Abstract
TLR2 plays a pivotal role in recognizing S. aureus, a common etiologic agent of CNS parenchymal infections, such as brain abscess. We previously reported that brain abscesses of TLR2 knockout (KO) mice exhibited elevated IL-17 levels, suggesting the presence of an alternative pathway available to respond to S. aureus infection that may involve Th17 cells. Both CD4+ and CD8+ T cell infiltrates were elevated in brain abscesses of TLR2 KO mice at days 3, 7, and 14 post-infection compared to wild type animals. Intracellular cytokine staining revealed a significant increase in the frequency of IL-17-producing Th17 cells in TLR2 KO mice with relatively few IFN-γ-positive cells.γδ T cells were also a source of IL-17 in brain abscesses. Microglia, astrocytes, and macrophages were shown to express both IL-17RA and IL-17RC. Despite receptor expression, IL-17 was relatively ineffective at eliciting glial activation, whereas the cytokine augmented the ability of TNF-α to induce CXCL2 and CCL2 expression by macrophages. Based on the ability of IL-17 to elicit the release of chemokines and other pro-inflammatory mediators, we propose that the exaggerated IL-17 response that occurs in TLR2 KO mice functions in a compensatory manner to control brain abscess pathogenesis, with cells other than glia as targets for IL-17 action. This is supported by our findings where innate immune infiltrates were not significantly different between TLR2 KO and WT mice in conjunction with the lack of prolonged alterations in the synthesis of other pro-inflammatory molecules during the course of infection.
Keywords: Toll-like receptor 2, Th17, IL-17, S. aureus, brain abscess, central nervous system
INTRODUCTION
Brain abscesses are serious CNS infections that develop in response to parenchymal colonization with pyogenic bacteria such as S. aureus or Streptococcus strains (1, 2). Abscesses begin as localized areas of cerebritis that evolve into suppurative lesions surrounded by a well-vascularized fibrotic capsule. While antibiotic and surgical treatment options are available, brain abscesses remain serious CNS infections with the potential for long-term complications including seizures, loss of mental acuity, and other neurologic defects (1, 2).
Toll-like receptors (TLRs) are an innate immune receptor family that is responsible for recognizing highly conserved microbial motifs (3, 4). In the CNS, TLRs are expressed in many cell types including microglia (5–10), astrocytes (10–13), and neurons (14, 15). TLR2 has been implicated as a critical receptor for eliciting responses to Gram-positive bacteria such as S. aureus (16, 17), which is mediated by its ability to recognize lipoproteins and peptidoglycan (PGN) associated with the bacterial cell wall. Earlier studies from our laboratory have demonstrated that TLR2 plays an important role in both microglial and astrocyte activation in response to S. aureus (7, 11). Although we previously reported that the expression of several pro-inflammatory mediators was transiently attenuated in brain abscesses of TLR2 KO mice compared to wild type (WT) animals, unexpectedly there were no significant adverse effects on survival or bacterial burdens in the former (18). One possibility that could have accounted for these results was the finding that brain abscesses of TLR2 KO mice exhibited elevated IL-17 levels (18). IL-17 is a potent inflammatory cytokine involved in immune amplification and is produced primarily by CD4+ Th17 cells, although other cellular sources of IL-17 have also been reported (19–26). IL-17 can stimulate the expression of chemokines and cytokines, either alone or in synergy with other pro-inflammatory mediators such as TNF-α 20, 27–30). Th17 cells play an important role in the management of extracellular bacterial infections (31, 32) and more recently these cells have been implicated as a key player in autoimmune diseases such as experimental autoimmune encephalomyelitis (EAE) (33–36).
There is limited information currently available regarding the expression of IL-17 in models of CNS bacterial infection. An earlier report described low levels of IL-17 expression in the brain following a systemic infection with Cryptococcus neoformans (37) and IL-17 expression is augmented in the CNS of mice chronically infected with Toxoplasma gondii (38). The finding that IL-17 levels were significantly exaggerated in brain abscesses of TLR2 KO mice led us to envision that these animals may exhibit enhanced Th17 infiltrates that could functionally compensate for the loss of TLR2-dependent signals through the pro-inflammatory effects of IL-17. Indeed, Th17 influx was increased in TLR2 KO mice compared to WT animals, whereas the recruitment of other innate immune effector cells (i.e. PMNs, macrophages, microglia, or dendritic cells) was not affected. Microglia and astrocytes were found to express both IL-17RA and IL-17RC; however, these cells were relatively non-responsive to IL-17 in terms of cytokine/chemokine production. In contrast, IL-17 potentiated chemokine release from TNF-α stimulated macrophages, suggesting that these cells may be a target of IL-17 in the infected CNS. We propose that the exaggerated IL-17 response that occurs in TLR2 KO mice functions as a compensatory mechanism to facilitate the effective control of brain abscess progression.
MATERIALS AND METHODS
Mouse strains
TLR2 KO mice (generously provided by Dr. Shizuo Akira, Osaka University, Japan) were backcrossed with C57BL/6 mice for a total of eight generations prior to use in these studies. Age- and sex-matched C57BL/6 mice (Harlan Labs, Indianapolis, IN) were used as WT controls. For all brain abscess studies, TLR2 KO and WT mice were used between 6 and 8 weeks of age.
Generation of experimental brain abscesses
Brain abscesses were induced by the stereotactic intracerebral injection of S. aureus strain RN6390 encapsulated in agarose beads as previously described (39). Briefly, mice were anesthetized with 2.5% avertin i.p. and a 1 cm longitudinal incision was made in the scalp to expose the underlying skull sutures to facilitate the identification of bregma. A rodent stereotaxic apparatus equipped with a Cunningham mouse adaptor (Stoelting, Kiel, WI) was used to implant S. aureus-encapsulated beads into the caudate-putamen region of the brain using the following coordinates relative to bregma: + 1.0 mm rostral, + 2.0 mm lateral, and −3.0 mm deep from the surface of the brain. A burr hole was made and a 5 μl Hamilton syringe fitted with a 26-gauge needle was used to slowly deliver 2 μl of S. aureus-laden beads (104 colony forming units [CFU]) into the brain parenchyma. The needle remained in place for 2.5 min following injection to minimize bead efflux and potential leakage into the meninges. The superficial skin incision was closed using surgical glue. Animals were closely monitored over the course of each study for clinical indices of infection. The animal use protocol, approved by the University of Nebraska Medical Center Animal Care and Use Committee, is in accord with the National Institutes of Health guidelines for the use of rodents.
Quantitation of T cells, microglia, macrophages, PMNs, and dendritic cells (DC) in brain abscesses
To determine whether the loss of TLR2-dependent signals influenced the influx of T cells, PMNs, macrophages, DCs, and/or microglia into lesions, abscess-associated cells were quantitated by FACS analysis as previously described (39–42). Briefly, mice were perfused to eliminate leukocytes from the vasculature, whereupon the entire infected hemisphere was collected to recover abscess-associated cells. This approach ensured that equivalent tissue regions were procured from both TLR2 KO and WT mice for downstream comparisons of leukocyte infiltrates. Following vascular perfusion, tissues were minced in HBSS (Mediatech, Herndon, VA) supplemented with 10% FBS (HyClone, Logan, UT) and filtered through a 70 μm nylon mesh cell strainer using a rubber policeman. The resulting slurry was digested for 30 min at 37° C in HBSS supplemented with 2 mg/ml collagenase type I (Sigma, St. Louis, MO) and 5000 U/ml DNAse I (Invitrogen, Carlsbad, CA) to obtain a single-cell suspension. Following enzyme neutralization, cells were layered onto a discontinuous Percoll gradient (1.03 to 1.088 g/ml) and centrifuged at 2,400 rpm for 20 min at room-temperature in a swinging bucket rotor. After centrifugation, myelin debris was carefully aspirated and the cell interface collected. Following extensive washes and incubation in Fc Block™(BD Biosciences, San Diego, CA) to minimize non-specific antibody binding to Fc receptors, cells were stained with directly conjugated antibodies for 4-color FACS to detect PMNs (Gr-1+, CD11b+, CD45hi), macrophages (Gr-1−, CD11b+, CD45hi), DCs (Gr-1−, CD11c+, CD45hi) and microglia (Gr-1−, CD11b+, CD45lo-intermediate). To enumerate abscess-associated T cell infiltrates, cells were analyzed by 3-color FACS using CD3, CD4, and CD8. All antibodies were purchased from BD Biosciences. Cells were analyzed using a BD FACSAria with compensation set based on the staining of each individual fluorochrome alone (CD11b-AlexaFluor488, CD11c-PECy7, Gr1-APC, CD45-FITC, CD3-FITC, CD4-PE, or CD8-APC) and correction for autofluorescence with unstained cells. Microglial, astrocyte, and macrophage IL-17 receptor A (IL-17RA) and IL-17RC expression was examined using goat anti-mouse primary antibodies directed against each receptor isoform (R & D Systems, Minneapolis, MN) followed by a donkey anti-goat IgG-FITC conjugate (Jackson Immunoresearch, West Grove, PA). Controls included cells stained with isotype control antibodies to assess the degree of non-specific staining.
For reporting differences in cellular influx between TLR2 KO and WT mice, one of two approaches was utilized. In the first, we normalized (i.e. divided) the numbers of FACS-purified T cells recovered from TLR2 WT mice by those collected from TLR2 KO animals to express the latter as a percentage of WT (set to 100%). This approach was required since it is difficult to achieve identical bacterial burdens in mice between independent brain abscess experiments. As a result, the absolute numbers of infiltrating cells within brain abscesses of TLR2 WT and KO mice differed between individual studies, requiring us to normalize our data within each independent replicate. Therefore, this method enabled us to pool the results from three independent experiments since the relative ratio of each cell type between TLR2 KO and WT mice remained consistent throughout our independent replicates. As an alternative approach, in some experiments to report immune cell influx into brain abscesses we used the percentage of positive cells for each innate immune cell population (i.e. PMN, macrophages, DCs, and microglia) and pooled data from a minimum of three independent experiments.
Intracellular cytokine staining
To characterize the cytokine expression profiles of infiltrating T cells into brain abscesses of TLR2 KO and WT mice, abscess-associated T cells were collected and evaluated by intracellular cytokine staining and FACS analysis. As described above, TLR2 KO and WT mice harboring brain abscesses were perfused to remove leukocytes from the vasculature and the entire infected hemispheres were collected. Brain tissues were manually disassociated as described above, followed by enzyme digestion and purification using a Percoll gradient. The abscess-associated cells were then incubated with CD90-conjugated magnetic beads and purified using MACS magnetic cell separation columns (Miltenyi Biotec, Auburn CA). Cells recovered from magnetic cell separation columns were stimulated with a cocktail of PMA + ionomycin (Leukocyte Activation Cocktail) in the presence of GolgiPlug™ for 12 h, whereupon cells were incubated with Fc Block™ (all from BD Biosciences) to minimize non-specific antibody binding. Subsequently, T cells were stained with directly-conjugated antibodies against CD4+, CD8+, or γδ TcR, permeabilized with a CytoFix/CytoPerm kit, and stained for intracellular IL-17 or IFN-γ (all from BD Biosciences). Cells were analyzed using a BD FACSAria with compensation set based on the staining of each individual fluorochrome alone (CD4-FITC, CD8-APC,γδ TcR-PE-Cy5, IL17-PE, IFNγ-PE-Cy7) and correction for autofluorescence with unstained cells. Controls included cells stained with directly-conjugated isotype control antibodies to assess the degree of non-specific staining.
Primary glial cell culture, macrophage isolation, and reagents
Primary microglia and astrocytes were isolated from the cerebral cortex of TLR2 KO and WT mice (2–4 days of age) as previously described (43, 44). Briefly, when mixed glial cultures reached confluency (7 to 10 days), flasks were shaken at 200 rpm overnight at 37° C to recover microglia. This was repeated for a minimum of three times, whereupon flasks were trypsinized and cells resuspended in culture medium supplemented with 0.1 mM of the microglial cytotoxic agent L-leucine methyl ester (L-LME; Sigma). Astrocytes were cultured in L-LME-containing medium for at least 2 weeks prior to use in experiments and were not utilized until the third passage in culture (approximately day 30 in vitro). Microglia and astrocyte cultures were evaluated by immunohistochemical staining using antibodies against CD11b (BD Biosciences) and glial fibrillary acidic protein (GFAP; Invitrogen), with purities approximating 98% and 95%, respectively. We acknowledge the fact that findings obtained with primary astrocyte cultures can often be confounded by the presence of contaminating microglia (45); however, several lines of evidence argue against this possibility using our culture paradigm as previously addressed (46).
Primary macrophages were elicited from the abdominal cavity of C57BL/6 mice four days following an i.p. injection of 4% Brewer’s thioglycollate broth. Cells were collected by peritoneal lavage with ice-cold PBS and maintained in RPMI medium (Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT).
To examine whether microglia, astrocytes, and/or macrophages were responsive to IL-17, cells were treated with varying doses of recombinant mouse IL-17 (R & D Systems) in the presence or absence of recombinant mouse IL-1β or TNF-α (both from Biosource, Camarillo, CA). After a 24 h stimulation period, cell-conditioned supernatants were evaluated for inflammatory mediator release by ELISA.
ELISAs
Brain abscess homogenates or cell culture supernatants from primary glia and macrophages stimulated with IL-17 alone or in combination with IL-1β or TNF-α were analyzed by ELISA for IL-17, IL-17F, CXCL1, and CXCL2 (all from R & D Systems) and IL-1β, IL-12 p40, and CCL2 (all from BD Biosciences) according to the manufacturer’s instructions. For quantitating IL-17 and IL-17F expression in brain abscess homogenates, cytokine levels were normalized to the amount of total protein extracted from tissues to correct for differences in sampling size using a BioRad protein assay kit (Hercules, CA).
Quantitative real-time RT-PCR (qRT-PCR)
Total RNA from TLR2 WT and KO glia was isolated using the TriZol reagent and treated with DNAse1 (both from Invitrogen, Carlsbad, CA) prior to use in qRT-PCR studies. The experimental procedure was performed as previously described (7). GAPDH primers and TAMRA Taqman probe were designed as previously described (7, 11) and synthesized by Applied Biosystems (ABI, Foster City, CA). ABI Assays-on-Demand™ Taqman kits were utilized to examine IL-17RA, IL-17RC, and β-actin expression. Comparisons in gene expression between TLR2 WT and KO primary glia were calculated after normalizing cycle thresholds against the housekeeping genes GAPDH or β-actin and are presented as the difference in cycle threshold (ΔCt).
Statistics
Significant differences between macrophages treated with IL-17 or TNF-α alone versus a combination of the two cytokines were determined using one-way ANOVA followed by the Holm-Sidak method for multiple pair-wise comparisons with Sigma Stat (SPSS Science, Chicago, IL). Significant differences between all other experimental groups were determined using the unpaired Student’s t test with Sigma Stat.
RESULTS
CD4+ and CD8+ T cell infiltrates are enhanced in TLR2 KO mice
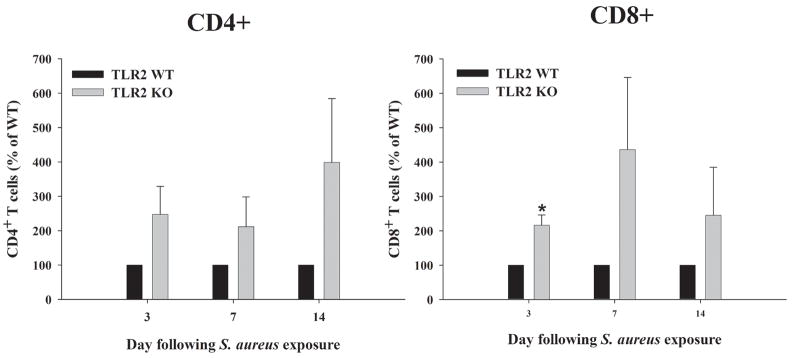
We have previously demonstrated that IL-17 levels were significantly elevated in TLR2 KO mice compared to WT animals from days 3 to 7 post-infection (18). In the current study this interval was extended out to 3 weeks following S. aureus infection, the latest time point examined (Figure 1). IL-17 is produced by Th17 cells and is known to play a role in granulopoiesis, PMN recruitment, and pro-inflammatory cytokine production in response to extracellular infections (20, 32). Given the elevated levels of a T cell-derived cytokine in brain abscesses of TLR2 knockout mice, it was expected that T cell infiltrates would be elevated in these mice compared to WT animals. To investigate this possibility, we quantitated T cell influx in the brains of infected TLR2 WT and KO mice by collecting abscess-associated cells at days 3, 7, and 14 following infection. Both CD4+ and CD8+ T cell influx into brain abscesses was greater in TLR2 KO mice compared to WT animals at all time points examined, although only CD8+ T cell infiltrates reached statistical significance at day 3 post-infection (Figure 2). The inevitable variability between individual experiments resulted in these differences not reaching statistical significance, although there was a strong trend towards elevated CD4+ and CD8+ infiltrates in TLR2 KO mice. The frequency of CD4+ T cells, in particular, increased from days 3 to 14 post-infection, corresponding with the elevated IL-17 expression detected throughout the course of brain abscess development in TLR2 KO mice (Figure 1). The increase in T cell infiltrates observed in mice deficient in TLR2 signaling may explain the enhanced IL-17 levels noted in these animals.
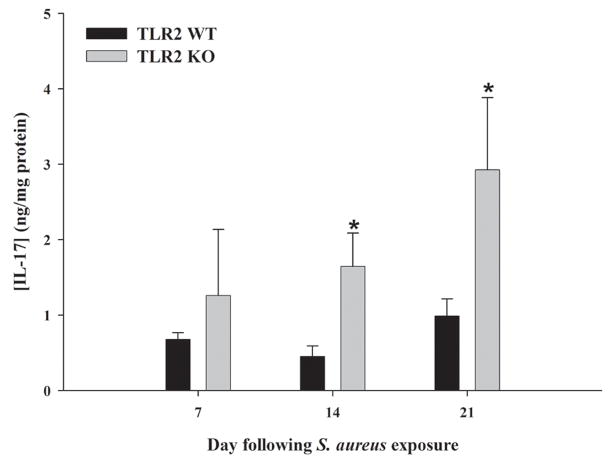
Figure 1. IL-17 production is augmented in brain abscesses of TLR2 KO mice out to 3 weeks post-infection.
TLR2 KO and WT mice (n = 4 to 6 per group) were infected with S. aureus intracerebrally as described in the Materials and Methods. Animals were euthanized at 7, 14, or 21 days following bacterial exposure, whereupon IL-17 expression was quantitated by ELISA. Results were normalized based on the amount of total protein recovered from abscesses to correct for differences in tissue sampling size. Significant differences in IL-17 expression between brain abscesses TLR2 KO and WT mice are indicated by asterisks (* p < 0.05). Results are representative of two independent experiments.
Figure 2. CD4+ and CD8+ T cell infiltrates are enhanced in TLR2 KO mice.
Abscess-associated cells were recovered from TLR2 KO and WT mice at the indicated days post-infection (n = 4 to 6 animals per group), whereupon CD3+CD4+ and CD3+CD8+ T cells were quantitated by FACS. Significant differences between TLR2 KO and WT mice are denoted with asterisks (*, p < 0.05). Results represent the mean ± SEM from 3–4 independent experiments.
IL-17 producing cells are more frequent in brain abscesses of TLR2 KO mice
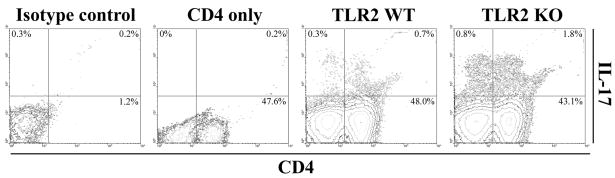
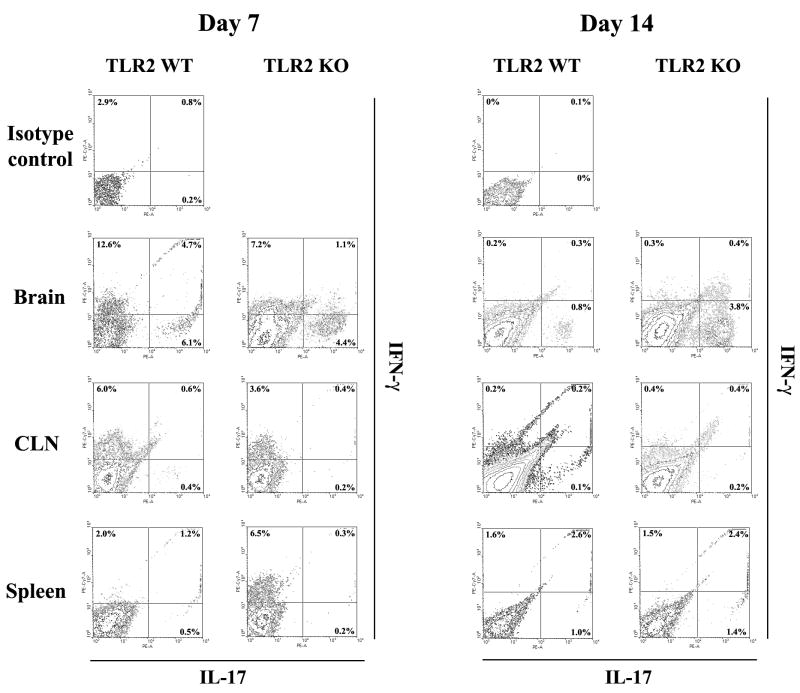
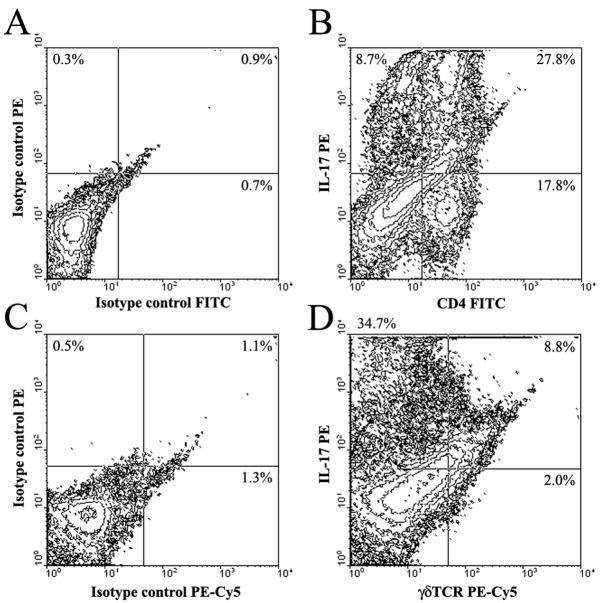
IL-17 is produced by Th17 cells, recently described as a distinct T cell subset involved in extracellular infections and autoimmune diseases (19, 47). Given the high levels of IL-17 and increased T cell infiltrates noted in infected TLR2 KO mice, we next examined the frequency of Th17 cells by intracellular cytokine staining to determine whether a shift in T cell subtypes was observed in brain abscesses of TLR2 KO animals. Abscess-associated T cells were recovered from TLR2 KO and WT mice using CD90-conjugated magnetic beads, whereupon Th17 (IL-17) and Th1 (IFN-γ) cells were quantitated by FACS. At day 14 post-infection, brain abscesses of TLR2 KO mice displayed an increase in the frequency of IL-17-producing Th17 cells (Figures 3 and 4), with relatively few IFN-γ-positive cells present (Figure 4). Although differences were observed between the percentages of IL-17+ and IFN-γ+ populations in TLR2 KO and WT mice at day 7 post-infection, the fold-differences at this earlier time point were not consistently reproducible, whereas changes in the frequency of IL-17+ and/or IFN-γ+ cells were consistent across several independent experiments after two weeks post-infection and typically reached a magnitude of several-fold higher in TLR2 KO mice (Figure 4). A small proportion of dual IL-17- and IFN-γ-producing T cells were also associated with brain abscesses of both TLR2 WT and KO animals (Figure 4) in agreement with earlier reports (48, 49). The majority of Th17 cells were observed in the brain, with relatively few Th17 cells detected in the draining cervical lymph nodes (CLNs) or spleens of infected TLR2 KO or WT animals (Figure 4). We also observed a population of IL-17 producing cells that were CD4− and CD8− and were more frequent in brain abscesses of TLR2 KO mice (Figure 3). Based on the recent literature, γδ T cells and NK cells have also been reported to express IL-17 (21); therefore, intracellular cytokine staining was repeated utilizing antibodies reactive with each subset (γδ TcR and NK1.1, respectively). From this analysis, we confirmed that CD4+ Th17 cells represent the majority of IL-17-producing cells in brain abscesses (Figure 5). However, γδ T cells were also found to express IL-17, albeit at a reduced frequency compared to CD4+ cells (i.e. ~ 8% versus 28%, respectively; Figure 5). We did not find any evidence for NK cell (NK1.1+) production of IL-17 at the time points examined in this study (data not shown); however, we cannot discount a potential contribution by NKT cells since recent studies have demonstrated IL-17 production by CD4−NK1.1− cells (25, 50, 51). The increased proportion of IL-17 producing cells detected in brain abscesses of TLR2 KO mice explains the elevated cytokine levels noted in previous experiments with these animals.
Figure 3. The frequency of CD4+ Th17 cells is increased in brain abscesses of TLR2 KO mice.
T cells were recovered from brain abscesses of TLR2 KO and WT mice at day 14 following S. aureus exposure using CD90-conjugated magnetic beads and stimulated with a cocktail of PMA + ionomycin for 12 h in the presence of GolgiBlock™, whereupon IL-17 production was quantitated by intracellular cytokine staining and FACS. The results presented are representative of three independent experiments.
Figure 4. IL-17-producing cells are more frequent in brain abscesses of TLR2 KO mice.
T cells were recovered from brain abscesses, draining cervical lymph nodes (CLN), and spleens of TLR2 KO and WT mice at days 7 and 14 following S. aureus exposure using CD90-conjugated magnetic beads and stimulated with a cocktail of PMA + ionomycin for 12 h in the presence of GolgiBlock™, whereupon IL-17 and IFN-γ production was quantitated by intracellular cytokine staining and FACS. The results presented are representative of three independent experiments.
Figure 5. γδ T cells are a source of IL-17 in brain abscesses.
Cells were recovered from brain abscesses of C57BL/6 wild type mice at day 14 following S. aureus exposure using CD90-conjugated magnetic beads and stimulated with a cocktail of PMA + ionomycin for 12 h in the presence of GolgiBlock™, whereupon IL-17 production in CD4+ Th17 or γδ T cells was quantitated by intracellular cytokine staining. The results presented are representative of two independent experiments.
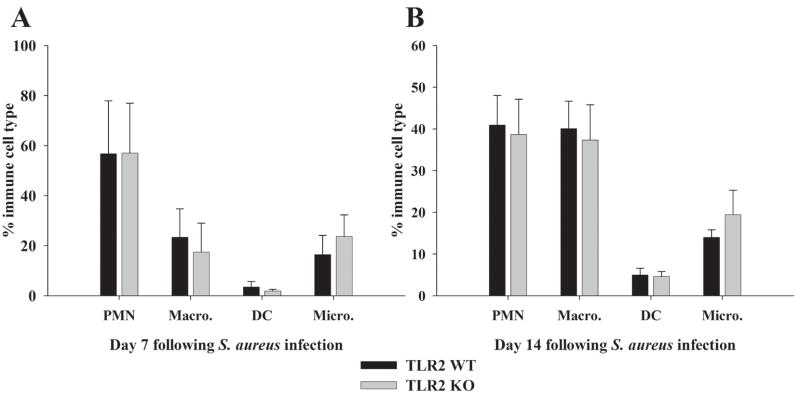
PMN, macrophage and dendritic cell (DC) infiltrates into brain abscesses remain unchanged in TLR2 KO mice compared to WT animals
One function of IL-17 is to recruit PMNs and other innate immune cells to sites of infection by eliciting chemokine production by many parenchymal and immune cell types (20, 31, 52, 53). In the S. aureus brain abscess model, augmented IL-17 levels observed in TLR2 KO mice may serve to facilitate the influx and activation of innate immune cells to control bacterial infection despite the lack of TLR2 signaling. To examine this possibility, we analyzed the brains of infected TLR2 KO and WT mice using 4-color FACS to determine the relative proportions of innate immune cells present. There were no significant differences in the numbers of PMNs, macrophages, DCs, or microglia associated with brain abscesses of TLR2 KO or WT mice at either 7 or 14 days post-infection (Figure 6A and B, respectively). To our knowledge, this study is the first to report the presence of myeloid DCs (CD11b+, CD11c+) in brain abscesses. DCs represented a small percentage of abscess-associated infiltrates; however, these cells did increase in number from day 7 to 14 post-infection (2.75% to 5% respectively, Figure 6). The fact that immune cell influx in TLR2 KO mice is comparable to WT animals in the face of relatively similar pro-inflammatory mediator levels likely explains the similarity in brain abscess severity between the two strains as demonstrated in our previous study (18).
Figure 6. Innate immune cell infiltrates into brain abscesses of TLR2 KO and WT mice are equivalent.
Abscess-associated cells were recovered from TLR2 KO and WT mice at days 7 and 14 following S. aureus infection using a Percoll gradient method and analyzed by FACS. Results are presented as the percent positive cells for each population pooled from three independent experiments (mean ± SEM).
IL-17 receptor expression patterns in primary glia and macrophages
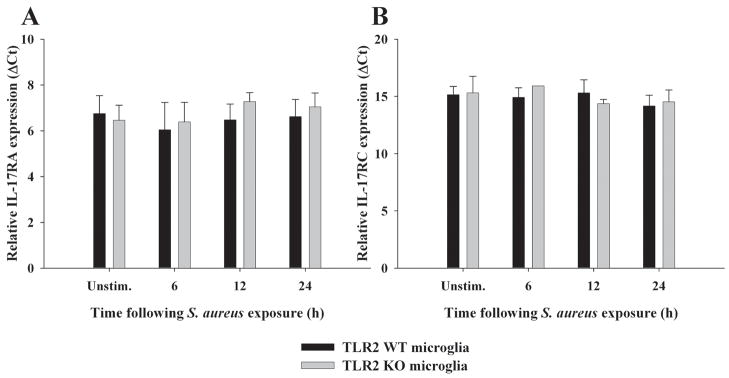
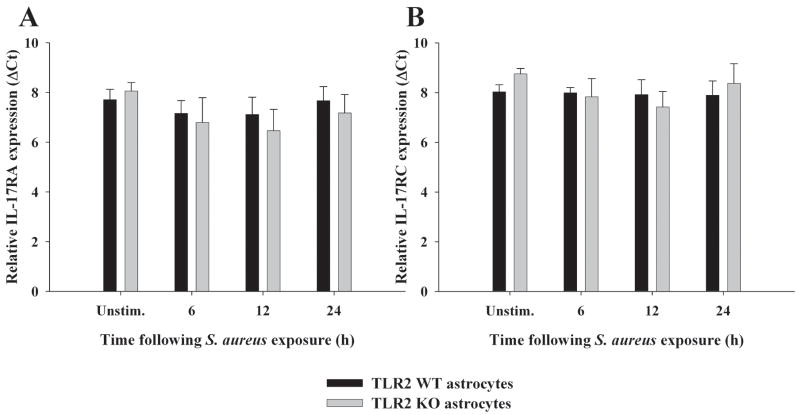
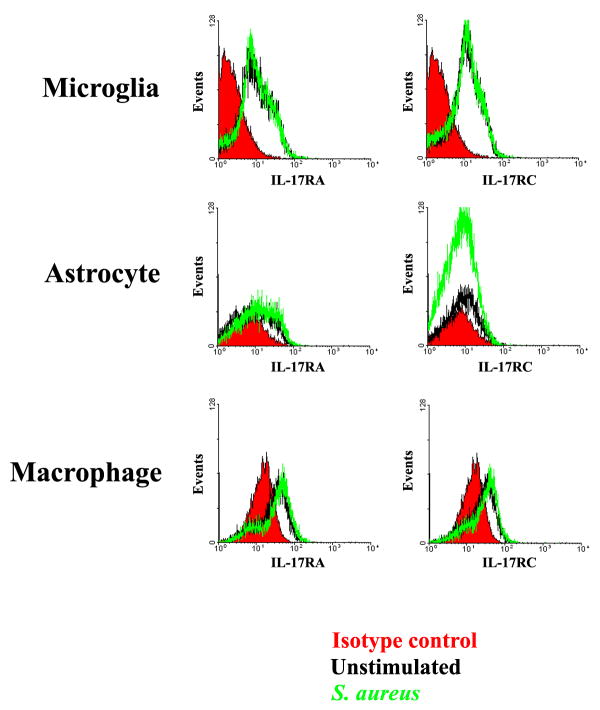
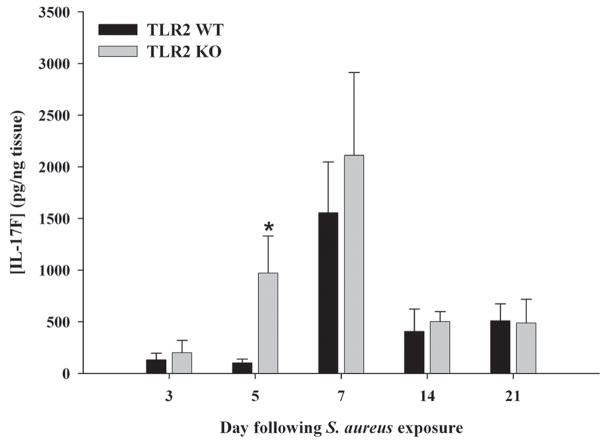
Unlike the more limited array of cells capable of producing IL-17, IL-17 receptors are present on multiple cell types, including myeloid lineages as well as parenchymal cells in a variety of mouse and human tissues (20). The main isoform of IL-17 (i.e. IL-17A) interacts with IL-17 receptor A (IL-17RA) to transduce activation signals (47, 54). Despite two studies demonstrating that IL-17 triggers pro-inflammatory mediator release from microglia and astrocytes (55, 56), to our knowledge no reports have described whether astrocytes express IL-17RA, whereas one study has demonstrated IL-17RA in microglia (55). Both primary microglia and astrocytes were found to express IL-17RA mRNA (Figures 7 and 8, respectively and Supplementary Figure 1), suggesting that these cells may be primed to respond to IL-17 in evolving brain abscesses. IL-17RA expression was confirmed at the protein level by FACS where equivalent levels were observed in both primary microglia and macrophages (Figure 9). In contrast, IL-17RA expression on astrocytes was low. Although human IL-17 is capable of binding to both IL-17RA and IL-17RC, recent studies have demonstrated that mouse IL-17 is only capable of engaging IL-17RA (54). Since we were interested in examining other IL-17R family members in glia, we evaluated IL-17RC expression in primary microglia and astrocytes, which to our knowledge, has not yet been reported in the literature. Similar to IL-17RA, IL-17RC expression was roughly equivalent in microglia and macrophages, whereas receptor levels on astrocytes were virtually undetectable (Figure 9). Neither IL-17RA nor IL-17RC expression was altered in response to S. aureus stimulation in any of the cell types examined (Figures 7–9). Expression of the ligand for IL-17RC, IL-17F, was transiently elevated in brain abscesses of TLR2 KO mice, albeit with different kinetics compared to IL-17 (Figure 10). Therefore, ligands for both IL-17RA and IL-17RC are upregulated during the course of brain abscess development in TLR2 KO mice.
Figure 7. IL-17R isoform expression in microglia.
TLR2 KO and WT microglia were seeded at 2 × 106 cells per well in 6-well plates and incubated overnight. The following day, cells were stimulated with 107 heat-inactivated S. aureus for 6, 12, or 24 h, whereupon total RNA was isolated and examined for IL-17RA (A) and IL-17RC (B) expression by qRT-PCR. Gene expression levels were calculated after normalizing IL-17R signals against GAPDH and are presented in relative mRNA expression units (mean ± SEM of two independent experiments).
Figure 8. IL-17R isoform expression in astrocytes.
TLR2 KO and WT astrocytes were seeded at 1 × 106 cells per well in 6-well plates and incubated overnight. The following day, cells were stimulated with 107 heat-inactivated S. aureus for 6, 12, or 24 h, whereupon total RNA was isolated and examined for IL-17RA (A) and IL-17RC (B) expression by qRT-PCR. Gene expression levels were calculated after normalizing IL-17R signals against GAPDH and are presented in relative mRNA expression units (mean ± SEM of two independent experiments).
Figure 9. Comparison of IL-17R isoform expression in microglia, astrocytes, and macrophages.
Primary microglia, astrocytes, and macrophages from C57BL/6 wild type mice were stimulated with 107 heat-inactivated S. aureus for 24 h, whereupon cell surface expression of IL-17RA and IL-17RC was analyzed by FACS.
Figure 10. IL-17F production is transiently elevated in brain abscesses of TLR2 KO mice.
TLR2 KO and WT mice (n = 3 to 5 per group) were infected with S. aureus intracerebrally as described in the Materials and Methods. Animals were euthanized at the indicated time points following bacterial exposure, whereupon IL-17F expression was quantitated by ELISA. Results were normalized based on the amount of total protein recovered from abscesses to correct for differences in tissue sampling size. Significant differences in IL-17F expression between brain abscesses TLR2 KO and WT mice are indicated by asterisks (* p < 0.05). Results are representative of two independent experiments.
IL-17 potentiates TNF-α-induced inflammatory mediator release in macrophages but not microglia
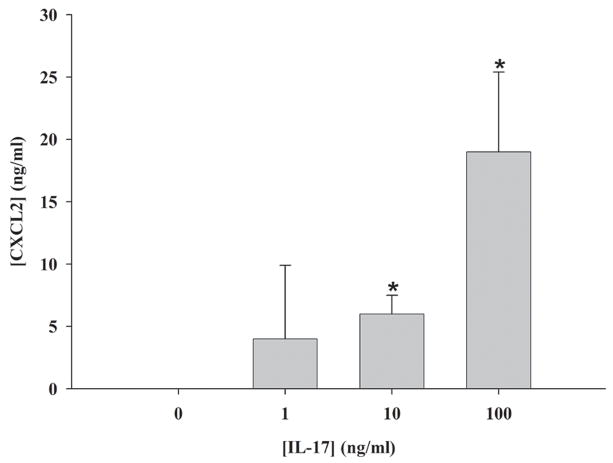
As noted above, IL-17 is known to induce pro-inflammatory mediator production both independently and in synergy with TNF-α and IL-1β in diverse parenchymal cell types (28–30, 47). In addition to demonstrating the presence of IL-17 receptors in glia, we attempted to establish the in vitro effects of IL-17 on astrocyte and microglial activation. Primary glial cultures were stimulated with various concentrations of IL-17 for 24 h, whereupon pro-inflammatory mediator expression was evaluated by ELISA. Of all the molecules examined (CXCL1, CXCL2, CCL2, IL-12 p40, and IL-1β), IL-17 was only capable of inducing the PMN chemokine CXCL1 (KC) in a dose-dependent manner by astrocytes (Figure 11). Interestingly, we did not observe any reproducible effects of IL-17 on the induction of inflammatory products by microglia or macrophages (i.e. CXCL2, CCL2, IL-1β, and TNF-α) despite the fact that they express IL-17RA.
Figure 11. IL-17 induces expression of the PMN chemokine CXCL2 in astrocytes.
C57BL/6 wild type astrocytes were treated with the indicated concentrations of recombinant mouse IL-17 for 24 h, whereupon CXCL1 (KC) levels were quantitated by ELISA. Significant differences between unstimulated and IL-17-treated astrocytes are denoted with asterisks (*, p < 0.05). Results are representative of two independent experiments.
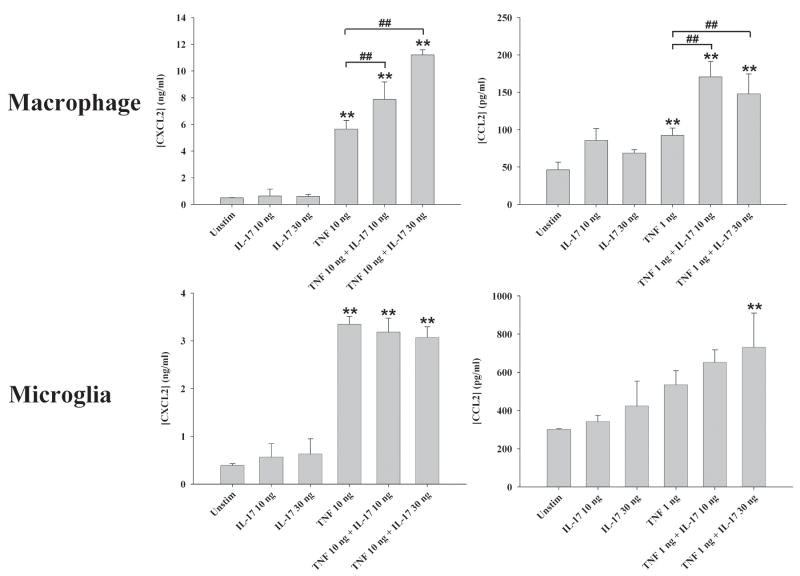
Since IL-17 has been reported to synergize with other pro-inflammatory cytokines such as TNF-α and IL-1β in other cell types (28–30, 47), we next examined whether this relationship would hold true for glia. Primary astrocytes and microglia were treated with IL-17 in combination with varying doses of either TNF-α or IL-1β, whereupon conditioned supernatants were evaluated by ELISA. Although TNF-α and IL-1β alone were capable of eliciting the production of a few inflammatory mediators by glia including CXCL2 and CCL2, the addition of IL-17 did not significantly alter the release of these factors (Figure 12 and data not shown). In contrast, IL-17 potentiated TNF-α-induced CXCL2 and CCL2 production in macrophages (Figure 12), which are also relevant to brain abscess pathogenesis since these cells represent a significant constituent of the inflammatory infiltrate in response to infection (39, 57, 58). Importantly, only a select number of mediators were augmented by IL-17 in TNF-α-stimulated macrophages (i.e. CXCL2 and CCL2), whereas others were not affected (i.e. IL-12, IL-1β; Figure 12 and data not shown). Interestingly, we failed to detect any synergy with IL-1β and IL-17 in macrophages, regardless of the concentrations examined, revealing specificity in the responses obtained (data not shown). Collectively, the limited response of glial cells to IL-17 stimulation suggests that the primary target of increased IL-17 levels in brain abscesses of TLR2 KO mice may be peripheral immune cells infiltrating the CNS such as macrophages and/or other parenchymal cell types.
Figure 12. IL-17 potentiates TNF-α-induced chemokine expression in macrophages.
Primary microglia or macrophages isolated from C57BL/6 wild type mice were treated with the indicated concentrations of IL-17 or TNF-α or in combination for 24 h, whereupon CXCL2 and CCL2 production was quantitated by ELISA. Significant differences between unstimulated versus cytokine-treated cells are indicated by asterisks (**, p < 0.001), whereas significant differences between cells exposed to TNF-α versus TNF-α IL-17 are denoted by hatched signs (##, p < 0.001).
DISCUSSION
Toll-like receptors initiate the production of pro-inflammatory mediators that influence the genesis of adaptive immunity against microbes, effectively bridging the innate and adaptive immune responses (4, 59). Despite an important role for TLR2 in mediating host defense during systemic Gram-positive infections (17, 60), our earlier study using TLR2 KO mice in the experimental brain abscess model did not reveal striking differences compared to WT animals, with one notable exception (18). Namely, IL-17 levels were consistently and markedly elevated in brain abscesses of TLR2 KO mice, a relationship that we extend out to three weeks following infection in the current study. Another novel finding was that a second IL-17 isoform, IL-17F, was also elevated during brain abscess development. These observations served as the basis for the hypothesis that elevated IL-17 expression in the absence of TLR2 could represent a compensatory signal to ensure the genesis of an adequate host anti-bacterial immune response based upon the numerous pro-inflammatory effects ascribed to IL-17 (20).
The major finding of this study was that Th17 influx was augmented in brain abscesses of TLR2 KO mice, which was demonstrated by enhanced CD4+ IL-17 producing cells by intracellular cytokine staining. Our findings demonstrating elevated CD4+ and CD8+ T cell infiltrates in brain abscesses of TLR2 KO mice is in agreement with a recent study by Stenzel et al. (61), providing further evidence that T cell dynamics are indeed altered in the infected CNS of mice lacking TLR2. However, these authors did not investigate IL-17 expression in their study, which remains a novel aspect of the current work. In addition to Th17 cells, γδ T cells were also found to produce IL-17 in brain abscesses, albeit at a much lower frequency than the former. It is worth noting that elevated Th17 influx in TLR2 KO animals appears to represent a non-traditional pathway since although Th17 cells are present in the CNS of infected WT mice, they do not predominate the adaptive response since Th1 cells producing IFN-γ are also a significant component of the T cell infiltrate. A few studies have reported that astrocytes (26, 62) and oligodendrocytes (26) produce IL-17 in the context of neuroinflammation. However, in our hands we were not able to detect any production of IL-17 in either microglia or astrocytes in response to S. aureus or PGN in vitro (data not shown). Possibilities to explain these discrepancies could relate to the context of the inciting stimulus, variations between in vitro and in vivo studies, and/or species differences since the reports describing IL-17 production by glia were all performed with human cells/tissues (26, 62).
One main issue that remains unresolved relates to the mechanism(s) by which TLR2 loss impacts exaggerated Th17 entry into the infected CNS. One possibility is that the absence of TLR2 may alter the cellular response to infection at the level of antigen presenting cells (APCs). For example, the inability to engage TLR2 may re-program APCs towards a phenotype that favors Th17 development by enhancing the production of positive regulators of Th17 cells such as TGF-β, IL-6, and/or IL-23 (33, 47, 63) or the overexpression of cytokines known to negatively impact Th17 development such as IL-27 (38, 64). Alternatively, a reduction in Th1 cytokines that normally antagonize Th17 development (i.e. IFN-γ) would also have a similar net effect in augmenting Th17 levels (33, 63). This possibility is supported by our data where IFN-γ-producing T cells were reduced in brain abscesses of TLR2 KO mice concomitant with an increase in Th17 infiltrates, a reciprocal regulatory relationship that has been described by other laboratories (33, 63). Another explanation to account for the skewing towards Th17 infiltrates during brain abscess development in TLR2 KO mice could be directly dictated by effects on the T cells themselves. Several studies have reported TLR2 expression on various T cell populations in the mouse and TLR2 engagement by microbial ligands is capable of T cell activation (65–67). By extension, it is possible that the lack of TLR2 signaling may lead to defects in the autocrine/paracrine regulation of Th17 cells, such as the failure to initiate a negative signal(s) to quell Th17 expansion. An alternative mechanism could be at the transcriptional level, where the absence of TLR2 signals may lead to the inability to trigger/negatively regulate certain transcription factors (TFs) that promote/repress Th17 development (49, 68). However, these possibilities have one important caveat; namely that although it seems clear that mouse T cells express TLR2, the evidence for TLR expression on human T cells is more controversial (65). Because of this apparent lack of TLR2 on human T cells coupled with the observation that IL-17 is a major constituent of the pro-inflammatory response following injury/infection in humans (69, 70), it is tempting to speculate that with the loss of TLR2 on T cells from KO mice we have in effect made these cells more like their human counterparts. A schematic summarizing the potential mechanisms of exaggerated IL-17 expression in brain abscesses of TLR2 KO mice is presented in Figure 13. The potential identity of a non-TLR2 receptor(s) for PGN in our experimental model is currently speculative. One candidate is the intracellular PRR nucleotide-binding oligomerization domain 2 (NOD2), which has been implicated in the recognition of PGN breakdown products (i.e. muramyl dipeptide; MDP) from Gram-positive bacteria such as S. aureus (71–73). Although NOD2 is a cytoplasmic PRR, studies have demonstrated that the receptor is capable of being engaged following the application of its ligand extracellularly (72, 73). Currently, the mechanism(s) by which NOD ligands enter the cell for direct engagement of intracellular PRRs such as NOD2 is unknown; however, possibilities including cytoplasmic translocation via a MDP-specific transporter on the plasma membrane (i.e. PepT1) or cytoplasmic release from phagolysosomes have been proposed (74–76). Since PGN is an extensively cross-linked insoluble polymer with a heterogeneous structure, it is conceivable that it is engulfed via a phagocytic pathway, whereupon degradation products could be liberated to engage intracellular PRRs such as NOD2. It is not known whether MDP is present in the extracellular milieu during brain abscess infection; however, this is plausible since S. aureus expresses a wide array of PGN hydrolases that serve to regulate bacterial cell death and lysis (77–79).
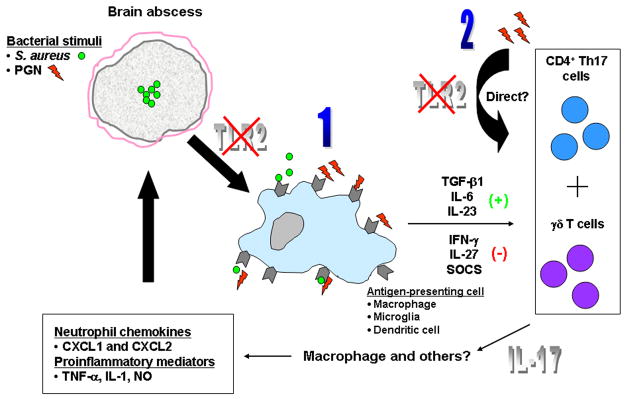
Figure 13. Potential mechanisms of exaggerated IL-17 expression in brain abscesses of TLR2 KO mice.
The absence of TLR2 signaling could lead to elevated IL-17 expression in a number of potential ways including (1) changes in APC properties or (2) direct effects on T cell populations. Based on the pro-inflammatory effects of IL-17, we propose that this response compensates for the loss of TLR2 signaling to result in an effective host anti-bacterial immune response.
An interesting relationship between Th17 and CD8+ T cells has emerged based on the production of IL-21 by the former, which serves as a potent regulator of CD8+ T cell proliferation (20, 80, 81). Since Th17 infiltrates are augmented in brain abscesses, IL-21 release by these cells may represent a positive feedback loop to augment CD8+ T cell expansion. This possibility would explain the finding that CD8+ T cell infiltrates were also increased in brain abscesses of TLR2 KO mice.
IL-17RA and IL-17RC are two receptor isoforms that are responsible for mediating effects via IL-17 and IL-17F, respectively (20, 47). To our knowledge, the current study represents the first report of IL-17RC expression in either microglia or astrocytes, despite a few previous reports describing glial responsiveness to IL-17 (55, 56). Despite earlier studies demonstrating that IL-17 was capable of inducing pro-inflammatory mediator release from microglia and astrocytes (55, 56), we observed very limited effects of IL-17 on glial activation, even in the context of other inflammatory molecules (i.e. TNF-α and IL-1β) where synergistic effects have been reported in other cell types (20, 27–30). The low levels of IL-17 responsiveness in primary glia suggest that alternative cell types within the infected CNS may be responsive to IL-17 such as infiltrating macrophages. Indeed, we demonstrated that unlike glia, IL-17 potentiated TNF-α-induced chemokine production in macrophages. Interestingly, IL-17RA was expressed at equivalent levels in both microglia and macrophages, although the former was not capable of producing inflammatory mediators in response to IL-17 in our hands. This suggests that receptor expression does not directly equate with cytokine responsiveness and the mechanism behind the relative inability of microglia to respond to IL-17 is unknown. Since IL-17 has been proposed to signal through a heterodimeric receptor composed of IL-17RA and IL-17RC (82), it is possible that there are differences in receptor combinations between glia and macrophages (83). Alternatively, signaling and/or adaptor proteins downstream of IL-17Rs may be differentially expressed within these cell types. However, we cannot discount a role for IL-17 in regulating other functions of glia that were not examined in this study including phagocytosis, chemotaxis, and/or increased responsiveness to other inflammatory signals.
The biological effects of IL-17 make it an ideal candidate for the amplification of CNS antibacterial immune responses in the absence of TLR2 signaling. For example, IL-17 is a potent inducer of PMN chemokines (20, 29, 84–86) and previous work from our laboratory has identified PMNs as an essential immune effector population during brain abscess development (87). Also IL-17 can act synergistically with other inflammatory mediators to induce the expression of a wide array of inflammatory mediators including cytokines, prostaglandin metabolites, and reactive oxygen/nitrogen intermediates (20). Alternative products produced by Th17 cells, such as IL-21 and IL-22 may provide further amplification of inflammatory networks in the brain. Collectively, these possibilities may contribute to the fact that in our hands, brain abscess severity is remarkably similar between TLR2 KO and WT mice (18), a finding that we attribute to the redundancy of infiltrating Th17 cells. Directly implicating the potential compensatory actions of IL-17 will require future studies where the cytokine is specifically neutralized in the CNS of TLR2 KO mice or by crossing TLR2 KO with IL-17R KO animals. This line of research is ongoing in our laboratory.
In summary, the current study demonstrates several novel aspects related to brain abscess pathogenesis in TLR2 KO mice. Namely, Th17 infiltrates are elevated in TLR2 KO animals as revealed by intracellular cytokine staining and the fact that primary microglia and astrocytes express both IL-17RA and IL-17RC, although not at equivalent levels.γδ T cells were also identified as another source of IL-17 in the infected brain. In addition, low levels of DC influx were observed in brain abscesses with numbers increasing progressively from day 7 to 14 post-infection. Although DC entry has been associated with other CNS infectious diseases (88, 89), to our knowledge, DC influx has not yet been described in brain abscesses. Collectively, we propose that the exaggerated Th17 influx into brain abscesses of TLR2 KO mice represents a compensatory mechanism to circumvent the loss of this important pattern recognition receptor to ensure the induction of an effective anti-bacterial immune response.
Supplementary Material
Supplementary Figure 1. IL-17RA isoform expression in glia. TLR2 KO and WT microglia and astrocytes were seeded at 2 × 106 or 1 × 106 cells per well in 6-well plates, respectively, and incubated overnight. The following day, cells were stimulated with 107 heat-inactivated S. aureus for 6, 12, or 24 h, whereupon total RNA was isolated and examined for IL-17RA expression by qRT-PCR. Gene expression levels were calculated after normalizing IL-17RA signals against β-actin and are presented in relative mRNA expression units (mean ± SEM of two independent experiments).
Acknowledgments
The authors would like to thank Gail Wagoner and Kelly McCastlain for excellent technical assistance and Dr. Charles Kuszynski, Victoria Smith, and Megan Michalak in the UNMC Cell Analysis Facility for assistance with FACS analysis.
Footnotes
This work was supported by the NIH National Institute of Neurological Disorders and Stroke (NINDS; RO1 NS055385) to T.K.
References
- 1.Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997;25:763–779. doi: 10.1086/515541. quiz 780–761. [DOI] [PubMed] [Google Scholar]
- 2.Townsend GC, Scheld WM. Infections of the central nervous system. Adv Intern Med. 1998;43:403–447. [PubMed] [Google Scholar]
- 3.Takeda K, Akira S. Toll-like receptors. Curr Protoc Immunol. 2007 doi: 10.1002/0471142735.im1412s77. Chapter 14:Unit 14 12. [DOI] [PubMed] [Google Scholar]
- 4.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 5.Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kielian T, Esen N, Bearden ED. Toll-like receptor 2 (TLR2) is pivotal for recognition of S. aureus peptidoglycan but not intact bacteria by microglia. Glia. 2005;49:567–576. doi: 10.1002/glia.20144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 9.Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J Neurosci Res. 2006;83:711–730. doi: 10.1002/jnr.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra BB, Mishra PK, Teale JM. Expression and distribution of Toll-like receptors in the brain during murine neurocysticercosis. J Neuroimmunol. 2006;181:46–56. doi: 10.1016/j.jneuroim.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esen N, Tanga FY, DeLeo JA, Kielian T. Toll-like receptor 2 (TLR2) mediates astrocyte activation in response to the Gram-positive bacterium Staphylococcus aureus. J Neurochem. 2004;88:746–758. doi: 10.1046/j.1471-4159.2003.02202.x. [DOI] [PubMed] [Google Scholar]
- 12.Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- 13.Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49:360–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- 14.Cameron JS, Alexopoulou L, Sloane JA, DiBernardo AB, Ma Y, Kosaras B, Flavell R, Strittmatter SM, Volpe J, Sidman R, Vartanian T. Toll-like receptor 3 is a potent negative regulator of axonal growth in mammals. J Neurosci. 2007;27:13033–13041. doi: 10.1523/JNEUROSCI.4290-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y, Li J, Chiu I, Wang Y, Sloane JA, Lu J, Kosaras B, Sidman RL, Volpe JJ, Vartanian T. Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J Cell Biol. 2006;175:209–215. doi: 10.1083/jcb.200606016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 18.Kielian T, Haney A, Mayes PM, Garg S, Esen N. Toll-Like Receptor 2 Modulates the Proinflammatory Milieu in Staphylococcus aureus-Induced Brain Abscess. Infect Immun. 2005;73:7428–7435. doi: 10.1128/IAI.73.11.7428-7435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roark CL, Simonian PL, Fontenot AP, Born WK, O’Brien RL. gammadelta T cells: an important source of IL-17. Curr Opin Immunol. 2008;20:353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 23.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 24.Lee KA, Kang MH, Lee YS, Kim YJ, Kim DH, Ko HJ, Kang CY. A distinct subset of natural killer T cells produces IL-17, contributing to airway infiltration of neutrophils but not to airway hyperreactivity. Cell Immunol. 2008;251:50–55. doi: 10.1016/j.cellimm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-de-Moraes MC. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witowski J, Pawlaczyk K, Breborowicz A, Scheuren A, Kuzlan-Pawlaczyk M, Wisniewska J, Polubinska A, Friess H, Gahl GM, Frei U, Jorres A. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J Immunol. 2000;165:5814–5821. doi: 10.4049/jimmunol.165.10.5814. [DOI] [PubMed] [Google Scholar]
- 28.Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, Gaffen SL. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 29.Maertzdorf J, Osterhaus AD, Verjans GM. IL-17 expression in human herpetic stromal keratitis: modulatory effects on chemokine production by corneal fibroblasts. J Immunol. 2002;169:5897–5903. doi: 10.4049/jimmunol.169.10.5897. [DOI] [PubMed] [Google Scholar]
- 30.Chabaud M, Page G, Miossec P. Enhancing effect of IL-1, IL-17, and TNF-alpha on macrophage inflammatory protein-3alpha production in rheumatoid arthritis: regulation by soluble receptors and Th2 cytokines. J Immunol. 2001;167:6015–6020. doi: 10.4049/jimmunol.167.10.6015. [DOI] [PubMed] [Google Scholar]
- 31.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 35.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 36.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleinschek MA, Muller U, Brodie SJ, Stenzel W, Kohler G, Blumenschein WM, Straubinger RK, McClanahan T, Kastelein RA, Alber G. IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12. J Immunol. 2006;176:1098–1106. doi: 10.4049/jimmunol.176.2.1098. [DOI] [PubMed] [Google Scholar]
- 38.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O’Shea J, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 39.Kielian T, Phulwani NK, Esen N, Syed MM, Haney AC, McCastlain K, Johnson J. MyD88-Dependent Signals Are Essential for the Host Immune Response in Experimental Brain Abscess. J Immunol. 2007;178:4528–4537. doi: 10.4049/jimmunol.178.7.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154:4309–4321. [PubMed] [Google Scholar]
- 41.Renno T, Krakowski M, Piccirillo C, Lin JY, Owens T. TNF-alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by Th1 cytokines. J Immunol. 1995;154:944–953. [PubMed] [Google Scholar]
- 42.Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22:72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 43.Phulwani NK, Feinstein DL, Gavrilyuk V, Akar C, Kielian T. 15-deoxy-Delta12,14-prostaglandin J2 (15d-PGJ2) and ciglitazone modulate Staphylococcus aureus-dependent astrocyte activation primarily through a PPAR-gamma-independent pathway. J Neurochem. 2006;99:1389–1402. doi: 10.1111/j.1471-4159.2006.04183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kielian T, McMahon M, Bearden ED, Baldwin AC, Drew PD, Esen N. S. aureus-dependent microglial activation is selectively attenuated by the cyclopentenone prostaglandin 15-deoxy-Delta12,14- prostaglandin J2 (15d-PGJ2) J Neurochem. 2004;90:1163–1172. doi: 10.1111/j.1471-4159.2004.02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saura J. Microglial cells in astroglial cultures: a cautionary note. J Neuroinflammation. 2007;4:26. doi: 10.1186/1742-2094-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phulwani NK, Esen N, Syed MM, Kielian T. TLR2 expression in astrocytes is induced by TNF-alpha- and NF-kappa B-dependent pathways. J Immunol. 2008;181:3841–3849. doi: 10.4049/jimmunol.181.6.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 48.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The Orphan Nuclear Receptor RORgammat Directs the Differentiation Program of Proinflammatory IL-17(+) T Helper Cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 50.Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, Berzins SP, Smyth MJ, Godfrey DI. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1-NKT cell population. Proc Natl Acad Sci U S A. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung DR, Kasper DL, Panzo RJ, Chitnis T, Grusby MJ, Sayegh MH, Tzianabos AO. CD4+ T cells mediate abscess formation in intra-abdominal sepsis by an IL-17-dependent mechanism. J Immunol. 2003;170:1958–1963. doi: 10.4049/jimmunol.170.4.1958. [DOI] [PubMed] [Google Scholar]
- 54.Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, Harder B, Okada S, Ostrander CD, Kreindler JL, Aujla SJ, Reardon B, Moore M, Shea P, Schreckhise R, Bukowski TR, Presnell S, Guerra-Lewis P, Parrish-Novak J, Ellsworth JL, Jaspers S, Lewis KE, Appleby M, Kolls JK, Rixon M, West JW, Gao Z, Levin SD. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol. 2007;179:5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawanokuchi J, Shimizu K, Nitta A, Yamada K, Mizuno T, Takeuchi H, Suzumura A. Production and functions of IL-17 in microglia. J Neuroimmunol. 2008;194:54–61. doi: 10.1016/j.jneuroim.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 56.Trajkovic V, Stosic-Grujicic S, Samardzic T, Markovic M, Miljkovic D, Ramic Z, Mostarica Stojkovic M. Interleukin-17 stimulates inducible nitric oxide synthase activation in rodent astrocytes. J Neuroimmunol. 2001;119:183–191. doi: 10.1016/s0165-5728(01)00391-5. [DOI] [PubMed] [Google Scholar]
- 57.Kielian T, Esen N, Liu S, Phulwani NK, Syed MM, Phillips N, Nishina K, Cheung AL, Schwartzman JD, Ruhe JJ. Minocycline Modulates Neuroinflammation Independently of Its Antimicrobial Activity in Staphylococcus aureus-Induced Brain Abscess. Am J Pathol. 2007;171:1199–1214. doi: 10.2353/ajpath.2007.070231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stenzel W, Soltek S, Miletic H, Hermann MM, Korner H, Sedgwick JD, Schluter D, Deckert M. An essential role for tumor necrosis factor in the formation of experimental murine Staphylococcus aureus-induced brain abscess and clearance. J Neuropathol Exp Neurol. 2005;64:27–36. doi: 10.1093/jnen/64.1.27. [DOI] [PubMed] [Google Scholar]
- 59.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 2004;6:1382–1387. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 60.Echchannaoui H, Frei K, Schnell C, Leib SL, Zimmerli W, Landmann R. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J Infect Dis. 2002;186:798–806. doi: 10.1086/342845. [DOI] [PubMed] [Google Scholar]
- 61.Stenzel W, Soltek S, Sanchez-Ruiz M, Akira S, Miletic H, Schluter D, Deckert M. Both TLR2 and TLR4 are required for the effective immune response in Staphylococcus aureus-induced experimental murine brain abscess. Am J Pathol. 2008;172:132–145. doi: 10.2353/ajpath.2008.070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meeuwsen S, Persoon-Deen C, Bsibsi M, Ravid R, van Noort JM. Cytokine, chemokine and growth factor gene profiling of cultured human astrocytes after exposure to proinflammatory stimuli. Glia. 2003;43:243–253. doi: 10.1002/glia.10259. [DOI] [PubMed] [Google Scholar]
- 63.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 64.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 65.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol. 2007;19:39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 66.Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci U S A. 2004;101:3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zanin-Zhorov A, Nussbaum G, Franitza S, Cohen IR, Lider O. T cells respond to heat shock protein 60 via TLR2: activation of adhesion and inhibition of chemokine receptors. Faseb J. 2003;17:1567–1569. doi: 10.1096/fj.02-1139fje. [DOI] [PubMed] [Google Scholar]
- 68.Moisan J, Grenningloh R, Bettelli E, Oukka M, Ho IC. Ets-1 is a negative regulator of Th17 differentiation. J Exp Med. 2007;204:2825–2835. doi: 10.1084/jem.20070994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Finnerty CC, Jeschke MG, Herndon DN, Gamelli R, Gibran N, Klein M, Silver G, Arnoldo B, Remick D, Tompkins RG. Temporal Cytokine Profiles in Severely Burned Patients: A Comparison of Adults and Children. Mol Med. 2008 doi: 10.2119/2007-00132.Finnerty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilmanski JM, Petnicki-Ocwieja T, Kobayashi KS. NLR proteins: integral members of innate immunity and mediators of inflammatory diseases. J Leukoc Biol. 2008;83:13–30. doi: 10.1189/jlb.0607402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Girardin SE, I, Boneca G, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 73.Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, Foster SJ, Moran AP, Fernandez-Luna JL, Nunez G. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 74.Vavricka SR, Musch MW, Chang JE, Nakagawa Y, Phanvijhitsiri K, Waypa TS, Merlin D, Schneewind O, Chang EB. hPepT1 transports muramyl dipeptide, activating NF-kappaB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology. 2004;127:1401–1409. doi: 10.1053/j.gastro.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 75.Ismair MG, Vavricka SR, Kullak-Ublick GA, Fried M, Mengin-Lecreulx D, Girardin SE. hPepT1 selectively transports muramyl dipeptide but not Nod1-activating muramyl peptides. Can J Physiol Pharmacol. 2006;84:1313–1319. doi: 10.1139/y06-076. [DOI] [PubMed] [Google Scholar]
- 76.Herskovits AA, Auerbuch V, Portnoy DA. Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system. PLoS Pathog. 2007;3:e51. doi: 10.1371/journal.ppat.0030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Komatsuzawa H, Sugai M, Nakashima S, Yamada S, Matsumoto A, Oshida T, Suginaka H. Subcellular localization of the major autolysin, ATL and its processed proteins in Staphylococcus aureus. Microbiol Immunol. 1997;41:469–479. doi: 10.1111/j.1348-0421.1997.tb01880.x. [DOI] [PubMed] [Google Scholar]
- 78.Oshida T, Sugai M, Komatsuzawa H, Hong YM, Suginaka H, Tomasz A. A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-L-alanine amidase domain and an endo-beta-N-acetylglucosaminidase domain: cloning, sequence analysis, and characterization. Proc Natl Acad Sci U S A. 1995;92:285–289. doi: 10.1073/pnas.92.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rice KC, Bayles KW. Molecular control of bacterial death and lysis. Microbiol Mol Biol Rev. 2008;72:85–109. doi: 10.1128/MMBR.00030-07. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 81.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, Berzofsky JA, Leonard WJ. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 83.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 84.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prause O, Laan M, Lotvall J, Linden A. Pharmacological modulation of interleukin-17-induced GCP-2-, GRO-alpha- and interleukin-8 release in human bronchial epithelial cells. Eur J Pharmacol. 2003;462:193–198. doi: 10.1016/s0014-2999(03)01341-4. [DOI] [PubMed] [Google Scholar]
- 86.Ruddy MJ, Shen F, Smith JB, Sharma A, Gaffen SL. Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: implications for inflammation and neutrophil recruitment. J Leukoc Biol. 2004;76:135–144. doi: 10.1189/jlb.0204065. [DOI] [PubMed] [Google Scholar]
- 87.Kielian T, Barry B, Hickey WF. CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses. J Immunol. 2001;166:4634–4643. doi: 10.4049/jimmunol.166.7.4634. [DOI] [PubMed] [Google Scholar]
- 88.Fischer HG, Bonifas U, Reichmann G. Phenotype and functions of brain dendritic cells emerging during chronic infection of mice with Toxoplasma gondii. J Immunol. 2000;164:4826–4834. doi: 10.4049/jimmunol.164.9.4826. [DOI] [PubMed] [Google Scholar]
- 89.Lauterbach H, Zuniga EI, Truong P, Oldstone MB, McGavern DB. Adoptive immunotherapy induces CNS dendritic cell recruitment and antigen presentation during clearance of a persistent viral infection. J Exp Med. 2006;203:1963–1975. doi: 10.1084/jem.20060039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. IL-17RA isoform expression in glia. TLR2 KO and WT microglia and astrocytes were seeded at 2 × 106 or 1 × 106 cells per well in 6-well plates, respectively, and incubated overnight. The following day, cells were stimulated with 107 heat-inactivated S. aureus for 6, 12, or 24 h, whereupon total RNA was isolated and examined for IL-17RA expression by qRT-PCR. Gene expression levels were calculated after normalizing IL-17RA signals against β-actin and are presented in relative mRNA expression units (mean ± SEM of two independent experiments).