Abstract
Nicotine is the addictive component of tobacco, and successful smoking cessation therapies must address the various processes that contribute to nicotine addiction. Thus, understanding the nicotinic acetylcholine receptor (nAChR) subtypes and subsequent molecular cascades activated after nicotine exposure is of the utmost importance in understanding the progression of nicotine dependence. One possible candidate is the calcium/calmodulin-dependent protein kinase II (CaMKII) pathway. Substrates of this kinase include the vesicle-associated protein synapsin I and the transcription factor cAMP response element-binding protein (CREB). The goal of these studies was to examine these postreceptor mechanisms after acute nicotine treatment in vivo. We first show that administration of nicotine increases CaMKII activity in the ventral tegmental area (VTA), nucleus accumbens (NAc), and amygdala. In β2 nAChR knockout (KO) mice, nicotine does not induce an increase in kinase activity, phosphorylated (p)Synapsin I, or pCREB. In contrast, α7 nAChR KO mice show nicotine-induced increases in CaMKII activity and pCREB, similar to their wild-type littermates. Moreover, we show that when animals are pretreated with the CaMKII inhibitors 4-[(2S)-2-[(5-isoquinolinylsulfonyl) methylamino]-3-oxo-3-(4-phenyl-1-piperazinyl)propyl]phenyl isoquinolinesulfonic acid ester (KN-62) and N-[2-[[[3-(4-chlorophenyl)-2 propenyl]methylamino]methyl]phenyl]-N-(2-hydroxyethyl)-4-methoxybenzenesulphonamide (KN-93), nicotine-induced increase in the kinase activity and pCREB was attenuated in the VTA and NAc, whereas pretreatment with (2-[N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine, phosphate) (KN-92), the inactive analog, did not alter the nicotine-induced increase in pCREB. Taken together, these data suggest that the nicotine-induced increase in CaMKII activity may correlate with the nicotine-induced increase in pSynapsin I and pCREB in the VTA and NAc via β2 subunit-containing nAChRs.
Tobacco dependence is the leading cause of preventable mortality around the world (Mackay and Eriksen, 2002). The primary factor responsible for tobacco's addictive properties is believed to be nicotine; however, the receptor and postreceptor mechanisms that occur after nicotine administration are still under investigation. Insight into these molecular cascades would prove useful in future research aimed at smoking cessation programs.
Neuronal nicotinic acetylcholine receptors (nAChRs) are located throughout the central nervous system. Nicotine activates presynaptic nAChRs located in the ventral tegmental area (VTA) and nucleus accumbens (NAc) (Dani and Heinemann, 1996) and increases dopamine levels in the NAc (Nisell et al., 1994). Although there are a variety of nAChRs in the central nervous system, the predominant subtypes are α4β2* and α7*, where * denotes the possible inclusion of additional subunits (Dani, 2001). Mice lacking the β2 nAChR subunit do not find nicotine rewarding in either the self-administration or the conditioned place preference paradigms (Picciotto et al., 1998; Walters et al., 2006). In contrast, α7 nAChR knockout (KO) mice do exhibit place preference to nicotine (Walters et al., 2006). These data suggest that the β2 and α7 nAChR subunits play different roles in mediating the effects of nicotine behaviorally and that they may differentially regulate downstream signaling pathways.
One of the molecular targets implicated in drug reward is the transcription factor cAMP response element-binding protein (CREB). Changes in CREB phosphorylation have been seen after morphine (Lane-Ladd et al., 1997), cocaine (Kano et al., 1995), and amphetamine chronic administration (Shaw-Lutchman et al., 2003). Animals with a decrease in CREB function do not find morphine rewarding (Walters and Blendy, 2001) but find cocaine more rewarding (Walters and Blendy, 2001). Taken together, CREB seems to play a major role in the cellular changes that occur in the brain after acute and chronic administration of several drugs of abuse.
Recently, several studies have examined changes in CREB after nicotine administration. Acute nicotine administration leads to increases in phosphorylated (p)CREB in the VTA and striatum (Walters et al., 2005). Chronic nicotine administration leads to a decrease in CREB phosphorylation in the NAc with an increase in CREB phosphorylation in the prefrontal cortex (PFc) (Brunzell et al., 2003). Withdrawal studies have shown a decrease in CREB, CREB phosphorylation, and cAMP response element-DNA binding in the cortex and amygdala (Pandey et al., 2001). Similar to morphine, CREBαΔ KO mice do not find nicotine rewarding (Walters et al., 2005). These reports suggest an important role for CREB in nicotine dependence. Although several signaling pathways could mediate CREB activation after exposure to drugs of abuse, calcium-dependent signaling pathways induced by nAChRs seem to be a possible target.
Indeed, neuronal nAChRs have high calcium permeability, and numerous studies have shown that activation of these receptors causes an increase in intracellular calcium concentration (Mulle et al., 1992). In PC12 cells, an increase in intracellular calcium after stimulation of nicotinic receptors activates calcium/calmodulin-dependent protein kinase II (CaMKII) (MacNicol and Schulman, 1992). Furthermore, data from our laboratory show that this kinase is involved in nicotine-induced antinociception at the spinal level after acute and chronic drug exposure (Damaj, 2000, 2005). Recent studies have revealed that alterations in the phosphorylation state of neuronal CaMKII occur after chronic exposure to various drugs of abuse such as amphetamine, cocaine, and morphine (Tan, 2002; Wang et al., 2003; Licata et al., 2004). Furthermore, mRNA levels and phosphorylation of synapsin I, a vesicle-associated CaMKII substrate essential for neurotransmitter release, were also shown to be increased after chronic morphine (Matus-Leibovitch et al., 1995) and amphetamine treatment (Iwata et al., 1996, 1997a,b). Although these studies suggest a role for CaMKII and synapsin I in the actions of drugs of abuse, the molecular characterization of the effects of nicotine on these proteins in the brain is still largely unknown.
The present study was undertaken to characterize the effect of in vivo acute nicotine exposure on a CaMKII-dependent pathway in different brain regions and to identify the main nAChR subtypes involved in the regulation of this protein by nicotine. We first investigated the effects of systemic subcutaneous injection of acute nicotine on activity and level of CaMKII and synapsin I in several brain regions involved in nicotine reward and dependence. We then identified the role of major nicotinic subtypes in mediating these changes by using nicotine antagonists and genetically modified mice. In addition, we investigated the link between CaMKII and CREB in the mesolimbic cortical pathways using biochemical and pharmacological approaches.
Materials and Methods
Subjects. Male C57BL/6 mice from The Jackson Laboratory (Bar Harbor, ME) were housed in a 21°C humidity-controlled Association for Assessment and Accreditation of Laboratory Animal Care-approved animal care facility with food and water available ad libitum. The rooms were on a 12-h light/dark cycle (lights on at 7:00 AM). Mice were approximately 8 to 10 weeks of age and weighed approximately 25 to 30 g at the start of an experiment. All experiments were performed during the light cycle described above and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University (Richmond, VA).
Mice lacking the α7 subunit of the nicotinic receptor (C57BL/6 background) and wild-type (WT) littermates were purchased from The Jackson Laboratory (B6.129S7-charna7tm1bay). Breeding pairs of mice lacking the β2 subunit of the nicotinic receptor (C57BL/6 background) and WT littermates were shipped from Institut Pasteur (Paris, France). Mice approximately 8 to 10 weeks of age (together with age- and sex-matched WT controls) were used. β2 and α7 nAChR KO mice are maintained on a C57BL/6 background, and both were backcrossed to at least 10 generations. For all experiments, mutant and WT controls were obtained from crossing heterozygote mice. This breeding scheme allows us to rigorously control for any anomalies that may occur with crossing solely mutant animals.
Drugs. (-)-Nicotine hydrogen tartrate salt was purchased from Sigma Chemical Co. (St. Louis, Mo). Mecamylamine hydrochloride was a gift from Merck Research Labs (West Point, PA). Dihydro-β-erythroidine (DHβE) and methyllycaconitine citrate (MLA) were purchased from Sigma/RBI (Natick, MA) and were dissolved in saline. Isopropyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate (nimodipine) was purchased from Sigma/RBI and was dissolved in a vehicle solution made of 5% ethyl alcohol, 5% Emulphor oil, and 90% saline. KN-62, KN-93, and KN-92 were purchased from Calbiochem (San Diego, CA) and were dissolved in dimethyl sulfoxide to a concentration of 4 mg/ml. For injection, the stock was diluted in artificial cerebrospinal fluid to a final concentration of 64 ng/μl. Systemically administered drugs were dissolved in saline (0.9% sodium chloride) and injected subcutaneously or intraperitoneally at a volume of 10 ml/kg body weight. The pH of the nicotine solution was checked and neutralized if necessary. All doses are expressed as the free base of the drug.
Treatment. For the dose-response studies, animals were given a subcutaneous injection of one of three doses of nicotine (0.5, 1.0, or 2.0 mg/kg). At various times after injection (15 min later for CaMKII and synapsin I studies and 20 min later for pCREB studies), mice were sacrificed by cervical dislocation, and the brains were rapidly removed and sliced into 1-mm-thick sections using a mouse brain matrix (Braintree Scientific Co., Braintree, MA) on ice. The VTA, NAc, PFc, hippocampus, and amygdala were identified using a stereotaxic atlas (Paxinos and Franklin, 2001) and dissected from the appropriate sections (approximate coordinates NAc and PFc: bregma, 1.10 mm; hippocampus and amygdala: bregma, -1.70; and VTA: bregma, -3.64 mm). Sections were frozen and stored at -80°C until the CaMKII assays and Western blots were performed. For the time course studies, mice were treated with a dose of 2 mg/kg nicotine and sacrificed 1, 2, and 5 h later, and CaMKII activity was measured in the different brain regions as described above. For studies with nicotinic antagonists [MLA (10 mg/kg s.c.) and DHβE(2 mg/kg s.c.)], drugs were given 10 min before nicotine, or 15 min before nicotine for studies with the L-type calcium channel blocker nimodipine (10 mg/kg i.p.).
Intracerebroventricular Injections. Mice were anesthetized with sodium pentobarbital (45 mg/kg i.p.) on the evening before testing, and a scalp incision was made to expose the bregma. Unilateral injection sites were prepared using a 26-gauge needle with a sleeve of polyurethane tubing to control depth of the needle at a site 2 mm rostral and 2 mm lateral to the bregma at a depth of 2 mm. The scalp was sutured in such a way to enable an injection volume of 5 μl using a 26-gauge needle with a sleeve of polyurethane tubing into the lateral ventricle on the morning of testing. Animals were allowed to recover overnight. For intracerebroventricular injections the next morning (test day), the needle was held in place for 30 s to ensure drug delivery.
CaMKII Assay. CaMKII activity was measured using a CaMKII-specific assay kit (Millipore, Billerica, MA). In brief, after experimental treatment, brain tissues were homogenized in calcium-free Tris buffer that contains 1 mM phenylmethylsulfonyl fluoride. Homogenates were normalized for protein concentration. Samples were centrifuged to separate the membrane and the cytosol-containing kinase. Standard phosphorylation reaction solutions contains 70 μg of protein, 10 mM MgCl2, 1 μCi of [32P]ATP, 10 mM PIPES, pH 7.4, 5 μM CaCl2, and 5 μg of calmodulin. Standard reactions were performed in a shaking water bath at 30°C. CaMKII activity was determined using the following calculation: [(count-specific binding) × (correcting factor)]/[(specific radioactivity) × time (10 min)].
Western Blot Assays for CaMKII, pSynapsin I, and pCREB. VTA, NAc, hippocampus, amygdala, and PFc sections were homogenized in extraction buffer containing phosphate-buffered saline, 0.2 mM EGTA, 0.5 mM EDTA, and 0.5% Triton X-100. Protein concentrations were determined using the Bradford assay (Bradford, 1976), and 50 μg of protein was incubated with sample buffer and heated for 5 min at 95°C. Samples were then separated by SDS-polyacrylamide gel electrophoresis on a 10% polyacrylamide gel and subjected to immunoblotting. pCREB primary antibody (1:500; Millipore), Synapsin I (1:2000; Millipore Bioscience Research Reagents, Temecula, CA), or pSynapsin I Ser603 (an antibody specific for the site phosphorylated by CaMKII) (1:2000; Sigma Chemical Co.) primary antibodies; CaMKII primary antibody (1:1000; Sigma Chemical Co.); pCaMKII primary antibody (1:1000; Thermo Fisher Scientific, Waltham, MA); or α-tubulin antibody (1:5000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was incubated overnight at 4°C, and secondary antibody (1:5000; Santa Cruz Biotechnology, Inc.) was incubated for 1 h at room temperature. Bound antibody was detected using enhanced chemiluminescence (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). Densitometric analysis was performed using a scanner and quantified with ImageQuant (GE Healthcare). pCREB bands were detected at 43 kDa, synapsin I bands were detected at 80 kDa, pSynapsin I bands were detected at 78 kDa, CaMKII bands were detected at 50 kDa, pCaMKII bands were detected at 52 kDa, and α-tubulin bands were detected at 55 kDa.
Statistics. For all data, statistical analyses were preformed using Statview (SAS Institute, Cary, NC). Data were analyzed using one-way analysis of variance with treatment as the between-subject factor or two-way analysis of variance for studies using KO mice and time course studies with strain and treatment or time and treatment, respectively, as between-subject factors. Significant results were further assessed using Newman-Keuls post hoc test. p values less than 0.05 (*) are considered significant.
Results
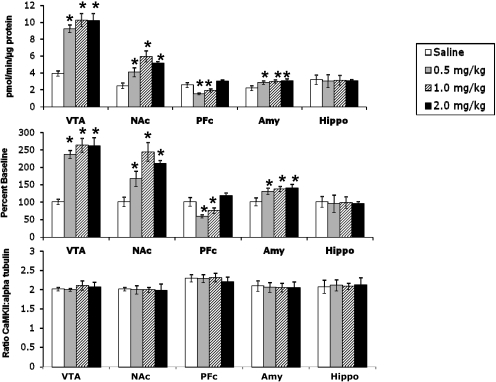
CaMKII Activity in Different Brain Regions after Acute Injection of Nicotine in Mice: Time Course and Dose-Response Effects. The activity of CaMKII was measured in several brain regions after acute subcutaneous administration of different doses of nicotine. Brain tissues were dissected, and the activity of CaMKII in the membrane was measured. Activity was expressed in Fig. 1 (top) as the number of picomoles of 32P incorporated into CaMKII substrate peptide per minute per microgram of protein in the presence or absence of calcium. For consistency, it is expressed as a percentage of saline baseline for the remaining figures. In the VTA, NAc, and amygdala, CaMKII activity was significantly increased (Fig. 1, top and middle). In contrast, CaMKII activity in the PFc was decreased at lower doses of nicotine but returned to baseline level at the highest dose (Fig. 1, top and middle). To ascertain that the changes in the kinase activity were not due to changes in levels of the protein, Western blot analysis for CaMKII levels was performed. The results of the study revealed no significant change in total kinase protein levels after acute nicotine administration (Fig. 1, bottom). Western blot analysis using a pCaMKII-specific antibody also revealed a significant increase in CaMKII phosphorylation in the VTA after acute nicotine (2 mg/kg s.c.), thus supporting our results using the CaMKII activity assay (Fig. 2).
Fig. 1.
Acute nicotine administration increases CaMKII activity in the VTA, NAc, and amygdala. Top, raw data showing an increase in CaMKII activity in the VTA, NAc, and amygdala (Amy); a decrease in the PFc; and no change in the hippocampus (Hippo). CaMKII activity was measured as picomoles per minute per microgram of protein. Middle, raw data from top graph represented as percentage of saline baseline. The changes seen from the raw data still persist; therefore, to compare graphs from different experiments, data are expressed as percentage of baseline for the remaining CaMKII assays. Bottom, levels of CaMKII protein are unchanged after acute nicotine treatment. The y-axis represents the ratio of CaMKII/α-tubulin. Each point represents the mean ± S.E.M. of six to eight mice per group. *, p < 0.05 from corresponding saline group.
Fig. 2.
Acute nicotine administration increases CaMKII phosphorylation in the VTA. Western blot analysis using a pCaMKII-specific antibody revealed a significant increase in level of CaMKII phosphorylation after acute nicotine administration (2 mg/kg s.c.). Data are expressed as percentage of baseline. Each point represents mean ± S.E.M. of four mice per group. *, p < 0.05 from corresponding saline group.
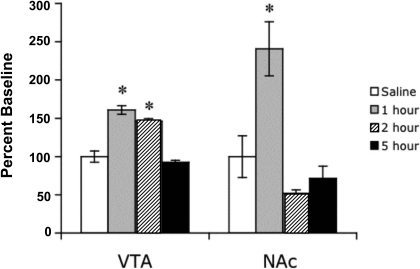
The time course of the action of nicotine (2 mg/kg s.c.) on CaMKII activity in the VTA and NAc was relatively long. Nicotine-induced increase in the activity of the kinase was still significant 2 h after injection in the VTA and 1 h in the NAc after drug exposure (Fig. 3). Five hours after nicotine injection, activity levels of CaMKII were no longer significantly different from baseline in any brain area. A similar time course was seen with the amygdala (data not shown).
Fig. 3.
Time course of increase in CaMKII activity in the VTA and NAc after acute subcutaneous injection of nicotine (2 mg/kg). Animals were sacrificed at different times after nicotine or saline injection. Each point represents the mean ± S.E.M. of six to eight mice. *, p < 0.05 from corresponding saline group.
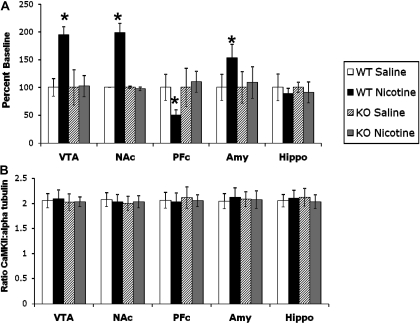
Nicotinic Receptor Subtypes Involved in Nicotine-Induced Changes in CaMKII Activity. Results show that the increased CaMKII activity induced by nicotine is mediated directly through nAChRs; thus, we examined the involvement of the two major nicotinic receptors in the brain, β2 and α7, in mediating this effect. The nicotine-induced increase in CaMKII activity was blocked by pretreatment with the β2-selective antagonist DHβE at a dose of 2 mg/kg (Table 1). The role of this subunit was further investigated by the use of β2 nAChR KO mice. Nicotine at 2 mg/kg was administered to mice lacking the β2 nAChR subunit. Animals were sacrificed, brains were dissected, and CaMKII assays were performed. Acute nicotine administration in β2 nAChR KO mice did not lead to the increase in CaMKII activity that was seen in the VTA, NAc, and amygdala of their WT littermates (Fig. 4A). To verify that the lack of increase in CaMKII activity was not due to a change in protein level of the kinase as a result of the mutation, we examined CaMKII protein levels with Western blots. The lack of increase in CaMKII activity in the β2 nAChR KO mice was not due to a change in protein level as a result of the mutation, because a Western blot for total protein showed no change in level in any of the brain areas studied (Fig. 4B).
TABLE 1.
Effects of DHβE (2 mg/kg) and MLA (10 mg/kg) on the nicotine-induced increase in CaMKII activity in the VTA and NAc Mice were pretreated with nicotinic antagonists 10 min before nicotine (2 mg/kg). Each number represents the mean ± S.E.M. of six to eight mice. The antagonists by themselves failed to alter CaMKII activity at the doses tested (data not shown).
| Treatment | VTA | NAc |
|---|---|---|
| % baseline ± S.E.M. | ||
| Veh/Veh | 100 ± 7 | 100 ± 3 |
| Veh/Nic (2 mg/kg) | 240 ± 22* | 202 ± 8* |
| Dihydro-β-erythroidine/Nic | 115 ± 18 | 105 ± 12 |
| MLA/Nic | 238 ± 25* | 198 ± 9* |
Nic, nicotine at 2 mg/kg; Veh, vehicle
p < 0.05 from Veh/Veh
Fig. 4.
Nicotine-induced increase in CaMKII activity is mediated by β2 nAChRs. A, WT mice show a nicotine-induced increase in CaMKII activity in the VTA, NAc, and amygdala, whereas this increase is absent in β2 nAChR KO mice. B, there is no change in CaMKII protein levels in any brain area examined either due to nicotine treatment or the β2 nAChR mutation. The y-axis represents the ratio of CaMKII/α-tubulin. Each point represents the mean ± S.E.M. of six to eight mice. *, p < 0.05 from corresponding saline group. Amy, amygdala; Hippo, hippocampus.
To examine the role of the α7 nAChR subunit, we first investigated the effect of MLA, an α7 antagonist. Pretreatment with MLA did not block the nicotine-induced increase in CaMKII activity (Table 1). To complement these pharmacological results, nicotine at 2 mg/kg was administered to α7 nAChR KO mice, and CaMKII assays were performed. In contrast to the β2 nAChR KO mice, α7 nAChR KO mice showed a significant nicotine-induced increase in CaMKII activity in the VTA, NAc, and amygdala while maintaining a decrease in the PFc (Fig. 5A). To ensure that there were no changes in CaMKII protein levels as a result of the mutation, Western blots for CaMKII were performed. The lack of the α7 nAChR subunit in these mice does not change the amount of total CaMKII protein levels (Fig. 5B). Taken together, these data implicate the β2 subunit but not the α7 nAChR subunit in the nicotine-induced increase in CaMKII activity.
Fig. 5.
The α7 nAChR subunit does not mediate the nicotine-induced increase in CaMKII activity. A, WT mice show a nicotine-induced increase in CaMKII activity in the VTA, NAc, and amygdala as do α7 nAChR KO mice. B, there is no change in CaMKII protein levels in any brain area examined either due to nicotine treatment or the α7 nAChR mutation. The y-axis represents the ratio of CaMKII/α-tubulin. Each point represents the mean ± S.E.M. of six to eight mice. *, p < 0.05 from corresponding saline group. Amy, amygdala; Hippo, hippocampus.
L-Type Calcium Channels Are Involved in Nicotine-Induced Changes in CaMKII Activity. The resulting nicotine-induced increase in intracellular calcium leads to an indirect calcium influx by calcium-induced calcium release from intracellular calcium stores and through voltage-gated calcium channels as a result of membrane depolarization after nAChR activation. This subsequent rise in intracellular calcium leads to activation of various downstream second messengers, including CaMKII. Thus, we also evaluated the role of L-type calcium channels in the acute nicotine-induced increase in CaMKII. Results show that the L-type calcium channel blocker nimodipine, at a dose of 10 mg/kg i.p., significantly blocked the nicotine-induced increase in CaMKII activity in the VTA and NAc as measured using the CaMKII activity assay (Fig. 6), implicating a potential role for indirect nicotine-induced sources of calcium influx. The dose of nimodipine used did not significantly affect CaMKII activity, because mice treated with nimodipine alone did not differ from vehicle controls (Fig. 6).
Fig. 6.
L-type calcium channels are involved in nicotine-induced increases in CaMKII activity. Nimodipine (10 mg/kg i.p.) significantly blocks the acute nicotine-induced (2 mg/kg s.c.) increase in CaMKII activity in the VTA and NAc. The dose of nimodipine used does not significantly affect CaMKII activity, suggesting that the observed effects on CaMKII activity are not attributed to actions of nimodipine alone. Each point represents the mean ± S.E.M. of six to eight mice. *, p < 0.05 from corresponding saline group.
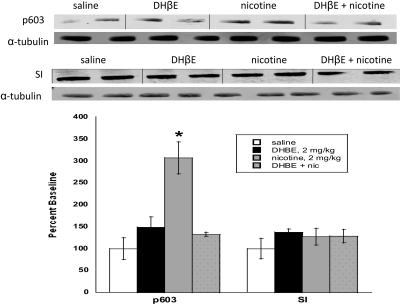
Nicotinic Receptor Subtypes Involved in Nicotine-Induced Changes in pSynapsin I. Our previous study suggests that nicotine-induced changes in the phosphorylated state of CaMKII are mediated through β2 subunit-containing nicotinic receptors. To evaluate the physiological relevance of this increase in CaMKII by nicotine, we investigated whether the phosphorylated state of synapsin I (pSynapsin I), a CaMKII substrate essential for neurotransmitter release, is altered after acute nicotine treatment, and whether this change is linked to the β2 nAChR receptor subtype. Using Western blot analysis, we examined pSynapsin I levels in the NAc and VTA using an antibody specific for the CaMKII site of phosphorylation (Ser603) after an acute (subcutaneous) injection of nicotine and after treatment with the β2 nAChR antagonist DHβE. As seen with CaMKII, acute nicotine induced a significant increase in pSynapsin I in the NAc, and this increase was significantly blocked by DHβE, indicating that the nicotine-induced increase in pSynapsin I is mediated through the β2 nicotinic receptor subtype (Fig. 7). Western blot analysis also revealed that there was no change in total synapsin I protein level after acute nicotine treatment. Similar results were observed in the VTA after acute nicotine (data not shown).
Fig. 7.
Nicotine-induced increases in synapsin I phosphorylation are mediated by β2 nAChRs. Western blot analysis using a synapsin I antibody specific for the Ser603 site phosphorylated by CaMKII (p603) revealed a significant increase in synapsin I (SI) phosphorylation after acute nicotine (nic) treatment (2 mg/kg s.c.). The increase was blocked by the β2 nAChR antagonist DHβE (2 mg/kg s.c.). Each point represents the mean ± S.E.M. of six to eight mice. *, p < 0.05 from corresponding saline group.
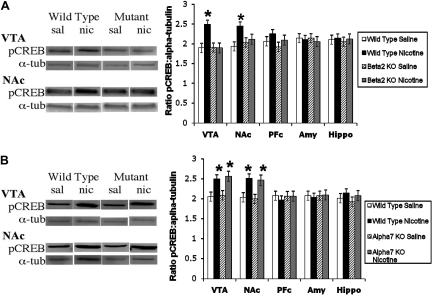
Nicotinic Receptor Subtypes Involved in Nicotine-Induced Changes in pCREB. To determine whether the nicotine-induced change in pCREB is linked to a β2 subunit-containing subtype, we examined pCREB levels after acute nicotine treatment in the VTA and NAc of β2 and α7 nAChR KO mice. Similar to CaMKII results, WT mice exhibit an increase in pCREB in the VTA and NAc after acute nicotine treatment. In contrast, mice lacking the β2 nAChR subunit do not exhibit this nicotine-induced increase in pCREB (Fig. 8A). Moreover, mice lacking the α7 nAChR do show a nicotine-induced increase in pCREB after acute exposure, similar to their WT littermates (Fig. 8B). Taken together, these data suggest that the nicotine-induced increase in pCREB is mediated by the β2 subtype and not the α7n-containing nAChR subtype.
Fig. 8.
Nicotine-induced changes in pCREB are mediated by β2, but not α7, nAChRs. A, increase in pCREB after acute nicotine treatment in the VTA and NAc is observed in WT mice. This increase is not observed in β2 nAChR KO mice. B, increase in pCREB after acute nicotine treatment in the VTA and NAc is observed in WT and α7 nAChR KO mice. The y-axis represents the ratio of pCREB/α-tubulin. Each point represents the mean ± S.E.M. of six to eight mice. *, p < 0.05 from corresponding saline group. Amy, amygdala; Hippo, hippocampus; nic, nicotine; sal, saline; α-tub, α-tubulin.
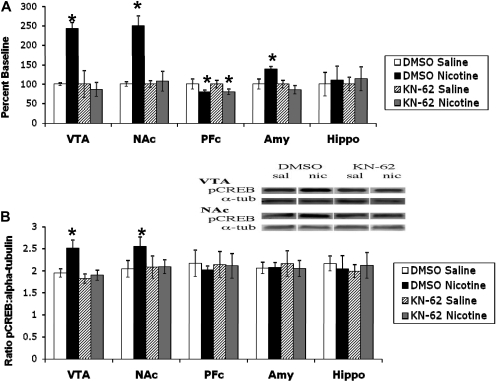
Effects of Blocking CaMKII Activity on pCREB after Nicotine Treatment. Because the nicotine-induced increase in CaMKII activity and pCREB seem to be linked to the same nAChR subtype, we determined whether the nicotine-induced CaMKII pathway would lead to pCREB activation. First, we verified that KN-62, a CaMKII inhibitor, would actually block the nicotine-induced increase in the kinase activity. To this end, we injected KN-62 (0.1 μg/animal i.c.v.) followed by systemic nicotine administration (2 mg/kg s.c.). The brains were dissected and assayed for CaMKII activity. KN-62 blocks the nicotine-induced increase in the kinase activity in the VTA, NAc, and amygdala (Fig. 9A). We then examined the effect of KN-62 on the nicotine-induced increase in pCREB. For this experiment, animals were treated with the same dose of KN-62 intracerebroventricularly followed by systemic nicotine (2 mg/kg s.c.). Brains were dissected, and Western blots for pCREB protein were performed. Similar to the blockade of CaMKII activity, KN-62 also blocks the nicotine-induced increase in pCREB in the VTA and NAc (Fig. 9B).
Fig. 9.
KN-62 blocks the nicotine-induced increase in CaMKII activity and pCREB. A, nicotine-induced increases in CaMKII activity in the VTA, NAc, and amygdala are attenuated by pretreatment with KN-62. The decrease in CaMKII activity in the PFc is not changed by pretreatment with KN-62. B, nicotine-induced increases in pCREB in the VTA and NAc are attenuated by pretreatment with KN-62. The y-axis represents the ratio of pCREB/α-tubulin. Each point represents the mean ± S.E.M. of six to eight mice. *, p < 0.05 from corresponding saline group. Amy, amygdala; Hippo, hippocampus; nic, nicotine; sal, saline; α-tub, α-tubulin.
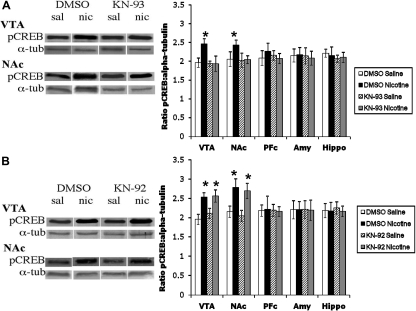
Finally, we examined the effect of a structurally different blocker of CaMKII (KN-93) and its inactive analog (KN-92) on pCREB induction after acute nicotine administration. KN-92 or KN-93 (0.1 μg/animal) was infused into the brain intracerebroventricularly followed by a systemic injection of nicotine. Brains were removed, and Western blots for pCREB were performed. Similar to KN-62, KN-93 blocked nicotine-induced increases in pCREB in the VTA and NAc (Fig. 10A). In contrast, the inactive analog KN-92 had no effect on pCREB induction after acute nicotine (Fig. 10B). Taken together, these studies suggest that nicotine-induced activation of CaMKII could lead to an increase in pCREB.
Fig. 10.
KN-93 blocks the nicotine-induced increase in pCREB. A, pretreatment with the CaMKII inhibitor KN-93 blocks the nicotine-induced increase in pCREB in the VTA and NAc. B, pretreatment with the inactive analog KN-92 has no effect on the nicotine-induced increase in pCREB in the VTA and NAc. Each point represents the mean ± S.E.M. of six to eight mice. *, p < 0.05 from corresponding saline group. Amy, amygdala; Hippo, hippocampus; nic, nicotine; sal, saline; α-tub, α-tubulin.
Discussion
CaMKII is the most abundant protein kinase in the brain. It is distributed throughout forebrain neurons, including the mesolimbic dopamine reward pathway, a region that plays a prominent role in the effects of nicotine, especially the rewarding properties of the drug. After acute injection of nicotine, we see a dose-dependent increase in the kinase activity in the VTA, NAc, and amygdala, whereas a decrease in kinase activity in the PFc was noted. Similarly, a nicotine-induced increase in CaMKII phosphorylation using a pCaMKII-specific antibody in Western blot analysis was observed in the VTA and NAc. It is interesting to note that the increase in CaMKII activity in the VTA was sustained until at least 2 h after a single injection of nicotine. This sustained activation could potentially have important pharmacological and molecular consequences, because CaMKII is a key element in neuronal plasticity and in calcium-dependent neurotransmitter release. In addition, this extended time course is consistent with an initiation of neuronal plasticity in the VTA after an acute exposure to nicotine (Mansvelder and McGehee, 2000).
The increase in CaMKII is mediated by the β2 subunit but not the α7 nAChR subunit. Indeed, DHβE, a β2-selective nicotinic antagonist, blocked the increase in the kinase activity. Furthermore, although WT mice show an increase in CaMKII activity after nicotine, β2 nAChR KO mice do not show such changes in any brain area examined. This lack of increase is not due to a variation in the amount of the kinase, because its protein levels in the β2 nAChR KO are similar to those of WT mice. In contrast, acute nicotine administration in mice lacking the α7 nAChR subunit leads to an increase in CaMKII activity in the VTA, NAc, and amygdala and a decrease in the PFc similar to WT littermates. Similar to the α7 nAChR KO mice data, MLA, an α7 nAChR antagonist, failed to block the nicotine-induced increase in kinase activity. These results suggest that the increase in CaMKII activity is mediated by β2 subunit-containing nAChRs in the VTA and NAc. Our data represent the first demonstration of the involvement of CaMKII in the mesolimbic dopamine reward system, suggesting that this kinase may play a role in the molecular signaling pathway underlying the effects of nicotine.
We also noted that blockade of L-type calcium channels by nimodipine significantly attenuates the nicotine-induced increase in CaMKII activity. Although our results suggest that the nicotine-induced increase in CaMKII activity is mediated directly through nicotinic receptors, these results also implicate the potential involvement of indirect sources in nicotine-induced calcium influx. Upon nicotine binding, there is a direct calcium influx through calcium-permeable nAChRs. The resulting increase in intracellular calcium leads to an indirect calcium influx through voltage-gated calcium channels as a result of membrane depolarization after nAChR activation. Because CaMKII activation is altered by L-type calcium channel pharmacological agents, this would suggest an important role for a mechanism of calcium influx that occurs as a result of nAChR activation.
We also examined the CaMKII substrate synapsin I after acute nicotine. As seen with CaMKII, there was a significant increase in synapsin I activity, but not total protein level, after acute nicotine treatment. This nicotine-induced increase in activity was significantly blocked by DHβEin the VTA and NAc, implying that acute nicotine-induced increases in synapsin I activity are mediated by β2 subunit-containing nAChRs. CaMKII phosphorylates synapsin I at two sites: Ser566 and Ser603 (De Camilli et al., 1990; Hilfiker et al., 1999). For our Western analysis, we used an antibody specific for synapsin I phosphorylation by CaMKII at Ser603, thus supporting our results indicating a link between CaMKII and synapsin I after acute nicotine administration. Taken together, these experiments suggest that synapsin I is also involved in mediating nicotine-induced effects on the mesolimbic dopamine reward system through the CaMKII pathway.
The transcription factor CREB has been implicated in mediating effects of multiple drugs of abuse. Recent work has shown that an acute injection of nicotine increases pCREB in the VTA and NAc in mice (Walters et al., 2005). Our studies confirm this increase and show that mice lacking the β2 nAChR subunit do not show an increase in pCREB in the VTA or NAc after treatment with acute nicotine, whereas this increase is still present in α7 nAChR KO mice. These results suggest that the nicotine-induced increase in pCREB is driven by β2- but not α7 nAChR subunit-containing nAChRs. Moreover, we show that there is a correlation between the increase in CaMKII activity and pCREB induction after acute nicotine. Both of these responses in the VTA and NAc after acute nicotine are linked to β2 subunit-containing nAChRs. Previous in vitro work suggests that CaMKII decreases levels of CREB (Wu and McMurray, 2001); however, more recent work in the spinal cord shows that CaMKII can increase CREB activity (Miyabe and Miletic, 2005).
Previous work by Brunzell et al. (2003) shows different responses to acute nicotine. Acute nicotine exposure in C57BL/6J mice resulted in no change in pCREB in the VTA or NAc; however, animals were exposed to nicotine in their drinking water with an acute exposure defined as a single 1.3-h session. In the present study, we administer nicotine with a single acute injection and measure changes in pCREB 20 min after this exposure; thus, it is possible that the different durations of exposure can lead to very different results.
To determine whether CREB is a potential downstream molecular target of the CaMKII pathway, we used the CaMKII inhibitors KN-62 and KN-93. Intracerebroventricular injections of KN-62 blocked the nicotine-induced increase in CaMKII activity in the VTA, NAc, and amygdala and the increase in pCREB in the VTA and NAc. Similarly, KN-93 also blocked the increase in pCREB in the VTA and NAc after acute nicotine, whereas the inactive analog KN-92 had no effect. These data lend support to the hypothesis that an increase in the activity of CaMKII leads to an increase in pCREB in the VTA and NAc after acute nicotine administration. Although KN-62 and KN-93 were reported previously to be selective inhibitors of CaMKII (Tokumitsu et al., 1990; Sumi et al., 1991), they were more recently shown to block other kinases such as calcium/calmodulin-dependent protein kinase IV (CaMKIV) (Enslen et al., 1994); thus, it is possible that increases in CaMKIV activity may be involved in acute nicotine-induced CREB activation. There are three different phosphorylation sites on the CREB protein (Ser133 and Ser142/143), each of which have differential effects on downstream activities associated with CREB activation. CaMKII phosphorylates primarily 142/143, whereas CaMKIV phosphorylates Ser133 (Deisseroth and Tsien, 2002). Further investigation into the role of CaMKIV is necessary to determine the function, if any, of this kinase in the effects of acute nicotine exposure.
It is noteworthy that there are different effects depending on which brain area is examined. In the VTA and NAc, both central players in the mesolimbic dopamine reward pathway, we see nicotine-induced increases in CaMKII activity, pSynapsin I, and pCREB that are mediated by the β2 nAChR subunit, and the increases in pCREB in both areas are blocked by two kinase inhibitors. In the amygdala, acute nicotine increases CaMKII activity, and this increase is also mediated by nAChRs containing the β2 nAChR subunit; however, nicotine does not increase pCREB in this brain area. Previous work suggests that the amygdala may be involved more in the associative learning and memory-enhancing processes (Levin et al., 2006); and, more recently, nicotine was shown to enhance responsiveness to auditory responses in single unit recordings in the amygdala (Cromwell and Woodward, 2007). Thus, although CaMKII activation in the amygdala is increased, the pathway that this increase affects is more likely one that is involved in the attention or memory-enhancing effects of nicotine. Finally, CaMKII activity is decreased in the PFc after acute nicotine, which is not present in β2 nAChR KO mice, and this decrease is not affected by administration of an inhibitor. In addition, the decrease in CaMKII activity does not have an affect on pCREB levels in this brain region. These data may support the hypothesis that the PFc is involved in inhibitory control over the VTA. This inhibitory control does not seem to be regulated by the CREB pathway after acute nicotine.
The second messenger systems involved after nicotine exposure are poorly understood. There is evidence for involvement of numerous pathways including the extracellular signal-regulated kinase (Brunzell et al., 2003) and CREB pathways (Brunzell et al., 2003; Walters et al., 2005). Our data suggest that in the VTA and NAc, nicotine works through β2 subunit-containing nAChRs to activate CaMKII-dependent pathways, which leads to the phosphorylation of molecular targets such as synapsin I and CREB. Our data need to be interpreted with caution because they do not provide a direct link between these activity changes in calcium/calmodulin-dependent protein kinases and addictive behavior after chronic nicotine exposure. The potential for engaging alternate signaling mechanisms is possible under more chronic nicotine exposure. Future investigation of the long-term effects of nicotine will aid in elucidation of potential target pathways in smoking cessation therapies.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA12610].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.153171.
ABBREVIATIONS: nAChR, nicotinic acetylcholine receptor; VTA, ventral tegmental area; NAc, nucleus accumbens; KO, knockout; CREB, cAMP-response element-binding protein; p, phosphorylated; PFc, prefrontal cortex; CaMKII, calcium/calmodulin-dependent protein kinase II; WT, wild type; DHβE, dihydro-β-erythroidine; MLA, methyllycaconitine citrate; KN-62, 4-[(2S)-2-[(5-isoquinolinylsulfonyl)methylamino]-3-oxo-3-(4-phenyl-1-piperazinyl)propyl]phenyl isoquinolinesulfonic acid ester; KN-93, N-[2-[[[3-(4-chlorophenyl)-2 propenyl]methylamino]methyl]phenyl]-N-(2-hydroxyethyl)-4-methoxybenzenesulphonamide; KN-92, (2-[N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine, phosphate); CaMKIV, calcium/calmodulin-dependent protein kinase IV.
References
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248-254. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Russell DS, and Picciotto MR (2003) In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. J Neurochem 84 1431-1441. [DOI] [PubMed] [Google Scholar]
- Cromwell HC and Woodward DJ (2007) Inhibitory gating of single unit activity in amygdala: effects of ketamine, haloperidol, or nicotine. Biol Psychiatry 61 880-889. [DOI] [PubMed] [Google Scholar]
- Damaj MI (2000) The involvement of spinal Ca(2+)/calmodulin-protein kinase II in nicotine-induced antinociception in mice. Eur J Pharmacol 404 103-110. [DOI] [PubMed] [Google Scholar]
- Damaj MI (2005) Calcium-acting drugs modulate expression and development of chronic tolerance to nicotine-induced antinociception in mice. J Pharmacol Exp Ther 315 959-964. [DOI] [PubMed] [Google Scholar]
- Dani JA (2001) Overview of nicotinic receptors and their roles in the central nervous system. Biol Psychiatry 49 166-174. [DOI] [PubMed] [Google Scholar]
- Dani JA and Heinemann S (1996) Molecular and cellular aspects of nicotine abuse. Neuron 16 905-908. [DOI] [PubMed] [Google Scholar]
- De Camilli P, Benfenati F, Valtorta F, and Greengard P (1990) The synapsins. Annu Rev Cell Biol 6 433-460. [DOI] [PubMed] [Google Scholar]
- Deisseroth K and Tsien RW (2002) Dynamic multiphosphorylation passwords for activity-dependent gene expression. Neuron 34 179-182. [DOI] [PubMed] [Google Scholar]
- Enslen H, Sun P, Brickey D, Soderling SH, Klamo E, and Soderling TR (1994) Characterization of Ca2+/calmodulin-dependent protein kinase IV. Role in transcriptional regulation. J Biol Chem 269 15520-15527. [PubMed] [Google Scholar]
- Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, and Greengard P (1999) Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci 354 269-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata S, Hewlett GH, and Gnegy ME (1997a) Amphetamine increases the phosphorylation of neuromodulin and synapsin I in rat striatal synaptosomes. Synapse 26 281-291. [DOI] [PubMed] [Google Scholar]
- Iwata S, Hewlett GH, Ferrell ST, Czernik AJ, Meiri KF, and Gnegy ME (1996) Increases in vivo phosphorylation state of neuromodulin and synapsin I in striatum from rats treated with repeated amphetamine. J Pharmacol Exp Ther 278 1428-1434. [PubMed] [Google Scholar]
- Iwata SI, Hewlett GH, Ferrell ST, Kantor L, and Gnegy ME (1997b) Enhanced dopamine release and phosphorylation of synapsin I and neuromodulin in striatal synaptosomes after repeated amphetamine. J Pharmacol Exp Ther 283 1445-1452. [PubMed] [Google Scholar]
- Kano T, Suzuki Y, Shibuya M, Kiuchi K, and Hagiwara M (1995) Cocaine-induced CREB phosphorylation and cFos expression are suppressed in Parkinsonism model mice. Neuroreport 6 2197-2200. [DOI] [PubMed] [Google Scholar]
- Lane-Ladd SB, Pineda J, Boundy VA, Pfeuffer T, Krupinski J, Aghajanian GK, and Nestler EJ (1997) CREB (cAMP response element-binding protein) in the locus coeruleus: biochemical, physiological, and behavioral evidence for a role in opiate dependence. J Neurosci 17 7890-7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, and Rezvani AH (2006) Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 184 523-539. [DOI] [PubMed] [Google Scholar]
- Licata SC, Schmidt HD, and Pierce RC (2004) Suppressing CaMKII activity in the ventral tegmental area enhances the acute behavioural response to cocaine but attenuates the initiation of cocaine-induced behavioural sensitization in rats. Eur J Neurosci 19 405-414. [DOI] [PubMed] [Google Scholar]
- Mackay J and Eriksen M (2002) The Tobacco Atlas, World Health Organization, Geneva, Switzerland.
- MacNicol M and Schulman H (1992) Multiple Ca2+ signaling pathways converge on CaM kinase in PC12 cells. FEBS Lett 304 237-240. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD and McGehee DS (2000) Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron 27 349-357. [DOI] [PubMed] [Google Scholar]
- Matus-Leibovitch N, Ezra-Macabee V, Saya D, Attali B, Avidor-Reiss T, Barg J, and Vogel Z (1995) Increased expression of synapsin I mRNA in defined areas of the rat central nervous system following chronic morphine treatment. Mol Brain Res 34 221-230. [DOI] [PubMed] [Google Scholar]
- Miyabe T and Miletic V (2005) Multiple kinase pathways mediate the early sciatic ligation-associated activation of CREB in the rat spinal dorsal horn. Neurosci Lett 381 80-85. [DOI] [PubMed] [Google Scholar]
- Mulle C, Choquet D, Korn H, and Changeux JP (1992) Calcium influx through nicotinic receptor in rat central neurons: its relevance to cellular regulation. Neuron 8 135-143. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, and Svensson TH (1994) Infusion of nicotine in the ventral tegmental area or the nucleus accumbens of the rat differentially affects accumbal dopamine release. Pharmacol Toxicol 75 348-352. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Xu T, and Mittal N (2001) Effects of protracted nicotine exposure and withdrawal on the expression and phosphorylation of the CREB gene transcription factor in rat brain. J Neurochem 77 943-952. [DOI] [PubMed] [Google Scholar]
- Paxinos G and Franklin K (2001) The Mouse Brain in Stereotaxic Coordinates, 2nd ed., Academic Press, San Diego, CA.
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, and Changeux JP (1998) Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391 173-177. [DOI] [PubMed] [Google Scholar]
- Shaw-Lutchman TZ, Impey S, Storm D, and Nestler EJ (2003) Regulation of CRE-mediated transcription in mouse brain by amphetamine. Synapse 48 10-17. [DOI] [PubMed] [Google Scholar]
- Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, and Hidaka H (1991) The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun 181 968-975. [DOI] [PubMed] [Google Scholar]
- Tan SE (2002) Impairing the amphetamine conditioning in rats through the inhibition of hippocampal CaMKII activity. Neuropharmacology 42 540-547. [DOI] [PubMed] [Google Scholar]
- Tokumitsu H, Chijiwa T, Hagiwara M, Mizutani A, Terasawa M, and Hidaka H (1990) KN-62, 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazine, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 265 4315-4320. [PubMed] [Google Scholar]
- Walters CL and Blendy JA (2001) Different requirements for cAMP response element binding protein in positive and negative reinforcing properties of drugs of abuse. J Neurosci 21 9438-9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, and Damaj MI (2006) The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 184 339-344. [DOI] [PubMed] [Google Scholar]
- Walters CL, Cleck JN, Kuo YC, and Blendy JA (2005) mu-Opioid receptor and CREB activation are required for nicotine reward. Neuron 46 933-943. [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Tang L, and Xin L (2003) Reversal of morphine antinociceptive tolerance by acute spinal inhibition of Ca2+/calmodulin-dependent protein kinase II. Eur J Pharmacol 465 199-200. [DOI] [PubMed] [Google Scholar]
- Wu X and McMurray CT (2001) Calmodulin kinase II attenuation of gene transcription by preventing cAMP response element-binding protein (CREB) dimerization and binding of the CREB-binding protein. J Biol Chem 276 1735-1741. [DOI] [PubMed] [Google Scholar]