Abstract
Thrombin induces platelet activation through an early, reversible stage of platelet aggregation, which is followed by a later, irreversible stage of platelet aggregation. Without intervention, events leading to pathological platelet activation can result in vessel occlusion, acute coronary syndrome, and stroke. Therefore, a better understanding of events leading to platelet-mediated clot formation may provide insight into new therapeutic targets. Once activated, protease activated receptors (PARs) are essential in regulating events leading to platelet aggregation. We have determined a signaling cascade through PAR1, which involves phosphatidylinositol (PI) kinases, phosphatidylinositol bisphosphate (PIP2), and Rap1 activation (independent of P2Y12) in the formation of a stable platelet aggregate. The putative phosphatidylinositol-3 kinase (PI3K) inhibitor LY294002 was found to reduce basal and PAR-stimulated PIP2 levels by mass spectrometry and to inhibit PAR1-mediated stable platelet aggregation. Rap1 activation in platelets (during time points corresponding to the late, irreversible phase of aggregation) was found to require the PI signaling pathway. Perturbation of PI3K signaling by isoform-selective inhibitors had differential effects on Rap1 activation through PAR1 and PAR4. Hence, it is possible to disrupt lipid signaling pathways involved in stable clot formation without inhibiting early clot formation, offering a new potential target for antiplatelet therapy.
Research spanning nearly three decades has firmly established the role of platelet activation in the pathophysiology of cardiovascular disease and acute coronary syndromes (Elwood et al., 1991; Ross, 1999; Gurbel and Bliden, 2003), because platelet activation plays a critical role in the formation of intravascular thrombus at the site of arterial injury or plaque rupture (Fuster et al., 1992). Under pathological conditions, a local increase in thrombin concentrations may result in a perpetual feed-forward activation of platelets, resulting in subsequent occlusion of coronary vessels and stroke. In platelets, thrombin signals through the activation of protease-activated receptors (PAR1 and PAR4). Inhibition of specific points in PAR signaling pathways leads to the attenuation of normal aggregation kinetics (Holinstat et al., 2007), suggesting that regulation of PAR1- and PAR4-mediated aggregation does not occur through identical signaling cascades.
Phosphatidylinositol phosphates PIP, PIP2, and PIP3 (PIPn) are produced upon the activation of PI-kinases. In human platelets, PI(4,5)P2 (PIP2) is the major substrate for PI(3,4,5)P3, (PIP3) and both of these have been implicated in thrombin-mediated signaling processes in platelets (Hartwig et al., 1995; Yang et al., 2004). A number of studies have investigated the role of PI3-kinase (PI3K) β in outside-in signaling, and studies suggest a potential role for other PI3K isoforms in inside-out signaling (Jackson et al., 2005; Schoenwaelder et al., 2007). PIP3 (and its precursor PIP2) may serve a critical role in platelet function. In platelets, activation with thrombin has been shown to stimulate PIP2 production (Nolan and Lapetina, 1990), and activation of the thrombin receptor with the thrombin receptor-activating peptide has been shown to cause redistribution of platelet phosphatidylinositol-4-phosphate-5-kinase type I (PI5K) to the cytoskeletal fraction (Yang et al., 2004). Consistent with its role in actin-mediated processes, thrombin has been shown to regulate PI5K association with cytoskeletal components (Grondin et al., 1991), and blocking cytoskeletal regulators such as Rho kinase has been shown to reduce PIP2 accumulation (Gratacap et al., 2001).
We have suggested previously a requirement for signaling through phospholipase D (PLD) in PAR1-but not PAR4-mediated aggregation (Holinstat et al., 2007). The previous work used primary alcohols to suppress the production of 1-heptadecanoyl-2-(9-tetradecenoyl)-sn-glycero-3-phosphate (PA) by PLD (via transphosphatidylation) (Singer et al., 1997) and propranolol, known to inhibit the lipid phosphatase involved in the conversion of PA to DAG (Koul and Hauser, 1987). These pharmacological techniques suggest that platelet aggregation through PAR1 is sensitive to agents involved in PLD-mediated PA production and DAG downstream of PA. This phospholipase C-independent pool of DAG may play a critical role in the regulation of Rap1. Furthermore, because PIP2 is an essential cofactor for PLD activation, this may render the PAR1 pathway particularly sensitive to agents that perturb PIP2 formation.
Rap1, a Ras family member, is highly expressed in human platelets. In vivo, activation of Rap1 has been shown to play a crucial role in the maintenance of hemostasis after vascular insult (Crittenden et al., 2004; Chrzanowska-Wodnicka et al., 2005). Furthermore, Rap1 activation is regulated through both G-protein-coupled receptor activation and outside-in signals via integrin activation (Brass, 2003). The temporal regulation of Rap1 activity may provide the key to understanding its role in platelet homeostasis and aggregation, because the biochemical signals that shift the aggregate from unstable (reversible) to stable (irreversible) (Ruggeri, 2002) most likely determine the extent of vessel occlusion under pathological conditions.
A multidisciplinary approach involving a combination of detailed biochemical and pharmacological studies is presented herein, which helps elucidate the pathway from PAR1 stimulation to stable aggregation, regulated by specific PI-kinases and characterized by persistent Rap1 activation. This study also highlights the interplay between Rap1, lipid signaling, and the mechanisms regulating transient versus irreversible platelet aggregation. Our findings (using activating peptides at their EC100) suggest that phosphatidylinositol signaling is intimately tied to PAR1-mediated stable platelet aggregation. We demonstrate that inhibition of PI-kinases interferes with sustained platelet activation mediated by PAR1 activation and attenuates PAR1-mediated activation of Rap1. PI3Kγ, -α, and possibly -β may play unique roles in the regulation of PAR1-mediated formation of stable platelet aggregates that are distinct from those activated by PAR4 stimulation. Furthermore, direct-infusion MS PIPn analysis reveals that LY294002 mediates reductions in basal and stimulated PIP2 levels, which is composed in large part of PI(4,5)P2 (Pettitt et al., 2006), suggesting that this compound has effects not only on PI3 kinases but also on other enzymes such as PI5 kinases. Hence, PIP2, PIP3, PI3K, and PI5K are implicated in the PAR1-mediated pathway, leading to persistent Rap1 activation and stable platelet aggregation.
Materials and Methods
Materials. Human α-thrombin (2700 NIH units/mg) was purchased from Enzyme Research Laboratories (South Bend, IN). PAR1 (PAR1-AP; SFLLRN) and PAR4 (PAR4-AP; AYPGKF) were purchased from GL Biochem (Shanghai, China). All solvents used for extraction, mass spectrometry, and nondeuterated and fully deuterated primary butanol were purchased from EM Scientific (Gibbstown, NJ) and Acros Organics (Fairlawn, NJ). 1,2-Dioctanoyl-sn-glycero-3-(phosphoinositol-4,5-bisphosphate) was purchased from Avanti Polar Lipids (Alabaster, AL). Anti-Rap1 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Blocking buffer and anti-rabbit IRDYE 800 antibody were purchased from LI-COR Biosciences (Lincoln, NE). Anti-PIP5Kα was purchased from AbCam Inc. (Cambridge, MA). Anti-phospho-AKT was purchased from Cell Signaling Technologies (Danvers, MA). Protein A Sepharose Beads were from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK). Aggregometers and supplies were purchased from Chrono-Log (Havertown, PA). Propranolol and 2-MeSAMP were purchased from Sigma-Aldrich (St. Louis, MO). 10-(4′-(N-diethylamino)butyl)-2-chlorophenoxazine (AKT inhibitor X; AKTi X) and PI-103 were purchased from Calbiochem (San Diego, CA). PI3K inhibitors (TGX-115, SW14, and SW30) were provided by Kevan Shokat (University of California San Francisco, San Francisco, CA).
Human Platelets. Human platelets were obtained from healthy volunteers within the Vanderbilt University community. Studies were approved by the Vanderbilt University Institutional Review Board. Informed consent was obtained from all individuals before blood donation. All experiments were performed on washed platelets that were prepared as described previously (Holinstat et al., 2007). Platelets for the PIPn analysis were washed three times and resuspended in buffer without acid citrate dextrose. Reported results are the data obtained using platelets from multiple subjects. Unless otherwise noted, all experiments were conducted with a platelet concentration of 3 × 108 platelets/ml and agonist concentrations corresponding to maximal receptor activation with thrombin, PAR1-AP, or PAR4-AP (10 nM, 20 μM, and 200 μM, respectively) based on previous studies (Holinstat et al., 2006, 2007).
Platelet Aggregation. Washed platelets were pretreated with inhibitors as indicated before the measurement of aggregation response to thrombin, PAR1-AP, or PAR4-AP using an aggregometer with stirring at 1000 rpm at 37°C.
Measurement of RAP1 Activity. Rap1 activity was measured using GST-RalGDS-Rap1-binding domain that specifically interacts with activated Rap1 as described elsewhere (van Triest et al., 2001; Holinstat et al., 2007). Activated Rap1 was detected by immunoblotting with the anti-Rap1 antibody. In parallel experiments using whole platelet lysate, Rap1 expression was analyzed to confirm equal protein loading.
Immunoprecipitation of Rap1-Associated Complexes. Washed platelets were stimulated with 20 μM PAR1-AP for the indicated times and lysed with an equal amount of 2× platelet lysis buffer (100 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2% Igepal CA-630, 1% sodium deoxycholate, 0.05% SDS, 2 mM Na3VO4, 2 mM phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, 2 μg/ml aprotinin, and 2 μg/ml pepstatin). Lysates were precleared with 20 μl of protein A beads for 1 h. Precleared samples were centrifuged at 4000 rpm at 4°C for 3 min, and supernatants were transferred to fresh 1.5-ml tubes containing 3 μg of anti-Rap1 antibody and rotated at 4°C for 4 h. Protein A beads (20 μl) were added to each tube, and the tubes were rotated at 4°C overnight. The next morning, the samples were washed four times with wash buffer (100 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM Na3VO4, 2 mM phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, 2 μg/ml aprotinin, and 2 μg/ml pepstatin), and Rap1-complexes were dissociated from Protein A beads with the addition of 2× Laemmli buffer, boiled for 5 min, and analyzed for PI5Kα by Western blot analysis.
Phosphatidylinositol Phosphate Determination. Phosphatidylinositol extraction was performed as described previously (Milne et al., 2005), with the following modifications: Washed platelets were resuspended in Tyrode's buffer, and 500-μl aliquots of the platelet suspension were treated as indicated. For LY294002- and PI-103-treated samples, platelet suspensions were incubated with inhibitor for 15 min in the dark at 37°C before stimulation with PAR1-AP agonists. After stimulation with PAR1-AP, platelets were immediately centrifuged at 4°C at 8000 rpm for 5 min. Samples were kept cold throughout the procedure. Supernatants were removed, followed by the addition of 400 μl of 1:1 CHCl3/CH3OH. Samples were vortexed, centrifuged at 8000 rpm for 5 min, and supernatants were discarded. Pellets were extracted into 200 μl of 2:1 chloroform/methanol containing 0.25% concentrated HCl. Supernatants were washed twice with 40 μl of 1 N HCl before drying in vacuo (CentriVap Concentrator; Labconco, Kansas City, MO). Dried lipid(s) were rapidly redissolved in 55 μl of 1:1:0.3 CHCl3/CH3OH/H2O. Just before analysis, 5 μl of 300 mM piperidine (Milne et al., 2005) and 1 μl of the internal standard 1,2-dioctanoyl-sn-glycero-3-(phosphoinositol-4,5-bisphosphate) (0.11 mg/ml) was added to each sample, and the sample was vortexed and centrifuged briefly before MS analysis by direct infusion. Results are normalized relative to PI 38:4 at 885 m/z in bar graphs to control for the efficiency of phospholipid extraction.
Analysis of PAR1- and PAR4-Mediated Changes in PI Fraction. Lipid extracts were prepared from platelet suspensions as described previously (Milne et al., 2006) with the following modifications. Washed platelets were stimulated with PAR1-AP, PAR4-AP, or the combination of both. After stimulation at 37°C for 15 min, 400 μl of ice-cold 0.1 N HCl/methanol (1:1) was added, vortexed, and maintained at 4°C. Chloroform (400 μl) was added, samples were vortexed again, and 40 μl of an internal standard containing equimolar amounts (8 μM) of 25:0 1-dodecanoyl-2-tridecanoyl-sn-glycero-3-phosphocholine and 31:1 PA was added to the organic layer before drying in vacuo. Dried lipids were resuspended in 80 μl of chloroform/methanol (9:1). Changes for 885 m/z PI fraction in lipid extracts (relative to internal standard) were analyzed by direct-infusion MS on a Finnigan TSQ Quantum triple quadrupole mass spectrometer (Thermo Fisher Scientific, Waltham, MA), and the identity of 885 m/z peak as 38:4 PI was confirmed by tandem mass spectrometry fragmentation.
Statistical Analysis. Comparison between experimental groups was made using a t test from the Prism software package (GraphPad Software Inc., San Diego, CA). Differences in mean values were considered significant at p < 0.05.
Results
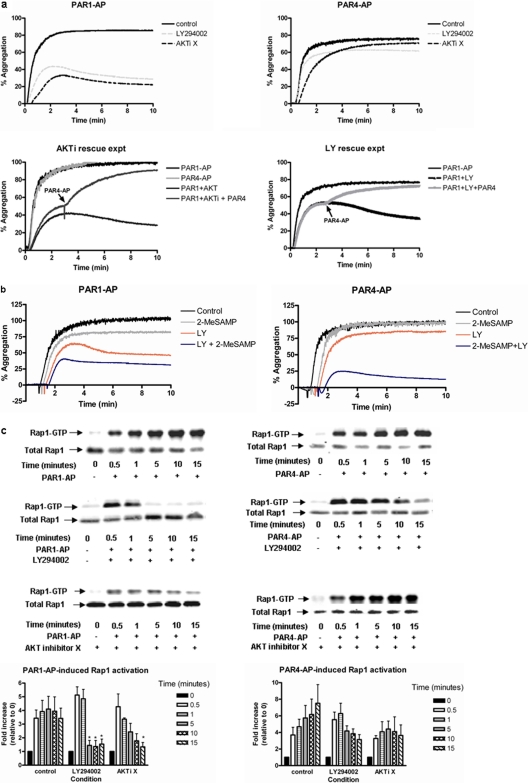
PI-Kinase Signaling Requirement for Normal PAR1-Mediated Platelet Aggregation. Several studies have uncovered important roles for PIPns in the regulation of platelet function (Hartwig et al., 1995; Yang et al., 2004; Voss et al., 2007). To determine the role PIPns play in PAR-mediated platelet aggregation, platelets were stimulated with PAR1-AP or PAR4-AP in the presence or absence of the PI3K inhibitor (LY294002), AKT inhibitor (AKTi X)(Thimmaiah et al., 2005), P2Y12 receptor antagonist (2-MeSAMP), or a combination of LY294002 and 2-MeSAMP (Fig. 1, a and b). Platelets treated with LY294002 or AKTi X resulted in a significant decrease in PAR1-AP-mediated maximal platelet aggregation that became unstable over a matter of minutes, whereas stimulation with PAR4-AP resulted in minimal inhibition of maximal aggregation with no instability observed for at least 10 min after stimulation (Fig. 1a).
Fig. 1.
PAR1-mediated platelet aggregation requires PI-kinase and AKT signaling in the human platelet. Washed platelets were treated with 100 μM LY294002 for 15 min, 50 μM 2-MeSAMP for 5 min, 20 μM AKTi X for 1 h, or both 2-MeSAMP and LY294002. After treatment, platelet aggregation and Rap1 activation were measured after stimulation with either 20 μM PAR1-AP or 200 μM PAR4-AP. a, treatment with LY294002 or AKTi X significantly inhibited PAR1-AP but not PAR4-AP-stimulated platelet aggregation (n = 4). PAR4-AP was able to rescue PAR1-AP-induced platelet aggregation treated with LY294002 or AKTi X (n = 3). b, PAR-induced platelet aggregation after treatment with 2-MeSAMP, LY294002, or 2-MeSAMP and LY294002 (n = 3). C, washed platelets were treated either with or without LY294002 or AKTi X. Platelets were then stimulated with either PAR1-AP or PAR4-AP for various times (0, 0.5, 1, 5, 10, or 15 min), and the level of Rap1 activity was determined relative to unstimulated conditions. Total Rap1 from platelet lysates was measured to confirm equal protein loading for each condition. PAR1-mediated Rap1 activation was significantly attenuated after treatment with either LY294002 (n = 6) or AKTi X (n = 3) for control; *, P < 0.05. D, washed platelets were treated either with or without 2-MeSAMP or LY294002 and 2-MeSAMP. Platelets were then stimulated with either PAR1-AP or PAR4-AP for various times (0, 0.5, 1, 5, 10, or 15 min), and the level of Rap1 activity was determined (n = 3). Total Rap1 from platelet lysates was measured to confirm equal protein loading for each condition. PAR-mediated Rap1 activation was significantly attenuated after treatment with LY294002 and 2-MeSAMP versus control conditions (*, P < 0.05). For analysis of PAR4-AP, Rap1 activation was compared in platelets treated with LY294002 versus those treated with both LY294002 and 2-MeSAMP (*, P < 0.05).
To determine whether the instability of PAR1-AP-mediated platelet aggregation resulting from treatment with LY294002 and AKTi X was due to direct or indirect PI-kinase signaling, the Gi/o-linked ADP receptor P2Y12 was blocked with 2-MeSAMP followed by stimulation with PAR agonists. We showed previously that the requirement for P2Y12 receptor activation for PAR-induced platelet aggregation diminishes as the effective concentration of agonist increases (Holinstat et al., 2006). Consistent with these findings, others have observed the attenuation of PAR-mediated platelet aggregation at submaximal agonist concentrations (Kim et al., 2004; Woulfe et al., 2004). 2-MeSAMP had a minimal effect on PAR-mediated platelet aggregation at maximal concentrations for PAR1-AP and PAR4-AP (Holinstat et al., 2006) (Fig. 1, a and b). In addition, treatment of platelets with both LY294002 and 2-MeSAMP followed by the stimulation with PAR1-AP resulted in a similar inhibition to what was observed with the treatment of LY294002 alone (p = 0.0863) (Fig. 1b). PAR4-AP-induced platelet aggregation was sensitive to dual treatment with LY294002 and 2-MeSAMP, whereas neither inhibitor alone significantly attenuated platelet aggregation (consistent with our earlier findings that inhibition of PAR4 signaling requires blocking multiple signaling pathways and inhibition of P2Y12 alone is not enough to perturb normal PAR4-induced platelet aggregation) (Holinstat et al., 2006). Furthermore, inhibition of PAR1-mediated platelet aggregation with LY294002 or AKTi X was rescued by the addition of PAR4-AP to form a stable platelet aggregate (Fig. 1a), suggesting the independent nature of PAR4 signaling relative to PAR1.
PAR1-Mediated Rap1 Activity Is Sensitive to Agents that Perturb PI-Kinase Signaling. Rap1 has been shown to play a crucial role in platelet hemostasis after vascular injury (Crittenden et al., 2004; Chrzanowska-Wodnicka et al., 2005). Because several groups report a temporal regulation of Rap1 after stimulation of the thrombin receptors in human platelets (Franke et al., 2000; Holinstat et al., 2007), Rap1 activation was measured at various time points after stimulation with PAR1 or PAR4, with or without various combinations of PI-kinase inhibitors (Fig. 1, c and d). Under control conditions, PAR1 stimulation resulted in an early activation of Rap1, which was sustained for all time points measured. Pretreatment with LY294002 had no observable effect on early Rap1 activation but resulted in significant inhibition of Rap1 activity at 5 min after stimulation (Fig. 1c). Likewise, pretreatment with AKTi X had no observable effect on the early activation of Rap1 but resulted in an attenuation of Rap1 at later time points after PAR1-AP, but not PAR4-AP.
Because treatment with 2-MeSAMP did not attenuate platelet aggregation, it is likely that the P2Y12 receptor is not required for PAR-induced Rap1 activation. Therefore, platelets treated with 2-MeSAMP or 2-MeSAMP plus LY294002 were stimulated with either PAR1-AP or PAR4-AP, and Rap1 activation was measured at various time points. Similar to what was observed for aggregation, 2-MeSAMP alone does not inhibit PAR-mediated Rap1 activation, whereas treatment with 2-MeSAMP and LY294002 results in the complete inhibition of PAR-mediated Rap1 activation within 5 min (Fig. 1d).
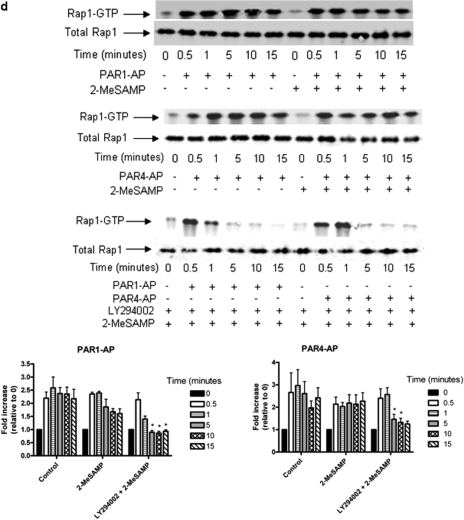
Formation of Stable Platelet Aggregates Requires Specific Isoform(s) of PI3K. PI3K has been shown to play a significant role in the formation of a stable platelet aggregate (Woulfe et al., 2004; Camps et al., 2005; Jackson et al., 2005). Because LY294002 inhibited PAR1-mediated stable platelet aggregation, we sought to determine the dose-dependence of this inhibition on PAR1, PAR4, and thrombin signaling (Fig. 2a). As expected, PAR1-induced platelet aggregation was the most sensitive to treatment with LY294002, with an IC50 of 100 μM (consistent with the effective inhibitory concentration known to inhibit PI3K activity in platelets), whereas PAR4-AP- and thrombin-mediated aggregation were only inhibited at higher concentrations of LY294002. We further identified the relative sensitivity of each PAR to the inhibition of PI3K by measuring the dose-dependence of PAR1-AP and PAR4-AP to aggregation in the absence and presence of 100 μM LY294002 (Fig. 2b). Although PAR4-AP showed little sensitivity to treatment with LY294002 at any point along the dose-response curve, PAR1-AP-mediated aggregation was significantly inhibited at 10 μM agonist and did not overcome LY294002-mediated inhibition even at very high concentrations of agonist (40 μM PAR1-AP). In agreement with others, we found that both PAR1 and PAR4 induce AKT phosphorylation in platelets (Kim et al., 2004; Woulfe et al., 2004) (Fig. 2c). Although only PAR1-mediated platelet aggregation exhibited sensitivity to LY294002 at concentrations typically used to perturb PI3K, PAR1- and PAR4-mediated AKT phosphorylation was eliminated in the presence of LY294002. This indicates that LY294002 exhibits differential effects on PAR1- and PAR4-mediated platelet aggregation in human platelets and is consistent with the PARs signaling through distinct pathways with differential dependence on PI-kinase signaling.
Fig. 2.
PI3K regulation of PAR1-mediated stable platelet aggregation. Washed platelets were treated with or without PI3K inhibitors to determine PAR1-AP-mediated aggregation sensitivity to the PI3K signaling pathway. a, sensitivity of PAR signaling to LY294002 reveals dose-dependent inhibition as measured by aggregation (n = 4; *, P < 0.05; **, P < 0.01). b, the dose response for platelet aggregation of PAR1-AP (0.5-40 μM) and PAR4-AP (10-400 μM) in the presence or absence of 100 μM LY294002 was measured (n = 5; **, P < 0.01). c, to confirm that LY294002 inhibited PI3K-mediated activation of AKT, AKT phosphorylation was measured in platelets in the absence or presence of 100 μM LY294002 followed by stimulation with PAR1-AP or PAR4-AP (n = 5).
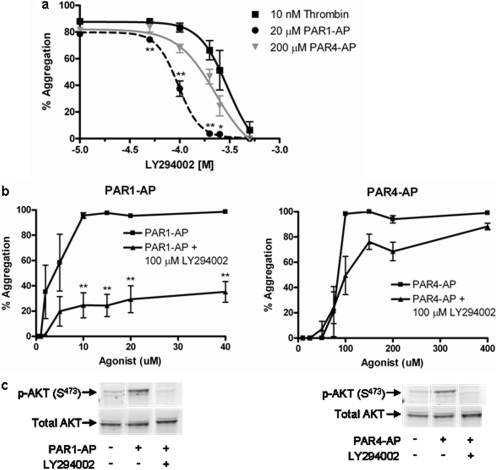
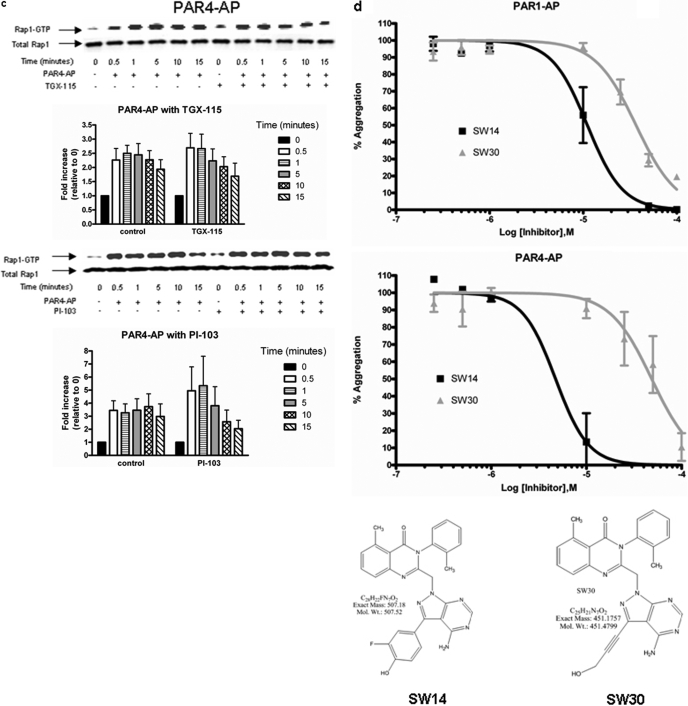
To identify which isoform(s) of PI3K mediate PAR1- and PAR4-induced stable platelet aggregation, PAR1-AP- and PAR4-AP-mediated platelet aggregation was measured in the presence of an inhibitor to either PI3Kα,-γ, and -δ (PI-103) or PI3Kβ and -δ (TGX-115) (Fig. 3a). PAR1-mediated aggregation was significantly attenuated by treatment with PI-103 but not TGX-115, whereas PAR4-induced aggregation was not appreciably affected by either compound. To further identify the biochemical intermediate(s) responsible for PI-103-mediated instability in PAR1-mediated platelet aggregation, Rap1 activation was measured after treatment with either PI-103 or TGX-115 (Fig. 3, b and c). Stimulation of Rap1 by PAR1-AP but not PAR4-AP was sensitive to PI-103 as early as 5 min after stimulation. In all conditions tested, attenuation of Rap1 signaling at time points later than 1 min was accompanied by defects in stable aggregate formation, underscoring the requisite nature of Rap1 signaling in stable platelet activation (Crittenden et al., 2004; Chrzanowska-Wodnicka et al., 2005). In addition, we treated the platelets with two novel small-molecule inhibitors of PI3K shown to have specificity toward PI3Kα and -γ (SW14 and SW30). The IC50 values for SW14 toward PI3Kα and PI3Kγ are 9 μM and 21 nM, respectively, whereas the IC50 values for SW30 toward PI3Kα and PI3Kγ are 85 and 1.3 μM, respectively. The observed IC50 values for PAR1-AP with SW14 and SW30 was 10 and 25 μM, respectively, whereas the IC50 values for PAR4-AP was 3 and 40 μM, respectively (Fig. 3d).
Fig. 3.
PAR-mediated aggregation and Rap1 activation show sensitivity to specific isoforms of PI3K. Washed platelets were treated with or without varying concentrations of the small-molecule PI3K inhibitors TGX-115, PI-103, SW-14, or SW-30 to determine isoform sensitivity to PAR-mediated platelet activation. a, washed platelets were treated with varying concentrations of PI-103 or TGX-115 followed by stimulation with 20 μM PAR1-AP or 200 μM PAR4-AP. Platelet aggregation 10 min after stimulation was recorded for each condition. PI-103 dramatically inhibits PAR1-mediated platelet aggregation at 10 μM. (n = 6; *, P < 0.05; **, P < 0.01). b, washed platelets were treated with or without 10 μM PI-103 or 10 μM TGX-115 for 15 min. PAR1-AP-mediated Rap1 activation was measured at various times after stimulation. PAR1-mediated Rap1 activation was sensitive to treatment with PI-103 (n = 6; *, <0.05) but not TGX-115 (n = 3). c, washed platelets were treated with or without 10 μM PI-103 or 10 μM TGX-115 for 15 min. PAR4-AP-mediated Rap1 activation was measured at various times after stimulation. PAR4-mediated Rap1 activation was insensitive to treatment with PI-103 (n = 6) or TGX-115 (n = 3). d, washed platelets were treated with varying concentrations of SW-14 or SW-30 followed by stimulation with PAR1-AP or PAR4-AP. Platelet aggregation 10 min after stimulation was recorded for each condition. PAR1 and PAR4 show sensitivity to both inhibitors in a dose-dependent manner (n = 6).
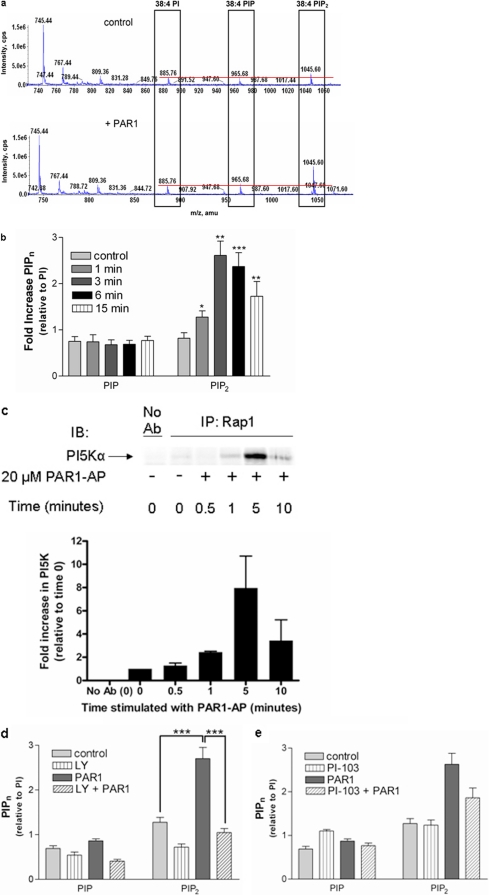
Lipid Regulation of PAR1-Mediated Platelet Function through PI-Kinases. To determine the relative levels of PIPns formed after stimulation of PAR1, washed platelets were treated with PAR1-AP versus buffer control for 10 min. Platelet extracts were examined by MS analysis using direct infusion of lipid extracts. Increases in 38:4 PIP2 levels were observed after treatment of platelets with PAR1-AP (Fig. 4a), shown relative to PI in the platelet extract, which controls for the efficiency of phospholipid extraction, given that the levels of 38:4 PI at 885 m/z were not perturbed by PAR1 stimulation. The levels of 38:4 PI at 885 m/z did not change significantly in seven separate experiments performed in triplicate upon stimulation with either PAR1-AP or PAR4-AP alone. In fact, changes in the PI fraction required the simultaneous activation with both PAR1-AP and PAR4-AP together (used as a positive control in this experiment) to elicit even a small change in the 38:4 PI fraction (p =<0.001), suggesting that the levels of PI are tightly regulated in platelets. Fragmentation of 38:4 PIPns (the most abundant PIPn species in platelets) revealed characteristic 20:4 and 18:0 fatty acyl chain constituents for all PI, PIP, and PIP2 species (data not shown), consistent with other published liquid chromatography/mass spectrometry analyses of platelet extracts (Pettitt et al., 2006). PIPn levels were examined at specific time points after stimulation (Fig. 4b). Relative increases in PIP2 levels in platelets were detected as early as 1 min after PAR1-AP treatment and reached a maximum level at 3 min. Significant increases in PIP2 were observed for at least 15 min after stimulation with PAR1-AP. Similar increases in PIP2 occurred upon stimulation with PAR4-AP (results not shown); however, because only PAR1-mediated aggregation was sensitive to perturbation by agents directed at PI-kinase signaling, we focused our investigation on PIPn production downstream of PAR1 activation.
Fig. 4.
PAR1-induced PIP2 formation is inhibited by LY294002. Washed platelets were analyzed for the formation of PIP2 after stimulation with 20 μM PAR1-AP. a, acidified extracts obtained from quiescent (top) or platelets stimulated with PAR1-AP for 5 min (bottom) were analyzed by direct-infusion MS (Milne et al., 2005). Levels of PIP and PIP2 relative to the stable PI fraction are indicated by a red line. b, time-dependence of PAR1-AP-mediated PIP2 increases in platelets (n = 2-4, in triplicate; *, P = 0.03; **, P = 0.003; ***, P =<0.0001). c, platelet lysates were stimulated with PAR1-AP for up to 10 min and immunoprecipitated with anti-Rap1 followed by immunoblotting with an anti-PI5Kα antibody. PI5Kα was found to be present after 5-min stimulation with PAR1-AP but not under unstimulated conditions (bar graph). d, MS analysis of platelet extracts reveals a significant increase in 38:4 PIP2 upon PAR1 stimulation (n = 2-4, in triplicate; ***, P = 0.007), which is eliminated by pretreatment with LY294002 for 15 min. e, MS analysis using direct infusion of platelet extracts reveals a minor decrease in PAR1-AP-mediated 38:4 PIP2 production (time) after pretreatment with PI-103 (500 nM) (n = 3-5, in triplicate; not significant, P = 0.049); Ab, antibody; IB, immunoblot; IP, immunoprecipitated.
Because agents known to perturb PI-kinase signaling also interfere with PAR1-mediated Rap1 activation, and because PI5K phosphorylates the predominant form of PIP in platelets (Pettitt et al., 2006), we wanted to determine whether PI5K formed a physical interaction with the Rap1 protein complex after PAR1-AP stimulation. Washed platelets were stimulated with PAR1-AP for up to 10 min, Rap1 was immunoprecipitated, and the immunoprecipitates were immunoblotted for PI5Kα. This analysis indicated that PI5Kα is present in complex with Rap1 at high levels after a 5-min stimulation with PAR1-AP but was absent in unstimulated Rap1 complexes (Fig. 4c).
LY294002 Effects on PAR1-AP-Stimulated Platelet Aggregation Is Linked to Decreases in PIP2. Given the sensitivity of PAR1-mediated aggregation to agents that perturb PI-kinase signaling, we investigated the effect of LY294002 on PAR1-mediated changes in PIP2 levels that, in human platelets, consist of mainly PI(4,5)P2 (Pettitt et al., 2006). Surprisingly, pretreatment with LY294002 blocked the PAR1-AP-mediated increase in PIP2 levels (Fig. 4d). Treatment of unstimulated platelets with LY294002 also caused a decrease in basal PIP2 levels. Although treatment of platelets with LY294002 also decreases PAR4-AP-mediated PIP2 levels (results not shown), it does not seem to have a marked effect on the ability of PAR4-AP to stimulate aggregation; therefore, we focused our investigation on the relevant signaling events stimulated by PAR1-AP.
These results suggest that LY294002 may indirectly decrease PIP3 formation in platelets by reducing substrate availability, because PIP2 is the substrate required for PI3K-mediated PIP3 formation. Our results demonstrate that pretreatment with LY294002 ablates PAR-mediated increases in PIP2 levels and reduces basal levels by nearly half, suggesting that LY294002 has effects on PI5K and on PI3K. Together with results from Fig. 4c, these results suggest that LY294002 may exert effects on aggregation via decreases in PIP2 mediated by the inhibition of PI5Kα.
Given the effects of LY294002 on PIP2 levels and its ability to interfere with PAR1-AP-mediated platelet activation, the effects of PI-103 on PIPn levels before and after PAR1 stimulation were examined. Although PI-103 had no effect on basal levels of PIP2, it moderately inhibited (Fig. 4e, p = 0.049) PAR1-stimulated PIP2 levels, an effect barely significant from a statistical standpoint. Nevertheless this suggests that PI-103 is a more specific PI3K inhibitor than LY294002 (Fig. 4d). This decrease may indicate a small effect on PI5Kα or may be due to effects on PI3K in the formation of the relatively minor PI(3,4)P2 and extremely minor PI(3,5)P2 fractions detected in platelets (Pettitt et al., 2006).
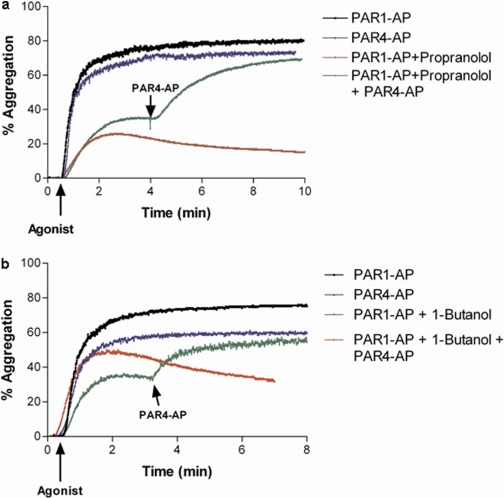
PAR1-Induced Irreversible Platelet Aggregation Requires PLD Component for Signaling. PAR1-mediated temporal regulation of Rap1 activity was shown previously to be influenced by primary alcohols (Holinstat et al., 2007) which, upon activation of PLD, has been shown to participate in transphosphatidylation to form phosphatidyl alcohols (Singer et al., 1997). PLD is also known to require PIP2 as an essential cofactor for activity (Brown et al., 1993; Liscovitch et al., 1994; Henage et al., 2006;). Because primary alcohols (but not tertiary ones) that perturb PLD-mediated PA formation (Singer et al., 1997) inhibited PAR1-mediated aggregation in a temporally similar manner as LY294002, we sought to determine whether effects mediated by these primary alcohols could be rescued by PAR4 in a manner similar to what was observed for the inhibition of PI-kinase and AKT in Fig. 1. Attenuation of PAR1-AP-mediated platelet aggregation by agents that either directly or indirectly reduce DAG formation (Koul and Hauser, 1987; Singer et al., 1997) was rescued by the addition of PAR4-AP (Fig. 5, a and b). This experiment implies that impairment in either PAR1-activated PI-kinase or DAG signaling downstream of PLD interferes with the formation of a stable platelet aggregate, whereas PAR4 potentially signals through a broader cascade of pathways and hence does not require either of these steps.
Fig. 5.
PAR4 activation rescues PAR1-mediated platelet aggregation in PLD inhibited conditions; 200 μM PAR4-AP rescues 20 μM PAR1-mediated aggregation impaired by 100 μM propranolol treatment for 10 min (a) or 0.3% 1-butanol treatment for 4 min (b) (n = 3; *, P < 0.05).
Discussion
PAR1-mediated stable platelet aggregation involves a set of lipid signals, which include the requisite activation of PI-kinases involved in the formation of PIP2, PIP3, and probably involves DAG downstream of PA production. This signaling network is operative in normal human platelets and may function under prothrombotic conditions. The current study has for the first time defined a pathway activated through PAR1 in the human platelet that delineates the roles of PI-kinases, regulation of PIPns, the importance of DAG downstream of PA, and prolonged Rap1 activation in the formation of a stable platelet aggregate.
The early phase, but not the later stable phase of thrombin-induced platelet activation, is known to be relatively insensitive to perturbation of a number of points in the signaling pathway leading to aggregation (Franke et al., 2000; Holinstat et al., 2007). In this report, PAR1-mediated platelet signaling shows sensitivity within 5 min after stimulation to agents that affect PIP2 levels and late Rap1 activity and to agents that block signaling downstream of PLD. PAR4-mediated activation of stable platelet aggregation, however, is insensitive to perturbation of most of these signaling pathways but is significantly inhibited after dual inhibition of platelet signaling with the broad-spectrum PI-kinase inhibitor LY294002 and 2-MeSAMP (a similar inhibition of PAR4-mediated platelet aggregation was observed upon simultaneous inhibition of both calcium mobilization and signaling through P2Y12; Holinstat et al., 2006). The underlying difference in signaling between these receptors may be explained by the relative difference in signaling to the Gαq pathway observed in PAR1- and PAR4-mediated calcium mobilization. PAR1-induced increases in calcium mobilization are short-lived (Covic et al., 2000) compared with prolonged calcium increases mediated by PAR4 stimulation. This difference in calcium signaling may indicate that PAR1 requires alternative signaling mechanisms (relative to those used by PAR4) to maintain a persistent level of activated Rap1 and subsequent integrin activation to reinforce the newly formed platelet aggregate. Hence, PAR1 regulation of PIP2 levels (which would probably affect PLD, because PLD requires PIP2 for activation) (Brown et al., 1993; Liscovitch et al., 1994) may play a critical role in feed-forward signal amplification. This is consistent with findings that both PIP2 and PA (shown here to be crucial for stable platelet aggregation) can bind to and activate PI5K in vitro (Jarquin-Pardo et al., 2007). PA-derived DAG formation may also play a role in feed-forward signal amplification through PKC (Henage et al., 2006), a cellular modulator of PLD activation, as well as CalDAG-GEF, an activator of Rap1 known to be highly expressed in platelets. Thus, PAR1 has a complex lipid-signaling mechanism by which it may maintain a relatively high level of Rap1 activation, resulting in a stable platelet aggregate. In comparison, PAR4, although having a lower affinity for thrombin, has a more persistent signal that is less sensitive to inhibition of any one point in its signaling cascade. Once activated, PAR4 may progress along multiple signaling cascades, and its blockade requires the inhibition of multiple signaling pathways (Fig. 1) (Holinstat et al., 2006).
LY294002 is commonly thought of as a relatively specific inhibitor of PI3K signaling. Mass spectrometry-based lipid analysis demonstrated that LY294002 also blocks other PI-kinases, particularly those involved in the formation of PIP2. The nonspecific nature of this pharmacological inhibitor necessitates the use of other, more specific pharmacological tools to identify the signaling pathways mediating PAR-induced physiological responses such as platelet aggregation. Our study showed that LY294002 significantly decreased levels of PIP2 (and probably PIP3 downstream from it), preventing the formation of a stable platelet aggregate after stimulation of PAR1. In addition, our laboratory showed recently that another PI3K inhibitor, wortmannin, significantly affects PAR1 but not PAR4 signaling in the platelet (Voss et al., 2007), with no effect on Ca2+ transients induced by either PAR1 or PAR4. Combined with data demonstrating the presence of PI5K in a protein complex with activated Rap1, the formation of PIP2 through PI5K activity seems to play a potentially important role in sustained PAR1-mediated platelet aggregation (Yang et al., 2004).
PI3K may also play a critical role, because blocking phosphorylation of AKT is sufficient to inhibit the later, stable phase of platelet aggregation through the PAR1 pathway. PI3Kγ or -α in particular are implicated by the ability of the isozyme specific PI3Kγ/α/δ inhibitor PI-103, but not the PI3Kβ/δ inhibitor TGX-115, to attenuate PAR1-mediated platelet activation at subtype-specific concentrations (Fig. 3).Because, even at high concentrations, TGX-115 was unable to inhibit PAR-mediated platelet activity, PI3Kβ and -δ can be ruled out as playing a crucial role in PAR-mediated formation of stable platelet aggregates. Combining the findings of PAR-mediated platelet activation with the use of TGX-115 and PI-103 limits the potential crucial PI3K isoform(s) to PI3Kα and/or PI3Kγ. The development of more specific PI-kinase inhibitors is essential to elucidating the specific contributions of the various PI-kinases involved in the formation of a stable platelet aggregate. Two small-molecule inhibitors recently developed in the lab of Kevan Shokat were used in this study to determine whether the PAR-mediated stable aggregate defects described here could be identified as PI3Kα versus PI3Kγ (SW14 and SW30; Fig. 3c). These inhibitors, combined with the data in Figs. 1, 2, 3, indicate that the PI3K signal regulating PAR1 and PAR4 is complex and differs for each receptor pathway. PAR4 seems to rely most heavily on PI3Kα, whereas PAR1 may rely on a complement of PI3Ks, including PI3Kα and PI3Kγ.
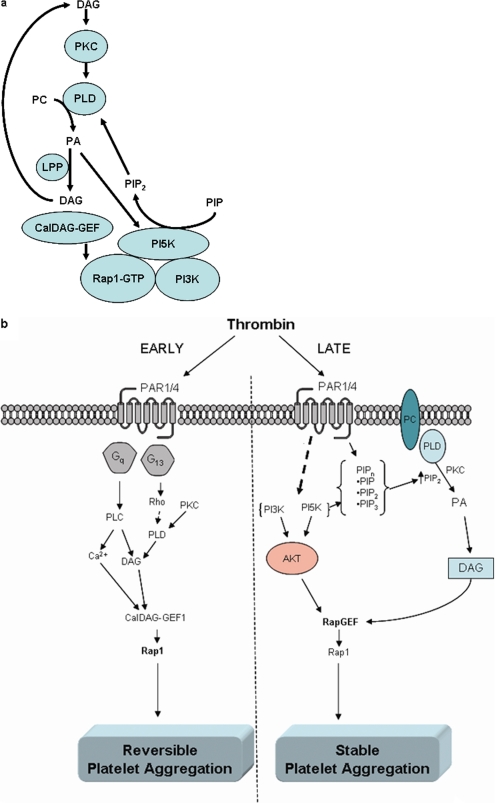
Although others have indicated a potential role for PI5K (Morris et al., 2000) and PI3K (Banfic et al., 1998; Jackson et al., 2005; Schoenwaelder et al., 2007) in the progression of platelet activation and thrombosis, this is the first report linking PAR1-mediated formation of a stable platelet aggregate to persistent Rap1 activation, activation of PI-kinases, and signaling through PLD. This novel signaling network requires a feed-forward activation of the lipid cycle by regulating both lipid kinases and lipid pools, which are directly or indirectly linked to PIPn levels (Fig. 6a).
Fig. 6.
Model for early- and late-phase platelet aggregation regulated through PAR1. Thrombin signals stable activation of human platelets through sequential PAR1-mediated pathways. a, initial DAG formation leads to activation of PKC, a potent activator of PLD, which converts 1-dodecanoyl-2-tridecanoyl-sn-glycero-3-phosphocholine to PA and subsequently DAG (with lipid phosphate phosphohydrolases). The cofactor for PLD, PIP2, is formed after the complex formation of active Rap1, PI3K, and PI5K, leading to a continuous generation of DAG formation and a continued positive feedback on RapGEF and subsequent Rap1 activation that can be inhibited by inhibiting any one of these processes. b, there are two phases to platelet activation: an early unstable (reversible), and a late stable (irreversible) phase, which signal through distinct pathways after activation of PAR1. The early phase of platelet activation results in activation primarily of the Gq and G13 G-protein pathways, which subsequently induce the activation of Rap1 through RapGEF (CalDAG-GEF). This mode of activating Rap1 induces early platelet aggregation. After this early activation phase, platelets require maintenance of the platelet aggregate through specific constituents of the PAR1 signaling pathway, including PI-kinase and PLD-signaling components, to attain irreversible platelet aggregation (a hallmark of second-phase activation of the human platelet). This reinforcement maintains Rap1 activity and platelet aggregation, which may allow for a stable platelet plug (and under pathological circumstances, complete vessel occlusion).
The inability of PKC or PI-kinase inhibitors to block early PAR1-mediated Rap1 activation (Franke et al., 2000) provides evidence for a positive feedback loop that initially activates Rap1 through a RapGEF (potentially CalDAG-GEF; Cifuni et al., 2008) but requires additional RapGEF activation through, perhaps, a different pool of CalDAG-GEF (Fig. 6a). Recent evidence supports a role for multiple RapGEFs regulating Rap1 function in platelets (Cifuni et al., 2008). Furthermore, the identification of a protein complex containing Rap1 and PI5K that forms after PAR1-mediated platelet activation gives evidence for potential regulation mediating continual Rap1 activation and resulting in stable platelet aggregation. Recruitment of these proteins to the Rap1 complex may allow for a fine-tuned regulation of local phosphatidylinositol synthesis, leading to continued inside-out signaling to integrin IIbIIIa and stability of a platelet plug. Future studies will be aimed at determining the nature of this positive feedback loop in regulation of the formation of stable platelet aggregates by PAR1.
The current study exemplifies the necessity for a physiologically regulated feed-forward system to achieve persistent platelet activation, because inhibition anywhere along the PAR1 signaling cascade will result in a loss of Rap1 activation accompanied by instability of the platelet plug. Stronger PAR4-mediated activation at higher thrombin concentrations in the platelet plug lead to stable platelet aggregation in the presence of inhibitors of PAR1-specific signaling pathways. Current antiplatelet drugs that target platelet receptors [clopidogrel and eptifibatide (CAPRIE Steering Committee, 1996; PURSUIT Trial Investigators, 1998)] or directly inhibit circulating thrombin (bivalirudin) (Di Nisio et al., 2005) result in an increased risk of bleeding, a complication that often leads to pathologies as serious as the thrombosis they were meant to treat, such as intracranial hemorrhage and gastrointestinal bleeding. Recent findings have begun to elucidate alternative targets for antiplatelet intervention, which preserve the ability to respond to vessel injury (Fig. 6b). Future investigation of the specific signaling pathways activated downstream of PAR1 in both the unstable and stable phases of platelet activation, or the yet-unknown signals downstream of PAR4 that do not require PI5K, PI3K, and PLD, may lead to the development of specific inhibitors with reduced side effects for treating disorders such as deep vein thrombosis, acute coronary syndrome, and stroke. This study highlights the rich complexity of lipid signaling underlying transient versus irreversible platelet aggregation and new potential therapeutic targets.
Acknowledgments
We thank Connie Childers for providing and drawing all of the blood donors for this study. We thank Dr. K. Shokat (University of California, San Francisco, CA) for providing specific PI3Kβ and PI3Kγ inhibitors, Dr. R. Anderson (University of Wisconsin, Madison, WI) for immunoblot confirmation of PI5K in the Rap1 complex, and Mark Byrne (Vanderbilt University, Nashville, TN) for compilation of PIPn data. We also thank Dr. J. Oates for critically reading the manuscript.
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants P50-HL081009, R01-HL084388, K99-HL089457, R00-HL089457] and the National Institutes of Health National Institute of General Medical Sciences [Grant U54-GM069338].
M.H. and A.M.P. contributed equally to this work.
ABBREVIATIONS: PAR, protease-activated receptor; CalDAG-GEF, calcium, diacylglycerol, guanine nucleotide exchange factor; PA, phosphatidic acid;, phosphatidylinositol; PI3K, phosphatidylinositol-3 kinase; PI5K, phosphatidylinositol-5 kinase; PIPns, phosphatidylinositol phosphates; PIP2, phosphatidylinositol bisphosphate; PIP3, phosphatidylinositol trisphosphate; PLD, phospholipase D; RapGEF, Rap1 guanine nucleotide exchange factor; DAG, diacylglycerol; PKC, protein kinase C; MS, mass spectrometry; TGX-115, 8-(2′-methylphenoxy)-2-morpholino-4-quinolone; PI-103, 3-(4-(4-morpholinyl)pyrido[3′,2′:4,5]furo[3,2-d]pyrimidin-2-yl)phenol; 2-MeSAMP, 2-methylthio-AMP; AKTi X, 10-(4′-(N-diethylamino)butyl)-2-chlorophenoxazine; LY294002, 2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride.
References
- Banfić H, Downes CP, and Rittenhouse SE (1998) Biphasic activation of PKBα/Akt in platelets. Evidence for stimulation both by phosphatidylinositol 3,4-bisphosphate, produced via a novel pathway, and by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273 11630-11637. [DOI] [PubMed] [Google Scholar]
- Brass LF (2003) Thrombin and platelet activation. Chest 124 18S-25S. [DOI] [PubMed] [Google Scholar]
- Brown HA, Gutowski S, Moomaw CR, Slaughter C, and Sternweis PC (1993) ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell 75 1137-1144. [DOI] [PubMed] [Google Scholar]
- Camps M, Rückle T, Ji H, Ardissone V, Rintelen F, Shaw J, Ferrandi C, Chabert C, Gillieron C, Françon B, et al. (2005) Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med 11 936-943. [DOI] [PubMed] [Google Scholar]
- CAPRIE Steering Committee (1996) A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 348 1329-1339. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, and White GC 2nd (2005) Rap1b is required for normal platelet function and hemostasis in mice. J Clin Invest 115 680-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifuni SM, Wagner DD, and Bergmeier W (2008) CalDAG-GEFI and protein kinase C represent alternative pathways leading to activation of integrin alphaIIbbeta3 in platelets. Blood 112 1696-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covic L, Gresser AL, and Kuliopulos A (2000) Biphasic kinetics of activation and signaling for PAR1 and PAR4 thrombin receptors in platelets. Biochemistry 39 5458-5467. [DOI] [PubMed] [Google Scholar]
- Crittenden JR, Bergmeier W, Zhang Y, Piffath CL, Liang Y, Wagner DD, Housman DE, and Graybiel AM (2004) CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med 10 982-986. [DOI] [PubMed] [Google Scholar]
- Di Nisio M, Middeldorp S, and Büller HR (2005) Direct thrombin inhibitors. N Engl J Med 353 1028-1040. [DOI] [PubMed] [Google Scholar]
- Elwood PC, Renaud S, Sharp DS, Beswick AD, O'Brien JR, and Yarnell JW (1991) Ischemic heart disease and platelet aggregation. The Caerphilly Collaborative Heart Disease Study. Circulation 83 38-44. [DOI] [PubMed] [Google Scholar]
- Franke B, van Triest M, de Bruijn KM, van Willigen G, Nieuwenhuis HK, Negrier C, Akkerman JW, and Bos JL (2000) Sequential regulation of the small GTPase Rap1 in human platelets. Mol Cell Biol 20 779-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster V, Badimon L, Badimon JJ, and Chesebro JH (1992) The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med 326 242-250. [DOI] [PubMed] [Google Scholar]
- Gratacap MP, Payrastre B, Nieswandt B, and Offermanns S (2001) Differential regulation of Rho and Rac through heterotrimeric G-proteins and cyclic nucleotides. J Biol Chem 276 47906-47913. [DOI] [PubMed] [Google Scholar]
- Grondin P, Plantavid M, Sultan C, Breton M, Mauco G, and Chap H (1991) Interaction of pp60c-src, phospholipase C, inositol-lipid, and diacyglycerol kinases with the cytoskeletons of thrombin-stimulated platelets. J Biol Chem 266 15705-15709. [PubMed] [Google Scholar]
- Gurbel PA and Bliden KP (2003) The stratification of platelet reactivity and activation in patients with stable coronary artery disease on aspirin therapy. Thromb Res 112 9-12. [DOI] [PubMed] [Google Scholar]
- Hartwig JH, Bokoch GM, Carpenter CL, Janmey PA, Taylor LA, Toker A, and Stossel TP (1995) Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell 82 643-653. [DOI] [PubMed] [Google Scholar]
- Henage LG, Exton JH, and Brown HA (2006) Kinetic analysis of a mammalian phospholipase D: allosteric modulation by monomeric GTPases, protein kinase C, and polyphosphoinositides. J Biol Chem 281 3408-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holinstat M, Voss B, Bilodeau ML, and Hamm HE (2007) Protease-activated receptors differentially regulate human platelet activation through a phosphatidic acid-dependent pathway. Mol Pharmacol 71 686-694. [DOI] [PubMed] [Google Scholar]
- Holinstat M, Voss B, Bilodeau ML, McLaughlin JN, Cleator J, and Hamm HE (2006) PAR4, but not PAR1, signals human platelet aggregation via Ca2+ mobilization and synergistic P2Y12 receptor activation. J Biol Chem 281 26665-26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SP, Schoenwaelder SM, Goncalves I, Nesbitt WS, Yap CL, Wright CE, Kenche V, Anderson KE, Dopheide SM, Yuan Y, et al. (2005) PI 3-kinase p110beta: a new target for antithrombotic therapy. Nat Med 11 507-514. [DOI] [PubMed] [Google Scholar]
- Jarquin-Pardo M, Fitzpatrick A, Galiano FJ, First EA, and Davis JN (2007) Phosphatidic acid regulates the affinity of the murine phosphatidylinositol 4-phosphate 5-kinase-Ibeta for phosphatidylinositol-4-phosphate. J Cell Biochem 100 112-128. [DOI] [PubMed] [Google Scholar]
- Kim S, Jin J, and Kunapuli SP (2004) Akt activation in platelets depends on Gi signaling pathways. J Biol Chem 279 4186-4195. [DOI] [PubMed] [Google Scholar]
- Koul O and Hauser G (1987) Modulation of rat brain cytosolic phosphatidate phosphohydrolase: effect of cationic amphiphilic drugs and divalent cations. Arch Biochem Biophys 253 453-461. [DOI] [PubMed] [Google Scholar]
- Liscovitch M, Chalifa V, Pertile P, Chen CS, and Cantley LC (1994) Novel function of phosphatidylinositol 4,5-bisphosphate as a cofactor for brain membrane phospholipase D. J Biol Chem 269 21403-21406. [PubMed] [Google Scholar]
- Milne S, Ivanova P, Forrester J, and Alex Brown H (2006) Lipidomics: an analysis of cellular lipids by ESI-MS. Methods 39 92-103. [DOI] [PubMed] [Google Scholar]
- Milne SB, Ivanova PT, DeCamp D, Hsueh RC, and Brown HA (2005) A targeted mass spectrometric analysis of phosphatidylinositol phosphate species. J Lipid Res 46 1796-1802. [DOI] [PubMed] [Google Scholar]
- Morris JB, Hinchliffe KA, Ciruela A, Letcher AJ, and Irvine RF (2000) Thrombin stimulation of platelets causes an increase in phosphatidylinositol 5-phosphate revealed by mass assay. FEBS Lett 475 57-60. [DOI] [PubMed] [Google Scholar]
- Nolan RD and Lapetina EG (1990) Thrombin stimulates the production of a novel polyphosphoinositide in human platelets. J Biol Chem 265 2441-2445. [PubMed] [Google Scholar]
- Pettitt TR, Dove SK, Lubben A, Calaminus SD, and Wakelam MJ (2006) Analysis of intact phosphoinositides in biological samples. J Lipid Res 47 1588-1596. [DOI] [PubMed] [Google Scholar]
- PURSUIT Trial Investigators (1998) Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. Platelet glycoprotein IIb/IIIa in unstable angina: receptor suppression using integrilin therapy. N Engl J Med 339 436-443.9705684 [Google Scholar]
- Ross R (1999) Atherosclerosis-an inflammatory disease. N Engl J Med 340 115-126. [DOI] [PubMed] [Google Scholar]
- Ruggeri ZM (2002) Platelets in atherothrombosis. Nat Med 8 1227-1234. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder SM, Ono A, Sturgeon S, Chan SM, Mangin P, Maxwell MJ, Turnbull S, Mulchandani M, Anderson K, Kauffenstein G, et al. (2007) Identification of a unique co-operative PI 3-kinase signaling mechanism regulating integrin αIIbβ3 adhesive function in platelets. J Biol Chem 282 28648-28658. [DOI] [PubMed] [Google Scholar]
- Singer WD, Brown HA, and Sternweis PC (1997) Regulation of eukaryotic phosphatidylinositol-specific phospholipase C and phospholipase D. Annu Rev Biochem 66 475-509. [DOI] [PubMed] [Google Scholar]
- Thimmaiah KN, Easton JB, Germain GS, Morton CL, Kamath S, Buolamwini JK, and Houghton PJ (2005) Identification of N10-substituted phenoxazines as potent and specific inhibitors of Akt signaling. J Biol Chem 280 31924-31935. [DOI] [PubMed] [Google Scholar]
- van Triest M, de Rooij J, and Bos JL (2001) Measurement of GTP-bound Ras-like GTPases by activation-specific probes. Methods Enzymol 333 343-348. [DOI] [PubMed] [Google Scholar]
- Voss B, McLaughlin JN, Holinstat M, Zent R, and Hamm HE (2007) PAR1, but not PAR4, activates human platelets through a Gi/o/phosphoinositide-3 kinase signaling axis. Mol Pharmacol 71 1399-1406. [DOI] [PubMed] [Google Scholar]
- Woulfe D, Jiang H, Morgans A, Monks R, Birnbaum M, and Brass LF (2004) Defects in secretion, aggregation, and thrombus formation in platelets from mice lacking Akt2. J Clin Invest 113 441-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SA, Carpenter CL, and Abrams CS (2004) Rho and Rho-kinase mediate thrombin-induced phosphatidylinositol 4-phosphate 5-kinase trafficking in platelets. J Biol Chem 279 42331-42336. [DOI] [PubMed] [Google Scholar]