Abstract
Plasma concentrations of protease inhibitors are lower in pregnant women than in nonpregnant women or men. Using nelfinavir as a model protease inhibitor, we have shown that this phenomenon can be reproduced in a representative non-human primate model, Macaca nemestrina (J Pharmacol Exp Ther 329:1016–1022, 2009). Nelfinavir is cleared from the body predominantly by CYP3A metabolism and P-glycoprotein (P-gp) efflux. Therefore, using midazolam (MDZ) as a CYP3A probe and digoxin (DIG) as a P-gp probe, we determined the antepartum (73–118 days) and postpartum (61–130 days) in vivo intestinal and hepatic CYP3A or P-gp activity in the macaque. Although the systemic clearance of MDZ was significantly increased (∼70%) during pregnancy after intra-arterial (IA) administration of the drug (15N-labeled MDZ; 40 μg/kg), pregnancy did not affect the oral clearance of the drug administered simultaneously (1 mg/kg p.o.) with the IA dose. In vitro studies in hepatic and intestinal S-9 fractions indicated no effect of pregnancy on CYP3A activity or protein expression in the small intestine or liver. In contrast, neither the oral (100 μg/kg) nor the IA (10 μg/kg) clearance of DIG was significantly altered by pregnancy, indicating no effect of pregnancy on P-gp activity. Assuming that MDZ and DIG are selective substrates of the macaque CYP3A enzymes and P-gp, respectively, these results suggest that factors other than increased CYP3A or P-gp activity contribute to the increased clearance of protease inhibitors during M. nemestrina pregnancy.
Anti-HIV protease inhibitors (PIs) are administered routinely to HIV-1-infected pregnant women (Hammer et al., 2006). However, the correct dose of the PIs that should be prescribed for this population is not clear, because the oral clearance of PIs is significantly increased during pregnancy, including the oral clearance of indinavir (Unadkat et al., 2007), saquinavir (Acosta et al., 2001), and nelfinavir (Angel et al., 2001; Nellen et al., 2004; van Heeswijk et al., 2004). As a result, on administration of standard doses of the PIs, the plasma concentrations of the PIs are lower than those achieved in nonpregnant women or men. These decreased concentrations are of significant clinical concern, because they have been strongly associated with virological treatment failure and the progression of disease (Back et al., 2002). In addition, they may result in resistant virus and the greater potential of maternal-fetal transmission of resistant virus.
Because the PIs are primarily cleared from the body by CYP3A metabolism and P-glycoprotein transport (Kim et al., 1998; Unadkat and Wang, 2000; Zhang et al., 2001), we have hypothesized that the increased oral clearance of the PIs during pregnancy is caused by increased activity of one or both of these proteins. Indeed, we have shown that oral clearance of midazolam, a CYP3A model drug, is greater in pregnant women compared with postpartum women (Hebert et al., 2008). However, these human studies did not reveal whether the CYP3A activity in the intestine, liver, or both was increased during pregnancy, and these studies could not reveal the molecular mechanisms of this observation. Therefore, we have embarked on a series of animal studies to identify the tissue in which CYP3A activity is increased during pregnancy and to elucidate the mechanistic basis of this phenomenon (Mathias et al., 2006; Zhang et al., 2008, 2009).
We replicated the phenomenon of increased clearance of PIs in the pregnant mouse (Mathias et al., 2006). We found that pregnancy significantly increased the hepatic expression and activity of Cyp3a enzymes in pregnant mice but did not affect hepatic and intestinal P-pg expression or intestinal Cyp3a activity (Mathias et al., 2006; Zhang et al., 2008). However, because of the multiplicity of Cyp3a and P-gp isoforms and the lack of specific reagents (isoform-specific antibody or purified proteins), determining the mechanistic basis for this increase in clearance of PIs during pregnancy is difficult in this mouse model. For this reason, we began studies in a more representative animal model that is genetically and physiologically closer to humans, the pregnant non-human primate, Macaca nemestrina (pig-tailed macaque). We confirmed that oral and systemic clearance of a model PI, nelfinavir, was increased in the pregnant M. nemestrina (Zhang et al., 2009). In addition, in vitro studies with hepatic and intestinal S-9 fractions obtained from pregnant and postpartum animals suggested that the increased oral clearance of NFV was caused by increased hepatic but not intestinal CYP3A activity. Here, we report on a study that tests this hypothesis in vivo and also determines whether in vivo intestinal and/or hepatic P-gp activity is increased during pregnancy.
Materials and Methods
Chemicals. Midazolam (5 mg/ml) was purchased from Roxane Laboratories (Ridgefield, CT). Stably labeled (15N) midazolam was purchased from F. Hoffman-La Roche (Basel, Switzerland) and 1′-OH midazolam from SAFC Corp. (St. Louis, MO). Deuteriumlabeled midazolam (D4-MDZ, 100 μg/ml) and deuterium-labeled hydroxyl midazolam (D4-OH MDZ, 100 μg/ml), internal standards for MDZ and 1′-OH MDZ analysis, were purchased from Cerilliant Corporation (Round Rock, TX). β-Glucuronidase was purchased from Sigma-Aldrich (St. Louis, MO). Digoxin (250 μg/ml) was purchased from Baxter (McGaw Park, IL), and digitoxin (internal standard for the digoxin assay) was purchased from IMP Biomedicals (Solon, OH). 3H-Labeled digoxin was purchased from American Radiolabeled Chemicals (St. Louis, MO). High-performance liquid chromatography-grade methanol, acetonitrile, water, and methyl-t-butyl-ether were purchased from Thermo Fisher Scientific (Waltham, MA). A BCA protein assay kit was purchased from Pierce Chemical (Rockford, IL). Primary antibodies anti-human CYP3A4, anti-P-gp C219, and anti-β-actin were purchased from Daiichi Pure Chemicals (Tokyo, Japan), Alexis Biomedicals (San Diego, CA), and Sigma-Aldrich, respectively. Secondary anti-mouse, anti-rabbit antibodies conjugated with different IRDyes were purchased from LI-COR Bioscience (Lincoln, NE). Polyvinylidene fluoride membrane was purchased from GE Healthcare (Little Chalfont, Buckinghamshire, UK). RNA extraction kit and RNase-free DNase were purchased from QIAGEN (Valencia, CA). Reverse-transcription kit and 2× master PCR mixture for real-time PCR were purchased from Applied Biosystems (Foster City, CA). All other reagents and materials were purchased from standard vendors and were of the highest available purity.
Animals. Four pregnant (antepartum, AP 73–118 day; term is ∼167 days) and four nonpregnant (postpartum, PP 61–130 day) macaques (M. nemestrina, 7.0–12 years, 6.5–10 kg) were studied. The nelfinavir pharmacokinetic studies that we reported previously were also conducted in these animals during the same pregnancies (Zhang et al., 2009).
Drug Administration and Blood Sampling. After at least 10 days of recovery from surgery, the animals were sequentially administered oral digoxin (DIG,100 μg/kg), oral midazolam (MDZ, 1 mg/kg), intra-arterial (IA) 15N-labeled MDZ (40 μg/kg), simultaneously, and IA DIG (10 μg/kg) each separated by at least 5 days. For safety reasons, the arterial catheter was used to administer the drug (rather than to draw samples), and blood samples were collected from the venous catheter. For MDZ studies, blood samples were collected from the femoral vein predose and at 3, 5, 15, and 30 min and at 1, 2, 4, 6, 8, 10, and 12 h after dose. For DIG oral study, blood samples were collected from the femoral vein before dose and at 30 min and at 1, 2, 4, 6, 8, 10, 12, 24, 36, 48, and 72 h after dose; for the DIG IA study, blood samples were collected before dose and at 5, 15, and 30 min and at 1, 2, 4, 6, 8, 10, 12, 24, 36, 48, and 72 h after dose. Urine was collected before dose and 24 h after dose for MDZ and 72 h after dose for DIG. Blood samples were immediately centrifuged, and plasma and urine samples were stored at -20°C until analysis. All dosing solutions were analyzed by LC-MS to confirm the concentrations of solutions administered.
MDZ and DIG Plasma Protein Binding. Plasma protein binding of MDZ and DIG was determined by ultrafiltration (Centrifree YM-30; Millipore Corporation, Billerica, MA). In brief, a total plasma concentration of 5 ng/ml MDZ (average plasma concentration) was used to determine the plasma protein binding in the predose samples. MDZ was dried in a cell culture tube, and then 900 μl of MDZ-free plasma collected from pregnant or nonpregnant macaques (predose sample) was added to each tube. After mixing at room temperature for 10 min, duplicate aliquots of 425 μl of the plasma were transferred to the filtration devices and incubated for 30 min at 37°C. The cartridges were then centrifuged in a swinging bucket centrifuge (preset to 37°C) at 1000g for 3 min (approximately 45 μl of filtrate was collected). Forty microliters of the ultrafiltrate or plasma was alkalinized with concentrated ammonium hydroxide (14 M) and extracted with 5 ml of methyl-t-butyl ether. Deuterium-labeled MDZ (5.0 ng/ml, D4-MDZ) was used as an internal standard. The extraction solvent was evaporated and reconstituted in 25 μl of 50:50 methanol/water. Fifteen microliters of each sample was injected to quantify MDZ and the internal standard D4-MDZ, following the UPLC-MS/MS method described below. Protein binding of MDZ was shown to be linear between 5 and 50 ng/ml using blank macaque plasma.
The same ultrafiltration technique as described above was used to determine plasma protein binding of DIG, with the exception that 3H-labeled compound was used. Twenty microliters of plasma (before filtration) and ultrafiltrate were analyzed for total radioactivity on a Packard Tri-Carb 1600RP liquid scintillation counter [PerkinElmer Life and Analytical Sciences (Boston, MA)]. The percentage unbound (fu) was calculated as the percentage of radioactive counts in the filtrate to percentage in the plasma sample. The percentage of DIG bound was determined to be constant over the range of 0.02 to 20 ng/ml using blank macaque plasma. The plasma protein binding was determined at an average plasma concentrations (IA, 0.2 ng/ml; oral, 1 ng/ml) observed in the studies. Nonspecific binding of [3H]DIG to the filtration cartridge was determined to be <15% (Hebert et al., 2008).
Tissue Collection and Isolation of S-9 Fractions. The procedures for collecting hepatic and proximal small-intestinal tissues and methods for S-9 fraction preparation were as described previously (Zhang et al., 2009).
1′-Hydroxylation of MDZ in S-9 Fractions. In brief, 0.2 mg/ml hepatic S-9 fractions or 0.5 mg/ml proximal small intestine S-9 fractions were preincubated for 5 min at 37°C with 100 mM potassium phosphate, pH 7.4, containing 0.1 mM EDTA and 10 μM MDZ in a shaking water bath. Incubation reactions were initiated by adding 1 mM NADPH (freshly prepared; final incubation volume, 100 μl) and terminated by the addition of 100 μl of ice-cold acetonitrile containing the internal standard D4-OH MDZ (5 ng/ml) at 10 min. Samples were vortexed and kept on ice for 30 min before centrifuging at 14,000g for 10 min at room temperature. Supernatant was then collected and injected directly onto LC-MS for analysis, following the method described below. All incubations were conducted in duplicate. For cDNA-expressed CYP3A64, 2 pmol of enzymes were used, and incubations were conducted in triplicate.
To determine whether 1′-OH MDZ formation was mediated by CYP3A, selective human CYP3A inhibitors, erythromycin (ERY, 0.5 mM), ketoconazole (KTZ, 0.5 μM), or troleandomycin (TAO, 0.01–1 mM), were included in the assay following the method described previously (Zhang et al., 2009). With the exception of TAO, all inhibitors were coincubated with substrate and S-9 fractions for 5 min before adding NADPH. TAO was coincubated with S-9 fractions and NADPH for 30 min before adding the substrate. Percentage of control activity was calculated as the activity in the incubation when the inhibitor was present compared with that when the inhibitor was absent.
MDZ Depletion in S-9 Fractions. In addition to 1′-OH MDZ formation, depletion of MDZ was also studied. Incubation conditions were the same as above, with the exception that MDZ concentrations used in depletion assays were 0.2 μM. All inhibition assays were carried out for 10 min for hepatic S-9 fractions and 20 min for intestinal S-9 fractions before termination with ice-cold acetonitrile containing internal standard D4-MDZ. The concentration of MDZ was quantified by use of the LC-MS method described below. The percentage of MDZ remaining in each incubation was calculated with respect to that in corresponding 0-min control.
TST 6β-Hydroxylation in S-9 Fractions. This was conducted essentially as described previously with only minor modifications (Mathias et al., 2006; Zhang et al., 2008). In brief, 0.2 mg/ml hepatic S-9 fractions or 0.5 mg/ml proximal small-intestine S-9 fractions were preincubated at 37°C for 5 min in 100 μl of phosphate buffer (100 mM potassium phosphate, pH 7.4, 0.1 mM EDTA) containing 150 μM testosterone (TST). Reaction was initiated by adding freshly prepared NADPH (final concentration, 1 mM) and terminated at 20 min by adding 100 μl of ice-cold acetonitrile containing 10 μl of internal standard 11α-OH progesterone (54 μg/ml). Samples were mixed and incubated on ice for 30 min before centrifugation at 15,000g for 10 min. Twenty microliters of the supernatant was directly injected onto LC-UV. All inhibition assays were performed as described in a previous section. 6β-OH TST was detected by use of the LC-UV method described previously (Zhang et al., 2008).
SDS-Polyacrylamide Gel Electrophoresis and Western Blot Analysis. Ten-microgram liver S-9 fractions or 20-μg small-intestinal S-9 fractions were separated by 10% precast Criterion Tris-HCl gel (Bio-Rad Laboratories, Hercules, CA) for CYP3A detection. Eighty-microgram liver homogenates were separated by 4 to 15% gradient precast Criterion Tris-HCl gel (Bio-Rad Laboratories) for P-gp detection. Proteins were transferred to polyvinylidene fluoride membrane (GE Healthcare). Western blot detection was performed according to the manufacturer's instructions (Odyssey, LI-COR Biosciences, Lincoln, NE). In brief, blots were soaked in phosphate-buffered saline for 10 min and then placed in blocking buffer (LI-COR Biosciences) overnight at 4°C. Anti-human CYP3A4 (1:2000), C219 (1:1000), and anti-β-actin antibody (1:5000) were added to the blocking solution and incubated for 1 h at room temperature on a rocking plate. The blots were then rinsed in washing buffer (0.05% Tween 20 in phosphate-buffered saline) and washed four times, 5 min each. The blots were then incubated in blocking buffer with secondary antibodies (1:10,000 anti-rabbit or anti-mouse IgG conjugated to IRDye; LI-COR Biosciences) for 45 min. Luminescent signal was then recorded on an Infrared Imaging System (Odyssey; LI-COR Biosciences). The relative intensity of each protein band was determined by the Odyssey program (LI-COR Biosciences) according to manufacturer's instructions.
Real-Time PCR Assay. Relative quantification of macaque CYP3A and P-gp transcript expression was performed according to the protocol described previously (Zhang et al., 2008), with the exception that Taqman primers and probes for macaque genes CYP3A64 (Rh02872540_m1), CYP3A66 (Rh02788718_m1), MDR1 (Rh01070639_m1), and β-actin (Rh03043379_gH) from Applied Biosystems were used. All of the other experimental procedures and methods for calculation of relative expression remained the same.
Analysis of MDZ and 1′-OH MDZ. MDZ and 1′-OH MDZ concentrations in the S-9 incubations were analyzed by a validated LC-MS method described previously (Kirby et al., 2006). To achieve greater sensitivity than that afforded by LC-MS, detection of MDZ and 1′-OH MDZ in the plasma and urine samples and plasma protein binding samples (described above) was conducted by UPLC-MS/MS. In brief, 50 μl of an internal standard mixture containing D4-MDZ and D4-OH MDZ (15 ng of each) was added to plasma samples followed by 100 μl of concentrated ammonium hydroxide and 5 ml of methyl-t-butyl-ether. Samples were mixed and agitated for 30 min and then centrifuged for 10 min at 2000g. The organic layer then was transferred to a culture tube and evaporated to dryness under vacuum. The residue was reconstituted in 100 μl of a 50:50 water/methanol solution containing 0.1% acetic acid. Fifteen microliters of the reconstituted solution was injected onto the UPLC-MS/MS for analysis. Quantification of 15N-MDZ and 15N-OH MDZ was based on the assumption that the detection efficiency of labeled and unlabeled compounds was the same. A calibration curve and controls were prepared in blank human plasma obtained from the Puget Sound Blood Bank. Urine samples were diluted either 1:10 or 1:50 with water and then pretreated with 1000 units of β-glucuronidase in a 100 mM acetic acid solution overnight (∼16 h) before extraction with the above-described method. A set of calibrators and controls were similarly subjected to the β-glucuronidase treatment for quantification of the urine samples. Precision and accuracy of the assay was <20% CV for precision and <20% error for accuracy. The level of quantification for MDZ was 0.1 ng/ml for a 0.5-ml sample. Detection of MDZ (m/z 326.0 > 291.2), 1′-OH MDZ (m/z 342.0 > 324.0), and the D4-labeled internal standards (m/z 330.0 > 296.2 and 346.0 > 329.0, respectively) was performed by use of MS-MS capabilities on a Waters Aquity UPLC with a binary solvent manager (Waters, Milford, Mass) coupled to a Micromass Quatro Premier XE tandem mass spectrometer (Waters) using positive electrospray ionization. The UPLC column was a Waters 2.1 × 50-mm 1.7-μm BEH C18 column with a Phenomenex 2.1 × 4-mm C18 guard column. Mobile phase flow was 0.3 ml/min with initial conditions of 95% aqueous (0.1% acetic acid in water) and 5% organic (0.1% acetic acid in methanol). The initial mobile phase conditions were held for 1 min and then increased linearly to 100% organic at 2.5 min and held until 3.2 min when the mobile phase was reverted to the original mobile conditions over 0.05 min. The column was equilibrated for 1.25 min before the next injection. Mass spectrometer conditions were as follows: desolvation temperature, 400°C; desolvation nitrogen gas flow, 1100 liters/h; source temperature, 120°C; capillary voltage, 3.0 kV; extractor, 6.0; collision cell argon gas flow, 0.1 ml/min; and multiplier voltage, 650 V. Cone voltages and collision energies were optimized for each transition. Multiple reaction-monitoring chromatograms were integrated with use of Masslynx version 4.0 (Waters). Sample concentrations were determined by linear regression of the ratio of analyte peak area to the internal standard peak area by use of Microsoft Excel.
Analysis of DIG. Plasma and urine samples were assayed for DIG concentrations by use of a validated LC-MS method published previously (Kirby et al., 2008). In brief, 1 ml of plasma and urine samples with 1 ng of digitoxin (internal standard) was alkalinized with 100 μl of ammonium hydroxide and extracted with 5 ml of methyl-t-butyl ether. Detection of sodium adducts of digoxin (m/z 802.4) and digitoxin (m/z 786.4) was performed on an Agilent 1100 series MSD with positive electrospray ionization. A gradient elution was used on an XDB-C8 analytical column (2.1 × 50 mm, 5 μm) coupled with a XDB-C18 guard column (2.1 × 12.5 mm, 5 μm) (Agilent Technologies, Santa Clara, CA) at a flow rate of 0.25 ml/min. A calibration curve and controls ranging from 0.05 to 1.0 ng/ml were analyzed in triplicate with all plasma and urine samples. The intra- and interday precision of the assay had a CV of ≤15%. The level of quantification for DIG was 0.1 ng/ml for a 0.5-ml sample.
Pharmacokinetic Data Analysis. Plasma concentration versus time profiles for MDZ and DIG were obtained for each individual animal, and noncompartmental analysis was performed with use of WinNonlin 5.0.1 (Pharsight Corp., Mountain View, CA) to recover area-under-the-curve (AUC0-∞) and other parameters. Bioavailability in each animal was estimated as the ratio of the systemic clearance and the corresponding oral clearance. Because pharmacokinetic parameters are typically log-normally distributed, statistical analysis was conducted on log-transformed pharmacokinetic parameters. The geometric mean ratio of pharmacokinetic parameters and their 95% confidence intervals were computed. If the 95% confidence interval encompassed the value of unity, the pharmacokinetic parameter of the drug was not considered significantly different antepartum versus postpartum. For clarity, data for each pharmacokinetic parameter are reported as arithmetic mean ± S.D.
Results
Pharmacokinetics of MDZ after Intra-Arterial and Oral Administration. After intra-arterial (IA) administration, 15N-MDZ plasma concentrations declined rapidly (Fig. 1A), with a terminal half-life of 1.2 to 1.3 h (Table 1). The antepartum dose-normalized AUC0-∞ (n = 4) was only 56% of that observed postpartum (n = 4) (Table 1; Fig. 1A). As a result, the MDZ total plasma clearance in pregnant macaques was significantly greater (by ∼70%) than that observed postpartum, regardless of whether it was normalized to body weight or not. The antepartum formation clearance to 1′-OH MDZ was 2.5-fold higher than that observed postpartum. The percentage of MDZ unbound in the plasma was similar antepartum and postpartum (Table 1). MDZ steady-state volume of distribution was not significantly different antepartum versus postpartum. Urinary recovery of MDZ as 1′-OH MDZ (after deconjugation of the glucuronide) was low (8.9–13.9%) and unaffected by pregnancy (Table 1).
Fig. 1.
The mean (± S.D.) MDZ dose-normalized plasma concentration-time profile in M. nemestrina is significantly lower antepartum (AP, n = 4, ○) than that obtained postpartum (PP, n = 4, •) after intra-arterial (40 μg/kg) (A) but not oral (1 mg/kg) (B) administration of the drug.
TABLE 1.
AP and PP pharmacokinetic parameters (arithmetic mean ± S.D., n = 4) of MDZ in M. nemestrina after simultaneous intra-arterial (40 μg/kg, 15N-labeled) and oral (1 mg/kg) administration of the drug
|
Parameters
|
Unit
|
Intra-Arterial
|
Oral
|
||||
|---|---|---|---|---|---|---|---|
| AP | PP | Geometric Mean Ratioa | AP | PP | Geometric Mean Ratio | ||
| C0/dose | ng/ml/μg | 0.36 ± 0.33 | 0.37 ± 0.16 | 0.81 (0.33–1.99) | N.A. | N.A. | N.A. |
| Cmax/dose | ng/ml/mg | N.A. | N.A. | N.A. | 3.52 ± 3.89 | 0.65 ± 0.20 | 3.47 (1.06–11.31)b |
| Tmax | h | N.A. | N.A. | N.A. | 0.25 ± 0.06 | 1.62 ± 0.47 | 0.16 (0.10–0.23)b |
| AUC0-∞/dose | h*ng/ml/mg | 0.05 ± 0.02 | 0.09 ± 0.02 | 0.56 (0.39–0.81)b | 3.19 ± 2.20 | 2.04 ± 0.96 | 1.40 (0.61–3.22) |
| CL | l/h | 21.1 ± 6.42 | 11.6 ± 2.25 | 1.77 (1.23–2.54)b | N.A. | N.A. | N.A. |
| CL | l/h/kg | 2.67 ± 0.76 | 1.62 ± 0.30 | 1.62 (1.15–2.27)b | N.A. | N.A. | N.A. |
| CL/F | l/h | N.A. | N.A. | N.A. | 452 ± 284 | 569 ± 232 | 0.71 (0.31–1.64) |
| CL/F | l/h/kg | N.A. | N.A. | N.A. | 58.6 ± 39.4 | 80.1 ± 33.4 | 0.65 (0.28–1.54) |
| Clf 1′OH MDZc | l/h | 3.13 ± 2.26 | 1.10 ± 0.60 | 2.53 (1.01–6.39)b | 68.3 ± 21.2 | 65.7 ± 15.0 | 1.02 (0.69–1.52) |
| Clf 1′OH MDZ | l/h/kg | 0.39 ± 0.29 | 0.15 ± 0.08 | 2.31 (0.98–5.44) | 8.66 ± 2.37 | 9.18 ± 2.11 | 0.93 (0.64–1.36) |
| fu | % | 3.65 ± 1.58 | 3.78 ± 0.72 | 0.90 (0.54–1.50) | 3.65 ± 1.58 | 3.78 ± 0.72 | 0.90 (0.54–1.50) |
| CLu | l/h | 611 ± 124 | 321 ± 107 | 1.96 (1.34–2.86)b | N.A. | N.A. | N.A. |
| CLu | l/h/kg | 78.0 ± 15.3 | 44.6 ± 13.5 | 1.79 (1.25–2.56)b | N.A. | N.A. | N.A. |
| CLu/F | l/h | N.A. | N.A. | N.A. | 14,243 ± 8937 | 14,737 ± 4627 | 0.79 (0.31–2.02) |
| CLu/F | l/h/kg | N.A. | N.A. | N.A. | 1860 ± 1302 | 2068 ± 683 | 0.72 (0.27–1.90) |
| Clf 1′OH MDZ unbound | l/h | 84.6 ± 40.7 | 32.2 ± 24.6 | 2.80 (1.13–6.92)b | 2248 ± 1219 | 1773 ± 450 | 1.13 (0.56–2.27) |
| Clf 1′OH MDZ unbound | l/h/kg | 10.6 ± 5.01 | 4.41 ± 3.14 | 2.56 (1.10–5.94)b | 286 ± 153 | 247 ± 57.8 | 1.03 (0.52–2.05) |
| Vss | liter | 17.6 ± 5.21 | 11.8 ± 4.52 | 1.55 (0.88–2.73) | N.A. | N.A. | N.A. |
| Vss | l/kg | 2.23 ± 0.61 | 1.63 ± 0.57 | 1.41 (0.85–2.35) | N.A. | N.A. | N.A. |
| F | % | N.A. | N.A. | N.A. | 7.15 ± 6.62 | 2.38 ± 1.31 | 2.48 (0.95–6.45) |
| t1/2 | h | 1.31 ± 0.36 | 1.17 ± 0.30 | 1.12 (0.78–1.56) | 1.74 ± 0.44 | 1.56 ± 0.44 | 1.14 (0.76–1.70) |
| Urinary recoveryd | % | 13.9 ± 6.88 | 8.93 ± 4.20 | 1.48 (0.74–2.99) | 18.6 ± 8.00 | 11.9 ± 4.55 | 1.51 (0.84–2.71) |
fu, percentage unbound in plasma; CLu, unbound plasma clearance; CLu/F, unbound oral plasma clearance; F, bioavailability; t1/2, terminal plasma half-life; N.A., not applicable
Geometric mean ratio was presented as the ratio of AP/PP with 95% confidence interval in parentheses
Significantly different antepartum versus postpartum
Clf 1′-OH MDZ was calculated as the ratio of the amount of MDZ excreted in the 24 h urine as 1′-OH MDZ over MDZ AUC0-∞
24-h urinary recovery of the dose as 1′-OH MDZ
After oral administration, MDZ pharmacokinetics were highly variable. MDZ was absorbed significantly faster during pregnancy (Tmax, 0.25 h) compared with postpartum (Tmax, 1.62 h), and the maximal plasma concentration (Cmax) achieved antepartum was significantly higher than that achieved postpartum (Fig. 1B; Table 1). However, the antepartum dose-normalized AUC0-∞ (n = 4) was not significantly different from that observed postpartum (n = 4) (Fig. 1B; Table 1). As a result, the total oral plasma clearance of MDZ in pregnant macaques was not significantly different from that observed postpartum. Likewise, formation clearance to 1′-OH MDZ was not statistically different antepartum versus postpartum. Bioavailability of MDZ was low in macaques both antepartum and postpartum and highly variable, especially in the pregnant macaques (Table 1). Terminal plasma half-life of MDZ was not significantly different antepartum versus postpartum.
Pharmacokinetics of DIG after Intra-Arterial and Oral Administration. After intra-arterial administration, the antepartum DIG AUC0-∞ (n = 3) was not different from that observed postpartum (n = 4) (Table 2; Fig. 2A). As a result, the DIG total plasma clearance was not affected by pregnancy. Likewise, renal clearance was not different between the two groups. DIG was mostly present in the plasma as the free drug (Table 2). The volume of distribution of DIG at steady state was large (>21 liters/kg) and not significantly different antepartum versus postpartum. Plasma terminal half-life (>20 h) was not significantly different between the two groups. The 72-h urinary recovery of DIG was approximately 35% of the dose and was unaffected by pregnancy.
TABLE 2.
AP and PP pharmacokinetic parameters (arithmetic mean ± S.D., n = 4) of DIG in M. nemestrina after intra-arterial (10 μg/kg) or oral (100 μg/kg) administration of the drug
|
Parameters
|
Unit
|
Intra-Arterial
|
Oral
|
||||
|---|---|---|---|---|---|---|---|
| APa | PP | Geometric Mean Ratiob | AP | PP | Geometric Mean Ratio | ||
| C0/dose | ng/ml/μg | 0.14 ± 0.12 | 0.12 ± 0.04 | 1.04 (0.65–1.46) | N.A. | N.A. | N.A. |
| Cmax/dose | pg/ml/μg | N.A. | N.A. | N.A. | 4.20 ± 1.43 | 3.70 ± 1.63 | 1.07 (0.82–1.48) |
| Tmax | h | N.A. | N.A. | N.A. | 1.75 ± 0.30 | 2.15 ± 2.59 | 0.89 (0.57–1.99) |
| AUC0-∞/dose | h*ng/ml/mg | 0.14 ± 0.05 | 0.17 ± 0.04 | 0.91 (0.74–1.12) | 0.14 ± 0.05 | 0.17 ± 0.04 | 1.09 (0.89–1.34) |
| CL | l/h | 7.53 ± 2.47 | 6.02 ± 1.46 | 1.09 (0.89–1.34) | N.A. | N.A. | N.A. |
| CL | l/h/kg | 0.93 ± 0.27 | 0.86 ± 0.21 | 1.03 (0.85–1.24) | N.A. | N.A. | N.A. |
| CL/F | l/h | N.A. | N.A. | N.A. | 10.5 ± 4.89 | 9.29 ± 0.67 | 1.02 (0.84–1.33) |
| CL/F | l/h/kg | N.A. | N.A. | N.A. | 1.28 ± 0.52 | 1.24 ± 0.08 | 0.99 (0.83–1.18) |
| Clrc | l/h | 2.75 ± 0.95 | 2.61 ± 1.23 | 1.04 (0.82–1.32) | 1.94 ± 1.32 | 2.25 ± 1.08 | 0.91 (0.64–1.70) |
| Clr | l/h/kg | 0.35 ± 0.15 | 0.37 ± 0.17 | 0.98 (0.75–1.28) | 0.23 ± 0.14 | 0.30 ± 0.16 | 0.88 (0.63–1.23) |
| fu | % | 79.1 ± 6.48 | 77.7 ± 4.91 | 0.98 (0.92–1.06) | 77.4 ± 4.64 | 73.4 ± 2.78 | 1.02 (0.99–1.05) |
| CLu | l/h | 9.38 ± 2.42 | 7.76 ± 1.92 | 1.00 (0.36–2.84) | N.A. | N.A. | N.A. |
| CLu | l/h/kg | 1.16 ± 0.27 | 1.11 ± 0.27 | 0.96 (0.82–1.14) | N.A. | N.A. | N.A. |
| CLu/F | l/h | N.A. | N.A. | N.A. | 13.5 ± 6.28 | 12.3 ± 0.89 | 1.00 (0.82–1.34) |
| CLu/F | l/h/kg | N.A. | N.A. | N.A. | 1.65 ± 0.67 | 1.69 ± 0.11 | 0.96 (0.81–1.15) |
| Clr unbound | l/h | 3.50 ± 1.23 | 3.40 ± 1.70 | 1.09 (0.76–1.58) | 2.50 ± 1.69 | 2.25 ± 1.08 | 0.89 (0.63–1.70) |
| Clr unbound | l/h/kg | 0.45 ± 0.19 | 0.49 ± 0.23 | 1.05 (0.58–1.87) | 0.32 ± 0.18 | 0.41 ± 0.20 | 0.85 (0.61–1.18) |
| Vss | l | 171 ± 44.3 | 177 ± 48.8 | 0.99 (0.84–1.16) | N.A. | N.A. | N.A. |
| Vss | l/kg | 21.6 ± 7.22 | 25.3 ± 6.36 | 0.93 (0.77–1.13) | N.A. | N.A. | N.A. |
| F | % | N.A. | N.A. | N.A. | 67.9 ± 29.1 | 65.8 ± 19.9 | 1.00 (0.81–1.39) |
| t1/2 | h | 22.4 ± 8.19 | 25.8 ± 7.53 | 0.93 (0.73–1.19) | 27.7 ± 5.27 | 32.5 ± 1.12 | 0.93 (0.84–1.17) |
| Urinary recoveryd | % | 37.3 ± 13.6 | 34.8 ± 8.97 | 1.02 (0.81–1.28) | 15.8 ± 13.2 | 20.9 ± 9.79 | 0.89 (0.67–1.54) |
N.A., not applicable; fu, percentage of unbound in plasma; CLu, unbound plasma clearance; CLu/F, unbound oral plasma clearance; F, bioavailability; t1/2, terminal plasma half-life
Intra-arterial DIG PK study was performed in three instead of four pregnant macaques (n = 3)
Geometric mean ratio was presented as the ratio of AP/PP with 95% confidence interval in parentheses
Clr, renal clearance was calculated as the ratio of the amount of DIG excreted in the urine in 72 h over AUC0–72 h
72-h urinary recovery of the dose
Fig. 2.
The mean (± S.D.) DIG dose-normalized plasma concentration-time profile in M. nemestrina is not significantly different AP (n = 4, ○) from that obtained PP (n = 4, •) after either intra-arterial (10 μg/kg) (A) or oral (100 μg/kg) (B) administration of the drug. For intra-arterial study, only three pregnant macaques were studied (AP, n = 3).
After oral administration, the pharmacokinetics of DIG, including the renal clearance, were not affected by pregnancy (Table 2; Fig. 2B). The bioavailability of DIG (∼65%) was also not significantly altered by pregnancy.
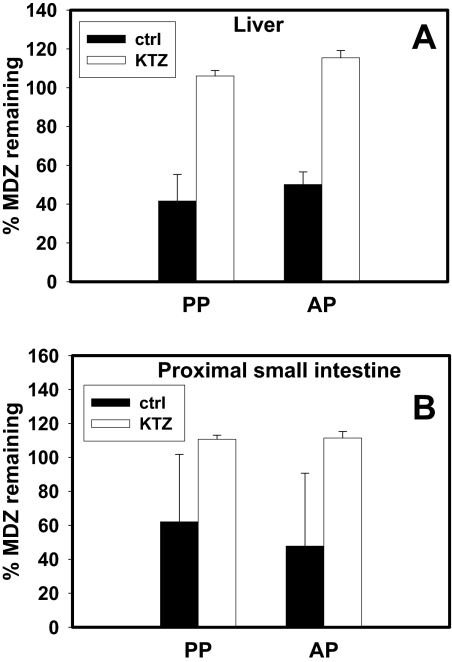
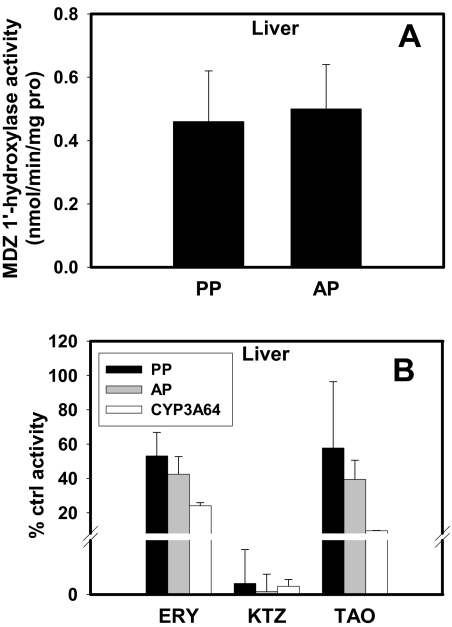
MDZ Metabolism in Hepatic and Intestinal S-9 Fractions. The rate of depletion of MDZ in hepatic or intestinal S-9 fractions of tissues obtained from both antepartum and postpartum animals was similar (Fig. 3). KTZ (0.5 μM) completely inhibited this depletion (Fig. 3, A and B). Likewise, MDZ 1′-hydroxylase activity in hepatic S-9 fraction was similar antepartum versus postpartum (Fig. 4A). KTZ (0.5 μM) almost completely inhibited this activity in hepatic S-9 fractions and in expressed CYP3A64 enzymes, whereas ERY (0.5 mM) and TAO (100 μM) inhibited this activity to a lesser extent (Fig. 4B).
Fig. 3.
No significant difference in MDZ depletion was observed AP (n = 6) and PP (n = 6) in hepatic (A) or proximal small-intestinal S-9 fractions (B; n = 4, AP or PP). This depletion was inhibited by 0.5 μM KTZ in both tissues (A and B). The percentage of MDZ remaining was compared with control incubations in the absence of inhibitor at 0 min.
Fig. 4.
A, no significant difference in 1′-OH MDZ formation was observed in hepatic S-9 fractions obtained AP (n = 6) and PP (n = 6). B, formation of 1′-OH MDZ was inhibited by 0.5 μM KTZ, 0.5 mM ERY, and 0.1 mM TAO in hepatic S-9 fractions and expressed CYP3A64 enzyme.
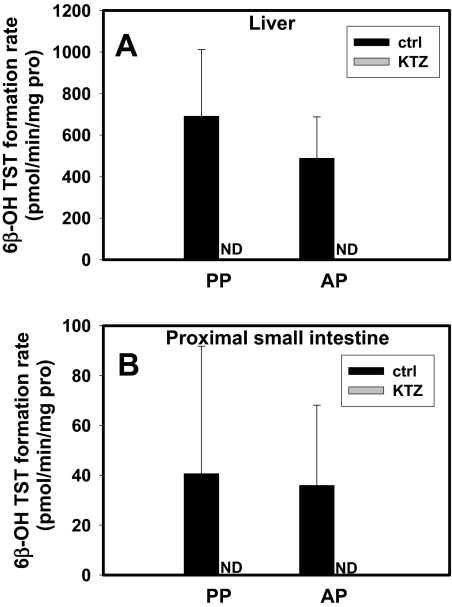
6β-OH Testosterone Formation in Hepatic and Intestinal S-9 Fractions. TST 6β-hydroxylation in hepatic and intestinal S-9 fractions obtained postpartum and antepartum was not significantly different (Fig. 5). KTZ (0.5 μM) abolished 6β-OH TST formation in these fractions (Fig. 5).
Fig. 5.
No significant difference in TST 6β-hydroxylation rate was observed AP (n = 6) and PP (n = 6) in hepatic (A) or proximal small-intestinal S-9 fractions (B; n = 4, AP or PP); 0.5 μM KTZ completely inhibited 6β-OH TST formation in both tissues (A and B). ND, not detected.
CYP3A and P-gp Expression in Macaque Liver and Small Intestine. With the use of human CYP3A4 antibody, two bands were detected in the Western blots of the macaque hepatic and intestinal tissue (data not shown). Because the lower band showed a molecular mass of approximately 42 kDa, quantification of only the top band (molecular mass ∼50 kDa) was conducted to represent the expression level of CYP3A. Hepatic or small-intestinal CYP3A protein expression was unaffected by pregnancy (Fig. 6, A and B). Likewise, pregnancy did not change hepatic P-gp protein expression (Fig. 6G).
Fig. 6.
Expression of CYP3A protein in hepatic (A) or intestinal (B) S-9 fractions was not significantly different AP versus PP. The hepatic expression of CYP3A64 transcript (C) was affected by pregnancy, whereas that in the proximal small intestine (D) was not. Expression of CYP3A66 transcript in the liver (E) or the proximal small intestine (F) was not affected by pregnancy. P-gp protein expression in macaque hepatic homogenates (G), transcript expression in the liver (H), or proximal small intestine (I) was not significantly different AP versus PP. The data are shown as mean ± S.D. of n = 4 for proximal intestine samples. Except for hepatic P-gp protein expression where n = 4, all other hepatic samples were n = 6. ** p < 0.01.
The sequence(s) of M. nemestrina CYP3A isoform(s) are currently unknown. Therefore, to determine the expression of CYP3A transcripts, we used the commercially available rhesus macaque CYP3A64 and CYP3A66 primers and probes. Pregnancy had an isoform- and tissue-specific effect on M. nemestrina CYP3A transcript expression. The hepatic expression of the CYP3A64-probed gene during pregnancy was lower (by ∼50%) than that observed postpartum (Fig. 6C), whereas its expression in the small intestine was unaffected by pregnancy (Fig. 6D). In contrast, the expression of CYP3A66-probed gene was not altered during pregnancy in the hepatic or the small-intestinal tissue (Fig. 6, E and F). Likewise, MDR1 transcript expression in the liver or small intestine was unaffected by pregnancy (Fig. 6, H and I). Based on the absolute Ct values, the rank order of the abundance of these genes in the liver was CYP3A64 > CYP3A66 > MDR1. In addition, by comparing the absolute Ct values, the CYP3A64 ortholog was expressed much more abundantly in the liver than in the proximal small intestine, whereas CYP3A66 and MDR1 orthologs were expressed at a similar level in both tissues (data not shown).
Discussion
Two major isoforms of CYP3A, CYP3A4 and CYP3A5, occur in human adults. However, the number of CYP3A isoforms present in the macaques is not clear. So far, three macaque CYP3A isoforms, CYP3A64 and CYP3A66 (rhesus macaque or M. mulatta) and CYP3A8 (cynomolgus macaque or M. fascicularis), have been identified (Komori et al., 1992). Of these, CYP3A64 is the best characterized. It exhibits 93% amino acid sequence identity to human CYP3A4 and 100% identity to CYP3A8 in the cynomolgus macaque (Carr et al., 2006). In vitro and in vivo studies strongly support the notion that, as in humans, MDZ is an excellent probe to determine in vivo CYP3A activity in the rhesus macaque (Heizmann and Ziegler, 1981; Kronbach et al., 1989; Kanazu et al., 2004; Carr et al., 2006; Prueksaritanont et al., 2006; Ogasawara et al., 2007). Because of the global genetic similarity between the Old-World macaques (e.g., pig-tailed and rhesus macaques) (Magness et al., 2005), and because the CYP enzymes of M. nemestrina have not been cloned, we assumed that MDZ is a selective in vivo substrate of the M. nemestrina CYP3A enzymes.
After intra-arterial administration, both systemic clearance of MDZ and formation clearance to 1′-OH MDZ were significantly increased by pregnancy (Table 1). This increase was not the results of changes in plasma protein binding of the drug (Table 1). Because very little MDZ was excreted unchanged in the urine (<1%, data not shown), we assumed that MDZ was cleared from the body primarily by hepatic metabolism. Based on the mean MDZ plasma clearance of 1.62 l/h/kg, the MDZ blood/plasma partition ratio of 0.6 (Prueksaritanont et al., 2006), and reported hepatic blood flow of 3.1 l/h/kg for the Old-World macaques (Boxenbaum 1980), the Fh of MDZ postpartum was calculated to be 0.13. This value is close to the value (0.16) reported in rhesus macaques (Prueksaritanont et al., 2006), indicating similar high hepatic CYP3A activities in the two macaque species.
In contrast to IA administration of MDZ, we found no effect of pregnancy on the oral clearance of MDZ or the formation clearance to 1′OH-MDZ (Table 1). Furthermore, the bioavailability (F) of MDZ was not significantly affected by pregnancy (Table 1). This bioavailability was much lower in the nonpregnant macaques (∼2%; Table 1) than in humans (∼24–36%) (Smith et al., 1981; Thummel et al., 1996). This was probably caused by high hepatic and intestinal extraction of the drug during the first pass in the macaques. Based on these data and assuming that MDZ was completely absorbed from the intestine (Fa = 1), the intestinal availability (Fg) in the nonpregnant macaques was estimated to be 0.15, indicating substantial intestinal metabolism.
The high MDZ clearance and hepatic extraction in the macaque suggests that the higher systemic clearance of MDZ during macaque pregnancy may be because of an increase in hepatic blood flow rather than an increase in intrinsic hepatic metabolism. Hepatic blood flow has been reported to be increased during human pregnancy (Nakai et al., 2002). This conclusion is consistent with our observation that the oral clearance of MDZ was not affected by pregnancy. If the intrinsic hepatic metabolism of MDZ were increased by pregnancy, this would have been apparent from the oral clearance of the drug. Based on fundamental pharmacokinetic principles, the oral clearance of a drug, even when it is highly extracted by the liver, is determined by the intrinsic metabolic clearance of the drug. However, the oral clearance of the drug was not increased during pregnancy suggesting that the hepatic metabolism of the drug was unaffected by pregnancy.
To confirm this conclusion, we measured the metabolism of MDZ in S-9 fractions of hepatic and small-intestinal tissues obtained from the animals that participated in the in vivo MDZ and DIG studies and from two additional animals. As expected, neither the rate of MDZ depletion (Fig. 3) nor the rate of 1′-OH MDZ formation (Fig. 4) in hepatic or small intestinal S-9 fractions was altered by pregnancy. Furthermore, we measured the testosterone 6β-hydroxylase activity (another measurement of CYP3A activity) in these S-9 fractions and the ability of ketoconazole, a selective human CYP3A inhibitor, to inhibit this metabolism and that of MDZ. As expected, testosterone 6β-hydroxylase activity in hepatic or intestinal S-9 fractions was not significantly altered by pregnancy (Fig. 5). Moreover, the metabolism of both testosterone and MDZ in S-9 fractions was completely inhibited by 0.5 μM ketoconazole. These data suggest that MDZ and testosterone metabolism reported macaque CYP3A activity and that this hepatic or intestinal activity was unchanged by pregnancy. Arguing against the former conclusion was the lack of significant correlation between MDZ 1′-hydroxylation and TST 6β-hydroxylation (PP, r2 = 0.16; AP, r2 = 0.43). Thus, it is possible that MDZ and TST are metabolized by different CYP enzymes or different CYP3A isoforms in the macaques. Nevertheless, neither MDZ 1′-hydroxylase nor TST 6β-hydroxylase activity was affected by pregnancy. The conclusion that pregnancy does not have an effect on CYP3A activity was supported by our measurement of CYP3A expression in these tissues by Western blot analysis and real-time PCR. CYP3A protein expression in the hepatic or intestinal tissues was unaffected by pregnancy (Fig. 6). The hepatic transcript expression of CYP3A64 ortholog was down-regulated by pregnancy (Fig. 6C), but this did not translate into changes in the expression of the protein or its activity.
The above-mentioned in vivo data on oral clearance of MDZ are in contrast to the data in pregnant women. In this population, we found that the oral clearance of MDZ is significantly increased during pregnancy (Hebert et al., 2008), suggesting that hepatic and/or intestinal CYP3A activity is increased during pregnancy. This conclusion is supported by other data in the literature that also suggest increased CYP3A activity during human pregnancy (Rey et al., 1979; Ohkita and Goto, 1990; Kosel et al., 2003; Tracy et al., 2005). The discrepancy between humans and macaques may be due to differences in the intrinsic rate of MDZ metabolism in the two species. In humans, MDZ is a low- to moderate-extraction drug with a hepatic extraction ratio of 0.35 (Thummel et al., 1996), but in macaques, this extraction ratio is much higher (0.87). The difference in the hepatic extraction ratio of MDZ between humans and macaques suggests that human hepatic CYP3A activity is lower than that in the macaque, although in humans, the lower CYP3A activity may be inducible by pregnancy-related factors, as suggested by our studies in CYP3A4-promoter-luciferase transgenic mice. If the baseline CYP3A enzyme expression/activity in the macaques is already very high, it may not be inducible by pregnancy-related factors (Zhang et al., 2008).
Compared with macaque CYP3A enzymes, even less is known about the macaque P-gp. Macaque P-gp shows 96% amino acid sequence identity to the human homolog and similar binding affinity toward common substrates of human P-gp such as DIG (Xia et al., 2006). Based on these data, we assumed that DIG, a well accepted in vivo probe of human P-gp activity, will serve as an in vivo probe of macaque P-gp activity. In contrast to MDZ, pregnancy did not affect the oral or IA pharmacokinetics of DIG, including its renal clearance. The systemic plasma clearance of DIG (Table 2) was comparable with that in cynomolgus macaques (0.49 ± 0.07 l/h/kg) (Manwaring et al., 2003) but was approximately 7-fold higher than that in humans (0.12 ± 0.06 l/h/kg) (Manwaring et al., 2003). The observed renal clearance of DIG (≈0.3 l/h/kg) after oral and IA administration was higher than the creatinine clearance (0.2 l/h/kg) in macaques (Boxenbaum, 1980), suggesting that DIG is actively secreted by the kidneys. Although the bioavailability (>65%) and plasma elimination half-life (>27 h) of DIG in the macaques were similar to that in humans, the percentage of IA dose excreted unchanged in the urine was quite different, approximately 35% in macaques versus 60% in humans (Manwaring et al., 2003). This suggests that, in the macaques, a larger fraction of the DIG dose is excreted in the feces or metabolized than that in humans. These data suggest that P-gp activity in the macaque (intestinal/hepatic or renal) was unaffected by pregnancy. In contrast, renal P-gp activity (as measured by net renal secretion of DIG) was increased in pregnant women (Hebert et al., 2008).
Based on these data, and assuming that MDZ and DIG are selective in vivo probes of macaque CYP3A and P-gp activity, respectively, we conclude that these activities are not affected by macaque pregnancy. This conclusion needs to be reconciled with our previous observation that systemic clearance of nelfinavir was increased, but its bioavailability was decreased by macaque pregnancy (Zhang et al., 2009). Moreover, NFV metabolism in hepatic (but not intestinal) S-9 fractions was enhanced by pregnancy, and was completely inhibited by ketoconazole, a selective inhibitor of human CYP3A enzymes. Based on these data, we hypothesized that NFV clearance in pregnant macaques was accelerated by increased hepatic CYP3A activity. However, the in vivo and in vitro data presented here do not support this hypothesis. It is important to note here that the in vivo MDZ and DIG studies were conducted in the same animals, during the same pregnancy, as the NFV in vivo studies. There are several possible explanations for this discrepancy. First, it is possible that nelfinavir is metabolized in the macaque by enzyme(s) other than CYP3A and that the activity of this enzyme(s) is enhanced by pregnancy and inhibited by ketoconazole. Alternatively, MDZ is not a selective substrate of macaque CYP3A enzymes and therefore does not faithfully report hepatic or intestinal CYP3A activity. Collectively, these data suggest that considerably more research needs to be conducted on macaque CYPs to determine their selective substrates and inhibitors. Until such studies are conducted, phenotyping studies in the macaques using selective substrates of human CYPs will be inconclusive. In addition, without these studies, it will be difficult to determine whether the activity of macaque CYPs is affected by pregnancy and the mechanistic basis of this phenomenon. In this regard, it should be noted here that the mouse may be a better model of the changes in human CYP3A activity than the macaques. As we have reported previously, the activity of mouse Cyp3a enzyme(s) is elevated by pregnancy, and this increase seems to be due to transcriptional up-regulation of selective hepatic Cyp3a isoforms. The pregnancy-related factors in the mouse that cause this transcriptional up-regulation, also transcriptionally up-regulate the human CYP3A4 promoter in the transgenic mouse expressing the human CYP3A4-promoter-luciferase transgene (CYP3A4-tg) (Zhang et al., 2008). Interestingly, this effect of pregnancy is observed only in the liver and not in the intestine. Therefore, the CYP3A4-tg mouse should be studied in more detail to determine the molecular mechanisms by which pregnancy up-regulates CYP3A activity in pregnant women.
Acknowledgments
We thank Keith Vogel, Mike Gough, and Carol Elliot for invaluable technical assistance in performing animal studies.
This work was supported by the National Institutes of Health [Grant P50-HD044404].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.153569.
ABBREVIATIONS: PI, protease inhibitor; NFV, nelfinavir; CYP3A, cytochrome P450 3A; P-gp, P-glycoprotein; MDZ, midazolam; DIG, digoxin; TST, testosterone; ERY, erythromycin; KTZ, ketoconazole; TAO, troleandomycin; ctrl, control; PP, postpartum; AP, antepartum; LC-MS, liquid chromatography-mass spectrometry; UPLC-MS/MS, ultra-performance liquid chromatography-mass spectrometry-mass spectrometry; PCR, polymerase chain reaction; MDR1, multidrug resistance gene.
References
- Acosta EP, Zorrilla C, Van Dyke R, Bardeguez A, Smith E, Hughes M, Huang S, Pitt J, Watts H, and Mofenson L (2001) Pharmacokinetics of saquinavir-SGC in HIV infected pregnant women. HIV Clin Trials 2 460-465. [DOI] [PubMed] [Google Scholar]
- Angel JB, Khaliq Y, Monpetit ML, Cameron DW, and Gallicano K (2001) An argument for routine therapeutic drug monitoring of HIV-1 protease inhibitors during pregnancy. AIDS 15 417-419. [DOI] [PubMed] [Google Scholar]
- Back D, Gatti G, Fletcher C, Garaffo R, Haubrich R, Hoetelmans R, Kurowski M, Luber A, Merry C, and Perno CF (2002) Therapeutic drug monitoring in HIV infection: current status and future directions. AIDS 16 S5-S37. [DOI] [PubMed] [Google Scholar]
- Boxenbaum H (1980) Interspecies variation in liver weight, hepatic blood flow, and antipyrine intrinsic clearance: extrapolation of data to benzodiazepines and phenytoin. J Pharmacokinet Biopharm 8 165-176. [DOI] [PubMed] [Google Scholar]
- Carr B, Norcross R, Fang Y, Lu P, Rodrigues AD, Shou M, Rushmore T, and Booth-Genthe C (2006) Characterization of the rhesus monkey CYP3A64 enzyme: species comparisons of CYP3A substrate specificity and kinetics using baculovirus-expressed recombinant enzymes. Drug Metab Dispos 34 1703-1712. [DOI] [PubMed] [Google Scholar]
- Hammer SM, Saag MS, Schechter M, Montaner JS, Schooley RT, Jacobsen DM, Thompson MA, Carpenter CC, Fischl MA, Gazzard BG, et al. (2006) Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-U S A Panel. JAMA 296 827-843. [DOI] [PubMed] [Google Scholar]
- Hebert MF, Easterling TR, Kirby B, Carr DB, Buchanan ML, Rutherford T, Thummel KE, Fishbein DP, and Unadkat JD (2008) Effects of pregnancy on CYP3A and P-glycoprotein activities as measured by disposition of midazolam and digoxin: a University of Washington Specialized Center of Research Study. Clin Pharmacol Ther 84 248-253. [DOI] [PubMed] [Google Scholar]
- Heizmann P and Ziegler WH (1981) Excretion and metabolism of 14C-midazolam in humans following oral dosing. Arzneimittelforschung 31 2220-2223. [PubMed] [Google Scholar]
- Kanazu T, Yamaguchi Y, Okamura N, Baba T, and Koike M (2004) Model for the drug-drug interaction responsible for CYP3A enzyme inhibition. I: evaluation of cynomolgus monkeys as surrogates for humans. Xenobiotica 34 391-402. [DOI] [PubMed] [Google Scholar]
- Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM, and Wilkinson GR (1998) The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest 101 289-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby B, Kharasch ED, Thummel KT, Narang VS, Hoffer CJ, and Unadkat JD (2006) Simultaneous measurement of in vivo P-glycoprotein and cytochrome P450 3A activities. J Clin Pharmacol 46 1313-1319. [DOI] [PubMed] [Google Scholar]
- Kirby BJ, Kalhorn T, Hebert M, Easterling T, and Unadkat JD (2008) Sensitive and specific LC-MS assay for quantification of digoxin in human plasma and urine. Biomed Chromatogr 22 712-718. [DOI] [PubMed] [Google Scholar]
- Komori M, Kikuchi O, Sakuma T, Funaki J, Kitada M, and Kamataki T (1992) Molecular cloning of monkey liver cytochrome P-450 cDNAs: similarity of the primary sequences to human cytochromes P-450. Biochim Biophys Acta 1171 141-146. [DOI] [PubMed] [Google Scholar]
- Kosel BW, Beckerman KP, Hayashi S, Homma M, and Aweeka FT (2003) Pharmacokinetics of nelfinavir and indinavir in HIV-1-infected pregnant women. AIDS 17 1195-1199. [DOI] [PubMed] [Google Scholar]
- Kronbach T, Mathys D, Umeno M, Gonzalez FJ, and Meyer UA (1989) Oxidation of midazolam and triazolam by human liver cytochrome P450IIIA4. Mol Pharmacol 36 89-96. [PubMed] [Google Scholar]
- Magness CL, Fellin PC, Thomas MJ, Korth MJ, Agy MB, Proll SC, Fitzgibbon M, Scherer CA, Miner DG, Katze MG, et al. (2005) Analysis of the Macaca mulatta transcriptome and the sequence divergence between Macaca and human. Genome Biol 6 R60-R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias AA, Maggio-Price L, Lai Y, Gupta A, and Unadkat JD (2006) Changes in pharmacokinetics of anti-HIV protease inhibitors during pregnancy: the role of CYP3A and P-glycoprotein. J Pharmacol Exp Ther 316 1202-1209. [DOI] [PubMed] [Google Scholar]
- Manwaring JD, Baker SJ, Karle JA, and Patel VS (2003) Pharmacokinetics of digoxin following oral (tablet and capsule) and intravenous administration in cynomolgus monkeys [abstract]. In: 17th Annual American Association of Pharmaceutical Scientists Meeting and Exposition; 2003 Oct 26–30; Salt Lake City, UT. p. A8260, American Association of Pharmaceutical Scientists, Arlington, VA.
- Nakai A, Sekiya I, Oya A, Koshino T, and Araki T (2002) Assessment of the hepatic arterial and portal venous blood flows during pregnancy with Doppler ultrasonography. Arch Gynecol Obstet 266 25-29. [DOI] [PubMed] [Google Scholar]
- Nellen JF, Schillevoort I, Wit FW, Bergshoeff AS, Godfried MH, Boer K, Lange JM, Burger DM, and Prins JM (2004) Nelfinavir plasma concentrations are low during pregnancy. Clin Infect Dis 39 736-740. [DOI] [PubMed] [Google Scholar]
- Ogasawara A, Kume T, and Kazama E (2007) Effect of oral ketoconazole on intestinal first-pass effect of midazolam and fexofenadine in cynomolgus monkeys. Drug Metab Dispos 35 410-418. [DOI] [PubMed] [Google Scholar]
- Ohkita C and Goto M (1990) Increased 6-hydroxycortisol excretion in pregnant women: implication of drug-metabolizing enzyme induction. DICP 24 814-816. [DOI] [PubMed] [Google Scholar]
- Prueksaritanont T, Kuo Y, Tang C, Li C, Qiu Y, Lu B, Strong-Basalyga K, Richards K, Carr B, and Lin JH (2006) In vitro and in vivo CYP3A64 induction and inhibition studies in rhesus monkeys: a preclinical approach for CYP3A-mediated drug interaction studies. Drug Metab Dispos 34 1546-1555. [DOI] [PubMed] [Google Scholar]
- Rey E, d'Athis P, Giraux P, de Lauture D, Turquais JM, Chavinie J, and Olive G (1979) Pharmacokinetics of clorazepate in pregnant and non-pregnant women. Eur J Clin Pharmacol 15 175-180. [DOI] [PubMed] [Google Scholar]
- Smith MT, Eadie MJ, and Brophy TO (1981) The pharmacokinetics of midazolam in man. Eur J Clin Pharmacol 19 271-278. [DOI] [PubMed] [Google Scholar]
- Thummel KE, O'Shea D, Paine MF, Shen DD, Kunze KL, Perkins JD, and Wilkinson GR (1996) Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther 59 491-502. [DOI] [PubMed] [Google Scholar]
- Tracy TS, Venkataramanan R, Glover DD, and Caritis SN (2005) Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A activity) during pregnancy. Am J Obstet Gynecol 192 633-639. [DOI] [PubMed] [Google Scholar]
- Unadkat J and Wang Y (2000) Antivirals, in Metabolic Drug Interactions (Levy RH, Thummel KE, Trager WF, Hansten PD, and Eichelbaum M eds) pp 421-433, Lippincott Williams & Wilkins: Philadelphia.
- Unadkat JD, Wara DW, Hughes MD, Mathias AA, Holland DT, Paul ME, Connor J, Huang S, Nguyen BY, Watts DH, et al. (2007) Pharmacokinetics and safety of indinavir in human immunodeficiency virus-infected pregnant women. Antimicrob Agents Chemother 51 783-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heeswijk RP, Khaliq Y, Gallicano KD, Bourbeau M, Seguin I, Phillips EJ, and Cameron DW (2004) The pharmacokinetics of nelfinavir and M8 during pregnancy and post partum. Clin Pharmacol Ther 76 588-597. [DOI] [PubMed] [Google Scholar]
- Xia CQ, Xiao G, Liu N, Pimprale S, Fox L, Patten CJ, Crespi CL, Miwa G, and Gan LS (2006) Comparison of species differences of P-glycoproteins in beagle dog, rhesus monkey, and human using Atpase activity assays. Mol Pharm 3 78-86. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wu X, Wang H, Mikheev AM, Mao Q, and Unadkat JD (2008) Effect of gestational age on cytochrome P450 3a and P-glycoprotein expression and activity in the mouse: mechanisms, tissue specificity, and time course. Mol Pharmacol 74 714-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wu X, Chung F, Naraharisetti SB, Whittington D, Mirfazaelian A, and Unadkat JD (2009) As in humans, pregnancy increases the clearance of the protease inhibitor, nelfinavir, in the non-human primate, M. nemestrina. J Pharmacol Exp Ther 329 1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang KE, Wu E, Patick AK, Kerr B, Zorbas M, Lankford A, Kobayashi T, Maeda Y, Shetty B, and Webber S (2001) Circulating metabolites of the human immunodeficiency virus protease inhibitor nelfinavir in humans: structural identification, levels in plasma and antiviral activities. Antimicrob Agents Chemother 45 1086-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]