Abstract
The lectin Siglec-8 (sialic acid-binding, immunoglobulin-like lectin), which is selectively expressed on eosinophil surfaces and regulates eosinophil survival, preferentially binds to the glycan 6′-sulfo-sialyl Lewis X (6′-sulfo-sLex). Antibody engagement of Siglec-8 on eosinophils causes their apoptosis, suggesting that engagement of Siglec-8 with its natural glycan ligands in vivo may control allergic inflammation. We report that a soluble synthetic polymer displaying 6′-sulfo-sLex glycan selectively binds to human eosinophils and human embryonic kidney 293 cells expressing Siglec-8. Binding was inhibited by anti-Siglec-8 antibody. In whole blood, eosinophils were the only leukocyte subtype to detectably bind polymeric 6′-sulfo-sLex. Interleukin-5-primed eosinophils underwent apoptosis when incubated with either anti-Siglec-8 monoclonal antibody or polymeric 6′-sulfo-sLex, although the glycan polymer was less effective. These data demonstrate that a soluble, multivalent glycan selectively binds to human eosinophils and induces their apoptosis in vitro and provide proof-of-concept that such a reagent could be used to selectively target eosinophils.
Siglecs are a family of single-pass cell surface receptors that contain N-terminal sialic acid-binding domains (Varki and Angata, 2006; Crocker et al., 2007; von Gunten and Bochner, 2008). Among all of the known human Siglecs, each has a unique pattern of cell surface expression ranging from a single cell type, such as sialoadhesin (Siglec-1) on macrophages, to a wide range of cell types, such as CD33 (Siglec-3) on most leukocytes (Varki and Angata, 2006; Crocker et al., 2007; von Gunten and Bochner, 2008). Somewhat more selective in cellular expression pattern is Siglec-8, found on eosinophils and mast cells and, to a lesser degree, on human basophils (Floyd et al., 2000; Kikly et al., 2000). Although all Siglecs bind sialic acid, each exhibits a distinct preference for the type of sialic acid, its glycosidic linkage, and the presence of underlying glycans (Varki and Angata, 2006; Crocker et al., 2007). However, whereas Siglec-8 binds weakly to 2,3-linked sialic acids (Floyd et al., 2000; Bochner et al., 2005), it shows a remarkable preference for 6′-sulfated-sialyl-Lewis X [6′-sulfo-sLex; NeuAcα2-3Gal(6-O-SO3)β1-4(Fucα1-3)GlcNAc] without recognizing closely related glycans like sLex and 6-sulfo-sLex (Bochner et al., 2005). Because Siglec-8 engagement with specific antibodies induces eosinophil apoptosis (Nutku et al., 2003, 2005; Nutku-Bilir et al., 2008), it remains unknown whether a glycan ligand-based material would have a similar effect. Therefore, we determined whether a synthetic polymer-based ligand decorated with the 6′-sulfo-sLex glycan ligand of Siglec-8 would have the same cellular binding specificity and biology as that seen with monoclonal antibodies.
Materials and Methods
Generation of Stable Siglec-8-Transfected Human Embryonic Kidney 293 Cells. Human embryonic kidney 293 (HEK 293) cell stable transfectants expressing levels of Siglec-8 similar to levels found on eosinophils were prepared and maintained as described previously (Kikly et al., 2000; Yokoi et al., 2008).
Purification of Human Eosinophils. Human eosinophils were isolated from peripheral blood by density gradient centrifugation, red cell hypotonic lysis, and immunomagnetic negative selection as described previously (Matsumoto et al., 1995).
Polyacrylamide Conjugates. Thirty-kilodalton label-free and biotinylated conjugates were obtained as described previously (Bovin et al., 1993) by coupling aminopropyl glycosides to poly(4-nitrophenyl acrylate) followed by quenching of the polymer active groups with ethanolamine. The 2000-kDa polymers were obtained similarly starting from poly(4-N-hydroxysuccinimidyl acrylate) (Shilova et al., 2005). Spacer-armed tetrasaccharides 6′-sulfo-sLex, sLex, and 3′-sulfo-sLex were synthesized as described previously (Zemlyanukhina et al., 1995; Pazynina et al., 2003; G. V. Pazynina, V. Severov, M. L. Maisel, I. M. Belyanchikov, and N. V. Bovin, submitted for publication).
Flow Cytometry. Binding of Siglec-8 monoclonal antibodies 2E2, 2C4, and 9G4 (all murine IgG1, generously provided by Dr. John White, GlaxoSmithKline, Uxbridge, Middlesex, UK), sLex IgM monoclonal antibody FH6 (Bochner et al., 1994), a polyclonal sheep anti-human Siglec-8 antibody (Tateno et al., 2005), and appropriate isotype controls (all used at 10 μg/ml concentrations unless otherwise indicated) was determined in heparinized whole blood or on purified eosinophils by use of indirect immunofluorescence and flow cytometry as described previously (Kikly et al., 2000; Nutku et al., 2003). Binding of saturating concentrations (10 μg/ml) of biotinylated 30- or 2000-kDa 6′-sulfo-sLex-containing polyacrylamide (PAA) polymers and controls [30-kDa 3′-sulfo-Lex-PAA and sLex-PAA; 2000-kDa Lac-Nac-PAA (Rapoport et al., 2006; Tateno et al., 2007)] was detected by use of saturating concentrations of fluorescein isothiocyanate-streptavidin (R&D Systems, Minneapolis, MN) and flow cytometry. In some experiments, cells were preincubated as described previously (Nimrichter et al., 2008) for 90 min at 37°C with sialidase [either from Clostridium perfringens, 1.6 U/ml from Sigma-Aldrich (St. Louis, MO), or from Vibrio cholerae, 10 mU/ml from Roche Diagnostics (Indianapolis, IN)] or monoclonal or polyclonal Siglec-8 antibodies (20 min, 4°C) before incubation with various PAA materials.
Adhesion Assays. Biotinylated glycan-conjugated PAA polymers (at an optimal concentration of 10 μg/ml) were immobilized on streptavidin-coated 96-well plates (Pierce Chemical, Rockford, IL), and adhesion of calcein-AM-labeled HEK 293 cell suspensions was tested under static conditions (30 min, room temperature) essentially as described previously for eosinophils (Matsumoto et al., 1997). In some experiments, cells were preincubated with either monoclonal or polyclonal Siglec-8 antibodies before testing cell adhesion as indicated.
Apoptosis Assays. Purified eosinophils were cultured for 24 or 72 h in interleukin 5 (IL-5) (10 ng/ml, R&D Systems), and then either 10 μg/ml 2C4 antibody or 10 μg/ml 2000-kDa PAA polymers were added for an additional 24 h. None of the cultures was performed without IL-5 because cytokine exposure is needed to see apoptosis to Siglec-8 antibodies alone (Nutku et al., 2003; von Gunten et al., 2007; Nutku-Bilir et al., 2008). Thus, no secondary antibodies were included. Cell survival was then assessed by flow cytometric analysis after labeling with annexin-V and propidium iodide as described previously (Nutku et al., 2003; Nutku-Bilir et al., 2008).
Statistical Analyses. Paired two-tailed Student's t test was used to compare values, and results were considered significant for p values <0.05.
Results
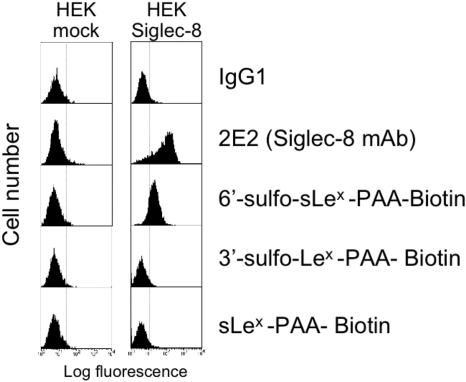
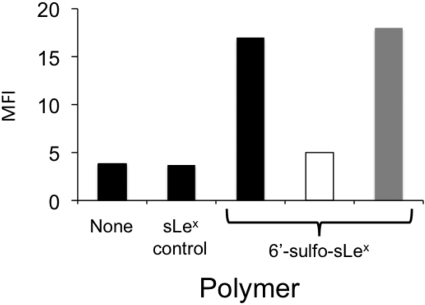
6′-Sulfo-sLex-PAA Polymer Selectively Binds to Siglec-8. Initial experiments used wild-type HEK 293 cells and HEK 293 cells stably transfected with Siglec-8. As shown in Fig. 1, such transfected cells prominently and selectively label with Siglec-8 antibody (2E2) and also bind 30-kDa 6′-sulfo-sLex-PAA but not the closely related control glycan-PAA polymers (sLex and 3-sulfo-sLex) that differ from 6′-sulfo-sLex only by the absence of the sulfate residue or the sulfate attachment site. Further evidence of specificity of binding is shown in Fig. 2, where a polyclonal sheep Siglec-8 antibody blocked 6′-sulfo-sLex-PAA binding to the Siglec-8-HEK 293 cell transfectants. This differs from results obtained by use of a mixture of all three Siglec-8 monoclonal antibodies, which failed to inhibit polymer binding, demonstrating that the polyclonal reagent, unlike the monoclonal antibodies, recognizes the carbohydrate binding site. Consistent with this conclusion is the observation that preincubation of Siglec-8-expressing HEK 293 cells with polyclonal antibodies does not reduce binding of any of the monoclonal antibodies, or vice versa (data not shown).
Fig. 1.
HEK 293 cells stably transfected with Siglec-8 have the ability to bind 30-kDa 6′-sulfo-sLex-PAA polymer. Shown are flow cytometric histograms comparing mock-transfected HEK 293 cells that fail to bind Siglec-8 antibody and 6′-sulfo-sLex-PAA polymer. Also shown is the failure of binding of several structurally similar glycan-conjugated polymers. Results are representative of two experiments with similar results.
Fig. 2.
Effect of different Siglec-8 antibodies on 30-kDa 6′-sulfo-sLex-PAA polymer binding to HEK 293 cells stably transfected with Siglec-8. Black bars represent data with no added antibodies. A polyclonal sheep Siglec-8 antibody had blocking activity (open bar), whereas a combination of mouse monoclonal antibodies (50 μg/ml each of 2E2, 2C4, and 9G4), had no blocking activity (gray bar). Results are from a single experiment representative of three experiments with similar results. MFI, mean fluorescence intensity.
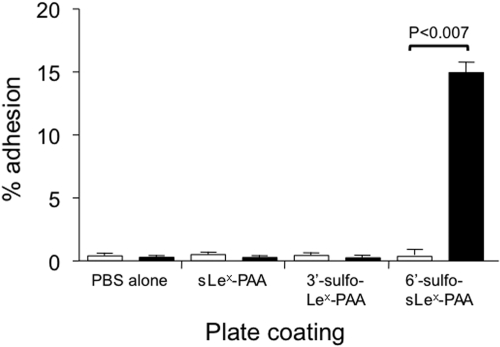
Further evidence of the functionality of 6′-sulfo-sLex-PAA binding to Siglec-8 is shown in Fig. 3 by use of an adhesion assay in which this or various control 30-kDa polymers were immobilized on plastic surfaces, and adhesion of Siglec-8-transfected or mock-transfected HEK 293 cells was tested under static conditions. Significant attachment was only observed with HEK 293 cells transfected with Siglec-8 and immobilized 30-kDa 6′-sulfo-sLex-PAA polymer (p < 0.007), and adhesion was eliminated by preincubation of the HEK 293 transfectants with polyclonal Siglec-8 antibody but not any of the monoclonals (Fig. 3; data not shown).
Fig. 3.
Adhesion of mock-transfected HEK 293 cells (open bars) and HEK 293 cells stably transfected with Siglec-8 (black bars) to various immobilized substrates. Attachment was only observed when using 30-kDa 6′-sulfo-sLex-PAA polymer and HEK 293 cells transfected with Siglec-8. Data are means ± S.E.M. of triplicate determinations and are from n = 2–4 experiments.
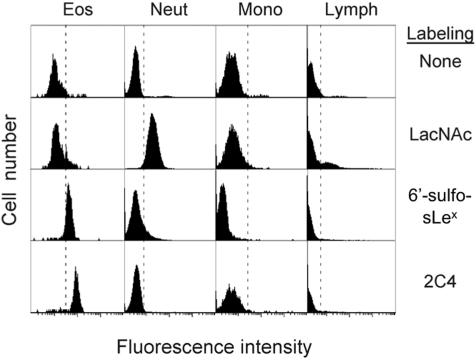
Surprisingly, and in marked contrast, when this same 6′-sulfo-sLex-PAA 30-kDa polymer was tested for its ability to bind purified eosinophils, either in flow cytometry or in static adhesion assays, no detectable binding was seen, even though these cells expressed levels of Siglec-8 virtually identical to the HEK 293 transfectants, as determined by antibody labeling and flow cytometry (data not shown). Because previous studies suggest that endogenous cell surface sialic acids can bind in so-called “cis” configuration and block Siglec ligand binding (Crocker et al., 2007), eosinophils were pretreated with sialidase to remove surface sialic acids (which was confirmed by complete loss of sLex surface expression by eosinophils), yet neither treated nor untreated eosinophils bound to the 30-kDa 6′-sulfo-sLex-PAA polymer (data not shown). However, when a much larger (2000-kDa) 6′-sulfo-sLex-PAA polymer was tested, binding to purified eosinophils was readily detected (net mean fluorescence of 14.9 ± 7.9, mean ± S.D., n = 11 with nine different donors), whereas a control 2000-kDa glycan polymer, LacNAc-PAA, did not bind (Fig. 4; data not shown). As was observed with the HEK 293 cell transfectants, binding of 2000-kDa 6′-sulfo-sLex-PAA polymer to human eosinophils was completely inhibited by polyclonal anti-Siglec-8 antibody (102 ± 20% inhibition, n = 11 with nine different donors), but not by any of the mouse monoclonal antibodies or the 30-kDa 6′-sulfo-sLex-PAA polymer (data not shown).
Fig. 4.
The 2000-kDa 6′-sulfo-sLex PAA polymer selectively binds to eosinophils among leukocytes in whole blood. Attachment of the 2000-kDa 6′-sulfo-sLex PAA polymer and the 2C4 Siglec-8 monoclonal antibody to eosinophils was readily apparent, whereas no labeling of monocytes, lymphocytes, neutrophils, or basophils was observed. Binding of the LacNAc control PAA polymer is seen for neutrophils and a subset of lymphocytes. Results are representative of at least eight experiments with similar results.
6′-Sulfo-sLex-PAA Polymer Uniquely Binds to Eosinophils in Whole Blood. Further evidence of the specificity of eosinophil binding to the 6′-sulfo-sLex glycan was provided by assays in which heparinized whole blood was incubated with biotinylated 2000-kDa 6′-sulfo-sLex-PAA or control polymer, and flow cytometry was performed after the addition of fluorochrome-conjugated streptavidin. As shown in Fig. 4, attachment of 6′-sulfo-sLex-PAA polymer to eosinophils was readily apparent, while no labeling of lymphocytes or neutrophils was observed and monocytes displayed a degree of reduced fluorescence. Interestingly, neutrophils and a subset of lymphocytes consistently and selectively bound the 2000-kDa control LacNAc polymer. As reported previously (Kikly et al., 2000), basophils were weakly labeled with Siglec-8 antibody, but no detectable labeling with 6′-sulfo-sLex-PAA polymer was seen (data not shown), presumably because of the low levels of basophil surface expression of Siglec-8.
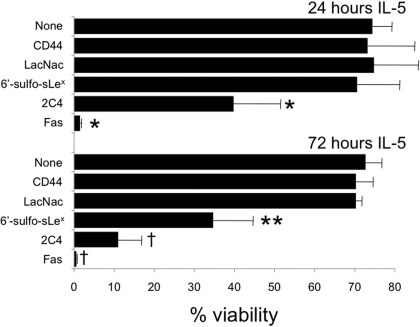
6′-Sulfo-sLex-PAA Polymer Induces Eosinophil Apoptosis in Vitro. Engagement of Siglec-8 with monoclonal antibodies causes eosinophil apoptosis (Nutku et al., 2003, 2005; Nutku-Bilir et al., 2008). Therefore, it was of interest to determine whether binding of the 2000-kDa 6′-sulfo-sLex-PAA polymer had the ability to induce eosinophil apoptosis. As shown in Fig. 5, IL-5-primed eosinophils, which are known to be particularly sensitive to Siglec-8 antibody-mediated apoptosis (von Gunten et al., 2007; Nutku-Bilir et al., 2008), underwent apoptosis when incubated with 6′-sulfo-sLex-PAA polymer after 72 h of culture with IL-5 (p < 0.03), but not after 24 h of culture. The magnitude of the effect was less than that seen with the 2C4 anti-Siglec-8 monoclonal antibody and much less than that seen with the Fas antibody, both of which caused significant apoptosis after either 24 or 72 h of culture with IL-5. Enhanced sensitivity to polymer was not the result of altered surface expression of cell surface Siglec-8 during the culture because levels only varied slightly and in no particular pattern (data not shown).
Fig. 5.
IL-5-primed eosinophils undergo apoptosis when exposed to Siglec-8 antibody or 6′-sulfo-sLex-PAA polymer. Results represent means ± S.D. from five experiments at 24 h and three experiments at 72 h. *, p < 0.02 compared with the CD44 control; **, p < 0.03 compared with the LacNac control; †, p < 0.005 compared with the CD44 control.
Discussion
Although we and others have previously demonstrated eosinophil apoptosis with Siglec-8 antibody engagement (Nutku et al., 2003, 2005; von Gunten et al., 2007; Nutku-Bilir et al., 2008), our subsequent identification of a selective and specific ligand for Siglec-8, namely 6′-sulfo-sLex (Bochner et al., 2005), made it possible to test the hypothesis that a synthetic polymer decorated with this glycan would have the same cellular binding specificity and biology as that seen with monoclonal antibodies. With use of a soluble glycan-conjugated PAA polymer for initial proof-of-concept in the development of a nonbiologically based agent for detecting eosinophils and inducing their apoptosis, experiments revealed both selective and specific 6′-sulfo-sLex binding to Siglec-8 on transfected cells and on either purified eosinophils or eosinophils in whole blood. Affinity and avidity were sufficient to also mediate cellular adhesion to 6′-sulfo-sLex PAA polymer-coated surfaces. Glycan binding was inhibited by a sheep polyclonal antibody but not by any of our available mouse monoclonal antibodies. Although it was not as effective as the monoclonal antibodies, binding of 6′-sulfo-sLex-PAA polymer also induced eosinophil apoptosis. To our knowledge, this is the first time a soluble glycan has been used to selectively recognize and kill eosinophils and raises the possibility of developing enhanced glycan-based Siglec-8 ligands for diagnostic or therapeutic purposes in which eosinophil-specific targeting is desired.
The reason that 30-kDa 6′-sulfo-sLex-PAA polymer bound HEK 293 cell transfectants, but not eosinophils, remains unknown but is probably not due to cell surface sialic acid interference because their removal from the surface of eosinophils with sialidase had no effect. One possibility is that the larger polymer has the potential to span Siglec-8 over a wider area of the eosinophil surface or fortuitously expresses the glycan ligand in groupings that match the grouping of Siglec-8 on the eosinophil surface.
Although the 2000-kDa polymer does induce eosinophil apoptosis, it is not as effective as the antibody. The reason for this difference is unclear, but it may be the result of differences in multivalent versus polyvalent binding and the reduced flexibility of a dimeric antibody for inducing cross-links compared with a large, highly flexible PAA polymer. Indeed, the density of carbohydrate decoration is high, on the order of several hundred per PAA molecule. Further optimization of the polymer rigidity, size, and density of decoration with 6′-sulfo-sLex glycan may enhance both potency and efficacy for binding and induction of apoptosis. However, the goal of the present experiments was to achieve proof-of-concept regarding the ability of a soluble glycan ligand of Siglec-8, specifically a 6′-sulfo-sLex glycan, to selectively bind eosinophils among all other cells in peripheral blood, and to induce their apoptosis. This was important because subsequent to our publication showing specificity of binding of 6′-sulfo-sLex for Siglec-8 (Bochner et al., 2005), additional glycan array screens performed by the Consortium for Functional Glycomics with other lectins (www.functionalglycomics.org) suggested that more structures, including Siglec-7, might be capable of binding this glycan. The fact that no detectable binding to any other leukocyte subtype was seen, including monocytes, CD8+ T lymphocytes, and NK cells that express Siglec-7 (Nicoll et al., 1999; Angata and Varki, 2000), suggests that 1) endogenous binding sites may be masked by cell surface sialic acids, 2) binding specificity or affinity is less than that for Siglec-8, or 3) levels of Siglec-7 on these cells do not permit detectable levels of polymer attachment.
The closest functional mouse paralog to Siglec-8 is Siglec-F, which is also expressed on eosinophils and, like human eosinophils, preferentially binds 6′-sulfo-sLex (Tateno et al., 2005). Previous work showing that Siglec-F antibody administration to mice leads to profound and selective eosinophil apoptosis and depletion (Kearley et al., 2007; Zimmermann et al., 2008; Song et al., 2009) suggests that in vivo administration of the 6′-sulfo-sLex PAA polymer might have a similar effect, but unfortunately sufficient quantities are not yet available to perform such experiments. Other in vitro experiments with murine eosinophils have shown, however, that this same glycan-decorated polymer binds to murine eosinophils via Siglec-F and triggers its internalization via a pathway involving ADP ribosylation factor 6 but independent of dynamin and clathrin (Tateno et al., 2007). Additional experiments in vivo suggest the presence of a lung ligand for Siglec-F (Zhang et al., 2007). Its exact molecular identity remains incompletely defined but is currently under investigation (Bochner, 2009). Regardless, selective and specific engagement of Siglec-8 on eosinophils by natural or synthetic 6′-sulfo-sLex-containing ligands, glycomimetics, or antibodies could be useful in controlling eosinophilic inflammatory responses in vivo.
Acknowledgments
We thank John White for providing the Siglec-8 monoclonal antibodies and Oksoon Choi for providing the stable Siglec-8 HEK transfectants.
This work was supported in part by the National Institutes of Health National Institute of Allergy and Infectious Diseases [Grants AI41472, AI72265]; the Human Immunology grant program of the Dana Foundation (to B.S.B); and the Russian Academy of Sciences Presidium Program entitled “Molecular and Cell Biology” (to N.V.B.).
B.S.B. received support as a Cosner Scholar in Translational Research from Johns Hopkins University.
Drs. Bochner and Schnaar are coauthors on existing and pending Siglec-8-related patents. If Siglec-8-related products are developed in the future, under a licensing agreement between GlaxoSmithKline and the Johns Hopkins University, Drs. Bochner and Schnaar may be entitled to a share of royalties received by the University on the potential sales of such products. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.152439.
ABBREVIATIONS: sLex, sialyl Lewis X; HEK, human embryonic kidney; MFI, mean fluorescence intensity; PAA, polyacrylamide.
References
- Angata T and Varki A (2000) Siglec-7: a sialic acid-binding lectin of the immunoglobulin superfamily. Glycobiology 10 431-438. [DOI] [PubMed] [Google Scholar]
- Bochner BS (2009) Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy 39 317-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, White JR, and Schnaar RL (2005) Glycan array screening reveals a candidate ligand for Siglec-8. J Biol Chem 280 4307-4312. [DOI] [PubMed] [Google Scholar]
- Bochner BS, Sterbinsky SA, Bickel CA, Werfel S, Wein M, and Newman W (1994) Differences between human eosinophils and neutrophils in the function and expression of sialic acid-containing counterligands for E-selectin. J Immunol 152 774-782. [PubMed] [Google Scholar]
- Bovin NV, Korchagina EYu, Zemlyanukhina TV, Byramova NE, Galanina OE, Zemlyakov AE, Ivanov AE, Zubov VP, and Mochalova LV (1993) Synthesis of polymeric neoglycoconjugates based on N-substituted polyacrylamides. Glycoconj J 10 142-151. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Paulson JC, and Varki A (2007) Siglecs and their roles in the immune system. Nat Rev Immunol 7 255-266. [DOI] [PubMed] [Google Scholar]
- Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, Steel J, and Crocker PR (2000) Siglec-8: a novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem 275 861-866. [DOI] [PubMed] [Google Scholar]
- Kearley J, Jones C, McMillan SJ, Cromie K, Crocker PR, and Lloyd CM (2007) Anti-Siglec-F antibody treatment during allergen-induced airway inflammation reduces eosinophil numbers but has no effect on airway hyperreactivity in vivo (Abstract). Am J Respir Crit Care Med 175 A690. [Google Scholar]
- Kikly KK, Bochner BS, Freeman SD, Tan KB, Gallagher KT, D'alessio KJ, Holmes SD, Abrahamson JA, Erickson-Miller CL, Murdock PR, et al. (2000) Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells and basophils. J Allergy Clin Immunol 105 1093-1100. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Schleimer RP, Saito H, Iikura Y, and Bochner BS (1995) Induction of apoptosis in human eosinophils by anti-fas antibody treatment in vitro. Blood 86 1437-1443. [PubMed] [Google Scholar]
- Matsumoto K, Sterbinsky SA, Bickel CA, Zhou DF, Kovach NL, and Bochner BS (1997) Regulation of α4 integrin-mediated adhesion of human eosinophils to fibronectin and vascular cell adhesion molecule-1 (VCAM-1). J Allergy Clin Immunol 99 648-656. [DOI] [PubMed] [Google Scholar]
- Nicoll G, Ni J, Liu D, Klenerman P, Munday J, Dubock S, Mattei MG, and Crocker PR (1999) Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J Biol Chem 274 34089-34095. [DOI] [PubMed] [Google Scholar]
- Nimrichter L, Burdick MM, Aoki K, Laroy W, Fierro MA, Hudson SA, Von Seggern CE, Cotter RJ, Bochner BS, Tiemeyer M, et al. (2008) E-selectin receptors on human leukocytes. Blood 112 3744-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutku E, Aizawa H, Hudson SA, and Bochner BS (2003) Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood 101 5014-5020. [DOI] [PubMed] [Google Scholar]
- Nutku E, Hudson SA, and Bochner BS (2005) Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem Biophys Res Commun 336 918-924. [DOI] [PubMed] [Google Scholar]
- Nutku-Bilir E, Hudson SA, and Bochner BS (2008) Interleukin-5 priming of human eosinophils alters siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol 38 121-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazynina GV, Sablina MA, Tuzikov AB, Chinarev AA, and Bovin NV (2003) Synthesis of complex α2–3 sialooligosaccharides, including sulfated and fucosylated ones, was done using Neu5Acα2–3Gal as a building block. Mendeleev Commun 13 245-248. [Google Scholar]
- Rapoport EM, Pazynina GV, Sablina MA, Crocker PR, and Bovin NV (2006) Probing sialic acid binding Ig-like lectins (siglecs) with sulfated oligosaccharides. Biochemistry (Mosc) 71 496-504. [DOI] [PubMed] [Google Scholar]
- Shilova NV, Galanina OE, Pochechueva TV, Chinarev AA, Kadykov VA, Tuzikov AB, and Bovin NV (2005) High molecular weight neoglycoconjugates for solid phase assays. Glycoconj J 22 43-51. [DOI] [PubMed] [Google Scholar]
- Song DJ, Cho JY, Miller M, Strangman W, Zhang M, Varki A, and Broide DH (2009) Anti-Siglec-F antibody inhibits oral egg allergen induced intestinal eosinophilic inflammation in a mouse model. Clin Immunol 131 157-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateno H, Crocker PR, and Paulson JC (2005) Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6′-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology 15 1125-1135. [DOI] [PubMed] [Google Scholar]
- Tateno H, Li H, Schur MJ, Bovin N, Crocker PR, Wakarchuk WW, and Paulson JC (2007) Distinct endocytic mechanisms of CD22 (Siglec-2) and Siglec-F reflect roles in cell signaling and innate immunity. Mol Cell Biol 27 5699-5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A and Angata T (2006) Siglecs—the major sub-family of I-type lectins. Glycobiology 16: 1R-27R. [DOI] [PubMed] [Google Scholar]
- von Gunten S and Bochner BS (2008) Basic and clinical immunology of Siglecs. Ann N Y Acad Sci 1143 61-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gunten S, Vogel M, Schaub A, Stadler BM, Miescher S, Crocker PR, and Simon HU (2007) Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. J Allergy Clin Immunol 119 1005-1011. [DOI] [PubMed] [Google Scholar]
- Yokoi H, Choi OH, Hubbard W, Lee HS, Canning BJ, Lee HH, Ryu SD, von Gunten S, Bickel CA, Hudson SA, et al. (2008) Inhibition of FcεRI-dependent mediator release and calcium flux from human mast cells by Siglec-8 engagement. J Allergy Clin Immunol 121 499-505.e1. [DOI] [PubMed] [Google Scholar]
- Zemlyanukhina TV, Nifantév NE, Shashkov AS, Tsvetkov YE, and N.V. Bovin (1995) Selectin receptors: synthesis of spacer-armed sulfated trisaccharides Lewis A and Lewis X and neoglycoconjugates thereof. Carbohydrate Lett 1 277-284. [Google Scholar]
- Zhang M, Angata T, Cho JY, Miller M, Broide DH, and Varki A (2007) Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood 109 4280-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann N, McBride ML, Yamada Y, Hudson SA, Jones C, Cromie KD, Crocker PR, Rothenberg ME, and Bochner BS (2008) Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy 63 1156-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]