Abstract
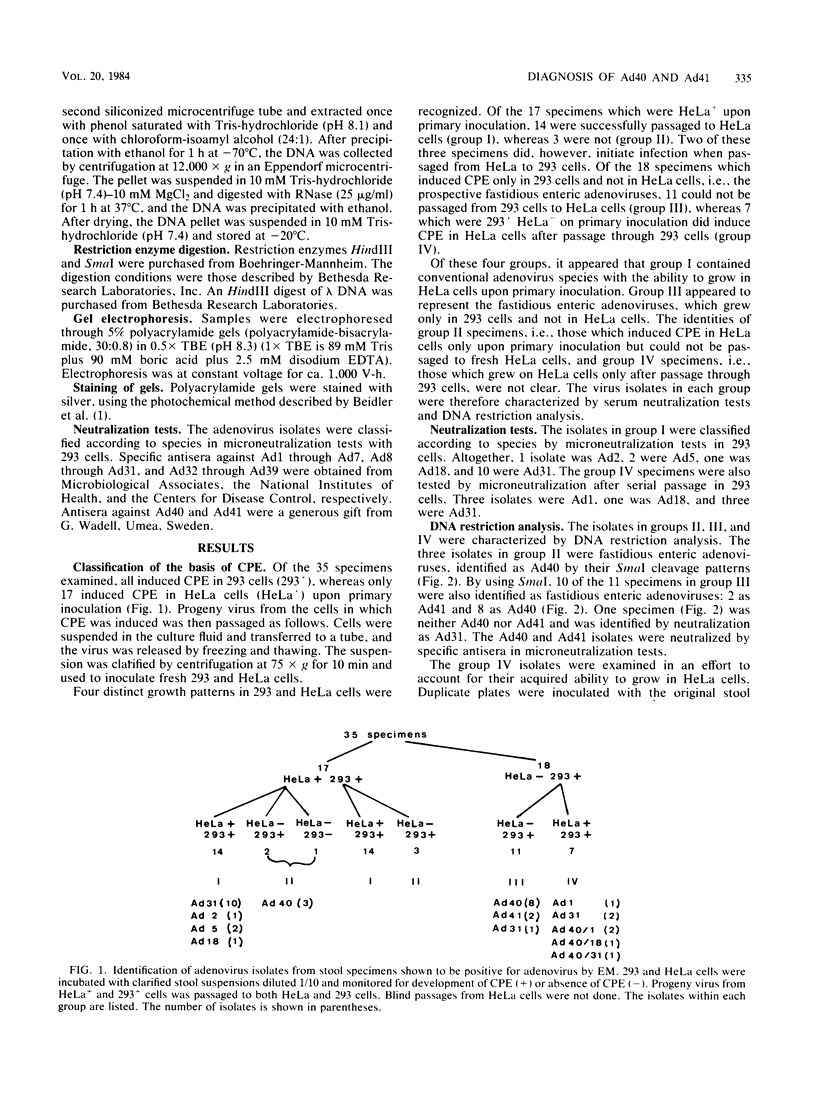
Thirty-five stool specimens, collected over a 14-week period from pediatric gastroenteritis patients and shown to contain adenovirus by electron microscopy, were inoculated onto 293 and HeLa cells. Virus isolates were characterized by serum neutralization and restriction endonuclease cleavage analysis of viral DNA from infected cells. Adenovirus was isolated upon primary inoculation of 293 cells from all 35 specimens shown to contain adenovirus by electron microscopy. Fastidious adenoviruses 40 and 41 (Ad40 and Ad41) were found in 17 (49%) of the stool specimens, and 4 of these specimens contained a conventional species (Ad1, Ad1, Ad18, Ad31) as well as Ad40. This was first manifest by the observation that four of the isolates which initially grew only in 293 cells acquired the capacity to grow in HeLa cells upon subsequent passage. In each case, the conventional species was undetectable by DNA analysis in the original inoculum but was selected in 293 cells and became the only one detectable by the second passage. Four other specimens, containing Ad1 or Ad31 alone, failed to grow initially in HeLa cells but did grow in 293 cells. The results of this study demonstrate therefore that (i) 293 cells are more sensitive than HeLa cells for the isolation of conventional as well as fastidious enteric adenovirus species and (ii) identification of viruses from patient specimens should involve minimal passage of the virus in cell culture, as a single passage can result in misdiagnosis of the virus associated with the infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beidler J. L., Hilliard P. R., Rill R. L. Ultrasensitive staining of nucleic acids with silver. Anal Biochem. 1982 Nov 1;126(2):374–380. doi: 10.1016/0003-2697(82)90530-9. [DOI] [PubMed] [Google Scholar]
- Chiba S., Nakata S., Nakamura I., Taniguchi K., Urasawa S., Fujinaga K., Nakao T. Outbreak of infantile gastroenteritis due to type 40 adenovirus. Lancet. 1983 Oct 22;2(8356):954–957. doi: 10.1016/s0140-6736(83)90463-4. [DOI] [PubMed] [Google Scholar]
- Fox J. P., Brandt C. D., Wassermann F. E., Hall C. E., Spigland I., Kogon A., Elveback L. R. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. VI. Observations of adenovirus infections: virus excretion patterns, antibody response, efficiency of surveillance, patterns of infections, and relation to illness. Am J Epidemiol. 1969 Jan;89(1):25–50. doi: 10.1093/oxfordjournals.aje.a120913. [DOI] [PubMed] [Google Scholar]
- Gary G. W., Jr, Hierholzer J. C., Black R. E. Characteristics of noncultivable adenoviruses associated with diarrhea in infants: a new subgroup of human adenoviruses. J Clin Microbiol. 1979 Jul;10(1):96–103. doi: 10.1128/jcm.10.1.96-103.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Johansson M. E., Uhnoo I., Kidd A. H., Madeley C. R., Wadell G. Direct identification of enteric adenovirus, a candidate new serotype, associated with infantile gastroenteritis. J Clin Microbiol. 1980 Jul;12(1):95–100. doi: 10.1128/jcm.12.1.95-100.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M. E., Wadell G., Jacobsson P. A., Svensson L. Preparation of specific antisera against adenoviruses by affinity bead immunization (ABI). J Immunol Methods. 1979;26(2):141–149. doi: 10.1016/0022-1759(79)90077-2. [DOI] [PubMed] [Google Scholar]
- Kidd A. H., Banatvala J. E., de Jong J. C. Antibodies to fastidious faecal adenoviruses (species 40 and 41) in sera from children. J Med Virol. 1983;11(4):333–341. doi: 10.1002/jmv.1890110409. [DOI] [PubMed] [Google Scholar]
- Kidd A. H., Cosgrove B. P., Brown R. A., Madeley C. R. Faecal adenoviruses from Glasgow babies. Studies on culture and identity. J Hyg (Lond) 1982 Jun;88(3):463–474. doi: 10.1017/s0022172400070327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd A. H., Madeley C. R. In vitro growth of some fastidious adenoviruses from stool specimens. J Clin Pathol. 1981 Feb;34(2):213–216. doi: 10.1136/jcp.34.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton P. J., Szymanski M. T., Petric M. Viruses associated with acute gastroenteritis in young children. Am J Dis Child. 1977 Jul;131(7):733–737. doi: 10.1001/archpedi.1977.02120200015004. [DOI] [PubMed] [Google Scholar]
- Retter M., Middleton P. J., Tam J. S., Petric M. Enteric adenoviruses: detection, replication, and significance. J Clin Microbiol. 1979 Oct;10(4):574–578. doi: 10.1128/jcm.10.4.574-578.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiff H. E., Straus S. E., Garon C. F. Propagation and in vitro studies of previously non-cultivable enteral adenoviruses in 293 cells. Lancet. 1981 Oct 17;2(8251):832–834. doi: 10.1016/s0140-6736(81)91104-1. [DOI] [PubMed] [Google Scholar]
- Uhnoo I., Wadell G., Svensson L., Johansson M. Two new serotypes of enteric adenovirus causing infantile diarrhoea. Dev Biol Stand. 1983;53:311–318. [PubMed] [Google Scholar]
- Wigand R., Baumeister H. G., Maass G., Kühn J., Hammer H. J. Isolation and identification of enteric adenoviruses. J Med Virol. 1983;11(3):233–240. doi: 10.1002/jmv.1890110306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Lawrence F., Leister F., Takiff H. E., Strauss S. E. Gastroenteritis associated with enteric type adenovirus in hospitalized infants. J Pediatr. 1982 Jul;101(1):21–26. doi: 10.1016/s0022-3476(82)80173-x. [DOI] [PubMed] [Google Scholar]
- de Jong J. C., Wigand R., Kidd A. H., Wadell G., Kapsenberg J. G., Muzerie C. J., Wermenbol A. G., Firtzlaff R. G. Candidate adenoviruses 40 and 41: fastidious adenoviruses from human infant stool. J Med Virol. 1983;11(3):215–231. doi: 10.1002/jmv.1890110305. [DOI] [PubMed] [Google Scholar]