Abstract
Under physiological circumstances, cellular responses often reflect integration of signaling by two or more different receptors activated coincidentally or sequentially. In addition to heterologous desensitization, there are examples in which receptor activation either reveals or potentiates signaling by a different receptor type, although this is perhaps less well explored. Here, we characterize one such interaction between endogenous receptors in human embryonic kidney 293 cells in which Gαq/11-coupled muscarinic M3 receptors facilitate Ca2+ signaling by Gαs-coupled β2-adrenoceptors. Measurement of changes in intracellular [Ca2+] demonstrated that noradrenaline released Ca2+ from thapsigargin-sensitive intracellular stores only during activation of muscarinic receptors. Agonists with low efficacy for muscarinic receptor-mediated Ca2+ responses facilitated cross-talk more effectively than full agonists. The cross-talk required Gαs and was dependent upon intracellular Ca2+ release channels, particularly inositol (1,4,5)-trisphosphate receptors. However, β2-adrenoceptor-mediated Ca2+ release was independent of measurable increases in phospholipase C activity and resistant to inhibitors of protein kinases A and C. Interestingly, single-cell imaging demonstrated that particularly lower concentrations of muscarinic receptor agonists facilitated marked oscillatory Ca2+ signaling to noradrenaline. Thus, activation of muscarinic M3 receptors profoundly influences the magnitude and oscillatory behavior of intracellular Ca2+ signaling by β2-adrenoceptors. Although these receptor subtypes are often coexpressed and mediate contrasting acute physiological effects, altered oscillatory Ca2+ signaling suggests that cross-talk could influence longer term events through, for example, regulating gene transcription.
Cells express a range of different receptors able to transduce extracellular signals and ultimately influence cellular behavior. Although receptor activation, intracellular signaling, and functional responses are often studied in isolation, such events in physiological settings are more likely to reflect the integration of signaling mediated by two or more different receptors that are activated either coincidentally or sequentially. For G protein-coupled receptors (GPCRs), such interactions have been explored, and there are many examples in which heterologous desensitization results in the loss of response to the challenge of one receptor type after activation of another receptor type linked to the same or a different signaling pathway. Perhaps less well explored is cross-talk in which activation of one receptor type either reveals or potentiates signaling by a different receptor type. One example of such cross-talk is that in which activation of a Gαq/11-coupled GPCR facilitates Ca2+ signaling by either Gαi/o- or Gαs-coupled GPCRs (Werry et al., 2003a). In most instances, the facilitated Ca2+ signaling is dependent upon an intracellular store, but the mechanisms through which additional Ca2+ is released are unclear. Indeed, many mechanisms have been suggested, and where experimental evidence is available this would suggest a variety of mechanisms are involved that may depend upon both the receptors and cell types involved (Werry et al., 2003a).
Although some examples of cross-talk are dependent upon enhanced activation of phospholipase C (PLC) and therefore increased generation of inositol (1,4,5)-trisphosphate [Ins(1,4,5)P3] to release Ca2+ from the intracellular stores (Dickenson and Hill, 1998; Chan et al., 2000; Werry et al., 2003b), others are independent of enhanced PLC activity, suggesting either increased sensitivity of Ca2+ release channels or alternative release mechanisms (Jiménez et al., 1999; Tanimura et al., 1999; Short and Taylor, 2000; Yeo et al., 2001). Irrespective of the mechanisms involved, the ability of cross-talk to influence intracellular Ca2+ signaling has profound implications for cell function given the diverse cellular events regulated by Ca2+. Here, we have explored interactions between Gαq/11-coupled muscarinic M3 receptors and Gαs-coupled β2-adrenoceptors that result in enhanced Ca2+ signaling, focusing particularly on the pharmacology of the cross-talk. These GPCRs are often coexpressed, for example, in airway smooth muscle, and an understanding of their potential interactions has important physiological and clinical implications.
Materials and Methods
Materials. Cell culture reagents were from Invitrogen (Paisley, UK). Cell culture plastics were from Nalgene (Hereford, UK). Poly-d-lysine-coated 96-well plates for fluorescence imaging plate reader (FLIPR) and other plate reader assays were from BD Biosciences (Oxford, UK). Cholera toxin (CTX), cAMP, Ins(1,4,5)P3, pertussis toxin, fluo-3-acetoxymethyl ester (AM), fluo-4-AM, mouse γ-tubulin antibody, horseradish peroxidase-conjugated secondary antibodies, and the protein kinase inhibitor H89 were from Sigma Chemical (Poole, UK). Forskolin was from Tocris Bioscience (Bristol, UK). Myristoylated peptide protein kinase A (PKA) 14-22 amide inhibitor and myristoylated peptide protein kinase C (PKC) 20-28 inhibitor were from Merck Bioscience (Nottingham, UK). Pluronic F-127 was obtained from Invitrogen. All other reagents were of analytical grade and were obtained from Sigma Chemical or Fisher Scientific (Loughborough, UK). ECL Plus reagents, Hyperfilm, and myo-[3H]inositol with PT6-271 (81Ci mmol-1) were from GE Healthcare (Little Chalfont, Buckinghamshire, UK). Gαs polyclonal antibodies and antibodies against both extracellular signal-regulated kinase (ERK) 1/2 and phospho-ERK were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Prestained molecular weight markers for Western blot (10–250-kDa range) were from Bio-Rad Laboratories (Hercules, CA). Protran nitrocellulose membrane was from Whatman Schleicher and Schuell (Dassel, Germany).
Cell Culture. HEK 293 cells were routinely cultured in minimal essential medium-α supplemented with fetal calf serum (10%, v/v), nonessential amino acids (1%), and l-glutamine (2 mM) and maintained at 37°C in a 95% air, 5% CO2 humidified environment.
Measurement of [Ca2+]i. Elevations of [Ca2+]i in cell populations were measured using fluo-3-AM-loaded cells in a FLIPR (Molecular Devices, Wokingham, UK). For the assay, 100 μl of HEK 293 at a density of 500,000 cells · ml-1 were added to each well of a poly-d-lysine-coated 96-well plate and incubated overnight. Where required, cells were plated in media with either 2 μg · ml-1 CTX or 100 ng · ml-1 pertussis toxin and incubated for 18 to 20 h. Cells were loaded in Hanks' balanced salt solution (HBSS; 10 mM HEPES, 136 mM NaCl, 5.3 mM KCl, 5 mM d-glucose, 0.8 mM MgSO4·7H2O, 1.2 mM CaCl2, and 4.1 mM NaHCO3, pH 7.4) containing 5 μM fluo-3-AM and Pluronic F-127 (0.044%) for 1 h at 37°C. Before the assay, the cells were washed twice with 100 μl of HBSS to remove any excess dye. The cells were finally resuspended in 100 μl of HBSS (± CaCl2 for some studies) and assayed. To examine cross-talk in HEK 293 cells, fluorescence (λex = 488 nm and λem = 540 nm) was initially measured for 5 to 10 s to establish a baseline. After this, a muscarinic receptor agonist or vehicle control (HBSS) was added (30–50 μl; 30–40 μl · s-1), and fluorescence was recorded for 130 to 180 s. In the continued presence of muscarinic receptor agonists or buffer control, an addition of either noradrenaline or buffer was made (30–50 μl; 30–40 μl · s-1), and fluorescence was recorded for a further 130 to 180 s. When the effects of other pharmacological tools were assessed on Ca2+ responses, these were added at the appropriate times before placing them in the FLIPR. In cases where the test agents were dissolved in dimethyl sulfoxide, this was included in control experiments at the appropriate concentrations and shown to be without any effect on Ca2+ responses at the highest concentration tested. The change in fluorescence units was taken as an index of the Ca2+ response. Concentration-response curves were fitted using nonlinear regression with a four-parameter logistic equation in Prism (GraphPad Software Inc., San Diego, CA). All data are presented as mean ± S.E.M. In a small number of experiments as indicated, population Ca2+ signaling experiments were performed in fluo-4-loaded cells using a NOVOstar microplate reader (BMG Labtech, Aylesbury, UK).
For measurement of changes in [Ca2+] by confocal microscopy, HEK 293 cells were plated onto 25-mm-diameter sterile borosilicate glass coverslips coated with 0.01% poly-d-lysine and incubated overnight. Cells were loaded as described above, washed twice with HBSS at 37°C, and images were collected at a rate of approximately one image per second on a laser-scanning confocal microscope (λex = 488 nm, with emitted light collected at >505 nm; Olympus UK Ltd., Watford, UK). The temperature of the chamber was maintained at 37°C using a temperature controller (Harvard Apparatus Inc., Edenbridge, Kent, UK). A region of interest was chosen within the cytoplasm of each cell, using purpose written software (FluoView software, version 4.3; Olympus). Fluorescence before agonist addition was regarded as baseline fluorescence, and the data obtained from each cell during the experiment are expressed as the fold change in cytosolic fluorescence (F/F0) relative to basal levels.
Western Blotting. To determine the effect of CTX on Gαs protein expression, HEK 293 cells were either untreated or treated with 2 μg · ml-1 CTX for 0.5, 1, 2, 4, or 20 h and then solubilized (10 mM Tris, 10 mM EDTA, 500 mM NaCl, 1% Igepal CA630, 0.1% SDS, 0.5% deoxycholate, 1 mM phenylmethylsulfonyl fluoride, 100 μg · ml-1 iodoacetamide, and 100 μg · ml-1 benzamidine, pH 7.4). Samples (30 μg of protein) were separated by SDS-PAGE with a 10% running gel. After transfer onto nitrocellulose, the membrane was blocked for 60 min at room temperature using skimmed milk powder (5%, w/v) in Tris-buffered saline/Tween 20 [150 mM NaCl, 50 mM Tris-HCl, and 0.05% (v/v) Tween 20, pH 7.4]. Nitrocellulose membranes were incubated overnight at 4°C with Gαs polyclonal antibody (1:1000), and visualization was achieved using anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:2000) with enhanced chemiluminescence detection and Hyperfilm. To ensure equivalent protein loading the nitrocellulose membranes were stripped (0.7% 2-β-mercaptoethanol, 2% SDS, and 62.5 mM Tris-HCl, pH 6.8, at 50°C for 30 min with constant agitation), washed extensively (TBST; 30 min), and blocked and probed for γ-tubulin (1:10,000) as described above. For assessment of ERK activity, cells were cultured for 24 h in a 12-well plate and placed in serum-free media for a further 24 h to reduce basal activity. Cells were then either untreated or preincubated with PKA inhibitors (10 μM H89 or 25 μM protein kinase A amide inhibitor 14-22) for 30 min and subsequently stimulated with 100 μM forskolin for 10 min. Whole-cell lysates were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with phospho-ERK or total ERK antibodies using bovine serum albumin (5%, w/v) as a blocking agent and visualized as described above.
Total [3H]Inositol Phosphate Generation. The generation of [3H]InsPx as an index of phospholipase C activity was determined as described previously (Werry et al., 2003b). In brief, cells were grown for 48 h in the presence of 3 μCi · ml-1 myo-[3H]inositol. After washing and preincubation (20 min at 37°C) with HBSS containing 10 mM Li+ to inhibit inositol monophosphatase activity, cells were stimulated for the required time before the reaction was terminated by addition of an equivalent volume of ice-cold 1 M trichloroacetic acid. The reaction mix (final volume, 1 ml) was added to 250 μl of 10 mM EDTA and subsequently 1 ml of a freshly prepared 1:1 (v/v) mixture of tri-n-octyl-amine and 1,1,2-trichloro-trifluoroethane was added. After thorough mixing, a 700-μl aliquot of the upper aqueous layer was removed and added to 50 μl of 250 mM NaHCO3. Soluble inositol phosphates in this aqueous fraction were subsequently isolated using strongly basic Dowex chloride anion exchange columns (8% cross-linkage, 100–200 dry mesh; Sigma 1 × 8-200) and quantified using liquid scintillation counting. Data are expressed as a fold increase in [3H]InsPx relative to basal levels for which the cells were challenged with agonist-free buffer for the longest time point of the experiment.
Data Analysis. All data are expressed as mean ± S.E.M. of three or more experiments as indicated in parentheses. Where representative data are shown, experiments were performed three or more times. Concentration-response curves were fit with Prism (GraphPad Software Inc.) using a standard four-parameter logistic equation. Statistical analysis was by unpaired two-tailed Student's t test, or where required either one-way or two-way analysis of variance (ANOVA), and where p < 0.05, an appropriate post hoc test for multiple comparisons. Statistical significance was accepted for all tests at p < 0.05. We assimilated values from experiments using the FLIPR for peak [Ca2+]i responses to 1 mM methacholine (n = 65) from across our study and tested for normality of distribution. There was no evidence that these data were not normally distributed (Kolmogorov-Smirnov normality test, D'Agostino and Pearson omnibus normality test, Shapiro-Wilk normality test), supporting the use of parametric descriptive and comparative statistics for these type of data.
Results
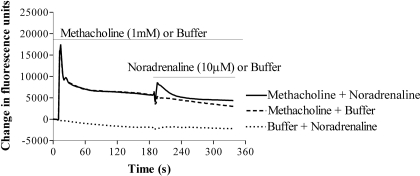
Demonstration of Cross-Talk. Challenge of HEK 293 cells with the muscarinic receptor agonist methacholine (1 mM) resulted in an increase in [Ca2+]i, consisting of a rapid transient peak, followed by a more sustained plateau phase. Addition of noradrenaline (10 μM) failed to elevate [Ca2+]i (Fig. 1). However, challenge of cells with noradrenaline (10 μM) in the continued presence of methacholine (1 mM) resulted in a rapid elevation of [Ca2+]i, which subsided over the subsequent few minutes (Fig. 1). Preaddition of buffer rather than methacholine did not result in a Ca2+ response to the subsequent addition of noradrenaline (Fig. 1).
Fig. 1.
Muscarinic receptor activation facilitates adrenoceptor-mediated Ca2+ signaling in HEK 293 cells. Representative FLIPR traces of cells challenged with 1 mM methacholine (top traces) or buffer (bottom trace), followed by either 10 μM noradrenaline (top-most and bottom traces) or buffer (middle trace) at the times indicated by the horizontal black bars. Neither the addition of buffer after methacholine nor the addition of noradrenaline after buffer elevated [Ca2+]i. However, in the continued presence of methacholine, addition of noradrenaline evoked an elevation of [Ca2+]i. Data are representative of four or more experiments.
Pharmacological Characterization of Cross-Talk. To determine the subtype of adrenoceptor mediating cross-talk, cells were incubated with a range of concentrations of the nonselective α-adrenoceptor antagonist phentolamine, the β1-adrenoceptor-selective antagonist atenolol, or the β2-adrenoceptor-selective antagonist ICI-118,551 (Hoffmann et al., 2004; Baker, 2005). Cells were incubated with the antagonists for 10 min before stimulation with a maximally effective concentration of methacholine (100 μM) and subsequently with a range of noradrenaline concentrations. In the continued presence of this fixed concentration of methacholine (100 μM), noradrenaline evoked a concentration-dependent elevation of [Ca2+]i, with a pEC50 value (-log10 of the EC50 molar concentration) of 6.65 ± 0.15 (n = 4). Phentolamine, over the concentration range of 1 nM to 10 μM, had no effect on either the concentration dependence or Emax values of these noradrenaline-evoked Ca2+ signals (e.g., at 10 μM phentolamine, the pEC50 value was 6.31 ± 0.32 and the Emax value was 117.6 ± 3.8% of the response in the absence of phentolamine; n = 4). Atenolol, over the range of 10 nM to 10 μM, caused a concentration-dependent dextral shift of the noradrenaline concentration-response curve in the presence of 100 μM methacholine (data not shown). However, the resulting Schild plot had a slope different from unity, perhaps as a consequence of the nonequilibrium conditions of the assay. ICI-118,551 caused a collapse of the noradrenaline concentration-response curve in the presence of 100 μM methacholine, with little effect on the pEC50 values where these could be determined. Thus, at 0.01 and 0.1 μM, ICI-118,551 inhibited the maximal responses to noradrenaline by 52 and 65%, respectively, whereas at 1 μM the responses to noradrenaline were abolished. Terbutaline (10 μM), a β2-adrenoceptor-selective agonist that has little or no efficacy at β1-adrenoceptors (Hoffmann et al., 2004), evoked Ca2+ responses that were 76 ± 14 (n = 3) and 86 ± 4% (n = 3) of the response evoked by noradrenaline (10 μM) in the presence of 1 mM methacholine and oxotremorine, respectively. Together, these data suggest that noradrenaline-mediated Ca2+ signaling in the presence of a muscarinic receptor agonist is mediated by β2-adrenoceptors.
Previous evidence suggests that HEK 293 cells express muscarinic M3 receptors (Ancellin et al., 1999), although there have been some reports of muscarinic M1 receptor-mediated effects (Mundell and Benovic, 2000). In the present study, pirenzepine and 4-diphenylacetoxy-N-methylpiperidine methiodide inhibited carbachol-mediated Ca2+ signaling, with pKi (-log10 of the Ki molar concentration) values of 6.84 ± 0.04 and 9.69 ± 0.09 (n = 3), respectively, which are consistent with muscarinic M3 receptor-mediated Ca2+ signaling (Dörje et al., 1991).
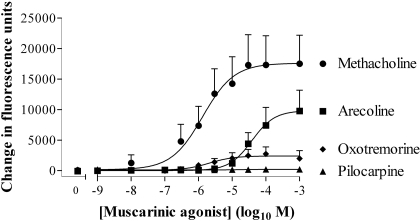
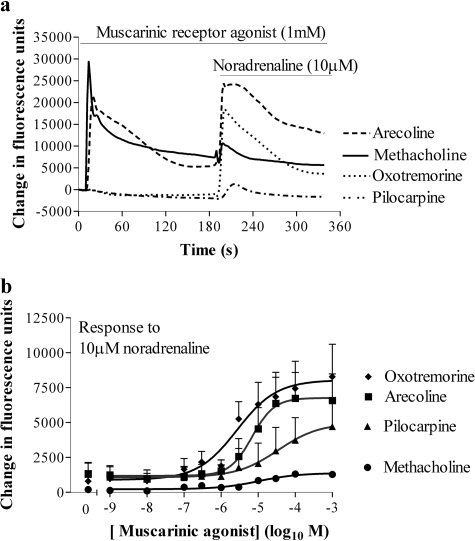
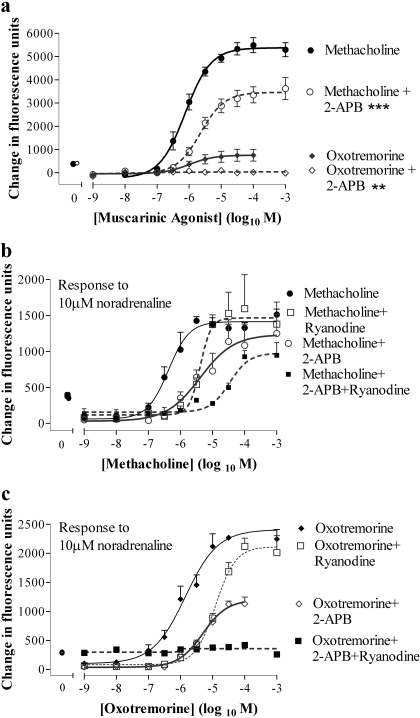
Partial Muscarinic Receptor Agonists Mediate Cross-Talk. In HEK 293 cells, the muscarinic receptor agonists methacholine, arecoline, and oxotremorine exhibited a range of intrinsic activities and potencies with respect to their abilities to elevate [Ca2+]i (Fig. 2; Table 1). Each of these muscarinic receptor agonists facilitated the elevation of [Ca2+]i in response to a subsequent addition of noradrenaline (10 μM) (Fig. 3, a and b; Table 1). Despite both oxotremorine and pilocarpine being relatively weak partial agonists of the muscarinic receptor-mediated Ca2+ response [Emax values of 13.0 ± 0.4 and 2.3 ± 0.6% (n = 4) of the methacholine response, respectively; Fig. 2], they were able to markedly facilitate Ca2+ signaling to a subsequent addition of noradrenaline (Fig. 3, a and b). The partial agonists were also slightly more potent in facilitating Ca2+ signaling by 10 μM noradrenaline than they were in elevating Ca2+, whereas methacholine was more potent in elevating Ca2+ than in facilitating Ca2+ signaling by 10 μM noradrenaline (Table 1). In a small number of experiments, there was a biphasic relationship between the concentration of methacholine and the response to 10 μM noradrenaline. Thus, increasing the concentration of methacholine to 1 μM resulted in an increased Ca2+ response to 10 μM noradrenaline, whereas above 1 μM the response to noradrenaline, in some instances, progressively decreased (data not shown).
Fig. 2.
Concentration dependence of muscarinic receptor-mediated Ca2+ signaling in HEK 293 cells. Using the FLIPR, cells were stimulated with muscarinic receptor agonists. The maximal change in fluorescence on agonist addition was determined and taken as an index of changes in the [Ca2+]i. The pEC50 values are given in Table 1. Data are mean + S.E.M., n = 4.
Table 1.
The pEC50 values of muscarinic receptor agonist-mediated Ca2+ responses and their ability to facilitate Ca2+ signaling by 10 μM noradrenaline
| Muscarinic Receptor Agonist | pEC50 of Muscarinic Receptor Agonist-Mediated Ca2+ Responses | pEC50 of Muscarinic Receptor-Agonists Facilitation of Ca2+ Responses by 10 μM Noradrenaline | n |
|---|---|---|---|
| Arecoline | 4.37 ± 0.21 | 5.03 ± 0.15 | 4 |
| Pilocarpine | 4.39 ± 0.16 | 4 | |
| Methacholine | 6.26 ± 0.52 | 5.78 ± 0.36 | 4 |
| Oxotremorine | 5.54 ± 0.23 | 5.94 ± 0.13 | 4 |
Fig. 3.
Partial agonists of the muscarinic receptor-mediated Ca2+ response facilitate greater Ca2+ responses to noradrenaline than the full agonist methacholine. a, cells were stimulated in a FLIPR (at 10 s) with maximal (1 mM) concentrations of muscarinic receptor agonists of different efficacies and fluorescence recorded every 1 s for a further 180 s. Noradrenaline (10 μM) was then added and fluorescence was recorded for a further 180 s. b, using the protocol described, cells were challenged with the indicated concentrations of muscarinic receptor agonist followed by noradrenaline (10 μM). The maximal change in fluorescence on addition of noradrenaline was quantified and is shown here as an index of changes in the [Ca2+]i. The pEC50 values are given in Table 1. Data are mean + S.E.M., n = 4.
In the presence of methacholine (1 mM), the β-adrenoceptor agonists salbutamol, terbutaline, procaterol, and fenoterol (all 10 μM) increased [Ca2+]i by 65 ± 3, 76 ± 14, 96 ± 4, and 100 ± 1% (n = 3) of the response to noradrenaline. Similarly, in the presence of oxotremorine, the β-adrenoceptor agonists salbutamol, terbutaline, procaterol, and fenoterol increased [Ca2+]i by 81 ± 10, 86 ± 4, 98 ± 18, and 103 ± 10%, respectively, of the response to noradrenaline. The potency (pEC50 values) of the noradrenaline-mediated calcium response in the continued presence of methacholine and oxotremorine was 4.82 ± 0.11 and 5.56 ± 0.09 (n = 3), respectively.
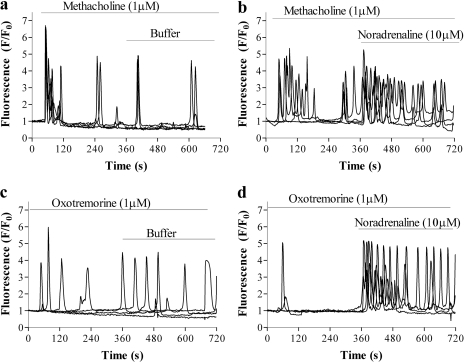
Effect of Cross-Talk on Oscillatory Ca2+ Signaling. Imaging of fluo-3-loaded HEK 293 cells using confocal microscopy confirmed that addition of 10 μM noradrenaline did not evoke Ca2+ signaling (data not shown). Addition of submaximal concentrations of either methacholine (1 μM) or oxotremorine (1 μM) caused oscillatory Ca2+ signaling [defined as a change in fluorescence (F/F0) >1.5 during the period from 60 to 360 s after agonist addition; i.e., one or more responses after any initial response to agonist addition] in 83 ± 4% of cells (n = 96 cells in three independent experiments with each experiment consisting of more than two coverslips) and 33 ± 5% of cells (n = 96 cells in three independent experiments with each experiment consisting of more than two coverslips), respectively (Fig. 4). The subsequent addition of noradrenaline (10 μM) in the continued presence of either muscarinic agonist markedly potentiated the oscillatory Ca2+ signaling (Fig. 4, b and d). Although the addition of noradrenaline in the continued presence of methacholine did not significantly increase the proportion of cells oscillating (from 83 ± 4 to 93 ± 5%; n = 96 cells in three independent experiments), it significantly increased the oscillatory frequency [0.61 ± 0.10 versus 1.13 ± 0.10 oscillations · min-1 (n = 96 in three independent experiments); p < 0.01, Student's t test]. Addition of noradrenaline in the continued presence of oxotremorine both increased the proportion of cells oscillating (from 33 ± 5 to 91 ± 3%; n = 96 cells in three independent experiments) and the oscillation frequency [0.10 ± 0.01 versus 0.84 ± 0.11 oscillations · min-1 (n = 96 cells in three independent experiments, with each experiment consisting of more than two coverslips; p < 0.001, Student's t test)].
Fig. 4.
Effect of noradrenaline on muscarinic receptor agonist-mediated Ca2+ oscillations. Cells were stimulated with approximately EC50 concentrations of either methacholine (1 μM) (a and b) or oxotremorine (1 μM) (c and d) and imaged for 360 s. These cells were subsequently challenged (at t = 360 s) with either buffer (a and c) or 10 μM noradrenaline (b and d). Data are represented as -fold increase over basal fluorescence (F/F0) and are representative of three independent experiments, with each experiment consisting of at least two coverslips, with 16 cells being analyzed from each coverslip.
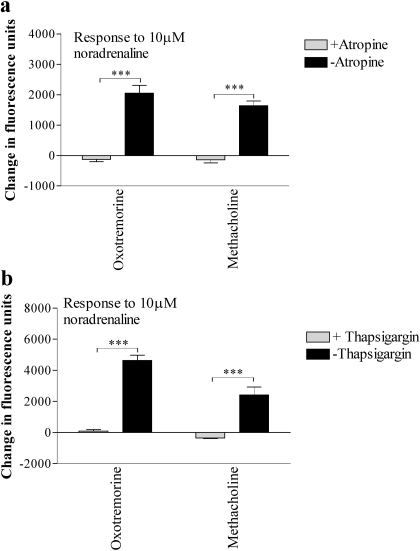
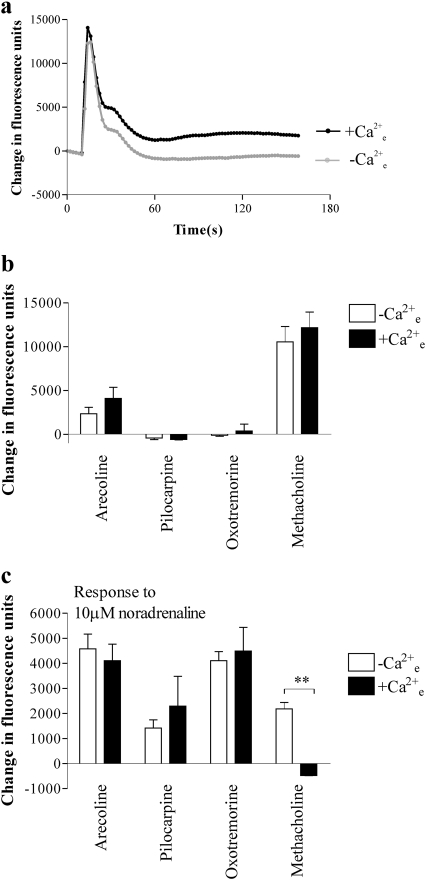
Further Characterization of Cross-Talk. After stimulation of the cells in the FLIPR with a maximal concentration (1 mM) of methacholine for 150 s, the addition of the muscarinic receptor antagonist atropine (10 μM; 5 min) abolished Ca2+ responses to the subsequent addition of 10 μM noradrenaline (Fig. 5a). Similar data were obtained using oxotremorine, arecoline, or pilocarpine as the muscarinic receptor agonists (Fig. 5a; data not shown). Preincubation (5 min) of cells with the sarco(endo)plasmic reticulum Ca2+-ATPase pump inhibitor thapsigargin (2 μM) abolished responses both to the muscarinic receptor agonists (data not shown) and to the subsequent addition of 10 μM noradrenaline (Fig. 5b; data not shown). Removal of extracellular Ca2+ (by use of a nominally Ca2+-free buffer) had no effect on the peak Ca2+ responses to either 1 mM methacholine or 1 mM arecoline but abolished their sustained, plateau phases (Fig. 6, a and b; data not shown). The absence of extracellular Ca2+ had no effect on the Ca2+ responses to noradrenaline (10 μM) after maximal concentrations of either arecoline, pilocarpine, or oxotremorine, whereas the response after methacholine was greater in the absence of [Ca2+]e (Fig. 6c). Although the use of nominally Ca2+-free buffer is often sufficient to investigate the impact of extracellular Ca2+ on GPCR-mediated Ca2+ entry (as reflected here by the abolition of the plateau phase), we also performed experiments using the NOVOstar plate reader in buffer in which the extracellular [Ca2+] was titrated to approximately 100 nM (determined using standard techniques with fura-2) using EGTA. Responses to noradrenaline (10 μM) in the presence of either methacholine (1 or 10 μM) or oxotremorine (1 or 10 μM) were not significantly different in the absence (1.3 mM [Ca2+]e) or presence (100 nM [Ca2+]e) of EGTA [an average of 92 ± 20% (n = 12) across all conditions].
Fig. 5.
Atropine and thapsigargin abolish noradrenaline-mediated Ca2+ responses in the presence of methacholine. a, using a FLIPR, cells were challenged with the indicated muscarinic receptor agonist (1 mM) in the presence or absence of 10 μM atropine before challenge with noradrenaline (10 μM). The graph shows the responses to noradrenaline in the presence of either muscarinic receptor agonists alone or muscarinic receptor agonists and atropine. b, cells were preincubated for 5 min in the presence or absence of thapsigargin (2 μM) before challenge with muscarinic receptor agonists (1 mM) and subsequently challenged with noradrenaline (10 μM). The graph shows only the responses to noradrenaline in the presence of muscarinic receptor agonists with or without thapsigargin treatment; the initial responses to methacholine are not shown. Data are mean + S.E.M., n = 3; ***, p < 0.0001, by Student's t test.
Fig. 6.
Effect of extracellular Ca2+ on maximal intracellular Ca2+ responses to either muscarinic receptor agonists or noradrenaline in the presence of muscarinic receptor agonists. Using a FLIPR, cells were challenged with methacholine (1 mM) in the presence or absence of [Ca2+]e a, from such experiments, the maximal (peak) change in fluorescence immediately after addition of the indicated muscarinic receptor agonist (1 mM) in the presence or absence of [Ca2+]e was determined as an index of the Ca2+ response (b). In the continued presence of the muscarinic receptor agonist, cells were stimulated with noradrenaline (10 μM) and the maximal change in fluorescence determined (c). Data are mean + S.E.M., n = 3; **, p < 0.002, by Student's t test.
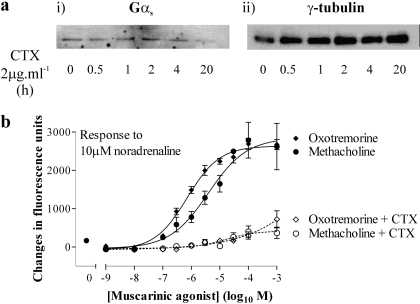
Pretreatment of cells with pertussis toxin (100 ng · ml-1; 18–20 h) to ADP-ribosylate Gαi and prevent GPCR-mediated activation had no effect on the magnitude or potency of either methacholine- or oxotremorine-mediated Ca2+ responses or Ca2+ responses to the addition of 10 μM noradrenaline in the presence of these muscarinic receptor agonists over their effective concentration ranges (data not shown). Although an activator of Gαs, CTX on extended exposure can down-regulate Gαs and abolish Gαs-mediated signaling (Seidel et al., 1999). Treatment of HEK 293 cells for 20 h with CTX (2 μg · ml-1) was sufficient to markedly reduce levels of Gαs (Fig. 7a), consistent with its ability to inhibit Gαs-mediated signaling in this cell background (Werry et al., 2002). This pretreatment with CTX had no effect on the magnitude or potency of oxotremorine- or methacholine-mediated Ca2+ signaling (data not shown). In contrast, Ca2+ responses to the addition of 10 μM noradrenaline in the presence of the muscarinic receptor agonists were abolished (Fig. 7b). In this particular experiment, Ca2+ responses to noradrenaline in the presence of either methacholine or oxotremorine were approximately equivalent rather than being greater in the presence of oxotremorine. This was the exception rather than the rule, and the reasons for this are unclear.
Fig. 7.
Noradrenaline-mediated Ca2+ signaling in the presence of muscarinic receptor agonists is dependent on Gαs. a, cells were incubated with or without 2 μg · ml-1 of CTX for the times indicated. i, cells were solubilized and the proteins were separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted for Gαs. ii, blots were stripped and reprobed for γ-tubulin expression to ensure equivalent protein loading. Data are representative of three different experiments. b, cells were incubated with or without 2 μg · ml-1 of CTX for 20 h and then challenged in a FLIPR with a range of concentrations of either methacholine or oxotremorine. In the continued presence of these muscarinic receptor agonists, cells were challenged with 10 μM noradrenaline (see protocol in Fig. 3a). Panel shows the maximal changes in fluorescence on addition of noradrenaline as an index of the Ca2+ responses. Data are mean ± S.E.M., n = 6.
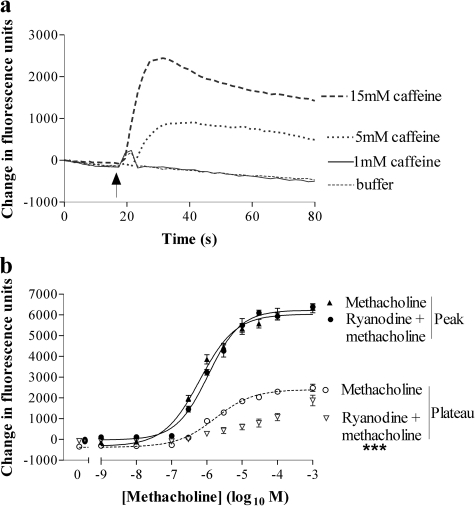
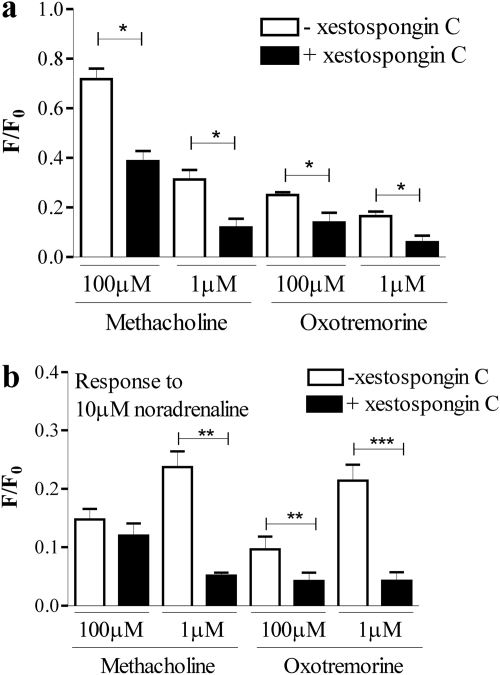
Role for Ryanodine and Ins(1,4,5)P3 Receptors in Cross-Talk. Addition of 1 mM caffeine to HEK 293 cells evoked a small transient elevation of [Ca2+]i (Fig. 8a). Addition of either 5 or 15 mM caffeine resulted in robust and more prolonged elevations of [Ca2+]i (Fig. 8a), indicative of functional ryanodine receptors. Pretreatment of cells with ryanodine (30 μM; 30 min) had no effect on the magnitude or potency of peak Ca2+ response to methacholine, but it significantly (p < 0.001, two-way ANOVA) reduced the plateau phase determined 50 s after agonist addition (Fig. 8b). In contrast, ryanodine enhanced the oxotremorine-mediated elevation of intracellular Ca2+, but it reduced its potency [Emax value of 330 ± 44% (n = 3) of response in the absence of ryanodine; pEC50 values of 4.68 ± 0.25 and 6.16 ± 0.01 (n = 3) in the presence or absence of ryanodine, respectively; p < 0.001 for the difference between concentration-response curves, two-way ANOVA]. Pretreatment of cells with the putative Ins(1,4,5)P3 receptor inhibitor 2-aminoethoxydiphenylborane (2-APB; 100 μM; 30 min) significantly inhibited methacholine-evoked Ca2+ responses and abolished responses to oxotremorine (Fig. 9a). To investigate the impact of ryanodine (30 μM) and 2-APB (100 μM) on noradrenaline-mediated Ca2+ signaling, cells were pretreated (30 min) with these compounds alone or in combination before challenge, with a range of concentrations of either methacholine or oxotremorine and subsequently 10 μM noradrenaline. Ryanodine reduced the potency of both methacholine and oxotremorine to facilitate Ca2+ signaling in response to 10 μM noradrenaline (Fig. 9, b and c). However, it had little effect on the maximal Ca2+ responses to noradrenaline at high concentrations of the muscarinic receptor agonists. 2-APB also reduced the potency of the facilitatory action of methacholine on noradrenaline-mediated Ca2+ signaling (Fig. 9b). In contrast, the magnitude of the Ca2+ responses to 10 μM noradrenaline in the presence of oxotremorine was reduced by 2-APB, although oxotremorine potency on this facilitatory activity was unaffected (Fig. 9c). Combined pretreatment of cells with both ryanodine and 2-APB reduced the potency of the facilitatory activity of methacholine (Fig. 9b). Furthermore, this combined treatment abolished noradrenaline-mediated Ca2+ signaling in the presence of oxotremorine (Fig. 9c). Preincubation of cells with xestospongin C (10 μM for 30 min) significantly reduced subsequent Ca2+ responses to maximal (100 μM) or approximate EC50 (1 μM) concentrations of either methacholine or oxotremorine when these agonists were added under conditions in which the external [Ca2+] was buffered with EGTA to approximately 100 nM (Fig. 10a). Responses to the subsequent addition of 10 μM noradrenaline were also reduced with the exception of addition in the presence of 100 μM methacholine (Fig. 10b).
Fig. 8.
Functional ryanodine receptor expression and the effect of ryanodine on muscarinic receptor-mediated Ca2+ signaling. a, using a FLIPR, cells were challenged (at 15 s as indicated by the arrow) with buffer or varying concentrations (1–15 mM) of caffeine. Data are representative of three experiments. b, cells were incubated with or without ryanodine (30 μM) for 10 min and then, using a FLIPR, they were challenged with a range of concentrations of methacholine. Data shown are the concentration-response curves for methacholine-mediated Ca2+ responses (change in fluorescence of fluo-3) in the presence or absence of ryanodine either immediately after addition of methacholine (peak) or 50 s after addition (plateau). Data are mean ± S.E.M., n = 3; ***, p < 0.001, by two-way ANOVA, plateau responses ± ryanodine.
Fig. 9.
Responses to muscarinic receptor agonists and noradrenaline in the presence of intracellular Ca2+ channel blockers. a, cells were incubated in the presence or absence of 100 μM 2-APB for 30 min before stimulation with varying concentrations of either methacholine or oxotremorine. Responses were determined as the maximal change in fluorescence after agonist addition an index of the Ca2+ responses. **, p < 0.01 and ***, p < 0.001 by two-way ANOVA in the absence and presence of 2-APB. b, cells were incubated in the presence or absence of either 100 μM 2-APB, 30 μM ryanodine or a combination of both for 30 min before challenge with a range of concentrations of methacholine. Cells were subsequently challenged with 10 μM noradrenaline and peak responses were determined. The pEC50 value (6.57 ± 0.39) was significantly reduced by 2-APB (5.49 ± 0.16), ryanodine (5.64 ± 0.16), or the two in combination (4.33 ± 0.04) (p < 0.05, by one-way ANOVA with Bonferroni's post test). c, cells were treated as described in b with the exception that the muscarinic receptor agonist was oxotremorine. Emax values were significantly reduced by 2-APB either alone (p < 0.05) or in combination with ryanodine (p < 0.001), whereas the pEC50 value (5.53 ± 0.04) was reduced (p < 0.05) by ryanodine (4.72 ± 0.17) but not 2-APB (5.30 ± 0.12) (one-way ANOVA with Bonferroni's post test). A pEC50 value could not be determined in the presence of both 2-APB and ryanodine. Data are mean ± S.E.M., n = 3.
Fig. 10.
Responses to muscarinic receptor agonists and noradrenaline in the presence of the Ins(1,4,5)P3 Ca2+ channel blocker xestospongin C. Cells were incubated in the presence or absence of 10 μM xestospongin C for 30 min. Immediately before experimentation, cell monolayers were washed with buffer containing no added Ca2+ and in which the [Ca2+] had been buffered with EGTA to ∼100 nM. Cells were then placed in this low [Ca2+] buffer, with or without xestospongin C as appropriate and immediately stimulated with an approximate EC50 or maximal concentration of either methacholine or oxotremorine in a plate reader (NOVOstar). Cells were subsequently challenged with 10 μM noradrenaline. Panels show the maximal changes in fluorescence on addition of either the muscarinic receptor agonist (a) or noradrenaline (b) as an index of the Ca2+ responses. Data are mean + S.E.M., n = 3; *, p < 0.05; **, p < 0.01; and ***, p < 0.001, by Student's t test.
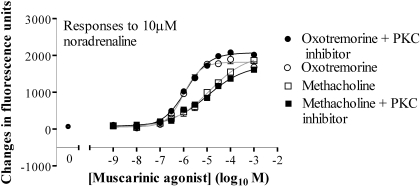
Cross-Talk Is Independent of Enhanced PLC Activity. As an index of total PLC activity, the accumulation of [3H]InsPx was determined in the presence of a Li+ block of inositol monophosphatase activity. Challenge of cells with methacholine for 20 min resulted in a concentration-dependent accumulation of [3H]InsPx, with a pEC50 value of 4.29 ± 0.24 and a maximal 4.40 ± 0.36-fold increase over basal levels (basal 2582 ± 655 dpm · well-1; n = 3). Costimulation with methacholine and 10 μM noradrenaline had no effect on the potency or magnitude of the response (pEC50 value of 4.05 ± 0.60 and a maximal 5.06 ± 0.69-fold increase over basal levels; n = 3). Oxotremorine (1 mM) was a weak partial agonist in respect of the [3H]InsPx response, generating a maximal increase of 1.34 ± 0.13 (n = 3)-fold increase over basal levels that was not affected by costimulation with 10 μM noradrenaline (1.46 ± 0.13; n = 3). To examine PLC activity with greater temporal resolution, cells were transfected with the enhanced green fluorescent protein (eGFP)-tagged pleckstrin homology (PH) domain of PLCδ1 (eGFP-PHPLCδ1). Under basal (unstimulated) conditions eGFP-PHPLCδ1 is localized to the plasma membrane, because it binds with high affinity and selectivity to phosphatidylinositol 4,5-bisphosphate (Nash et al., 2001). Ins(1,4,5)P3 is able to bind to it with high affinity and displace it from the membrane (Nash et al., 2001). This can be monitored in real time by confocal imaging (Nash et al., 2001; Tovey and Willars, 2004) and increased cytosolic fluorescence reflects PLC activation and Ins(1,4,5)P3 generation. Transfection of cells with the eGFP-PHPLCδ1 construct resulted in fluorescence located predominantly at the plasma membrane. However, challenge of cells with either 1 mM methacholine alone or 10 μM noradrenaline in the continued presence of 1 mM methacholine did not consistently result in an increase of the cytosolic fluorescence (data not shown), presumably reflecting very low/localized changes in Ins(1,4,5)P3. Treatment of cells with the cell-permeable PKC inhibitor myristoylated protein kinase C 20-28 (100 μM; 30 min) had no effect on the Ca2+ responses to either methacholine or oxotremorine across the concentration ranges of the muscarinic receptor agonists (data not shown). Furthermore, the magnitude of the subsequent responses to 10 μM noradrenaline were also unaffected by the PKC inhibitor across the range of concentrations of these muscarinic receptor agonists (Fig. 11).
Fig. 11.
Cross-talk is unaffected by inhibition of PKC. Cells were incubated with 100 μM PKC inhibitor myristoylated protein kinase C 20-28 for 30 min before stimulation with the indicated concentrations of muscarinic receptor agonists, and fluorescence was recorded for 150 s. These cells were subsequently challenged with 10 μM noradrenaline, and fluorescence was recorded for an additional 150 s. Maximal changes in fluorescence on the addition of noradrenaline are shown. Data are mean ± S.E.M., n = 3.
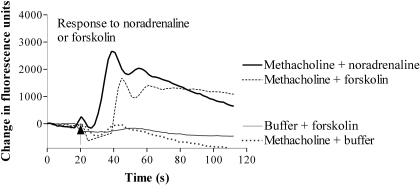
Cross-Talk Is Dependent on cAMP but Independent of PKA Activity. In the presence of 1 mM methacholine, treatment of cells with forskolin (100 μM) to directly activate adenylyl cyclases resulted in a Ca2+ response that was comparable with the responses evoked by 10 μM noradrenaline in the presence of 1 mM methacholine (Fig. 12; 1133 ± 158 versus 1877 ± 399 fluorescence units, respectively; n = 3). Addition of forskolin in the absence of methacholine did not cause a Ca2+ response (Fig. 12). Similar data were obtained by treating cells with forskolin in the presence of oxotremorine.
Fig. 12.
Muscarinic receptor activation facilitates forskolin-mediated Ca2+ signaling. Using a FLIPR, cells were challenged with either methacholine (1 mM) or buffer and ∼60 s later they were challenged with forskolin (100 μM) as indicated by the arrow. Forskolin evoked a Ca2+ response only in the presence of methacholine. For comparison, cells challenged initially with methacholine and subsequently with either noradrenaline or buffer are shown. For clarity, only the responses to the second addition (noradrenaline, forskolin, or buffer) are shown. Data are representative of three or more experiments.
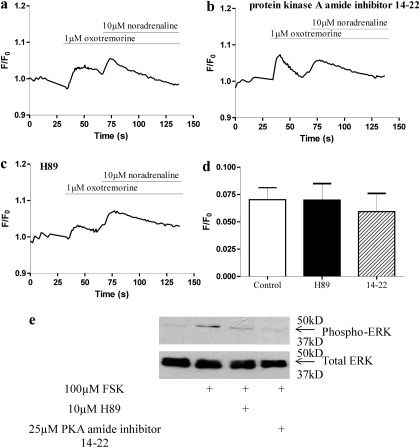
Cells were incubated with the PKA inhibitors H89 (10 μM) or 14-22 myristoylated amide PKA inhibitor (25 μM) for 30 min before stimulation with an approximate EC50 concentration of oxotremorine (1 μM) and a subsequent stimulation with a maximal concentration of noradrenaline (10 μM) to evoke a robust cross-talk. Preincubation with these inhibitors did not affect the Ca2+ response to either oxotremorine or noradrenaline (Fig. 13). Similar data were obtained using a maximal concentration of methacholine (1 mM) to facilitate the Ca2+ responses to noradrenaline (data not shown). The efficacy of the PKA inhibitors was demonstrated by their ability to inhibit forskolin-mediated activation of ERK, as assessed by levels of phospho-ERK (Fig. 13e).
Fig. 13.
Lack of effect of PKA inhibitors on muscarinic receptor- and noradrenaline-mediated Ca2+ responses. Cells were either untreated (a) or preincubated with the PKA inhibitor H89 (b) or protein kinase A amide inhibitor 14-22 (c) for 30 min. Using a plate reader (NOVOstar), cells were then stimulated with approximately an EC50 concentration of oxotremorine (1 μM) and subsequently with noradrenaline (10 μM) to provide robust cross-talk. d, maximal responses to noradrenaline immediately after addition of noradrenaline in the absence or presence of the PKA inhibitors. e, cells were cultured for 24 h in a 12-well plate and placed in serum-free media for a further 24 h. Cells were either untreated (control) or preincubated with the PKA inhibitors H89 (10 μM) or protein kinase A amide inhibitor 14-22 (25 μM) for 30 min and subsequently stimulated with forskolin (100 μM) for 10 min. Whole cell lysates were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with phospho-ERK and total ERK antibodies, visualized with ECL+ and exposure to film. Data are either representative of three independent experiments (a–c and e) or mean + S.E.M., n = 3 (d).
Discussion
Ca2+ responses to muscarinic receptor agonists in our HEK 293 cells are mediated by Gαq/11-coupled muscarinic M3 receptors. Furthermore, noradrenaline elevates [Ca2+]i in these cells via Gαs-coupled β2-adrenoceptors only in the presence of muscarinic receptor activation. This β2-adrenoceptor-mediated Ca2+ signaling is facilitated by both full and partial muscarinic receptor agonists, with partial agonists often being more effective than full agonists. Ca2+ responses to noradrenaline, although dependent on Gαs, are independent of enhanced PLC activity or PKC and PKA activity.
The lack of effect of pertussis toxin on muscarinic receptor-mediated Ca2+ signaling or facilitated noradrenaline Ca2+ signaling excludes roles for Gαi-coupled muscarinic M2 and M4 receptors. Furthermore, pKi values of pirenzepine and 4-diphenylacetoxy-N-methylpiperidine methiodide for inhibition of muscarinic receptor-mediated Ca2+ signaling suggest that, although muscarinic M1 (Mundell and Benovic, 2000) and M3 (Ancellin et al., 1999; Tovey and Willars, 2004) receptors are reported in HEK 293 cells, muscarinic M3 receptors are responsible in our clone. The pharmacology of facilitated adrenoceptor Ca2+ signaling indicates mediation by β2-adrenoceptors. Although ICI-118,551 is considered a selective, competitive antagonist of β2-adrenoceptors (Skeberdis et al., 1997), behavior here is consistent with a noncompetitive interaction. This has been observed previously with low receptor expression, possibly due to a higher affinity of ICI-118,551 for G protein-uncoupled receptors, thereby effectively reducing receptor number and decreasing maximal responses (Hopkinson et al., 2000).
Methacholine, arecoline, oxotremorine, and pilocarpine showed intrinsic activities ranging from full agonism (methacholine) to exceptionally weak partial agonism (pilocarpine). At higher concentrations, the partial agonists arecoline and oxotremorine facilitated Ca2+ signaling by noradrenaline equivalent to or greater than that of methacholine. Thus, the extent of cross-talk is not directly related to peak Ca2+ elevations by the muscarinic receptor agonist. This may reflect competing factors; first, agonist efficacy on the cross-talk pathway and second, the remaining store Ca2+. At higher concentrations of methacholine, Ca2+ stores are likely to be substantially depleted, thereby limiting Ca2+ available for subsequent release. This might explain the reduced response to noradrenaline sometimes seen at concentrations of methacholine >1 μM. It is interesting that concentrations of methacholine and arecoline matched for peak Ca2+ responses (1 and 100 μM, respectively; Fig. 2) facilitate very different noradrenaline Ca2+ responses (Fig. 3b). However, due to different release rates, differential activation of extrusion mechanisms or oscillatory Ca2+ signaling, the relationship between muscarinic receptor-mediated peak Ca2+ responses and the extent of store depletion may not be identical for different agonists. Furthermore, at 1 μM methacholine and 100 μM arecoline, receptor occupancy will be different, and this could influence cross-talk. It is therefore unclear whether aspects other than Ca2+ store depletion contribute to greater facilitation of β2-adrenoceptor-mediated signaling by partial compared with full muscarinic receptor agonists.
Ca2+ signaling by β2-adrenoceptors was abolished after antagonism of muscarinic receptors with atropine, indicating that concurrent activation of both receptors is required. This is consistent with many examples of cross-talk resulting in enhanced Ca2+ signaling (Dickenson and Hill, 1998; Jiménez et al., 1999; Tanimura et al., 1999; Chan et al., 2000; Short and Taylor, 2000; Yeo et al., 2001; Werry et al., 2003b). Treatment of our cells with CTX for 24 h down-regulated Gαs and substantially inhibited noradrenaline-mediated Ca2+ signaling, thereby demonstrating its role in cross-talk. Furthermore, consistent with other examples of such cross-talk (Werry et al., 2002; Tovey et al., 2003; Werry et al., 2003a), noradrenaline responses during muscarinic receptor activation were independent of extracellular Ca2+. Cross-talk also required a replete, thapsigargin-sensitive intracellular Ca2+ store, and although our data suggest that Ins(1,4,5)P3 receptors are particularly involved in release, interpretation of inhibitor studies can be problematic. Thus, both 2-APB and xestospongin C inhibit a range of plasma membrane channels, including store-operated Ca2+ channels (Liu and Ambudkar, 2001; Bootman et al., 2002; Ozaki et al., 2002). However, cross-talk was independent of extracellular Ca2+, and xestospongin C reduced noradrenaline responses even when the trans-plasmalemmal [Ca2+] gradient was abolished, indicating that these compounds reduced cross-talk through inhibition of intracellular channels. The mechanism by which cross-talk enhances Ca2+ release by these receptors is not clear. Although enhanced PLC activity and increased Ins(1,4,5)P3 generation underlies cross-talk between a variety of Gαq/11- and Gαi-coupled receptors (Selbie et al., 1995; Yang et al., 2001), we were unable to find evidence for enhanced PLC activity. Although such measurements of PLC activity are sufficiently sensitive to demonstrate enhanced PLC activity as a means of cross-talk (Selbie et al., 1995; Yang et al., 2001; Werry et al., 2003b), we cannot exclude the possibility that localized, transient increases in Ins(1,4,5)P3 occur that are below the sensitivity of our technique. However, a lack of enhanced PLC activity is consistent with other examples of cross-talk involving Gαq/11- and Gαs-coupled receptors (Jiménez et al., 1999; Tanimura et al., 1999), and alternative mechanisms must be considered.
After activation of Gαs and subsequent cAMP generation by adenylyl cyclase, transduction occurs through PKA or an exchange protein directly activated by cAMP (EPAC), making these likely mediators of cross-talk. Although PKA-dependent sensitization of Ins(1,4,5)P3 receptors may potentiate Gαq/11-coupled receptor-mediated Ca2+ responses by isoproterenol, in rat parotid cells (Tanimura et al., 1999), the cross-talk described here is independent of PKA activation. EPAC has been linked to Ca2+ signaling through either sensitization of intracellular Ca2+ channels (Kang et al., 2001, 2003, 2005; den Dekker et al., 2002) or PLCε activation (Schmidt et al., 2001). Although activation of PLCε is unlikely to be responsible (see above), sensitization of intracellular Ca2+ release channels or inhibition of the sarco(endo)-plasmic reticulum Ca2+-ATPase (den Dekker et al., 2002) via EPAC and Rap-dependent mechanisms could underlie cross-talk.
Of relevance to the present study are observations in HEK 293 cells demonstrating that either ATP or the muscarinic receptor full agonist carbachol enhances Ca2+ signaling by recombinant Gαs-coupled type 1 parathyroid hormone receptors independently of enhanced PLC activity or PKA (Short and Taylor, 2000; Tovey et al., 2003). Although these previous studies suggested that cAMP was not responsible, recent work by this group revealed that low-affinity binding of cAMP to the Ins(1,4,5)P3 receptor (or associated protein) increases sensitivity to Ins(1,4,5)P3 sufficiently to account for cross-talk (Tovey et al., 2008). Indeed, a specific association between adenylyl cyclase 6 and the type 2 Ins(1,4,5)P3 receptor forms “cAMP junctions” that ensure sufficiently high local concentrations of cAMP to influence Ins(1,4,5)P3 receptor sensitivity. Although alternative mechanisms for cross-talk exist (Werry et al., 2003a), this is consistent with the current study, which shows lack of direct dependence on Gαs (forskolin activation of adenylyl cyclase is effective), independence from PKA, and requirement for coactivation of Gαq/11 and Gαs.
Muscarinic M3 receptors and β2-adrenoceptors are often coexpressed, for example, in smooth muscle in which muscarinic receptors evoke Ca2+-dependent contraction and adrenoceptors cause cAMP- and PKA-dependent relaxation. In airways, these receptors are critical regulators of airway diameter and drug targets in the management of respiratory diseases such as asthma. Thus, selective β2-adrenoceptor agonists are remarkably effective for acute relief of airway narrowing, and anticholinergics can also be useful. Indeed, acetylcholine release may be exaggerated in asthma, potentially contributing to the clinical efficacy of anticholinergic therapy (Hai, 2007). However, asthma is a chronic airway disease characterized by hyperactivity, inflammation, altered contractile properties, and remodelling through hypertrophy and hyperplasia. Airway smooth muscle cells play prominent roles in these pathological features, although mechanisms remain to be precisely defined (Baroffio et al., 2008). Therefore, it is noteworthy that muscarinic receptors also promote inflammatory gene expression and mitogenesis in these cells (Hai, 2007) and that this could contribute to disease development and progression, particularly with exaggerated acetylcholine release. Ca2+ is central to muscarinic receptor signaling, and prominent roles for the Ca2+-regulated transcription factors, nuclear factor of activated T cells and cAMP-response element-binding protein, have been demonstrated in smooth muscle (Barlow et al., 2006; Pulver-Kaste et al., 2006). Thus, although any Ca2+ signaling by β2-adrenoceptors during muscarinic receptor activation could offset adrenoceptor-mediated relaxation, it is important to recognize that Ca2+ plays other roles. In this respect, the pattern of Ca2+ signaling is likely to be critical as at least in other cell types, Ca2+ oscillations encode information about gene expression (Dolmetsch et al., 1998; Li et al., 1998). Thus, remodelling of Ca2+ responses by β2-adrenoceptors could contribute to altered gene expression. Such an influence of cross-talk has been established in UMR-106 rat osteosarcoma cells, in which interaction between Gαs-coupled parathyroid hormone receptors and Gαq/11-coupled P2Y1 receptors enhances Ca2+ signaling, cAMP-response element-binding protein phosphorylation, and induction of the c-fos gene in a cAMP- and PKA-independent manner (Buckley et al., 2001).
It is interesting that although selective β2-adrenoceptor agonists provide acute relief of airway narrowing, long-term use may be associated with a deterioration of control and an increased morbidity and mortality (Bond et al., 2007). The mechanisms underlying this are unclear. One possibility is that this is a direct consequence of enhanced β2-adrenoceptor signaling because overexpression of β2-adrenoceptors in mice enhances tracheal sensitivity to acetylcholine through up-regulation of phospholipase Cβ1 (McGraw et al., 2003). Whether cross-talk as described in the present study occurs in airway smooth muscle in health and/or disease and whether this contributes to regulation of airway structure and function remain to be established.
This work was supported in part by AstraZeneca (UK); and the Research Councils UK [Grant BBS/S/N/2006/13051] (Biotechnology and Biological Sciences Research Council).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.153619.
ABBREVIATIONS: GPCR, G protein-coupled receptor; PLC, phospholipase C; Ins(1,4,5)P3, inositol 1,4,5-trisphosphate; FLIPR, fluorescent imaging plate reader; AM, acetoxymethyl ester; H89, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride; PKA, protein kinase A; PKC, protein kinase C; ERK, extracellular signal-regulated kinase; HEK, human embryonic kidney; CTX, cholera toxin; HBSS, Hanks' balanced salt solution; PAGE, polyacrylamide gel electrophoresis; InsPx, inositol phosphates; ANOVA, analysis of variance; ICI-118,551, (±)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-2-butanol hydrochloride; 2-APB, 2-aminoethoxydiphenyl borane; eGFP, enhanced green fluorescent protein; PH, pleckstrin homology; EPAC, exchange proteins directly activated by cAMP.
References
- Ancellin N, Preisser L, Le Maout S, Barbado M, Créminon C, Corman B, and Morel A (1999) Homologous and heterologous phosphorylation of the vasopressin V1a receptor. Cell Signal 11 743-751. [DOI] [PubMed] [Google Scholar]
- Baker JG (2005) The selectivity of β-adrenoceptor antagonists at the human β1, β2 and β3 adrenoceptors. Br J Pharmacol 144 317-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow CA, Rose P, Pulver-Kaste RA, and Lounsbury KM (2006) Excitation-transcription coupling in smooth muscle. J Physiol 570 59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroffio M, Crimi E, and Brusasco V (2008) Airway smooth muscle as a model for new investigative drugs in asthma. Ther Adv Respir Dis 2 129-139. [DOI] [PubMed] [Google Scholar]
- Bond RA, Spina D, Parra S, and Page CP (2007) Getting to the heart of asthma: can “β blockers” be useful to treat asthma? Pharmacol Ther 115 360-374. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, and Peppiatt CM (2002) 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J 16 1145-1150. [DOI] [PubMed] [Google Scholar]
- Buckley KA, Wagstaff SC, McKay G, Gaw A, Hipskind RA, Bilbe G, Gallagher JA, and Bowler WB (2001) Parathyroid hormone potentiates nucleotide-induced [Ca2+]i release in rat osteoblasts independently of Gq activation or cyclic monophosphate accumulation. A mechanism for localizing systemic responses in bone. J Biol Chem 276 9565-9571. [DOI] [PubMed] [Google Scholar]
- Chan JS, Lee JW, Ho MK, and Wong YH (2000) Preactivation permits subsequent stimulation of phospholipase C by Gi-coupled receptors. Mol Pharmacol 57 700-708. [DOI] [PubMed] [Google Scholar]
- den Dekker E, Heemskerk JW, Gorter G, van der Vuurst H, Donath J, Kroner C, Mikoshiba K, and Akkerman JW (2002) Cyclic AMP raises intracellular Ca2+ in human megakaryocytes independent of protein kinase A. Arterioscler Thromb Vasc Biol 22 179-186. [DOI] [PubMed] [Google Scholar]
- Dickenson JM and Hill SJ (1998) Human 5-HT1B receptor stimulated inositol phospholipid hydrolysis in CHO cells: synergy with Gq-coupled receptors. Eur J Pharmacol 348 279-285. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Xu K, and Lewis RS (1998) Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392 933-936. [DOI] [PubMed] [Google Scholar]
- Dörje F, Wess J, Lambrecht G, Tacke R, Mutschler E, and Brann MR (1991) Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J Pharmacol Exp Ther 256 727-733. [PubMed] [Google Scholar]
- Hai CM (2007) Airway smooth muscle cell as therapeutic target of inflammation. Curr Med Chem 14 67-76. [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Leitz MR, Oberdorf-Maass S, Lohse MJ, and Klotz KN (2004) Comparative pharmacology of human β-adrenergic receptor subtypes-Characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol 369 151-159. [DOI] [PubMed] [Google Scholar]
- Hopkinson HE, Latif ML, and Hill SJ (2000) Non-competitive antagonism of β2-agonist-mediated cyclic AMP accumulation by ICI 118551 in BC3H1 cells endogenously expressing constitutively active β2-adrenoceptors. Br J Pharmacol 131 124-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez AI, Castro E, Mirabet M, Franco R, Delicado EG, and Miras-Portugal MT (1999) Potentiation of ATP calcium responses by A2B receptor stimulation and other signals coupled to Gs proteins in type-1 cerebellar astrocytes. Glia 26 119-128. [PubMed] [Google Scholar]
- Kang G, Chepurny OG, and Holz GG (2001) cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic beta-cells. J Physiol 536 375-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, and Holz GG (2003) Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic β-cells. J Biol Chem 278 8279-8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Chepurny OG, Rindler MJ, Collis L, Chepurny Z, Li WH, Harbeck M, Roe MW, and Holz GG (2005) A cAMP and Ca2+ coincidence detector in support of Ca2+-induced Ca2+ release in mouse pancreatic β-cells. J Physiol 566 173-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Llopis J, Whitney M, Zlokarnik G, and Tsien RY (1998) Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature 392 936-941. [DOI] [PubMed] [Google Scholar]
- Liu X and Ambudkar IS (2001) Characteristics of a store-operated calcium-permeable channel: sarcoendoplasmic reticulum calcium pump function controls channel gating. J Biol Chem 276 29891-29898. [DOI] [PubMed] [Google Scholar]
- McGraw DW, Almoosa KF, Paul RJ, Kobilka BK, and Liggett SB (2003) Antithetic regulation of beta-adrenergic receptors of Gq receptor signalling via phospholipase C underlies the airway beta-agonist paradox. J Clin Invest 112 619-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundell SJ and Benovic JL (2000) Selective regulation of endogenous G protein-coupled receptors by arrestins in HEK293 cells. J Biol Chem 275 12900-12908. [DOI] [PubMed] [Google Scholar]
- Nash MS, Young KW, Challiss RA, and Nahorski SR (2001) Receptor-specific messenger oscillations. Nature 413 381-382. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Hori M, Kim YS, Kwon SC, Ahn DS, Nakazawa H, Kobayashi M, and Karaki H (2002) Inhibitory mechanism of xestospongin-C on contraction and ion channels in the intestinal smooth muscle. Br J Pharmacol 137 1207-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver-Kaste RA, Barlow CA, Bond J, Watson A, Penar PL, Tranmer B, and Lounsbury KM (2006) Ca2+ source-dependent transcription of CRE-containing genes in vascular smooth muscle. Am J Physiol Heart Circ Physiol 291 H97-H105. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW, and Jakobs KH (2001) A new phospholipase-C–calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nature Cell Biol 3 1020-1024. [DOI] [PubMed] [Google Scholar]
- Seidel MG, Klinger M, Freissmuth M, and Höller C (1999) Activation of mitogen-activated protein kinase by the A2A-adenosine receptor via a rap1-dependent and via a p21ras-dependent pathway. J Biol Chem 274 25833-25841. [DOI] [PubMed] [Google Scholar]
- Selbie LA, Darby K, Schmitz-Peiffer C, Browne CL, Herzog H, Shine J, and Biden TJ (1995) Synergistic interaction of Y1-neuropeptide Y and α1b-adrenergic receptors in the regulation of phospholipase C, protein kinase C, and arachidonic acid production. J Biol Chem 270 11789-11796. [DOI] [PubMed] [Google Scholar]
- Short AD and Taylor CW (2000) Parathyroid hormone controls the size of the intracellular Ca2+ stores available to receptors linked to inositol trisphosphate formation. J Biol Chem 275 1807-1813. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Jurevicius J, and Fischmeister R (1997) Pharmacological characterization of the receptors involved in the β-adrenoceptor-mediated stimulation of the L-type Ca2+ current in frog ventricular myocytes. Br J Pharmacol 121 1277-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A, Nezu A, Tojyo Y, and Matsumoto Y (1999) Isoproterenol potentiates α-adrenergic and muscarinic receptor-mediated Ca2+ response in rat parotid cells. Am J Physiol 276 C1282-C1287. [DOI] [PubMed] [Google Scholar]
- Tovey SC, Goraya TA, and Taylor CW (2003) Parathyroid hormone increases the sensitivity of inositol trisphosphate receptors by a mechanism that is independent of cyclic AMP. Br J Pharmacol 138 81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey SC and Willars GB (2004) Single-cell imaging of intracellular Ca2+ and phospholipase C activity reveals that RGS 2, 3, and 4 differentially regulate signaling via the Gαq/11-linked muscarinic M3 receptor. Mol Pharmacol 66 1453-1464. [DOI] [PubMed] [Google Scholar]
- Tovey SC, Dedos SG, Taylor EJ, Church JE, and Taylor CW (2008) Selective coupling of type 6 adenylyl cyclase with type 2 IP3 receptors mediates direct sensitization of IP3 receptors by cAMP. J Cell Biol 183 297-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werry TD, Christie MI, Dainty IA, Wilkinson GF, and Willars GB (2002) Ca2+ signalling by recombinant human CXCR2 chemokine receptors is potentiated by P2Y nucleotide receptors in HEK cells. Br J Pharmacol 135 1199-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werry TD, Wilkinson GF, and Willars GB (2003a) Mechanisms of cross-talk between G-protein-coupled receptors resulting in enhanced release of intracellular Ca2+. Biochem J 374 281-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werry TD, Wilkinson GF, and Willars GB (2003b) Cross talk between P2Y2 nucleotide receptors and CXC chemokine receptor 2 resulting in enhanced Ca2+ signaling involves enhancement of phospholipase C activity and is enabled by incremental Ca2+ release in human embryonic kidney cells. J Pharmacol Exp Ther 307 661-669. [DOI] [PubMed] [Google Scholar]
- Yang CM, Chien CS, Wang CC, Hsu YM, Chiu CT, Lin CC, Luo SF, and Hsiao LD (2001) Interleukin-1β enhances bradykinin-induced phosphoinositide hydrolysis and Ca2+ mobilization in canine tracheal smooth-muscle cells: involvement of the Ras/Raf/mitogen-activated protein kinase (MAPK) kinase (MEK)/MAPK pathway. Biochem J 354 439-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo A, Samways DS, Fowler CE, Gunn-Moore F, and Henderson G (2001) Coincident signalling between the Gi/Go-coupled δ-opioid receptor and the Gq-coupled m3 muscarinic receptor at the level of intracellular free calcium in SH-SY5Y cells. J Neurochem 76 1688-1700. [DOI] [PubMed] [Google Scholar]