Abstract
This study tested whether history of depression is associated with an increased likelihood of having dementia, and to verify whether a first depressive episode earlier in life is associated with an increased likelihood of dementia, or whether only depressive episodes occurring close in time to dementia diagnosis are related to dementia. Depression information was collected from national hospital discharge registries, medical history, and medical records. Dementia was clinically diagnosed using DSM-IV criteria. Case-control results showed that individuals with recent registry-identified depression were 3.9 times more likely than those with no registry-identified depression history to have dementia, while registry-identified depression earlier in life was not associated with an increased dementia risk. Each 1-year increase in the difference between depression onset and dementia onset or censored age decreased the likelihood of dementia by 8.4%. Co-twin control analyses found that individuals with prior depression were 3.0 times more likely to have dementia than their non-depressed twin partner, with a similar gradient of age of depression onset. Taken together, these findings suggest that after partially controlling for genetic influences, late-life depression for many individuals may be a prodrome rather than a risk factor for dementia.
Keywords: dementia, depression, risk factor, twin study
Studies have shown that depression and dementia frequently coexist (Jorm, 2001). Whether or not a history of depression leads to an increased risk of dementia, however, remains controversial. Several case-control studies have tested the association between dementia and depression. An early analysis (Agbayewa, 1986) found that patients with Alzheimer's disease were 2.8 times more likely than nondemented patients to have a history of psychiatric illness, with depression being the most frequent diagnosis. A later meta-analysis by Jorm (2001) suggested that a history of depression nearly doubles the risk of dementia, as found by both case-control studies, RR=2.01, 1.16-3.50, and prospective cohort studies, RR=1.87, 1.09-3.20. Of the 14 studies, seven were able to show a statistically significant association between history of depression and increased dementia risk (Buntinx, Kester, Bergers, & Knottnerus, 1996; Cooper & Holmes, 1998; Devanand et al., 1996; Geerlings et al., 2000; Jorm et al., 1991; Kessing, Mortensen, & Bolwig, 1998; Steffens et al., 1997). The other seven studies did not find a statistically significant relationship (Agbayewa, 1986; Chen, Ganguli, Mulasnt, & DeKosky, 1999; Henderson et al., 1997; Palsson, Aevarsson, & Skoog, 1999; Speck et al., 1995; Wetherell, Gatz, Johansson, & Pedersen, 1999; Zalsman et al., 2000). The meta-analysis yielded a gain in power that separate small studies would obscure.
Many studies have found a higher risk of dementia only for depressive episodes developing for the first time closer in proximity to dementia onset (Berger, Fratiglioni, Forsell, Winblad, & Backman, 1999; Chen et al., 1999; Wetherell et al., 1999; Yaffe et al., 1999). These authors therefore concluded that depression is a prodromal feature of dementia, rather than a risk factor for dementia, suggesting that depression manifests itself as an early symptom of dementia.
Other studies, however, have found the opposite. A meta-analysis by Ownby, Crocco, Acevedo, John, and Loewenstein (2006) used the results from 13 studies (2 case-control, 11 cohort) to analyze the relationship between the interval length between diagnosis of depression and Alzheimer's disease, and the risk for developing Alzheimer's disease. They found that the length of the interval between the diagnoses of depression and Alzheimer's disease was positively associated with an increased risk of developing Alzheimer's disease in later life. They concluded therefore, that depression was a risk factor, rather than a prodrome of dementia. Of these 13 studies, however, only two showed that a history of depression more than 10 years prior to the onset of dementia or Alzheimer's disease was associated with a higher risk for developing dementia or Alzheimer's disease (Palsson, Aevarsson, & Skoog, 1999; Speck et al., 1995). While Palsson, Aevarsson, and Skoog (1999) found that early-onset depression was associated with a higher risk of developing dementia, this finding only held true among individuals with a low level of education. The study by Speck et al. (1995) relied on informants' retrospective reports of depression and specifically looked at risk of developing Alzheimer's disease among those whose depression was not related to loss or grief. They observed an odds ratio of 2.4, 95% CI = 1.2, 4.5, for depression occurring more than 10 years before the onset of dementia, but an odds ratio of 0.9, 95% CI = 0.3, 2.8, for depression occurring within 10 years before dementia onset. Other studies from the meta-analysis did not individually support the conclusion that an early history of depression, rather than a later-life diagnosis, was more associated with the risk of developing dementia.
In a review of research on depression in Alzheimer's disease and other dementias, Boland (2000) concluded that depression arises from anatomic damages that are part of the neuropathological course of dementia. In other words, the depression is caused by the dementia. This neuropathological argument could also account for the appearance of depression shortly before dementia is clinically diagnosed, i.e., as a prodrome of dementia (Palmer et al., 2007). In fact many longitudinal studies have found that a greater number of depressive symptoms at baseline predicts cognitive decline (Berger et al., 1999; Devanand et al., 1996; Yaffe et al., 1999). A recent prospective study, however, found neither an increase in the number of depressive symptoms across a four-year period before the onset of Alzheimer's disease (Wilson, Arnold, Beck, Bienias, & Bennett, 2008) nor an association between psychological distress and the neuropathology associated with dementia (Wilson, Arnold, Schneider, Li, & Bennett, 2007).
The current study tests whether a history of depression is associated with the likelihood of having dementia and if this association is dependent on the timing of an individual's first depressive episode. Using both case-control and co-twin control designs, we tested whether a history of depression significantly predicted the likelihood of having dementia later in life. Additionally, using the case-control design, we examined the difference between age of first depressive episode, as recorded in the national registries of inpatient discharges and psychiatric hospitalizations, and age of dementia onset. In other words, we tested whether those with a greater time difference between onset of registry-identified depression and onset of dementia (or determination that the individual was not demented) were more likely to have dementia compared with those who either had no prior depressive episodes or those who had a first registry-identified depressive episode occurring closer in time to dementia onset. On the one hand, an earlier depression diagnosis implicates depression as a risk factor for dementia. On the other hand, if depression predominantly occurs close in time to dementia onset, then the hypothesis of depression as a prodromal feature of dementia cannot be refuted.
Research Design and Methods
Research Design
Two research designs were employed in this study—the co-twin control method (also called internal control) and the case-control method (also called external control). The co-twin control method is used to examine the importance of a risk factor controlling for those genetic background and early environmental experiences that are shared by twins, through comparing twins with a disorder to their unaffected twin siblings (Lichtenstein et al., 2002). Both monozygotic (MZ) and dizygotic (DZ) twin pairs discordant for dementia were used, and the non-demented co-twin served as the control. In the case-control design, twins diagnosed with dementia are compared with unrelated “external” controls, and the design is in essence a typical case-control design, with the results indicating the association between the exposure, in this case registry-identified depression, and the outcome, dementia. If case-control results indicate a significant association, while co-twin control results show less association or a non-significant result, this pattern would suggest that genetic or early environmental factors mediated the association.
Participants
Participants in the study took part in the Study of Dementia in Swedish Twins, HARMONY (described in Gatz et al., 2005). The acronym is taken from the Swedish words for “health” (Hälsa), “genes” (ARv), “environment” (Miljö), “and” (Och), and “new” (NY). Dementia cases were identified by a two-stage process—a telephone-screening of all members in the Swedish Twin Registry (STR) aged 65 and older, followed by a clinical workup. The STR consists of all Swedish twins born between 1886 and 2001 (Pedersen, Lichtenstein, & Svedberg, 2002). In 1963 and 1967 all like-sexed twin pairs born before 1925 were mailed a questionnaire. Like-sexed twins born 1926 through 1958 were sent a similar questionnaire in 1973. Opposite-sexed pairs were identified for the latter cohort, but not invited to participate. In March 1998, telephone screening was begun of all living twins, same-sex and opposite-sex pairs, regardless of gender composition or vital status of the pair, and regardless of whether the individual had responded to the earlier questionnaires. All members of the STR aged 65 and older at the time of the telephone call were considered part of the HARMONY population (N = 20,269). Screening took place over a period of 2 ½ years. The response rate was 71%.
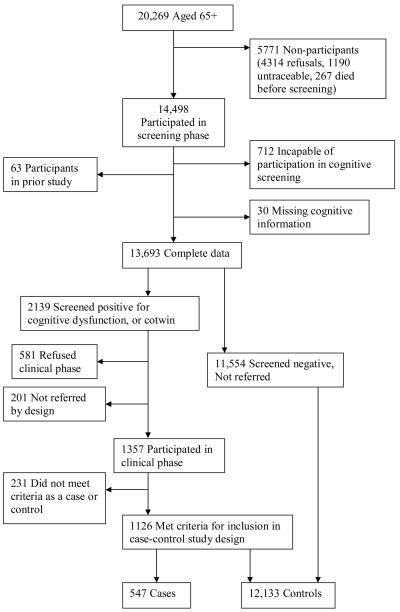
For our analyses, the case-control (external control) sample consisted of all individuals aged 65 and older who completed the telephone screening process and had information about both their lifetime history of depression and their dementia status (N = 12,680). Those who screened positive for cognitive dysfunction during the screening phase and later were diagnosed with dementia during the clinical phase were considered cases (N=547) and those who screened negative for dementia and were either diagnosed as nondemented or not referred for a clinical workup were controls (N=12,133). See Figure 1. The co-twin control (internal control) sample consisted of 146 twin pairs discordant for clinically diagnosed dementia, where there is information about depression for both individuals.
Figure 1.
Case-control sample.
Measures
Dementia Diagnosis
Dementia was diagnosed in two phases, a screening phase and a clinical phase. In the screening phase, the TELE cognitive screening instrument (Gatz, Reynolds, Nikolic, Lowe, & Karel, 1995) was used to screen for cognitive dysfunction. If an individual performed poorly on the TELE, a relative was subsequently interviewed with the Blessed Dementia Rating Scale (BDRS; Blessed, Tomlinson, & Roth, 1968) to determine if the twin's cognitive status affected functioning on a daily basis. When cognitive dysfunction sufficient to interfere with daily functioning was confirmed, the twin was referred to the clinical phase. After the assessment team made a preliminary assessment that a twin was demented, the co-twin was referred for clinical evaluation, regardless of whether the co-twin had screened positive or negative for cognitive dysfunction. The remaining participants did not proceed to the clinical phase either because they screened negative for cognitive dysfunction and thus were not referred (N = 11,554), refused to participate in the clinical phase (N = 581), or by the design consideration that their co-twin had died before age 65, and thus the pair would not be informative (N = 201).
The clinical phase entailed an in-home evaluation by an assessment team comprised of a nurse and a physician. Clinical diagnoses of dementia followed the DSM-IV criteria (American Psychiatric Association, 1994) and the evaluation protocol generally followed the Consortium to Establish a Registry for Alzheimer's disease (CERAD; Morris et al., 1989). The diagnostic procedure of the clinical phase followed three steps. First, based on the in-home clinical workup and review of medical records, the assessment team completed an initial diagnosis. Next, the assessment information was given to a diagnostic review board, comprised of a psychologist and neurologist, who independently constructed a clinical dementia diagnosis, including differential diagnosis of type of dementia. Differential diagnoses were made using the NINCDS/ADRDA criteria for Alzheimer's disease (McKhann et al., 1984) and the NINDS-AIREN criteria for vascular dementia (Roman et al., 1993). Finally, disagreements between board members as well as disagreements between assessment teams and board members were submitted back to the diagnostic review board for resolution, which was determined by consensus. The consensus diagnosis is used in this study.
The HARMONY study used two sources of information to estimate the age of dementia onset: informant reporting and medical records (Fiske, Gatz, Aadnoy, & Pedersen, 2005). During the clinical phase, to assess age of onset and course of dementia, an in-depth, semi-structured interview, designed specifically for the HARMONY study was administered to an informant by trained nurses and physicians. Informants were usually either children or spouses, had a long history with the proband, and were at least weekly in contact with the proband. Information was also extracted from medical records, including the dates of any prior workup conducted to assess dementia, which indicated some level of cognitive impairment. Based on the informant interview and the information from medical records, the assessment team determined the age of onset for dementia.
Lifetime History of Depression
Registry-identified depression information, including occurrence and age of onset, was available from two sources for all individuals in the study: 1) the inpatient hospital discharge registry, and 2) the inpatient psychiatric hospital discharge registry. For cases and co-twin controls who received the in-person visit from the assessment team, two additional sources of depression information were collected: 1) a medical history, and 2) medical records. The latter two sources were not available for external controls as they were collected during the clinical workup phase, so this information was only used for comparing cases with co-twin controls. See Table 1. Depression was considered to be present if indicated by any source and considered to be absent if the earliest onset of depression was after the onset of dementia. Additionally, both hospital discharge registries indicate the specific type of depression diagnosed. Current classification systems endorse the separation of bipolar disorder from major depressive disorder (Gruenberg, Goldstein, & Pincus, 2005). Therefore, in order to provide greater consistency in type of depression being assessed, individuals who only received diagnoses of bipolar disorder depression (case-control analyses: N = 4; co-twin control analyses: N = 0) were not considered to have a lifetime history of depression. However, individuals who had both a past diagnosis of bipolar disorder depression in addition to a separate past diagnosis of depression were considered to have a lifetime history of depression.
Table 1.
Number of Individuals With a History of Depression as Identified by Each Source of Information for Case-Control and Co-Twin Control Study Designs
| Study Design | ||||
|---|---|---|---|---|
| Number of Cases | Case-Control (N = 317) |
Co-Twin Control (N = 40) |
||
| Onset of Depression Data Source |
Recent Onseta |
Early Onsetb |
Recent Onseta |
Early Onsetb |
| Inpatient Discharge Registry | 96 | 188 | 3 | 2 |
| Psychiatric Discharge Registry | 4 | 182 | 1 | 2 |
| Medical History | 12 | 14 | ||
| Medical Records | 21 | 10 | ||
| Total Cases | 96 | 221 | 23 | 17 |
Recent refers to depression onset within 10 years of dementia onset age or censored age.
Early refers to depression onset more than 10 years prior to dementia onset age or censored age.
Inpatient hospital discharge registry
The STR is linked to the national Inpatient Discharge Registry (IDR) that records inpatient discharge diagnoses every time an individual leaves a hospital in Sweden. The IDR was begun in 1964, with coverage increasing each year until 100% coverage throughout Sweden was achieved in 1987, three years after reporting to the IDR became mandatory. Sweden's National Board of Health and Welfare estimates that about 99% of all public hospitalizations are included in the registry (Centre for Epidemiology, 1997). The discharge diagnosis is given in terms of an International Classification of Disease (ICD) code. The ICD-7 was used prior to 1969, the ICD-8 was used from 1969 to 1986, the ICD-9 was used from 1987 to 1996, and beginning in 1997 the ICD-10 was used. A total of 284 participants in this study had at least one depression-related discharge diagnosis in the IDR between the years of 1964 and 2002, a span of 38 years. The most common depression-related discharge diagnosis was depressive neurosis, followed by depression NOS and recurrent depressive disorder.1
Psychiatric hospital discharge registry
The STR has also been linked to a national registry of inpatient psychiatric hospital admissions between 1967 and 1983. For each person entered in this registry, there is a record of the discharge diagnosis and the date of hospitalization. All discharge diagnoses are given in terms of an ICD-8 diagnosis. A total of 186 study participants had a depression-related discharge diagnosis recorded in the psychiatric hospital discharge registry, with a large majority of the diagnoses being depressive neurosis. Of these, 153 appeared in both registries, while 33 were in the psychiatric registry only.
Thus, a total of 317 individuals had at least one depression-related diagnosis. This indicates that the morbid risk for being hospitalized for depression in this sample is 2.5%, which is not significantly different from the morbid risk estimation (2.96%) for the entire STR population (Kendler, Pedersen, Johnson, Neale, & Mathe, 1993).
Medical history for cases and co-twin controls (cases and internal controls only)
Medical history was collected during the clinical evaluation. Information was obtained from an informant for each twin, in order to avoid biases that might come from comparing self-report for controls to proxy-report for cases. Twins were never informants for each other, and different informants were sought for each twin, so the same informant was not reporting on both members of a pair. During the administration of the medical history interview, the assessment team asked whether the proband had any history of depression. They recorded the date of onset for any episode that would be regarded as major depressive disorder, dysthymia, “depressive neurosis,” or reactive depression. Also collected during the medical history interview was information regarding the past and current use of medication, including antidepressants. If the medical history indicated any depressive disorder or the use of antidepressants, the participant was considered to have a history of depression. The first depressive episode according to the medical history was determined by taking the earliest date recorded for either the onset of a depressive disorder or the start date for initiating the use of an antidepressant medication treatment.
Medical records for cases and co-twin controls (cases and internal controls only)
During the clinical evaluation phase, copies of medical records were ordered from each twin's local healthcare clinic. Because of Sweden's system of universal healthcare, medical records were available for all twins included in the co-twin control analyses. The records were coded by the physicians on the assessment team, who were instructed to code only from diagnosis and not infer from symptoms. Records typically go back approximately ten years prior to the onset of symptoms of cognitive dysfunction. In addition to listing whether there had ever been a diagnosis for depression, the coder also listed the onset and dates of the depressive illnesses and information regarding the past or current use of antidepressant medication, including the date of first recorded usage. The medical records and the medical history also contained information on whether or not a twin had received electroconvulsive therapy (ECT). However, none of the twins included in the co-twin control analyses had received ECT prior to the age of dementia onset or censored age. The first depressive episode according to the medical records was determined by taking the earliest date recorded for either onset of depression or the start date for the use of antidepressant medication.
Years Since Age of Onset of Depression
In the case-control analyses, a case was regarded as having a history of depression if the registry-identified depression occurred prior to dementia onset. In order to avoid a potential bias, wherein controls had a longer time frame in which they could be considered to have a history of registry-identified depression, we created a censored age for controls to establish a comparable timeframe for manifesting a depressive episode that required hospitalization. Because the median length of time between age of onset of dementia and study age for cases was four years, we censored registry-identified depression in the control group at four years prior to their study age Thus, for cases, years since first episode of registry-identified depression was determined by discerning the age at earliest recorded hospitalization for depression and calculating the number of years from that age until the age of dementia onset. For external controls, years since first registry-identified depression was calculated as the difference between the age at first registry-identified depression and four years prior to study age, at which age registry-identified depression was censored.
For the co-twin control analysis, depression for cases had to occur before the age of dementia onset. The co-twin control was considered to have a history of depression if depression occurred prior to the proband's dementia onset. Thus, for the co-twin control analysis, the number of years since the first episode of depression for the co-twin controls was determined by discerning the earliest reported occurrence of depression and calculating the number of years from that date until the date of the demented twin's age of dementia onset. If different sources indicated different dates or ages for the earliest reported occurrence of depression, the earliest date or age was used.
Combining Sources of Depression for Co-Twin Control Analyses
Of the 40 participants with a history of depression prior to dementia onset or prior to the co-twin's onset of dementia, only five had ever been hospitalized for depression. Three of these five individuals also had evidence of a history of depression in their medical records and medical history. Among the remaining 35 participants with a history of depression but not hospitalization, four had evidence of depression only in their medical history, 12 had evidence of depression only in their medical records, and 19 had evidence of depression history in both their medical records and medical history. In most of the cases where one source indicated a history of depression, but the medical records did not, it was because the depression occurred prior to earliest available medical record. These numbers are consistent with the fact that most people who have a history of depression are not hospitalized for the disorder, and therefore will only have evidence of a history of depression in a source that is not limited to information from hospitalization records.
There were 28 people who had data in either their medical records or medical history indicating that they had taken antidepressants prior to age of dementia onset or censored age. Of these individuals, everyone except two participants had another source (e.g. the IDR, medical history, medical records) corroborating a diagnosis of depression.
Comparability between medical history and medical records
Among all individuals in the co-twin control analysis, medical history and medical records showed a moderate level of agreement (kappa value = 0.47), with 70.6% of the disagreement being instances where the medical records indicated depression, but the medical history did not. When stratified by dementia status, kappa values indicated that the agreement between sources was higher for the controls (kappa value = 0.60) than for the probands (kappa value = 0.37). For the controls, there was no difference between which source was more likely to indicate depression when there was disagreement between sources. However, for the probands, 75.7% of the disagreements between sources were instances where the medical records indicated depression, but the medical history did not. This suggests that for dementia cases, depression may have been underreported in the informant-reported medical history.
Sources of information for probands and co-twin controls
Another consideration when combining sources of information regarding history of depression was to ensure that probands and controls within a pair had the same sources of information. For 39 of the 146 twin pairs, informant-reported medical histories were conducted for the proband, but not for the co-twin control. Though self-reported medical histories were available for these 39 co-twin controls, self-reported data were not used in this study. To avert the potential information bias, for these pairs depression history was coded using only the inpatient discharge registries and the medical records, both of which were available for both proband and co-twin control.
Covariates
Potential confounders included age, gender, zygosity, and number of years of education completed. All of these variables were primarily measured during the telephone screening phase, which began in March 1998 (Lichtenstein et al., 2002). These data were supplemented with information from other sources (i.e. other studies based on the STR) if available. Zygosity was determined by a developed algorithm that was based on the answers to questions such as, “During childhood, were you and your twin partner as like as ‘two peas in a pod’ or not more alike than siblings in general” and “How often did strangers have difficulty in distinguishing between you and your twin partner when you were children.” The algorithm was validated by testing 13 DNA markers on 199 adult pairs, proving to be correct in 99% of the pairs. Only one pair identified as MZ by the algorithm was misclassified (Lichtenstein et al., 2002). In our analyses we collapsed zygosity into two levels: MZ and not MZ (same sex or opposite sex dizygotic or indeterminate). Information regarding years of education was missing for 164 individuals (41 cases; 123 controls). Missing values for education were replaced by estimated values using a regression model, which imputed years of education as predicted by gender and year of birth.
Analyses
Case-Control (External Control) Analyses
Logistic regression models (LOGISTIC procedure in SAS; version 8.2; SAS Institute, Inc., Cary, North Carolina) were used to assess the association between history of registry-identified depression and presence of dementia. Because members of twin pairs were included in the analyses in the same way as single participants, confidence intervals were corrected using robust standard errors for having two members from the same family included (Moradi et al., 2002).
Additionally we conducted analyses comparing recent onset of registry-identified depression to early onset of registry-identified depression, using 10 years to differentiate between early and recent onset to parallel previous studies (Speck et al., 1995). We also performed a survival analysis using a Cox regression model (PHREG procedure in SAS) to study the timing of the onset of registry-identified depression as a continuous variable. Though analysis was also performed using a logistic regression model, the Cox regression model was chosen as the superior model due to its simplified assumptions and thus, greater power (Singer & Willett, 2003). Finally we conducted analyses to assess the association between a history of registry-identified depression and risk of Alzheimer's disease. Preliminary analyses indicated that age, gender, and education differed significantly by dementia status. Therefore, we included age, education, gender, and zygosity as covariates in all models.
Co-Twin Control (Internal Control) Analyses
Conditional logistic regression models (PHREG procedure in SAS) were used to investigate the association between history of depression and risk of dementia in twin pairs discordant for dementia. We also examined the association between history of depression and Alzheimer's disease in pairs discordant for Alzheimer's disease. Using conditional logistic regression with twin pair as the stratum compares each dementia case to his or her co-twin. Gender and level of education were considered as possible covariates and were included in the final conditional logistic regression model. Finally, in order to estimate whether there was differential misclassification by source of information about depression, we re-ran these models using each depression information source independently.
Results
Characteristics of Case-Control Sample
Dementia cases
There were 547 individuals diagnosed with dementia who also had information about their lifetime history of registry-identified depression. Sample demographics are shown in Table 2. Alzheimer's disease was the most common diagnosis (62%), followed by vascular dementia (23.5%), dementia NOS (7%), and secondary dementia (4%). The remaining 3.5% of the cases had diagnoses of frontotemporal dementia, dementia with Lewy bodies, or alcoholic dementia. Twenty-one individuals with dementia had records in the inpatient discharge registry indicating that they had been hospitalized for depression. The average age at first hospitalization for depression was 66.6 (SD = 11.8). The mean age for the first episode of registry-identified depression was 9.7 years prior to the onset of the dementia (SD = 9.6), and ranged from 31.0 years prior to dementia onset to the same year as dementia onset. Finally the mean number of separate hospitalizations for separate episodes of depression among this group was 2.9 (SD = 4.4).
Table 2.
Sample Demographics for Case-Control Analysis Covariates
| Dementia | No Dementia | |
|---|---|---|
| N | 547 | 12,133 |
| Age at Assessment for Dementia (SD)* | 77.0 (8.1) | 72.8 (6.2) |
| Years of Education (SD)* | 7.3 (2.2) | 8.7 (3.0) |
| % Female* | 68.0 % | 55.7 % |
| % MZ Twins* | 29.1% | 23.5% |
p < .01.
Controls
Among the 12,133 participants who were determined to be non-demented, 296 individuals had a history of registry-identified depression. The average age of first hospitalization for depression among controls was 52.4 years (SD = 9.5). The mean for the earliest hospitalization of depression was 15.4 years prior to censored age (SD = 7.6), and ranged from 30.0 years prior to censored age to the same year as censored age. Finally, the mean number of separate hospitalizations for depression among this group was 4.1 (SD = 5.3).
Bivariate analyses indicated that age at first hospitalization was significantly earlier for the controls compared with the cases (p < .0001). Additionally, the time between the first reported episode of registry-identified depression and the censored age for the controls was significantly longer than the time between the first reported episode of registry-identified depression and onset of dementia for cases (p = .0011). In other words controls, compared with cases, had an earlier onset of registry-identified depression. The mean number of separate hospitalizations for depression and the type of registry-identified depression diagnosis, however, did not differ in cases versus controls.
Case-Control Analyses
An initial logistic regression model without covariates indicated that individuals with a history of registry-identified depression were 1.60 times more likely to have dementia than individuals without a history of registry-identified depression, CI = 1.02, 2.51. After controlling for gender, age, education, and zygosity in our multivariate model, the effect remained significant, OR = 1.72, CI = 1.07, 2.76. See Table 3. To determine whether timing of registry-identified depression acted as an effect modifier, we ran a logistic regression model comparing the reference group (individuals with no history of registry-identified depression) with individuals having an onset of registry-identified depression within the previous 10 years and individuals with an onset of registry-identified depression more than 10 years prior to either the onset of dementia or censored age. These analyses indicated that individuals with a recent onset of registry-identified depression (within the past 10 years) were 3.87 times more likely than those with no registry-identified depression to have dementia, CI = 2.10, 7.14. In contrast, an earlier onset of registry-identified depression (more than 10 years prior to age of dementia onset of censored age) was not associated with an increased risk of dementia, OR = 0.90, CI = 0.44, 1.85.
Table 3.
Risk for Dementia Stratified by Early and Recent Depression Onset for Case-Control Analysis
| Cases | Controls | |||||
|---|---|---|---|---|---|---|
| Depression | Depression | |||||
| Yes | No | Yes | No | OR | 95% CI | |
| All Depression vs. No Depression | 21 | 526 | 296 | 11,837 | 1.72 | 1.07 – 2.76* |
| Recenta Onset of Depression vs. No Depression |
13 | 526 | 83 | 11,837 | 3.87 | 2.10 – 7.14** |
| Earlyb Onset of Depression vs. No Depression |
8 | 526 | 213 | 11,837 | 0.90 | 0.44 – 1.85 |
Note. Covariates for all case-control group models included age, education, gender, and zygosity. OR = odds ratio. CI = confidence interval.
Recent refers to depression onset within 10 years of dementia onset age or censored age.
Early refers to depression onset more than 10 years prior to dementia onset age or censored age.
p < .05.
p < .0001.
We used the same multivariate models to test whether the effect remained if the group with dementia was narrowed to include only individuals with AD. As shown in Table 4, the model with covariates showed no association between lifetime history of registry-identified depression and a diagnosis of AD, OR = 1.20, CI = 0.63, 2.30. We then compared the reference group (with no history of registry-identified depression) to individuals having an onset of registry-identified depression within the previous 10 years and to individuals having an onset of registry-identified depression more than 10 years prior to either the onset of AD or censored age. We found that the individuals with recent onset of registry-identified depression were 2.62 times more likely that those without a history of registry-identified depression to be diagnosed with AD, CI = 1.12, 6.17. In contrast, AD was not associated with an earlier history of registry-identified depressive episodes, OR = 0.66, CI = 0.24, 1.81.
Table 4.
Risk for AD Stratified by Early and Recent Depression Onset for Case-Control Analysis
| AD Only | Controls | |||||
|---|---|---|---|---|---|---|
| Depression | Depression | |||||
| Yes | No | Yes | No | OR | 95% CI | |
| All Depression vs. No Depression | 10 | 360 | 296 | 11,821 | 1.20 | 0.63, 2.30 |
| Recenta Onset of Depression vs. No Depression |
6 | 360 | 83 | 11,821 | 2.62 | 1.12, 6.17* |
| Earlyb Onset of Depression vs. No Depression |
4 | 360 | 213 | 11,821 | 0.66 | 0.24, 1.81 |
Note. OR = odds ratio. CI = confidence interval.
Recent refers to depression onset within 10 years of dementia onset age or censored age.
Early refers to depression onset more than 10 years prior to dementia onset age or censored age.
p < .05.
In order to analyze the timing of the onset of registry-identified depression as a continuous construct, survival analysis using a Cox regression model was used. Controlling for age, education, gender, and zygosity, we found that each one-year increase in the length of time between first registry-identified depressive episode and age of dementia onset or censored age decreased the hazard of having dementia by 8.4% (p = .0031).2
In a post-hoc analysis we sought to determine whether the estimated age of dementia onset was significantly earlier in cases with a history of registry-identified depression compared to cases without a history of registry-identified depression. We found that there was no difference between cases with and cases without a history of registry-identified depression in the estimated age of dementia onset (p = .6880), suggesting that registry-identified depression is not associated with an earlier age of dementia onset.
A series of post-hoc analyses were run to determine whether including individuals with bipolar disorder and psychotic disorders in addition to depression confounded our results. There were 66 individuals with histories of registry-identified depression as well as bipolar disorder and/or psychosis (24 with bipolar disorder, 30 with psychosis, and 12 with both bipolar disorder and psychosis). When the 36 individuals with both registry-identified depression and bipolar disorder were recoded as nondepressed, our results were not changed. Individuals with a later onset of registry-identified depression were 3.91, CI = 2.07, 7.40 times more likely than those without a history of registry-identified depression to be diagnosed with dementia, while an earlier onset of registry-identified depression was not associated with dementia, OR = 0.78, CI = 0.34, 1.77. Findings were similar when people with registry-identified psychotic disorders in addition to registry-identified depression were recoded as nondepressed (N = 42, OR = 3.65, CI = 1.82, 7.30), and when people with a psychotic disorder and/or bipolar disorder as well as registry-identified depression were recoded as nondepressed (N = 66, OR = 3.81, CI = 1.90, 7.66). As the strength of our findings did not change, it is unlikely that people with diagnoses of bipolar disorder or psychotic disorders as well as depression confounded our results.
Co-Twin Control Analysis
There were 146 pairs of twins discordant for dementia (Table 5). MZ twins made up 22.6% of the pairs, DZ same sex twins made up 42.4% of the pairs, DZ opposite sex twins made up 33.6% of the pairs, and 1.4% of the pairs were of indeterminate zygosity. Among the probands, the average age of first episode of depression was 62.9 years (SD = 14.3) and the average age among the controls for the first episode of depression was 56.6 (SD = 16.9). Finally, the mean for the first episode of depression was 11.6 years (SD = 13.5) prior to dementia onset for the probands and 20.6 years (SD = 15.0) prior to the proband's onset of dementia for the controls.
Table 5.
Sample Demographics for Co-Twin Control Covariates
| Dementia | No Dementia | |
|---|---|---|
| N | 146 | 146 |
| % Female | 59.6% | 58.9% |
| Years of Education (SD | 7.9 (2.5) | 8.2 (2.9) |
p < .01.
There were 27 twins with a diagnosis of dementia who also had a history of depression and 13 co-twin controls with a history of depression. Without controlling for any covariates, an initial conditional logistic regression model indicated that individuals with a history of depression were 3.00 times more likely than their co-twin to have dementia, CI = 1.27, 7.06. After controlling for gender and education, we found that individuals with a history of depression were 2.98 times more likely than their co-twin to have dementia, CI = 1.26, 7.03 (Table 6).
Table 6.
Risk for Dementia and AD for Co-twin Control Analysis
| History of Depression | |||||
|---|---|---|---|---|---|
| Both Twins |
Case Only |
Co-twin Control Only |
OR | 95% CI | |
| Depression vs. No Depression | 6 | 21 | 7 | 2.97 | 1.26, 7.03* |
| Depression vs. No Depression (AD Only) |
4 | 7 | 3 | 2.33 | 0.60, 9.11 |
Note. Covariates for all co-twin control models included gender, education, and number of sources of depression information. OR = odds ratio. CI = confidence interval.
p < .01.
We used the same conditional logistic regression model to test whether the effect remained if the group with dementia was narrowed to only include individuals with AD. When we ran a model to determine if ever having a history of depression was associated with the likelihood of having AD, we found that there was not a significant association between a history of depression and the risk of AD, OR = 2.33, CI = 0.60, 9.11.
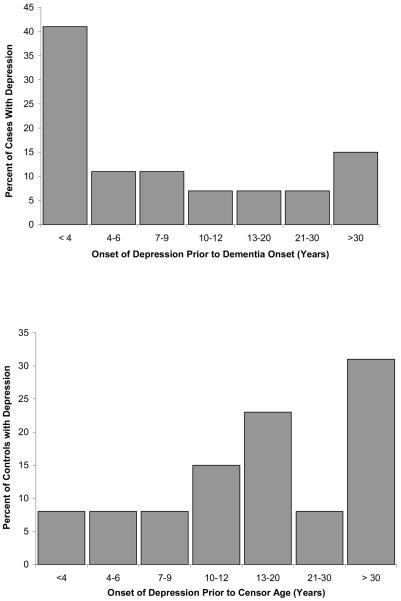
It was not feasible to use a survival approach in analyzing the co-twin control sample. For descriptive purposes, Figure 2 shows the distribution of age of onset of depression in cases and in controls. We found that 66.7% of the probands with a history of depression had their first episode of depression sometime during the 10 years preceding dementia onset, and 40.0% had their first episode within five years prior to dementia onset, with the median being 6 years prior to dementia onset. Among the controls with a history of depression, 38.6% had their first episode of depression within the 10 years preceding their twin partner's dementia onset, and 15.4% had their first episode of depression within five years prior to the twin partner's dementia onset, with the median being 17 years prior to dementia onset.
Figure 2.
Years since first episode of depression for cases and controls.
In a post-hoc analysis, we re-ran our co-twin control conditional logistic regression models using each source of depression data independently as a measure of depression. The association remained statistically significant for medical records OR=2.54, CI = 1.12, 5.84. While the point estimates for medical histories, OR=2.50, CI = 0.97, 6.46 and hospital discharge registries, OR=3.37, CI = 0.35, 32.24 were consistent with our previous analyses, the confidence interval widened and the association was no longer significant. Also, there were two individuals who were considered to have a history of depression solely on the basis of having used antidepressant medication prior to age of dementia onset or censored age. Thus it is possible that these individuals took these medications for reasons other than depression. However, when these individuals were re-coded as non-depressed, the findings remained significant. Finally, we recoded as non-depressed the four individuals whose history of depression was identified only through the informant-reported medical history. When we re-ran the analysis, we found that the association remained significant, OR = 2.79, CI = 1.23, 6.33. These post-hoc results suggest that our findings were not dependent upon which source was used as a measure for depression.
Discussion
This study provides further evidence for a relationship between dementia and depression. Specifically we found that individuals with a history of registry-identified depression were more than one and a half times more likely to have dementia than those with no history of depression. In the co-twin control analysis, which controlled for genetic and early environmental risk factors shared by twins, we found that twins with a history of depression were three times more likely to have dementia compared to their co-twin. Thus, the finding of increased likelihood of dementia associated with depression is not explained by shared genes or shared early life influences. Furthermore the association between dementia and registry-identified depression in the case control analysis was strengthened when the timing of onset of registry-identified depression was considered. Registry-identified depression onset occurring more than 10 years before dementia onset or censored age was no longer associated with an increased likelihood of dementia, but registry-identified depression onset occurring within 10 years of dementia onset or censored age was associated with a nearly four times greater likelihood of having dementia. We also found that registry-identified depression occurring within 10 years of dementia onset or censored age was associated with a more than two and a half times greater likelihood of having AD. Rather than being a risk factor for dementia, these findings suggest that for many individuals late-life depression may be a prodrome of dementia.
The hypothesis that depression is a prodromal feature of dementia is further supported by our findings from the case-control group, in which controls had a much earlier average age of first registry-identified depressive episode (approximately 20 years before censored age) compared with the cases (approximately 11 years before dementia onset). Furthermore as indicated by our proportional hazard regression model, with each one-year increase in registry-identified depression onset prior to dementia onset or censored age, the likelihood of having dementia decreased by eight percent. Therefore, individuals having an onset of registry-identified depression less than five years prior to dementia onset or censored age have an even greater likelihood of having dementia than those having an onset of registry-identified depression between five and 10 years prior to dementia onset or censored age.
Our findings are consistent with much of the prior literature (e.g., Steffens et al., 1997), although at variance with Wilson et al. (2008), who found that the number of depressive symptoms do not increase in the four years preceding the onset of AD. Also contrary to our findings, Speck et al. (1995) found that an early, rather than a recent, history of depression was more associated with the risk of developing AD. In their study, however, Speck et al. (1995) relied solely on retrospective interviews with informants to determine the proband's history of depression and excluded depression that was attributed to grief or loss.
In Jorm's meta-analysis (2001), he suggested that there were three hypotheses that may explain the association between depression and risk of dementia: 1) depression is a prodrome of dementia, 2) depression and dementia are independent illnesses, but depression affects the threshold for developing dementia, or in other words the cognitive deficits caused by depression might lead to an earlier diagnosis for dementia and 3) depression causes damage to the hippocampus, leading to dementia, via a glucocorticoid cascade. While these explanations are not mutually exclusive, they do lead to some different predictions about the relative timing of onset of depression and onset of dementia. Among these hypothesized explanations, our results most strongly support the prodromal hypothesis. Because the age of dementia onset for cases did not differ significantly by depression status, it is less likely that our results indicate that depression affects the threshold for developing dementia, leading to an earlier diagnosis.
Jorm (2001) and others (e.g. Sheline, Gado, & Kraemer, 2003) have suggested that dementia might be the result of damage to the hippocampus through a glucocorticoid cascade brought on by depression. Our results are not consistent with this hypothesis, for two reasons specifically. First, controls had a longer standing history of registry-identified depression, including on average, more hospitalizations for depression. Secondly, although we did not specifically test for varying levels of depression severity, depression was measured solely by hospitalization in the case-control analyses, and depression that requires hospitalization is no doubt severe. Therefore, there is no reason to think that the depression in the controls was less severe and caused less hippocampal damage.
However, depression could be due to changes caused by dementia, rather than the other way around. One study by Almeida, Burton, Ferrier, McKeith, and O'Brien (2003) found that participants with late-onset depression (onset after age 60) had more frontal lobe atrophy compared to participants with early-onset depression (before age 60) and controls. These findings are consistent with the types of atrophy observed in patients with AD (van der Flier et al., 2002). Another study found that older adult patients with late-onset depression had significantly more white matter hyperintensities than older adult patients with early-onset depression or no depression (Lesser et al., 1996). As changes in white matter hyperintensities are also associated with dementia, particularly vascular dementia (Capizzano et al., 2004), these studies indicate a neurobiological basis for suggesting that depression may be a prodrome of dementia.
It is important to note, however, that if depression is a prodromal sign of dementia, then there should be a clinical-pathological correlation as well as an association between advancing pathology related to dementia and number of depressive symptoms (Wilson et al., 2007). Two recent prospective studies (Wilson et al., 2007; Wilson et al, 2008) found an association between chronic distress and risk of dementia, but failed to find either of these related associations. They concluded therefore that psychological distress is associated with other brain changes that are not directly related to dementia pathology.
Strengths
This study has several advantages over past investigations. First, because in the co-twin control design each person with a dementia diagnosis was paired with a non-demented twin, this study controls for some genetic and early environmental risk factors. Also, sample sizes for previous twin studies have been substantially smaller than in the current study. This study additionally has the advantage of having several sources from which to gain information regarding the history of depression for each person, including sources that do not rely on the self-reports of participants.
Limitations
Inherent in any cross-sectional study that relies on retrospective exposure data is the potential for measurement error, which can lead to information bias (Morgenstern & Thomas, 1993). In this study, however, the data on depression came from sources that did not rely on self-report, and therefore were less likely to contribute to information bias. Indeed all of our depression data for the case-control analyses came from the hospital discharge registries, which were created at the time of the hospitalization and not retrospectively. Thus, the differences in the associations based on the timing of depression are unlikely due to forward telescoping bias. For hospital discharge data, the potential bias lies in changing patterns of hospitalization. The informant-reported medical histories used in the co-twin control analyses retrospectively ascertained depression over several decades. These reports, therefore, are subject to forward telescoping bias. However, telescoping bias would not be relevant in these analyses because we did not statistically test whether the association between depression and dementia was dependent upon the timing of the first depressive episode. In our co-twin control group, we also tried to minimize the risk of information bias by using several sources of information regarding depression history. For example, all except four medical histories that indicated depression had other sources that corroborated the informant-reported history of depression. When these individuals were subsequently recoded as non-depressed in our sensitivity analysis, our findings remained both robust and significant.
Because the measures of depression were dichotomous, it was infeasible to assess the effects of depression severity. One approximation of severity could be inferred from whether or not an individual was hospitalized for their depression, as it is likely that only severe depression would necessitate inpatient care. The case-control measure of depression was based solely on those who were hospitalized for depression, which yielded a lifetime prevalence of registry-identified depression of only 3.8% in cases and 2.4% in controls. As the lifetime prevalence of depression among Swedes is 13.2% (Kendler et al., 1993), our measure clearly results in an underestimation of lifetime depression. It may be difficult to accurately estimate the association between rare and often under-diagnosed conditions. Thus it is possible that our findings would not hold if the full spectrum of depression (including depression that did not require hospitalization) had been measured.
In the co-twin control analyses, depression was assessed by medical records (from outpatient care settings) and medical history, in addition to hospital discharge records. In these analyses, depression continued to be associated with a higher risk of dementia. Thus, our results do indicate at least some spectrum of depression severity that is associated with a higher risk of dementia.
Dementia has an insidious onset, which leads to imprecision in determining exact age of onset. Additionally, symptoms of depression can often mimic symptoms of dementia. Whereas depression earlier in life would not be confused with dementia, early symptoms of dementia in older adults could be misdiagnosed as depression. Therefore, the association between recent history of depression and dementia may be an artifact due to diagnostic error. Furthermore, it is possible that the estimated age of dementia onset for those with depression was earlier than it would have been had they not been depressed. However, we did not find any difference between cases with and cases without depression in age of dementia onset.
A limitation inherent in any study involving proxy reporting is that an informant may not always be aware of the proband's full medical history. In fact, we did find that depression was more likely to have been reported in the medical records, rather than in the medical history, for the individuals diagnosed with dementia. For this reason, we only included medical history as a source of depression information in twin pairs where both proband and co-twin control had informant-reported medical histories.
It is also possible that antidepressant treatment may mediate the relationship between history of depression and risk of dementia. In fact, animal studies have suggested that chronic treatment with serotonergic antidepressants prevents (Shakesby, Anwyl, & Rowan, 2002), and, with enough time, even reverses (Sairanen, O'Leary, Knuuttila, & Castren, 2007), stress-induced hippocampal changes and disruption to the neural pathways implicated in depression, by increasing synaptic plasticity and connectivity in the hippocampus and prefrontal cortex. Thus, treated depression may not be a risk marker (Kazdin et al., 1997) for dementia. Finally, it is possible that the association we observed between dementia and depression is not specific to a diagnosis of depression. Future studies need to examine the impact of psychiatric history in general, or account for co-morbid disorders such as anxiety.
Conclusions
Our results are consistent with previous findings that a later-life diagnosis of depression is associated with an increased likelihood of having dementia, suggesting that late-life depression is a prodromal feature of dementia. At the same time, it is possible that both prodromal and risk factor associations may be differentially relevant for different individuals. The association between depression and dementia was similar in both case-control and co-twin control analyses. Therefore, confounding by genetic or shared early environmental influences is unlikely. On a clinical level, late-life depression should be carefully assessed and treated. Treatment is not only important for attending to the depressive symptoms per se, but it may also be important in the improvement of associated symptoms, such as fatigue, lack of concentration, and memory problems, that may exacerbate cognitive impairment. Clinicians should keep this association in mind when assessing for depression as well as dementia.
Acknowledgments
Research and preparation of this article was funded by NIA grants R01 AG08724 and T32 AG00037, NIH grant F31 MH078331, and a Zenith Award (ZEN-02-3895) from the Alzheimer's Association.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/pag.
Discharge diagnoses in both the IDR and the psychiatric hospital discharge registry that were regarded as not depression-related included bipolar affective disorder and manic-depressive reaction, manic or unspecified type, schizoaffective disorder, and unspecified mood disorders.
A logistic regression model yielded similar results, indicating that each one-year increase in the length of time between first depressive episode and age of dementia onset or censored age decreased the likelihood of having dementia by 8.6% (p = .0032).
Contributor Information
Jessica A. Brommelhoff, University of Southern California
Margaret Gatz, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Sweden, and Department of Psychology, University of Southern California.
Boo Johansson, Göteborg University, Sweden.
John J. McArdle, University of Southern California
Laura Fratiglioni, Aging Research Center, Karolinska Institutet, Sweden.
Nancy L. Pedersen, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Sweden, and Department of Psychology, University of Southern California
References
- Agbayewa MO. Earlier psychiatric morbidity in patients with Alzheimer's disease. Journal of the American Geriatrics Society. 1986;34:561–564. doi: 10.1111/j.1532-5415.1986.tb05759.x. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Burton EJ, Ferrier N, McKeith G, O'Brien JT. Depression with late onset is associated with right frontal lobe atrophy. Psychological Medicine. 2003;33:675–681. doi: 10.1017/s003329170300758x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. DSM-IV. Fourth ed. American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- Bassuck SS, Berkman LF, Wypij D. Depressive symptomatology and incident cognitive decline in an elderly community sample. Archives of General Psychiatry. 1998;55:1073–1081. doi: 10.1001/archpsyc.55.12.1073. [DOI] [PubMed] [Google Scholar]
- Berger AK, Fratiglioni L, Forsell Y, Winblad B, Backman L. The occurrence of depressive symptoms in the preclinical phase of AD: A population-based study. Neurology. 1999;53:1998–2002. doi: 10.1212/wnl.53.9.1998. [DOI] [PubMed] [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. British Journal of Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Bolland RJ. Depression in Alzhiemer's disease and other dementias. Current Psychiatry Reports. 2000;2:427–433. doi: 10.1007/s11920-000-0028-0. [DOI] [PubMed] [Google Scholar]
- Buntinx F, Kester A, Bergers J, Knottnerus JA. Is depression in elderly people followed by dementia? A retrospective cohort study based in general practice. Age and Ageing. 1996;25:231–233. doi: 10.1093/ageing/25.3.231. [DOI] [PubMed] [Google Scholar]
- Capizzano AA, Acion L, Bekinschtein T, Furman M, Gomila H, Martinez A, et al. White matter hyperintensities are significantly associated with cortical atrophy in Alzheimer's disease. Journal of Neurology, Neurosurgery & Psychiatry. 2004;75:822–827. doi: 10.1136/jnnp.2003.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centre for Epidemiology, National Board of Health and Welfare . The Swedish Hospital Discharge Register 1987-1995. Author; Stockholm: 1997. [Google Scholar]
- Chen P, Ganguli M, Mulsant BH, DeKosky ST. The temporal relationship between depressive symptoms and dementia. Archives of General Psychiatry. 1999;56:261–266. doi: 10.1001/archpsyc.56.3.261. [DOI] [PubMed] [Google Scholar]
- Cooper B, Holmes C. Previous psychiatric history as a risk factor for late-life dementia: A population-based case-control study. Age and Ageing. 1998;27:181–188. doi: 10.1093/ageing/27.2.181. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Sano M, Tang M-X, Taylor S, Gurland BJ, Wilder D, et al. Depressed mood and the incidence of Alzheimer's disease in the elderly living in the community. Archives of General Psychiatry. 1996;53:175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- Fiske A, Gatz M, Aadnoy B, Pedersen NL. Assessing age of dementia onset: Validity of informant reports. Alzheimer's Disease and Associated Disorders. 2005;19:128–134. doi: 10.1097/01.wad.0000174947.76968.74. [DOI] [PubMed] [Google Scholar]
- Gatz M, Fratiglioni L, Johansson B, Berg S, Mortimer JA, Reynolds CA, et al. Complete ascertainment of dementia in the Swedish Twin Registry: The HARMONY study. Neurobiology of Aging. 2005;26:439–447. doi: 10.1016/j.neurobiolaging.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Gatz M, Reynolds C, Nikolic J, Lowe B, Karel M. An empirical test of telephone screening to identify potential dementia cases. International Psychogeriatrics. 1995;9:429–437. doi: 10.1017/s1041610295002171. [DOI] [PubMed] [Google Scholar]
- Geerlings MI, Schoevers RA, Beekman AT, Jonker C, Deeg DJ, Schmand B, et al. Depression and risk of cognitive decline and Alzheimer's disease. British Journal of Psychiatry. 2000;176:568–575. doi: 10.1192/bjp.176.6.568. [DOI] [PubMed] [Google Scholar]
- Gruenberg AM, Goldstein RD, Pincus HA. Classification of depression: Research and diagnostic criteria: DSM-IV and ICD-10. In: Licinio J, Wong M-L, editors. Biology of depression. From novel insights to therapeutic strategies. Wiley-VCH Verlag GmbH and Company; Weinheim, Germany: 2005. pp. 1–12. [Google Scholar]
- Henderson AS, Korten AE, Jacomb PA, Mackinnon AJ, Jorm AF, Christensen H, et al. The course of depression in the elderly: A longitudinal community-based study in Australia. Psychological Medicine. 1997;27:119–129. doi: 10.1017/s0033291796004199. [DOI] [PubMed] [Google Scholar]
- Jorm AF. History of depression as a risk factor for dementia: An updated review. Australian and New Zealand Journal of Psychiatry. 2001;35:776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- Jorm AF, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, et al. Psychiatric history and related exposures as risk factors for Alzheimer's disease: A collaborative re-analysis of case-control studies. International Journal of Epidemiology. 1991;20:S43–S47. doi: 10.1093/ije/20.supplement_2.s43. [DOI] [PubMed] [Google Scholar]
- Kazdin AE, Kraemer HC, Kessler RC, Kupfer DJ, Offord DR. Contributions of risk-factor research to developmental psychopathology. Clinical Psychology Review. 1997;17:375–406. doi: 10.1016/s0272-7358(97)00012-3. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Pedersen N, Johnson L, Neale MC, Mathe AA. A pilot Swedish twin study of affective illness, including hospital- and population-ascertained subsamples. Archives of General Psychiatry. 1993;50:699–700. doi: 10.1001/archpsyc.1993.01820210033004. [DOI] [PubMed] [Google Scholar]
- Kessing LV, Mortensen PB, Bolwig TG. Clinical consequences of sensitization in affective disorder: A case register study. Journal of Affective Disorders. 1998;47:41–47. doi: 10.1016/s0165-0327(97)00128-6. [DOI] [PubMed] [Google Scholar]
- Lesser IM, Boone KB, Mehringer CM, Wohl MA, Miller BL, Berman NG. Cognition and white matter hyperintensities in older depressed patients. American Journal of Psychiatry. 1996;153:1280–1287. doi: 10.1176/ajp.153.10.1280. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, DeFaire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: A unique resource for clinical, epidemiological and genetic studies. Journal of Internal Medicine. 2002;252:184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA workgroup under the auspices of Department of Health and Human Services task force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Moradi T, Adami H-O, Ekrom A, Wendren, Terry P, Floderus B, et al. Physical activity and risk for breast cancer: A prospective cohort study among Swedish twins. International Journal of Cancer. 2002;100:76–81. doi: 10.1002/ijc.10447. [DOI] [PubMed] [Google Scholar]
- Morgenstern H, Thomas D. Principles of study design in environmental epidemiology. Environmental Health Perspectives Supplements. 1993;101:23–38. doi: 10.1289/ehp.93101s423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD): 1. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: Systematic review, meta-analysis, and metaregression analysis. Archives of General Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer K, Berger AK, Monastero R, Winblad B, Backman L, Fratiglioni L. Predictors of progression from mild cognitive impairment to Alzheimer's disease. Neurology. 2007;68:1596–1602. doi: 10.1212/01.wnl.0000260968.92345.3f. [DOI] [PubMed] [Google Scholar]
- Palsson S, Aevarsson O, Skoog I. Depression, cerebral atrophy, cognitive performance and incidence of dementia. British Journal of Psychiatry. 1999;174:249–253. doi: 10.1192/bjp.174.3.249. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, Lichtenstein P, Svedberg P. The Swedish Twin Registry in the third millennium. Twin Research. 2002;5:427–432. doi: 10.1375/136905202320906219. [DOI] [PubMed] [Google Scholar]
- Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN international workshop. Neurology. 1993;43:256–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- Sairanen M, O'Leary OF, Knuuttila JE, Castren E. Chronic antidepressant treatment selectively increases expression of plasticity-related proteins in the hippocampus and medial prefrontal cortex of the rat. Neuroscience. 2007;144:368–374. doi: 10.1016/j.neuroscience.2006.08.069. [DOI] [PubMed] [Google Scholar]
- Shakesby AC, Anwyl R, Rowan MJ. Overcoming the effects of stress on synaptic plasticity in the intact hippocampus: Rapid actions of serotonergic and antidepressant agents. The Journal of Neuroscience. 2002;22:3638–3644. doi: 10.1523/JNEUROSCI.22-09-03638.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. American Journal of Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal analysis: Modeling change and event occurrence. Oxford University Press; New York: 2003. [Google Scholar]
- Speck CE, Kukull WA, Brenner DE, Bowen JD, McCormick WC, Teri L, et al. History of depression as a risk factor for Alzheimer's disease. Epidemiology. 1995;6:366–369. doi: 10.1097/00001648-199507000-00006. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Plassman BL, Helms MJ, Welsh-Bohmer KA, Saunders AM, Breitner JC. A twin study of late-onset depression and apolipoprotein E epsilon 4 as risk factors for Alzheimer's disease. Biological Psychiatry. 1997;41:851–856. doi: 10.1016/S0006-3223(96)00247-8. [DOI] [PubMed] [Google Scholar]
- van der Flier WM, van den Heuval DMJ, Weverling-Rijnsburger AWE, Bollen ELEM, Westendorp RGJ, van Buchem MA, et al. Magnetization transfer imaging in normal aging, mild cognitive impairment, and Alzheimer's disease. Annals of Neurology. 2002;52:62–67. doi: 10.1002/ana.10244. [DOI] [PubMed] [Google Scholar]
- Wetherell JL, Gatz M, Johansson B, Pedersen NL. History of depression and other psychiatric illness as risk factors for Alzheimer disease in a twin sample. Alzheimer Disease and Associated Disorders. 1999;13:47–52. doi: 10.1097/00002093-199903000-00007. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Beck TL, Bienias JL, Bennett DA. Change in depressive symptoms during the prodromal phase of Alzheimer's disease. Archives of General Psychiatry. 2008;65:439–446. doi: 10.1001/archpsyc.65.4.439. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Li Y, Bennett DA. Chronic distress, age-related neuropathology, and late-life dementia. Psychosomatic Medicine. 2007;69:47–53. doi: 10.1097/01.psy.0000250264.25017.21. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Blackwell T, Gore R, Sands L, Reus V, Browner WS. Depressive symptoms and cognitive decline in nondemented elderly women. Archives of General Psychiatry. 1999;1999:425–430. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]
- Zalsman G, Aizenberg D, Sigler M, Nahshony E, Karp L, Weizman A. Increased risk for dementia in elderly psychiatric inpatients with late-onset major depression. Journal of Nervous and Mental Disease. 2000;188:242–243. doi: 10.1097/00005053-200004000-00010. [DOI] [PubMed] [Google Scholar]