behavioral efficacy, so it is not known whether these differences can be attributed to the selection of asymmetrical doses or to efficacy differences between the drugs in terms of their ability to produce sensitization. Regardless, these data extend numerous studies showing that repeated administration of dopamine agonists leads to functional alterations in mesolimbic and mesocortical signaling and enhanced sensitivity to the effects of psychomotor stimulants (Lett, 1989; Vanderschuren et al., 1999a,b).
Effects of μ, κ, δ, and Mixed μ/κ Opioids. The μ opioids morphine and buprenorphine produced cross-sensitization to the locomotor effects of cocaine. These effects were dose-dependent and blocked by coadministration of a low dose of the opioid receptor antagonist naltrexone. μ Opioid agonists inhibit GABAergic interneurons in the ventral tegmental area, thus releasing dopamine cell bodies from inhibition and inducing the release of dopamine in the nucleus accumbens (Spanagel et al., 1992). When administered acutely, μ agonists synergistically enhance the elevations of extracellular dopamine concentrations induced by cocaine (Smith et al., 2006) and potentiate its effects on locomotor activity (Smith et al., 2003). Repeated administration of μ agonists increases the firing rate of mesolimbic dopamine neurons and produces cross-sensitization to the effects of cocaine (Lett, 1989; Spanagel et al., 1993; Cunningham et al., 1997). Similar to that reported with psychomotor stimulants (e.g., Vanderschuren et al., 1999a), a single injection of morphine is sufficient to augment electrically evoked dopamine release in the nucleus accumbens, produce sensitization to its locomotor effects, and produce cross-sensitization to the locomotor effects of amphetamine (Vanderschuren et al., 2001). Such findings have led investigators to propose that the expression of μ opioid sensitization and cross-sensitization is due, in part, to neuroadaptive changes in mesolimbic dopamine neurons (Vanderschuren and Kalivas, 2000).
A high dose, but not a low dose, of the δ agonist BW373U86 produced cross-sensitization to cocaine that was similar in magnitude to that produced by morphine and buprenorphine. Cross-sensitization between BW373U86 and cocaine was not blocked by coadministration of the δ-selective antagonist naltrindole, suggesting that agonist activity at δ receptors was not responsible for this effect. BW373U86 has only a 15-fold selectivity for δ over μ receptors (Chang et al., 1993), and it is possible that the ability of BW373U86 to produce cross-sensitization to cocaine was due to a μ component of action at a high dose. Supporting this possibility, coadministration of a low dose of naltrexone prevented the development of cross-sensitization in BW373U86-treated rats. Although previous studies have reported that blockade of δ receptors inhibits the development of cocaine-induced sensitization (e.g., Heidbreder et al., 1993a), the present findings suggest that agonist activity at δ receptors alone is not sufficient to produce cross-sensitization to cocaine in opioid-treated subjects.
The selective κ agonist spiradoline did not produce cross-sensitization to cocaine but blocked the ability of morphine and buprenorphine to produce cross-sensitization. The ability of spiradoline to block cross-sensitization was mediated by κ receptors because this effect was itself blocked by the selective κ antagonist nor-binaltorphimine. κ Agonists inhibit dopamine release in the nucleus accumbens (Spanagel et al., 1992), block the biochemical and behavioral effects of cocaine when administered acutely (Heidbreder et al., 1993b; Maisonneuve et al., 1994), and prevent the development of sensitization to cocaine and other psychomotor stimulants (Heidbreder et al., 1993b; Spanagel, 1995). Furthermore, κ agonists block heroin-induced dopamine release (Xi et al., 1998) and prevent the development of sensitization to morphine on measures of locomotor activity and conditioned reward (Spanagel, 1995). A previous study reported that the κ agonist U69593 failed to block cross-sensitization to cocaine in rats treated with morphine, even though it blocked cross-sensitization to morphine in rats treated with cocaine (Shippenberg et al., 1998). Although the reasons for the discrepancies between that study and the present findings are not known, a number of important procedural factors differed between the two studies, including the study drug, induction protocol, and dependent measure.
The mixed μ/κ opioids butorphanol, nalbuphine, and nalorphine failed to produce cross-sensitization to cocaine at all of the doses examined. All three of these drugs possess agonist activity at both μ and κ receptors, and it is possible that their κ component of action was blocking the development of cross-sensitization. To examine this possibility, nor-binaltorphimine was coadministered with these drugs during the induction period of the sensitization protocol. Even at very high doses, these drugs failed to produce cross-sensitization when their κ component of action was blocked. We have shown previously that butorphanol and nalbuphine increase the locomotor effects of cocaine when administered acutely (Smith et al., 2003); thus, the failure of these drugs to produce cross-sensitization to cocaine was somewhat surprising. It is important to note that although these drugs possess μ agonist activity, their relative efficacy at μ receptors is much less than that possessed by morphine and buprenorphine (Morgan and Picker, 1998). The ability of low-efficacy μ agonists to produce cross-sensitization to cocaine has not been examined extensively, but the present findings suggest that a minimal level of intrinsic activity may be necessary to produce the types of compensatory neuroadaptations that are necessary for cross-sensitization to occur.
Implications for the Etiology and Treatment of Substance Use Disorders. The present findings have a number of implications for the etiology of substance use disorders. Sensitization is believed to play a role in the transition from casual drug use to the dysregulated patterns of abuse that characterize substance-dependent populations (Robinson and Berridge, 2000; Morgan and Roberts, 2004), and cross-sensitization between drugs may play a role in the escalation of substance use in polydrug-abusing individuals. Perhaps the most direct clinical implication of these findings is that individuals with a history of prescription opioid use and/or misuse may be at a higher risk for developing problems with cocaine and other psychomotor stimulants. Epidemiological studies reveal that a history of prescription opioid misuse is a predictor of recent cocaine abuse (Tetrault et al., 2008), but whether these findings reflect a causal role of sensitization or simply the tendency of this population to abuse multiple substances is not known. Given the concern regarding the diversion of opioid analgesics and the growing popularity of some μ opioid formulations among recreational drug users, there is a continuing need to identify analgesic medications that are clinically effective but with limited abuse liability. In addition to these characteristics, we would also argue that new and existing medications should be limited in their ability to produce cross-sensitization to other drugs likely to be abused. The present findings suggest that opioids with agonist activity at both μ and κ receptors, or opioids possessing only limited agonist activity at μ receptors, would satisfy these criteria.
One additional implication of these findings regards the development of pharmacotherapies for cocaine dependence. Ideally, such medications would have low abuse liability, limited side effects, and decrease cocaine self-administration at doses that do not disrupt other types of behavior. Direct and indirect dopamine agonists, such as d-amphetamine, have demonstrated some efficacy in clinical populations, and their potential utility represents a promising advance in the treatment of substance use disorders (Grabowski et al., 2004). We caution, however, that d-amphetamine and related drugs produce sensitization and cross-sensitization to a number of drugs with significant abuse liability (Lett, 1989; Vanderschuren et al., 1999b; present study), and the types of neuroadaptations produced by these drugs in vulnerable populations are not fully understood. Mixed μ/κ opioids reduce cocaine self-administration in animal models at doses that do not disrupt other types of operant behavior (Bowen et al., 2003). Furthermore, as shown in the present study, these drugs do not produce cross-sensitization to the behavioral effects of cocaine after repeated administration. Although clinical reports describing their efficacy have been disappointing (e.g., Walsh et al., 2001; Preston et al., 2004), they still may be an effective adjunct in combination with other types of pharmacotherapies. Indeed, their κ component of action may serve to limit the degree of sensitization and cross-sensitization that may develop with other types of agonist substitution therapies.
Acknowledgments
We thank Amy Becton for expert animal care and technical assistance and the National Institute on Drug Abuse (cocaine, d-amphetamine, methamphetamine, naltrindole hydrochloride naltrindole, nor-binaltorphimine, morphine, buprenorphine) and Mitchell J. Picker (BW373U86) for supplying the study drugs.
This work was supported in part by the National Institutes of Health [Grant DA14255]; the Howard Hughes Medical Institute [Grant 52006292]; the National Science Foundation; the Duke Endowment; and Davidson College.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.150037.
ABBREVIATIONS: U69593, (+)-(5α,7α,8β)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]benzeneacetamide; BW373U86, (±)-4-[(R*)-[(2S*,5R*)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-hydroxyphenyl)methyl]-N,N-diethylbenzamide hydrochloride; ANOVA, analysis of variance; AUC, area(s) under the curve.
References
- Beyer CE and Steketee JD (2001) Characterization of the role of medial prefrontal cortex dopamine receptors in cocaine-induced locomotor activity. Behav Neurosci 115 1093-1100. [DOI] [PubMed] [Google Scholar]
- Bowen CA, Negus SS, Zong R, Neumeyer JL, Bidlack JM, and Mello NK (2003) Effects of mixed-action kappa/mu opioids on cocaine self-administration and cocaine discrimination by rhesus monkeys. Neuropsychopharmacology 28 1125-1139. [DOI] [PubMed] [Google Scholar]
- Chang KJ, Rigdon GC, Howard JL, and McNutt RW (1993) A novel, potent and selective nonpeptidic delta opioid receptor agonist BW373U86. J Pharmacol Exp Ther 267 852-857. [PubMed] [Google Scholar]
- Crombag HS, Jedynak JP, Redmond K, Robinson TE, and Hope BT (2002) Locomotor sensitization to cocaine is associated with increased Fos expression in the accumbens, but not in the caudate. Behav Brain Res 136 455-462. [DOI] [PubMed] [Google Scholar]
- Cunningham ST, Finn M, and Kelley AE (1997) Sensitization of the locomotor response to psychostimulants after repeated opiate exposure: role of the nucleus accumbens. Neuropsychopharmacology 16 147-155. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, and Vanderschuren LJ (1998) Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci 10 3565-3571. [DOI] [PubMed] [Google Scholar]
- Erdtmann-Vourliotis M, Mayer P, Riechert U, and Höllt V (2000) Prior experience of morphine application alters the c-fos response to MDMA (`ecstasy') and cocaine in the rat striatum. Brain Res Mol Brain Res 77 55-64. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, and Negus SS (2004) Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav 29 1439-1464. [DOI] [PubMed] [Google Scholar]
- Heidbreder C, Goldberg SR, and Shippenberg TS (1993a) Inhibition of cocaine-induced sensitization by the delta-opioid receptor antagonist naltrindole. Eur J Pharmacol 243 123-127. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Goldberg SR, and Shippenberg TS (1993b) The kappa-opioid receptor agonist U-69593 attenuates cocaine-induced behavioral sensitization in the rat. Brain Res 616 335-338. [DOI] [PubMed] [Google Scholar]
- Jackson HC and Nutt DJ (1993) A single preexposure produces sensitization to the locomotor effects of cocaine in mice. Pharmacol Biochem Behav 45 733-735. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Taylor S, and Miller JS (1985) Sensitization to repeated enkephalin administration into the ventral tegmental area of the rat. I. Behavioral characterization. J Pharmacol Exp Ther 235 537-543. [PubMed] [Google Scholar]
- Kim JA, Pollak KA, Hjelmstad GO, and Fields HL (2004) A single cocaine exposure enhances both opioid reward and aversion through a ventral tegmental area-dependent mechanism. Proc Natl Acad Sci U S A 101 5664-5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Flores J, Rajabi H, and Stewart J (2003) Effects of cocaine in rats exposed to heroin. Neuropsychopharmacology 28 2102-2116. [DOI] [PubMed] [Google Scholar]
- Lett BT (1989) Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology 98 357-362. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Archer S, and Glick SD (1994) U50,488, a kappa opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats. Neurosci Lett 181 57-60. [DOI] [PubMed] [Google Scholar]
- Maldonado R (2003) The neurobiology of addiction. J Neural Transm Suppl 66 1-14. [DOI] [PubMed] [Google Scholar]
- McDaid J, Dallimore JE, Mackie AR, Mickiewicz AL, and Napier TC (2005) Cross-sensitization to morphine in cocaine-sensitized rats: behavioral assessments correlate with enhanced responding of ventral pallidal neurons to morphine and glutamate, with diminished effects of GABA. J Pharmacol Exp Ther 313 1182-1193. [DOI] [PubMed] [Google Scholar]
- Morgan D and Picker MJ (1998) The mu opioid irreversible antagonist beta-funaltrexamine differentiates the discriminative stimulus effects of opioids with high and low efficacy at the mu opioid receptor. Psychopharmacology 140 20-28. [DOI] [PubMed] [Google Scholar]
- Morgan D and Roberts DC (2004) Sensitization to the reinforcing effects of cocaine following binge-abstinent self-administration. Neurosci Biobehav Rev 27 803-812. [DOI] [PubMed] [Google Scholar]
- Preston KL, Umbricht A, Schroeder JR, Abreu ME, Epstein DH, and Pickworth WB (2004) Cyclazocine: comparison to hydromorphone and interaction with cocaine. Behav Pharmacol 15 91-102. [DOI] [PubMed] [Google Scholar]
- Robinson TE and Kolb B (2004) Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47 33-46. [DOI] [PubMed] [Google Scholar]
- Robinson TE and Berridge KC (2000) The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 95 S91-S117. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, and Zahniser NR (2003) Individual differences in cocaine-induced locomotor sensitization in low and high cocaine locomotor-responding rats are associated with differential inhibition of dopamine clearance in nucleus accumbens. J Pharmacol Exp Ther 305 180-190. [DOI] [PubMed] [Google Scholar]
- Schenk S and Partridge B (2000) Sensitization to cocaine's reinforcing effects produced by various cocaine pretreatment regimens in rats. Pharmacol Biochem Behav 66 765-770. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, LeFevour A, and Thompson AC (1998) Sensitization to the conditioned rewarding effects of morphine and cocaine: differential effects of the kappa-opioid receptor agonist U69593. Eur J Pharmacol 345 27-34. [DOI] [PubMed] [Google Scholar]
- Smith JE, Co C, Coller MD, Hemby SE, and Martin TJ (2006) Self-administered heroin and cocaine combinations in the rat: additive reinforcing effects-supra-additive effects on nucleus accumbens extracellular dopamine. Neuropsychopharmacology 31 139-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Gordon KA, Craig CK, Bryant PA, Ferguson ME, French AM, Gray JD, McClean JM, and Tetirick JC (2003) Interactions between opioids and cocaine on locomotor activity in rats: influence of an opioid's relative efficacy at the mu receptor. Psychopharmacology 167 265-273. [DOI] [PubMed] [Google Scholar]
- Smith MA, Greene-Naples JL, Lyle MA, Iordanou JC, and Felder JN (2009) The effects of repeated opioid administration on locomotor activity: I. Opposing actions of μ and κ receptors. J Pharmacol Exp Ther 330 468-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R (1995) Modulation of drug-induced sensitization processes by endogenous opioid systems. Behav Brain Res 70 37-49. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Almeida OF, and Shippenberg TS (1993) Long lasting changes in morphine-induced mesolimbic dopamine release after chronic morphine exposure. Synapse 14 243-245. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, and Shippenberg TS (1992) Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A 89 2046-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetrault JM, Desai RA, Becker WC, Fiellin DA, Concato J, and Sullivan LE (2008) Gender and non-medical use of prescription opioids: results from a national U.S. survey. Addiction 103 258-268. [DOI] [PubMed] [Google Scholar]
- Vaccarino FJ and Corrigall WA (1987) Effects of opiate antagonist treatment into either the periaqueductal grey or nucleus accumbens on heroin-induced locomotor activation. Brain Res Bull 19 545-549. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, De Vries TJ, Wardeh G, Hogenboom FA, and Schoffelmeer AN (2001) A single exposure to morphine induces long-lasting behavioural and neurochemical sensitization in rats. Eur J Neurosci 14 1533-1538. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ and Kalivas PW (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology 151 99-120. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Schmidt ED, De Vries TJ, Van Moorsel CA, Tilders FJ, and Schoffelmeer AN (1999a) A single exposure to amphetamine is sufficient to induce long-term behavioral, neuroendocrine, and neurochemical sensitization in rats. J Neurosci 19 9579-9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Schoffelmeer AN, Mulder AH, and De Vries TJ (1999b) Dopaminergic mechanisms mediating the long-term expression of locomotor sensitization following pre-exposure to morphine or amphetamine. Psychopharmacology 143 244-253. [DOI] [PubMed] [Google Scholar]
- Vezina P, Kalivas PW, and Stewart J (1987) Sensitization occurs to the locomotor effects of morphine and the specific mu opioid receptor agonist, DAGO, administered repeatedly to the ventral tegmental area but not to the nucleus accumbens. Brain Res 417 51-58. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Geter-Douglas B, Strain EC, and Bigelow GE (2001) Enadoline and butorphanol: evaluation of kappa-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J Pharmacol Exp Ther 299 147-158. [PubMed] [Google Scholar]
- Xi ZX, Fuller SA, and Stein EA (1998) Dopamine release in the nucleus accumbens during heroin self-administration is modulated by kappa opioid receptors: an in vivo fast-cyclic voltammetry study. J Pharmacol Exp Ther 284 151-161. [PubMed] [Google Scholar]