Abstract
The respiratory tract poses a substantial challenge to the immune system due to its large surface area, an extensive vasculature that is in very close proximity to the external environment, and repeated exposure to potentially pathogenic organisms in the air. Yet many lung pathogens are controlled by appropriate immune responses. The underlying mechanisms of the adaptive cellular immune response in protecting the respiratory tract are poorly understood. Recently, it has emerged that memory CD4+ and CD8+ T cells are present in the lung airways, and evidence is mounting that these cells play a key role in pulmonary immunity to pathogen challenge by immediately engaging the pathogen at the site of infection when pathogen loads are low. For example, in the case of respiratory virus infections, there is evidence that both CD4+ and CD8+ memory cells in the lung airways mediate substantial control of a secondary respiratory virus infection in the lungs. Here we address recent developments in our understanding of lung airway memory T cells and their role in infectious disease.
Keywords: CD8-positive T lymphocytes; immunity, mucosal; immunologic memory; paramyxovirus
The respiratory system is a major portal of entry for many pathogens, including a wide array of respiratory viruses. Influenza virus alone is responsible for nearly 36,000 deaths and 110,000 hospitalizations each year in the United States, and this number is likely to increase as the population ages. Moreover, the emergence of highly pathogenic respiratory viruses, such as the H5 avian influenza virus and the coronavirus associated with severe acute respiratory syndrome, highlight the critical need for the generation of vaccines that elicit protective immunity against pulmonary pathogens. For viral infections, in which antibody determinants evolve rapidly, effective vaccines are required that generate strong cellular immune responses to conserved viral epitopes. However, a significant hurdle in the development of these vaccines is our poor understanding of cell-mediated immunity in the lung. We don't know which subpopulations of memory T cells are critical for influenza virus clearance, how these subpopulations are established and subsequently maintained, or how these cells can be effectively generated by vaccination strategies. Moreover, we have little understanding of the circumstances under which some vaccines promote the development of detrimental cellular immune responses in the lung.
The lungs are an anatomically and structurally complex organ system. An important feature is the large surface area of epithelium that is continually exposed to the external environment and, at the same time, is highly vascularized. Over the last few years, there has been a growing appreciation of the role that cells of the innate and adaptive immune systems play in maintaining the health of the lungs. With respect to the adaptive immune system, it has emerged that pools of memory T cells are able to persist for prolonged periods of time in the lung airways and that these cells play an important role in mediating immune responses to pathogen challenge. In this article, we review the properties of memory T cells in the lung airways and their role in cellular immune responses to respiratory virus infections. We also discuss how pools of memory T cells might be maintained in the lung—a question of major relevance for vaccine development.
THE GENERATION OF T-CELL MEMORY IN THE LUNG
Mouse models have proven to be invaluable tools for demonstrating the central role of major histocompatibility complex–restricted T lymphocytes in the clearance of influenza and parainfluenza viruses from the lungs of infected mice (1–7). These models suggest that, upon virus exposure, dendritic cells in the lungs mature and traffic to the local draining lymph nodes (cervical and mediastinal lymph nodes), where they display peptide antigen to T cells (8, 9). Antigen-specific T cells then become activated and initiate a program of proliferation and differentiation, resulting in the production of effector cells that have the capacity to migrate to the lung and terminate the infection (10). Effector T cell numbers typically peak in the lung around Day 10 after infection, mediate viral clearance via cytokine production, direct cytolytic mechanisms (either perforin- or Fas-mediated) (1, 4, 6, 11–14).
During the course of a respiratory virus infection, pools of memory T cells are established that persist for the life of the animal (15). These cells differ substantially from their naive precursors in that they persist at a high frequency, generate rapid effector functions in response to antigen exposure, have distinct cytokine production profiles, have low requirements for costimulation, and have reduced susceptibility to apoptosis (16, 17). Many memory cells can be found in secondary lymphoid organs, such as the draining lymph nodes and spleen. However, it has recently emerged that large numbers of antigen-specific T cells can also be found in nonlymphoid tissues (18–22). For example, it has been reported that as many as half of the long-lived memory CD8+ T cells in animals that had recovered from an influenza virus infection were located at peripheral sites (21). These observations have led to the conclusion that memory T cells can be divided into two major subsets depending on their ability to traffic to either secondary lymphoid organs or peripheral tissues. In this regard, studies by a number of investigators have shown that this dichotomy can be primarily ascribed to adhesion molecules and chemokine receptors that target them to different tissues (23, 24). Central memory T cells express CD62L, which allows them to cross high endothelial venules and enter lymph nodes, and CCR7, which mediates chemoattraction to and localization within the T zone of lymphoid tissue via CCL19 and CCL21. Effector memory T cells typically lack the expression of CD62L and CCR7, but express alternative adhesion molecules and chemokine receptors, promoting a wider distribution of these cells into the peripheral organs and tissues. The effector memory and central memory paradigm is still relatively new and is not the last word on the classification of memory T cells. However, it represents a useful framework for developing experimental hypotheses. Thus, we use the effector and central memory terminology throughout this article.
One of the more surprising peripheral sites shown to harbor substantial numbers of effector memory cells in both animal models and human clinical studies is the airways of the respiratory tract. The conditions in the airways are relatively hostile given the proximity to the external environment and the presence of surfactants and other molecules involved in maintaining lung integrity. Mouse models have demonstrated that these memory T cells are established after resolution of a respiratory tract infection and can be recovered from this site for over a year after the initial infection has resolved. The absolute numbers of these cells can be surprisingly high. For example, we have shown that as many as 40,000 antigen-specific CD8+ T cells can be recovered from the lung airways of mice a month after resolution of influenza and parainfluenza virus infections (18). However, these numbers tend to decline over the first few months after infection before stabilizing at much lower numbers (typically only a few thousand cells per animal). As will be discussed subsequently here, this decline and stabilization in the number of memory T cells correlates with a progressive decline in the overall efficacy of cellular memory in terms of its ability to clear a secondary virus challenge (25). In addition, substantial numbers of memory T cells can also be recovered from the lung parenchyma and pleural cavity long after resolution of the infection. Thus, it appears that prior exposure to a respiratory pathogen establishes a network of memory T cells that persist in several distinct compartments of the lung. The number of antigen-specific memory T cells present in the lung is typically greater than the number in the local draining lymph nodes, and their role in host defense will be discussed below.
PROPERTIES OF AIRWAY MEMORY CELLS
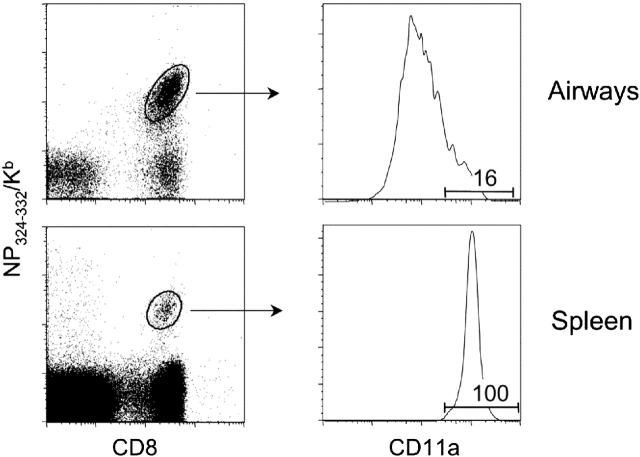
Lung airway memory CD8+ T cells express low levels of CD62L and CCR7, identifying them as “effector memory” cells (Table 1). In addition, these cells express other markers associated with effector memory cells, such as interleukin-7Rα, CXCR3, and high levels of CD44 (18, 26, and unpublished data). In addition, memory cells in the lung airways express the acute activation marker CD69, which is normally associated with activated T cells (18). However, unlike T cell blasts, lung memory T cells are small, nonproliferating cells that are in the G0-G1 phase of the cell cycle (27). A particularly interesting feature of lung airway memory cells is that the majority of these cells express low levels of the lymphocyte function–associated antigen (LFA)-1 (CD11a/CD18) integrin (28). For example, approximately 80% of antigen-specific T cells in the lung airways of mice that have recovered from a Sendai virus are CD11alo (Figure 1). This is in marked contrast to memory T cells from all other lymphoid and nonlymphoid sites, and is especially surprising because LFA-1 is involved in T cell activation and migration into tissues (29). We have evidence that LFA-1 is downregulated after effector or memory T cells enter the airways (30, 31). Thus, LFA-1 expression may be used as a marker to identify T cells that have been recently recruited into the lung airways, as discussed subsequently here.
TABLE 1.
Properties of effector and memory t cells
| Marker | Effector Cells | Central Memory Cells | Effector Memory Cells | Airway Memory Cells |
|---|---|---|---|---|
| CD44 | Hi | Hi | Hi | Hi |
| CD62L | Lo | Hi | Lo | Lo |
| CD69 | Variable | — | Mixed (≅10% +) | Mixed (≅70% +) |
| CD25 | Mixed (≅10–50% +) | Mixed (≅10–50% +) | Mixed (≅10–50% +) | Mixed (≅50% +) |
| Ly6C | + | + | + | Mixed (≅50% +) |
| LFA-1 (CD11a) | Hi | Hi | Hi | Mixed (≅20% Hi) |
| IL-7Rα | — | + | + | — |
| CXCR3 | + | + | + | + |
| CCR7 | Lo | Hi | Lo | Lo |
| Size | Large | Small | Small | Small |
| Cell cycle | Cycling | Most in G0-G1 | Most in G0-G1 | 100% in G0-G1 |
Definition of abbreviations +/— = expressed/not expressed; cycling = cells that are in various stages of the cell cycle; Hi/Lo = high/low levels of expression; IL = interleukin; LFA = lymphocyte function–associated antigen; mixed = mixed levels of expression on subsets of cells.
Figure 1.
Memory T cells in the lung airways are CD11alo. Mice were infected with Sendai virus and lymphocytes isolated from the lung airways and spleen on Day 40 after infection for flow cytometric analysis. Data in the left panels identify CD8+ T cells specific for the dominant NP324-332/Kb epitope of Sendai virus. Data in the right panels are gated on NP324-332/Kb-specific T cells and show the distinct patterns of CD11a expression on these cells depending on whether they are isolated from the spleen or lung airways.
Lung airway CD8+ T cells not only have novel phenotypic characteristics, but we have also described important functional differences between them and other effector memory cells. One key difference between lung airway memory CD8+ T cells and effector memory CD8+ T cells from other peripheral sites is that these cells lack constitutive cytolytic activity. This is most likely due to the loss of LFA-1 expression, as this integrin is involved in the adhesion of cytotoxic T cells to their targets. In addition, memory T cells in the lung airways do not to proliferate in situ, either as part of a homeostatic maintenance mechanism or in response to cognate antigen (27, 32). However, cytolytic activity, LFA-1 expression and proliferative capacity recover rapidly when cells are removed from the airway environment (18, 33). These observations suggest that factors within the lung airways mediate tight control over T cell function, allowing only necessary and appropriate responses. In this regard, it is well established that molecules, such as surfactants, and cells, such as alveolar macrophages, maintain an immunosuppressive environment in the lung. Another interesting feature of lung memory T cells is that they are able to produce antiviral cytokines, such as IFN-γ, in response to proinflammatory cytokines produced by dendritic cells (34). Thus, the response of these cells may operate effectively against pathogens for which they are not specific. Under certain conditions, airway memory T cells may also produce cytokines involved in airway inflammation and airway hyperresponsiveness, such as interleukin-13 (35). In a mouse model of allergen-induced airway hyperresponsiveness, this property was specifically attributed to effector memory T cells that accumulated in the lungs (35).
Most of our research into pulmonary T cell memory has focused on CD8+ T cells. However, we have also extended our studies to the CD4+ memory T cell population. In this regard, we have generated a major histocompatibility complex/peptide multimeric reagent that can detect T cells specific for a dominant hemagglutinin epitope that we had identified in Sendai virus (HN419-433/Ab) (36–38). Using this reagent, we have shown that HN419-433/Ab-specific T cells persist in the lung airways, lung parenchyma, and secondary lymphoid organs after resolution of a Sendai virus infection. The general phenotypic and functional characteristics of these cells are similar to those of equivalent populations of memory CD8+ T cells. However, the absolute numbers of memory CD4+ T cells are much lower than those for memory CD8+ T cells.
LUNG AIRWAY MEMORY T CELLS MEDIATE PROTECTION AGAINST VIRAL CHALLENGE
As noted previously here, studies in influenza and parainfluenza models have shown that the number of antigen-specific CD8+ T cells in the lung airways is high in the first few months after recovery from infection, but then declines before subsequently stabilizing 6 months after infection (18, 25). There is an even more rapid decline in the number of antigen-specific memory CD4+ T cells in the lung airways, and these cells become undetectable within 2 months after infection (36). The decline in the absolute number of memory T cells in the airways correlates with a decline in the overall efficacy of the cellular immune response to viral challenge (25). Thus, there is a correlation between the numbers of cells in the airways and the relative efficacy of the immune response despite stable numbers of memory T cells being present in the spleen (18). Although circumstantial, this was the first evidence that lung airway memory cells play an important role in recall responses. To address this issue in more detail, we directly analyzed the capacity of airway memory cells to mediate control of respiratory virus infections. In these experiments, lung airway memory cells specific for Sendai virus were transferred intratracheally to naive mice. These mice were then infected intranasally with Sendai virus, and viral loads were assessed in the lung 4 days later. These studies showed that there was a substantial reduction in viral loads in the experimental mice compared with mice that had received irrelevant memory T cells specific for influenza virus (26). To our knowledge, these are the only data demonstrating that peripheral effector memory T cells actually play a direct role in mediating immune control of a respiratory virus infection. It should be noted that airway memory T cells were not able to clear virus completely, but did significantly reduce viral loads at early time points during the infection. Thus, it has been suggested that effector memory T cells at peripheral sites, such as the lung airways, act as a first line of defense against infection, and effectively divide memory recall responses into two major phases. First, there is the immediate response of effector memory T cells at the site of infection. Importantly, these cells are able to respond at the first signs of infection when viral loads are very low. Although they are unable to proliferate in response to infection due to the constraints of the airway environment, they can produce cytokines that may limit viral replication and spread in the epithelium (27, 31). Second, there is the response of central memory cells that divide in response to antigen in local draining lymph nodes and are recruited to the lung airways as full-fledged effector T cells (24). These cells generate a prolonged supply of new effector cells.
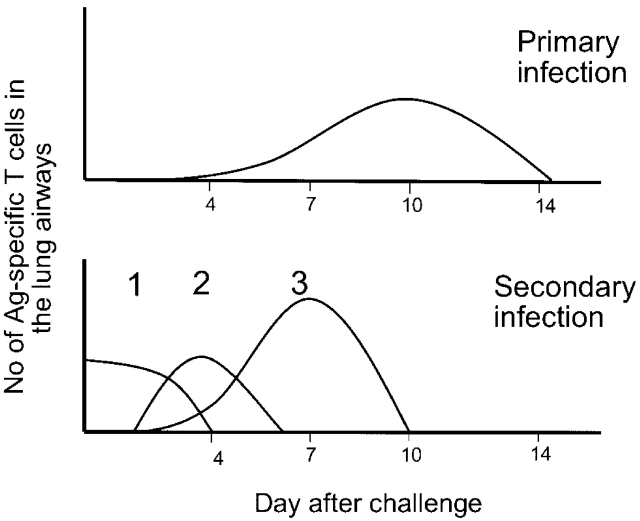
We have further modified this two-phase recall response to propose that there is also an intermediate phase, in which nonproliferating memory cells are recruited to the lung airways during the first week of the infection. This idea is based on studies showing that there is a rapid increase in the number of effector memory T cells expressing high levels of CD11a between Days 3 and 7 of infection (Figure 2). This recruitment is mediated by inflammation in the lung, but is antigen-independent. For example, when mice that have recovered from a prior Sendai virus infection are challenged with influenza virus, Sendai-specific CD11ahi memory T cells are recruited to the lung airways before the arrival of influenza virus–specific T cells. A key feature of the three-phase response model is that these distinct phases are integrated to produce a sustained response to the infection in the lung airways. Although the first two phases of the responses are nonrenewing (i.e., do not involve T cell expansion), they nevertheless engage the pathogen at the site of infection when viral loads are relatively low. Presumably, this reduces the amount of virus encountered by T cells comprising the third phase of the response. Because this latter phase involves proliferating cells, it is able to mediate a sustained response to the infection.
Figure 2.
T-cell response in the lung airways occurs in distinct phases. T cells responding to a primary respiratory virus infection begin to accumulate in the lung airways around Day 6 after infection and peak numbers are typically present on Day 10 after infection. T cells responding to a secondary respiratory virus infection are comprised of three distinct phases. The first phase involves memory T cells that are already in the lung airways. These cells respond immediately to the infection and their numbers decline rapidly. The second phase involves memory T cells that are rapidly recruited into the lung airways at early stages of the infection (Days 3–6). These cells are nonproliferating. The third phase involves memory T cells that have proliferated in response to antigen and are then recruited into the airways (peaking at Day 7). Taken together, the secondary response is characterized by a sustained T cell response to the infection. In addition, the accumulation of proliferating cells in the lung airways (phase 3 cells) occurs earlier, and is of greater magnitude, than in the primary response.
THE MAINTENANCE OF MEMORY T CELLS IN THE LUNG AIRWAYS
As we have discussed, it appears that resident lung-airway memory T cells are protective in the face of viral challenge. In terms of vaccine development, it is therefore important to understand how to establish and maintain this population. One hypothesis is that effector cells entering the airways during the infection are important in establishing this pool. This would be consistent with the large numbers of memory cells that are present in the lung airways immediately after viral clearance and the subsequent decline in this number with time. However, recent data suggest that infection is not required to draw effector cells into the airways. For example, transgenic T cells that have been activated in vitro and transferred to mice intravenously can be subsequently recovered from the lung airways (18, 32). These cells are recruited into the airways in the absence of any kind of pulmonary infection. One possible explanation is that the lung airways have a specific mechanism to continually recruit effector T cells. An alternative possibility is that there is always a low level of inflammation in the upper respiratory tract (perhaps due to exposure to the environment) that acts to continually recruit effector cells into the site. If this is the case, one might expect the number of effector cells recruited into the airways and the corresponding numbers of memory T cells established to be higher after the strong inflammatory response of a virus infection compared with resting conditions. This appears to be the case. For example, the absolute number of effector cells that migrate into the lung airways during a primary Sendai virus infection is much greater than during a secondary Sendai virus infection. Whereas both primary and secondary infections drive strong T-cell responses and generate large numbers of effector T cells, inflammation in the lung is greatly reduced in the secondary infection due to the presence of neutralizing antibody. Thus, there is a correlation between the level of inflammation and the numbers of cells recruited (39).
Although inflammatory processes can be evoked to explain the recruitment of effector cells into peripheral sites, the situation is less clear with memory T cells. It is known that memory T cell populations in secondary lymphoid organs are maintained by a poorly understood process of homeostatic proliferation driven by interleukin-7 and interleukin-15 (40–44). Interestingly, bromodeoxyuridine incorporation rates of memory CD8+ T cells in the lung airways match precisely those of memory cells in secondary lymphoid tissues. Yet we, and others (18, 32), have shown that T cells don't proliferate in the airways, presumably due to the inhibitory effects of surfactants and the inappropriate cytokine milieu. This suggests that bromodeoxyuridine-positive memory cells in the airways initially acquire bromodeoxyuridine during homeostasis-driven division in the secondary lymph nodes and only subsequently traffic into the airways. Importantly, this provides evidence for a process of continual recruitment of memory T cells to the lung airways.
There is additional evidence for a process of continual recruitment of memory T cells into the lung airways. As discussed previously here, an interesting characteristic of lung airway memory CD4+ and CD8+ T cells established by respiratory virus infections is the fact that the majority of cells express low levels of LFA-1 (CD11a/CD18). This feature distinguishes lung airway memory cells from other memory T cell populations in the animal, including those in the lung parenchyma and pleural cavity. We believe that this low level of LFA-1 expression may be due to the downregulation of the molecule after recruitment into the airways. Thus, high expression levels of LFA-1 may serve as a natural marker of cells that have been recently recruited into the airways. Because 20% of CD4+ and CD8+ memory T cells in the lung airways are LFA-1hi, this suggests that effector memory cell populations in the lung airways are maintained by a dynamic process of continual recruitment of new cells. We are actively investigating this possibility.
Downregulation of LFA-1 on lung airway memory T cells may have several important functional consequences. LFA-1 undergoes a conformational change after TCR ligation or exposure to specific cytokines or chemokines into an active form with increased ligand-binding affinity and lateral mobility (45–47). This promotes receptor clustering and the firm adhesion of migrating effector memory cells to the endothelial wall before extravasation into inflammatory sites (48, 49). Therefore, LFA-1 downregulation in the lung airways could serve to trap memory cells within the airways. Consistent with this, effector/memory cells that have entered the lung airways cannot re-enter the circulation (32). In addition, downregulation of LFA-1 may allow other adhesion molecules, such as very late antigen–1, to enhance localization of memory cells to the airway matrix (50). An alternative possibility is that LFA-1 downregulation may serve to limit lymphocyte effector functions, such as cytolytic activity, and so reduce lung immunopathology.
FUTURE DIRECTIONS AND THERAPEUTIC IMPLICATIONS
Recent studies have shown that memory T cells within the lung airways have unique phenotypic and functional features, likely reflecting the need to protect the tissue from damage. In addition, these memory T cells are able to significantly reduce lung viral loads following infection, potentially protecting mice from lethal infection. Thus, it will be important to clarify the mechanisms underlying the generation and maintenance of this T cell pool. A key question is whether recruitment is stochastic, or whether discrete peripheral memory cell subsets selectively populate the lung airways. Identifying the contributing subset will allow us to measure its decay, an important consideration in measuring vaccine efficacy. In addition, functional comparisons between the cells in the lung airways and those in the periphery should clarify the constraints imposed on T cell function by the airway environment.
It is unclear by which effector mechanisms airway memory T cells control viral replication and mediate protection. As we have stated previously here, lung airway CD8+ T cells appear to have reduced cytolytic activity in comparison with effector cells of other sites. It is possible, however, that cytokine production by antigen-specific memory T cells helps initiate an antiviral state within the lung. Alveolar macrophages mediate suppressive functions in the uninfected lung and IFN-γ produced by stimulated T cells is likely to activate antiviral activities in these cells. Similarly, T cell–derived granulocyte macrophage-colony stimulating factor is likely to stimulate maturation, differentiation, and trafficking of respiratory tract dendritic cells, thus enhancing antigen presentation to virus-specific central memory and naive T cells. Finally, effector T cells are potent producers of inflammatory chemokines, such as regulated on activation, normal T cell expressed and secreted, and their production by lung airway memory cells may be vital in the early recruitment of many antiviral immune cells, including immature dendritic cells, natural killer cells, and activated and effector memory T cells.
We have also described the early recruitment of nonproliferating memory T cells into the lung and lung airways. These cells include significant numbers of antigen-specific cells in mice that have recovered from a prior infection, and so may exert additional control on viral replication supplementing resident airway T cell activity before the arrival of T cells generated by antigen-driven proliferation. Currently, little is known of their origin, their mode of recruitment during inflammation, or their exact function during infection. It is important to investigate this aspect of the immune response in order to understand why protective memory T cell immune responses to respiratory virus infection wane over time.
In summary, a successful T cell vaccine should generate T cells capable of participating in all three phases of the memory T cell response; namely, airway resident memory T cells, nonproliferating memory T cells recruited early by inflammatory signals, and central memory T cells able to proliferate rapidly differentiate and mediate viral clearance. To do this, we clearly need to better understand the generation and maintenance requirements of each subpopulation. This knowledge will likely help guide vaccine formulation, adjuvant selection, and route of delivery.
Acknowledgments
The authors thank Drs. Kenneth Ely, Sherry Crowe, and Marcia Blackman for critically reading the manuscript.
Supported by funds from the Trudeau Institute and the PHS (HL63925, HL69502, AI055500, AG021600, and AI057158).
Conflict of Interest Statement: Neither of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Hou S, Doherty PC, Zijlstra M, Jaenisch R, Katz JM. Delayed clearance of Sendai virus in mice lacking class I MHC–restricted CD8+ T cells. J Immunol 1992;149:1319–1325. [PubMed] [Google Scholar]
- 2.Epstein SL, Lo CY, Misplon JA, Bennink JR. Mechanism of protective immunity against influenza virus infection in mice without antibodies. J Immunol 1998;160:322–327. [PubMed] [Google Scholar]
- 3.Graham MB, Braciale TJ. Resistance to and recovery from lethal influenza virus infection in B lymphocyte–deficient mice. J Exp Med 1997;186:2063–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichelberger M, Allan W, Zijlstra M, Jaenisch R, Doherty PC. Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex–restricted CD8+ T cells. J Exp Med 1991;174:875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doherty PC, Allan W, Eichelberger M, Carding SR. Roles of alpha beta and gamma delta T cell subsets in viral immunity. Annu Rev Immunol 1992;10:123–151. [DOI] [PubMed] [Google Scholar]
- 6.Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity 1998;8:683–691. [DOI] [PubMed] [Google Scholar]
- 7.Lukacher AE, Braciale VL, Braciale TJ. In vivo effector function of influenza virus–specific cytotoxic T lymphocyte clones is highly specific. J Exp Med 1984;160:814–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blandford G, Heath RB. Studies on the immune response and pathogenesis of Sendai virus infection of mice. II: the immunoglobulin class of plasma cells in the bronchial sub-mucosa. Immunology 1974;26:667–671. [PMC free article] [PubMed] [Google Scholar]
- 9.Harmsen AG, Muggenburg BA, Snipes MB, Bice DE. The role of macrophages in particle translocation from lungs to lymph nodes. Science 1985;230:1277–1280. [DOI] [PubMed] [Google Scholar]
- 10.Cerwenka A, Morgan TM, Dutton RW. Naive, effector, and memory CD8 T cells in protection against pulmonary influenza virus infection: homing properties rather than initial frequencies are crucial. J Immunol 1999;163:5535–5543. [PubMed] [Google Scholar]
- 11.Allan W, Tabi Z, Cleary A, Doherty PC. Cellular events in the lymph node and lung of mice with influenza: consequences of depleting CD4+ T cells. J Immunol 1990;144:3980–3986. [PubMed] [Google Scholar]
- 12.Hou S, Doherty PC. Clearance of Sendai virus by CD8+ T cells requires direct targeting to virus-infected epithelium. Eur J Immunol 1995;25:111–116. [DOI] [PubMed] [Google Scholar]
- 13.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol 1997;159:5197–5200. [PubMed] [Google Scholar]
- 14.Kagi D, Hengartner H. Different roles for cytotoxic T cells in the control of infections with cytopathic versus noncytopathic viruses. Curr Opin Immunol 1996;8:472–477. [DOI] [PubMed] [Google Scholar]
- 15.Woodland DL, Hogan RJ, Zhong W. Cellular immunity and memory to respiratory virus infections. Immunol Res 2001;24:53–67. [DOI] [PubMed] [Google Scholar]
- 16.Dutton RW, Swain SL, Bradley LM. The generation and maintenance of memory T and B cells. Immunol Today 1999;20:291–293. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science 1996;272:54–60. [DOI] [PubMed] [Google Scholar]
- 18.Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, Woodland DL. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol 2001;166:1813–1822. [DOI] [PubMed] [Google Scholar]
- 19.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature 2001;410:101–105. [DOI] [PubMed] [Google Scholar]
- 20.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 2001;291:2413–2417. [DOI] [PubMed] [Google Scholar]
- 21.Marshall DR, Turner SJ, Belz GT, Wingo S, Andreansky S, Sangster MY, Riberdy JM, Liu T, Tan M, Doherty PC. Measuring the diaspora for virus-specific CD8+ T cells. Proc Natl Acad Sci USA 2001;98:6313–6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostler T, Hussell T, Surh CD, Openshaw P, Ehl S. Long-term persistence and reactivation of T cell memory in the lung of mice infected with respiratory syncytial virus. Eur J Immunol 2001;31:2574–2582. [DOI] [PubMed] [Google Scholar]
- 23.Sallusto F, Langenkamp A, Geginat J, Lanzavecchia A. Functional subsets of memory T cells identified by CCR7 expression. Curr Top Microbiol Immunol 2000;251:167–171. [DOI] [PubMed] [Google Scholar]
- 24.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999;401:708–712. [DOI] [PubMed] [Google Scholar]
- 25.Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice: effector mechanisms and their longevity. J Immunol 1994;152:1653–1661. [PubMed] [Google Scholar]
- 26.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4+ T cells that persist in the lungs. J Exp Med 2001;193:981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogan RJ, Cauley LS, Ely KH, Cookenham T, Roberts AD, Brennan JW, Monard S, Woodland DL. Long-term maintenance of virus-specific effector memory CD8+ T cells in the lung airways depends on proliferation. J Immunol 2002;169:4976–4981. [DOI] [PubMed] [Google Scholar]
- 28.Lefrancois L. Dual personality of memory T cells. Trends Immunol 2002;23:226–228. [DOI] [PubMed] [Google Scholar]
- 29.Hviid L, Odum N, Theander TG. The relation between T-cell expression of LFA-1 and immunological memory. Immunology 1993;78:237–243. [PMC free article] [PubMed] [Google Scholar]
- 30.Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrancois L. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol 2004;172:4875–4882. [DOI] [PubMed] [Google Scholar]
- 31.Ely KH, Cauley LS, Roberts AD, Brennan JW, Cookenham T, Woodland DL. Nonspecific recruitment of memory CD8+ T cells to the lung airways during respiratory virus infections. J Immunol 2003;170:1423–1429. [DOI] [PubMed] [Google Scholar]
- 32.Harris NL, Watt V, Ronchese F, Le Gros G. Differential T cell function and fate in lymph node and nonlymphoid tissues. J Exp Med 2002;195:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ely KH, Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T cells in the lung airways retain the potential to mediate recall responses. J Immunol 2003;171:3338–3342. [DOI] [PubMed] [Google Scholar]
- 34.Marsland BJ, Harris NL, Camberis M, Kopf M, Hook SM, Le Gros G. Bystander suppression of allergic airway inflammation by lung resident memory CD8+ T cells. Proc Natl Acad Sci USA 2004;101:6116–6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyahara N, Swanson BJ, Takeda K, Taube C, Miyahara S, Kodama T, Dakhama A, Ott VL, Gelfand EW. Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness. Nat Med 2004;10:865–869. [DOI] [PubMed] [Google Scholar]
- 36.Cauley LS, Cookenham T, Miller TB, Adams PS, Vignali KM, Vignali DA, Woodland DL. Cutting edge: virus-specific CD4+ memory T cells in nonlymphoid tissues express a highly activated phenotype. J Immunol 2002;169:6655–6658. [DOI] [PubMed] [Google Scholar]
- 37.Arnold PY, Vignali KM, Miller TB, La Gruta NL, Cauley LS, Haynes L, Scott Adams P, Swain SL, Woodland DL, Vignali DA. Reliable generation and use of MHC class II:gamma2aFc multimers for the identification of antigen-specific CD4+ T cells. J Immunol Methods 2002;271:137–151. [DOI] [PubMed] [Google Scholar]
- 38.Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T cells play a prominent role in recall responses to secondary viral infection in the lung. J Immunol 2004;172:6533–6537. [DOI] [PubMed] [Google Scholar]
- 39.Cauley L, Cookenham T, Hogan R, Crowe S, Woodland D. Renewal of peripheral CD8 memory T cells during secondary viral infection of antibody sufficient mice. J Immunol 2003;170:5597–5606. [DOI] [PubMed] [Google Scholar]
- 40.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol 2000;1:426–432. [DOI] [PubMed] [Google Scholar]
- 41.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science 2000;288:675–678. [DOI] [PubMed] [Google Scholar]
- 42.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med 2002;195:1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med 2002;195:1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8+ T cells. J Exp Med 2002;196:935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature 1989;341:619–624. [DOI] [PubMed] [Google Scholar]
- 46.Oppenheimer-Marks N, Brezinschek RI, Mohamadzadeh M, Vita R, Lipsky PE. Interleukin 15 is produced by endothelial cells and increases the transendothelial migration of T cells in vitro and in the SCID mouse-human rheumatoid arthritis model in vivo. J Clin Invest 1998;101:1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Constantin G, Majeed M, Giagulli C, Piccio L, Kim JY, Butcher EC, Laudanna C. Chemokines trigger immediate beta2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity 2000;13:759–769. [DOI] [PubMed] [Google Scholar]
- 48.Dixon AE, Mandac JB, Martin PJ, Hackman RC, Madtes DK, Clark JG. Adherence of adoptively transferred alloreactive Th1 cells in lung: partial dependence on LFA-1 and ICAM-1. Am J Physiol Lung Cell Mol Physiol 2000;279:L583–L591. [DOI] [PubMed] [Google Scholar]
- 49.Thatte J, Dabak V, Williams MB, Braciale TJ, Ley K. LFA-1 is required for retention of effector CD8 T cells in mouse lungs. Blood 2003;101:4916–4922. [DOI] [PubMed] [Google Scholar]
- 50.Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, Sprague AG, Doherty PC, de Fougerolles AR, Topham DJ. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell–mediated immune protection against heterologous influenza infection. Immunity 2004;20:167–179. [DOI] [PubMed] [Google Scholar]