Abstract
Protein kinase A (PKA)-dependent phosphorylation of troponin (Tn)I represents a major physiological mechanism during β-adrenergic stimulation in myocardium for the reduction of myofibrillar Ca2+ sensitivity via weakening of the interaction with TnC. By taking advantage of thin filament reconstitution, we directly investigated whether or not PKA-dependent phosphorylation of cardiac TnI (cTnI) decreases Ca2+ sensitivity in different types of muscle: cardiac (porcine ventricular) and fast skeletal (rabbit psoas) muscles. PKA enhanced phosphorylation of cTnI at Ser23/24 in skinned cardiac muscle and decreased Ca2+ sensitivity, of which the effects were confirmed after reconstitution with the cardiac Tn complex (cTn) or the hybrid Tn complex (designated as PCRF; fast skeletal TnT with cTnI and cTnC). Reconstitution of cardiac muscle with the fast skeletal Tn complex (sTn) not only increased Ca2+ sensitivity, but also abolished the Ca2+-desensitizing effect of PKA, supporting the view that the phosphorylation of cTnI, but not that of other myofibrillar proteins, such as myosin-binding protein C, primarily underlies the PKA-induced Ca2+ desensitization in cardiac muscle. Reconstitution of fast skeletal muscle with cTn decreased Ca2+ sensitivity, and PKA further decreased Ca2+ sensitivity, which was almost completely restored to the original level upon subsequent reconstitution with sTn. The essentially same result was obtained when fast skeletal muscle was reconstituted with PCRF. It is therefore suggested that the PKA-dependent phosphorylation or dephosphorylation of cTnI universally modulates Ca2+ sensitivity associated with cTnC in the striated muscle sarcomere, independent of the TnT isoform.
INTRODUCTION
In the mammalian heart, β-adrenergic stimulation activates PKA coupled with a rise of cAMP, resulting in phosphorylation of several membrane- and sarcomere-based proteins in the cardiomyocyte (for reviews with original citations see Bers, 2001, 2002). Accordingly, myocardial function is associated with increased developed force and increased rates of rise and fall of developed force. It is generally considered that changes in the intracellular Ca2+ concentration are primarily responsible for these effects (Kurihara and Konishi, 1987; Endoh and Blinks, 1988; Li et al., 2000; Bers, 2001, 2002). In the sarcomere, PKA principally targets the thin filament protein troponin (Tn)I, the thick filament protein myosin-binding protein C (MyBP-C) (e.g., Bers, 2001, 2002), and the giant elastic protein titin (connectin) (Yamasaki et al., 2002; Fukuda et al., 2005, 2008). Phosphorylation of cardiac TnI (cTnI) and MyBP-C has been reported to decrease myofibrillar Ca2+ sensitivity and to enhance cross-bridge cycling, which likely contributes to the increased rate of relaxation and decreased twitch duration (Hoh et al., 1988; Hongo et al., 1993; Kentish et al., 2001). Diastolic properties are also affected during β-adrenergic stimulation, with a reduction in myocardial stiffness due to titin phosphorylation at the N2B segment in the I-band (both N2B and N2BA titins: Yamasaki et al., 2002; Fukuda et al., 2005, 2008).
Because cardiac myofilaments are activated by Ca2+ in a graded fashion, in the range of the submaximal Ca2+ concentration, the relationship between the Ca2+ concentration and active force is of fundamental importance (Bers, 2001, 2002). In this process, Tn switches the thin filament state to “off” or “on” (two-state model; e.g., Solaro and Rarick, 1998), or to “blocked, “closed,” or “open” (three-state model; e.g., Gordon et al. 2000). Tn is a heterotrimer of the following distinct gene products: i.e., TnC (a Ca2+ receptor), TnI (an inhibitor of actomyosin interaction), and TnT (an anchor that binds to tropomyosin [Tm]) (e.g., Solaro and Rarick, 1998; Ohtsuki and Morimoto, 2008). The cardiac isoform of TnI has a far N-terminal extension consisting of ∼30 amino acids, which is unique to this molecule (e.g., Solaro and Rarick, 1998). It is well documented that two Ser residues (Ser23 and Ser24) in the N-terminal extension are phosphorylated by PKA, resulting in enhanced relaxation via a decrease in myofibrillar Ca2+ sensitivity (for review see Solaro and Rarick, 1998; Layland et al., 2005). Recently, Howarth et al. (2007) demonstrated a clear conformational transition in the cardiac Tn complex (cTn) upon Ser23/24 phosphorylation in cTnI. They proposed that Ser23/24 phosphorylation extends and stabilizes the C-terminal helix in the N-terminal extension of cTnI, and weakens interactions with the N-lobe of cTnC. This may result in bending of cTnI and positioning of the acidic N terminus for electrostatic interactions with the electrostatic inhibitory region of cTnI. Skeletal TnI, from either fast or slow muscle, does not possess PKA phosphorylation sites in the N-terminal region and, therefore, does not exhibit a decrease in Ca2+ sensitivity upon PKA treatment (e.g., Solaro and Rarick, 1998). Indeed, it has been reported that cardiac muscle from a mouse model expressing slow skeletal TnI in the heart does not exhibit a decrease in Ca2+ sensitivity when exposed to β-adrenergic stimulation (Fentzke et al., 1999). More recent studies using mutant forms of cTnI with either pseudo-phosphorylated PKA sites (cTnI-S23D, S24D) or nonphosphorylatable mutant cTnI-S23A, S24A strongly support a critical role of cTnI in cardiac contractility during β-adrenergic stimulation (for review see Solaro et al., 2008).
Here, we tested whether the PKA-dependent control of phosphorylation or dephosphorylation of cTnI modulates Ca2+ sensitivity in different types of muscle by using our Tn reconstitution technique (Terui et al., 2008). We found that PKA decreases Ca2+ sensitivity not only in cardiac muscle, but also in fast skeletal muscle after thin filaments had been reconstituted with cTn or the hybrid Tn complex (PCRF) consisting of cTnI and cTnC and fast skeletal TnT (sTnT). Our findings demonstrate a universal role of cTnI via PKA activation in the modulation of Ca2+ sensitivity associated with cTnC in the striated muscle sarcomere.
MATERIALS AND METHODS
All experiments performed in this study conform to the Guide for the Care and Use of Laboratory Animals (1996. National Academy of Sciences, Washington D.C.).
Preparation of skinned muscles
Skinned cardiac muscles were prepared based on our recent article (Terui et al., 2008). In brief, porcine hearts (animals, ∼1.0 years old) were obtained from a local slaughterhouse. Muscle strips (1–2 mm in diameter and ∼10 mm in length) were dissected from the papillary muscle of the left ventricle in Ca2+-free Tyrode's solution (see Terui et al., 2008 for composition containing 30 mM 2,3-butanedione monoxime [BDM] to inhibit actomyosin interaction), with 10 µM propranolol to block nonspecific β-adrenergic stimulation and 10 µM carbamoylcholine to enhance phosphatase activity (Gupta et al., 1994). BDM is also known to enhance dephosphorylation of myofibrillar proteins (Venema et al., 1993; Turnbull et al., 2002), including cTnI and MyBP-C. The presence of propranolol, carbamoylcholine, and BDM, however, does not exclude basal levels of protein phosphorylation (Fukuda et al., 2005 and this study). Psoas muscles were obtained from white rabbits (2–3 kg). The muscle strips were skinned in relaxing solution (5 mM MgATP, 40 mM N,N-bis(2-hydroxy-ethyl)-2-aminoethanesulfonic acid [BES], 1 mM Mg2+, 10 mM EGTA, 1 mM dithiothreitol, 15 mM phosphocreatine, 15 U/ml creatine phosphokinase, and 180 mM ionic strength [adjusted by K-propionate], pH 7.0) containing 1% (wt/vol) Triton X-100 and 10 mM BDM overnight at ∼3°C. They were stored for up to 3 wk at −20°C in relaxing solution containing 50% (vol/vol) glycerol. All solutions contained protease inhibitors (0.5 mM PMSF, 0.04 mM leupeptin, and 0.01 mM E64) to avoid protein degradations (see Fukuda et al., 2003).
Skinned muscle mechanics
Isometric force was measured at 15°C based on our previous reports with either thin cardiac bundles (porcine left ventricular [PLV]; ∼100 µm in diameter and ∼2 mm in length; see Terui et al., 2008) or single skeletal fibers (rabbit psoas [RP]; see Udaka et al., 2008). In brief, sarcomere length (SL) was measured by laser diffraction during relaxation (as in Fukuda et al., 2000, 2001a,b) and set at 2.0 µm for both PLV and RP. To avoid the possible effects of PKA-dependent passive force modulation on active force, we conducted all experiments at SL 2.0 µm, where passive force is barely produced in both PLV and RP (e.g., Cazorla et al., 2000; Prado et al., 2005; Terui et al., 2008). Just before contraction, the preparation was immersed in low-EGTA (0.5 mM) relaxing solution to avoid slowing of contraction (Fukuda et al., 2001a). The preparation was first activated at pCa 4.5 to obtain maximal Ca2+-activated force, followed by relaxation. The preparation was then activated at various pCa's (from high to low pCa, and lastly at pCa 4.5) to construct the force-pCa curve (i.e., force-pCa protocol). The curves were fitted to the Hill equation, and the value of the midpoint of the force-pCa curve (i.e., pCa50) was used as an index of myofibrillar Ca2+ sensitivity (Fukuda et al., 2000). The Hill coefficient (nH) was used as an index of thin filament cooperative activation (Fukuda et al., 2000). Here, forces at submaximal Ca2+ levels were normalized to the maximal force obtained at the end of the force-pCa protocol (rundown in active force during the protocol was <10%). With the values of pCa50, nH, and maximal force being similar in the presence of a higher concentration of creatine phosphokinase (225 U/ml; not depicted), we used 15 U/ml throughout the study for both PLV and RP.
Next, the preparation was incubated for 50 min at 22°C with purified PKA (catalytic subunit from bovine heart; Sigma-Aldrich) at a concentration of 1 U/µl (Fukuda et al., 2005). PKA-specific inhibitor (PKI; Sigma-Aldrich) was used in control experiments at 50 µM (Fukuda et al., 2005). Then, the force-pCa protocol was repeated and the force-pCa curve was obtained. The difference between the values of pCa50 (ΔpCa50) before and after PKA treatment was used as an index of the PKA-induced Ca2+ desensitization (PKA effect).
To reduce the background phosphorylation of myofibrillar proteins, we used protein phosphatase 1 (PP1; Sigma-Aldrich) (see Jideama et al., 2006). After conducting the force-pCa protocol, cardiac muscle preparations were immersed in relaxing solution containing PP1 at 900 U/ml for 1 h at 25°C. Then, the preparations were immersed in normal relaxing solution at 15°C for 10 min with gentle agitation to remove PP1 for subsequent PKA treatment.
Tn extraction
The fast skeletal Tn complex (sTn) was purified from rabbit fast skeletal muscle based on our previously published procedure (Shiraishi et al., 1993) and stored at −80°C.
Porcine ventricular and rabbit fast skeletal Tn subunits were prepared by the method of Ojima and Nishita (1986), with slight modifications. In brief, Tn components were separated by the two-step ion-exchange chromatography in the presence of 6 M urea and 1 mM EDTA, with Toyopearl DEAE-650M and CM-650M (Tosoh) columns (2.0 cm i.d. × 20 cm) (see Ojima and Nishita, 1988 for details). To reconstitute the cardiac Tn and sTnT-substituted Tn, the three Tn components (60 µM final of each) were mixed at a 1:1:1 molar ratio and dialyzed as follows: (1) The mixtures were dialyzed for 3 h against 6 M urea, 0.5 M KCl, 10 mM Tris-HCl, pH 7.6, and 5 mM 2-mercaptoethanol. (2) The concentration of urea in the outer dialysate was decreased stepwise to 3 and 1 M. (3) The urea was removed by dialysis for 6 h against 0.5 M KCl, 10 mM Tris-HCl, pH 7.6, 2 mM MgCl2, 0.1 mM CaCl2, 0.01% NaN3, and 5 mM 2-mercaptoethanol. (4) The KCl concentration of the outer dialysate was decreased stepwise to 0.25 and 0.15 M. The precipitate formed was removed by centrifugation (10,000 g, 15 min). The reconstituted Tn complexes were concentrated by centrifugal ultrafiltration with an Apollo concentrator (Orbital Biosciences) and finally dialyzed against 1 mM NaHCO3 and 5 mM 2-mercaptoethanol. Reconstituted Tn complexes were stored at −80°C.
Tn exchange
Tn exchange was performed based on our previously published procedure (Terui et al., 2008). In brief, after performing the force-pCa protocol without any treatment (as described above), the skinned muscle preparation, either PLV or RP, was bathed in rigor solution (10 mM BES, 150 mM K-propionate, 2.5 mM EGTA, and 5 mM MgCl2, pH 7.0) containing cTn or PCRF (2 and 6 mg/ml for PLV and RP, respectively) and 80 mM BDM for 60 min at 25°C. sTn reconstitution was performed on PLV at 2 mg/ml for 20, 40, or 60 min at 25°C (60 min for quasi-complete reconstitution; see Terui et al., 2008). The time-dependent change in cTnI upon sTn reconstitution was assessed using the ratio cTnI/(cTnI + sTnI). As noted in our previous report (Terui et al., 2008), the presence of BDM was essential to avoid contraction upon exchange and the ensuing damage to the sarcomere structure. Next, the preparation was washed with normal relaxing solution at 15°C for 10 min with gentle agitation to remove excess Tn molecules. In experiments with cTn- or PCRF-reconstituted RP, the fibers were further treated with sTn at 2 mg/ml for 60 min at 25°C after PKA treatment.
Electrophoresis
SDS-PAGE was performed based on our previous studies (Terui et al., 2008; Udaka et al., 2008). Muscle preparations were solubilized and electrophoresed on Laemmli gels (acrylamide, 3.5% in stack and 15% resolving gel, pH 9.3; stained with Coomassie brilliant blue). Tn isoforms were identified by commigration with known standards, as performed in our previous study (Terui et al., 2008). We quantified the reconstitution ratio by comparing the ratio of TnI or TnT to actin with that obtained for the original muscle (i.e., either RP or PLV muscle). Due to the technical difficulty in the quantitative analysis of TnC, especially in RP where cTnC and sTnC coexist after reconstitution (with only a slight difference in molecular weight), we present only the analyzed data on TnT and TnI, based on our previous study (Terui et al., 2008).
Western blotting
Immunoblotting was performed based on our previous report (O-Uchi et al., 2008). After separation on 20% SDS-PAGE, proteins were transferred to a polyvinylidene difluoride membrane. For the detection of PKA-dependent Ser23/24 phosphorylation in cTnI (pig cTnI; GenBank accession no. DQ534449.1), the transferred membranes were blocked by Blocking One-P solution (Nakalai Tesque, Inc.), which is free of phosphate groups and endogenous phosphates, and incubated with cardiac phospho-TnI (Ser23/24) polyclonal antibody (Cell Signaling Technology). Then, the membranes were incubated with horseradish peroxidase–conjugated secondary antibody (GE Healthcare), and the bands were visualized by using an ECL-plus detection kit (GE Healthcare). Sequentially, after gently stripping these primary and secondary antibodies to allow reprobings on the same membrane, the membranes were blocked by Tris-based buffer containing 5% nonfat skim milk (BD) and 0.1% tween-20. For the detection of total cTnI, we used anti-cTnI monoclonal antibody (Millipore) and secondary horseradish peroxidase–conjugated antibody (GE Healthcare). Radiographs were scanned and digitized (ES-2200; Epson), and densitometry was performed using One-D-Scan (v. 2.03; BD).
Statistics
Significant differences were assigned using the paired or unpaired Student's t test as appropriate. Data are expressed as mean ± SEM, with “n” representing the number of muscles. Statistical significance was assumed to be P < 0.05. “N.S.” indicates P > 0.05.
Online supplemental material
Table S1 shows the reproducibility of the values of pCa50, nH, and maximal force after repeated measurements of the force-pCa curve in PLV and RP under the control condition with no treatment. Fig. S1 shows that (1) PKA phosphorylates MyBP-C in PLV, and (2) cTnI is the only protein among various Tn subunits that shows phosphorylation upon PKA treatment. Table S1 and Fig. S1 are available at http://www.jgp.org/cgi/content/full/jgp.200910206/DC1.
RESULTS
Effect of PKA on Ca2+ sensitivity in PLV muscle with and without sTn reconstitution
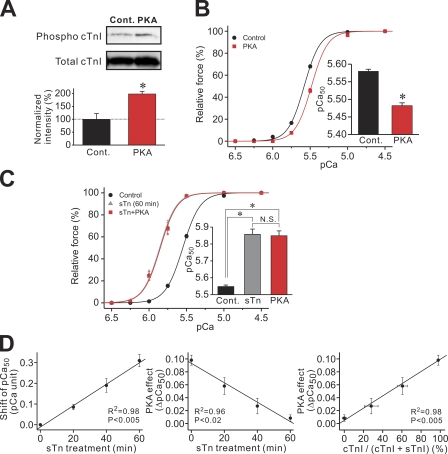
First, we tested how PKA-dependent phosphorylation of cTnI modulates Ca2+ sensitivity in PLV under the control condition without Tn reconstitution. We found that our PKA treatment nearly doubled Ser23/24 phosphorylation in cTnI (Fig. 1 A). Fig. 1 B shows the effect of PKA on myofibrillar Ca2+ sensitivity. Consistent with a previous study on porcine ventricular preparations (Zhang et al., 1995), the force-pCa curve was shifted to the right by 0.10 ± 0.01 pCa units (as indexed by pCa50), exhibiting a decrease in Ca2+ sensitivity. PKA did not affect maximal force or thin filament cooperative activation (as indexed by nH; Table I). Ca2+ desensitization was not observed when the preparation was treated with PKA in the presence of the PKI (pCa50: 5.57 ± 0.02 and 5.54 ± 0.03, respectively, before and after PKA+PKI; P > 0.05; n = 6).
Figure 1.
Effects of PKA on cTnI phosphorylation and Ca2+ sensitivity in PLV. (A; Top) Western blotting showing cTnI phosphorylation (12.5 µg protein/well). Cont., control without PKA treatment. (Bottom) Comparison of the intensity of cTnI Ser23/24 phosphorylation normalized with total cTnI content in control and PKA-treated PLV. *, P < 0.05. n = 3. (B) Force-pCa curves showing the effect of PKA on Ca2+ sensitivity. (Inset) pCa50 values before and after PKA treatment. *, P < 0.05. n = 7. (C) Force-pCa curves showing the effects of quasi-complete sTn reconstitution (shown in gray) and subsequent treatment with PKA on Ca2+ sensitivity. (Inset) pCa50 values. *, P < 0.05. n = 7. (D; Left) Plot of sTn treatment time versus sTn-induced shift of pCa50. (Middle) Plot of sTn treatment time versus PKA-induced shift of pCa50. (Right) Plot of residual cTnI ratio (i.e., cTnI/(cTnI + sTnI)) versus PKA-induced shift of pCa50. n = 7.
TABLE I.
Summary of the values of maximal active force and nH before and after PKA treatment in PLV
| Maximal force (mN/mm2) | nH | |
| Fig. 1 B | ||
| Control PLV | 52.89 ± 3.24 | 4.06 ± 0.20 |
| PKA | 52.23 ± 3.70 | 4.02 ± 0.22 |
| Control PLV | 51.89 ± 3.16 | 3.98 ± 0.21 |
| PKA + PKI | 50.23 ± 3.78 | 3.97 ± 0.18 |
| Fig. 1 C | ||
| Control PLV | 52.06 ± 3.77 | 3.74 ± 0.24 |
| sTn | 47.71 ± 4.59 | 3.87 ± 0.09 |
| sTn + PKA | 46.92 ± 4.85 | 3.84 ± 0.12 |
Maximal force was obtained by activating muscle at pCa 4.5 before plotting of the force-pCa curve at each condition. No significant difference was observed in each case.
Next, we tested the effect of PKA on Ca2+ sensitivity in PLV after reconstitution with sTn (Fig. 1 C). Quasi-complete reconstitution with sTn (for 60 min) increased Ca2+ sensitivity by 0.31 ± 0.03 pCa units (see Terui et al., 2008) and completely abolished the PKA-induced Ca2+ desensitization. Increasing the time for sTn reconstitution (up to 60 min) increased Ca2+ sensitivity (Fig. 1 D, left) and diminished the PKA effect (Fig. 1 D, middle), both in a linear fashion. A linear relationship was found between the residual amount of cTnI and the PKA effect (Fig. 1 D, right).
Effect of PKA on Ca2+ sensitivity in PLV muscle reconstituted with cTn or PCRF
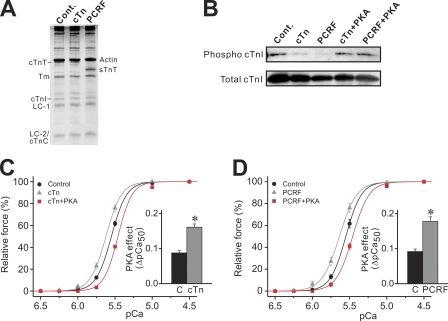
Next, we tested whether or not PKA exerts its Ca2+-desensitizing effect in PLV after thin filament reconstitution with cTn or PCRF. The representative 15% gel showed that the band for cTnT or cTnI was unaffected by cTn reconstitution (cTn reconstitution ratio: cTnI, 94.21 ± 9.63%; cTnT, 93.22 ± 5.33%; n = 6) (Fig. 2 A; see Terui et al., 2008). Upon PCRF reconstitution, endogenous cTnT was effectively replaced by sTnT, with cTnI appearing similarly as in control PLV (PCRF reconstitution ratio: cTnI, 86.79 ± 8.81%; sTnT, 130.28 ± 4.09%; residual cTnT, 4.91 ± 1.62%; n = 6) (Fig. 2 A). Western blotting demonstrated that cTnI Ser23/24 phosphorylation was clearly less in cTn- or PCRF-reconstituted PLV than in control PLV, and that PKA treatment increased the phosphorylation level in both (Fig. 2 B; see legend for quantitative analysis).
Figure 2.
Effects of PKA on cTnI phosphorylation and Ca2+ sensitivity in PLV reconstituted with cTn or PCRF. (A; Left) SDS-PAGE for control, cTn-, and PCRF-reconstituted PLV. Cont., control with no reconstitution; cTn, cTn reconstitution; PCRF, PCRF reconstitution; Tm, tropomyosin; LC-1, myosin light chain 1; LC-2, myosin light chain 2. (B) Western blotting showing the effect of PKA on cTnI Ser23/24 phosphorylation in cTn- and PCRF-reconstituted PLV (12.5 µg protein/well). Cont., control with no treatment. Normalized intensity compared with control (n = 3): cTn, 10.12 ± 6.53%; PCRF, 13.83 ± 5.23%; cTn + PKA (PKA phosphorylation after cTn reconstitution), 214.94 ± 30.82% (P < 0.05 compared with cTn); PCRF + PKA (PKA phosphorylation after PCRF reconstitution), 228.89 ± 20.13% (P < 0.05 compared with PCRF). (C) Force-pCa curves showing the effects of cTn reconstitution and subsequent PKA treatment on Ca2+ sensitivity. (Inset) Difference between the values of the PKA-induced shift of pCa50 (C, control minus cTn + PKA; cTn, cTn minus cTn + PKA). *, P < 0.05. n = 7. (D) Force-pCa curves showing the effects of PCRF reconstitution and subsequent PKA treatment on Ca2+ sensitivity. (Inset) Difference between the values of the PKA-induced shift of pCa50 (C, control minus PCRF + PKA; PCRF, PCRF minus PCRF + PKA). *, P < 0.05. n = 7. PKA treatment did not significantly affect maximal force or nH in both C and D (see Table II).
As with sTn reconstitution (Table I; see also Terui et al., 2008), maximal force was decreased by a small magnitude (∼5%) upon cTn or PCRF reconstitution in PLV (Table II). However, the decrease was not statistically significant and, therefore, we consider that the damage, if at all, to the sarcomere structure (or the regulatory system) was minimal in our protocol, as reported in our earlier study (Terui et al., 2008).
TABLE II.
Summary of the values of maximal active force, pCa50 and nH before and after PKA treatment in PLV after reconstitution with cTn or PCRF or treatment with PP1
| Maximal force (mN/mm2) | pCa50 | nH | |
| Fig. 2 C | |||
| Control PLV | 55.99 ± 5.92 | 5.55 ± 0.01 | 4.23 ± 0.25 |
| cTn | 52.52 ± 5.48 | 5.62 ± 0.01a | 4.15 ± 0.35 |
| cTn+PKA | 51.17 ± 5.42 | 5.46 ± 0.01ab | 4.56 ± 0.30 |
| Fig. 2 D | |||
| Control PLV | 53.85 ± 6.03 | 5.55 ± 0.02 | 4.16 ± 0.16 |
| PCRF | 51.42 ± 5.35 | 5.64 ± 0.03a | 3.69 ± 0.16 |
| PCRF + PKA | 49.86 ± 5.32 | 5.46 ± 0.01ab | 3.92 ± 0.18 |
| Fig. 3 B | |||
| Control PLV | 59.32 ± 4.51 | 5.55 ± 0.01 | 4.12 ± 0.16 |
| PP1 | 56.47 ± 5.70 | 5.64 ± 0.02a | 4.24 ± 0.35 |
| PP1 + PKA | 54.10 ± 5.66 | 5.46 ± 0.01ab | 4.05 ± 0.20 |
Maximal force was obtained by activating muscle at pCa 4.5 before plotting of the force-pCa curve at each condition.
P < 0.05 compared with the corresponding values for control PLV (before thin filament reconstitution or PP1 treatment).
P < 0.05 compared with the corresponding values for thin filament reconstitution or PP1 treatment (before PKA treatment).
Ca2+ sensitivity was slightly but significantly (0.07 ± 0.01 pCa units) increased upon cTn reconstitution, and it was decreased by 0.16 ± 0.01 pCa units after PKA treatment (Fig. 2 C). Similarly, Ca2+ sensitivity was increased by 0.09 ± 0.01 pCa units upon PCRF reconstitution, and it was decreased by 0.18 ± 0.01 pCa units after PKA treatment (Fig. 2 D). Accordingly, the PKA effect was more pronounced with exogenous cTnI (compare with Fig. 1 B). Reconstitution with cTn or PCRF did not significantly affect the maximal force or nH (Table II).
Effect of PKA on Ca2+ sensitivity in PP1-treated PLV muscle
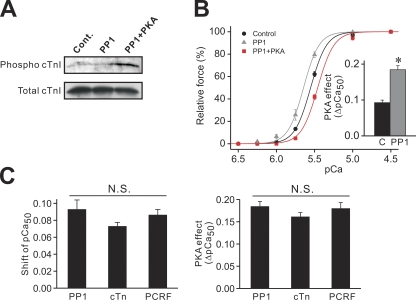
The apparently greater effect of PKA on Ca2+ sensitivity in cTn- or PCRF-reconstituted PLV (Fig. 2, C and D) may be coupled with reconstitution of the dephosphorylated form of cTnI and, therefore, a greater magnitude of PKA-dependent phosphorylation of it. We therefore tested the effect of PKA on Ca2+ sensitivity in PLV that had been pretreated with PP1 to reduce background phosphorylation of cTnI. Consistent with a previous report (Jideama et al., 2006), PP1 effectively reduced background cTnI Ser23/24 phosphorylation, and the subsequent PKA treatment increased the phosphorylation level (Fig. 3 A; see legend for quantitative analysis).
Figure 3.
Effects of PKA on cTnI phosphorylation and Ca2+ sensitivity in PP1-treated PLV. (A) Western blotting showing the effect of PP1 or PKA on the phosphorylation level of Ser23/24 in cTnI (12.5 µg protein/well). Cont., control with no treatment. Normalized intensity compared with control (n = 5): PP1, 40.16 ± 10.48%; PP1 + PKA (PKA phosphorylation after PP1 treatment), 207.76 ± 53.85% (P < 0.05 compared with PP1). (B) Force-pCa curves showing the effects of PP1 and subsequent PKA treatment on Ca2+ sensitivity. (Inset) Difference between the values of the PKA-induced shift of pCa50 (C, control minus PP1 + PKA; PP1, PP1 minus PP1 + PKA). *, P < 0.05. n = 7. PKA treatment did not significantly affect maximal force or nH (see Table II). (C; Left) Comparison of Ca2+ sensitization as indexed by the shift of pCa50 after PP1 treatment and cTn or PCRF reconstitution in PLV. (Right) Comparison of the PKA-induced shift of pCa50 in PP1-treated and cTn- or PCRF-reconstituted PLV. Statistical significance was not observed between groups in either graph.
Ca2+ sensitivity was slightly but significantly (0.09 ± 0.01 pCa units) increased upon PP1 treatment, with no significant change in maximal force or nH (Table II), and it was decreased by 0.18 ± 0.01 pCa units after PKA treatment. Fig. 3 C compares the magnitude of Ca2+ sensitization upon PP1 treatment, cTn, and PCRF reconstitution, and the subsequent PKA effect obtained in these preparations. All maneuvers similarly increased Ca2+ sensitivity (Fig. 3 C, left; P > 0.05), and the subsequent PKA effect was similar (Fig. 3 C, right; P > 0.05).
Effect of PKA on Ca2+ sensitivity in cTn-reconstituted RP muscle
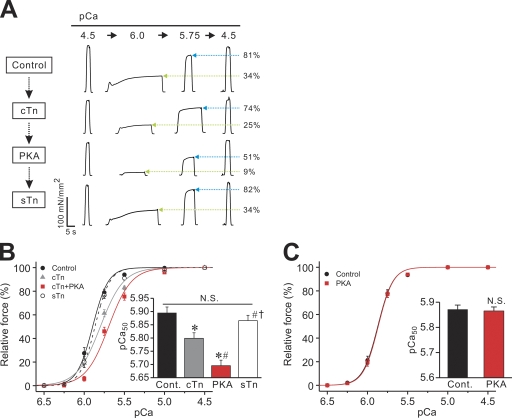
We next investigated whether PKA exerts its Ca2+-desensitizing effect in RP after cTn reconstitution (reconstitution ratio, ∼50%; see Terui et al., 2008). As shown in the chart recording (Fig. 4 A), submaximal forces (at pCa 6.0 and 5.75) were decreased upon cTn reconstitution by a magnitude greater than that of maximal force, resulting in a decrease in the percentage to the maximum (see Terui et al., 2008). PKA treatment further decreased submaximal forces with little or no change in maximal force. Lastly, sTn reconstitution restored submaximal forces to levels similar to those obtained before cTn reconstitution, accompanied by a slight increase in maximal force.
Figure 4.
Effect of PKA on Ca2+ sensitivity in cTn-reconstituted RP. (A) Typical chart recording showing force-pCa protocols. A fiber was treated with cTn, PKA, and sTn, in this order (see Terui et al., 2008 for exchange ratio of Tn subunits under the same condition; i.e., ∼50% with cTn reconstitution). Green arrows (blue arrows) indicate percentage of force at pCa 6.0 (pCa 5.75) compared with the maximum obtained at the end of each force-pCa protocol. (B) Force-pCa curves showing the effects of cTn reconstitution, PKA treatment, and sTn reconstitution on Ca2+ sensitivity in RP. Black solid line, control with no treatment; gray solid line, cTn reconstitution; red solid line, PKA treatment; black dashed line, sTn reconstitution. (Inset) Summary of pCa50 values. *, P < 0.05 compared with control; #, P < 0.05 compared with cTn reconstitution; †, P < 0.05 compared with PKA. n = 9. (C) Force-pCa curves showing the effect of PKA on Ca2+ sensitivity in RP with no Tn reconstitution. (Inset) pCa50 values before and after PKA treatment. Maximal force: 175.35 ± 6.32 and 176.25 ± 5.31 mN/mm2 (P > 0.05) before and after PKA, respectively; nH: 4.61 ± 0.34 and 4.67 ± 0.30 (P > 0.05) before and after PKA, respectively. n = 7.
Force-pCa curves in Fig. 4 B demonstrate that Ca2+ sensitivity is decreased by 0.10 ± 0.01 pCa units with cTn reconstitution (see Terui et al., 2008) and is further decreased by 0.10 ± 0.01 pCa units with PKA treatment. As evident in the chart recording (Fig. 4 A), sTn reconstitution almost completely restored Ca2+ sensitivity to the original level before cTn reconstitution. In a control experiment without cTn reconstitution, PKA treatment did not affect Ca2+ sensitivity in RP (Fig. 4 C).
Maximal force and nH were decreased by cTn reconstitution (Terui et al., 2008), but not by PKA treatment, and both were restored with sTn reconstitution to levels not significantly different than those obtained before cTn reconstitution (Table III).
TABLE III.
Summary of the values of maximal active force and nH before and after PKA treatment in RP after reconstitution with cTn or PCRF
| Maximal force (mN/mm2) | nH | |
| Fig. 4 B | ||
| Control RP | 179.71 ± 7.04 | 4.47 ± 0.15 |
| cTn | 157.86 ± 4.30a | 3.00 ± 0.12a |
| cTn + PKA | 157.57 ± 4.22a | 3.07 ± 0.15a |
| sTn | 171.86 ± 5.18bc | 4.71 ± 0.36bc |
| Fig. 5 C | ||
| Control RP | 182.14 ± 7.98 | 4.50 ± 0.17 |
| PCRF | 158.14 ± 5.84a | 3.20 ± 0.25a |
| PCRF + PKA | 157.03 ± 5.71a | 3.13 ± 0.23a |
| sTn | 174.43 ± 5.05bc | 4.42 ± 0.16bc |
Maximal force was obtained by activating muscle at pCa 4.5 before plotting of the force-pCa curve at each condition.
P < 0.05 compared with the corresponding values for control RP (before thin filament reconstitution).
P < 0.05 compared with the corresponding values for thin filament reconstitution (before PKA treatment).
P < 0.05 compared with the corresponding values for PKA treatment (before sTn reconstitution).
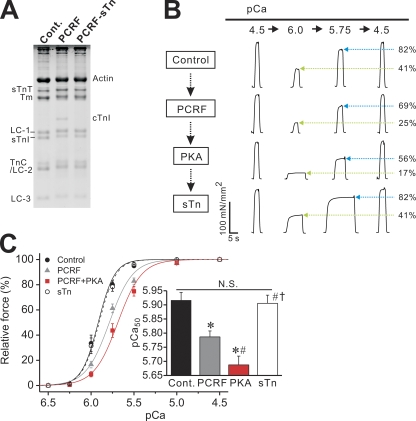
Effect of PKA on Ca2+ sensitivity in PCRF-reconstituted RP muscle
Finally, by using PCRF reconstitution, we investigated whether the PKA-induced decrease in Ca2+ sensitivity in cTn-reconstituted RP is coupled with the phosphorylation of cTnI or, alternatively, cTnT. As shown in Fig. 5 A, our protocol with PCRF allowed for ∼50% replacement of sTnI with cTnI (PCRF reconstitution ratio: cTnI, 47.80 ± 2.54%; sTnT, 128.52 ± 8.80%; residual sTnI, 43.52 ± 6.51%; n = 6). Subsequent treatment with sTn completely replaced cTnI with sTnI, and the ratio of sTnT or sTnI to actin was similar to that observed in control RP before PCRF reconstitution (sTn reconstitution ratio after PCRF reconstitution: sTnI, 107.04 ± 10.54%; sTnT, 85.97 ± 11.76%; residual cTnI, ∼0%; n = 6).
Figure 5.
Effect of PKA on Ca2+ sensitivity in PCRF-reconstituted RP. (A; Left) 15% gel showing reconstitution of RP with PCRF. Cont., control fibers; PCRF, PCRF-reconstituted fibers; PCRF-sTn, PCRF-reconstituted fibers treated with sTn; LC-3, myosin light chain 3. For other abbreviations, see legend to Fig. 2 A. (B) Typical chart recording showing force-pCa protocols. A fiber was treated with PCRF, PKA, and sTn, in this order. Green arrows (blue arrows) indicate percentage of force at pCa 6.0 (pCa 5.75) compared with the maximum obtained at the end of each force-pCa protocol. (C) Force-pCa curves showing the effects of PCRF reconstitution, PKA treatment, and sTn reconstitution in RP. Black solid line, control with no treatment; gray solid line, PCRF reconstitution; red solid line, PKA treatment; black dashed line, sTn reconstitution. (Inset) Summary of pCa50 values. *, P < 0.05 compared with control; #, P < 0.05 compared with PCRF; †, P < 0.05 compared with PKA. n = 10.
As shown in the chart recording (Fig. 5 B), submaximal forces (at pCa 6.0 and 5.75) were decreased upon PCRF treatment by a magnitude greater than that of maximal force, resulting in a decrease in the percentage to the maximum. PKA treatment further decreased submaximal forces with little or no change in maximal force. Finally, sTn reconstitution restored submaximal forces to levels similar to those obtained before PCRF reconstitution, accompanied by a slight increase in maximal force.
Force-pCa curves in Fig. 5 C show that Ca2+ sensitivity is decreased by 0.13 ± 0.02 pCa units with PCRF reconstitution, and it is further decreased by 0.10 ± 0.02 pCa units with PKA treatment, which is similar to what was observed in cTn reconstitution in RP (see Fig. 4 B). Also, sTn reconstitution almost completely restored Ca2+ sensitivity to the original level before PCRF reconstitution. As with cTn reconstitution, maximal force and nH were decreased by PCRF reconstitution, but not by PKA treatment, and both were restored with sTn reconstitution to levels not significantly different than those obtained before PCRF reconstitution (Table III).
DISCUSSION
The goal of this study was to provide direct evidence that PKA-based phosphorylation of cTnI has the potential to decrease myofibrillar Ca2+ sensitivity associated with cTnC in different types of the striated muscle sarcomere. To test this, we investigated whether PKA induces Ca2+ desensitization in PLV and RP muscles, by using thin filament reconstitution. We demonstrated that PKA-dependent cTnI phosphorylation decreased Ca2+ sensitivity not only in PLV, but also in RP after reconstitution with the cTn complex. Moreover, the Ca2+-desensitizing effect was independent of the TnT isoform in both types of muscle. The present findings are summarized in Table IV.
TABLE IV.
Summary of this work describing whether PKA exerts its Ca2+-desensitizing effect in PLV and RP with various combinations of Tn subunits
| PLV | RP | ||||||
| Muscle type | Original | Reconstituted (cTn) | Reconstituted (PCRF) | Reconstituted (sTn) | Original | Reconstituted (cTn) | Reconstituted (PCRF) |
| TnI-TnC complex | C | C | C | S | S | C | C |
| TnT | C | C | S | S | S | C | S |
| Ca2+ desensitization (+PKA) | + | + | + | − | − | + | + |
“C” and “S” indicate that the Tn subunit (or the complex) is of cardiac or fast skeletal origin, respectively. +, Ca2+ desensitization present; −, Ca2+ desensitization absent (see Results).
In both cardiac and skeletal sarcomeres, TnT is the largest component of the Tn complex. Within the cTn complex, the N-terminal half of cTnT anchors the complex to Tm, and the C-terminal half forms a complex with cTnC, cTnI, and Tm (e.g., Solaro and Rarick, 1998; Ohtsuki and Morimoto, 2008). Despite extensive regions of homology among the various forms of cTnT and sTnT, there are important regions of structural diversity, especially in the N-terminal region, which is highly charged and essentially absent in sTnT (Solaro and Rarick, 1998). In the present study, however, we found that the force-pCa curve was shifted leftward (rightward) by a similar magnitude upon cTn and PCRF reconstitution in PLV (in RP) (Fig. 2, C and D, PLV, and Figs. 4 B and 5 C, RP). Therefore, the TnT isoform variance of adult ventricular versus adult fast skeletal isoform is unlikely to account for the altering of Ca2+ sensitivity, as demonstrated earlier in rat (Chandra et al., 2006). Rather, it is likely that the “on–off” equilibrium of the thin filament state is more strongly governed by the TnC–TnI interaction within the Tn molecule.
cTnI is phosphorylated not only by PKA, but also by other kinases, such as various types of PKC, cGMP-dependent protein kinase (PKG), and p21-activated kinases (Pak) (e.g., Layland et al., 2005). Despite conflicting reports regarding the physiological effects of PKC-based phosphorylation of cTnI at Ser43/45 (and Thr144), an emerging consensus based on recent findings is that Ser43/45 phosphorylation reduces maximal Ca2+-activated force, and in concert with Thr144 phosphorylation, it reduces maximal sliding velocity in in vitro motility assays (Layland et al., 2005). PKG phosphorylates cTnI at Ser23/24 at a ∼100-fold slower rate than PKA, with a physiological effect similar to that by PKA (Blumenthal et al., 1978; Yuasa et al., 1999; Layland et al., 2005). Pak reportedly phosphorylates cTnI at Ser149, resulting in an increase (not a decrease) in Ca2+ sensitivity with no change in maximal force (Buscemi et al., 2002). Also, cTnT, but not sTnT, is phosphorylated by PKC in the C-terminal half of this molecule (Solaro and Rarick, 1998). However, PKC-based cTnT phosphorylation does not reportedly influence Ca2+ sensitivity (Noland et al., 1995). Collectively, it is likely that PKA-dependent phosphorylation of cTnI at Ser23/24 is predominant in Ca2+ desensitization among the various phosphorylation sites in cTnI and cTnT.
Reconstitution with cTn (Terui et al., 2008) or PCRF in PLV slightly but significantly increased Ca2+ sensitivity (Fig. 2, C and D). Because cTnI Ser23/24 phosphorylation was less in cTn- or PCRF-reconstituted PLV than in control PLV (Fig. 2 B), the Ca2+ sensitization may result at least in part from lowering of the background phosphorylation of these residues. Consistent with this view, PP1 treatment reduced the cTnI phosphorylation level (Fig. 3 A) and increased Ca2+ sensitivity (Fig. 3 B). Therefore, the Ca2+ sensitization after cTn or PCRF reconstitution and PP1 treatment in PLV may result predominantly from dephosphorylation of Ser23/24 in cTnI. This is supported by the finding that PKA increased cTnI phosphorylation (Figs. 2 B and 3 A) and caused a similar magnitude of Ca2+ desensitization after cTn or PCRF reconstitution and PP1 treatment (Fig. 3 C), with a position of the force-pCa curve similar to that of control PLV after PKA treatment (Fig. 1 B).
This study revealed that RP became respondent to PKA treatment showing Ca2+ desensitization after thin filaments had been reconstituted with cTn (Fig. 4, A and B) or PCRF (Fig. 5, B and C). Because in a control experiment PKA failed to alter Ca2+ sensitivity in RP without thin filament reconstitution (Fig. 4 C), the Ca2+-desensitizing effect after cTn or PCRF reconstitution likely results from Ser23/24 phosphorylation in cTnI and subsequent reduction in the affinity of cTnI for cTnC, as well as in the affinity of cTnC for Ca2+. This interpretation is supported by the result that subsequent treatment with sTn after PKA treatment (∼100% reconstitution for PCRF [Fig. 5 A] as well as for cTn [Terui et al., 2008]) almost completely restored Ca2+ sensitivity to the original level. It is also worthwhile noting that the magnitude of the PKA-induced shift of pCa50 was similar with cTn and PCRF reconstitution not only in PLV (Fig. 2, C and D), but also in RP (Figs. 4 B and 5 C). Howarth et al. (2007) proposed that there are “extended” and “bent” conformations in Tn (see Solaro et al., 2008); phosphorylation or dephosphorylation at Ser23/24 in cTnI modulates the equilibrium between the two conformations (“bent” with phosphorylation), similar to that proposed for regulation of smooth muscle myosin by phosphorylation at the N terminus of the regulatory light chain. It is therefore suggested that the phosphorylation-induced “bending” and the ensuing reduction in Ca2+ sensitivity occur regardless of the TnT isoform; viz., regardless of the presence or absence of the N-terminal extension of TnT.
cTn or PCRF reconstitution significantly decreased maximal Ca2+-activated force and nH in RP (Table III), but the effects were not significant in PLV (Table II). This suggests that the cTnI–cTnC complex shifts the equilibrium of the thin filament state toward the “off” state to a relatively greater degree than its fast skeletal counterpart (Terui et al., 2008; Fukuda et al., 2009). In fact, upon sTn reconstitution, both maximal force and nH were restored to levels not significantly different than those obtained before reconstitution in RP (Table III). PKA treatment, however, decreased Ca2+ sensitivity with no change in maximal force or nH in both RP (Table III) and PLV (Tables I and II), suggesting a different mode of action between Tn reconstitution and PKA treatment in the thin filament regulation. We consider that Tn reconstitution can potentially alter the near-neighbor regulatory unit interactions within thin filaments (see Terui et al., 2008), whereas PKA-based Ser23/24 phosphorylation in cTnI simply reduces Ca2+ binding to cTnC with no change in signaling between neighboring regulatory units. Clearly, future studies are needed to clarify the differences in the effects between isoform switching of Tn subunits and phosphorylation by various kinases.
The magnitude of Ca2+ desensitization by PKA in cTn- or PCRF-reconstituted RP was ∼0.1 pCa units (Figs. 4 B and 5 C), ∼50% of the effect observed in reconstituted PLV (Fig. 2, C and D). This is probably due to the fact that in RP the thin filament reconstitution ratio with either cTn or PCRF cannot technically exceed ∼50% to avoid irreversible damages to the regulatory system (see Terui et al., 2008), whereas in PLV thin filament reconstitution can be achieved by ∼100% with cTn or PCRF (see Terui et al., 2008 and Fig. 2 A). Given the finding that the magnitude of PKA-based Ca2+ desensitization is linearly correlated to the amount of cTnI (Fig. 1 D), it can be considered that the magnitude of Ca2+ desensitization is similar in both PLV and RP; i.e., by potentially ∼0.2 pCa units with a full amount of the cTnI–cTnC complex.
One may point out that MyBP-C phosphorylation has to be taken into account in the interpretation of the data on PLV due to its potential impact on Ca2+ sensitivity. MyBP-C, a large protein associated with the thick filament of striated muscle, constrains cross-bridges to the thick filament backbone (see Granzier and Campbell, 2006; Oakley et al., 2007 and references therein). A binding site for the light meromyosin domain of myosin is present near the C terminus of MyBP-C, and a second site that binds the S2 domain of myosin is present near the N terminus of MyBP-C. The cardiac MyBP-C isoform contains PKA phosphorylation sites within the S2 binding site that are absent in skeletal isoforms. A recent study demonstrated that the primary effect of MyBP-C phosphorylation is to accelerate the cross-bridge cycling rate, and that of cTnI phosphorylation is to reduce Ca2+ sensitivity (Stelzer et al., 2007). Our findings that PKA exerted no effect on Ca2+ sensitivity after sTn reconstitution in PLV (Fig. 1 C), despite clear enhancement of MyBP-C phosphorylation (Fig. S1), appear to be consistent with the conclusion of the work by Stelzer et al. (2007). It is therefore safe to consider that the PKA-induced Ca2+ desensitization in PLV is not modulated by MyBP-C phosphorylation, with and without thin filament reconstitution.
We performed all of the mechanical experiments at SL 2.0 µm, where passive force was barely produced in both PLV and RP. In PLV at longer SL, the decrease in titin-based passive force via PKA-dependent phosphorylation of the N2B segment may weaken titin's effect on the actomyosin interaction (e.g., Cazorla et al., 2001; Fukuda et al., 2001a, 2003), resulting presumably in the enhancement of Ca2+ desensitization. However, this possible SL dependence is unlikely to occur in RP due to the absence of the N2B segment (e.g., Prado et al., 2005).
In conclusion, this study for the first time directly demonstrated that PKA-dependent cTnI phosphorylation universally causes Ca2+ desensitization in cardiac and fast skeletal sarcomeres, in association with cTnC. Future studies are warranted to investigate whether or not the mechanical properties are altered in response to β-adrenergic stimulation in intact skeletal muscle expressed with the cTnI–cTnC complex.
Acknowledgments
We thank Ms. Naoko Tomizawa (The Jikei University School of Medicine, Tokyo, Japan) for technical assistance.
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to N. Fukuda and S. Kurihara), and by grants from the Japan Science and Technology Agency (Core Research for Evolutional Science and Technology; to N. Fukuda) and from the Vehicle Racing Commemorative Foundation (to S. Kurihara).
Footnotes
Abbreviations used in this paper: BDM, 2,3-butanedione monoxime; cTn, cardiac troponin complex; cTnI, cardiac troponin I; MyBP-C, myosin-binding protein C; nH, Hill coefficient; PCRF, hybrid troponin complex (fast skeletal troponin T with cardiac troponin I and C); PKI, PKA inhibitor; PLV, porcine left ventricular; PP1, protein phosphatase 1; RP, rabbit psoas; SL, sarcomere length; sTn, fast skeletal troponin complex; sTnT, fast skeletal troponin T; Tm, tropomyosin; Tn, troponin.
References
- Bers D.M. 2001. Excitation-Contraction Coupling and Cardiac Contractile Force. Second edition. Kluwer-Academic, Dordrecht, Netherlands: 427 pp [Google Scholar]
- Bers D.M. 2002. Cardiac excitation-contraction coupling.Nature. 415:198–205 [DOI] [PubMed] [Google Scholar]
- Blumenthal D.K., Stull J.T., Gill G.N. 1978. Phosphorylation of cardiac troponin by guanosine 3′:5′-monophosphate-dependent protein kinase.J. Biol. Chem. 253:334–336 [PubMed] [Google Scholar]
- Buscemi N., Foster D.B., Neverova I., Van Eyk J.E. 2002. p21-activated kinase increases the calcium sensitivity of rat triton-skinned cardiac muscle fiber bundles via a mechanism potentially involving novel phosphorylation of troponin I.Circ. Res. 91:509–516 [DOI] [PubMed] [Google Scholar]
- Cazorla O., Freiburg A., Helmes M., Centner T., McNabb M., Wu Y., Trombitás K., Labeit S., Granzier H. 2000. Differential expression of cardiac titin isoforms and modulation of cellular stiffness.Circ. Res. 86:59–67 [DOI] [PubMed] [Google Scholar]
- Cazorla O., Wu Y., Irving T.C., Granzier H. 2001. Titin-based modulation of calcium sensitivity of active tension in mouse skinned cardiac myocytes.Circ. Res. 88:1028–1035 [DOI] [PubMed] [Google Scholar]
- Chandra M., Tschirgi M.L., Rajapakse I., Campbell K.B. 2006. Troponin T modulates sarcomere length-dependent recruitment of cross-bridges in cardiac muscle.Biophys. J. 90:2867–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh M., Blinks J.R. 1988. Actions of sympathomimetic amines on the Ca2+ transients and contractions of rabbit myocardium: reciprocal changes in myofibrillar responsiveness to Ca2+ mediated through α- and β-adrenoceptors.Circ. Res. 62:247–265 [DOI] [PubMed] [Google Scholar]
- Fentzke R.C., Buck S.H., Patel J.R., Lin H., Wolska B.M., Stojanovic M.O., Martin A.F., Solaro R.J., Moss R.L., Leiden J.M. 1999. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart.J. Physiol. 517:143–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda N., Kajiwara H., Ishiwata S., Kurihara S. 2000. Effects of MgADP on length dependence of tension generation in skinned rat cardiac muscle.Circ. Res. 86:E1–E6 [DOI] [PubMed] [Google Scholar]
- Fukuda N., Sasaki D., Ishiwata S., Kurihara S. 2001a. Length dependence of tension generation in rat skinned cardiac muscle: role of titin in the Frank-Starling mechanism of the heart.Circulation. 104:1639–1645 [DOI] [PubMed] [Google Scholar]
- Fukuda N., O-Uchi J., Sasaki D., Kajiwara H., Ishiwata S., Kurihara S. 2001b. Acidosis or inorganic phosphate enhances the length dependence of tension in rat skinned cardiac muscle.J. Physiol. 536:153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda N., Wu Y., Farman G., Irving T.C., Granzier H. 2003. Titin isoform variance and length dependence of activation in skinned bovine cardiac muscle.J. Physiol. 553:147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda N., Wu Y., Nair P., Granzier H.L. 2005. Phosphorylation of titin modulates passive stiffness of cardiac muscle in a titin isoform-dependent manner.J. Gen. Physiol. 125:257–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda N., Granzier H.L., Ishiwata S., Kurihara S. 2008. Physiological functions of the giant elastic protein titin in mammalian striated muscle.J. Physiol. Sci. 58:151–159 [DOI] [PubMed] [Google Scholar]
- Fukuda N., Terui T., Ohtsuki I., Ishiwata S., Kurihara S. 2009. Titin and troponin: central players in the Frank-Starling mechanism of the heart.Curr. Cardiol. Rev. 5:119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A.M., Homsher E., Regnier M. 2000. Regulation of contraction in striated muscle.Physiol. Rev. 80:853–924 [DOI] [PubMed] [Google Scholar]
- Granzier H.L., Campbell K.B. 2006. New insights in the role of cardiac myosin binding protein C as a regulator of cardiac contractility.Circ. Res. 99:795–797 [DOI] [PubMed] [Google Scholar]
- Gupta R.C., Neumann J., Boknik P., Watanabe A.M. 1994. M2-specific muscarinic cholinergic receptor-mediated inhibition of cardiac regulatory protein phosphorylation.Am. J. Physiol. 266:H1138–H1144 [DOI] [PubMed] [Google Scholar]
- Hoh J.F., Rossmanith G.H., Kwan L.J., Hamilton A.M. 1988. Adrenaline increases the rate of cycling of crossbridges in rat cardiac muscle as measured by pseudo-random binary noise-modulated perturbation analysis.Circ. Res. 62:452–461 [DOI] [PubMed] [Google Scholar]
- Hongo K., Tanaka E., Kurihara S. 1993. Alterations in contractile properties and Ca2+ transients by β- and muscarinic receptor stimulation in ferret myocardium.J. Physiol. 461:167–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth J.W., Meller J., Solaro R.J., Trewhella J., Rosevear P.R. 2007. Phosphorylation-dependent conformational transition of the cardiac specific N-extension of troponin I in cardiac troponin.J. Mol. Biol. 373:706–722 [DOI] [PubMed] [Google Scholar]
- Jideama N.M., Crawford B.H., Hussain A.A., Raynor R.L. 2006. Dephosphorylation specificities of protein phosphatase for cardiac troponin I, troponin T, and sites within troponin T.Int. J. Biol. Sci. 2:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish J.C., McCloskey D.T., Layland J., Palmer S., Leiden J.M., Martin A.F., Solaro R.J. 2001. Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle.Circ. Res. 88:1059–1065 [DOI] [PubMed] [Google Scholar]
- Kurihara S., Konishi M. 1987. Effects of β-adrenoceptor stimulation on intracellular Ca transients and tension in rat ventricular muscle.Pflugers Arch. 409:427–437 [DOI] [PubMed] [Google Scholar]
- Layland J., Solaro R.J., Shah A.M. 2005. Regulation of cardiac contractile function by troponin I phosphorylation.Cardiovasc. Res. 66:12–21 [DOI] [PubMed] [Google Scholar]
- Li L., Desantiago J., Chu G., Kranias E.G., Bers D.M. 2000. Phosphorylation of phospholamban and troponin I in β-adrenergic-induced acceleration of cardiac relaxation.Am. J. Physiol. 278:H769–H779 [DOI] [PubMed] [Google Scholar]
- Noland T.A., Jr., Guo X., Raynor R.L., Jideama N.M., Averyhart-Fullard V., Solaro R.J., Kuo J.F. 1995. Cardiac troponin I mutants: phosphorylation by protein kinases C and A and regulation of Ca2+-stimulated MgATPase of reconstituted actomyosin S-1.J. Biol. Chem. 270:25445–25454 [DOI] [PubMed] [Google Scholar]
- Oakley C.E., Chamoun J., Brown L.J., Hambly B.D. 2007. Myosin binding protein-C: enigmatic regulator of cardiac contraction.Int. J. Biochem. Cell Biol. 39:2161–2166 [DOI] [PubMed] [Google Scholar]
- Ohtsuki I., Morimoto S. 2008. Troponin: regulatory function and disorders.Biochem. Biophys. Res. Commun. 369:62–73 [DOI] [PubMed] [Google Scholar]
- Ojima T., Nishita K. 1986. Troponin from Akazara scallop striated adductor muscles.J. Biol. Chem. 261:16749–16754 [PubMed] [Google Scholar]
- Ojima T., Nishita K. 1988. Separation of Akazara scallop and rabbit troponin components by a single-step chromatography on CM-Toyopearl.J. Biochem. 104:9–11 [DOI] [PubMed] [Google Scholar]
- O-Uchi J., Sasaki H., Morimoto S., Kusakari Y., Shinji H., Obata T., Hongo K., Komukai K., Kurihara S. 2008. Interaction of α1-adrenoceptor subtypes with different G proteins induces opposite effects on cardiac L-type Ca2+ channel.Circ. Res. 102:1378–1388 [DOI] [PubMed] [Google Scholar]
- Prado L.G., Makarenko I., Andresen C., Krüger M., Opitz C.A., Linke W.A. 2005. Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles.J. Gen. Physiol. 126:461–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi F., Nakamura Y., Ohtsuki I. 1993. Replacement of troponin in bullfrog skeletal myofibrils by rabbit skeletal and bovine cardiac troponins.Biomed. Res. 14:93–97 [Google Scholar]
- Solaro R.J., Rarick H.M. 1998. Troponin and tropomyosin: proteins that switch on and tune in the activity of cardiac myofilaments.Circ. Res. 83:471–480 [DOI] [PubMed] [Google Scholar]
- Solaro R.J., Rosevear P., Kobayashi T. 2008. The unique functions of cardiac troponin I in the control of cardiac muscle contraction and relaxation.Biochem. Biophys. Res. Commun. 369:82–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer J.E., Patel J.R., Walker J.W., Moss R.L. 2007. Differential roles of cardiac myosin-binding protein C and cardiac troponin I in the myofibrillar force responses to protein kinase A phosphorylation.Circ. Res. 101:503–511 [DOI] [PubMed] [Google Scholar]
- Terui T., Sodnomtseren M., Matsuba D., Udaka J., Ishiwata S., Ohtsuki I., Kurihara S., Fukuda N. 2008. Troponin and titin coordinately regulate length-dependent activation in skinned porcine ventricular muscle.J. Gen. Physiol. 131:275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull L., Hoh J.F.Y., Ludowyke R.I., Rossmanith G.H. 2002. Troponin I phosphorylation enhances crossbridge kinetics during β-adrenergic stimulation in rat cardiac tissue.J. Physiol. 542:911–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udaka J., Ohmori S., Terui T., Ohtsuki I., Ishiwata S., Kurihara S., Fukuda N. 2008. Disuse-induced preferential loss of the giant protein titin depresses muscle performance via abnormal sarcomeric organization.J. Gen. Physiol. 131:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema R.C., Raynor R.L., Noland T.A., Jr., Kuo J.F. 1993. Role of protein kinase C in the phosphorylation of cardiac myosin light chain 2.Biochem. J. 294:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki R., Wu Y., McNabb M., Greaser M., Labeit S., Granzier H. 2002. Protein kinase A phosphorylates titin's cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes.Circ. Res. 90:1181–1188 [DOI] [PubMed] [Google Scholar]
- Yuasa K., Michibata H., Omori K., Yanaka N. 1999. A novel interaction of cGMP-dependent protein kinase I with troponin T.J. Biol. Chem. 274:37429–37434 [DOI] [PubMed] [Google Scholar]
- Zhang R., Zhao J., Mandveno A., Potter J.D. 1995. Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation.Circ. Res. 76:1028–1035 [DOI] [PubMed] [Google Scholar]