Abstract
The health effects of cell phone radiation exposure are a growing public concern. This study investigated whether expression of genes related to cell death pathways are dysregulated in primary cultured neurons and astrocytes by exposure to a working GSM (Global System for Mobile Communication) cell phone rated at a frequency of 1900 MHz. Primary cultures were exposed to cell phone emissions for 2 hrs. We used array analysis and real-time RT-PCR to show up-regulation of caspase-2, caspase-6 and Asc (apoptosis associated speck-like protein containing a card) gene expression in neurons and astrocytes. Upregulation occurred in both “on” and “stand-by” modes in neurons, but only in “on” mode in astrocytes. Additionally, astrocytes showed up-regulation of the Bax gene. The effects are specific since up-regulation was not seen for other genes associated with apoptosis, such as caspase-9 in either neurons and astrocytes, or Bax in neurons. The results show that even relatively short-term exposure to cell phone radiofrequency emissions can up-regulate elements of apoptotic pathways in cells derived from the brain, and that neurons appear to be more sensitive to this effect than astrocytes.
Keywords: cell phone radiation, apoptosis, caspase, gene expression
Cell phones transmit and receive electromagnetic waves, mainly at frequencies of 800–1900 MHz. Cell phone usage is a public health concern because of the potential risk of chronic exposure to the low levels of radiofrequency and microwave (RF/MW) radiation that pulse off the phone antenna, in close proximity to the user’s head. Cell phones transmit electromagnetic waves in all directions, increasing the region of cells within the brain that are at risk for damage by RF/MW radiation penetrating the skull. Although RF/MW radiation can result in thermal damage if energy absorption rates are high, it is more likely that deleterious effects of RF/MW radiation on cells of the brain would be due to non-thermal effects induced by lower intensities of exposure[5].
Effects of RF/MW radiation on human health have been widely investigated using an epidemiological approach. Several reports showed no association between cell phone use and brain tumors [4, 8, 25] while others came to the opposite conclusion [17, 20]. Headache has been reported among cell phone users compared to non-users [7]. When rats were exposed to RF/MW radiation to reproduce normal human exposure, the life span and tumorigenicity of rats were unchanged [1, 21] but evidence of oxidative damage was found in brain tissues [19, 29]. RF/MW radiation induced damage can lead to death in single cell organisms [3], inhibit cell proliferation [9], cause DNA damage [11, 32], and alter gene expression in different cell types including brain cells as measured by gene microarrays [6, 14, 15]. Although the methods and target cells differ among the report cited above, the existing literature taken as a whole suggests that the expression of specific genes and proteins in cultured cells and intact animals can be affected by RF/MW radiation exposure [10].
In spite of previous studies, knowledge about the adverse effects of RF/MW radiation on human health, or the biological responses to RF/MW radiation exposure is still limited. There is a paucity of investigation into the cells that are most at risk, central nervous system (CNS) neurons and glia. The present experiments have used both gene array and real time RT-PCR to determine whether exposure to emissions from an operational cell phone might regulate expression of genes associated with apoptosis in cells derived from murine brains.
Primary neuron cultures were prepared using E15, timed-pregnant female ICR mice (Jackson Laboratories, Bar Harbor, ME) following a published protocol [30]. All procedures involving animals were approved by the University of Kentucky IACUC Committee. The neurons were counted and re-suspended in 2.0 ml neurobasal medium with 25 μM glutamate, B27 additive, and antibiotics, then plated at 4.5 x 105 cells per 35 mm petri-dish and cultured for 3 days (37ºC, 5% CO2). The depth of the medium at the center of the meniscus was approximately 4 mm. Viability was routinely assessed after attachment in sister culture dishes using a fluorescent live-dead assay (Molecular Probes, Eugene, OR). Cultures with viability less than 95% were not used.
Primary astrocytes were prepared as previously published [12]. Astrocytes were plated in 2.0 ml Dulbecco’s modified Eagle’s medium with 10% fetal calf serum (HyClone, Logan, UT) and antibiotics at a density of 1 x 105 cells per 35 mm petri-dish and cultured for 3 days (37ºC, 5% CO2). At this time the cultures were ≥95% viable, approximately 60% confluent, and consisted of about 5–8 x 105 astrocytes attached to the culture surface. Purity as assessed by immunostaining for glial fibrillary acidic protein was ≥95%. The depth of the medium at the center of the meniscus was approximately 4 mm.
For RF/MW radiation exposure, the cover was removed from a petri-dish containing neurons or astrocytes, and a cell phone either in the “on” mode (exposed) or in the “stand-by” mode (sham) was placed on top of the petri-dish with its antenna over the center of the dish (Fig. 1). The cell phone was manufactured by Samsung (model SGH-E105; Ridgefield Park, NJ) (GSM signal; 1900 MHz). The signal provider was T-Mobile. After 2 hr, the cells were harvested for RNA isolation.
Fig. 1.

Photograph of the method used to expose cultured cells to cell phone RF/MW radiation. The photograph shows a primary neuron culture in a 35 mm Petri-dish containing 2.0 ml medium. A cell phone in “stand-by” or “on” mode was placed on top of the petri-dish as shown.
Array analysis (GEArray Q series Mouse Apoptosis Arrays, SuperArray Bioscience Corp., Frederick, MD) was used to profile the expression of genes related to apoptosis in neuron cultures. Total RNA was isolated from control and cell phone-exposed cells using the GenEluteTM Mammalian Total RNA kit (Sigma). cDNA probes were synthesized with Biotin-16 dUTP [13] and using an AmpoLabeling-LPR kit (SuperArray Bioscience). Hybridization, washing and chemiluminescent detection of signals were performed following the manufacturer’s recommendations. Images were scanned using a Kodak 440CF Image Station with 1D software and automatic background correction. The signal intensity of each spot on the array is indicative of gene expression level. Arrays were analyzed using SuperArray on line software (GEArray Expression Analysis Suite; http://geasuite.superarray.com). The fold-change of gene expression level was calculated as the ratio of expression in exposed cells to expression in control cells. Because of their known variability, arrays were used only as screening tools to indicate genes or gene families of potential interest. Genes showing an increase or decrease of ≥35% in two arrays were further examined and changes were validated by a more quantitative method, real time RT-PCR.
Real time RT-PCR was performed on control, sham, and exposed cells using the ABI Prism 7500 system. SYBR Green PCR Master Mix kit was purchased from Applied Biosystems (Warrington, UK). cDNA was synthesized from 2 μg of total RNA using the High-Capacity cDNA Archive Kit (Applied Biosystems). cDNAs from all samples were mixed and then diluted 2, 4, 8, and 16-fold to generate a standard curve for each gene. The mRNA level of all genes tested by real time RT-PCR was normalized against 18S mRNA. The RT2 primer sets for all genes except 18S used in real time RT-PCR were purchased from SuperArray Bioscience. The primers for 18S used in real time RT-PCR were designed based on its cDNA sequence (forward primer: CCCCCCGTGGCGGCGACGAC; reverse primer: TTGCCCTCCAATGGATCCTC). Real time RT-PCR data was analyzed by ANOVA with post-hoc Scheffe’s test (StatView 5.0, Abacus Concepts, Berkeley, CA).
Results of array analyses on neurons are presented in Table 1. The GEArray Q series mouse apoptosis array contains 96 genes involved in regulating apoptosis. When primary cultures of neurons were exposed to RF/MW radiation from a cell phone in the “on” mode for 2 hrs, the expression of eight genes was potentially up-regulated and one gene was potentially down-regulated compared to unexposed, control cells (Table 1). The expression of the majority of genes on the array was unchanged. Among those that were changed, caspase-2 and Asc are known to be involved in the early stages of apoptosis, and caspase-6 is known to be an apoptosis executor. These initial array data were further validated by real time RT-PCR (Fig. 2A) and, in most cases, gene array and real time RT-PCR results were similar. As observed in the arrays, mRNAs for Asc, caspase-2 and caspase-6 were significantly increased in neurons exposed to the cell phone in the “on” mode over values in control, non-exposed, neurons. In addition, we found that exposure to the cell phone in the “stand-by” mode also significantly increased expression of these same genes. Results in the “stand-by” and “on” modes were indistinguishable. To validate the specific regulation of caspase-2, caspase-6 and Asc genes, the expression of other genes within the apoptotic pathway was also examined. Real time RT-PCR analysis confirmed gene array results showing that expression of caspase-9 and Bax genes was not up-regulated by cell phone exposure (Fig. 2A). Taken together, these results suggest that a 2 hr exposure to the cell phone used in these experiments, either in the “on” or “stand-by” position, is sufficient to induce significant changes in expression of specific pro-apoptotic genes in cultured mouse neurons.
Table 1.
Regulation of apoptosis-associated gene expression in mouse striatal neurons by exposure to cell phone radiation
| Gene name | Genebank # | Description | Fold change (vs. control) | |
|---|---|---|---|---|
| Up-regulated | Asc | NM_023258 | Apoptosis-associated speck-like protein containing a CARD | +1.66 |
| Casp2 | NM_007610 | Caspase 2 | +1.75 | |
| Casp6 | NM_009801 | Caspase 6 | +1.69 | |
| I-TRAF | NM_011529 | TRAF family member-associated Nf-kappa B activator | +1.69 | |
| TRAF2 | NM_009422 | Tnf receptor-associated factor 2 | +1.61 | |
| TRAF3 | NM_011632 | Tnf receptor-associated factor 3 | +1.55 | |
| TRAF5 | NM_011633 | Tnf receptor-associated factor 2 | +2.40 | |
| TRAF6 | NM_009424 | Tnf receptor-associated factor 6 | +2.76 | |
| Down-regulated | Rpa3 | NM_026632 | Replication protein A3 | −0.61 |
Neurons grown in culture were exposed to a working cell phone. Gene array analysis was used to examine changes in expression of genes related to apoptosis. Data presented are the means of two separate experiments. The fold change values represent gene expression levels detected in cells exposed to a cell phone in the “on” mode (exposed) relative to that of non-exposed, control cells.
Fig. 2.
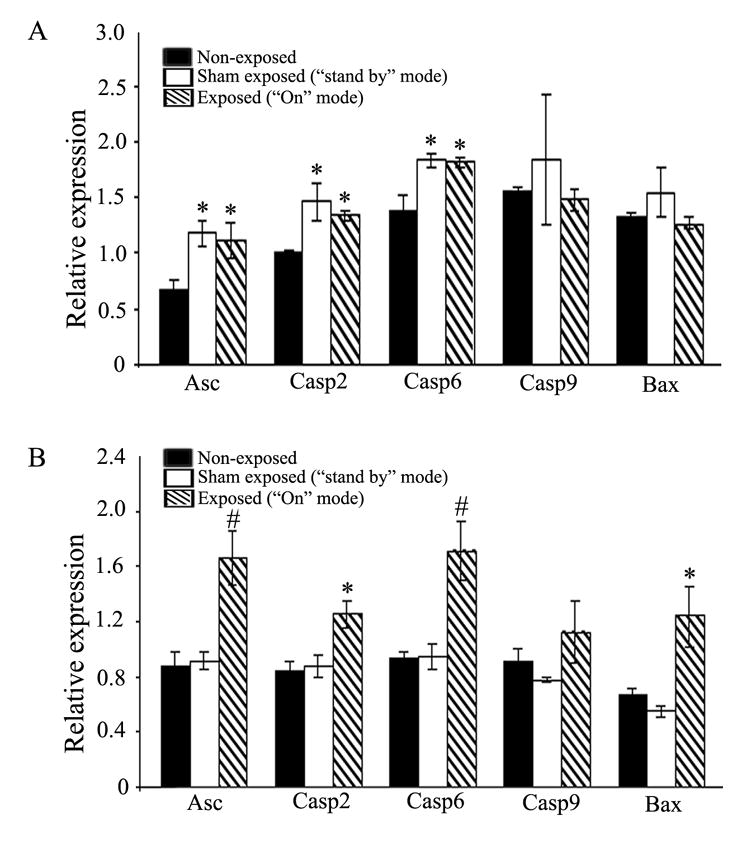
Regulation of apoptosis pathway gene expression in cultured neurons (A) or astrocytes (B) exposed to cell phone RF/MW radiation. Cells were placed proximate to a cell phone for 2 hrs, as shown in Fig. 1, then analyzed for mRNA expression by real time RT-PCR. mRNA levels are shown relative to expression of 18S mRNA. (A) Neurons show upregulation of Asc, caspase-2, and caspase-6 gene expression after exposure to a cell phone either in the “on” mode (exposed) or “stand-by” mode (sham exposed) versus non-exposed, control cells (*p<0.05). Caspase-9 and Bax mRNA levels are unchanged. (B) Astrocytes show upregulated expression of Asc, caspase-2, caspase-6 and Bax genes after exposure to a cell phone in the “on” mode (exposed) but not in the “stand-by” mode (sham exposed) versus non-exposed, control cells (*p<0.05; #p<0.01). Caspase-9 gene expression was unchanged. For both (A) and (B), values are the mean ± SEM (n=3).
To determine whether the responses described above were specific to neurons, the expression of the same genes was also examined by real time RT-PCR in control, sham-exposed (“stand-by” mode), and exposed (“on” mode) primary astrocytes. Similar to the case in neurons, the caspase-2, caspase-6, and Asc genes were found to be significantly up-regulated in astrocytes exposed to the cell phone in the “on”position, while the caspase-9 gene was unchanged. However, unlike the situation in neurons, the Bax gene was also up-regulated in astrocytes (Fig. 2B). Exposure to the cell phone in “stand-by” mode did not increase expression of any gene above control levels.
Apoptosis is a cellular suicide mechanism that occurs in mammalian cells during normal development and also as a response to situations of injury or disease. Caspases, a family of cysteine proteases, have been identified as important effectors of the intrinsic cell death machinery. Both caspase-2 and caspase-6 were significantly up-regulated in primary mouse neurons and astrocytes after RF/MW radiation exposure. Caspase-2 was recently shown to be an initiator caspase when DNA damage occurs [28]. Agents causing DNA damage were shown to first activate caspase-2, which in turn initiated both mitochondrial and post mitochondrial events. Caspase-2 promotes Bax translocation to the mitochondria, increases mitochondrial permeability, releases cytochrome-c into the cytosol, and finally activates caspase-9 [18, 26]. For this reason, we also analyzed Bax and caspase-9 mRNA levels in experimental neurons and astrocytes by real time RT-PCR. Exposure to cell phone RF/MW radiation did not alter the transcription of caspase-9 in either cell type (Fig. 2). This suggests that in the timeframe of these experiments, any activation of caspase-9 which occurs does not involve new gene transcription. Bax transcription was increased in astrocytes but unchanged in neurons, suggesting that the responses of neurons and glia to cell phone exposure differs. RF/MW radiation is capable of inducing DNA damage in human cells [2, 11, 32]. The activation of caspase-2 in our experiments may reflect cellular DNA damage caused by cell phone exposure. Unlike other caspases, caspase-2 also has a non-enzymatic function. Activation of caspase-2 can induce NF-κB and p38 MAPK in a TRAF2-mediated manner [22], and could result in activation of apoptotic effectors through these downstream elements. Our gene arrays indicated a 60% upregulation in TRAF2 mRNA in mouse neurons after exposure to cell phone RF/MW radiation compared to control cells (Table 1). We also found that other TRAF family members, (TRAF3, 5, 6) were up-regulated (Table 1). p38 MAPK was also activated by cell phone RF/MW radiation in the human endothelial cell line EA.hy926 [24], suggesting this as a potential common response to cell phone exposure.
Cell phone exposure also significantly up-regulated expression of the caspase-6 gene in both mouse neurons and astrocytes. Caspase-6 can play a critical role in human neuronal degeneration through induction of Bid dependent cytochrome-c release and activation of caspase-8 [16, 23]. Even though we did not analyze the levels of caspase-6 protein or activity, the increase in mRNA indicates that cell phone use may adversely affect CNS function through this pathway. Although caspase-3 is the responsible effector for apoptosis in many situations, array data showed that caspase-3 transcription was not significantly increased in neurons exposed to cell phone RF/MW radiation (data not shown). Thus, in terms of caspases and immediate upstream members of caspase signaling pathways, the increases seen in activity of the caspase-2 and -6 genes appear to be relatively specific.
Asc, a member of the CARD (caspase recruitment domain)-containing adaptor protein family [27] involved in caspase-1 activation [31], was also significantly up-regulated in mouse primary neurons and astrocytes after cell phone exposure (Table 1 and Figure 2). The caspase-1 gene was not activated in exposed neurons (data not shown), suggesting that procaspase-1 might be cleaved by Asc independent of any additional transcription.
Our results suggest that specific CNS cells may activate different genes in response to cell phone emissions, and that there is a variable threshold sensitivity depending on cell type. The variations in culture conditions that could contribute to the observed differences were minimized since both cells were grown in an attached manner, in the same size culture dish, and in the same volume of medium. Still, some technical differences are impossible to avoid and might create disparities in the amount of total radiation received by the two cell types. For example, the culture media for astrocytes and neurons is slightly different. Astrocytes, which are highly proliferative, were also plated at a lower density compared to neurons to allow for expansion of the cell population between plating and RF/MW radiation exposure. Inherent differences in cell size and shape, composition of cell membranes, organelle distribution, junctional coupling between adjacent cells, stage of the cell cycle, and other parameters that cannot be controlled will also contribute to different amounts of energy absorption by the cells.
Our experiments were designed to test whether exposure to a cell phone under ambient conditions altered the expression of apoptotic genes, regardless of the frequencies within the electromagnetic field that caused the effects. For this reason, we did not measure or attempt to control the electromagnetic field components. Much of the radiation emitted by GSM cell phones is in the frequency range of radiofrequency (3kHz – 300 MHz) and microwave (300mHz – 300 Ghz) radiation. The biological consequences of RF/MW radiation can include both thermal and non-thermal effects, depending on a number of electromagnetic field parameters, including the power, duration or intermittency of exposure, pulse shape, and ambient field strength [5]. The specific absorption rate (SAR) is often used to describe thermal effects of absorbed RF/MW radiation, and to provide a basis of comparison between experiments. We did not measure the SAR, since our studies were designed to compare effects between two very different types of cells. The SAR reflects the additive effects of all parameters that contribute to thermal absorption, including both inherent cell structure differences as well as differences in culture conditions and field strength. Since the SAR for neurons and astrocytes is likely to be different because of their distinct cell structure, it would not be a practical basis on which to compare exposure. Some cell phones also emit radiation in the extremely low frequency (ELF) (50–60Hz) range, which can contribute to non-thermal biological effects. Although our cell phone was rated at 1900 MHz, ELF emission was detected and measured at 50–100 nT (equivalent to 0.5–1 mGauss) in “on” mode, and 5 nT (0.05 mGauss) in “stand-by” mode (Multidetector II, Less EMF Inc., Germany). Both measurements were higher than the background environmental level of 2 nT. Since the phone used in these experiments had emissions over a wide frequency range, is not clear which component(s) of the electromagnetic field were responsible for induction of apoptotic gene activity in neurons and astrocytes. Similarly, the induction may result from thermal effects, non-thermal effects, or some combination thereof.
To our knowledge, this study is the first to examine the effects of RF/MW emissions from a working cell phone on elements of cell death signaling pathways in primary neurons and astrocytes. Overall, there have been few published studies that explore the responses of primary cells to an actual mobile communication signal, which may be quite different from those in a standardized and tightly controlled electromagnetic exposure chamber. Our results indicate that short-term exposure to cell phone RF/MW radiation emissions can up-regulate specific intermediaries of apoptotic pathways, with neurons appearing to have a lower threshold for apoptotic activation than astrocytes. Cell phone emissions thus have the potential to cause dysfunction or death through activation of specific intracellular cell death signaling pathways.
Abbreviations used
- Asc
apoptosis associated speck-like protein containing a card
- CARD
caspase recruitment domain
- CNS
central nervous system
- ELF
extremely low frequency
- GSM
global system for mobile communication
- MAPK
mitogen activated protein kinase
- NF-κB
nuclear factor kappa B
- nt
nanotesla
- RT-PCR
reverse transcriptase polymerase chain reaction
- RF/MW
radiofrequency and microwave
- TRAF
tumor necrosis factor receptor-associated factor
- SAR
specific absorption rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Adey WR, Byus CV, Cain CD, Higgins RJ, Jones RA, Kean CJ, Kuster N, MacMurray A, Stagg RB, Zimmerman G. Spontaneous and nitrosourea-induced primary tumors of the central nervous system in Fischer 344 rats exposed to frequency-modulated microwave fields. Cancer Res. 2000;60:1857–63. [PubMed] [Google Scholar]
- 2.Aitken RJ, Bennetts LE, Sawyer D, Wiklendt AM, King BV. Impact of radio frequency electromagnetic radiation on DNA integrity in the male germline. Int J Androl. 2005;28:171–9. doi: 10.1111/j.1365-2605.2005.00531.x. [DOI] [PubMed] [Google Scholar]
- 3.Aksoy U, Sahin S, Ozkoc S, Ergor G. The effect of electromagnetic waves on the growth of Entamoeba histolytica and Entamoeba dispar. Saudi Med J. 2005;26:1388–90. [PubMed] [Google Scholar]
- 4.Auvinen A, Hietanen M, Luukkonen R, Koskela RS. Brain tumors and salivary gland cancers among cellular telephone users. Epidemiology. 2002;13:356–9. doi: 10.1097/00001648-200205000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Belyaev I. Non-thermal biological effects of microwaves. Microwave Review. 2005;11:13–23. [Google Scholar]
- 6.Belyaev IY, Koch CB, Terenius O, Roxstrom-Lindquist K, Malmgren LO, W HS, Salford LG, Persson BR. Exposure of rat brain to 915 MHz GSM microwaves induces changes in gene expression but not double stranded DNA breaks or effects on chromatin conformation. Bioelectromagnetics. 2006;27:295–306. doi: 10.1002/bem.20216. [DOI] [PubMed] [Google Scholar]
- 7.Chia SE, Chia HP, Tan JS. Prevalence of headache among handheld cellular telephone users in Singapore: a community study. Environ Health Perspect. 2000;108:1059–62. doi: 10.1289/ehp.001081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen HC, Schuz J, Kosteljanetz M, Poulsen HS, Thomsen J, Johansen C. Cellular telephone use and risk of acoustic neuroma. Am J Epidemiol. 2004;159:277–83. doi: 10.1093/aje/kwh032. [DOI] [PubMed] [Google Scholar]
- 9.Cleary SF, Du Z, Cao G, Liu LM, McCrady C. Effect of isothermal radiofrequency radiation on cytolytic T lymphocytes. Faseb J. 1996;10:913–9. doi: 10.1096/fasebj.10.8.8666169. [DOI] [PubMed] [Google Scholar]
- 10.Cotgreave IA. Biological stress responses to radio frequency electromagnetic radiation: are mobile phones really so (heat) shocking? Arch Biochem Biophys. 2005;435:227–40. doi: 10.1016/j.abb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Diem E, Schwarz C, Adlkofer F, Jahn O, Rudiger H. Non-thermal DNA breakage by mobile-phone radiation (1800 MHz) in human fibroblasts and in transformed GFSH-R17 rat granulosa cells in vitro. Mutat Res. 2005;583:178–83. doi: 10.1016/j.mrgentox.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 12.El-Hage N, Wu G, Wang J, Ambati J, Knapp PE, Reed JL, Bruce-Keller AJ, Hauser KF. HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia. 2006;53:132–46. doi: 10.1002/glia.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friso S, Girelli D, Martinelli N, Olivieri O, Lotto V, Bozzini C, Pizzolo F, Faccini G, Beltrame F, Corrocher R. Low plasma vitamin B-6 concentrations and modulation of coronary artery disease risk. Am J Clin Nutr. 2004;79:992–8. doi: 10.1093/ajcn/79.6.992. [DOI] [PubMed] [Google Scholar]
- 14.Fritze K, Wiessner C, Kuster N, Sommer C, Gass P, Hermann DM, Kiessling M, Hossmann KA. Effect of global system for mobile communication microwave exposure on the genomic response of the rat brain. Neuroscience. 1997;81:627–39. doi: 10.1016/s0306-4522(97)00228-5. [DOI] [PubMed] [Google Scholar]
- 15.Goswami PC, Albee LD, Parsian AJ, Baty JD, Moros EG, Pickard WF, Roti Roti JL, Hunt CR. Protooncogene mRNA levels and activities of multiple transcription factors in C3H 10T 1/2 murine embryonic fibroblasts exposed to 835.62 and 847.74 MHz cellular phone communication frequency radiation. Radiat Res. 1999;151:300–9. [PubMed] [Google Scholar]
- 16.Guo H, Albrecht S, Bourdeau M, Petzke T, Bergeron C, LeBlanc AC. Active caspase-6 and caspase-6-cleaved tau in neuropil threads, neuritic plaques, and neurofibrillary tangles of Alzheimer's disease. Am J Pathol. 2004;165:523–31. doi: 10.1016/S0002-9440(10)63317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardell L, Carlberg M, Hansson Mild K. Use of cellular telephones and brain tumour risk in urban and rural areas. Occup Environ Med. 2005;62:390–4. doi: 10.1136/oem.2004.017434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Q, Huang Y, Sheikh MS. Bax deficiency affects caspase-2 activation during ultraviolet radiation-induced apoptosis. Oncogene. 2004;23:1321–5. doi: 10.1038/sj.onc.1207212. [DOI] [PubMed] [Google Scholar]
- 19.Ilhan A, Gurel A, Armutcu F, Kamisli S, Iraz M, Akyol O, Ozen S. Ginkgo biloba prevents mobile phone-induced oxidative stress in rat brain. Clin Chim Acta. 2004;340:153–62. doi: 10.1016/j.cccn.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Kundi M, Mild K, Hardell L, Mattsson MO. Mobile telephones and cancer--a review of epidemiological evidence. J Toxicol Environ Health B Crit Rev. 2004;7:351–84. doi: 10.1080/10937400490486258. [DOI] [PubMed] [Google Scholar]
- 21.La Regina M, Moros EG, Pickard WF, Straube WL, Baty J, Roti Roti JL. The effect of chronic exposure to 835.62 MHz FDMA or 847.74 MHz CDMA radiofrequency radiation on the incidence of spontaneous tumors in rats. Radiat Res. 2003;160:143–51. doi: 10.1667/rr3028. [DOI] [PubMed] [Google Scholar]
- 22.Lamkanfi M, D'Hondt K, Vande Walle L, van Gurp M, Denecker G, Demeulemeester J, Kalai M, Declercq W, Saelens X, Vandenabeele P. A novel caspase-2 complex containing TRAF2 and RIP1. J Biol Chem. 2005;280:6923–32. doi: 10.1074/jbc.M411180200. [DOI] [PubMed] [Google Scholar]
- 23.LeBlanc A, Liu H, Goodyer C, Bergeron C, Hammond J. Caspase-6 role in apoptosis of human neurons, amyloidogenesis, and Alzheimer's disease. J Biol Chem. 1999;274:23426–36. doi: 10.1074/jbc.274.33.23426. [DOI] [PubMed] [Google Scholar]
- 24.Leszczynski D, Joenvaara S, Reivinen J, Kuokka R. Non-thermal activation of the hsp27/p38MAPK stress pathway by mobile phone radiation in human endothelial cells: molecular mechanism for cancer- and blood-brain barrier-related effects. Differentiation. 2002;70:120–9. doi: 10.1046/j.1432-0436.2002.700207.x. [DOI] [PubMed] [Google Scholar]
- 25.Lonn S, Ahlbom A, Hall P, Feychting M. Long-term mobile phone use and brain tumor risk. Am J Epidemiol. 2005;161:526–35. doi: 10.1093/aje/kwi091. [DOI] [PubMed] [Google Scholar]
- 26.Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM. Overexpression of the anti-apoptotic caspase-2 short isoform in macrophage-derived foam cells of human atherosclerotic plaques. Am J Pathol. 2003;162:731–6. doi: 10.1016/S0002-9440(10)63869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masumoto J, Taniguchi S, Ayukawa K, Sarvotham H, Kishino T, Niikawa N, Hidaka E, Katsuyama T, Higuchi T, Sagara J. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem. 1999;274:33835–8. doi: 10.1074/jbc.274.48.33835. [DOI] [PubMed] [Google Scholar]
- 28.Robertson JD, Enoksson M, Suomela M, Zhivotovsky B, Orrenius S. Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptosis. J Biol Chem. 2002;277:29803–9. doi: 10.1074/jbc.M204185200. [DOI] [PubMed] [Google Scholar]
- 29.Salford LG, Brun AE, Eberhardt JL, Malmgren L, Persson BR. Nerve cell damage in mammalian brain after exposure to microwaves from GSM mobile phones. Environ Health Perspect. 2003;111:881–3. doi: 10.1289/ehp.6039. discussion A408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh IN, El-Hage N, Campbell ME, Lutz SE, Knapp PE, Nath A, Hauser KF. Differential involvement of p38 and JNK MAP kinases in HIV-1 Tat and gp120-induced apoptosis and neurite degeneration in striatal neurons. Neuroscience. 2005;135:781–90. doi: 10.1016/j.neuroscience.2005.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stehlik C, Lee SH, Dorfleutner A, Stassinopoulos A, Sagara J, Reed JC. Apoptosis-associated speck-like protein containing a caspase recruitment domain is a regulator of procaspase-1 activation. J Immunol. 2003;171:6154–63. doi: 10.4049/jimmunol.171.11.6154. [DOI] [PubMed] [Google Scholar]
- 32.Tice RR, Hook GG, Donner M, McRee DI, Guy AW. Genotoxicity of radiofrequency signals. I. Investigation of DNA damage and micronuclei induction in cultured human blood cells. Bioelectromagnetics. 2002;23:113–26. doi: 10.1002/bem.104. [DOI] [PubMed] [Google Scholar]


