Abstract
Accumulation of a large body of evidence during the past two decades testifies to the complexity of the renin–angiotensin system (RAS). The incorporation of novel enzymatic pathways, resulting peptides, and their corresponding receptors into the biochemical cascade of the RAS provides a better understanding of its role in the regulation of cardiovascular and renal function. Hence, in recent years, it became apparent that the balance between the two opposing effector peptides, angiotensin II and angiotensin-(1-7), may have a pivotal role in determining different cardiovascular pathophysiologies. Furthermore, our recent studies provide evidence for the functional relevance of a newly discovered rat peptide, containing two additional amino acid residues compared to angiotensin I, first defined as proangiotensin-12 [angiotensin-(1-12)]. This review focuses on angiotensin-(1-7) and its important contribution to cardiovascular function and growth, while introducing angiotensin-(1-12) as a potential novel angiotensin precursor.
Keywords: (3-6): Renin–angiotensin system, Novel angiotensins, Angiotensin-(1-7), Angiotensin-(1-12), Hypertension, Cardiovascular growth
Introduction
It seemed for a long time that all components of the renin–angiotensin system (RAS) and their physiological roles were well defined. In this traditional view, the RAS is viewed as a classical endocrine system with the octapeptide angiotensin II (Ang II; Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8) as an effector hormone expressing its vasoconstrictor, sodium retention, mitogenic, and proliferative effects upon its binding to Ang II type 1 receptors (AT1). Renin and angiotensin converting enzyme (ACE) were thought to be the only enzymes responsible for angiotensin I (Ang I; Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8-His9-Leu10) and Ang II synthesis, respectively. However, over the last two decades, increasing evidence has accumulated that indicates an exceeding complexity of the system, particularly in tissues such as the heart and kidneys. The evidence for a fully operational RAS in local tissues with tissue-specific enzymatic pathways for the processing of Ang I and Ang II has been detailed in a number of publications from this laboratory [1–6]. Furthermore, the pleiotropic actions of the resulting fragment of Ang I or Ang II, the heptapeptide Ang-(1-7), have been gradually appreciated over the last decade. In general, Ang-(1-7) [Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7] counterbalances biological actions of Ang II, and in that way, an inadequate balance between these two peptides may determine different cardiovascular pathophysiological states. Interestingly, the spectrum of novel peptides within RAS continues to expand showing that a peptide containing two amino acids more than Ang I, the dodecapeptide angiotensin-(1-12) [Ang-(1-12); rat sequence: Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8-His9-Leu10-Leu11-Tyr12], could also be a key player in the regulation of cardiovascular function. This review will therefore focus on Ang-(1-7), its important contribution to cardiovascular function and growth, while introducing Ang-(1-12) as a potential novel angiotensin precursor.
Angiotensin-(1-7)
Biochemical pathways for Ang-(1-7) synthesis and degradation
Ang-(1-7) may be derived from either Ang I or Ang II (Fig. 1). Different tissue-specific endopeptidases, including neprilysin (NEP), thimet oligopeptidase (TOP), and prolyl oligopeptidase (POP) catalyze the hydrolysis of the decapeptide Ang-I at the Pro7-Phe8 bond to release the three terminal amino acids and Ang-(1-7) [7–9]. Both POP [10] and TOP [7] have been reported by us to mediate Ang-(1-7) formation in cultures of vascular endothelial and smooth muscle cells. Neprilysin has been shown to be particularly abundant in the kidney [11]. Importantly, as NEP is a membrane-bound enzyme, its localization on the luminal side of the endothelium makes it accountable for most of the Ang-(1-7) production in the circulation [12–14]. Neprilysin degrades vasodilatory atrial natriuretic peptide as well, but its high substrate specificity for Ang-(1-7) formation in hypertensive humans may explain, at least in part, the lack of significant beneficial effects of its inhibitors in the treatment of hypertension [15]. However, neprilysin also degrades Ang-(1-7) into Ang-(1-4) [11], and further studies are necessary to clarify its role in hypertensive disease.
Fig. 1.
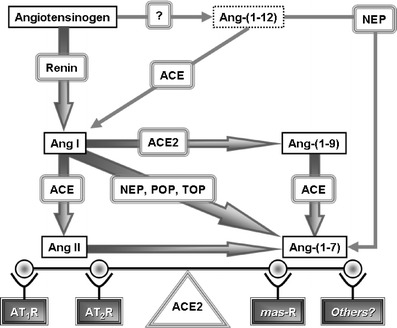
Biochemical pathways for Ang-(1-7) formation. Adapted from Trask and Ferrario [110]
It has been only recently that direct conversion of Ang II into Ang-(1-7) by a newly discovered homolog of ACE, angiotensin converting enzyme 2 (ACE2), was demonstrated (Fig. 1) [16, 17]. As a carboxypeptidase, ACE2 also mediates the conversion of Ang I into Ang-(1-9), which can be further metabolized into Ang-(1-7) by ACE. However, the higher substrate preference of ACE2 towards Ang II than Ang I underscores the significance of this enzyme in the regulation of tissue Ang II/Ang-(1-7) balance [16, 17]. Consequently, higher cardiac Ang II level was associated with genetic deletion of ACE2 in mice and resulted in the development of severe cardiac dysfunction [18]. On the other hand, local ACE2 overexpression by systemic lentiviral delivery was followed by an attenuation of cardiac remodeling in hypertensive rats [19]. Furthermore, a recent report from our laboratory showed that the hypertensive heart predominantly depends on ACE2 for the production of Ang-(1-7) [20]. Together with evidence for increased ACE2 expression in failing human [21] and rat [22] hearts, our study suggests a preserved compensatory response of injured hearts to maintain Ang-(1-7) levels even in the advanced stage of the disease, although it was obviously not sufficient to counteract the deleterious effects of Ang II.
Besides breaking down bradykinin and Acetyl-Ser-Asp-Lys-Pro, ACE hydrolyzes Ang-(1-7) as well. It acts upon the Ile5-His6 bond to form the inactive metabolite Ang-(1-5) [23–25], and ACE inhibitors increase the short half-life of Ang-(1-7) in the circulation [26]. On the other hand, neprilysin hydrolysis of the Tyr4-Ile5 bond of Ang-(1-7) to form Ang-(1-4) seems to be the predominant pathway for Ang-(1-7) metabolism in the kidney [23, 27–29].
Ang-(1-7) receptor and signaling mechanisms
Prior to the identification of a specific Ang-(1-7) receptor, a modified form of Ang-(1-7), D-Ala7-Ang-(1-7) was designed as a selective antagonist for the putative Ang-(1-7) receptor. Thus, D-Ala7-Ang-(1-7) inhibited Ang-(1-7)-induced systemic and renal vasodilation, did not block pressor or contractile response to Ang-II, and did not compete for binding of 125I-Ang II to rat adrenal AT1 or AT2 receptors [30]. Subsequent studies from our group identified specific non-AT1/AT2 Ang-(1-7) binding sites on bovine aortic endothelial cells [31] and endothelium of coronary artery rings [1] that were selectively competed by D-Ala7-Ang-(1-7). This finding was in agreement with nitric oxide (NO) release from bovine aortic endothelial cells stimulated by Ang-(1-7) that was blocked by D-Ala7-Ang-(1-7) [32]. It was also consistent with previously demonstrated Ang-(1-7)-induced vasodilation of endothelium-intact coronary arteries through release of kinins and NO [33, 34]. However, it was not before the discovery that endothelium-mediated vasodilation by Ang-(1-7) was abolished in mas-knockout mice that the “orphan” mas proto-oncogene receptor was linked to the intracellular signaling of Ang-(1-7) [35–37]. More recent studies revealed that Ang-(1-7), acting on this G protein-coupled receptor, activated endothelial nitric oxide synthase and NO production via Akt-dependent pathways [38]. Furthermore, we showed recently that the presence of an antisense probe directed against mas abolished the Ang-(1-7)-induced inhibition of protein synthesis in cardiomyocytes [39]. This study also revealed that Ang-(1-7) decreased serum-stimulated ERK1/ERK2 mitogen-activated protein kinase activity, a response that was blocked by D-Ala7-Ang-(1-7). These findings agree with the observation that genetic deletion of mas elicits cardiac dysfunction [37, 40]. Thus, it is clear that a reduction in the counterbalancing arm of the renin–angiotensin system via the ACE2/Ang-(1-7)/mas axis may have a major influence in determining cardiac structural and functional development [18, 37, 40]. In addition, a recent report suggests another Ang-(1-7) receptor subtype sensitive to the Ang-(1-7) antagonist [D-Pro7]-Ang-(1-7) but not D-Ala7-Ang-(1-7) [41]. This finding, as well as an intriguing interaction between AT1 and mas [42, 43], clearly warrants further investigation.
Pleiotropy of Ang-(1-7) biological actions
Cardiovascular and renal effects of Ang-(1-7)
A series of studies after our initial characterization of Ang-(1-7) actions in brain [44] established the basis for exploring the systemic and regional vasodilatory and hypotensive effects of this peptide [33, 34, 45]. In these studies, it was demonstrated that the vasodilator effect of Ang-(1-7) was mediated through different vasoactive autocoid release [2, 14, 46–52]. Moreover, it was also shown that Ang-(1-7) potentiated bradykinin vasodilatory action [49, 53] and that this interaction was exaggerated after ACE inhibition. Although the precise mechanisms of this potentiation remains controversial [54, 55], data suggest that the release of prostaglandins, NO, endothelium-derived hyperpolarizing factor [56–58] as well as the ability of Ang-(1-7) to inhibit ACE activity [24, 25, 59] may be involved.
Early studies from our laboratory strongly suggested that Ang-(1-7) may represent an intrinsic counterbalancing factor to the pressor and trophic actions of Ang II [1]. This unique concept was confirmed in the experiments in which hyperreninemia was stimulated through induction of renovascular hypertension [51] or a low-salt diet [47]. Despite increased levels of Ang II during salt depletion, blood pressure remained unchanged, at least in part, due to the opposing actions of Ang-(1-7). Indeed, Ang-(1-7) blockade by either the selective Ang-(1-7) receptor antagonist D-Ala7-Ang-(1-7) or specific Ang-(1-7) antibodies caused a dose-dependent increase in arterial pressure in salt-restricted rats [47], underscoring the importance of Ang-(1-7) in counterbalancing the effects of Ang II.
The significance of the alternative arm of the RAS comprising ACE2, Ang-(1-7), and mas in blood pressure regulation was further underscored by the demonstration of a considerable contribution of Ang-(1-7) to the hypotensive effects of RAS blockade [14, 46, 48, 60–62]. Importantly, chronic antihypertensive effects of captopril or omapatrilat in hypertensive patients were also associated with increased urinary levels of Ang-(1-7) [28, 63]. The importance of this observation was magnified by the concurrent observation that plasma and urinary excretion levels of Ang-(1-7) are reduced in untreated essential hypertensive subjects [64]. More recently, we showed that chronic administration of irbesartan to normotensive subjects was associated with large increases in plasma Ang-(1-7) [65, 66]. These results suggest an important contribution of Ang-(1-7) in mediating the antihypertensive effects of both ACE inhibitors and AT1 receptor antagonists.
It was then in our laboratory that the effects of RAS blockade on the Ang-(1-7)-forming enzyme, ACE2, were evaluated for the first time [67]. From the preceding study, we knew that heart failure due to coronary artery ligation was associated with compensatory increase in cardiac Ang-(1-7) levels [68]. It was in this experimental model that we subsequently showed that AT1 receptor antagonism further augmented plasma Ang-(1-7)/Ang II ratio suggesting increased formation of Ang-(1-7) from Ang II [67]. Indeed, AT1 receptor antagonism attenuated cardiac remodeling and dysfunction, and these changes were associated with a threefold increase in ACE2 mRNA expression in the left ventricle. The changes in the cardiac ACE2 gene activity and the profile of plasma angiotensin peptides after RAS inhibition were confirmed in following experiments including different strains of normotensive and hypertensive animals [60–62]. The pathophysiological relevance of Ang-(1-7) in the heart was further highlighted by studies demonstrating that chronic infusion of either Ang-(1-7) [69] or its stable non-peptide analog AVE-0991 [70] was cardioprotective in experimental heart failure. Finally, several studies demonstrated that Ang-(1-7) was protective against cardiac ischemia-induced injury and arrhythmias [71–73]. The beneficial antiarrhythmic effects of Ang (1-7) on the failing heart result from the combined effect of the peptide on the sodium pump, hyperpolarization of cardiac cell membranes, and increased conduction velocity [74]. However, in isolated hearts, supra-pharmacological concentrations of Ang-(1-7) enhanced reperfusion arrhythmias [75]. We also showed that Ang-(1-7) at higher concentrations (10−7 M), induces early-after depolarization [74]; therefore, an optimal tissue concentration of Ang (1-7) must be achieved to permit a protective role of the heptapeptide on cardiac arrhythmias.
Numerous studies indicate that the Ang-(1-7) effects on the kidney are opposite to those of Ang II. Thus, Ang-(1-7) infusion induced vasodilation of pre-constricted afferent arterioles [45], increased glomerular filtration rate, and induced natriuresis and diuresis [76–78] by inhibiting the Na+–K+–ATPase [79]. These vascular and tubular effects were attenuated by the selective Ang-(1-7) antagonist D-Ala7-Ang-(1-7). Interestingly, these counterbalancing effects of Ang-(1-7) were noticeable under conditions of RAS activation, such as during salt depletion or renal hypertension, but not in the salt-replete state [80].
Ang-(1-7) functions in the brain
Ang-(1-7) is present in brain tissue, and its distribution throughout the hypothalamus, medulla oblongata, and amygdala underlines its importance in the regulation of blood pressure, fluid balance, and osmoregulation [81]. Although the action of Ang-(1-7) in the brain sometimes mimics the action of Ang II, such as stimulation of vasopressin release [44], their overall effects are in general opposite. The involvement of different receptors, neurotransmitter pathways, and complex integrative regulatory brain mechanisms implicated in the action of the two angiotensins have been already reviewed elsewhere [4]. In brief, intracerebroventricular administration of an Ang-(1-7) antibody elevated arterial pressure, while endogenous neutralization of Ang II had an opposite effect [50]. Ang-(1-7) at the nucleus of the solitary tract evoked bradycardic and depressor response [82], augmented baroreceptor reflex control of heart rate [83–85], and these effects were enhanced in hypertensive animals when compared to the controls [86, 87]. In the rostral ventrolateral medulla, Ang-(1-7) elicited pressor responses [88]; however, in the caudal ventrolateral medulla, Ang-(1-7) lowered arterial pressure by inhibiting the pressor action of the rostral ventrolateral medulla [89, 90]. More unexpected actions of Ang-(1-7) include its ability to enhance long-term potentiation, a process thought to be involved in learning and memory [91].
Ang-(1-7) relevance in cardiovascular and cancerous growth
Similar to their actions in the circulation and the control of blood pressure, Ang II and Ang-(1-7) elicit opposing effects on tissue growth as well. Ang-(1-7) inhibited proliferation of aortic vascular smooth muscle cells in culture [92], and this antiproliferative effect was later confirmed in in vivo studies. Indeed, Ang-(1-7) infusion reduced neointimal proliferation after vascular injury in rat carotid arteries [93]. Recent reports demonstrated that Ang-(1-7) also inhibited Ang II-induced protein synthesis in neonatal cardiomyocytes by activating mas [39]. Consistently, Ang-(1-7) infusion reduced myocyte surface area in rats subjected to coronary artery ligation [94]. These results are in keeping with the beneficial effects of RAS blockade on cardiac remodeling and dysfunction after myocardial infarction where activation of ACE2/Ang-(1-7) system has been verified [67]. In addition, Ang-(1-7) inhibited collagen synthesis in adult rat cardiac fibroblasts acting on receptors that are distinct from the AT1 and AT2 receptors [95]. Subsequent studies confirmed that Ang-(1-7) prevented an excessive accumulation of cardiac collagen fibrils in different models of experimental hypertension [69, 96]. Excitingly, the antiproliferative and antiangiogenic ability [97] of Ang-(1-7) found an important application in inhibiting cancerous growth as well. Thus, experimental evidence that Ang-(1-7) inhibited lung [98] and breast cancer growth in vitro [99] as well as in vivo [100] now provides a solid foundation for the initiation of clinical trials in which the chemotherapeutic potential of Ang-(1-7) is being tested.
Ang-(1-7) in pregnancy
All components of the RAS are expressed in placenta including Ang-(1-7) and ACE2 [101], and activation of the RAS during normal pregnancy has been described in plasma and urine [102, 103]. Adequate balance between the two opposing arms of RAS might be of extreme importance in normal pregnancy, as the predominance or deficit of either one might lead toward adverse outcomes. For example, unopposed antiangiogenic properties of Ang-(1-7) may have a harmful effect, particularly in early pregnancy during which vascularization of tissue beds is critical. On the other hand, decreased plasma levels of Ang-(1-7) were associated with preeclamptic pregnancies characterized by elevated arterial pressure and proteinuria [102]. To further confirm this relationship, the most recent study from our group related an experimental model of preeclampsia with failure to increase Ang-(1-7) in kidney as well [104].
Further expansion of the complexity of the renin–angiotensin system: angiotensin-(1-12)
In line with expanding data on the newer angiotensin peptide, Ang-(1-7), Nagata and colleagues [105] recently identified another new angiotensin peptide, the dodecapeptide Ang-(1-12). The authors were probing for analogs of Ang II when they discovered an unidentified immunoreactive peak by high-performance liquid chromatography (HPLC), which the authors found to be a 12-amino acid derivative of angiotensinogen, two amino acids larger than the traditional intermediate peptide Ang I. The dodecapeptide produced pressor responses both in isolated rat aorta and acutely in intact Wistar rats—a finding that was abrogated by coadministration of both an ACE inhibitor or an angiotensin receptor blocker (ARB). These data suggested that “proangiotensin-12,” as the authors named it, was exerting its actions through rapid metabolism into Ang II.
Recent data from our laboratory provided further evidence for a biological role of Ang-(1-12) as a new endogenous peptide of the RAS. Because Ang-(1-12) was identified endogenously by RIA in different organs and tissues [105] (Fig. 2), we first undertook studies that investigated the immunolocalization of the dodecapeptide in the hearts and kidneys of normal Wistar–Kyoto (WKY) and spontaneously hypertensive rats (SHR). Jessup et al. [106] found that Ang-(1-12) was localized by immunohistochemistry predominantly in cardiac myocytes, while staining in the medial and endothelial layers of the coronary arteries appeared more faint (Fig. 3) and failed to be detected in all vessels examined. The distribution of Ang-(1-12) within the hearts of SHR was more robust than that found in WKY. This observation was confirmed by tissue content analysis, which revealed significantly higher levels of cardiac Ang-(1-12) in SHR compared to WKY. Renal Ang-(1-12) was localized to the proximal and distal tubules and the collecting duct, but it was scantily observed in glomeruli or intra-renal vessels. These data, in accordance with those from Nagata and colleagues [105] show that Ang-(1-12) is indeed localized endogenously within tissues, and the distribution of the new angiotensin peptide may reflect the state of the health of that tissue, as shown by differences in distribution between WKY and SHR.
Fig. 2.
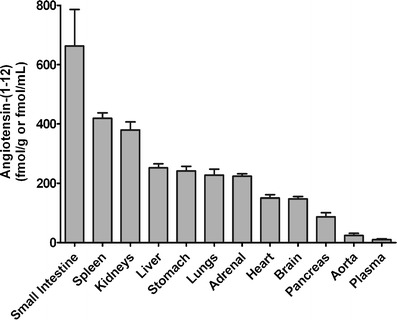
Ang-(1-12) peptide levels by radioimmunoassay in several tissues from male Wistar rats. Adapted from Nagata et al. [105]
Fig. 3.
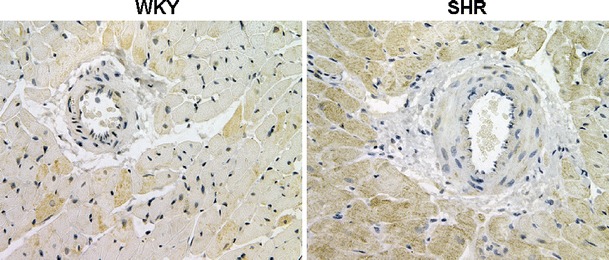
Immunohistochemical localization of Ang-(1-12) in the heart of WKY and SHR rats. Note the more robust distribution of Ang-(1-12) within the hearts of SHR than that assessed in WKY. This observation was confirmed by tissue content analysis, which revealed significantly higher levels of cardiac Ang-(1-12) in SHR compared to WKY. Adapted from Jessup et al. [106]
Further enhancements towards the understanding for a biological role for Ang-(1-12) were made by studies from our laboratory which illustrated the metabolic capacity for Ang-(1-12) to yield known downstream bioactive angiotensin peptides. Intriguingly, Chappell et al. [107] found that serum exclusively formed Ang II from Ang-(1-12) by ACE, and renal neprilysin activity converted Ang-(1-12) to Ang-(1-7). Both of these pathways were independent of renin activity. Moreover, we [108] showed that Ang-(1-12) could be metabolized into Ang I, Ang II, and Ang-(1-7) in isolated hearts from five different normotensive and hypertensive rat strains. Collectively, these data provide strong evidence that Ang-(1-12) may be an alternate precursor substrate for the formation of bioactive angiotensin peptides in the heart, kidney, and circulation that may depend on the localization of one of its processing enzymes, ACE, but not renin.
Conclusions
In conclusion, a large body of evidence emerging from experimental and human studies clearly reveals pathophysiological importance of novel peptides and related enzymes incorporated recently into the biochemical RAS cascade. The consequences of an altered ACE2/Ang-(1-7)/mas axis in hypertensive disease and heart failure is now well recognized. In addition to the potential therapeutic application in the treatment of cardiovascular disease, the ACE2/Ang-(1-7)/mas axis emerges further as a prospective therapeutic target in cancer and preeclamptic patients. Thus, there is a great potential for genetic and pharmacological modulation of the ACE2/Ang-(1-7)/mas axis in the treatment of various diseases that, however, warrants further meticulous investigation. Future studies will certainly provide us with better understanding of the relevance of ACE2 function as a receptor for severe acute respiratory syndrome (SARS) virus. They will also expand our comprehension of the signaling mechanisms and a potential angiotensin receptor interaction.
Building upon the complexity unveiled thus far on the RAS as documented by data on the ACE2/Ang-(1-7)/mas axis, the demonstration of endogenous Ang-(1-12) is indeed novel and important. Although the data are still limited to the rat, forthcoming research may provide insight onto anomalies within RAS physiology that we cannot yet explain. Conceptually, Ang-(1-12) may serve as an alternate substrate for the production of bioactive angiotensin peptides as shown in our preliminary studies. Moreover, in lieu of the specific sequence requirements for the generation of Ang I by renin, Ang-(1-12) may be formed directly from angiotensinogen in a renin-independent manner. In support of this notion, Oparil and colleagues [109] found that coadministration of aliskiren and the ARB valsartan produced additive reduction in blood pressure in patients when compared to each drug administered alone—a finding not expected if renin is the limiting step in the formation of angiotensin peptides from angiotensinogen. Identification of a biological role for Ang-(1-12) requires further work; however, expression of the peptide throughout the body argues strongly that this angiotensin intermediate may add further unrecognized complexity to the renin–angiotensin system.
Acknowledgments
The studies summarized here were supported by awards from the National Institutes of Health (HL-51952 to CMF, HL-56973 to MCC), American Heart Association, Mid-Atlantic Affiliate (0765308U to JV and 0715249U to AJT), and WFUSM Venture Fund (to JV). In addition, the authors gratefully acknowledge grant support in part provided by Unifi, Inc., Greensboro, NC, and Farley-Hudson Foundation, Jacksonville, NC.
References
- 1.Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI. Counterregulatory actions of angiotensin-(1-7) Hypertension. 1997;30:535–541. doi: 10.1161/01.hyp.30.3.535. [DOI] [PubMed] [Google Scholar]
- 2.Ferrario CM. Angiotensin-(1-7) and antihypertensive mechanisms. J Nephrology. 1998;11:278–283. [PubMed] [Google Scholar]
- 3.Ferrario CM, Iyer SN. Angiotensin-(1-7): a bioactive fragment of the renin–angiotensin system. Regul Pept. 1998;78:13–18. doi: 10.1016/s0167-0115(98)00134-7. [DOI] [PubMed] [Google Scholar]
- 4.Ferrario CM, Averill DB, Brosnihan KB, Chappell MC, Diz DI, Gallagher PE, Tallant EA. Angiotensin-(1-7). Its contributions to arterial pressure control mechanisms. In: Unger T, Scholkens BA, editors. Handbook of experimental pharmacology: angiotensin, vol. 1. Germany: Heidelberg; 2004. pp. 478–518. [Google Scholar]
- 5.Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol. 2005;289:H2281–H2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrario CM. Angiotensin-converting enzyme 2 and angiotensin-(1-7): an evolving story in cardiovascular regulation. Hypertension. 2006;47:515–521. doi: 10.1161/01.HYP.0000196268.08909.fb. [DOI] [PubMed] [Google Scholar]
- 7.Chappell MC, Tallant EA, Brosnihan KB, Ferrario CM. Conversion of angiotensin I to angiotensin-(1-7) by thimet oligopeptidase (EC 3.4.24.15) in vascular smooth muscle cells. J Vasc Med Biol. 1994;5:129–137. [Google Scholar]
- 8.Welches WR, Santos RAS, Chappell MC, Brosnihan KB, Greene LJ, Ferrario CM. Evidence that prolyl endopeptidase participates in the processing of brain angiotensin. J Hypertens. 1991;9:631–638. doi: 10.1097/00004872-199107000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Welches WR, Brosnihan KB, Ferrario CM. A comparison of the properties, and enzymatic activity of three angiotensin processing enzymes: angiotensin converting enzyme, prolyl endopeptidase and neutral endopeptidase 24.11. Life Sci. 1993;52:1461–1480. doi: 10.1016/0024-3205(93)90108-f. [DOI] [PubMed] [Google Scholar]
- 10.Santos RAS, Brosnihan KB, Jacobsen DW, DiCorleto PE, Ferrario CM. Production of angiotensin-(1-7) by human vascular endothelium. Hypertension. 1992;19(Suppl II):II–56-II-61. doi: 10.1161/01.hyp.19.2_suppl.ii56. [DOI] [PubMed] [Google Scholar]
- 11.Turner AJ. Exploring the structure and function of zinc metallopeptidases: old enzymes and new discoveries. Biochem Soc Trans. 2003;31:723–727. doi: 10.1042/bst0310723. [DOI] [PubMed] [Google Scholar]
- 12.Campbell DJ, Anastasopoulos F, Duncan AM, James GM, Kladis A, Briscoe TA. Effects of neutral endopeptidase inhibition and combined angiotensin converting enzyme and neutral endopeptidase inhibition on angiotensin and bradykinin peptides in rats. J Pharmacol Exp Ther. 1998;287:567–577. [PubMed] [Google Scholar]
- 13.Chappell MC, Gomez MN, Pirro NT, Ferrario CM. Release of angiotensin-(1-7) from the rat hindlimb: influence of angiotensin-converting enzyme inhibition. Hypertension. 2000;35:348–352. doi: 10.1161/01.hyp.35.1.348. [DOI] [PubMed] [Google Scholar]
- 14.Iyer SN, Ferrario CM, Chappell MC. Angiotensin-(1-7) contributes to the antihypertensive effects of blockade of the renin–angiotensin system. Hypertension. 1998;31:356–361. doi: 10.1161/01.hyp.31.1.356. [DOI] [PubMed] [Google Scholar]
- 15.Favrat B, Burnier M, Nussberger J, Lecomte JM, Brouard R, Waeber B, Brunner HR. Neutral endopeptidase versus angiotensin converting enzyme inhibition in essential hypertension. J Hypertens. 1995;13:797–804. [PubMed] [Google Scholar]
- 16.Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383:45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 18.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santo AJ, da Costa J, Zhang L, Pei Y, Scholey J, Bray MR, Ferrario CM, Backx PH, Manoukian AS, Chappell MC, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 19.Huentelman MJ, Grobe J, Vazquez J, Stewart JM, Mecca AP, Katovich MJ, Ferrario CM, Raizada MK. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol. 2005;90:783–790. doi: 10.1113/expphysiol.2005.031096. [DOI] [PubMed] [Google Scholar]
- 20.Trask AJ, Averill DB, Ganten D, Chappell MC, Ferrario CM. Primary role of angiotensin-converting enzyme-2 in cardiac production of angiotensin-(1-7) in transgenic Ren-2 hypertensive rats. Am J Physiol. 2007;292:H3019–H3024. doi: 10.1152/ajpheart.01198.2006. [DOI] [PubMed] [Google Scholar]
- 21.Zisman LS, Keller RS, Weaver B, Lin Q, Speth R, Bristow MR, Canver CC. Increased angiotensin-(1-7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme homologue ACE2. Circulation. 2003;108:1707–1712. doi: 10.1161/01.CIR.0000094734.67990.99. [DOI] [PubMed] [Google Scholar]
- 22.Averill DB, Jessup JA, Ishiyama Y, Ferrario CM. Augmented ACE2 expression in ischemic cardiomyopathy. Am J Hypertens. 2005;18:118A–118A. [Google Scholar]
- 23.Allred AJ, Diz DI, Ferrario CM, Chappell MC. Pathways for angiotensin-(1-7) metabolism in pulmonary and renal tissues. Am J Physiol. 2000;279:F841–F850. doi: 10.1152/ajprenal.2000.279.5.F841. [DOI] [PubMed] [Google Scholar]
- 24.Chappell MC, Pirro NT, Sykes A, Ferrario CM. Metabolism of angiotensin-(1-7) by angiotensin converting enzyme. Hypertension. 1998;31:362–367. doi: 10.1161/01.hyp.31.1.362. [DOI] [PubMed] [Google Scholar]
- 25.Deddish PA, Jackman HL, Wang H-Z, Skidgel RA, Erdos EG. An N-domain specific substrate and C-domain specific inhibitor of angiotensin converting enzyme: angiotensin-(1-7) and Keto-ACE. Hypertension. 1997;31:912–917. doi: 10.1161/01.hyp.31.4.912. [DOI] [PubMed] [Google Scholar]
- 26.Yamada K, Iyer SN, Chappell MC, Ganten D, Ferrario CM. Converting enzyme determines the plasma clearance of angiotensin-(1-7) Hypertension. 1998;98:496–502. doi: 10.1161/01.hyp.32.3.496. [DOI] [PubMed] [Google Scholar]
- 27.Chappell MC, Allred AJ, Ferrario CM. Pathways of angiotensin-(1-7) metabolism in the kidney. Nephrol Dial Transplant. 2001;16:22–26. doi: 10.1093/ndt/16.suppl_1.22. [DOI] [PubMed] [Google Scholar]
- 28.Ferrario CM, Smith RD, Brosnihan KB, Chappell MC, Campese VM, Vesterqvist O, Liao W, Ruddy MC, Grim CE. Effects of omapatrilat on the renin angiotensin system in salt sensitive hypertension. Am J Hyperten. 2002;15:557–564. doi: 10.1016/s0895-7061(02)02268-9. [DOI] [PubMed] [Google Scholar]
- 29.Ferrario CM, Averill DB, Brosnihan KB, Chappell MC, Iskandar SS, Dean RH, Diz DI. Vasopeptidase inhibition and Ang-(1-7) in the spontaneously hypertensive rat. Kid Int. 2002;62:1349–1357. doi: 10.1111/j.1523-1755.2002.kid559.x. [DOI] [PubMed] [Google Scholar]
- 30.Santos RAS, Campagnole-Santos MJ, Baracho NCV, Fontes MAP, Silva LCS, Neves LAA, Oliveira DR, Caligiorne SM, Rodrigues ARV, Gropen C, Jr., Carvalho WS, Silva ACSE, Khosla MC. Characterization of a new angiotensin antagonist selective for angiotensin-(1-7): evidence that the actions of angiotensin -(1-7) are mediated by specific angiotensin receptors. Brain Res Bull. 1994;35:293–398. doi: 10.1016/0361-9230(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 31.Tallant EA, Lu X, Weiss RB, Chappell MC, Ferrario CM. Bovine aortic endothelial cells contain an angiotensin-(1-7) receptor. Hypertension. 1997;29:388–392. doi: 10.1161/01.hyp.29.1.388. [DOI] [PubMed] [Google Scholar]
- 32.Heitsch H, Brovkovych S, Malinski T, Wiemer G. Angiotensin-(1-7)-stimulated nitric oxide and superoxide release from endothelial cells. Hypertension. 2001;37:72–76. doi: 10.1161/01.hyp.37.1.72. [DOI] [PubMed] [Google Scholar]
- 33.Brosnihan KB, Li P, Ferrario CM. Angiotensin-(1-7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension. 1996;27:523–528. doi: 10.1161/01.hyp.27.3.523. [DOI] [PubMed] [Google Scholar]
- 34.Porsti I, Bara AT, Busse R, Hecker M. Release of nitric oxide by angiotensin-(1-7) from porcine coronary endothelium: implications for a novel angiotensin receptor. Br J Pharmacol. 1994;111:652–654. doi: 10.1111/j.1476-5381.1994.tb14787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santos RAS, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Bul I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss H-P, Speth R, Walther T. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP. Evidence for a functional interaction of the angiotensin-(1-7) receptor Mas with AT1 and AT2 receptors in the mouse heart. Hypertension. 2005;46:937–942. doi: 10.1161/01.HYP.0000175813.04375.8a. [DOI] [PubMed] [Google Scholar]
- 37.Santos RA, Castro CH, Gava E, Pinheiro SV, Almeida AP, Paula RD, Cruz JS, Ramos AS, Rosa KT, Irigoyen MC, Bader M, Alenina N, Kitten GT, Ferreira AJ. Impairment of in vitro and in vivo heart function in angiotensin-(1-7) receptor MAS knockout mice. Hypertension. 2006;47:996–1002. doi: 10.1161/01.HYP.0000215289.51180.5c. [DOI] [PubMed] [Google Scholar]
- 38.Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49:185–192. doi: 10.1161/01.HYP.0000251865.35728.2f. [DOI] [PubMed] [Google Scholar]
- 39.Tallant EA, Ferrario CM, Gallagher PE. Angiotensin-(1-7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol. 2005;289:H1560–H1566. doi: 10.1152/ajpheart.00941.2004. [DOI] [PubMed] [Google Scholar]
- 40.Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP. Effects of genetic deletion of angiotensin-(1-7) receptor Mas on cardiac function during ischemia/reperfusion in the isolated perfused mouse heart. Life Sci. 2006;80:264–268. doi: 10.1016/j.lfs.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Silva DM, Vianna HR, Cortes SF, Campagnole-Santos MJ, Santos RA, Lemos VS. Evidence for a new angiotensin-(1-7) receptor subtype in the aorta of Sprague-Dawley rats. Peptides. 2007;28:702–707. doi: 10.1016/j.peptides.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Canals M, Jenkins L, Kellett E, Milligan G. Up-regulation of the angiotensin II type 1 receptor by the MAS proto-oncogene is due to constitutive activation of Gq/G11 by MAS. J Biol Chem. 2006;281:16757–16767. doi: 10.1074/jbc.M601121200. [DOI] [PubMed] [Google Scholar]
- 43.Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM, Gembardt F, Kellett E, Martini L, Vanderheyden P, Schultheiss HP, Walther T. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation. 2005;111:1806–1813. doi: 10.1161/01.CIR.0000160867.23556.7D. [DOI] [PubMed] [Google Scholar]
- 44.Schiavone MT, Santos RAS, Brosnihan KB, Khosla MC, Ferrario CM. Release of vasopressin from the rat hypothalamo-neurohypophysial system by angiotensin-(1-7) heptapeptide. Proc Natl Acad Sci USA. 1988;85:4095–4098. doi: 10.1073/pnas.85.11.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1-7) on isolated rabbit afferent arterioles. Hypertension. 2002;39:799–802. doi: 10.1161/hy0302.104673. [DOI] [PubMed] [Google Scholar]
- 46.Iyer SN, Chappell MC, Averill DB, Diz DI, Ferrario CM. Vasodepressor actions of angiotensin-(1-7) unmasked during combined treatment with lisinopril and losartan. Hypertension. 1998;31:699–705. doi: 10.1161/01.hyp.31.2.699. [DOI] [PubMed] [Google Scholar]
- 47.Iyer SN, Averill DB, Chappell MC, Yamada K, Jones AG, Ferrario CM. Contribution of angiotensin-(1-7) to blood pressure regulation in salt-depleted hypertensive rats. Hypertension. 2000;36:417–422. doi: 10.1161/01.hyp.36.3.417. [DOI] [PubMed] [Google Scholar]
- 48.Iyer SN, Yamada K, Diz DI, Ferrario CM, Chappell MC. Evidence that prostaglandins mediate the antihypertensive actions of angiotensin-(1-7) during chronic blockade of the renin-angiotensin system. J Cardiovasc Pharmacol. 2000;36:109–117. doi: 10.1097/00005344-200007000-00015. [DOI] [PubMed] [Google Scholar]
- 49.Li P, Chappell MC, Ferrario CM, Brosnihan KB. Angiotensin-(1-7) augments bradykinin-induced vasodilation by competing with ACE and releasing nitric oxide. Hypertension. 1997;29:394–400. doi: 10.1161/01.hyp.29.1.394. [DOI] [PubMed] [Google Scholar]
- 50.Moriguchi A, Tallant EA, Matsumura K, Reilly TM, Walton H, Ganten D, Ferrario CM. Opposing actions of Angiotensin-(1-7) and Angiotensin II in the brain of transgenic hypertensive rats. Hypertension. 1995;25:1260–1265. doi: 10.1161/01.hyp.25.6.1260. [DOI] [PubMed] [Google Scholar]
- 51.Nakamoto H, Ferrario CM, Fuller SB, Robaczwski DL, Winicov E, Dean RH. Angiotensin-(1-7) and nitric oxide interaction in renovascular hypertension. Hypertension. 1995;25:796–802. doi: 10.1161/01.hyp.25.4.796. [DOI] [PubMed] [Google Scholar]
- 52.Neves LA, Averill DB, Ferrario CM, Chappell MC, Aschner JL, Walkup MP, Brosnihan KB. Characterization of angiotensin-(1-7) receptor subtype in mesenteric arteries. Peptides. 2003;24:455–462. doi: 10.1016/s0196-9781(03)00062-7. [DOI] [PubMed] [Google Scholar]
- 53.Paula RD, Lima CV, Khosla MC, Santos RA. Angiotensin-(1-7) potentiates the hypotensive effect of bradykinin in conscious rats. Hypertension. 1995;26:1154–1159. doi: 10.1161/01.hyp.26.6.1154. [DOI] [PubMed] [Google Scholar]
- 54.Greco AJ, Master RG, Fokin A, Jr., Baber SR, Kadowitz PJ. Angiotensin-(1-7) potentiates responses to bradykinin but does not change responses to angiotensin I. Can J Physiol Pharmacol. 2006;84:1163–1175. doi: 10.1139/y06-053. [DOI] [PubMed] [Google Scholar]
- 55.Carvalho MB, Duarte FV, Faria-Silva R, Fauler B, da Mata Machado LT, de Paula RD, Campagnole-Santos MJ, Santos RA. Evidence for Mas-mediated bradykinin potentiation by the angiotensin-(1-7) nonpeptide mimic AVE 0991 in normotensive rats. Hypertension. 2007;50:762–767. doi: 10.1161/HYPERTENSIONAHA.107.094987. [DOI] [PubMed] [Google Scholar]
- 56.Fernandes L, Fortes ZB, Nigro D, Tostes RC, Santos RA, Carvalho MHC. Potentiation of bradykinin by angiotensin-(1-7) on arterioles of spontaneously hypertensive rats studied in vivo. Hypertension. 2001;37:703–709. doi: 10.1161/01.hyp.37.2.703. [DOI] [PubMed] [Google Scholar]
- 57.Gorelik G, Carbini LA, Scicli AG. Angiotensin 1-7 induces bradykinin-mediated relaxation in porcine coronary artery. J Pharmacol Exp Ther. 1998;286:403–410. [PubMed] [Google Scholar]
- 58.Oliveira MA, Fortes ZB, Santos RA, Kosla MC, De CM. Synergistic effect of angiotensin-(1-7) on bradykinin arteriolar dilation in vivo. Peptides. 1999;20:1195–1201. doi: 10.1016/s0196-9781(99)00123-0. [DOI] [PubMed] [Google Scholar]
- 59.Tom B, de VR, Saxena PR, Danser AH. Bradykinin potentiation by angiotensin-(1-7) and ACE inhibitors correlates with ACE C- and N-domain blockade. Hypertension. 2001;38:95–99. doi: 10.1161/01.hyp.38.1.95. [DOI] [PubMed] [Google Scholar]
- 60.Ferrario CM, Jessup JA, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 61.Ferrario CM, Jessup JA, Gallagher PE, Averill DB, Brosnihan KB, Tallant EA, Smith RD, Chappell MC. Effects of renin angiotensin system blockade on renal angiotensin-(1-7) forming enzymes and receptors. Kidney Int. 2005;68:2189–2196. doi: 10.1111/j.1523-1755.2005.00675.x. [DOI] [PubMed] [Google Scholar]
- 62.Jessup JA, Gallagher PE, Averill DB, Brosnihan KB, Tallant EA, Chappell MC, Ferrario CM. Effect of angiotensin II blockade on a new congenic model of hypertension derived from transgenic Ren-2 rats. Am J Physiol. 2006;291:H2166–H2172. doi: 10.1152/ajpheart.00061.2006. [DOI] [PubMed] [Google Scholar]
- 63.Luque M, Martin P, Martell N, Fernandez C, Brosnihan KB, Ferrario CM. Effects of captopril related to increased levels of prostacyclin and angiotensin-(1-7) in essential hypertension. J Hypertens. 1996;14:799–805. doi: 10.1097/00004872-199606000-00017. [DOI] [PubMed] [Google Scholar]
- 64.Ferrario CM, Martell N, Yunis C, Flack JM, Chappell MC, Brosnihan KB, Dean RH, Fernandez A, Novikov SV, Pinillas C, Luque M. Characterization of angiotensin-(1-7) in the urine of normal and essential hypertensive subjects. Am J Hypertens. 1998;11:137–146. doi: 10.1016/s0895-7061(97)00400-7. [DOI] [PubMed] [Google Scholar]
- 65.Schindler C, Brosnihan KB, Ferrario CM, Bramlage P, Maywald U, Koch R, Oertel R, Kirch W. Comparison of inhibitory effects of irbesartan and atorvastatin treatment on the renin angiotensin system (RAS) in veins: a randomized double-blind crossover trial in healthy subjects. J Clin Pharmacol. 2007;47:112–120. doi: 10.1177/0091270006294280. [DOI] [PubMed] [Google Scholar]
- 66.Schindler C, Bramlage P, Kirch W, Ferrario CM. Role of the vasodilator peptide angiotensin-(1-7) in cardiovascular drug therapy. Vasc Health Risk Manag. 2007;3:125–137. [PMC free article] [PubMed] [Google Scholar]
- 67.Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 68.Averill DB, Ishiyama Y, Chappell MC, Ferrario CM. Cardiac angiotensin-(1-7) in ischemic cardiomyopathy. Circulation. 2003;108:2141–2143. doi: 10.1161/01.CIR.0000092888.63239.54. [DOI] [PubMed] [Google Scholar]
- 69.Grobe JL, Mecca AP, Lingis M, Shenoy V, Bolton TA, Machado JM, Speth RC, Raizada MK, Katovich MJ. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1-7) Am J Physiol. 2007;292:H736–H742. doi: 10.1152/ajpheart.00937.2006. [DOI] [PubMed] [Google Scholar]
- 70.Ferreira AJ, Oliveira TL, Castro MC, Almeida AP, Castro CH, Caliari MV, Gava E, Kitten GT, Santos RA. Isoproterenol-induced impairment of heart function and remodeling are attenuated by the nonpeptide angiotensin-(1-7) analogue AVE 0991. Life Sci. 2007;81:916–923. doi: 10.1016/j.lfs.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 71.De Mello WC. Angiotensin (1-7) re-establishes impulse conduction in cardiac muscle during ischaemia-reperfusion. The role of the sodium pump. J Renin Angiotensin Aldosterone Syst. 2004;5:203–208. doi: 10.3317/jraas.2004.041. [DOI] [PubMed] [Google Scholar]
- 72.Ferreira AJ, Santos RA, Almeida AP. Angiotensin-(1-7): cardioprotective effect in myocardial ischemia/reperfusion. Hypertension. 2001;38:665–668. doi: 10.1161/01.hyp.38.3.665. [DOI] [PubMed] [Google Scholar]
- 73.Ferreira AJ, Santos RA, Almeida AP. Angiotensin-(1-7) improves the post-ischemic function in isolated perfused rat hearts. Braz J Med Biol Res. 2002;35:1083–1090. doi: 10.1590/s0100-879x2002000900009. [DOI] [PubMed] [Google Scholar]
- 74.De Mello WC, Ferrario CM, Jessup JA. Beneficial versus harmful effects of Angiotensin (1-7) on impulse propagation and cardiac arrhythmias in the failing heart. J Renin Angiotensin Aldosterone Syst. 2007;8:74–80. doi: 10.3317/jraas.2007.015. [DOI] [PubMed] [Google Scholar]
- 75.Neves LA, Almeida AP, Khosla MC, Campagnole-Santos MJ, Santos RA. Effect of angiotensin-(1-7) on reperfusion arrhythmias in isolated rat hearts. Braz J Med Biol Res. 1997;30:801–809. doi: 10.1590/s0100-879x1997000600016. [DOI] [PubMed] [Google Scholar]
- 76.DelliPizzi A, Hilchey SD, Bell-Quilley CP. Natriuretic action of angiotensin (1-7) Br J Pharmacol. 1994;111:1–3. doi: 10.1111/j.1476-5381.1994.tb14014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heller J, Kramer HJ, Maly J, Cervenka L, Horacek V. Effect of intrarenal infusion of angiotensin-(1-7) in the dog. Kidney Blood Press Res. 2000;23:89–94. doi: 10.1159/000025959. [DOI] [PubMed] [Google Scholar]
- 78.Vallon V, Heyne N, Richter K, Khosla MC, Fechter K. [7-D-ALA]-Angiotensin 1-7 blocks renal actions of angiotensin 1-7 in the anesthetized rat. J Cardiovasc Pharmacol. 1998;32:164–167. doi: 10.1097/00005344-199807000-00025. [DOI] [PubMed] [Google Scholar]
- 79.Handa RK, Ferrario CM, Strandhoy JW. Renal actions of angiotensin-(1-7) in vivo and in vitro studies. Am J Physiol. 1996;270:F141–F147. doi: 10.1152/ajprenal.1996.270.1.F141. [DOI] [PubMed] [Google Scholar]
- 80.Burgelova M, Kramer HJ, Teplan V, Thumova M, Cervenka L. Effects of angiotensin-(1-7) blockade on renal function in rats with enhanced intrarenal Ang II activity. Kidney Int. 2005;67:1453–1461. doi: 10.1111/j.1523-1755.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 81.Chappell MC, Brosnihan KB, Diz DI, Ferrario CM. Identification of angiotensin-(1-7) in rat brain: evidence for differential processing of angiotensin peptides. J Biol Chem. 1989;264:16518–16523. [PubMed] [Google Scholar]
- 82.Campagnole-Santos MJ, Diz DI, Santos RAS, Khosla MC, Brosnihan KB, Ferrario CM. Cardiovascular effects of angiotensin-(1-7) injected into the dorsal medulla of rats. Am J Physiol. 1989;257:H324–H329. doi: 10.1152/ajpheart.1989.257.1.H324. [DOI] [PubMed] [Google Scholar]
- 83.Benter IF, Diz DI, Ferrario CM. Pressor and reflex sensitivity is altered in spontaneously hypertensive rats treated with angiotensin-(1-7) Hypertension. 1995;26:1138–1144. doi: 10.1161/01.hyp.26.6.1138. [DOI] [PubMed] [Google Scholar]
- 84.Campagnole-Santos MJ, Heringer SB, Batista EN, Khosla MC, Santos RAS. Differential baroreceptor reflex modulation by centrally infused angiotensin peptides. Am J Physiol. 1992;263:R89–R94. doi: 10.1152/ajpregu.1992.263.1.R89. [DOI] [PubMed] [Google Scholar]
- 85.Oliveira DR, Santos RAS, Santos GFP, Khosla MC, Campagnole-Santos MJ. Changes in the baroreflex control of heart rate produced by central infusion of selective angiotensin antagonists in hypertensive rats. Hypertension. 1996;27:1284–1290. doi: 10.1161/01.hyp.27.6.1284. [DOI] [PubMed] [Google Scholar]
- 86.Chaves GZ, Caligiorne SM, Santos RAS, Khosla MC, Campagnole-Santos MJ. Modulation of the baroreflex control of heart rate by angiotensin-(1-7) at the nucleus tractus solitarii of normotensive and spontaneously hypertensive rats. J Hypertens. 2000;18:1841–1848. doi: 10.1097/00004872-200018120-00019. [DOI] [PubMed] [Google Scholar]
- 87.Diz DI, Westwood BM. Deficiency of endogenous angiotensin-(1-7) in the nucleus tractus solitarii of (mREN2)27 transgenic rats may account for diminished baroreceptor reflex function. Hypertension. 2000;36:681. [Google Scholar]
- 88.Fontes MA, Pinge MC, Naves V, Campagnole-Santos MJ, Lopes OU, Khosla MC, Santos RA. Cardiovascular effects produced by microinjection of angiotensins and angiotensin antagonists into the ventrolateral medulla of freely moving rats. Brain Res. 1997;750:305–310. doi: 10.1016/s0006-8993(96)01476-x. [DOI] [PubMed] [Google Scholar]
- 89.Potts PD, Horiuchi J, Coleman MJ, Dampney RA. The cardiovascular effects of angiotensin-(1-7) in the rostral and caudal ventrolateral medulla of the rabbit. Brain Res. 2000;877:58–64. doi: 10.1016/s0006-8993(00)02626-3. [DOI] [PubMed] [Google Scholar]
- 90.Potts PD, Allen AM, Horiuchi J, Dampney RA. Does angiotensin II have a significant tonic action on cardiovascular neurons in the rostral and caudal VLM? Am J Physiol. 2000;279:R1392–R1402. doi: 10.1152/ajpregu.2000.279.4.R1392. [DOI] [PubMed] [Google Scholar]
- 91.Hellner K, Walther T, Schubert M, Albrecht D. Angiotensin-(1-7) enhances LTP in the hippocampus through the G-protein-coupled receptor Mas. Mol Cell Neurosci. 2005;29:427–435. doi: 10.1016/j.mcn.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 92.Freeman EJ, Chisolm GM, Ferrario CM, Tallant EA. Angiotensin-(1-7) inhibits vascular smooth muscle cell growth. Hypertension. 1996;28:104–108. doi: 10.1161/01.hyp.28.1.104. [DOI] [PubMed] [Google Scholar]
- 93.Strawn WB, Ferrario CM, Tallant EA. Angiotensin-(1-7) reduces smooth muscle growth after vascular injury. Hypertension. 1999;33:207–211. doi: 10.1161/01.hyp.33.1.207. [DOI] [PubMed] [Google Scholar]
- 94.Loot AE, Roks AJM, Henning RH, Tio RA, Suurmeijer AHH, Boomsma F, vanGilst WH. Angiotensin-(1-7) attenuates the development of heart failure after myocardial infarction in rats. Circulation. 2002;105:1548–1550. doi: 10.1161/01.cir.0000013847.07035.b9. [DOI] [PubMed] [Google Scholar]
- 95.Iwata M, Cowling RT, Gurantz D, Moore C, Zhang S, Yuan JX, Greenberg BH. Angiotensin-(1-7) binds to specific receptors on cardiac fibroblasts to initiate antifibrotic and antitrophic effects. Am J Physiol. 2005;289:H2356–H2363. doi: 10.1152/ajpheart.00317.2005. [DOI] [PubMed] [Google Scholar]
- 96.Wang LJ, He JG, Ma H, Cai YM, Liao XX, Zeng WT, Liu J, Wang LC. Chronic administration of angiotensin-(1-7) attenuates pressure-overload left ventricular hypertrophy and fibrosis in rats. Di Yi Jun Yi Da Xue Xue Bao. 2005;25:481–487. [PubMed] [Google Scholar]
- 97.Machado RD, Santos RA, Andrade SP. Opposing actions of angiotensins on angiogenesis. Life Sci. 2000;66:67–76. doi: 10.1016/s0024-3205(99)00562-7. [DOI] [PubMed] [Google Scholar]
- 98.Gallagher PE, Tallant EA. Inhibition of human lung cancer cell growth by angiotensin-(1-7) Carcinogenesis. 2004;25:2045–2052. doi: 10.1093/carcin/bgh236. [DOI] [PubMed] [Google Scholar]
- 99.Pantoja DS, Tallant AE, Gallagher PE (2004) Inhibition of human breast cancer cell growth by angiotensin-(1-7). Proc Am Assoc Cancer Res 45 [DOI] [PubMed]
- 100.Menon J, Soto-Pantoja DR, Callahan MF, Cline JM, Ferrario CM, Tallant EA, Gallagher PE. Angiotensin-(1-7) inhibits growth of human lung adenocarcinoma xenografts in nude mice through a reduction in cyclooxygenase-2. Cancer Res. 2007;67:2809–2815. doi: 10.1158/0008-5472.CAN-06-3614. [DOI] [PubMed] [Google Scholar]
- 101.Valdes G, Neves LA, Anton L, Corthorn J, Chacon C, Germain AM, Merrill DC, Ferrario CM, Sarao R, Penninger J, Brosnihan KB. Distribution of angiotensin-(1-7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta. 2006;27:200–207. doi: 10.1016/j.placenta.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 102.Merrill DC, Karoly M, Chen K, Ferrario CM, Brosnihan KB. Angiotensin-(1-7) in normal and preeclamptic pregnancy. Endocrine. 2002;18:239–245. doi: 10.1385/ENDO:18:3:239. [DOI] [PubMed] [Google Scholar]
- 103.Valdes G, Germain AM, Corthorn J, Berrios C, Foradori AC, Ferrario CM, Brosnihan KB. Urinary vasodilator and vasoconstrictor angiotensins during menstrual cycle, pregnancy, and lactation. Endocrine. 2001;16:117–122. doi: 10.1385/ENDO:16:2:117. [DOI] [PubMed] [Google Scholar]
- 104.Joyner J, Neves LA, Granger JP, Alexander BT, Merrill DC, Chappell MC, Ferrario CM, Davis WP, Brosnihan KB. Temporal-spatial expression of angiotensin-(1-7) and angiotensin converting enzyme 2 in the kidney of normal and hypertensive pregnant rats. Am J Physiol. 2007;239:R169–R177. doi: 10.1152/ajpregu.00387.2006. [DOI] [PubMed] [Google Scholar]
- 105.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin–angiotensin system. Biochem Biophys Res Commun. 2006;350:1026–1031. doi: 10.1016/j.bbrc.2006.09.146. [DOI] [PubMed] [Google Scholar]
- 106.Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K, Ferrario CM (2008) Localization of the novel angiotensin peptide, angiotensin-12 [Ang-(1–2)], in the heart and kidney of hypertensive and normotensive rats. Am J Physiol (in press) [DOI] [PMC free article] [PubMed]
- 107.Chappell MC, Westwood BM, Pendergrass KD, Jessup JA, Ferrario CM. Distinct processing pathways for the novel peptide angiotensin-(1-12) in the serum and kidney of the hypertensive mRen2. Lewis Rat. Hypertension. 2007;50:e139. [Google Scholar]
- 108.Trask AJ, Jessup JA, Chappell MC, Ferrario CM (2008) Angiotensin-(1-12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol (in press) [DOI] [PMC free article] [PubMed]
- 109.Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet. 2007;370:221–229. doi: 10.1016/S0140-6736(07)61124-6. [DOI] [PubMed] [Google Scholar]
- 110.Trask AJ, Ferrario CM. Angiotensin-(1-7): pharmacology and new perspectives in cardiovascular treatments. Cardiovasc Drug Rev. 2007;25:162–174. doi: 10.1111/j.1527-3466.2007.00012.x. [DOI] [PubMed] [Google Scholar]


