Abstract
Objective
To compare weekend atropine augmented by a plano lens for the sound eye with weekend atropine alone for moderate amblyopia in children 3 to <7 years old.
Methods
In a multi-center clinical trial, 180 children with moderate amblyopia (20/40 to 20/100) were randomized to weekend atropine augmented by a plano lens or weekend atropine alone.
Main Outcome Measure
Masked assessment of amblyopic eye visual acuity using the Amblyopia Treatment Study HOTV testing protocol at 18 weeks.
Results
At 18 weeks, amblyopic eye improvement averaged 2.8 lines in the atropine plus plano lens group and 2.4 lines in the atropine alone group (mean difference between groups adjusted for baseline acuity 0.3 lines; 95% confidence interval, −0.2 to 0.8). Amblyopic eye acuity was 20/25 or better in 24 (29%) patients in the atropine-only group and 35 (40%) patients in the atropine plus plano lens group (P=0.03). More patients in the atropine plus plano lens group had reduced sound eye acuity at 18 weeks; however, there were no cases of persistent reverse amblyopia.
Conclusions
As an initial treatment for moderate amblyopia, the augmentation of weekend atropine with a plano lens does not substantially improve amblyopic eye acuity when compared with atropine alone.
Application to Clinical Practice
Treatment of children with unilateral amblyopia
Trial Registry Name
Trial Comparing Atropine to Atropine Plus a Plano Lens for the Sound Eye for Amblyopia in Children 3 to <7 Years Old
Introduction
Amblyopia is the most common cause of monocular visual impairment in children. Generally accepted treatment consists of providing the optimal refractive correction followed by occlusion or blurring of the sound eye. Historically, occlusion therapy has been the mainstay of treatment and the most commonly prescribed amblyopia therapy. However in recent years, pharmacological penalization of the sound eye with atropine has gained increased use as an alternative to patching. For patients whose sound eyes are optically corrected for distance, atropine results in blur of the sound eye at near only. In patients with hypermetropia, distance vision also will be blurred when the hypermetropic refractive error is left uncorrected or undercorrected.
Two clinical trials conducted by the Pediatric Eye Disease Investigator Group (PEDIG) have evaluated atropine as a primary treatment for moderate amblyopia in children 3 to < 7 years of age. In a randomized trial of 419 children with moderate amblyopia, one drop of atropine 1% administered daily was shown to be equivalent to 6 or more hours per day of prescribed patching after 6 months of treatment.1 While patching resulted in a more rapid improvement, atropine had the advantage of easier administration, lower cost, and was found to be a more acceptable treatment in terms of compliance and social stigma.2 In a subsequent randomized trial of 168 young children with moderate amblyopia, the use of atropine on weekends only was found to be as effective as atropine administered daily.3
It has been suggested that the treatment effect of atropine can be enhanced by undercorrecting hypermetropic refractive error in the atropine-treated sound eye;4 however, others have noted that the combination of atropine therapy and optical penalization can result in severe treatment-related amblyopia developing in the sound eye when parental noncompliance occurs.5 In the PEDIG trial comparing patching and atropine as treatments for moderate amblyopia,1 a plano lens was prescribed for hypermetropic sound eyes of patients randomized to atropine who were not successfully treated with atropine alone at the 16-week visit. Although the presumption was that removing the corrective lens of hypermetropic sound eyes would more effectively blur these eyes at both near and distance fixation, and thus increase the intensity of treatment, the study was not designed to determine whether the treatment effect was enhanced by this modification to treatment.
To evaluate whether it is beneficial and safe to reduce the optical correction of hypermetropia in sound eyes at the time of initiation of atropine therapy, we conducted a randomized trial in children 3 to < 7 years of age with moderate amblyopia (20/40 to 20/100) and at least 1.50 D of hypermetropia in the sound eye.
Methods
The study was supported through a cooperative agreement with the National Eye Institute of the National Institutes of Health, Department of Health and Human Services and was conducted by the Pediatric Eye Disease Investigator Group (PEDIG) at 30 clinical sites. The protocol and Health Insurance Portability and Accountability Act (HIPAA) compliant informed consent forms were approved by the institutional review boards for participating sites, and a parent or guardian (referred to subsequently as “parent”) of each study patient gave written informed consent. Study oversight was provided by an independent data and safety monitoring committee. The study is listed on www.clinicaltrials.gov, under identifier NCT00315302. The protocol is available on the PEDIG website (www.pedig.net) and is summarized below.
Patient Selection
The major eligibility criteria were: age 3 to < 7 years, best-corrected visual acuity in the amblyopic eye between 20/40 and 20/100 inclusive, sound eye best-corrected visual acuity of 20/40 or better, interocular acuity difference of ≥ 3 logarithm of the minimum angle of resolution (logMAR) lines, the presence of or history of an amblyogenic factor meeting study-specified criteria for strabismus and/or anisometropia, hypermetropia ≥ +1.50 diopters (D) spherical equivalent in the sound eye, and the wearing of optimal spectacle correction for a minimum of 16 weeks or until stability of visual acuity was documented (no improvement in amblyopic eye visual acuity at 2 consecutive visits at least 4 weeks apart). Table 1 (website) provides a complete listing of eligibility and exclusion criteria.
Table 1.
Eligibility and Exclusion Criteria (for online)
Eligibility Criteria
|
| Exclusion Criteria |
|
D = diopters; logMAR = logarithm of the minimum angle of resolution
Randomization and Treatment Protocols
After eligibility was confirmed and informed consent was obtained, data were entered on the PEDIG website to randomly assign each patient (using a permuted-blocks design stratified by site) with equal probability to one of two treatment groups: atropine alone (atropine-only group) or atropine augmented with a plano lens for the sound eye (atropine plus plano group).
At enrollment, patients were provided with atropine sulfate 1% solution, with instructions to place one drop in the sound eye each Saturday and Sunday for 16 weeks. New spectacles were provided with refractive correction such that: (1) in the atropine-only group, neither eye was underplused by more than 0.50 D spherical equivalent and (2) in the atropine plus plano group, the amblyopic eye was underplused by no more than 0.50 D spherical equivalent and the sound eye was prescribed a plano lens. Sunglasses were provided, which were to be worn with a brimmed hat when the child was in sunlight. If an allergy to atropine developed, one drop of homatropine 5% solution each morning for 5 days per week was substituted. Before the 18-week outcome examination, patching or alternate therapies for amblyopia were not to be prescribed, even if there was no response to treatment. If reverse amblyopia was suspected, or strabismus developed or worsened, continuation of study treatment was at investigator discretion.
At each visit, the parent was queried about side effects of treatment and adherence to the treatment protocol. Adherence was assessed by having the parent record on a calendar the days atropine was administered. Based on discussion with the parents and review of the calendars brought in at each visit, the investigator made an assessment of adherence to the prescribed treatment (excellent, 76%–100%; good, 51%–75%; fair, 26%–50%; or poor, ≥ 25%).
Follow-up Schedule
Follow-up visits were performed at 5 weeks (± 1 week), 10 weeks (± 1 week), and 18 weeks (± 1 week), the latter being the primary outcome visit. Parents were instructed to discontinue the use of atropine 2 weeks prior to the 18-week outcome examination. At each protocol-specified visit, visual acuity was measured in each eye using the Amblyopia Treatment Study (ATS) visual acuity testing protocol.6 Ocular alignment and stereoacuity were assessed at the 18-week outcome examination.
Visual acuity testing at the 18-week outcome examination was conducted by an examiner masked to treatment group. Following the masked testing, visual acuity was retested in the amblyopic eye by the same or another tester if acuity was worse than 20/25 and either not improved from baseline by two or more lines or not improved from the last visit by at least one line. The better of the two amblyopic eye acuity measures was used to determine the need for continued follow up in the study. Visual acuity was retested in the sound eye if reduced by 1 or more lines from baseline. If acuity in the sound eye was still reduced after retesting, a cycloplegic refraction was performed and acuity was tested again. If visual acuity was still reduced and there was a change in refraction, a change in spectacles was prescribed. Regardless of whether a change in spectacle correction was made, the patient remained off treatment and returned for a reevaluation of visual acuity in 1 to 4 weeks.
After the 18-week outcome examination, patients were discontinued from the study if amblyopia had either (1) resolved (amblyopic eye visual acuity either ≥ 20/25 or equal to or better than the sound eye acuity) or (2) not improved at least 2 lines from baseline and at least one line from the 10-week visit. Patients in whom amblyopia had improved but not resolved remained on the randomization-assigned treatment and were followed every 8 weeks (± 1 week) until one of the following occurred: (1) amblyopic eye acuity was 20/25 or better, (2) there was no further improvement in amblyopic eye acuity (defined as no better acuity than that at the prior visit on a test and retest of acuity), or (3) worsening of sound eye acuity occurred.
Examination Procedures
At the enrollment visit and at each protocol-specified visit, visual acuity was measured in each eye using the ATS visual acuity testing protocol (consisting of single-surrounded HOTV optotypes),6 administered by a study-certified vision examiner using an electronic visual acuity tester.7 Additional baseline testing included a cycloplegic refraction, an ocular examination, measurement of ocular alignment with a simultaneous prism and cover test at distance and near, and assessment of binocularity with the Titmus (fly only) and the Randot Preschool Stereoacuity Tests (Stereo Optical Co., Chicago, IL). Ocular alignment and stereoacuity testing were repeated at the 18-week outcome examination.
Statistical Methods
The trial was designed to evaluate whether one treatment regimen was superior to the other. The primary outcome was the change in amblyopic eye visual acuity from baseline to 18 weeks. A sample size of 172 patients was planned to have 90% power to detect a difference in the primary outcome between groups, assuming a population difference of 0.075 logMAR, a 2-sided type 1 error rate of 5%, no more than 5% loss to follow up, standard deviation of 18-week visual acuity scores of 0.16 logMAR, and correlation between 18-week and baseline visual acuity scores of 0.40 (the latter two assumptions based on prior PEDIG studies using atropine for the treatment of amblyopia1, 3).
The primary analysis was a treatment group comparison of logMAR visual acuity scores in the amblyopic eye obtained 18 weeks from randomization in an analysis of covariance (ANCOVA) model adjusting for baseline acuity. When the protocol mandated retesting of amblyopic eye visual acuity at the 18-week outcome examination, only the initial masked acuity measure was used for analysis. All analyses followed the intent-to-treat principle. To be included in the primary analysis, the 18-week outcome examination could be performed no earlier than 14 weeks from randomization and no later than 27 weeks. Results were also obtained from the following alternative analyses: (1) including only visits completed in the visit window of 18 ± 1 weeks from randomization, (2) using the last-observation-carried-forward method to impute for missing data, and (3) including in the ANCOVA model(s) baseline variables found to be imbalanced between groups.
The proportion of patients in each treatment group with amblyopic eye visual acuity ≥ 20/25 at 18 weeks was compared using logistic regression adjusting for baseline acuity, as was the proportion of patients whose amblyopic eye visual acuity improved at least 3 lines from enrollment. The exact Wilcoxon rank sum test was used for treatment group comparison of change in stereoacuity scores.
Methods used to analyze the maximum visual acuity at the 18-week outcome visit or subsequent visits paralleled the analysis conducted on the 18-week data. The difference between treatment groups in additional improvement for patients continuing on treatment beyond the 18-week outcome exam was evaluated with an independent samples t-test. The difference between groups in the proportion of patients with 2 or more lines improvement with continued treatment was evaluated with a Fisher’s Exact Test.
The treatment effect in subgroups based on baseline characteristics (cause of amblyopia, age, prior treatment, baseline amblyopic eye visual acuity, and refractive error in the sound eye) was assessed by including interaction terms in the ANCOVA models.
The difference between groups in the proportion of patients with sound eye visual acuity worse than 20/20 and reduced from baseline by 1 or more lines at 18 weeks was evaluated with a Fisher’s Exact Test.
To determine whether the treatments differed with respect to rate of improvement in visual acuity, a straight line was fit to the amblyopic eye visual acuity over follow-up time for each patient and the average slope for patients on each treatment was compared using a random effects model. A log transformation for time was used to form a linear relationship between visual acuity and time.
Results
Between February 2005 and May 2007, 180 patients entered the trial at 30 sites, with 90 assigned to the atropine-only group and 90 assigned to the atropine plus plano lens group. The average age of the patients was 5.1 years; 52% were female, and 81% were white. The mean visual acuity in the amblyopic eye at enrollment was 0.48 logMAR (approximately 20/63), with a mean difference in acuity between eyes of 4.1 lines. The mean spherical equivalent refractive error in the sound and amblyopic eyes were 3.62 D and 4.97 D of hypermetropia, respectively. Additional baseline characteristics of the two groups are provided in Table 2.
Table 2.
Baseline Data for all Randomized Patients
| Atropine Only N=90 | Atropine + Plano Lens N=90 | |
|---|---|---|
| n (%) | n (%) | |
| Gender: Female | 47 (52) | 47 (52) |
|
| ||
| Race/Ethnicity | ||
| White | 75 (83) | 70 (78) |
| African-American | 1 (1) | 4 (4) |
| Hispanic or Latino | 12 (13) | 14 (16) |
| Asian | 1 (1) | 2 (2) |
| Unknown/Not reported | 1 (1) | 0 |
|
| ||
| Age at Enrollment | ||
| 3 to <4 years | 14 (16) | 11 (12) |
| 4 to <5 years | 24 (27) | 30 (33) |
| 5 to <6 years | 29 (32) | 29 (32) |
| 6 to <7 years | 23 (26) | 20 (22) |
| Mean (SD) | 5.2 (1.1) | 5.1 (1.0) |
|
| ||
| Prior Treatment for Amblyopia at Enrollment | ||
| None | 76 (84) | 72 (80) |
| Patching | 9 (10) | 12 (13) |
| Atropine | 1 (1) | 2 (2) |
| Patching and Atropine* | 4 (4) | 4 (4) |
|
| ||
| Cause of Amblyopia | ||
| Strabismus | 38 (42) | 37 (41) |
| Anisometropia | 32 (36) | 30 (33) |
| Strabismus and anisometropia | 20 (22) | 23 (26) |
|
| ||
| Distance Visual Acuity in Amblyopic Eye | ||
| 20/100 | 7 (8) | 15 (17) |
| 20/80 | 14 (16) | 15 (17) |
| 20/63 | 29 (32) | 28 (31) |
| 20/50 | 24 (27) | 17 (19) |
| 20/40 | 16 (18) | 15 (17) |
| Mean (SD) logMAR | 0.47 (0.12) | 0.50 (0.13) |
| Snellen Equivalent | 20/63 | 20/63 |
|
| ||
| Distance Visual Acuity in Sound Eye | ||
| 20/40 | 3 (3) | 3 (3) |
| 20/32 | 18 (20) | 18 (20) |
| 20/25 | 27 (30) | 28 (31) |
| 20/20 | 31 (34) | 33 (37) |
| 20/16 | 11 (12) | 8 (9) |
| Mean (SD) logMAR | 0.07 (0.10) | 0.07 (0.10) |
| Snellen Equivalent | 20/25 | 20/25 |
|
| ||
| Intereye Acuity Difference | ||
| Mean (SD) logMAR lines | 4.0 (1.2) | 4.3 (1.3) |
|
| ||
| Refractive Error in Amblyopic Eye (sph equiv) | ||
| 0 to < +1.00D | 1 (1) | 0 |
| +1.00 to <+2.00D | 0 | 6 (7) |
| +2.00 to <+3.00D | 8 (9) | 6 (7) |
| +3.00 to <+4.00D | 13 (14) | 15 (17) |
| ≥+4.00D | 68 (76) | 63 (70) |
| Mean (SD) D | + 4.99 (1.66) | +4.94 (1.81) |
|
| ||
| Refractive Error in Sound Eye (sph equiv) | ||
| +1.50 to <+2.00D | 17 (19) | 23 (26) |
| +2.00 to <+3.00D | 14 (16) | 19 (21) |
| +3.00 to <+4.00D | 20 (22) | 12 (13) |
| ≥+4.00D | 39 (43) | 36 (40) |
| Mean (SD) D | +3.71 (1.71) | +3.54 (1.86) |
One of these patients received atropine + patching + fogging SD = standard deviation; D = diopters; logMAR = logarithm of the minimum angle of resolution; sph equiv = spherical equivalent
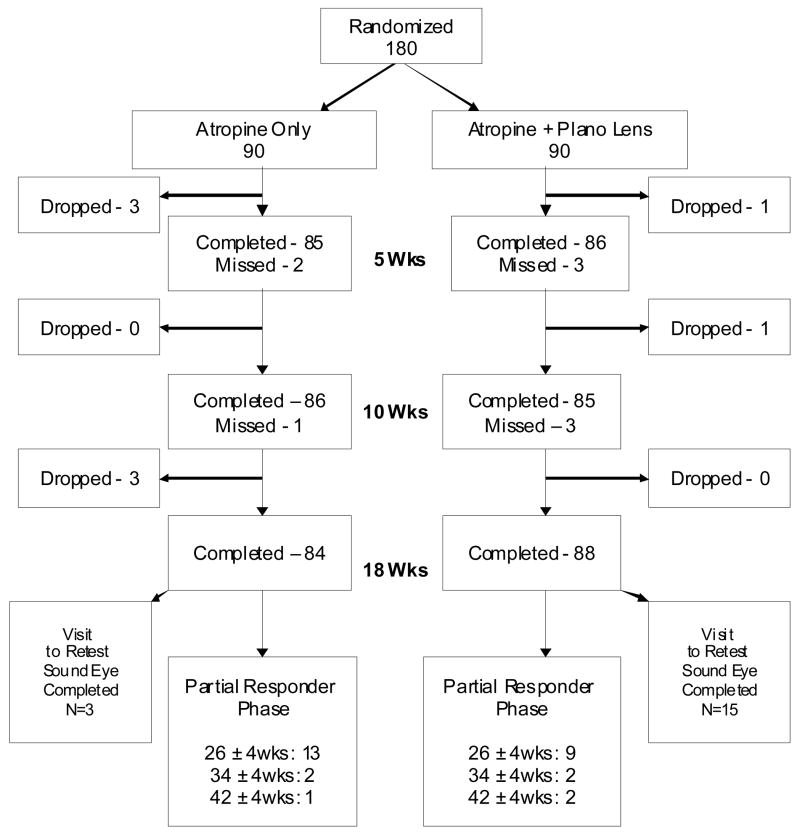
Visit Completion
Figure 1 provides the visit completion rates for the 5-week, 10-week, and 18-week visits. The 18-week outcome examination was completed by 84 (93%) of the 90 patients in the atropine-only group and 88 (98%) of the 90 patients in the atropine plus plano lens group. The vision tester was masked to treatment group for 100% of the atropine-only group examinations and 98% the atropine plus plano lens group examinations.
Figure 1. Visit Completion.
Flow chart showing study completion in each treatment group.
Treatment
Among the patients completing the 18-week outcome examination, the randomization-assigned treatment was prescribed throughout follow up for 80 (95%) patients in the atropine-only group and for 78 (89%) patients in the atropine plus plano lens group. Of the 4 patients in the atropine-only group who did not remain in their assigned treatment group, two switched to patching and two were switched to homatropine 5%. Of the 10 patients in the atropine plus plano lens group who did not remain on assigned treatment, one switched to patching, one was decreased to once per week atropine administration, one had the plano lens replaced with a corrective lens when the amblyopia resolved, one was increased to daily atropine, one was switched to homatropine 5%, and five had their plano lens replaced with the appropriate hypermetropic spectacle lens due to suspected reverse amblyopia.
During the 18 weeks of the study, patient adherence to the prescribed treatment was judged by the investigator to be excellent in 94%, good in 4%, fair in 1%, and poor in 1% of patients in the atropine-only group, and to be excellent in 89%, good in 7%, fair in 2%, and poor in 1% of patients in the atropine plus plano lens group.
Effect of Treatment on Visual Acuity in the Amblyopic Eye
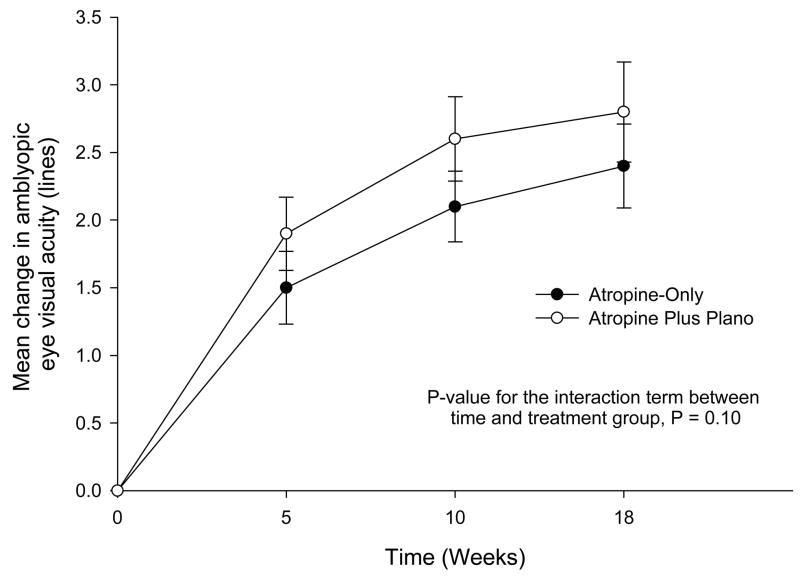
At the 18-week primary outcome examination, improvement in amblyopic eye visual acuity averaged 2.4 lines in the atropine-only group and 2.8 lines in the atropine plus plano lens group (Table 3). The treatment group difference in mean logMAR acuity adjusted for baseline visual acuity was 0.3 lines (95% confidence interval (CI), −0.2 to 0.8). Results at 18-weeks from alternative analyses were similar (data not shown). Results at the 5-week and 10-week visits were also similar, with adjusted mean differences being 0.3 lines (95% CI, 0.0 to 0.7) and 0.4 lines (95% CI, 0.0 to 0.8), respectively (Figure 2).
Table 3.
Amblyopic Eye Visual Acuity at 18-Week Outcome Examination
| 18-Weeks
|
||
|---|---|---|
| Atropine Only N=84 n(%) | Atropine + Plano Lens N=88 n(%) | |
| Lines change from baseline | ||
| −3 | 0 | 1 (1) |
| −2 | 0 | 0 |
| −1 | 2 (2) | 2 (2) |
| 0 | 4 (5) | 5 (6) |
| +1 | 18 (21) | 11 (13) |
| +2 | 18 (21) | 19 (22) |
| +3 | 22 (26) | 18 (20) |
| +4 | 17 (20) | 18 (20) |
| ≥ +5 | 3 (4) | 14 (16) |
| Mean (SD) lines change | 2.4 (1.4) | 2.8 (1.8) |
|
| ||
| Distribution of visual acuity | ||
| 20/160 | 0 | 1 (1) |
| 20/125 | 0 | 1 (1) |
| 20/100 | 2 (2) | 1 (1) |
| 20/80 | 2 (2) | 4 (5) |
| 20/63 | 4 (5) | 6 (7) |
| 20/50 | 6 (7) | 7 (8) |
| 20/40 | 18 (21) | 9 (10) |
| 20/32 | 28 (33) | 24 (27) |
| 20/25 | 12 (14) | 19 (22) |
| 20/20 | 11 (13) | 12 (14) |
| 20/16 | 1 (1) | 4 (5) |
| 20/25 or better | 24 (29) | 35 (40) |
| Mean (SD) logMAR | 0.23 (0.16) | 0.22 (0.20) |
| (Snellen equivalent) | (20/32) | (20/32) |
|
| ||
| Mean difference between treatment groups inacuity lines, adjusted for baseline acuity (95% confidence interval) | 0.3 (−0.2, 0.8) | |
SD = standard deviation; logMAR = logarithm of the minimum angle of resolution
Figure 2. Rate of Improvement in Amblyopic Eye Visual Acuity.
Amblyopic eye visual acuity in each group at baseline, 5 weeks, 10 weeks, and 18 weeks. The point estimates and 95% confidence intervals for the mean change from baseline are shown. A log transformation for time (plus 0.5) was used to form a linear relationship between visual acuity and time. SD = standard deviation; logMAR = logarithm of the minimum angle of resolution; A = Atropine-only group; A + P = Atropine plus plano lens group.
| 20/160 | 1%† | |||||||
| 20/125 | 2%* | 1%* | 1%* | |||||
| 20/100 | 8% | 17% | 2% | 1% | 2% | 2% | 1% | |
| 20/80 | 16% | 17% | 4% | 4% | 2% | 2% | 2% | 5% |
| 20/63 | 32% | 31% | 12% | 11% | 6% | 8% | 5% | 7% |
| 20/50 | 27% | 19% | 27% | 15% | 17% | 7% | 7% | 8% |
| 20/40 | 18% | 17% | 20% | 24% | 24% | 19% | 21% | 10% |
| 20/32 | 21% | 33% | 27% | 31% | 33% | 27% | ||
| 20/25 | 11% | 11% | 13% | 21% | 15% | 22% | ||
| 20/20 | 4% | 9% | 9% | 13% | 14% | |||
| 20/16 | 1% | 5% | ||||||
| Mean logMAR | 0.47 | 0.50 | 0.32 | 0.31 | 0.26 | 0.24 | 0.23 | 0.22 |
| (SD) | 0.12 | 0.13 | 0.16 | 0.15 | 0.15 | 0.16 | 0.16 | 0.20 |
| Mean Lines | -- | -- | 1.5 | 1.9 | 2.1 | 2.6 | 2.4 | 2.8 |
| Change | ||||||||
| (SD) | 1.3 | 1.3 | 1.2 | 1.5 | 1.4 | 1.8 | ||
| Treatment Group | A | A + P | A | A + P | A | A + P | A | A + P |
| Visit | Baseline | 5 Weeks | 10 Weeks | 18 Weeks | ||||
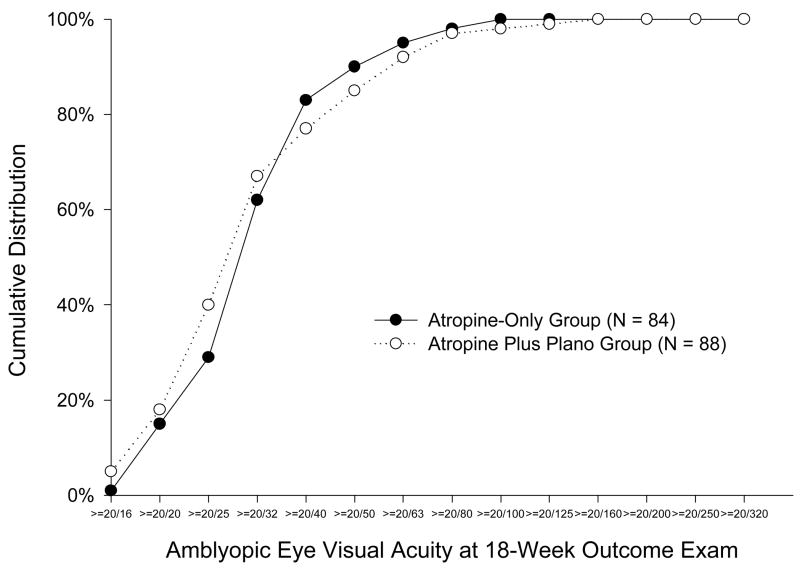
Amblyopic eye acuity met the pre-specified secondary outcome of 20/25 or better in 24 (29%) patients in the atropine-only group and 35 (40%) patients in the atropine plus plano lens group (P = 0.03, Table 3, Figure 3). For 41 (49%) patients in the atropine-only group and 50 (57%) patients in the atropine plus plano lens group, amblyopic eye visual acuity at 18 weeks improved at least 3 lines from enrollment (P = 0.39). There were 4 patients (2 in each treatment group) whose amblyopic eye acuity at 18 weeks was one line worse than at baseline, who then tested better than or equal to baseline upon retesting during the same visit; however, by protocol the first acuity test was counted as the “outcome examination” (Figure 2). One other patient’s (in atropine plus plano lens group but not on treatment because of lost spectacles) amblyopic eye visual acuity decreased from 20/80 at baseline to 20/160 at 18weeks for an unknown reason.
Figure 3. Cumulative Distribution of Amblyopic Eye Visual Acuity Scores at the 18-Week Outcome Examination According to Treatment Group.
Cumulative distribution of amblyopic eye visual acuity scores at 18-week outcome exam according to treatment group.
Post 18-week Follow-up
At the 18-week outcome examination, 16 (18%) patients in the atropine-only group and 17 (19%) patients in the atropine plus plano lens group met the study criteria for continued treatment (amblyopic eye visual acuity improved at least 2 lines from baseline and 1 line from the previous visit, but still worse than 20/25 and worse than sound eye acuity). Three of the 17 patients in the atropine plus plano lens group did not continue in follow up. Among the remaining 30 patients, follow up continued for one 8-week cycle for 12 in the atropine only group and 11 in the atropine plus plano lens group, and two 8-week cycles for 4 and 3 patients, respectively. The mean additional improvement in visual acuity among these patients was 0.2 lines in the atropine only group and 1.1 lines in the atropine plus plano lens group (P=0.01). One patient in the atropine only group and six patients in the atropine plus plano lens group tested two or more lines better with continued treatment (P=0.03). The mean maximum visual acuity improvement measured at the 18-week visit or a subsequent visit was 2.5 lines in the atropine-only group and 3.0 lines in the atropine plus plano group (treatment group difference in mean logMAR acuity adjusted for baseline visual acuity 0.4 lines, 95% confidence interval −0.1 to 0.9). Maximum visual acuity was 20/25 or better in 24 (29%) patients in the atropine-only group and 39 (44%) patients in the atropine plus plano lens group (P = 0.006). At the visit of maximum improvement in amblyopic eye visual acuity, 44 (52%) patients in the atropine-only group and 54 (61%) patients in the atropine plus plano lens group had improved at least 3 logMAR lines from enrollment (P = 0.38).
Influence of Baseline Factors
At 18 weeks, results in subgroups based on cause of amblyopia (strabismus, anisometropia, or combined-mechanism), age, prior treatment, baseline amblyopic eye visual acuity, and refractive error in the sound eye were similar to the overall results (Table 4 -website).
Table 4.
Mean Lines Change in Visual Acuity in the Amblyopic Eye at 18-week Outcome Examination According to Baseline Patient Characteristics (for online)
| Baseline Characteristic | N (Atropine, Atropine + Plano) | Mean Lines Improvement from Baseline to 18 weeks
|
|||
|---|---|---|---|---|---|
| Atropine | Atropine + Plano Lens | P-Value for interaction* | |||
| All Patients | (84,88) | 2.4 | 2.8 | ||
|
| |||||
| Age | 0.51 | ||||
| <5 years | (34, 41) | 2.5 | 2.7 | ||
| ≥ 5 years | (50, 47) | 2.4 | 2.8 | ||
|
| |||||
| Baseline Amblyopic Eye Acuity | 0.71 | ||||
| ≥ 20/50 | (38, 30) | 2.1 | 2.5 | ||
| < 20/50 | (46, 58) | 2.6 | 2.9 | ||
|
| |||||
| Prior Amblyopia Treatment | 0.53 | ||||
| Yes | (14, 18) | 2.1 | 3.1 | ||
| No | (70, 70) | 2.5 | 2.7 | ||
|
| |||||
| Cause of Amblyopia | 0.79 | ||||
| Strabismus | (36, 36) | 2.5 | 2.8 | ||
| Anisometropia | (29, 30) | 2.4 | 2.7 | ||
| Strabismus and Anisometropia | (19, 22) | 2.2 | 2.9 | ||
|
| |||||
| Refractive Error in Sound Eye | 0.40 | ||||
| < +3.00D | (28, 40) | 2.3 | 2.9 | ||
| ≥ +3.00D | (56, 48) | 2.5 | 2.7 | ||
The P-values are for the interaction between the characteristic and treatment, adjusted by baseline amblyopic eye visual acuity
D = diopters
Stereoacuity
There was no difference between treatment groups in stereoacuity at the 18-week outcome visit (P=0.39 overall and P=0.90 for patients with purely anisometropic amblyopia, Table 5 - website)
Table 5.
Randot Preschool Stereoacuity at Baseline and 18 Weeks (for online)
| All Patients
| ||||
|---|---|---|---|---|
| Randot Preschool Stereoacuity | Baseline – N (%)
|
18 Weeks – N (%)
|
||
| Atropine only (N=84) | Atropine+Plano (N=88) | Atropine only (N=84) | Atropine+Plano (N=88) | |
| Failed pretest | 8 (10) | 6 (7) | 3 (4) | 4 (5) |
| >800 | 39 (46) | 47 (53) | 40 (48) | 41 (47) |
| 800 | 12 (14) | 10 (11) | 7 (8) | 10 (11) |
| 400 | 10 (12) | 8 (9) | 11 (13) | 12 (14) |
| 200 | 8 (10) | 8 (9) | 10 (12) | 6 (7) |
| 100 | 4 (5) | 5 (6) | 4 (5) | 10 (11) |
| 60 | 2 (2) | 3 (3) | 5 (6) | 1 (1) |
| 40 | 1 (1) | 1 (1) | 2 (2) | 1 (1) |
| Not done | 0 | 0 | 2 (2) | 3 (3) |
|
Anisometropia Only
| ||||
| Randot Preschool Stereoacuity |
Baseline – N (%)
|
18 Weeks – N (%)
|
||
| Atropine only (N=29) | Atropine+Plano (N=30) | Atropine only (N=29) | Atropine+Plano (N=30) | |
|
| ||||
| Failed pretest | 1 (3) | 1 (3) | 0 | 0 |
| >800 | 8 (28) | 11 (37) | 7 (24) | 9 (30) |
| 800 | 3 (10) | 5 (17) | 2 (7) | 3 (10) |
| 400 | 6 (21) | 4 (13) | 5 (17) | 6 (20) |
| 200 | 5 (17) | 3 (10) | 8 (28) | 3 (10) |
| 100 | 4 (14) | 3 (10) | 3 (10) | 6 (20) |
| 60 | 1 (3) | 2 (7) | 1 (3) | 1 (3) |
| 40 | 1 (3) | 1 (3) | 2 (7) | 1 (3) |
| Not done | 0 | 0 | 1 (3) | 1 (3) |
P = 0.39 from exact Wilcoxon Rank Sum Test comparing treatment groups with respect to levels of change from baseline to 18 weeks
P = 0.90 from exact Wilcoxon Rank Sum Test comparing treatment groups with respect to levels of change from baseline to 18 weeks
Effect of Treatment on Visual Acuity in the Sound Eye
At the 18-week outcome examination, sound eye visual acuity changed from baseline by an average of +0.4 lines in the atropine-only group and 0.0 lines in the atropine plus plano lens group (Table 6). For 3 (4%) patients in the atropine-only group and 15 (17%) patients in the atropine plus plano lens group, sound eye acuity was worse than 20/20 and reduced by 1 or more lines from baseline at the 18-week visit (P=0.005). These patients had an additional study visit from 1 to 4 weeks after the 18-week outcome examination to recheck the sound eye acuity. Within this group, sound eye visual acuity returned to baseline or better in 1 of the 3 patients in the atropine-only group and in 8 of 14 patients in the atropine plus plano lens group (one patient in the atropine plus plano lens group dropped from the study after the 18-week visit). Post-study examinations on the remaining 8 patients whose acuity was 1 or more lines worse than baseline (2 of whom were actively treated for suspected reverse amblyopia) indicated that in 7 of the 8 patients the sound eye acuity subsequently was measured to be the same or better than at baseline and 1 patient who tested 20/20 at baseline was one line worse (20/25) at the last follow-up visit.
Table 6.
Lines Change in Sound Eye Visual Acuity from Baseline to 18 Weeks
| Atropine Only N= 84 n(%) | Atropine + Plano Lens N= 88 n(%) | |
|---|---|---|
| Lines change from baseline | ||
| −4 | 1 (1) | 1 (1) |
| −3 | 0 | 1 (1) |
| −2 | 0 | 3 (3) |
| −1 | 3 (4) | 12 (14) |
| 0 | 49 (58) | 50 (57) |
| +1 | 24 (29) | 17 (19) |
| +2 | 6 (7) | 4 (5) |
| +3 | 1 (1) | 0 |
| Mean (SD) lines change | 0.4 (0.9) | 0.0 (1.0) |
|
| ||
| Distribution of visual acuity | ||
| 20/63 | 1 (1) | 1 (1) |
| 20/50 | 0 | 3 (3) |
| 20/40 | 2 (2) | 3 (3) |
| 20/32 | 10 (12) | 16 (18) |
| 20/25 | 14 (17) | 22 (25) |
| 20/20 | 36 (43) | 28 (32) |
| 20/16 | 21 (25) | 15 (17) |
| Mean (SD) logMAR | 0.0 (0.1) | 0.1 (0.1) |
| (Snellen Equivalent) | 20/20 | 20/25 |
SD = standard deviation; logMAR = logarithm of the minimum angle of resolution
Other Adverse Effects
A new-onset manifest strabismus occurred in 3 patients in the atropine-only group and 4 patients in the atropine plus plano lens group, while manifest strabismus present at baseline resolved in 5 and 12 patients in the two groups, respectively. Ocular side effects, most commonly light sensitivity, were reported by 13 (7%) patients. Facial flushing was reported by six patients, three of whom were switched to homatropine 5%.
Discussion
We compared the effectiveness of prescribing weekend atropine augmented with a plano lens for the sound eye with weekend atropine alone for the treatment of moderate amblyopia in 180 children 3 to < 7 years old. By protocol all patients had +1.50 D or greater spherical equivalent hypermetropia in the sound eye. Therefore, those in the atropine plus plano lens group were blurred at both distance and near and those in the atropine-only group (who were undercorrected by no more than 0.50 D spherical equivalent) were effectively blurred at near only. Despite the atropine plus plano lens group having sound eyes presumably blurred full time and having increased blur with near viewing relative to the atropine-only group, the magnitude of visual acuity improvement in the amblyopic eye in this group at the 18-week outcome visit was only marginally better (approximately 0.3 lines), a difference that was neither statistically significant nor clinically compelling. With continuation of treatment in patients who showed an improvement from baseline at 18 weeks, there was more post-18 week acuity improvement in the atropine plus plano lens group (mean = 1.1 lines additional improvement) than in the atropine-only group (mean = 0.2 lines additional improvement), with the difference between treatment groups in the maximum acuity at the 18-week visit or a subsequent visit being about half a line. In a preplanned secondary analysis, a higher proportion (40%) of patients in the atropine plus plano group did achieve 20/25 or better visual acuity at 18 weeks in the amblyopic eye than those receiving atropine alone (29%). This modest difference should be considered when recommending the addition of a plano lens when initiating treatment of amblyopia with atropine. We did not find this effect persuasive for choosing to use a plano lens.
Since the current study did not evaluate residual amblyopia we do not know if there may be value in prescribing a plano lens in conjunction with continued atropine use in patients with an incomplete response to treatment with atropine alone. A randomized trial evaluating the benefit of adding a plano lens for residual atropine-treated amblyopia may have merit.
We analyzed treatment outcome with regard to age, baseline visual acuity in the amblyopic eye, cause of amblyopia, prior amblyopia treatment, and refractive error in the sound eye to determine whether any of these might be predictive of a response to treatment. No difference in the treatment response related to any of these factors was found.
Regarding the safety of atropine in treating amblyopia, similar to our previous studies of atropine,1, 3 side effects associated with treatment were mild and infrequent. When side-effects occurred, the patients were satisfactorily changed to homatropine without recurrence. At the 18-week visit, a reduction in sound eye acuity was more common in the atropine plus plano lens group than in the atropine only group (17% vs. 4%). Although the sound eye acuity subsequently was measured to be the same or better than at baseline after treatment was discontinued for all but one child, two of these children were actively treated for suspected reverse amblyopia with one still testing one line worse at the last follow-up visit than at baseline. A 1-line difference is within the variability of testing,6,7 and while we had no definitive cases of persistent reverse amblyopia, it has been reported to occur in children undergoing atropine penalization both with and without a reduced hypermetropic lens,5, 8, 9 including instances of continued treatment with inadequate follow up.5 Thus, sound eye acuity should be carefully monitored, particularly when atropine treatment is augmented with the undercorrection of hypermetropia in the sound eye with a plano lens. Another potential concern when prescribing a plano lens for the sound eye to augment atropine treatment is the effect on eye alignment in children with accommodative esotropia. However, in this study there was no difference between treatment groups in the development, worsening, or improvement of strabismus. Likewise, an adverse effect on stereoacuity was not found.
We could identify no sources of bias or confounding to explain our findings. The follow-up rate was uniformly high, missing data from patients who dropped out of the study did not influence the interpretation of results and spherical equivalent refractive error was similar in the two groups. There was a slight imbalance in baseline visual acuity between the groups (more children in the atropine plus plano group had 20/100), but this was accounted for in analysis. With few exceptions, children stayed on assigned treatment and there were no imbalances by treatment group for the few who switched treatment. Masking to the primary outcome measure of visual acuity was achieved in 99% of cases. Because the patients in this study had only moderate amblyopia, we caution against extrapolating these findings to patients with more severe amblyopia where augmentation with a plano lens might be more effective.
We are aware of only one published study of pharmacologic penalization using atropine plus a plano lens for the sound eye with which to compare our findings. In this retrospective and uncontrolled report of 42 children (mean age of 4.7 years) who had failed patching treatment and had at least 1.75 D of sound eye hypermetropia, Kaye and colleagues4 found a mean improvement in amblyopic eye visual acuity from 20/113 to 20/37 after 10 weeks of treatment with daily atropine and a plano lens for the sound eye. However, the absence of a control group treated with atropine alone makes it impossible to determine what proportion of the treatment effect resulted from adding the plano lens to the atropine treatment. Other differences in amblyopia severity, dosage, follow-up, and prior treatment also limit our ability to compare this study to ours.
Conclusion
Prescribing one-drop of atropine each weekend day augmented with a plano lens over the sound eye is not substantially better than prescribing weekend atropine alone when initiating treatment in 3 – < 7 year old children with hypermetropia ≥ 1.50D and moderate amblyopia.
Acknowledgments
Writing Committee: Lead authors: Susan A. Cotter OD, David R. Weakley Jr. MD, and Samara F. Strauber MS. Additional writing committee members (alphabetical): Roy W. Beck MD, PhD, Eileen E. Birch PhD, Sean Donahue MD, Jonathan M. Holmes BM, BCh, Darren L. Hoover MD, Pamela A. Huston, B. Michele Melia ScM, Michael X. Repka MD, David T. Wheeler MD
The Pediatric Eye Disease Investigator Group
Clinical Sites that Participated in this Protocol:
Sites are listed in order by number of patients enrolled into the study. Personnel are listed as (I) for Investigator, (C) for Coordinator, and (V) for Visual Acuity Tester.
Cranberry TWP PA - Everett and Hurite Ophthalmic Association (39)
Darren L. Hoover, (I); Pamela A. Huston, (C); Jody L. Parker, (V); Pamela M. Racan, (V); Barbara R. Fuchs, (V); Christine J. Deifel, (V); Joan M. Addison, (V)
Miami FL - Bascom Palmer Eye Institute (25)
Susanna M. Tamkins, (I); Adam S. Perlman, (I); Erin X. Goga, (V); Eva M. Olivares, (C); Ana C. Rosa, (C); Nidia Y. Rosado, (C); Candice Robinson, (V); Mirna Garcia, (V); Gloria Chow, (V); Effie Lilas, (V); Lesley L. Bursey, (V); Garnet Yokoi, (V)
Erie PA - Pediatric Ophthalmology of Erie (17)
Nicholas A. Sala, (I); Rhonda M. Hodde, (C); Veda L. Zeto, (C); Cindy E. Tanner, (V); Benjamin H. Whitling, (V)
Lancaster PA - Family Eye Group (12)
David I. Silbert, (I); Noelle S. Matta, (C); Diane M. Jostes, (V); Darlene R. Crick, (N); Laura Heisey, (V)
Concord NH - Concord Eye Care P.C. (11)
Christie L. Morse, (I); Maynard B. Wheeler, (I); Caroline C. Fang, (C); Linda E. Smith, (V)
Nashville TN - Vanderbilt Eye Center (11)*
Sean Donahue, (I); David G. Morrison, (I); Kamila M. Kinder, (C); Gini B. Taylor-Ward, (C); Christine C. Franklin, (C); Neva J. Palmer, (C); Sandy A. Owings, (C); Ronald J. Biernacki, (V); Joseph M. Martin, (V)
Rockville MD - Stephen R. Glaser, M.D., P.C. (11)
Stephen R. Glaser, (I); Allison A. Jensen, (I); Jenifer Ann Aventuro Luck, (I); Jill R. Mason, (C); Christen Y. Addison, (C)
Birmingham AL - University of Alabama at Birmingham School of Optometry (5)
Robert P. Rutstein, (I); Wendy L. Marsh-Tootle, (I); Michelle L. Anderson, (I); Katherine K. Weise, (I); Marcela Frazier, (I); Kristine B. Hopkins, (I); Michael P. Hill, (C)
Dallas TX - Pediatric Ophthalmology (5)
David R. Stager, (I); Joost Felius, (C); Mary K. Alters, (C); June M. Gartlir, (V)
Boise ID - Katherine Ann Lee, M.D., Ph.D., Intermountain Eye Centers (4)
Katherine A. Lee, (I); Bonita R. Schweinler, (C); Larry W. Plum, (V)
Englewood CO - Children’s Eye Physicians (4)
Anna Steele, (I); Jamie Kilpatrick, (C); Kate L Shivley, (V)
Albuquerque NM - Goldblum Family Eye Care Center, P.C. (3)
Todd A. Goldblum, (I); Antoinette Ramirez, (C); Angela Alfaro, (C)
Fullerton CA - Southern California College of Optometry (3)
Susan A. Cotter, (I); Carmen N. Barnhardt, (I); Raymond H. Chu, (I); Susan Shin, (I); Kristine Huang, (I); Erin Song, (I); Monique M. Nguyen, (I); Sue M. Parker, (C); Jamie Morris (C)
Minneapolis MN - University of Minnesota (3)*
C. Gail Summers, (I); Stephen P. Christiansen, (I); Erick D. Bothun, (I); Ann M. Holleschau, (C); Kim S. Merrill, (V); Kathy M. Hogue, (V); Sara J. Downes, (V)
Salt Lake City UT - Rocky Mountain Eye Care Associates (3)
David B. Petersen, (I); Kristin L. Sylvester, (C); J. Ryan McMurtrey, (V)
South Charleston WV - Children’s Eye Care & Adult Strabismus Surgery (3)
Deborah L. Klimek, (I); Bounthavy Lisa Greenlee, (C); Lisa L. Winter, (C); Diana K. Brandon, (V)
Waterbury CT - Eye Care Group, PC (3)
Andrew J. Levada, (I); Christina Brassett, (C); Cheryl Schleif, (C); Tabitha L. Walker, (C); Gina Silva, (V); LeAnne J. Ingala, (V)
Anchorage AK - Ophthalmic Associates (2)
Robert W. Arnold, (I); Mary Diane Armitage, (C); Nancy H. Brusseau, (V)
Atlanta GA - The Emory Eye Center (2)
Scott R. Lambert, (I); Rachel A. Robb, (C); Kathy M. Brown, (V)
Portland OR - Casey Eye Institute (2)*
David T. Wheeler, (I); Kimberley A. Beaudet, (C); Annika S. Joshi, (V)
Saint Paul MN - Associated Eye Care (2)
Susan Schloff, (I); Valori E. Host, (C); Rebecca A. Wolf, (V)
Calgary - Alberta Children’s Hospital (1)
William F. Astle, (I); Heather J. Peddie, (C); Charlene Dawn Gillis, (V)
Cincinnati OH - Children’s Hospital Medical Center (1)
Dean J. Bonsall, (I); Corey S Bowman, (C); Jennefier L. Duncan, (V)
Durham NC - Duke University Eye Center (1)
Laura B. Enyedi, (I); Sarah K. Jones, (C); Melinda K. Robinson, (V); Courtney Fuller (V)
Durham NC - North Carolina Eye Ear & Throat (1)
Joan Therese Roberts, (I); Lynelle Gregory Perez, (C); Marguerite J. Sullivan, (C)
Madison WI - University of Wisconsin, Dept. of Ophthalmology & Visual Sciences (1)
Yasmin S. Bradfield, (I); Barbara H. Soderling, (C); Gail V. Morton, (V); Kristin A. Anderson, (V); Jacque W. Shimko, (V)
Norfolk VA - Eastern Virginia Medical School (1)
Eric R. Crouch III, (I); Gaylord G. Ventura, (C); Kristen D. Ruark, (V)
Portland OR - Pacific University College of Optometry (1)
Richard London, (I); Jayne L. Silver, (C)
Rochester MN - Mayo Clinic (1)*
Jonathan M. Holmes, (I); Jan M. Sease, (C); Virginia Karlsson, (V); Rose M. Kroening, (V)
Rochester NY - University of Rochester Eye Institute (1)
Matthew D. Gearinger, (I); Nancy M. Fedick, (C); Lynne M. Addams, (V)
*Center received support utilized for this project from an unrestricted grant from Research to Prevent Blindness Inc., New York, New York.
Amblyopia Treatment Study Steering Committee:
Roy W. Beck, Eileen E. Birch, Susan A. Cotter, Sean Donahue (2005), Allison R. Edwards (2005), Donald F. Everett, Stephen R. Glaser (2006), Richard W. Hertle (2006–2007), Michael Hill (2007), Rhonda Hodde (2005), Jonathan M. Holmes, Pamela Huston (2006), Deborah L. Klimek (2006), Don W. Lyon, Graham Quinn (2007), Michael X. Repka, Robert Rutstein (2007), Nicholas Sala (2005), Mitchell M. Scheiman (2006–2007), David K. Wallace, David R. Weakley.
PEDIG Coordinating Center:
Roy W. Beck, Brian D. Becker, Nicole M. Boyle, Christina M. Cagnina-Morales, Debora A. Cagnina, Danielle L. Chandler, Laura E. Clark, Katrina L. Dawson, Quayleen Donahue, Mitchell Dupre, Heidi A. Gillespie, Raymond T. Kraker, Stephanie V. Lee, Lee Anne Lester, Shelly T. Mares, B. Michele Melia, Pamela S. Moke, Jim L. Pyner, Diana E. Rojas, Sydney L. Shrader, Heidi J. Strayer
National Eye Institute - Bethesda, MD:
Donald F. Everett
PEDIG Executive Committee:
Roy W. Beck, Eileen E. Birch, Stephen P. Christiansen (2007), Susan A. Cotter (2005–2006), Sean Donahue (2005–2006), Donald F. Everett, Jonathan M. Holmes, Darren Hoover (2007), Pamela Huston (2007), Raymond T. Kraker, Michael X. Repka, Nicholas Sala (2006), Mitchell M. Scheiman (2007), David K. Wallace (2006–2007)
PEDIG Data and Safety Monitoring Committee:
William Barlow (2005), Edward G. Buckley (2005–2006), Barry Davis, Marie Diener-West (2007), Velma Dobson, Donald Everett, Stephen Poff, Dale L. Phelps, Richard Saunders, Lawrence Tychsen (2007)
Supported by National Eye Institute of National Institutes of Health, Department of Health and Human Services EY011751 (PEDIG).
Footnotes
Registration Number: NCT00315302
References
- 1.Pediatric Eye Disease Investigator Group. A randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmol. 2002;120(3):268–78. doi: 10.1001/archopht.120.3.268. [DOI] [PubMed] [Google Scholar]
- 2.Pediatric Eye Disease Investigator Group. Impact of patching and atropine treatment on the child and family in the amblyopia treatment study. Arch Ophthalmol. 2003;121(11):1625–32. doi: 10.1001/archopht.121.11.1625. [DOI] [PubMed] [Google Scholar]
- 3.Pediatric Eye Disease Investigator Group. A randomized trial of atropine regimens for treatment of moderate amblyopia in children. Ophthalmology. 2004;111(11):2076–85. doi: 10.1016/j.ophtha.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 4.Kaye SB, Chen SI, Price G, et al. Combined optical and atropine penalization for the treatment of strabismic and anisometropic amblyopia. J AAPOS. 2002;6:289–93. doi: 10.1067/mpa.2002.127920. [DOI] [PubMed] [Google Scholar]
- 5.Morrison DG, Palmer NJ, Sinatra RB, Donahue S. Severe amblyopia of the sound eye resulting from atropine therapy combined with optical penalization. J Pediatr Ophthalmol Strabismus. 2005;42(1):52–3. doi: 10.3928/01913913-20050101-07. [DOI] [PubMed] [Google Scholar]
- 6.Holmes JM, Beck RW, Repka MX, et al. The amblyopia treatment study visual acuity testing protocol. Arch Ophthalmol. 2001;119(9):1345–53. doi: 10.1001/archopht.119.9.1345. [DOI] [PubMed] [Google Scholar]
- 7.Moke PS, Turpin AH, Beck RW, et al. Computerized method of visual acuity testing: adaptation of the amblyopia treatment study visual acuity testing protocol. Am J Ophthalmol. 2001;132(6):903–9. doi: 10.1016/s0002-9394(01)01256-9. [DOI] [PubMed] [Google Scholar]
- 8.von Noorden GK. Amblyopia caused by unilateral atropinization. Ophthalmology. 1981;88:131–3. doi: 10.1016/s0161-6420(81)35063-5. [DOI] [PubMed] [Google Scholar]
- 9.North RV, Kelly ME. Atropine occlusion in the treatment of strabismic amblyopia and its effect upon the non-amblyopic eye. Ophthalmic Physiol Opt. 1991;11:113–7. doi: 10.1111/j.1475-1313.1991.tb00209.x. [DOI] [PubMed] [Google Scholar]