Abstract
An enzyme-linked immunosorbent assay has been established to measure anthrax antibody titers. The protective antigen component of anthrax toxin was used as the capture antigen. Two types of conjugates (protein A-horseradish peroxidase and anti-human immunoglobulin G plus immunoglobulin A plus immunoglobulin M-horseradish peroxidase) were tested. Results from enzyme-linked immunosorbent assay testing were compared with those from indirect hemagglutination titers on serum from vaccinees. The overall trend of enzyme-linked immunosorbent assay and indirect hemagglutination titers was significant. The enzyme-linked immunosorbent assay offered speed, precision, and reduced cost per test.
Full text
PDF
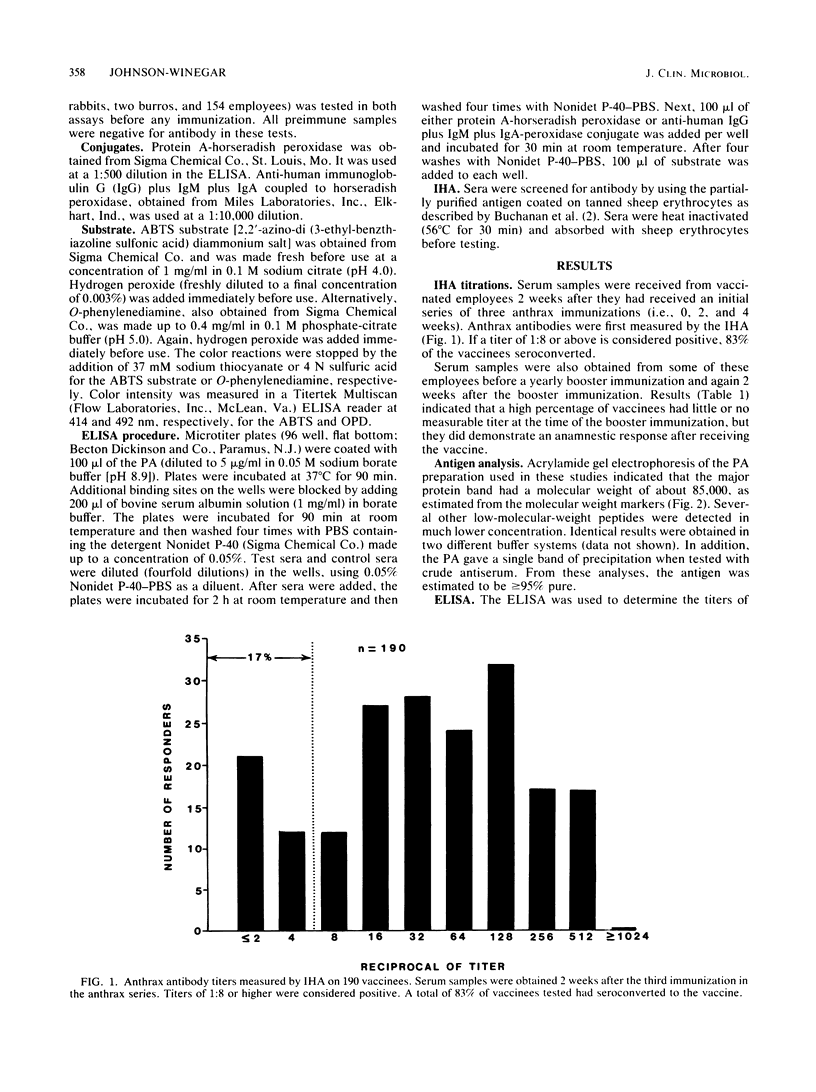


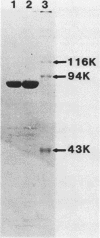
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brachman P. S., Gold H., Plotkin S. A., Fekety F. R., Werrin M., Ingraham N. R. Field Evaluation of a Human Anthrax Vaccine. Am J Public Health Nations Health. 1962 Apr;52(4):632–645. doi: 10.2105/ajph.52.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Feeley J. C., Hayes P. S., Brachman P. S. Anthrax indirect microhemagglutination test. J Immunol. 1971 Dec;107(6):1631–1636. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- NORMAN P. S., RAY J. G., Jr, BRACHMAN P. S., PLOTKIN S. A., PAGANO J. S. Serologic testing for anthrax antibodies in workers in a goat hair processing mill. Am J Hyg. 1960 Jul;72:32–37. doi: 10.1093/oxfordjournals.aje.a120132. [DOI] [PubMed] [Google Scholar]
- PUZISS M., MANNING L. C., LYNCH J. W., BARCLAYE, ABELOW I., WRIGHT G. G. Large-scale production of protective antigen of Bacillus anthracis in anaerobic cultures. Appl Microbiol. 1963 Jul;11:330–334. doi: 10.1128/am.11.4.330-334.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUZISS M., WRIGHT G. G. Studies on immunity in anthrax. X. Gel-adsorbed protective antigen for immunization of man. J Bacteriol. 1963 Jan;85:230–236. doi: 10.1128/jb.85.1.230-236.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAY J. G., Jr, KADULL P. J. AGAR-GEL PRECIPITIN TECHNIQUE IN ANTHRAX ANTIBODY DETERMINATIONS. Appl Microbiol. 1964 Jul;12:349–354. doi: 10.1128/am.12.4.349-354.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNE C. B., BELTON F. C. An agar-diffusion method for titrating Bacillus anthracis immunizing antigen and its application to a study of antigen production. J Gen Microbiol. 1957 Oct;17(2):505–516. doi: 10.1099/00221287-17-2-505. [DOI] [PubMed] [Google Scholar]
- WRIGHT G. G., GREEN T. W., KANODE R. G., Jr Studies on immunity in anthrax. V. Immunizing activity of alum-precipitated protective antigen. J Immunol. 1954 Dec;73(6):387–391. [PubMed] [Google Scholar]