Abstract
It is now clear that the β2-adrenergic receptor continuously oscillates between various conformations in the basal state, and that agonists act to stabilize one or more conformations. It is conceivable that synthetic agonists might be engineered to preferentially confine the receptor to certain conformations deemed clinically important while having a less stabilizing effect on unwanted conformations. In addition, studies of genetically engineered mice have revealed previously unrecognized cross-talk between the β2-receptor and phospholipase C, such that removal of the primary dilating pathway results in downregulation of constrictive pathways and overactivity of the dilating pathway increases the contractile response. These results indicate a dynamic interaction between β2-receptor activity and Gq-coupled receptors that constrict the airway. Potentially, then, during chronic β-agonist therapy, expression of phospholipase C is increased, the functions of Gq-coupled constrictive receptors are enhanced, and there may be an increased tendency for clinical decompensation due to asthma and chronic obstructive pulmonary disease triggers. Antagonists to these receptors might be able to act synergistically with chronic β-agonists to block the effect of phospholipase C. Alternatively, perhaps novel phospholipase C antagonists would provide the most efficacious approach to blocking the physiologic sequelae of cross-talk between the β2-receptor and phospholipase C.
Keywords: asthma, β-agonist, chronic obstructive pulmonary disease, G protein–coupled receptor, phospholipase C
G PROTEIN–COUPLED RECEPTOR SUPERFAMILY
G protein–coupled receptors (GPCRs) represent the largest signaling family in the human genome. They transduce signals from the cell exterior to the interior from a host of systems, including the sight, smell, hormonal, neurotransmitter, autocrine, and paracrine systems. The β2-adrenergic receptor (β2AR) was one of the first receptors to be identified by radioligand binding, which solidified the notion that a receptor was in fact a bona fide entity rather than a theoretical concept that was useful for describing physiologic processes. Subsequent studies revealed that agonists for β2AR led to activation of adenylyl cyclase, and thus an increase in cyclic adenosine monophosphate (cAMP), via receptor interaction with a third protein, now termed the stimulatory guanine nucleotide–binding protein, Gs. Gs is a heterotrimer, consisting of an α subunit (the dissociated form stimulates adenylyl cyclase) and βγ subunits (which also transduce signals). Each of the aforementioned components of the β2AR pathway has been cloned and its amino acid sequences delineated. On the basis of structure–function studies and the ability to express these components in various cell types and genetically altered mice, concepts about β2AR signaling have changed fundamentally.
STRUCTURE OF THE β2AR
Like all GPCRs, the β2AR has seven transmembrane-spanning segments, which are α helices. As a consequence, there are three extracellular and three intracellular loops. The amino terminus is extracellular and the carboxy terminus is intracellular. Several posttranslational modifications are noteworthy. The human β2AR is N-glycosylated at amino acids 6, 15, and 187; these are important for proper insertion of the receptor into the membrane as well as for agonist trafficking (1, 2). Notably, the glycosylation site at amino acid 187 is restricted to higher order primates (1). At amino acid position 341, the cysteine of the human β2AR is palmitoylated (3). This acts to anchor this part of the carboxy terminus to the cell membrane and imparts several functional features (3). The region between the seventh transmembrane-spanning domain and the palmitoylated cysteine has also been shown to be an α helix in the homologous protein rhodopsin. Therefore, this region is sometimes denoted as the fourth intracellular loop or the eighth α helix. Agonist-promoted phosphorylation of the β2AR occurs via protein kinase A at serines in the third intracellular loop and the proximal cytoplasmic tail. Such phosphorylation decreases the coupling of the receptor to the G protein Gs and is one mechanism of agonist-promoted desensitization (4). A family of kinases termed G protein–coupled receptor kinases (GRKs) phosphorylates the β2AR at multiple serines and threonines in the cytoplasmic tail (4). Subsequent binding of β-arrestins to the phosphorylated receptor serves to (1) uncouple the receptor from Gs and thus desensitize receptor function (4), (2) promote receptor internalization, and (3) by virtue of the scaffolding action of β-arrestins, bring other proteins into the receptor's microdomain (5). An example of the latter is phosphodiesterase-4, which is recruited by β-arrestin and acts to metabolize the locally generated cAMP (6). Another posttranslational modification of the β2AR is ubiquitination, which occurs via an E3 ligase and is a prerequisite for receptor degradation (7).
AGONIST INTERACTION WITH THE β2AR: WHAT IS THE “ACTIVE” STATE?
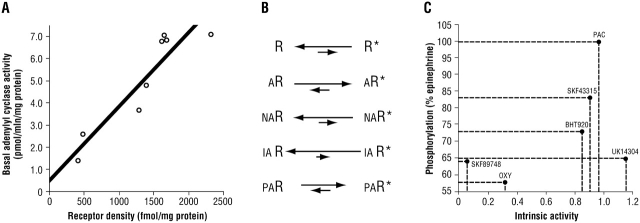
The traditional concept of agonist activation of the β2AR was akin to a lock and key: the agonist fit into the receptor and caused it to adopt a conformation that was favorable for coupling to Gs. However, it is now clear that the receptor is continuously “toggling” between various conformations in the absence of agonist. This is readily appreciated by measuring basal adenylyl cyclase activity in membranes from transfected cells expressing various levels of the β2AR (8) (Figure 1A). As expression increases, so does basal (nonagonist) adenylyl cyclase activity. In this model, agonist occupancy increases the probability that the receptor will be in the active state (Figure 1B). Neutral antagonists do not affect the equilibrium and thus adenylyl cyclase activities are not altered. (Of course, in a clinical setting in which the levels of synthetic or endogenous agonist are elevated, neutral antagonists nevertheless have a physiologic effect, because they block agonist access to the receptor.) Inverse agonists actually lower basal adenylyl cyclase activities because they preferentially bind to receptor in the inactive state. Data have suggested that most antagonists probably have the properties of an inverse agonist, but their efficacy in decreasing adenylyl cyclase is low and is probably clinically insignificant. Partial agonists either stabilize a different conformation of the receptor compared with full agonists or stabilize the same active conformation as a full agonist but do so less frequently.
Figure 1.
Features of the activation states of G protein–coupled receptors (GPCRs). (A) Spontaneous activation of the β2-adrenergic receptor (β2AR) in the absence of agonist is shown by the increase in basal adenylyl cyclase activity as β2AR expression increases (8). (B) Actions of various ligands on the equilibrium between inactive receptor (R) and active receptor (R*). A = agonist; NA = neutral antagonist; IA = inverse agonist; PA = partial agonist. (C) Lack of correlation between the intrinsic activity of an agonist and agonist-promoted receptor phosphorylation by GRK2 of the α2AAR. PAC = p-aminoclonidine; OXY = oxymetazoline.
Oscillation of the β2AR between various conformations brings into question whether there is a single “active” conformation. Traditionally, the active state was defined by a single function, which was coupling to Gs, with the “readout” being cAMP production or adenylyl cyclase activities. However, it is now clear that multiple signals are promoted by β2AR, and that the “ideal” conformation for one effector signal may not be the same as that for another. For example (9), the β2AR activates the sodium–hydrogen exchanger regulatory factor by direct interaction with the carboxy-terminal domain of the receptor (no G protein is required). Conceivably, an agonist might preferentially activate the Gs pathway compared with the sodium–hydrogen exchanger regulatory factor pathway. Other conformations, such as those that favor GRK-mediated phosphorylation, β-arrestin binding, mitogen-activated protein kinase activation, or Gi coupling, might also be selectively activated, or not activated, by synthetic agonists. Indeed, for the closely related α2ARs, it has been shown that there is a poor correlation between agonist-promoted phosphorylation of α2AR by GRKs and the intrinsic activity (compared with the prototypic full agonist epinephrine) of the given synthetic agonist (10) (Figure 1C). Similarly, some agonists show a relative preference for α2AR–Gs coupling over α2AR–Gi coupling (11). Taken together, the data suggest that GPCRs, including the β2AR, oscillate between many conformations in the basal state, and that agonists act to stabilize one or more conformations. Thus, it is conceivable that synthetic agonists might be engineered to preferentially confine the receptor to certain conformations deemed clinically important (e.g., coupling to Gs) while having a less stabilizing effect on potentially unwanted conformations such as phosphorylation by GRKs.
AFTER RECEPTOR ACTIVATION: DEEP PATHWAY EFFECTS
With prolonged agonist activation, β2ARs undergo processes that limit function, generically termed desensitization. These events are critical for the cell to integrate the myriad signals being received, and for adaptation to physiologic and pathologic states. Desensitization may also limit the therapeutic effectiveness of prolonged agonist exposure (tachyphylaxis), although this may be highly dependent on the structure of the agonist, the cell type, the disease being treated, and the outcome measures deemed to be relevant. Long-term desensitization of the β2AR is the result of the net effect of several processes, including the short-term events of protein kinase A and GRK phosphorylation, Gi coupling, and the long-term events leading to a decrease in receptor expression (termed downregulation), which is due to transcriptional as well as protein degradation mechanisms.
Several studies (12, 13) have begun to investigate the effects of long-term β2AR activation on signaling elements far removed from the receptor, G protein, and effector. The bases for such investigations with β2AR in asthma and chronic obstructive pulmonary disease (COPD) were intriguing clinical observations made during chronic β-agonist therapy that were not readily reconciled by the traditional desensitization paradigm. For example, some, but certainly not all, studies have shown that β-agonists administered on a regular schedule result in a decrease in bronchodilator function. β2AR desensitization presumably causes this decrease in bronchodilator function. In addition, chronic use of β-agonists causes an increase in sensitivity (i.e., a decrease in the provocative concentration of an agonist causing a 20% fall in FEV1) to the bronchoconstrictive effects of agents such as methacholine and histamine. This phenomenon appears to be observed more consistently than tachyphylaxis of bronchodilatation from chronic β-agonists. It has also been ascribed to β2AR desensitization, supposedly because of less opposed bronchoconstriction, and thus increased hyperreactivity.
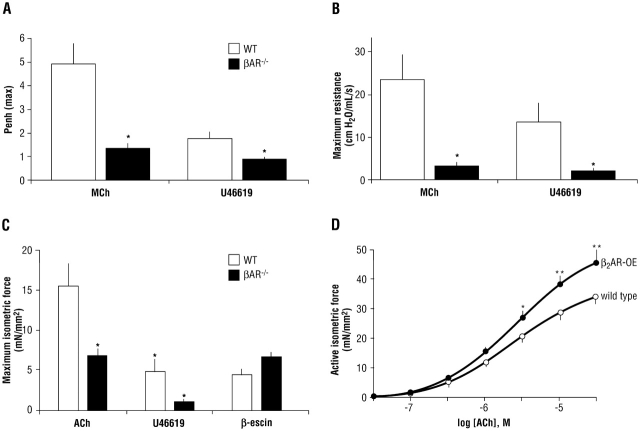
We hypothesized that these events might be explained by regulation of other signaling elements apart from the receptor (12). To explore this possibility, we studied genetically altered mice in which the β2AR was overexpressed in airway smooth muscle (mimicking constant β2AR activation) and mice in which the β1- and β2AR genes were ablated (equivalent to an absolute lack of airway βAR activity). In the βAR knockout (βAR−/−) mice we were surprised to find that removal of this bronchodilating pathway resulted in a paradoxical decrease in the bronchoconstricting effect of methacholine and the thromboxane mimetic U46619, as studied by whole-animal plethysmography (Figure 2A). This finding was confirmed by measuring airway resistance in an intubated mouse model, in which the contractile responses to methacholine and serotonin were also found to be decreased (Figure 2B). Finally, tracheal rings were studied ex vivo, and the phenomenon was again observed (Figure 2C). Here we were able to expose the rings to β-escin, an agent that makes the cell permeable, allowing an influx of extracellular calcium and subsequent contraction via non-GPCR mechanisms. We found that contraction was the same for βAR−/− rings compared with rings from wild-type mice, which indicated that the basic contractile apparatus was intact in βAR−/− mice.
Figure 2.
Functional consequences of altered βAR activity in the airways of genetically modified mice. (A) βAR–/– mice display a paradoxical decrease in the bronchoconstricting effect of methacholine (MCh) and the thromboxane mimetic U46619, as assessed by whole-body plethysmography. Penh indicates enhanced pause, a function of the maximum inspiratory and maximum expiratory pressures and the timing of expiration. * p < 0.001 compared with wild type (WT). (B) In vivo airway resistance responses to contractile ligands are also decreased in βAR–/– mice. * Not different (p > 0.05) from baseline resistance. (C) Airway smooth muscle contractions in response to Gq-coupled receptor agonists are impaired in βAR–/– tracheal rings. ACh = acetylcholine. * p = 0.005. (D) β2AR-overexpressing (β2AR-OE) mice have increased contractile responses in airway smooth muscle. * p = 0.02; ** p < 0.001 (12).
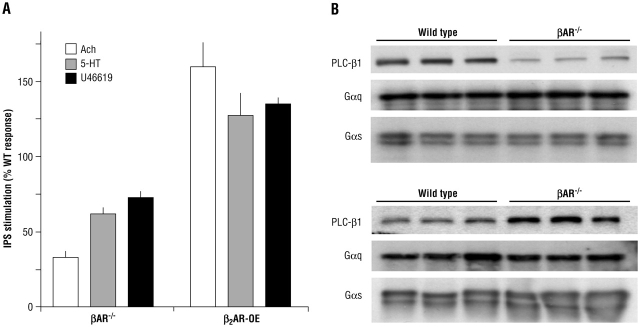
To isolate the mechanism of regulation of these constrictive Gq-coupled receptors by the absence of βAR, we generated primary airway smooth muscle cells from each line. As shown in Figure 3, inositol 1,4,5-trisphosphate (IP3) production was decreased in βAR−/− mice. In addition, consistent with what we term antithetic regulation, IP3 production was increased in β2AR-overexpressing (β2AR-OE) mice. This, along with the β-escin data, indicated that the mechanism of this regulation was likely to be at the level of a receptor, Gq, or the effector phospholipase C (PLC), rather than IP3 precursors, the endoplasmic reticulum IP3 receptor, or altered intracellular Ca2+ stores. We believed it unlikely that the Gq-coupled receptors that we activated (M3-muscarinic, 5HT2, and thromboxane) were all downregulated in βAR−/− mice and upregulated in βAR-OE mice. We thus concentrated on elements shared by these receptors. Immunoblotting of airway smooth muscle cell lysates revealed no change in Gαq content or in the levels of the cognate G protein for the β2AR, Gαs. However, in βAR−/− mice, the PLC-β1 content of βAR−/− airway smooth muscle cells was markedly decreased compared with cells from wild-type mice (Figure 3). And, in β2AR-OE cells, PLC-β1 was increased about threefold over wild type. The decrease in PLC-β1 in βAR−/− mice was entirely consistent with the decreases in bronchoconstriction and IP3 production observed in response to Gq-coupled receptor agonists. Tracheal ring studies from β2AR-OE mice revealed what might be predicted from the IP3 results and the immunoblotting: acetylcholine evoked greater contraction and had greater potency in these mice compared with wild type (Figure 2D). These data revealed a previously unrecognized interaction between β2AR and PLC. It appears that there is a mechanism in place to cross-regulate bronchodilating and bronchoconstricting responses. Removal of the primary dilating pathway resulted in a downregulation of constrictive pathways, and overactivity of the dilating pathway had the effect of increasing the contractile response. Data from Callaerts-Vegh have shown in a mouse model of asthma that chronic treatment (28 days) with β-blockers decreases contractile responses (13), analogous to the results we found in βAR−/− mice (12).
Figure 3.
Signaling consequences of altered βAR activity in airway smooth muscle cells derived from genetically modified mice. (A) IP3 responses to the indicated Gq-coupled receptor agonists. ACh = acetylcholine; β2AR-OE = β2AR-OE cells; 5-HT = serotonin. (B) Immunoblots indicating regulation of phospholipase C (PLC)-β1 by β2AR activity (12).
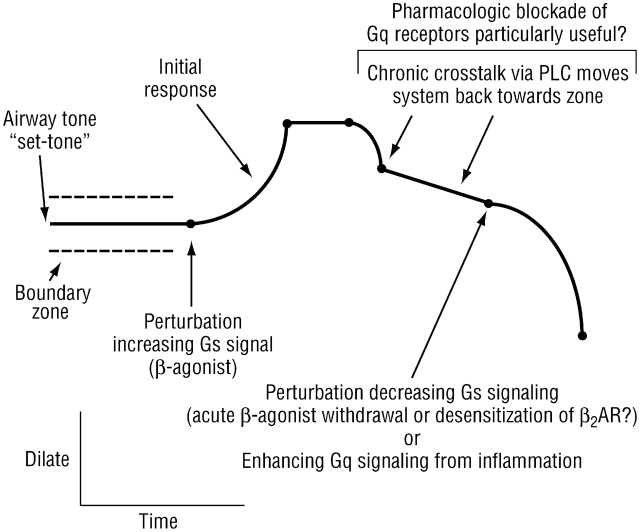
These results indicate a dynamic interaction between β2AR activity and receptors that act to constrict the airway. The local concentrations of agonists for these receptors, such as acetylcholine, serotonin, thromboxane, histamine, certain prostaglandins, and certain leukotrienes (acting at the cysteinyl leukotriene-1 receptor) are increased in asthma, and potentially in chronic bronchitis. Thus, during chronic β-agonist therapy, when PLC expression is increased and the functions of these Gq-coupled constrictive receptors are enhanced, there may be an increased tendency for clinical decompensation due to infection, allergy, or other asthma/COPD triggers. As such, antagonists to these receptors may be particularly useful under these circumstances. Interestingly, as monotherapy in asthma, these antagonists (such as ipratropium, montelukast, and seratrodast) have limited efficacy. However, they could act synergistically with chronic β-agonists to block the PLC effect and thus achieve a more satisfactory clinical response. Still, because there are so many Gq-coupled constrictive receptors, it will be difficult to fully block this pathway with receptor antagonists. Perhaps new pharmacologic agents that antagonize PLC-β1 would provide the most efficacious approach to blocking the physiologic sequelae of the chronic β-agonist cross-talk. The events discussed above are also expected in patients with COPD who are treated long term with β-agonists. However, we know less about which endogenous Gq-coupled receptors are activated in this disease. Certainly the favorable response to ipratropium in COPD and the synergy of this anticholinergic with β-agonists are consistent with the cholinergic pathway as an active participant. The potential clinical relevance of β2AR–PLC cross-talk is illustrated in Figure 4.
Figure 4.
Potential clinical relevance of β2AR–phospholipase C (PLC) cross-talk. β-Agonists initially dilate the airway but also evoke cross-talk with constrictive receptors, essentially moving airway tone back toward the “set point” after prolonged treatment. A perturbation of contraction–relaxation signaling under these conditions could lead to a substantial decrease in airway caliber. Such a perturbation might be abrupt withdrawal of β-agonist (unopposed contraction) or further activation of Gq signaling from constrictive ligands released during inflammation.
It is intriguing to consider this paradigm in the light of several studies that show associations between β2AR polymorphisms (14, 15) and worsening asthma control (16–18). Because the trigger for this PLC-based constrictive pathway cross-talk is the β2AR, any genetic variation that alters expression, function, or regulation of the β2AR will alter the degree of augmentation of Gq receptor signaling. As such, a β2AR polymorphism might also influence the response to a Gq receptor antagonist such as ipratropium. This indeed appears to be the case in the beta-adrenergic response by genotype (BARGE) trial (18). Patients with asthma who were homozygous for Arg-16, when withdrawn from β-agonist therapy and provided with ipratropium for rescue purposes, had a significant increase in morning peak expiratory flow. Gly-16 homozygotes showed no such improvement under the same conditions. Although the trial was not specifically designed to address ipratropium responsiveness, the results are nevertheless consistent with the concept of chronic β2AR cross-talk with M3-muscarinic receptor signaling of the airway.
Supported by NIH grants HL045967 and HL071609.
Conflict of Interest Statement: D.W.M. received $1,500 for being on an advisory board for GlaxoSmithKline. S.B.L. received $20,000 in 2004 for speaking at conferences sponsored by Merck & Co., Inc., and $3,000 for being on an advisory board for Novartis.
References
- 1.Mialet-Perez J, Green SA, Miller WE, Liggett SB. A primate-dominant third glycosylation site of the β2-adrenergic receptor routes receptors to degradation during agonist regulation. J Biol Chem 2004;279:38603–38607. [DOI] [PubMed] [Google Scholar]
- 2.Rands E, Candelore MR, Cheung AH, Hill WS, Strader CD, Dixon RA. Mutational analysis of β-adrenergic receptor glycosylation. J Biol Chem 1990;265:10759–10764. [PubMed] [Google Scholar]
- 3.O'Dowd BF, Hnatowich M, Caron MG, Lefkowitz RJ, Bouvier M. Palmitoylation of the human β2-adrenergic receptor: mutation of Cys341 in the carboxyl tail leads to an uncoupled nonpalmitoylated form of the receptor. J Biol Chem 1989;264:7564–7569. [PubMed] [Google Scholar]
- 4.Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein–coupled receptor regulation. Annu Rev Pharmacol Toxicol 1998;38:289–319. [DOI] [PubMed] [Google Scholar]
- 5.Benovic JL. Novel β2-adrenergic receptor signaling pathways. J Allergy Clin Immunol 2002;110:S229–S235. [DOI] [PubMed] [Google Scholar]
- 6.Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL, Miller WE, McLean AJ, Conti M, Houslay MD, et al. Targeting of cyclic AMP degradation to β2-adrenergic receptors by β-arrestins. Science 2002;298:834–836. [DOI] [PubMed] [Google Scholar]
- 7.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated β2-adrenergic receptor and β-arrestin. Science 2001;294:1307–1313. [DOI] [PubMed] [Google Scholar]
- 8.Green SA, Cole G, Jacinto M, Innis M, Liggett SB. A polymorphism of the human β2-adrenergic receptor within the fourth transmembrane domain alters ligand binding and functional properties of the receptor. J Biol Chem 1993;268:23116–23121. [PubMed] [Google Scholar]
- 9.Hall RA, Premont RT, Chow CW, Blitzer JT, Pitcher JA, Claing A, Stoffel RH, Barak LS, Shenolikar S, Weinman EJ, et al. The β2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature 1998;392:626–630. [DOI] [PubMed] [Google Scholar]
- 10.Jewell-Motz EA, Small KM, Theiss CT, Liggett SB. α2A/α2C-Adrenergic receptor third loop chimera show that agonist interaction with receptor subtype backbone establishes G protein-coupled receptor kinase phosphorylation. J Biol Chem 2000;275:28989–28993. [DOI] [PubMed] [Google Scholar]
- 11.Eason MG, Jacinto MT, Liggett SB. Contribution of ligand structure to activation of α2-adrenergic receptor subtype coupling to Gs. Mol Pharmacol 1994;45:696–702. [PubMed] [Google Scholar]
- 12.McGraw DW, Almoosa KF, Paul RJ, Kobilka BK, Liggett SB. Antithetic regulation by β-adrenergic receptors of Gq receptor signaling via phospholipase C underlies the airway β-agonist paradox. J Clin Invest 2003;112:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callaerts-Vegh Z, Evans KL, Dudekula N, Cuba D, Knoll BJ, Callaerts PF, Giles H, Shardonofsky FR, Bond RA. Effects of acute and chronic administration of β-adrenoceptor ligands on airway function in a murine model of asthma. Proc Natl Acad Sci USA 2004;101:4948–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Small KM, McGraw DW, Liggett SB. Pharmacology and physiology of human adrenergic receptor polymorphisms. Annu Rev Pharmacol Toxicol 2003;43:381–411. [DOI] [PubMed] [Google Scholar]
- 15.Small KM, Rathz DA, Liggett SB. Identification of adrenergic receptor polymorphisms. Methods Enzymol 2002;343:459–475. [DOI] [PubMed] [Google Scholar]
- 16.Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli VM, Cooper DM, Fahy JV, Fish JE, Ford JG, et al. The effect of polymorphisms of the β2-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med 2000;162:75–80. [DOI] [PubMed] [Google Scholar]
- 17.Taylor DR, Drazen JM, Herbison GP, Yandava CN, Hancox RJ, Town GI. Asthma exacerbations during long term β-agonist use: influence of β2-adrenoceptor polymorphism. Thorax 2000;55:762–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Israel E, Chinchilli VM, Ford JG, Boushey A, Cherniack R, Craig TJ, Deykin A, Fagan JK, Fahy JV, Fish J, et al. Use of regularly scheduled albuterol treatment in asthma: genotype stratified, randomised, placebo controlled cross-over trial. Lancet 2004;364:1505–1512. [DOI] [PubMed] [Google Scholar]