Abstract
Opioid antagonists can be classified as inverse agonists and neutral antagonists. In the opioid-dependent state, neutral antagonists are significantly less potent in precipitating withdrawal than inverse agonists. Consequently, neutral opioid antagonists may offer advantages over inverse agonists in the management of opioid overdose. In this study, the relative potency of three opioid antagonists to block opioid analgesia and toxicity and precipitate withdrawal was examined. First, the potency of two opioid inverse agonists (naltrexone and naloxone) and a neutral antagonist (6β-naltrexol) to antagonize fentanyl-induced analgesia and lethality was determined. The order of potency to block analgesia was naltrexone > naloxone > 6β-naltrexol (17, 4, 1), which was similar to that to block lethality (13, 2, 1). Next, the antagonists were compared using withdrawal jumping in fentanyl-dependent mice. The order of potency to precipitate withdrawal jumping was naltrexone > naloxone ⋙ 6β-naltrexol (1107, 415, 1). The relative potencies to precipitate withdrawal for the inverse agonists compared with the neutral antagonist were dramatically different from that for antagonism of analgesia and lethality. Finally, the effect of 6β-naltrexol pretreatment on naloxone-precipitated jumping was determined in morphine and fentanyl-dependent mice. 6β-Naltrexol pretreatment decreased naloxone precipitated withdrawal, indicating that 6β-naltrexol is a neutral antagonist. These data demonstrate that inverse agonists and neutral antagonists have generally comparable potencies to block opioid analgesia and lethality, whereas the neutral opioid antagonist is substantially less potent in precipitating opioid withdrawal. These results support suggestions that neutral antagonists may have advantages over inverse agonists in the management of opioid overdose.
Antagonists can display a spectrum of efficacy from zero to negative (Milligan and Bond, 1997; Kenakin, 2001). Antagonists that have negative efficacy can suppress basal signaling (constitutive) activity of receptors and are termed inverse agonists or negative antagonists (Milligan et al., 1997; Kenakin, 2001; Prather, 2004). Antagonists with zero efficacy generally only block agonist-induced effects without altering basal receptor signaling and are termed neutral antagonists, although in the absence of constitutive activity inverse agonists behave as neutral antagonists (Milligan et al., 1997; Kenakin, 2001; Prather, 2004).
Like many G protein-coupled receptors, opioid receptors can display basal signaling activity. Constitutive activity has been reported for μ, δ, and κ opioid receptors (Costa and Herz, 1989; Becker et al., 1999; Burford et al., 2000) as well as for some opioid receptor mutants (e.g., Huang et al., 2001). In addition, studies have demonstrated that chronic exposure to opioid agonists can increase constitutive signaling activity of μ, δ, and κ opioid receptors (Costa and Herz, 1989; Becker et al., 1999; Liu and Prather, 2001). This increase in constitutive activity has been suggested to be associated with the development of tolerance and dependence (Wang et al., 1994, 2001; Sadée et al., 2005; Walker and Sterious, 2005).
In behavioral studies in opioid-dependent mice, inverse opioid agonists (e.g., naltrexone and naloxone) precipitate withdrawal jumping, whereas neutral antagonists (e.g., 6β-naltrexol and d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2) are dramatically less potent (Wang et al., 2001; Raehal et al., 2005; Walker and Sterious, 2005; Sirohi et al., 2007). In biochemical studies, inverse opioid agonists increase cyclic AMP levels and inhibit guanosine 5′-O-(3-thio)triphosphate binding in cells previously exposed to opioid agonists (Liu and Prather, 2001; Wang et al., 2001; Raehal et al., 2005). In contrast, neutral opioid antagonists do not affect cAMP levels or guanosine 5′-O-(3-thio)triphosphate binding (e.g., Wang et al., 2001). The behavioral and biochemical effects of inverse agonists have been attributed to reductions in basal signaling activity induced by prior opioid agonist exposure, whereas neutral antagonists are less effective because they lack the ability to inhibit constitutive receptor signaling (Liu and Prather, 2001; Wang et al., 2001). Taken together, opioid agonist pretreatment seems to reveal the inverse agonist properties of some antagonists, whereas the effects of neutral opioid antagonists remain mostly independent of prior opioid treatment.
The utility of opioid antagonists to reverse the action of opioid agonists is well established. Opioid antagonists (e.g., naltrexone and naloxone) have a long clinical history in the management of opioid overdose (Ling and Wesson, 1990; Gutstein and Akil, 2001; Clarke et al., 2005). However, in opioid overdose situations, administration of an opioid antagonist can induce an acute withdrawal syndrome that could be life-threatening (e.g., van Dorp et al., 2007). Consequently, precipitated withdrawal by opioid antagonists may be a concern in the management of opioid overdose and treatment of opioid dependence (Ling and Wesson, 1990; van Dorp et al., 2007). In light of these issues, recent data on inverse agonists and neutral antagonists in opioid dependence may be of pragmatic importance. Specifically, neutral antagonists may be preferable to inverse agonists in the clinical management of opioid overdose and dependence.
To explore the utility of using a neutral opioid antagonist in the management of opioid overdose, the present study compared the activity of inverse agonists (e.g., naltrexone and naloxone) and a neutral antagonist (e.g., 6β-naltrexol) in blocking analgesia and lethality induced by fentanyl in the mouse. We also examined the relative potencies of these antagonists to precipitate withdrawal jumping in mice dependent on fentanyl. The data suggest that both the neutral antagonist and the inverse opioid agonists exhibit generally similar potency to block analgesia and lethality. However, the neutral opioid antagonist was dramatically less potent in precipitating withdrawal in fentanyl-dependent mice. These results suggest that neutral antagonists retain efficacy to block or reverse opioid toxicity with reduced potency to precipitate withdrawal and support suggestions (e.g., Wang et al., 2001) that neutral opioid antagonists may be preferred in the management of opioid overdose.
Materials and Methods
Subjects. Male Swiss-Webster mice (25–33 g) obtained from Taconic Farms (Germantown, NY) were used throughout this study. Animals were housed 10 per cage for at least 24 h after arrival, with food and water available ad libitum. All protocols were approved by the St. John's University Institutional Animal Care and Use Committee.
General Procedure. The time-to-peak effect (15 min), dose-response function, and analgesic (tail-flick; see below) ED50 value for fentanyl were determined previously (preliminary studies; Sirohi et al., 2008). The ED50 value to block fentanyl subcutaneously induced analgesia (100 μg/kg) was estimated for subcutaneous naltrexone, naloxone, and 6β-naltrexol. The peak effect for all antagonists was determined previously (Sirohi et al., 2007; (naltrexone and naloxone, 40 min; 6β-naltrexol, 70 min). Next, the dose-response function and LD50 value for fentanyl were determined. Mice were injected with naltrexone, naloxone, and 6β-naltrexol 5 min before a lethal subcutaneous dose of fentanyl (80 mg/kg), and the ED50 value to block lethality was determined. To examine dependence, mice were infused subcutaneously for 72 h with fentanyl (1 mg/kg/day) by using osmotic minipumps. The ED50 value for naltrexone, naloxone, and 6β-naltrexol to precipitate withdrawal jumping was then determined. Finally, to examine the effect of 6β-naltrexol pretreatment on naloxone-induced withdrawal jumping, mice were implanted with a minipump infusing fentanyl (1 mg/kg/day) or a morphine pellet (25 mg) for 72 h. At the end of fentanyl or morphine treatment, mice were injected with saline or 6β-naltrexol, and 70 min (peak effect of 6β-naltrexol) later they were injected with naloxone and withdrawal jumping was determined. In all studies, pump and pellet implantation were conducted while mice were lightly anesthetized with isoflurane/oxygen (4:96).
Analgesia Assay. Analgesia (antinociception) was determined using the tail-flick assay (model TF6; Emdie Instrument Co., Maidens, VA), in which a beam of light was focused on the dorsal surface of the tail of the mouse, approximately 2 cm from the tip of the tail. The intensity of the light was adjusted so that baseline tail-flick latency was typically 1 to 3 s. If a mouse did not remove its tail from the heat source by 10 s, the test was terminated, a latency of 10 s was recorded, and the mouse was defined as analgesic. Analgesia results are presented as both graded (mean tail-flick latency) and quantal data. All testing was conducted by an experimenter who was unaware of the treatment of an individual mouse.
Antagonism of Analgesia and Lethality. Mice (5–20/dose) were injected subcutaneously with naltrexone (0.01–0.4 mg/kg), naloxone (0.1–1.0 mg/kg), or 6β-naltrexol (0.2–2.0 mg/kg). Fentanyl (100 μg/kg) was injected subcutaneously 25 min after naltrexone and naloxone and 55 min after 6β-naltrexol. Mice were tested for analgesia 15 min after fentanyl administration at the time of peak analgesic effect for fentanyl and the time of peak antagonist effect. Controls were injected with saline and then with fentanyl (100 μg/kg) and tested for analgesia 15 min later.
For lethality studies, the dose-response function and the LD50 value for fentanyl were determined over a 4-h observation period. Other mice (5–15/dose) were injected subcutaneously with saline, naltrexone (0.1–10.0 mg/kg), naloxone (1.0–20.0 mg/kg), or 6β-naltrexol (5.0–80.0 mg/kg) 5 min before a lethal fentanyl dose (80 mg/kg s.c.). Mice were observed for lethality for 4 h after fentanyl administration, and time of death was recorded.
Dependence Studies. Mice were implanted subcutaneously with osmotic minipumps (Alzet model 2001; Durect Corporation, Cupertino, CA) delivering fentanyl (1 mg/kg/day) for 72 h. At the end of treatment, with the pumps in place, mice (5–10/dose) were injected subcutaneously with saline, naltrexone (0.01–1.0 mg/kg), naloxone (0.01–10.0 mg/kg), or 6β-naltrexol (2.0–200 mg/kg). Immediately after saline or antagonist injections, mice were placed in a clear container (5 l) and observed for withdrawal jumping for 15 min (see below).
Other mice were implanted subcutaneously with a morphine pellet (25 mg) or a minipump infusing fentanyl (1 mg/kg/day) for 72 h, and then with pumps and pellets in place, they were injected subcutaneously (5–10/dose) with saline or 6β-naltrexol (0.5–2.0 mg/kg) and 70 min (peak effect of 6β-naltrexol) later injected subcutaneously with naloxone (1.0 mg/kg). Immediately after naloxone injections, mice were observed for withdrawal jumping for 15 min.
Jumping was defined as all four paws leaving the bottom of the container. All jumping was observed by an experimenter who was unaware of the treatment of an individual mouse. For the purpose of quantal dose-response analysis, mice that jumped 50 or more times in the 15-min observation period were defined as positive for withdrawal jumping. The ED50 value for each antagonist to precipitate withdrawal jumping was estimated. In addition, the mean number of jumps was determined for each condition.
Drugs. Naltrexone HCl, naloxone HCl, 6β-naltrexol HCl, fentanyl citrate, and 25-mg morphine pellets were obtained from the Research Triangle Institute (Research Triangle Park, NC) through the Research Technology Branch of the National Institute on Drug Abuse (Bethesda, MD) or Spectrum Laboratory Products Inc. (Gardena, CA). Fentanyl and all antagonists were dissolved in 0.9% saline, and doses are expressed as the free base.
Data Analysis. Quantal dose-response data were analyzed using the BLISS-21 computer program (Department of Statistics, University of Edinburgh, Edinburgh, UK). This program uses Probit analysis (Finney, 1973) to calculate ED50 values, potency estimates, S.E. values, and 95% confidence intervals from quantal data. Graded dose-response data were analyzed using the four-parameter logistic equation to calculate the EC50, potency estimates, S.E. values, and 95% confidence limits (Prism, version 5.02; GraphPad Software Inc., San Diego, CA). Other data were analyzed by analysis of variance and t tests.
Results
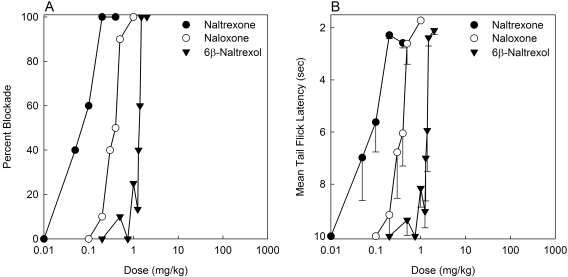
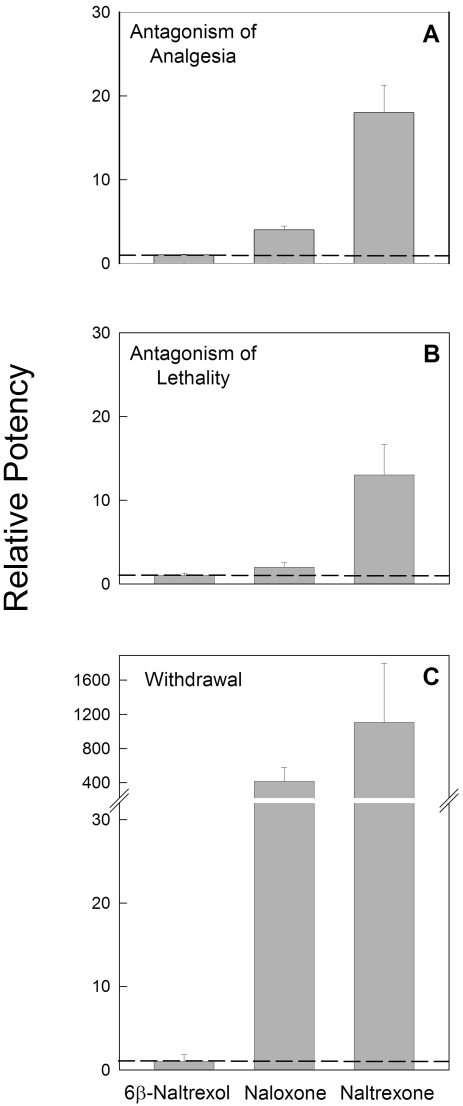
Fentanyl (100 μg/kg) produced analgesia in 100% of saline-pretreated mice. This fentanyl dose is approximately 5 times the analgesic ED50 of fentanyl (Sirohi et al., 2008). The estimated quantal ED50 values (95% CL) for naltrexone, naloxone, and 6β-naltrexol to block fentanyl-induced analgesia (100 μg/kg) were 0.08 (0.05–0.10), 0.35 (0.28–0.44), and 1.38 mg/kg (1.18–1.69), respectively (Fig. 1A). The graded (mean tail-flick latency) EC50 values (95% CL) for naltrexone, naloxone, and 6β-naltrexol were 0.08 (0.02–0.27), 0.37 (0.26–0.51), and 1.37 mg/kg (1.28–1.47), respectively (Fig. 1B). The order of potency for quantal data relative to 6β-naltrexol was naltrexone (17) > naloxone (4) > 6β-naltrexol (1) (Fig. 4A). Graded relative potency data were similar: naltrexone (17) > naloxone (4) > 6β-naltrexol (1).
Fig. 1.
Dose-response functions for antagonism of fentanyl-induced analgesia by naltrexone, naloxone, and 6β-naltrexol. Mice (5–20/dose/drug) were injected subcutaneously with naltrexone (0.01–0.4 mg/kg), naloxone (0.1–1.0 mg/kg), or 6β-naltrexol (0.2–2.0 mg/kg). Fentanyl (100 μg/kg) was injected subcutaneously 25 min after naltrexone and naloxone and 55 min after 6β-naltrexol. Mice were tested for analgesia 15 min after fentanyl administration at the time of peak effect for fentanyl and each antagonist. Each mouse was tested only once. A tail-flick latency of less than 10 s was recorded as blockade of fentanyl analgesia. Percentage of mice with blockade of fentanyl analgesia (A) and mean (-S.E.M.) tail-flick latency (B) are plotted versus dose for each antagonist. For ease of comparison with the quantal data, mean tail-flick latency data are plotted on an inverted ordinate.
Fig. 4.
Relative potencies of naltrexone, naloxone, and 6β-naltrexol for antagonism of analgesia, lethality, and to precipitate withdrawal jumping. The data in this figure are based on quantal dose-response results presented in Figs. 1, 2, 3, and potencies were calculated using Probit analyses. A, mice were treated as described in Fig. 1 and tested for antagonism of fentanyl-induced analgesia. Quantal relative potencies (±S.E.) of naltrexone, naloxone, and 6β-naltrexol to block fentanyl (100 μg/kg)-induced analgesia are plotted. B, mice were treated as described in Fig. 2 and tested for antagonism of fentanyl-induced lethality. Relative potencies (±S.E.) of naltrexone, naloxone, and 6β-naltrexol to block fentanyl (80 mg/kg) induced lethality are plotted. C, mice were treated as described in Fig. 3 and tested for precipitated withdrawal after fentanyl treatment. Quantal relative potencies (±S.E.) of naltrexone, naloxone, and 6β-naltrexol to induce withdrawal jumping in mice dependent on fentanyl (1 mg/kg/day) are plotted.
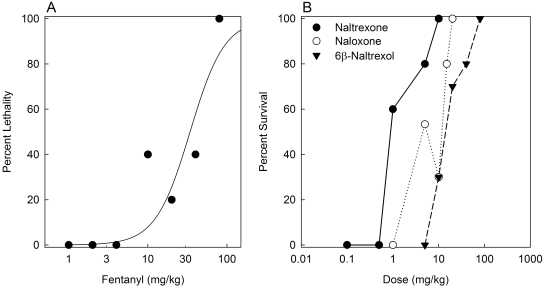
The dose-response function for fentanyl-induced lethality is presented in Fig. 2A. The LD50 (95% CL) value was 28.5 mg/kg (14.5–74.1). In all subsequent lethality antagonism studies, 80 mg/kg fentanyl was used, and the ED50 for each antagonist to block fentanyl-induced lethality was determined. Mice were observed for 4 h to determine the time course of toxicity. In each experiment, 100% lethality was observed in control groups in which saline was injected 5 min before fentanyl. The estimated ED50 values (95% CL) for naltrexone, naloxone, and 6β-naltrexol to block fentanyl-induced lethality were 1.18 (0.69–1.95), 7.19 (4.60–11.22), and 15.34 mg/kg (9.31–24.98), respectively (Fig. 2B). The order of potency relative to 6β-naltrexol was naltrexone (13) > naloxone (2) > 6β-naltrexol (1) (Fig. 4B). The time (mean ± S.E.M.) to death in controls (46.7 ± 3.6 min) was not significantly different (F3, 97 = 1.00; p > 0.05) from that for 6β-naltrexol- (45.0 ± 2.2 min), naltrexone- (62.2 ± 13.3 min), or naloxone (52.8 ± 8.1 min)-pretreated mice. These data suggest that the potency differences among the three antagonists to antagonize opioid overdose were not due to delayed effects of fentanyl.
Fig. 2.
Dose-response functions for fentanyl-induced lethality and blockade of fentanyl-induced lethality by naltrexone, naloxone, and 6β-naltrexol. A, mice (five/dose) were injected with fentanyl (1–80 mg/kg) and observed for 4 h. The percentage of lethality is plotted versus fentanyl dose. The LD50 (95% CL) was 28.5 mg/kg (14.5–74.1). B, mice (5–15/dose/drug) were injected subcutaneously with saline, naltrexone (0.1–10.0 mg/kg), naloxone (1.0–20.0 mg/kg), or 6β-naltrexol (5.0–80.0 mg/kg) 5 min before a lethal dose of fentanyl (80 mg/kg s.c.), and mice were observed for 4 h. The percentage of mice that survived is plotted versus antagonist dose.
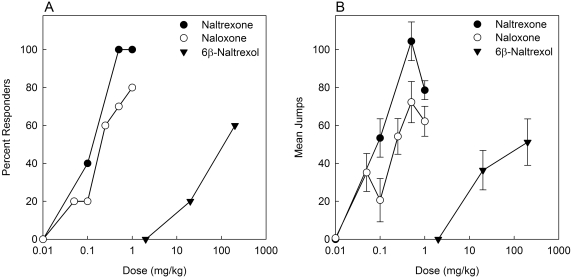
To determine the ED50 of each antagonist to precipitate withdrawal jumping, mice were infused with fentanyl (1 mg/kg/day) for 72 h and then injected with saline or an antagonist and observed (see Materials and Methods). Naltrexone, naloxone, and 6β-naltrexol all produced dose-dependent withdrawal jumping in mice dependent on fentanyl (Fig. 3). Naloxone at doses higher than 1.0 mg/kg produced overt signs of ataxia and shaking that were distinctly different behaviors from the doses that elicit jumping; therefore, doses higher than 1.0 mg/kg for naloxone were excluded from the analysis. Similar results were obtained when mice implanted with a 25-mg morphine pellet for 72 h were injected with higher doses of naloxone (data not shown), indicating that this was not specifically related to fentanyl dependence. In controls, no withdrawal jumping was observed when saline was injected in mice dependent on fentanyl (data not shown). The quantal ED50 values (95% CL) for naltrexone, naloxone, and 6β-naltrexol to precipitate withdrawal jumping were 0.09 (0.03–0.26), 0.24 (0.12–0.47), and 99.65 mg/kg (28.74–383.40), respectively (Fig. 3A). The graded (mean number of jumps) EC50 values (95% CL) for naltrexone, naloxone, and 6β-naltrexol to precipitate withdrawal jumping were 0.08 (0.02–0.40), 0.25 (0.12–0.51), and 119.1 mg/kg (6.83–2078), respectively (Fig. 3B). The order of potency for quantal data relative to 6β-naltrexol was naltrexone (1107) > naloxone (415) > 6β-naltrexol (1) (Fig. 4C). Graded relative potency data were similar: naltrexone (1489) > naloxone (476) > 6β-naltrexol (1).
Fig. 3.
Dose-response functions of naltrexone, naloxone, and 6β-naltrexol to precipitate withdrawal jumping. Mice were implanted subcutaneously with osmotic minipumps delivering fentanyl (1 mg/kg/day) for 72 h. At the end of treatment, mice (5–10/dose/drug) were injected subcutaneously with naltrexone (0.01–1.0 mg/kg), naloxone (0.01–1.0 mg/kg), or 6β-naltrexol (2.0–200 mg/kg) and observed for withdrawal jumping for 15 min (see Materials and Methods). Percentage of responders (50 or more jumps) (A) and mean number of jumps (±S.E.M.) (B) are plotted versus antagonist dose.
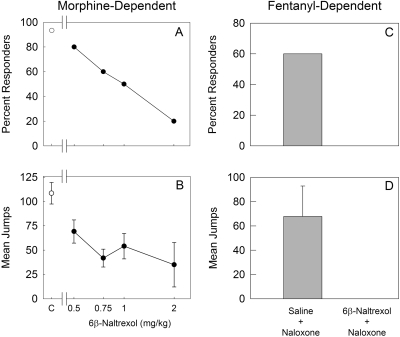
Because 6β-naltrexol is believed to function as a neutral antagonist, we examined the effect of 6β-naltrexol pretreatment on naloxone-precipitated withdrawal jumping. Mice were implanted with a 25-mg morphine pellet for 72 h and injected first with saline or 6β-naltrexol (0.5–2.0 mg/kg) and 70 min later (peak effect of 6β-naltrexol) injected with naloxone 1.0 mg/kg (see Materials and Methods). In the saline pretreatment group, withdrawal jumping was observed in 93% of mice. In contrast, 6β-naltrexol pretreatment before naloxone administration dose-dependently decreased naloxone-precipitated withdrawal jumping (Fig. 5, A and B). Other mice were treated with fentanyl (1 mg/kg/day) for 72 h and then injected with saline or 6β-naltrexol (2.0 mg/kg), followed by naloxone (1.0 mg/kg). 6β-Naltrexol completely blocked naloxone-precipitated withdrawal in fentanyl-dependent mice (Fig. 5, C and D).
Fig. 5.
Effect of 6β-naltrexol on naloxone-precipitated withdrawal jumping in morphine and fentanyl-dependent mice. A and B, mice (5–10/dose) were implanted subcutaneously with a morphine pellet (25 mg) for 72 h. At the end of treatment, mice were injected subcutaneously with saline (control; C) or 6β-naltrexol (0.5–2.0 mg/kg) and 70 min later (peak antagonist effect of 6β-naltrexol) injected subcutaneously with naloxone (1.0 mg/kg). The doses of 6β-naltrexol used in this study do not produce a withdrawal response (see Fig. 3 and Sirohi et al., 2007). Control (C) mice were injected with saline followed by naloxone. Mice were observed for withdrawal jumping for 15 min following naloxone treatment. Percentage of responders for withdrawal jumping (50 or more jumps) (A) and mean number of jumps (±S.E.M.) (B) are plotted versus antagonist dose. C and D, mice (five/group) were implanted with subcutaneously with osmotic minipumps that infused fentanyl (1.0 mg/kg/day) for 72 h. At the end of treatment, mice were injected with saline or 6β-naltrexol (2.0 mg/kg) and 70 min later injected with naloxone (1.0 mg/kg). Mice were observed for withdrawal jumping for 15 min following naloxone treatment. Percentage of responders for withdrawal jumping (50 or more jumps) (C) and mean number of jumps (+S.E.M.) (D) are presented for each group.
Discussion
The activity of opioid antagonists has been shown to range from neutral efficacy to negative efficacy. Studies strongly suggest that the commonly used opioid antagonists naloxone and naltrexone display negative efficacy and are therefore classified as inverse agonists (Costa and Herz, 1989; Wang et al., 2001; Marczak et al., 2007). Although all opioid antagonists are capable of reversing or blocking the effects of opioid agonists, only inverse agonists inhibit signaling of constitutively active opioid receptors (Wang et al., 2004; Sadée et al., 2005; Sirohi et al., 2007). However, in the absence of constitutive receptor activity, inverse agonists are generally indistinguishable from neutral antagonists.
Previous studies have demonstrated that opioid agonist pretreatment can increase the constitutive activity of opioid receptors, and it has been suggested that this may play an important role in opioid tolerance and dependence (Wang et al., 2001; Sadée et al., 2005). In the opioid-dependent state, inverse opioid agonists are substantially more potent in precipitating withdrawal and activating putative biochemical pathways that mediate dependence compared with neutral opioid antagonists (Wang et al., 2001; Sadée et al., 2005; Sirohi et al., 2007). Based on these findings, it seems plausible that neutral opioid antagonists may offer advantages over inverse agonists in the clinical management of opioid overdose and dependence (Wang et al., 2004; Raehal et al., 2005). To date, there has been no comparison between inverse agonists and neutral antagonists in terms of reversal of opioid toxicity. In in vivo studies, the most widely studied neutral opioid antagonist is 6β-naltrexol. Therefore, the present study compared the potency of two opioid inverse agonists (i.e., naltrexone and naloxone) and a neutral antagonist (i.e., 6β-naltrexol) to block fentanyl-induced analgesia and lethality. The relative potency of these antagonists to precipitate withdrawal jumping in mice dependent on fentanyl was also determined.
Initially, the potencies of each antagonist to block fentanyl-induced analgesia (Figs. 1 and 4A) and lethality (Figs. 2B and 4B) were determined. The order of potency to block fentanyl-induced analgesia, relative to 6β-naltrexol, was naltrexone > naloxone > 6β-naltrexol. The rank order of potencies to block analgesia was similar to that to block lethality. Next, the potency to precipitate withdrawal jumping in fentanyl-dependent mice was estimated for each antagonist. Naltrexone and naloxone were >1000 and >400 times, respectively, more potent than 6β-naltrexol in precipitating withdrawal (Figs. 3 and 4C). Overall, there was good agreement for the relative potencies of the antagonists for quantal and graded analyses of the data. These results are consistent with previous studies (Wang et al., 2001; Sirohi et al., 2007) in which 6β-naltrexol was found to be substantially less potent in precipitating withdrawal jumping than antagonizing morphine analgesia. In the present study, we extend this observation to fentanyl and to the reversal of opioid toxicity. The neutral opioid antagonist was dramatically less potent in precipitating withdrawal jumping in fentanyl-dependent mice compared with antagonism of fentanyl-induced analgesia and toxicity. Taken together, these results suggest that a neutral opioid antagonist such as 6β-naltrexol might be preferable to the commonly used inverse agonists (i.e., naloxone and naltrexone) in the clinical management of opioid overdose.
The current data suggest that 6β-naltrexol might be useful clinically in treating opioid overdose, while limiting the risk of precipitating withdrawal. If neutral antagonists have a role in reversing opioid overdose clinically, it is important that activity be demonstrated in primates. In light of this suggestion, it is notable that Ko et al. (2006) and Li et al. (2008) propose that 6β-naltrexol does not act as a neutral antagonist in the rhesus monkey. Li et al. (2008) found that naltrexone, 6β-naltrexol, and 6α-naltrexol did not differ in pA2 values in dependent and nondependent monkeys. Ko et al. (2006) report that pretreatment with 6β-naltrexol did not shift the dose-response function for naltrexone-induced increases in respiration, which is a model of morphine dependence. Although these results suggest caution in concluding that 6β-naltrexol, and neutral opioid antagonists in general, might be useful in humans, there are substantial procedural differences between our studies and the primate studies. An important difference is that morphine dependence was produced using intermittent injections (Ko et al., 2006; Li et al., 2008), and monkeys were tested for withdrawal 18 h (Ko et al., 2006) after the final injection, unlike the present results in which mice were tested in the presence of morphine or fentanyl. It is worth noting that the protocol used in the present study is analogous to what might occur in clinical opioid overdose situations. In addition, Ko et al. (2006) used a cumulative-dose approach that might reduce the effect of 6β-naltrexol. Specifically, it might be anticipated that the effects of 6β-naltrexol would be waning as the experiment using cumulative dosing continues. These studies may be best conducted using a single pretreatment 6β-naltrexol dose, followed by a single naltrexone dose. Nevertheless, it is important that the potential translational value of 6β-naltrexol and other neutral antagonists be thoroughly studied. Finally, Divin et al. (2008) report that 6β-naltrexol did not block withdrawal precipitated by the putative inverse agonist naltrexone in the mouse. The reason for this outcome is not clear, but there are several studies, including the present report, that have demonstrated that inverse agonist effects, including precipitated withdrawal, are inhibited by neutral antagonists such as 6β-naltrexol (Raehal et al., 2005; Walker and Sterious, 2005; Marczak et al., 2007; Wang et al., 2007). Furthermore, in the present study 6β-naltrexol was effective in inhibiting withdrawal in both morphine- and fentanyl-dependent mice, which suggests that this effect is likely to extend to dependence on other opioid agonists.
In a recent study, 6β-naltrexol was reported to be equipotent to naltrexone and naloxone to up-regulate μ-opioid receptor density and to produce functional supersensitivity (Sirohi et al., 2007). Thus, these data indicate that naloxone, naltrexone, and 6β-naltrexol share similar efficacy to block opioid analgesia and lethality, and to induce opioid receptor up-regulation. These results strongly indicate that these effects depend only upon occupancy of the receptor and do not require changes in receptor signaling. In contrast, that 6β-naltrexol is substantially less potent than naloxone and naltrexone in precipitating withdrawal jumping suggests that this outcome is dependent to some degree on changes (reductions) in opioid receptor activity by inverse agonists. These data support the idea of functional (protean) selectivity of ligands and extend this idea to antagonist effects (e.g., Urban et al., 2007). Thus, a given antagonist acting at a receptor can produce a profile of effects that differs from that of another antagonist acting at the same receptor in terms of the relative potencies to produce these effects. That 6β-naltrexol functions as a lower efficacy antagonist is supported by blockade of inverse agonist-mediated effects. 6β-Naltrexol pretreatment dose-dependently blocked naloxone-precipitated withdrawal jumping in mice dependent on morphine and fentanyl (Fig. 5). These data, taken together with results reported previously (Wang et al., 2001; Raehal et al., 2005; Sirohi et al., 2007), substantiate suggestions that 6β-naltrexol is a neutral antagonist.
In summary, inverse agonists and the neutral antagonist have relatively similar potencies to block opioid analgesia and lethality. Conversely, inverse agonists are substantially more potent in precipitating opioid withdrawal compared with the neutral antagonist. These results suggest that neutral antagonists may be preferred over inverse agonists in the management of opioid overdose. Furthermore, the reduced potency of neutral antagonists to precipitate withdrawal in the opioid-dependent state may allow the initiation of antagonist treatment for addiction without the opioid-free interval that is typically required in the case of inverse opioid agonists. Overall, the present results support suggestions (e.g., Wang et al., 2001) that neutral opioid antagonists may have advantages in the clinical management of opioid overdose, addiction, and dependence.
Supplementary Material
Acknowledgments
We are very grateful to H. H. Sant Rajinder and Singh Ji Maharaj. Dr. M. T. Turnock provided continuous encouragement during this study. We thank Subit Barua, Sunil Kumar, Harsh Mehta, Abhejeet Patel, Nishit Patel, Virender Beriwal, Sumit Khanna, Kalyan Kathala, Maitreyi Muralidhar, and Shilpa Raut for technical assistance.
This work was supported in part by the National Institutes of Health National Institute on Drug Abuse [Grant DA19959] (to B.C.Y.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.152678.
ABBREVIATIONS: CL, confidence limits.
References
- Becker JA, Wallace A, Garzon A, Ingallinella P, Bianchi E, Cortese R, Simonin F, Kieffer BL, and Pessi A (1999) Ligands for kappa-opioid and ORL1 receptors identified from a conformationally constrained peptide combinatorial library. J Biol Chem 274 27513-27522. [DOI] [PubMed] [Google Scholar]
- Burford NT, Wang D, and Sadée W (2000) G-protein coupling of mu-opioid receptors (OP3): elevated basal signaling activity. Biochem J 348 531-537. [PMC free article] [PubMed] [Google Scholar]
- Clarke SF, Dargan PI, and Jones AL (2005) Naloxone in opioid poisoning: walking the tightrope. Emerg Med J 22 612-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa T and Herz A (1989) Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci U S A 86 7321-7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divin MF, Holden Ko MC, and Traynor JR (2008) Comparison of the opioid receptor antagonist properties of naltrexone and 6 beta-naltrexol in morphine-naïve and morphine-dependent mice. Eur J Pharmacol 583 48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney DJ (1973) Probit Analysis, 3rd ed., Cambridge University Press, London, UK.
- Gutstein HB and Akil H (2001) Opioid Analgesics, in Goodman & Gilman's The Pharmacological Basis of Therapeutics, 10th ed., The McGraw-Hill Book Companies, Columbus, OH.
- Huang P, Li J, Chen C, Visiers I, Weinstein H, and Liu-Chen LY (2001) Functional role of a conserved motif in TM6 of the rat mu opioid receptor: constitutively active and inactive receptors result from substitutions of Thr 6.34(279) with Lys and Asp. Biochemistry 40 13501-13509. [DOI] [PubMed] [Google Scholar]
- Kenakin T (2001) Inverse, protean, and ligand-selective agonism: matters of receptor conformation. FASEB J 15 598-611. [DOI] [PubMed] [Google Scholar]
- Ko MC, Divin MF, Lee H, Woods JH, and Traynor JR (2006) Differential in vivo potencies of naltrexone and 6β-naltrexol in the monkey. J Pharmacol Exp Ther 316 772-779. [DOI] [PubMed] [Google Scholar]
- Li JX, McMahon LR, and France CP (2008) Comparison of naltrexone, 6alpha-naltrexol, and 6beta-naltrexol in morphine-dependent and in nondependent rhesus monkeys. Psychopharmacology (Berl) 195 479-486. [DOI] [PubMed] [Google Scholar]
- Ling W and Wesson DR (1990) Drugs of abuse–opiates. West J Med 152 565-572. [PMC free article] [PubMed] [Google Scholar]
- Liu JG and Prather PL (2001) Chronic exposure to mu-opioid agonists produces constitutive activation of mu-opioid receptors in direct proportion to the efficacy of the agonist used for pretreatment. Mol Pharmacol 60 53-62. [DOI] [PubMed] [Google Scholar]
- Marczak ED, Jinsmaa Y, Li T, Bryant SD, Tsuda Y, Okada Y, and Lazarus LH (2007) [N-allyl-Dmt1]-endomorphins are micro-opioid receptor antagonists lacking inverse agonist properties. J Pharmacol Exp Ther 323 374-380. [DOI] [PubMed] [Google Scholar]
- Milligan G and Bond RA (1997) Inverse agonism and the regulation of receptor number. Trends Pharmacol Sci 18 468-474. [DOI] [PubMed] [Google Scholar]
- Milligan G, MacEwan DJ, Mercouris M, and Mullaney I (1997) Inverse agonism at adrenergic and opioid receptors: studies with wild type and constitutively active mutant receptors. Receptors Channels 5 209-213. [PubMed] [Google Scholar]
- Prather PL (2004) Inverse agonists: tools to reveal ligand-specific conformations of G protein-coupled receptors. Sci STKE 2004 pe1. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Lowery JJ, Bhamidipati CM, Paolino RM, Blair JR, Wang D, Sadée W, and Bilsky EJ (2005) In vivo characterization of 6β-naltrexol, an opioid ligand with less inverse agonist activity compared with naltrexone and naloxone in opioid-dependent mice. J Pharmacol Exp Ther 313 1150-1162. [DOI] [PubMed] [Google Scholar]
- Sadée W, Wang D, and Bilsky EJ (2005) Basal opioid receptor activity, neutral antagonists, and therapeutic opportunities. Life Sci 76 1427-1437. [DOI] [PubMed] [Google Scholar]
- Sirohi S, Dighe SV, Walker EA, and Yoburn BC (2008) The analgesic efficacy of fentanyl: relationship to tolerance and mu-opioid receptor regulation. Pharmacol Biochem Behav 91 115-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi S, Kumar P, and Yoburn BC (2007) mu-Opioid receptor up-regulation and functional supersensitivity are independent of antagonist efficacy. J Pharmacol Exp Ther 323 701-707. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320 1-13. [DOI] [PubMed] [Google Scholar]
- van Dorp EL, Yassen A, and Dahan A (2007) Naloxone treatment in opioid addiction: the risks and benefits. Expert Opin Drug Saf 6 125-132. [DOI] [PubMed] [Google Scholar]
- Walker EA and Sterious SN (2005) Opioid antagonists differ according to negative intrinsic efficacy in a mouse model of acute dependence. Br J Pharmacol 145 975-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Raehal KM, Bilsky EJ, and Sadée W (2001) Inverse agonists and neutral antagonists at μ opioid receptor (MOR): possible role of basal receptor signaling in narcotic dependence. J Neurochem 77 1590-1600. [DOI] [PubMed] [Google Scholar]
- Wang D, Raehal KM, Lin ET, Lowery JJ, Kieffer BL, Bilsky EJ, and Sadée W (2004) Basal signaling activity of μ opioid receptor in mouse brain: role in narcotic dependence. J Pharmacol Exp Ther 308 512-520. [DOI] [PubMed] [Google Scholar]
- Wang Z, Bilsky EJ, Porreca F, and Sadée W (1994) Constitutive mu opioid receptor activation as a regulatory mechanism underlying narcotic tolerance and dependence. Life Sci 54: PL339-PL350. [DOI] [PubMed] [Google Scholar]
- Wang D, Sun X, and Sadee W (2007) Different effects of opioid antagonists on μ-, δ-, and κ-opioid receptors with and without agonist pretreatment. J Pharmacol Exp Ther 321 544-552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.