Abstract
Cryoablation of a solitary tumor mass releases intact tumor antigens and can induce protective antitumor immunity but has limited efficacy in the treatment of established metastatic cancer. Cyclophosphamide (Cy), an anticancer drug, selectively depletes regulatory T cells (Tregs) and attenuates suppression of antitumor immunity. We used a BALB/c mouse model of metastatic colon cancer to investigate the systemic antitumor effects of in situ cryotherapy alone or in combination with 200 mg/kg i.p. Cy. When combined with Cy, cryoablation was significantly more effective than either surgical excision or cautery at inducing systemic antitumor immunity, resulting in the cure of a fraction of animals with established metastatic disease and resistance to tumor rechallenge. Lymphocytes from cured animals contained an expanded population of tumor-specific, interferon-γ producing T cells and transferred antitumor immunity to naive recipients. Depletion of CD8+ cells significantly impaired the adoptive transfer of antitumor immunity. Furthermore, treatment with Cy and cryoablation was associated with a significant decrease in the ratio of regulatory to effector CD4+ T cells. The combination of tumor cryoablation and Cy induces potent, systemic antitumor immunity in animals with established metastatic disease.
Cryoablation, or tissue destruction by freezing, is a widely used treatment of localized cancer. The procedure induces tumor cell death directly by damage to cell membranes and organelles through formation of ice crystals and indirectly by causing vascular compromise through thrombosis of small vessels (Fraser and Gill, 1967; Whittaker, 1984; Hoffmann and Bischof, 2002). Cryoablation is used as an alternative to surgical resection in the treatment of cancers of the breast, prostate, kidney, liver, and skin (Korpan, 2007). With the advent of third generation units and a decrease in the complications associated with cryoablation, this modality has become even more popular.
In addition to its effects on localized cancers, anecdotal reports from the clinic demonstrate that cryoablation can induce a systemic antitumor immune response resulting in regression of metastatic disease. The immunological effects of cryosurgery were first documented by Shulman and co-workers (Yantorno et al., 1967; Shulman et al., 1968) when they demonstrated the production of antibodies against rabbit male accessory tissues after freezing. Ablin and co-workers observed regression of metastatic lesions in prostate cancer patients whose primary tumors were treated with cryoablation (Soanes et al., 1970) and coined the term “cryo-immunotherapy” (Ablin, 1972). Further studies in animals confirmed the development of an antitumor response when cryotherapy was used for destruction of neoplasms (Tanaka, 1982; Sabel et al., 2005, 2006). It is postulated that the freezing of cells disrupts cell membranes and releases intact tumor antigens, which are captured by antigen-presenting cells for presentation to antitumor lymphocytes in tumor-draining lymph nodes (den Brok et al., 2006). Multiple studies have also demonstrated that cryoablation induces a vigorous inflammatory response to the necrotic tissue that is produced during the procedure (Sabel, 2009). This cell necrosis can be a potent stimulus for an adaptive (T cell-mediated) immune response through liberation of endogenous tumor antigens and “danger signals.” Taken together, these studies suggest that cryoablation of cancer tissue can potentially generate a clinically meaningful antitumor immune response.
The ability of the tumor-bearing host to mount an immune response to tumor antigens is down-regulated by regulatory T cells (Tregs), a subset of CD4+ T cells. These cells express the interleukin 2 receptor α chain (CD25) and the forkhead box P3, or Foxp3, transcription factor. These cells have a potent ability to suppress immunity by inhibiting both cytotoxic T lymphocytes and natural killer (NK) cells and are thought to play a role in tolerance to tumor and self-antigens (Smyth et al., 2006; Wang and Wang, 2007). When Treg number or function was reduced in experimental models, a surge in antitumor response was shown (Onizuka et al., 1999; Golgher et al., 2002; den Brok et al., 2006; Imai et al., 2007). Cyclophosphamide (Cy), an alkylating agent used to treat cancer, has been shown to mitigate suppression of antitumor immunity through effects on Tregs (Ghiringhelli et al., 2004; Lutsiak et al., 2005; Brode and Cooke, 2008).
In this study, we use a mouse model of metastatic cancer to investigate the systemic antitumor effects of in situ cryotherapy with or without cyclophosphamide, with an aim to devise a clinical strategy to induce the regression of metastatic disease. To establish the efficacy of the cryoimmunologic response, we have compared cryoablation with surgery and high-temperature ablation in our tumor model.
Materials and Methods
Animals. Female BALB/c mice were purchased from Harlan (Indianapolis, IN) and kept under pathogen-free conditions at the Johns Hopkins animal care facility. All experiments were conducted on protocols approved by the institutional animal care and use committee. Six- to 12-week-old mice were used for the ablation and passive transfer experiments.
Tumor Cell Line. The mouse colon cancer cell line CT26 was obtained from the American Type Culture Collection (Manassas, VA). Cells were maintained in RPMI 1640 supplemented with heat-inactivated fetal bovine serum [10% (v/v)] at 37°C and 5% CO2. The cells were allowed to grow to 80 to 90% confluence and split at 1:10 dilution.
Tumor Model. Tumor cells were reconstituted with phosphate-buffered saline to a concentration of 1 × 107 cells/ml for subcutaneous injection and 2 × 105 cells/ml for intravenous injection. Mice were injected with 1 × 106 cells into the right flank for subcutaneous tumor. At the same time, 1 × 105 tumor cells were injected intravenously via the tail vein for establishment of metastatic tumor. Animals were monitored daily for the establishment of subcutaneous tumor. On day 14 after tumor implantation, the tumor was cryoablated, surgically resected, or thermally ablated.
Cy. Animals were given 200 mg/kg Cy in an injection of 1 ml or an equivalent volume of saline intraperitoneally on the day before the cryoablation or surgery.
Cryoablation. Animals were anesthetized with intraperitoneal ketamine and xylazine. The flank was shaved, and the skin overlying the tumor was disinfected with alcohol. The tumor was lifted up from the muscle layer and clamped underneath with a hemostat. An incision was made on the tumor site, and the cryoprobe was inserted. The tumor was frozen until ice ball formation was observed all around the tumor (approximately 30–80 s depending on the size of the tumor) using the CRYOcare Cryo-20 argon/helium research unit (Endocare, Irvine, CA). The hemostat was removed, and the animal was kept under a heat lamp for 2 to 5 min to prevent hypothermia from the cryotherapy.
Surgery. The animals were prepared similarly for surgery as outlined above. A skin incision was made over the tumor site, and the tumor was resected using a scalpel. The skin was stapled, and an antiseptic was applied.
Tumor Electrocautery. The animals were anesthetized and shaved both at the tumor site and the contralateral flank. The animals were placed with the contralateral flank in contact with conducting pad. The tumor area was disinfected, a tiny incision was made, and the tip of the electrocautery unit was pushed into the tumor tissue. The tumor was coagulated.
Tumor Rechallenge. At day 150, surviving animals and naive 6- to 8-week-old BALB/c mice were injected with 2 × 105 CT26 cells intravenously via tail vein at a concentration of 4 × 105 cells/ml. This was twice the amount we have demonstrated in previous experiments to cause 100% mortality because of metastatic tumor.
Adoptive Transfer of Antitumor Immunity. Spleen, axillary, and inguinal lymph node cells used in adoptive transfer experiments were taken from CT26-bearing BALB/c mice that were treated with Cy and cryotherapy and survived 125 to 150 days after tumor inoculation. This time interval was chosen because no animals succumbed to tumor past day 125. Single-cell suspensions were prepared using a Falcon Strainer (40 μm), and graded doses from 105 to 107 were mixed with 2 × 105 CT26 cells and injected intravenously in a volume of 0.5 ml into naive 6- to 8-week-old BALB/c mice.
Lymphocyte Depletions. T cells were depleted from a mixture of donor splenocytes and lymph node cells by incubation of the cell suspension with antibodies to CD4 (RL172.4; gift of Dr. Albert Bendelac, University of Chicago, Chicago, IL) and/or CD8 (3.155; American Type Culture Collection) and guinea pig complement (Invitrogen, Carlsbad, CA) at 37°C. Depletion of >98% of the relevant T cell subset(s) was confirmed by flow cytometry. Ten million spleen and lymph node cells, either untreated or depleted of CD4+ cells, CD8+ cells, or both, were mixed with 2 × 105 CT26 cells and injected intravenously in a volume of 0.5 ml into naive BALB/c mice.
Flow Cytometry Analysis. Spleen and tumor-draining lymph nodes were harvested from animals 10 days after tumor removal by surgery or cryoablation. One hundred microliters of prepared cells (1 × 106 cells) was used for each analysis. Cells were first incubated for surface staining with antimouse antibodies for CD25 (allophycocyanin anti-mouse CD25 (interleukin-2 receptor α, p55; eBioscience, San Diego, CA) and CD4 [fluorescein isothiocyanate anti-mouse CD4 (L3T4); eBioscience]. After appropriate washing steps, intracellular staining for FoxP3 was done by incubating the cells with antimouse antibody for FoxP3 (phycoerythrin anti-mouse/rat Foxp3; eBioscience). Cell sorting was then performed using the BD FACS Calibur system (BD Biosciences, San Jose, CA). Tregs were defined as CD4+, FoxP3+, and CD25+ cells, whereas the effector cells were defined as FoxP3-negative and CD25+ cells.
Intracellular Cytokine Staining for Interferon-γ. For intracellular interferon (IFN)-γ staining, 1 × 106 splenocytes were stimulated by incubating with AH1 (10 μg), the immunodominant H-2Ld-restricted tumor peptide present on CT26 cells for 6 h at 37°C. The cells were then stained with antimouse IFN-γ (phycoerythrin-conjugated anti-mouse IFN-γ; BD Biosciences Pharmingen, San Diego, CA) and CD4 [fluorescein isothiocyanate anti-mouse CD4 (L3T4); eBioscience]. The sorting was done by flow cytometry for cells with intracellular IFN-γ.
Statistical Analysis. The statistical analysis was done using GraphPad Prism version 4.0 for Windows XP (GraphPad Software Inc., San Diego, CA). Animal survival is presented using Kaplan-Meier survival curves and was statistically analyzed using the log rank test. Numerical data were expressed as mean ± S.D., and the differences were assessed using the Student's t test. A value of p < 0.05 was considered statistically significant.
Results
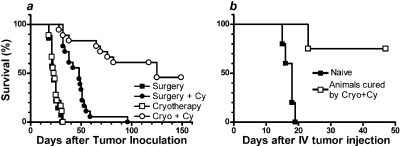
Cyclophosphamide Unmasks a Systemic Antitumor Effect of Cryoablation. Animals injected with 1 × 106 CT26 cells subcutaneously and 1 × 105 CT26 cells intravenously were randomized into four groups. Two groups were injected with Cy on day 13, and the other two were given an equivalent volume of saline. Tumors were removed the next day by cryoablation or surgical resection, with the mice having a subcutaneous tumor ranging from 3 to 8 mm in greatest dimension. There were no local recurrences of tumor after either surgery or cryoablation, but neither procedure alone prevented death from systemic disease (Fig. 1A). Cyclophosphamide administration prolonged survival of animals treated with surgery [median survival time (MST) of surgery + Cy versus surgery, 48 versus 23 days; hazard ratio (HR) 5.24, 95% CI 14.54–171.7; p < 0.0001] or with cryoablation (MST 125 versus 24 days; HR = 6.96, 95% CI = 8.14 to 61.67; p < 0.0001). It is interesting that, although there was no significant difference in survival of animals treated with cryoablation alone versus surgery alone (p = 0.46), recipients of cyclophosphamide plus cryoablation survived significantly longer than recipients of cyclophosphamide plus surgery (MST 125 versus 48 days; HR 4.89, 95% CI 3.4–21.4; p < 0.0001). These results suggest that cyclophosphamide effectively unmasks a systemic antitumor effect of cryoablation. The next experiments were conducted to determine whether anamnestic antitumor immunity mediated the systemic effects of cryoablation.
Fig. 1.
A, cyclophosphamide unmasks a systemic antitumor response induced by cryoablation. Animals injected with 1 × 106 CT26 cells subcutaneously and 1 × 105 CT26 cells intravenously were randomized into four groups: surgery (n = 14), surgery + cyclophosphamide (n = 18), cryoablation (n = 18), and cryoablation + cyclophosphamide (n = 18). Median survival times of animals in each arm were 23.0, 48.5, 23.5, and 125.0 days, respectively. B, surviving animals resist tumor rechallenge. Surviving animals (n = 4) from first experiment and naive controls were challenged with 2 × 105 CT26 cells intravenously.
Animals Cured with Cryoablation and Cyclophosphamide Resist Tumor Rechallenge. Four of the nine CT26-bearing animals treated with a combination of cryoablation and Cy were apparently cured (Fig. 1A) and were rechallenged with 2 × 105 CT26 cells intravenously. As controls, five naive BALB/c mice were challenged with the same tumor dose. Although all of the control animals died (MST 18 days), only one of the previously cured animals died at 23 days after tumor injection (Fig. 1B). No macroscopic tumor was found on autopsy of this animal, although microscopic metastases could not be ruled out. This suggests the development of antitumor effects in these animals, which prevented tumor cells from establishing, thus significantly prolonged their survival in comparison with naive mice (p = 0.0051, HR = 9.2, 95% CI = 2.25–100.1).
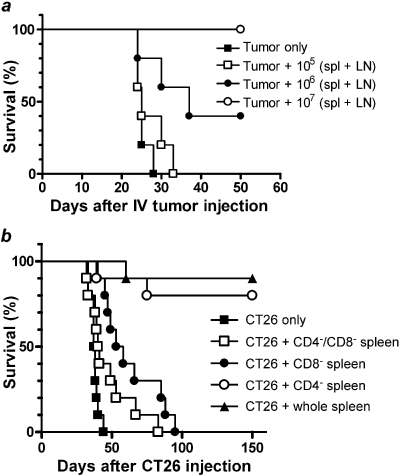
Lymphocytes from Cured Animals Transfer Antitumor Immunity to Naive Animals. Because T and B cells are the mediators of anamnestic antitumor immunity, we sought to determine whether lymphocytes from cured animals could transfer tumor resistance to naive animals. Naive BALB/c mice (five per group) received 2 × 105 CT26 cells intravenously along with graded doses, from 0 to 107, of a mixture of spleen and lymph node cells from mice cured of CT26 by Cy plus cryoablation (Fig. 2A). Compared with naive BALB/c recipients of CT26 tumor cells only, recipients of tumor cells plus either 106 or 107 lymphocytes from cured animals survived significantly longer (MST 25 versus 37 versus >50 days, respectively; p = 0.0005 for trend by log-rank test).
Fig. 2.
A, antitumor immunity generated by the combination of cyclophosphamide plus cryoablation can be passively transferred by lymphocytes in a dose-dependent manner. Surviving animals from the first two experiments were sacrificed at day 150. Spleen and draining lymph nodes were harvested, and a single-cell suspension was prepared. Naive animals were injected with 2 × 105 CT26 cells intravenously along with 0, 105, 106, or 107 spleen and lymph node cells (n = 5 per group). B, CD8+ T cells are effectors of antitumor response. Splenic suspensions from surviving animals of previous experiments were depleted of CD4+ or CD8+ T cells or both by incubating with respective antibodies. Naive animals were injected with 2 × 105 CT26 cells intravenously along with 107 cells obtained from whole spleen, CD4+ T cell depleted, CD8+ T cell depleted, or double-depleted splenic suspension (n = 10 in each arm).
CD8+ T Cells Are Important in Adoptive Transfer of Tumor Immunity. To determine the role of specific T cell subsets in antitumor immunity induced by Cy plus cryoablation, lymphocytes from cured animals were left untreated or were depleted of CD4+ cells, CD8+ cells, or both before intravenous cotransfer with CT26 cells into naive BALB/c mice (Fig. 2B). Recipients of CD8+ cell-depleted lymphocytes survived significantly shorter than recipients of undepleted lymphocytes (MST 56 versus >150 days, p < 0.0001). In contrast, depletion of CD4+ cells from the lymphocyte infusion had no significant effect on the survival of animals given tumor (p = 0.54). These results suggest that CD8+ T cells are the major effectors of antitumor immunity in mice cured of disseminated CT26 by Cy plus cryoablation. Recipients of lymphocytes depleted of both CD4+ and CD8+ cells had a small but significant survival advantage over recipients of CT26 cells only (41 versus 38 days; p = 0.03). This slight survival advantage may reflect a beneficial effect of non-T cells such as natural killer cells or may reflect activity of residual contaminating T cells.
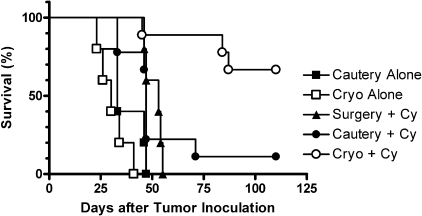
Cryoablation Is More Effective Than Cautery at Promoting Systemic Antitumor Immunity. Radiofrequency ablation and high-intensity focused ultrasound are used clinically to kill tumor cells by high temperature. In contrast to freezing of tissue, which preserves the tertiary structure of proteins, burning of tissue induces protein denaturation, which may compromise immunogenicity. Because radiofrequency ablation and high-intensity focused ultrasound are not easily performed in mice with small tumors (M. Y. Levy and W. H. Chowdhury, unpublished observations), electrocautery was used to compare the immunologic effects of burning versus freezing tumors (Fig. 3). When combined with Cy, cryoablation was significantly more effective than cautery in prolonging survival (MST >125 days versus 47 days; HR 4.57, 95% CI 1.71–25.71; p = 0.0006). In fact, animals treated with Cy plus cautery did not survive significantly longer than animals treated with Cy plus surgery (MST 47 versus 53 days; HR 1.01, 95% CI 0.27–3.73; p = 0.99), suggesting no immunologic benefit to burning tumor tissue.
Fig. 3.
Cryoablation is more effective than high-temperature ablation in augmenting host's antitumor response.
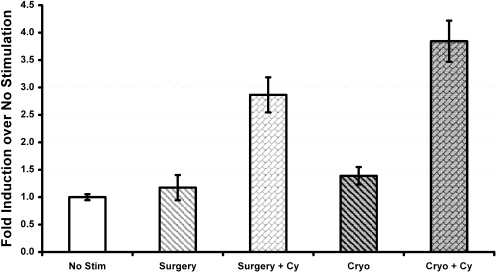
Cyclophosphamide Plus Cryoablation Increases Tumor-Specific T Cell Production of IFN-γ. Mice with local and metastatic disease were treated with surgery, cryoablation, Cy plus surgery, or Cy plus cryoablation. Ten days after local therapy, mice were sacrificed, spleen and lymph node cells were harvested, CD4+ T cells were tested for the intracellular production of IFN-γ in response to AH1, and the immunodominant H-2Ld-restricted tumor peptide was encoded by an endogenous retrovirus present in CT26 cells. The percentage of IFN-γ-positive CD4+ cells was significantly higher in groups treated with cyclophosphamide irrespective of the procedure performed for local tumor removal (Fig. 4). CD4+ T cells from mice treated with Cy plus cryoablation contained a higher proportion of IFN-γ-positive cells compared with CD4+ T cells from mice treated with Cy plus surgery, but the difference failed to reach statistical significance (p = 0.08).
Fig. 4.
Cryoablation plus cyclophosphamide increases IFN-γ release. Animals were divided into four groups: surgery, cyclophosphamide plus surgery, cryoablation, and cyclophosphamide plus cryoablation. They were sacrificed 10 days after local tumor removal. Splenic cell suspensions were prepared, and 1 × 106 cells were stimulated with 10 μg of AH1, the immunodominant peptide of CT26, for 6 h before surface staining with anti-mouse CD4 antibody and intracellular staining with anti-mouse IFN-γ antibody. Flow cytometric data are shown as percentage of CD4+, IFN-γ-positive cells in response to AH1 stimulation.
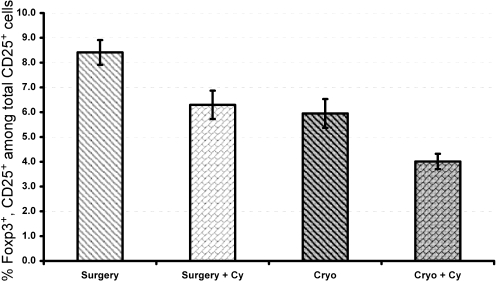
Cyclophosphamide Suppresses Tregs. FACS analysis of fresh spleen and draining lymph node cells, harvested from the previous experiment, was done to determine whether there was a change in the T cell populations after therapy. Animals with cyclophosphamide pretreatment had a significantly lower ratio of regulatory T cells to effector cells compared with animals with only local tumor removal (p < 0.05; Fig. 5). Treg numbers were significantly lower in mice treated with Cy plus cryoablation compared with mice treated with Cy plus surgery (p < 0.05). Thus, the enhanced antitumor efficacy of Cy plus cryoablation may reflect, in part, the effect of therapy on reducing Tregs, which are known to suppress antitumor immunity in animals with advanced tumors.
Fig. 5.
Cyclophosphamide suppresses Tregs. Spleen cell suspensions used in the experiment of Fig. 4 were stained on the cell surface with anti-mouse CD4 and CD25 antibodies and intracellularly with anti-mouse Foxp3. Flow cytometry was done for Tregs and T effector cells. Data are shown as proportion of Tregs to Teffector cells.
Discussion
Cryotherapy is used frequently to eradicate localized cancers in patients who are poor candidates for standard surgical resection. It utilizes freezing temperatures to damage tumor cells by multiple mechanisms, including destruction of local microvasculature, breakdown of cellular microtubular networks, conversion of lipid layers of cell membrane into semisolids, and the grinding action of irregular ice crystal formation (Fraser and Gill, 1967; Whittaker, 1984; Hoffmann and Bischof, 2002). Cryoablation in situ results in the generation of tumor debris in the context of an inflammatory background. This tumor debris contains endogenous danger signals required for the activation of antigen-presenting cells that are rendered competent to sensitize adaptive antitumor immunity (den Brok et al., 2006).
Cancers escape immunosurveillance by different mechanisms including anergy induction, loss of tumor antigen expression, and suppression of tumor-specific immunity by CD4+CD25+Foxp3+ Tregs. These cells play an important role in immune evasion mechanisms employed by cancer (Sakaguchi et al., 2001; Terabe and Berzofsky, 2004; Wang and Wang, 2007). Tregs are actively recruited and induced by tumors to block the priming and/or effector function of antitumor T cells, thereby inhibiting the efficacy of therapeutic cancer vaccines. Treatment with cyclophosphamide enhances the apoptosis and decreases homeostatic proliferation of these cells. Expression of Foxp3, the lineage-directing transcription factor of Tregs, is down-regulated after cyclophosphamide administration (Brode and Cooke, 2008; Matsushita et al., 2008).
In this report, we have developed a treatment of metastatic cancer focusing on these principles. First, in situ cryotherapy is used to generate a shower of tumor antigens in the context of tissue destruction and distress. Therefore, cryoablation effectively converts tumor tissue into an autologous tumor vaccine. Furthermore, Cy is administered to enhance apoptosis of Tregs and allow for a more vigorous antitumor response. A previous study has shown that Cy pretreatment of tumor-bearing animals augments the local antitumor effect of cryoablation (Cooper et al., 1981). However, the effect of this combination on metastatic cancer had not been explored previously.
Interest in the immunostimulatory potential of cryoablation was first stimulated by anecdotal reports of regression of metastases in patients whose primary tumors were treated by cryosurgery (Soanes et al., 1970; Gursel et al., 1972). Studies in animals of the antitumor potential of cryoablation have yielded disparate results, often depending upon the read-out system. On the one hand, cryoablation of a subcutaneous tumor retards the simultaneous growth of untreated tumor on the contralateral side and induces resistance to tumor rechallenge (Neel et al., 1970; Tanaka, 1982; Sabel et al., 2005; den Brok et al., 2006). On the other hand, cryoablation of the primary tumor alone is usually, but not always (El-Shakhs et al., 1999; Joosten et al., 2001), ineffective or deleterious in controlling the growth or spread of hematogenous metastases (Javadpour et al., 1979; Yamashita et al., 1982; Wing et al., 1988; Yan et al., 2006). Our studies confirm that cryoablation of local tumor as sole therapy is unable to retard the progression of metastatic cancer. The addition of cyclophosphamide was able to unmask a systemic antitumor response and resulted in complete cure in approximately half of the animals. Cyclophosphamide selectively reduced Tregs as demonstrated by flow cytometry analysis. Reduction of Treg number and function mitigates the suppression of NK cells and CD8+ cytotoxic T lymphocytes (CTLs), providing a boost to both innate and adaptive antitumor immunity. Activation of NK cell-mediated immunity provides added protection against tumor cells that escape tumor-specific CD8+ T cells through down-regulation of surface expression of MHC class I molecules.
We have demonstrated an increased secretion of IFN-γ by CD4+ T cells against AH1 peptide in spleens of animals treated with the combination of cyclophosphamide plus cryoablation. IFN-γ is a proinflammatory cytokine secreted by both activated lymphocytes and natural killer cells and is considered as an antitumor agent (Doherty et al., 1996; Karimi et al., 2008). Besides the direct apoptotic effects of IFN-γ on various cancer cells, it also up-regulates the expression of MHC class I on tumor cells, thus making them more susceptible to CTLs (Wadler and Schwartz, 1990; Boehm et al., 1997). The ability of the combination of cryoablation and Cy to induce IFN-γ secretion, a mediator of Th1 immune responses (Mosmann and Coffman, 1989), in reaction to tumor antigen demonstrates the effectiveness of our treatment strategy in augmenting an antitumor response.
The ability of cured animals to resist tumor rechallenge suggests the development of immunologic memory in these mice. Furthermore, this antitumor immunity can be passively transferred to naive animals, offering them a dose-dependent protection against tumor implantation. Similar results of the adoptive transfer of cells or serum from tumor-bearing animals subjected to cryoablation have been shown previously (Sabel et al., 2006). Our adoptive transfer studies reveal CD8+ T cells as critical effectors of antitumor immunity in animals cured by cyclophosphamide plus cryoablation. This result is in accordance with the available literature on the role of CD8+ CTLs in antitumor responses (Barth et al., 1991; Paglia et al., 1996).
We also compared the immunogenicity of burning versus freezing. In our model, cryoablation was more effective than cautery at promoting a systemic antitumor immune response. Although both procedures leave tumor debris inside the host, the differential response could be due to greater damage to antigens caused by the denaturing effect of high temperature compared with the release of intact antigens by cryoablation.
The combination of cryoablation and Cy serves as an efficacious tool in our model for the development of antitumor immunity sufficient for prolongation of survival from and even cure of systemic, metastatic disease. Further studies using different cancer models and using immunomodulatory approaches such as monoclonal antibodies and/or adoptive cell therapy in combination with cryoablation and cyclophosphamide are required to devise an immunotherapeutic strategy that can affect the progression of metastatic disease in patients.
This work was supported in part by the National Institutes of Health [Grants R01-CA105148, P01-CA15396]; and the Patrick C. Walsh Prostate Cancer Research Fund.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.152603.
ABBREVIATIONS: Treg, regulatory T cell; NK, natural killer; Cy, cyclophosphamide; MST, median survival time; HR, hazard ratio; CI, confidence interval; CTL, cytotoxic T lymphocyte.
References
- Ablin RJ (1972) Cryoimmunotherapy. Br Med J 3 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth RJ Jr, Mulé JJ, Spiess PJ, and Rosenberg SA (1991) Interferon gamma and tumor necrosis factor have a role in tumor regressions mediated by murine CD8+ tumor-infiltrating lymphocytes. J Exp Med 173 647-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm U, Klamp T, Groot M, and Howard JC (1997) Cellular responses to interferon-gamma. Annu Rev Immunol 15 749-795. [DOI] [PubMed] [Google Scholar]
- Brode S and Cooke A (2008) Immune-potentiating effects of the chemotherapeutic drug cyclophosphamide. Crit Rev Immunol 28 109-126. [DOI] [PubMed] [Google Scholar]
- Cooper AJ, Powell JM, Perry S, and Fraser JD (1981) Cyclophosphamide pretreatment in tumor cryotherapy: a murine model. Cryobiology 18 577-584. [DOI] [PubMed] [Google Scholar]
- den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Frielink C, Toonen LW, Boerman OC, Figdor CG, Ruers TJ, and Adema GJ (2006) Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer 95 896-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty GM, Tsung K, McCluskey B, and Norton JA (1996) Endogenous interferon-gamma acts directly on tumor cells in vivo to suppress growth. J Surg Res 64 68-74. [DOI] [PubMed] [Google Scholar]
- El-Shakhs SA, Shimi SA, and Cuschieri A (1999) Effective hepatic cryoablation: Does it enhance tumor dissemination? World J Surg 23 306-310. [DOI] [PubMed] [Google Scholar]
- Fraser J and Gill W (1967) Observations on ultra-frozen tissue. Br J Surg 54 770-776. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, and Martin F (2004) CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 34 336-344. [DOI] [PubMed] [Google Scholar]
- Golgher D, Jones E, Powrie F, Elliott T, and Gallimore A (2002) Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol 32 3267-3275. [DOI] [PubMed] [Google Scholar]
- Gursel E, Roberts M, and Veenema RJ (1972) Regression of prostatic cancer following sequential cryotherapy to the prostate. J Urol 108 928-932. [DOI] [PubMed] [Google Scholar]
- Hoffmann NE and Bischof JC (2002) The cryobiology of cryosurgical injury. Urology 60 40-49. [DOI] [PubMed] [Google Scholar]
- Imai H, Saio M, Nonaka K, Suwa T, Umemura N, Ouyang GF, Nakagawa J, Tomita H, Osada S, Sugiyama Y, et al. (2007) Depletion of CD4+CD25+ regulatory T cells enhances interleukin-2-induced antitumor immunity in a mouse model of colon adenocarcinoma. Cancer Sci 98 416-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadpour N, Bagley DH, and Zbar B (1979) Failure of cryosurgical treatment of experimental intradermal tumors to eradicate microscopic lymph node metastases in guinea pigs. J Natl Cancer Inst 62 1479-1481. [PubMed] [Google Scholar]
- Joosten JJ, Muijen GN, Wobbes T, and Ruers TJ (2001) In vivo destruction of tumor tissue by cryoablation can induce inhibition of secondary tumor growth: an experimental study. Cryobiology 42 49-58. [DOI] [PubMed] [Google Scholar]
- Karimi K, Boudreau JE, Fraser K, Liu H, Delanghe J, Gauldie J, Xing Z, Bramson JL, and Wan Y (2008) Enhanced antitumor immunity elicited by dendritic cell vaccines is a result of their ability to engage both CTL and IFN gamma-producing NK cells. Mol Ther 16 411-418. [DOI] [PubMed] [Google Scholar]
- Korpan NN (2007) A history of cryosurgery: its development and future. J Am Coll Surg 204 314-324. [DOI] [PubMed] [Google Scholar]
- Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, and Sabzevari H (2005) Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 105 2862-2868. [DOI] [PubMed] [Google Scholar]
- Matsushita N, Pilon-Thomas SA, Martin LM, and Riker AI (2008) Comparative methodologies of regulatory T cell depletion in a murine melanoma model. J Immunol Methods 333 167-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann TR and Coffman RL (1989) TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 7 145-173. [DOI] [PubMed] [Google Scholar]
- Neel HB 3rd, Ketcham AS, and Hammond WG (1970) Comparison of tumor immunity after complete excision, cryonecrosis, and in the presence of persistent tumor. Surg Forum 21 120-122. [PubMed] [Google Scholar]
- Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, and Nakayama E (1999) Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res 59 3128-3133. [PubMed] [Google Scholar]
- Paglia P, Chiodoni C, Rodolfo M, and Colombo MP (1996) Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J Exp Med 183 317-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabel MS (2009) Cryo-immunology: A review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology 58 1-11. [DOI] [PubMed] [Google Scholar]
- Sabel MS, Arora A, Su G, and Chang AE (2006) Adoptive immunotherapy of breast cancer with lymph node cells primed by cryoablation of the primary tumor. Cryobiology 53 360-366. [DOI] [PubMed] [Google Scholar]
- Sabel MS, Nehs MA, Su G, Lowler KP, Ferrara JLM, and Chang AE (2005) Immunologic response to cryoablation of breast cancer. Breast Canc Res Treat 90 97-104. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, and Takahashi T (2001) Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev 182 18-32. [DOI] [PubMed] [Google Scholar]
- Shulman S, Brandt EJ, and Yantorno C (1968) Studies in cryo-immunology. II. Tissue and species specificity of the autoantibody response and comparison with iso-immunization. Immunology 14 149-158. [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, and Hayakawa Y (2006) CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol 176 1582-1587. [DOI] [PubMed] [Google Scholar]
- Soanes WA, Ablin RJ, and Gonder MJ (1970) Remission of metastatic lesions following cryosurgery in prostatic cancer: immunologic considerations. J Urol 104 154-159. [DOI] [PubMed] [Google Scholar]
- Tanaka S (1982) Immunological aspects of cryosurgery in general surgery. Cryobiology 19 247-262. [DOI] [PubMed] [Google Scholar]
- Terabe M and Berzofsky JA (2004) Immunoregulatory T cells in tumor immunity. Curr Opin Immunol 16 157-162. [DOI] [PubMed] [Google Scholar]
- Wadler S and Schwartz EL (1990) Antineoplastic activity of the combination of interferon and cytotoxic agents against experimental and human malignancies: a review. Cancer Res 50 3473-3486. [PubMed] [Google Scholar]
- Wang HY and Wang RF (2007) Regulatory T cells and cancer. Curr Opin Immunol 19 217-223. [DOI] [PubMed] [Google Scholar]
- Whittaker DK (1984) Mechanisms of tissue destruction following cryosurgery. Ann R Coll Surg Engl 66 313-318. [PMC free article] [PubMed] [Google Scholar]
- Wing MG, Goepel JR, Jacob G, Rees RC, and Rogers K (1988) Comparison of excision versus cryosurgery of an HSV-2-induced fibrosarcoma: I. Survival, extent of metastatic disease and host immunocompetence following surgery. Cancer Immunol Immunother 26 161-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Hayakawa K, Hosokawa M, Kodama T, Inoue N, Tomita K, and Kobayashi H (1982) Enhanced tumor metastases in rats following cryosurgery of primary tumor. Gann 73 222-228. [PubMed] [Google Scholar]
- Yan TD, Chiang G, Zhao J, Chan D, and Morris DL (2006) Lung metastases after liver resection or cryotherapy for hepatic metastasis from colorectal cancer: there is a difference!. HPB (Oxford) 8 124-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantorno C, Soanes WA, Gonder MJ, and Shulman S (1967) Studies in cryoimmunology: I. The production of antibodies to urogenital tissue in consequence of freezing treatment. Immunology 12 395-410. [PMC free article] [PubMed] [Google Scholar]