Abstract
Solid organ and hematopoietic stem cell transplantation are definitive therapies for a variety of end-stage diseases. Immunosuppression has improved graft survival but leaves the patient susceptible to infectious complications. Of these, pulmonary infections are the leading cause of morbidity and mortality in the transplant recipient. Allograft rejection is mediated primarily by T cells, with B cells playing a role via antibody production. Depending on the transplant type, rejection can be hyperacute, acute, or chronic. Hyperacute rejection occurs as an immediate response to preformed antibodies to donor human leukocyte antigens. Acute cellular rejection involves recipient T-cell recognition of human leukocyte antigen molecules expressed on donor-derived, antigen-presenting cells (direct allorecognition) or presentation of donor-derived peptides by recipient antigen-presenting cells to recipient T cells (indirect allorecognition). Once the alloantigens are recognized as foreign, the activation, proliferation, and production of cytokines by T lymphocytes and other immune cells lead to the amplification of the alloimmune response. This complex process involves the generation of effector T cells, antibody production by activated B cells, and macrophage activation. Alloimmunity is facilitated by the production of many cytokines, chemokines, and other effector molecules, such as complement. The immunosuppressants involve many classes of drugs, including antibody therapies that eliminate specific groups of cells or alter signaling pathways used by effector cells. The article reviews the agents and associated infections.
Keywords: hematopoietic stem cell transplant, immunosuppression and pulmonary infections, solid organ transplant
Solid organ and hematopoietic stem cell transplantation (HSCT) are definitive therapies for a variety of end-stage diseases. Immunosuppression has improved graft survival but leaves the patient susceptible to infectious complications, of which pulmonary infections are the leading cause of morbidity and mortality.
Allograft rejection is mediated primarily by T cells, with B cells playing a role via antibody production. Depending on the transplant type, rejection can be hyperacute, acute, or chronic. Hyperacute rejection occurs as an immediate response to preformed antibodies to donor human leukocyte antigens (HLA). Acute cellular rejection involves recipient T-cell recognition of HLA molecules expressed on donor-derived, antigen-presenting cells (direct allorecognition) or presentation of donor-derived peptides by recipient antigen-presenting cells to recipient T cells (indirect allorecognition). Once the alloantigens are recognized as foreign, the activation and production of cytokines by T lymphocytes and other immune cells lead to the amplification of the alloimmune response. This complex process involves the generation of effector T cells, antibody production by activated B cells, and macrophage activation. Alloimmunity is facilitated by the production of many cytokines, chemokines, and other effector molecules, such as complement (1, 2). Chronic rejection, a slow but unrelenting process, is characterized by remodeling of the graft extracellular matrix. This process produces scarring by elaborating collagen, fibronectin, and proteoglycans, which leads to progressive graft dysfunction and loss.
The immunosuppressants used to prevent and treat rejection involve many classes of drugs, including antibody therapies that eliminate specific groups of cells or alter signaling pathways used effector cells. The following section discusses the effects of these agents on pathways involved in regulating immunity to transplanted organs.
IMMUNOSUPPRESSANTS
Corticosteroids
Although glucocorticoids are the first immunosuppressants used in transplantation.Although glucocorticoids are potent, they are the least selective agents and affect multiple leukocyte cell lines, including T and B cells, macrophages, granulocytes, and monocytes. When not protein bound, glucocorticoids are highly lipophilic and readily cross cell membranes where they bind to cytoplasmic receptors and influence transcription in a positive or negative manner.
In lymphocytes, glucocorticoids exert their negative regulatory effect on cytokine gene expression by directly inhibiting two transcription factors: activator protein-1 (AP-1) and nuclear factor–κB. They seem to inhibit the interaction between AP-1 and transcriptional regulatory proteins—specifically members of the c-Fos/c-Jun family—thus blocking IL-2 production. Nuclear factor–κB is a key component in the induction of several immunoregulatory genes, including those for IL-1, -2, -3, and -6; γ-IFN; CD40 ligand; tumor necrosis factor (TNF)-α; granulocyte/ macrophage colony-stimulating factor; and MHC molecules. AP-1 is also involved in the regulation of these genes. Glucocorticoids exert their effect on monocytes in a similar manner. They also inhibit other sites in the T-cell activation pathway by blocking the breakdown of cytokine mRNA and the tyrosine phosphorylation of intracellular proteins. Glucocorticoids cause a transient but significant lymphocytopenia by redistributing circulating lymphocytes, changing the expression of adhesion molecules, and causing the lysis of immature T lymphocytes. Their effect on B cells is in part mediated by a reduction in T-cell help required for antibody production.
Glucocorticoids interact with many nonlymphoid cell lines and retard inflammatory responses by inhibiting vasoactive and chemo-attractant substances. They inhibit the ability of neutrophils to adhere to endothelium by decreasing the expression of endothelial leukocyte adhesion molecule-1 and intercellular cell adhesion molecule-1, thus decreasing extravasation and chemotaxis. These agents also interfere with MHC class II expression on antigen-presenting cells in part by suppressing production of IFN-γ, IL-1 and -6, TNF-α, prostaglandins, and leukotrienes (3).
Calcineurin Inhibitors: Cyclosporine A and Tacrolimus (FK506)
The calcineurin inhibitors cyclosporine and tacrolimus have greatly decreased the incidence of allograft rejection. Because they are more T-cell selective, use of these agents has allowed more for preservation of other myeloid-derived cell lines and has reduced the overall incidence of infection by facilitating the lowering of corticosteroid doses (4). These immunosuppressants inhibit T-cell activation by binding to intracellular immunophilins; cyclophilin A binds cyclosporine, and FK–binding protein-12 binds tacrolimus. Although they are structurally unrelated, both have a cis-trans prolyl-peptidyl isomerase activity that increases the binding affinity of the immunophilins to calcineurin, which inhibits its action. Calcineurin, a calmodulin-activated serine threonine phosphatase found in T lymphocytes, dephosphorylates inactive nuclear factor of T cells (NFAT), which leads to its translocation to the nucleus and the subsequent activation of T cells. NFAT 1, NFAT 2, and NFAT 4 are responsible for the activation of IL-2, IL-4, and CD40 ligand. By inhibiting calcineurin, cyclosporine and tacrolimus also interfere with the activation of IκB, Na-K-ATPase, IL-3, granulocyte/macrophage colony-stimulating factor, and nitric oxide synthase.
Despite their similarities, cyclosporine and tacrolimus have different effects on multiple molecules, including IL-10, transforming growth factor–β, p-glycoprotein expression, anti-endothelial antibody production, and apoptotic potential. Both inhibit production of IL-1β, IL-2, IL-6, IL-8, IFN-γ, and TNF-α. Tacrolimus preferentially suppresses helper T 1 cells over helper T 2 cells. It also suppresses other functions in T cells and macrophages. Both agents have been shown to upregulate transforming growth factor–β, which, although it is an immunosuppressive molecule, promotes matrix formation and may contribute to allograft fibrosis. This effect may play a role in the development of chronic rejection. Tacrolimus also seems to lead to less anti-HLA antibody formation and augments glucocorticoid-induced apoptosis of antigen stimulated T cells. Both agents inhibit the Jun N terminal kinase and p38 pathways, which are important in the activation of AP-1. These drugs do not seem to interfere with mononuclear phagocytosis (5, 6). Recent reports indicate that both agents suppress antigen presentation by dendritic cells (7). Thus, by inhibiting T-cell activation and proliferation, the calcineurin inhibitors are indeed potent immunosuppressants.
Antimetabolites: Azathioprine and Mycophenolate Mofetil
Azathioprine (AZA), a pro-drug of 6-mercaptopurine (6-MP), was first developed in the 1950s. Mycophenolate mofetil (MMF) was later developed in an attempt to replace AZA by being more potent and selective. Both are classified as antimetabolite agents because of their ability to inhibit DNA and RNA production. Although most cell lines use the salvage and de novo pathways for purine synthesis, lymphocytes rely almost exclusively upon the de novo pathway. By blocking the de novo synthesis of purine, which is required for T- and B-cell proliferation, they prevent clonal expansion. An imidazolyl derivative of 6-MP, AZA exerts its effects by several mechanisms, including the inhibition of DNA synthesis, purine metabolism, nucleotide synthesis, and blocking the CD28 costimulation pathway (3). These actions result in inhibition of T-cell activation, reduced antibody production, and decreased levels of circulating monocytes and granulocytes. AZA releases the bioactive 6-MP, which is converted to 6-thioinosine-5′-monophosphate, which in turn converts into several thioguanine nucleotides leading to the inhibition of DNA synthesis. AZA, via 6-MP, also inhibits critical enzymes of the de novo pathway of purine synthesis. One of the enzymes involved in the purine salvage pathway, hypoxanthine-guanine phosphoribosyl transferase, participates in the activation of 6-MP. Hypoxanthine-guanine phosphoribosyl transferase transforms 6-MP into thioinosinic mercaptopurine, which inhibits the de novo pathway enzymes phosphoribosyl pyrophosphate synthase and inosinate monophosphate dehydrogenase (IMPDH). Thus, by preventing the formation of adenosine monophosphate (AMP) and the pivotal guanosine monophosphate (GMP), the de novo purine pathway is inhibited. Therefore, AZA's mechanism of action results in suppression of all hematopoietic cell lines.
MMF, the morpholinoethyl ester pro-drug of mycophenolic acid (MPA), is a more potent and selective inhibitor of the de novo purine pathway without significant affect on hematopoietic or neutrophil populations. MMF more profoundly inhibits the proliferation of T and B lymphocytes, blocks antibody production (including anti-HLA), and decreases the generation of cytotoxic natural killer (NK) cells and delayed-type hypersensitivity (DTH) response. MMF, via MPA, inhibits IMPDH by binding to the cofactor site (NAD/H2O) located next to the substrate site for inosine monophosphate (8). This is noncompetitive inhibition; MPA is not a purine analog but rather inhibits cofactor binding. It prevents the rate-limiting enzyme of GMP production, IMPDH, from converting IMP to xanthosine 5′-monophosphate, which is converted to GMP. With IMPDH inhibited, an imbalance between GMP and AMP ensues with the accumulation of AMP and, via negative feedback, downregulates more proximal enzymes within the de novo pathway. MMF has also been shown to inhibit the glycosylation of leukocyte adhesion molecules, thereby decreasing the recruitment of lymphocytes and monocytes to areas of inflammation, and reduces cytokine production through the inhibition of clonal expansion (3, 9–13).
Target of Rapamycin Inhibitors: Sirolimus and Everolimus
Rapamycin (or sirolimus), which is structurally related to tacrolimus, is a lipophilic macrolide antibiotic that binds the FK-binding proteins. However, it does not bind to or inhibit calcineurin or cytokine transcription. Instead, it binds to a kinase, named target of rapamycin, preventing the translation of mRNA responsible for cell cycle regulation. When cytokines such as IL-2 bind to T-cell receptors, they activate intracellular phosphatidyl inositol 3 kinase, which activates protein kinase B. Protein kinase B activates target of rapamycin, which, in association with PP2A (protein phosphatase 2A), controls the rate of phosphorylation of regulatory proteins, specifically, translational inhibitor 4E-BP1 (needed for cell division), eukaryotic translation initiator protein 4G1 (eIF4G1), and p70s6 kinase (active on ribosomal protein S6). Inhibition of these pathways results in failure of the cell cycle to progress from G1 to the S phase. Thus, by blocking IL-2 postreceptor signaling and arresting the cell cycle, T-cell proliferation is prevented (3, 14). Everolimus, a new synthetic derivative of sirolimus, has the same mechanism of action (15). These two drugs have also been shown to inhibit growth factor–driven proliferation of smooth muscle, fibroblast cells, and endothelial cells (16), which may account for the unusually high incidence of airway anastomotic dehiscence seen with sirolimus use in lung transplantation (17).
Polyclonal Antilymphocyte Antibodies
Developed early in the 1900s as antiinflammatory agents, the polyclonal antilymphocyte antibodies have a long history of use in transplantation and have been used for induction and to treat acute rejection. These polyclonal agents are purified monomeric anti-human gamma globulins created by immunizing animals with human lymphocytes or thymocytes. They are usually made from horse or rabbit and can be made to target different cell lines, such as lymphocytes, thymocytes, or specific T-cell lines. These antibodies result in direct lymphocyte depletion due to complement-mediated opsonization or Fas-dependent apoptosis. Profound, prolonged lymphopenia can result from the use of these agents. These polyclonal agents eliminate preactivated uncirculating memory lymphocytes. In general, they are very nonspecific immunosuppressive agents because these preparations contain antibodies to a wide variety of T- and B-cell antigens, NK surface antigens, adhesion and co-stimulatory molecules, and class I and II MHC antigens (3).
Monoclonal Antibodies
Antilymphocyte antibodies.
The first monoclonal antibody (MoAb) used in organ transplantation, OKT3 or Muromonab-CD3 (M-CD3), is a murine IgG2a monoclonal antibody produced in vitro by a B-cell/myeloma cell hybridoma directed against the epsilon subunit of CD3, which is closely linked to 90% of the T-cell receptors (TcR). CD3 facilitates the translational expression of the TcR-α and -β chains on the cell surface to intracellular signaling. The TcR is critical in CD4+ cell activation by alloantigen and in the ability of CD8+ cells to bind and lyse targeted cells. M-CD3 initially activates T cells, causing a massive release of cytokines, but within hours of administration, TcR-α and -β chains are internalized, rendering the T cell unable to respond to antigens. Binding of M-CD3 also causes T-cell opsonization and the removal of T cells from the circulation by mononuclear phagocytes in the liver and spleen. Apoptosis of activated T cells and NK/antibody dependent cytotoxicity of T cells has also been reported (3). Therefore, as with the polyclonal antibodies, M-CD3 causes nonspecific T-cell depletion.
Anticytokine receptor antibodies.
Daclizumab is a humanized MoAb, whereas basiliximab is a human/mouse chimeric MoAb directed against the α-chains of the CD25 molecule, a key unit of the IL-2 receptor. IL-2 has a central role in regulating T-cell activation, differentiation, and apoptosis. Accordingly, anti-CD25 antibodies inhibit IL-2 induced T-cell proliferation. IL-2 also regulates elimination of activated T cells by regulating the interaction between the Fas ligand and the Fas receptor (activation-induced cell death) (8). Because it targets primarily CD25+ T cells, there is no significant depletion of other T-cell populations. Binding to IL-2α also causes the downregulation of IL-2β/IL-15Rβ expression resulting in suppression of IL-15–dependent proliferation. There is no effect on IL-7–dependent mononuclear cell proliferation (18–20).
With the initial success of the MoAb class of immunosuppressant, many other drugs are under investigation or used in transplantation. Efalizumab, a humanized IgG1 MoAb against the CD11α chain of the leukocyte-function-associated antigen 1, is a nonlymphocyte-depleting antibody that, by blocking the binding of leukocyte-function-associated antigen 1–intercellular cell adhesion molecule-1, prevents T-cell adhesion, activation, and trafficking. Costimulatory signals are another potential target for antibody blockade. Antigens require a costimulatory signal to activate T cells. The B7 molecules (CD80 and CD86) are expressed on many antigen-presenting cells, B cells, and activated endothelial cells. LEA29Y, a MoAb against CD80 and CD86, is in clinical trials (18). Rituximab, a human-murine chimeric anti-CD20 antibody, is active on all cells in B-cell lines except Pro-B cells and plasma cells. It has been successfully used to treat humoral-mediated rejection in cardiac, renal, and liver transplantation (21).
Alemtuzumab (anti-CD52 MoAb/Campath-1H), first used in 1986, is a humanized IgG1 MoAb that causes profound T-cell depletion through compliment-mediated cell lysis. CD52 is expressed on T, B, NK, and dendritic cells but not on hematopoietic stem cells. Therefore, alemtuzumab causes depletion of peripheral T cells and markedly reduced levels of B cells, NK cells, monocytes, and CD34 stem cells (22, 23).
Inhibitors of cell emigration.
FTY720, the first in a new class, is a sphingosine-1 phosphate receptor agonist that reduces the recirculation of lymphocytes from lymph nodes back to the circulation, causing a peripheral lymphopenia. It has not been shown to impair T-cell activation, proliferation, or memory in response to viral infection (24, 25).
PULMONARY IMMUNITY
Once a pathogen has entered the lung, it encounters many innate local host defenses, including surfactant proteins, immunoglobulins, lysozymes, lactoferrin, peroxidase, and low-molecular-weight peptides (defensins). The most abundant immune cells are macrophages. Normal bronchoalveolar lavage fluid contains 85% macrophages, 7 to 12% lymphocytes, and 3% neutrophils. Infectious agents may reach the lung via inhalation, aspiration, or hematogenous routes. Once encountered in the lung, the organism or antigen undergoes phagocytosis by alveolar macrophages, mast cells, and neutrophils, which initiate or suppress inflammatory responses. Dendritic cells process and present the foreign antigen to memory and naive T cells, thus activating the adaptive cell-mediated immune response. Within the lungs, T-cell–mediated immunity is normally suppressed except after the initiation of an inflammatory event that leads to the expression of various proinflammatory cytokines and chemoattractants. CD4+ T cells activate B cells to form antibodies and divide and activate cytolytic CD8+ T cells and NK cells. Within this framework, extracellular bacteria (e.g., Staphylococcus, Streptococcus, Pseudomonas, and Haemophilus) are eliminated by phagocytosis, and cytotoxic products (e.g., lysosomal enzymes, nitrogen/O2 reactive species). Cytokines (e.g., IFN-α, TNF-α, IL-1, IL-6, and IL-8) and soluble mediators that include complement contribute to immune defense. Intracellular pathogens (e.g., Mycoplasma, Mycobacterium, Legionella, fungi, and respiratory viruses) are attacked via cell-mediated immunity after antigen-presenting cell stimulation of CD8+ T cells and, to a lesser extent, CD4+ cells. Within the lungs, innate and adaptive immune responses are intricately intertwined with overlapping signaling pathways (26, 27). Therefore, exogenous immunosuppression can inhibit multiple steps within the pulmonary immune cascade.
SPECIFIC INFECTIONS
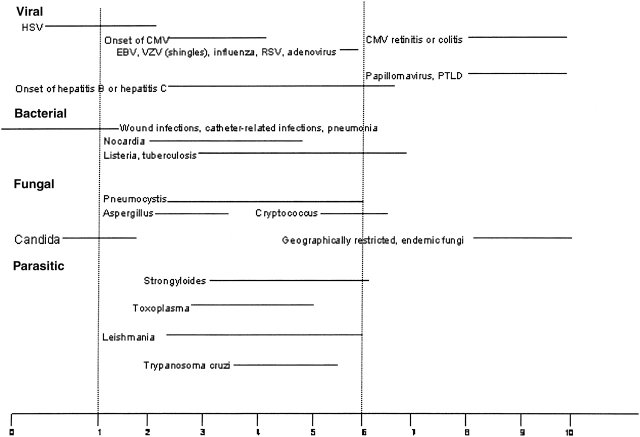
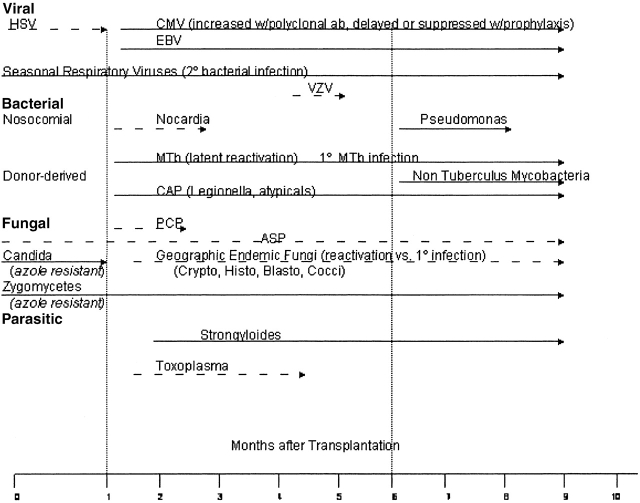
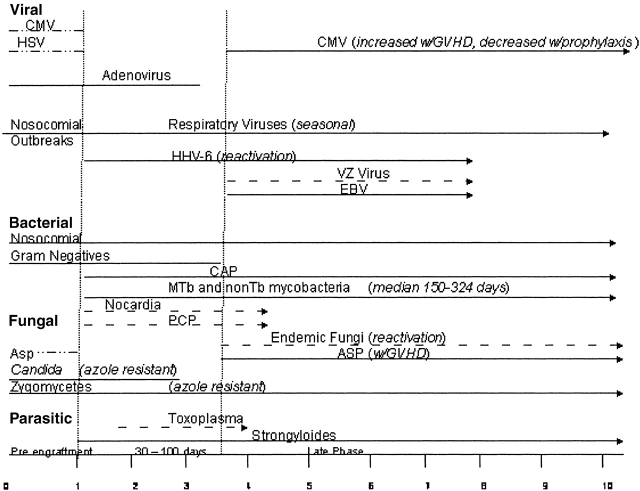
The overall risk of pulmonary infection in transplant recipients is dependent upon a number of factors that result in a “net state of immunosuppression,” including the degree of exogenous immunosuppression, anatomic location and type of transplant, and the intensity of exposure to a particular pathogen (28). Most transplant patients have comorbid conditions that include diabetes, renal insufficiency, and malnutrition; these conditions increase infection risk. Investigators have also determined that coinfection with viruses such as Epstein-Barr virus, cytomegalovirus (CMV), HIV, and hepatitis C virus can predispose transplant patients to bacterial and fungal infections (29). Local disruption of host defenses also plays a significant role. For example, lung transplant is associated with denervation of the graft with decreased cough reflexes, ischemic injury of the bronchial mucosa with impaired mucociliary clearance, bronchial anastomotic narrowing, and interruption of lymphatic drainage. Passive transfer of occult pneumonia from donor to recipient can also occur. The organisms responsible for lung infections in all organ transplant recipients are similar, and the timing of their appearance post-transplant has historically been predictable (Figure 1). However, with the use of modern prophylactic regimens that use sulfonamides, azoles, and antivirals, the classic timing for pathogen presentation can no longer be reliably predicted. This is further complicated by anti–T-cell strategies that increase the risk of viral infections at multiple time points not observed before the use of such treatments. Viral infections and T-cell depletion increase the risk of bacterial and fungal infections. Finally, there is a trend for the emergence of fungi that are resistant to the azoles. Cisneros and colleagues (30) reported that up to 23% of diagnosed pneumonias in heart transplant recipients are polymicrobial. Thus, the previous paradigm of predicting pathogens in certain time points post-transplantation is no longer applicable. Figures 2 and 3 propose modified timelines that account for the use of prophylactic regimens and their effect on the onset of infections post-transplantation.
Figure 1.
Infection timeline for recipients of solid organ transplants and HSCT. Data represent the traditional time to onset of infection and may not account for the effect induced by the use of antimicrobial prophylaxis. Adapted with permission from Reference 28.
Figure 2.
Proposed infection timeline based on the use of common prophylactic antimicrobials such as sulfa, azoles, and antivirals in recipients of solid organ transplants. Dotted lines denote onset of infection that would occur without prophylaxis, as reported in Figure 1. Solid lines indicate the most common times to onset of infection for each pathogen. Asp = Aspergillus; Blasto = Blastomyces; CAP = community-acquired pneumonia; CMV = cytomegalovirus; Cocci = Coccidioides; Crypto = Cryptococcus; EBV = Ebstein-Barr virus; Histo = Histoplasma; HSV = herpes simplex virus; MTb = Mycobacteria tuberculosis; PCP = Pneumocystis carinii; Toxo = Toxoplasmosis; VZV = Varicella zoster virus. Zero denotes the time of transplantation.
Figure 3.
Proposed infection timeline based on the use of common prophylactic antimicrobials such as sulfa, azoles, and antivirals in recipients of HSCT. Dotted lines denote onset of infection that would occur without prophylaxis, as reported in Figure 1. Solid lines indicate the most common times to onset of infection for each pathogen. Asp = Aspergillus; CAP = community-acquired pneumonia; CMV = cytomegalovirus; EBV = Ebstein-Barr virus; GVHD = graft versus host disease; HHV 6 = human herpes virus 6; HSV = herpes simplex virus; MTb = Mycobacteria tuberculosis; PCP = Pneumocystis carinii; VZV = Varicella zoster virus. Zero denotes the time of transplantation.
As in solid organ transplant, multiple factors, other than immunosuppressive drugs, increase infection risk in recipients of hematopoietic stem cell transplants. These include chemotherapy/radiation-induced neutropenia (which can be prolonged), lung injury induced by the conditioning regimen, and rejection in the form of graft versus host disease (GVHD).
The recipient of HSCT is burdened further by the need to develop a functional immune system from donor-derived cells. The production of granulocytes, red blood cells, and platelets occurs soon after HSCT; however, the production of lymphocytes (especially T cells) is significantly delayed. Serious infection in the first 2 yr post-transplant occurs in 50% of uncomplicated transplants from histocompatible sibling donors and in 80–90% of matched unrelated donors or histocompatible recipients who develop GVHD. Patients undergoing HSCT are at risk for the same infections as solid organ transplant recipients, but because of the differing timing of the immune deficits, the presentation post-transplant is altered. The pre-engraftment phase (0–30 d post–transplant) is characterized by prolonged neutropenia and breaks in the mucocutaneous barrier. The postengraftment phase (30–100 d post-transplant) is noted for impairment in the patient's cellular immunity, which in part is determined by the degree of exogenous immunosuppression and GVHD. During the late phase (> 100 d post-transplant), immune recovery and function is variable, depending on the type of HSCT; autologous HSCT recover more rapidly than allogeneic HSCT. Infections caused by poor lymphocyte function include the viral pathogens, whereas inadequate cellular immunity results in the same fungal pathogens seen in solid organ transplant (SOT). Patients undergoing HSCT are at risk for infections by encapsulated organisms due to delayed production of antipolysaccharide antibodies (31, 32).
Bacterial Pathogens
Bacterial pneumonia is a major cause of morbidity and mortality in all transplant patients. The first post-transplant month is notable for pneumonias caused by the usual nosocomial pathogens. Although the reported incidence of nosocomial pneumonia has declined to < 10% in liver and heart transplant recipients, it remains at 15% in lung transplant patients, with a similar incidence in patients undergoing HSCT (33). Because the transplant patient frequently requires recurrent hospitalization, they are at risk for acquiring nosocomial pathogens throughout their clinical course. Reported organisms include gram negatives, such as Pseudomonas, Klebsiella, Escherichia, Legionella, Acinetobacter, Stenotrophomonas, Enterobacter, Serratia, Proteus, Nocardia, and Citrobacter species. Notable nosocomial gram positives include Staphylococcus, Corynebacterium, Enterococcus, and Streptococcus species. Anaerobes, though rare, include Bacteroides and Fusobacterium species (30, 34–36). Lung transplant patients with cystic fibrosis who are colonized with Burckholderia cepacia, Stenotrophomonus maltopilia, and Alcaligenes Xylosoxidans deserve special mention. Patients colonized with certain strains of B. cepacia are at increased risk for morbidity and mortality from pneumonia and sepsis (37).
Community pathogens can emerge after the immediate post-transplant period. Haemophilus influenzae is the most common isolate, followed by Streptococcus pneumoniae and Legionella species (33). Lung transplant patients who develop bronchiolitis obliterans syndrome (BOS) with bronchiectasis are prone to Pseudomonas aeruginosa (33). Infection with Nocardia species was commonly reported before sulfonamide prophylaxis regimens (33) but is now rare. Chlamydia pneumoniae and Mycoplasma pneumoniae are also reported. C. pneumoniae infection has been associated with early graft dysfunction, rejection, BOS, and early mortality in lung transplant patients (38).
Tuberculous and atypical mycobacterial infections are rare, comprising less than 2% of pneumonias in the transplant recipient (33, 39). However, depending on the patient, the risk for tuberculous infection is nearly 70-fold greater in the transplant recipient compared with the general population (33, 39).
Viruses
Beyond the first months post-transplant, viral pathogens emerge as the most important group of infections affecting the solid organ recipients. They are the second most common cause of infection in the lung transplant population, accounting for 23 to 31% of all infections, but the incidence varies in recipients of nonlung grafts (40). CMV infection is considered by many to be the single most important pathogen affecting transplant recipients because 50% of the adult population harbor latent virus. Accordingly, reactivation of latent infections accounts for virtually all transplant-related CMV disease (33). It is estimated that 75% of solid organ transplant patients have some evidence of CMV infection (41). In lung transplantation, CMV has been implicated in chronic allograft rejection–BOS (42), but the relationship of CMV to chronic rejection is not a uniform phenomenon in recipients of other allografts (43).
Patients undergoing HSCT are at increased risk for CMV pneumonia due to effects related to delayed reconstitution of cytotoxic T cells and immunosuppressants. Without prophylaxis, the incidence is 20–35% in allogeneic transplants and is 1–6% in recipients of autologous HSCT. Recipients of allogeneic grafts are at the greatest risk, presumably due to increased requirements for immunosuppression. Anti-CMV prophylaxis has changed the usual onset of disease from the first 100 d (decreased from 35–6%) to beyond the first 100 d (up from 4–15%) (33). Patients with chronic GVHD are particularly vulnerable to CMV due to an increased need for immunosuppression. GVHD also causes an immunodeficient state by involving mucosal surfaces, reticuloendothelial system, and bone marrow (33). Epstein-Barr virus infections usually manifest as post-transplant lymphoproliferative disorder. Usually a B-cell lymphoma, its onset is thought to be related to T-cell depletion or suppression strategies (28). Without prophylaxis, herpes simplex virus infection occurs in up to 18% of transplant recipients, with severe pneumonia observed in up to 10% of patients; mortality may reach 20% (37). Herpes simplex virus may also cause a severe tracheobronchitis associated with endobronchial ulcers (44).
Infection by the community-acquired viruses (influenza A and B, parainfluenza, respiratory syncytial virus, and adenovirus) often leads to significant pneumonitis (up to 66%) and respiratory failure. Of these, parainfluenza, adenovirus, and respiratory syncytial virus have been directly linked to chronic rejection (BOS) in lung transplant patients (45–48). Human herpes virus 6 has been associated with the onset of idiopathic pneumonia syndrome post HSCT (33).
Fungal Pathogens, Protozoa, and Parasites
The incidence of invasive fungal infection in solid organ transplantation is 5–50%. Colonization with Candida species is most common, occurring in the early period post-transplant but rarely causing pneumonia. In contrast, Aspergillus species, with an incidence of 18–22%, represents the classic opportunistic fungal infection encountered in organ transplantation and has a clinical presentation similar to Mucormycosis. Strongly associated with neutropenia in patients undergoing HSCT, Aspergillus or Mucormycosis infections occur in solid organ recipients despite adequate numbers of circulating neutrophils. Invasive disease is usually encountered, with a mortality of 50–100%. Localized Aspergillus infections of the bronchial anastomosis and tracheobronchitis are unique causes of significant morbidity in the lung transplant patient (49–53).
Encountered infrequently, Cryptococcus, Histoplasma, and Coccidioides usually occur as reactivation of latent infection, whereas Blastomyces dermatitidis infection usually represents primary disease. Pneumocystis carinii prophylaxis that includes sulfonamides has significantly reduced the incidence of P. carinii pneumonia and Toxoplasma in all transplant recipients (33, 54). Other emerging fungi include the Trichoderma species, Pseudallescheria boydii, Torulopsis species, Microascus species, Penicillium species, and the Zygomycetes Absidia and Rhizopus (55–57). Rare causes of pulmonary infection include Microsporidia and Strongyloides (28, 58).
CONCLUSIONS
Advances in solid organ and HSC transplantation continue to be hindered by infection and rejection. The optimal immunosuppressive regimen maintains graft function, minimizing rejection while limiting the potential for infection. Pulmonary infections remain a leading cause of morbidity and mortality in solid organ and HSC transplantation despite prophylactic antibiotics. Because transplant patients tolerate established infection poorly, prevention is of paramount importance. The future of transplantation lies in the ability to more selectively create immune tolerance of the graft while preserving the patient's ability to mount an immune response to infection.
Supported by National Institutes of Health grants HL60797, HL67177, and HL081350 to D.S.W.
Conflict of Interest Statement: Neither of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Lindenfeld J, Miller GG, Shakar SF, Zolty R, Lowes BD, Wolfel EE, Mestroni L, Page RL II, Kobashigawa J. Drug therapy in the heart transplant recipient: part I: cardiac rejection and immunosuppressive drugs. Circulation 2004;110:3734–3740. [DOI] [PubMed] [Google Scholar]
- 2.Thorsby E, Pfeffer P, Leivestad T. Role of HLA molecules in the induction of alloimmune responses: clinical significance in the cyclosporine era. Transplant Proc 2004;36:16S–21S. [DOI] [PubMed] [Google Scholar]
- 3.Norman D, Turka L. Primer on transplantation, 2nd ed. Mt. Laurel, NJ: American Society of Transplantation; 2001.
- 4.Haberal M, Emiroglu R, Dalgic A, Karakayli H, Moray G, Bilgin N. The impact of cyclosporine on the development of immunosuppressive therapy. Transplant Proc 2004;36:143S–147S. [DOI] [PubMed] [Google Scholar]
- 5.Kapturczak MH, Meier-Kriesche HU, Kaplan B. Pharmacology of calcineurin antagonists. Transplant Proc 2004;36:25S–32S. [DOI] [PubMed] [Google Scholar]
- 6.Reichenspurner H. Overview of tacrolimus-based immunosuppression after heart or lung transplantation. J Heart Lung Transplant 2005; 24:119–130. [DOI] [PubMed] [Google Scholar]
- 7.Lee YR, Yang IH, Lee YH, Im SA, Song S, Li H, Han K, Kim K, Eo SK, Lee CK. Cyclosporin A and tacrolimus, but not rapamycin, inhibit MHC-restricted antigen presentation pathways in dendritic cells. Blood 2005;105:3951–3955. [DOI] [PubMed] [Google Scholar]
- 8.Baan CC, Balk AH, van Riemsdijk IC, Vantrimpont PJ, Maat AP, Niesters HG, Zondervan PE, van Gelder T, Weimar W. Anti-CD25 monoclonal antibody therapy affects the death signals of graft-infiltrating cells after clinical heart transplantation. Transplantation 2003;75:1704–1710. [DOI] [PubMed] [Google Scholar]
- 9.David KM, Morris JA, Steffen BJ, Chi-Burris KS, Gotz VP, Gordon RD. Mycophenolate mofetil vs. azathioprine is associated with decreased acute rejection, late acute rejection, and risk for cardiovascular death in renal transplant recipients with pre-transplant diabetes. Clin Transplant 2005;19:279–285. [DOI] [PubMed] [Google Scholar]
- 10.Eisen HJ, Kobashigawa J, Keogh A, Bourge R, Renlund D, Mentzer R, Alderman E, Valantine H, Dureau G, Mancini D, et al. Three-year results of a randomized, double-blind, controlled trial of mycophenolate mofetil versus azathioprine in cardiac transplant recipients. J Heart Lung Transplant 2005;24:517–525. [DOI] [PubMed] [Google Scholar]
- 11.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med 2004;351:2715–2729. [DOI] [PubMed] [Google Scholar]
- 12.Lederer SR, Friedrich N, Banas B, Welser G, Albert ED, Sitter T. Effects of mycophenolate mofetil on donor-specific antibody formation in renal transplantation. Clin Transplant 2005;19:168–174. [DOI] [PubMed] [Google Scholar]
- 13.Sollinger HW. Mycophenolates in transplantation. Clin Transplant 2004;18:485–492. [DOI] [PubMed] [Google Scholar]
- 14.Ingle GR, Sievers TM, Holt CD. Sirolimus: continuing the evolution of transplant immunosuppression. Ann Pharmacother 2000;34:1044–1055. [DOI] [PubMed] [Google Scholar]
- 15.Neumayer HH. Introducing everolimus (Certican) in organ transplantation: an overview of preclinical and early clinical developments. Transplantation 2005;79:S72–S75. [DOI] [PubMed] [Google Scholar]
- 16.Mohacsi PJ, Tuller D, Hulliger B, Wijngaard PL. Different inhibitory effects of immunosuppressive drugs on human and rat aortic smooth muscle and endothelial cell proliferation stimulated by platelet-derived growth factor or endothelial cell growth factor. J Heart Lung Transplant 1997;16:484–492. [PubMed] [Google Scholar]
- 17.King-Biggs MB, Dunitz JM, Park SJ, Kay Savik S, Hertz MI. Airway anastomotic dehiscence associated with use of sirolimus immediately after lung transplantation. Transplantation 2003;75:1437–1443. [DOI] [PubMed] [Google Scholar]
- 18.Chapman TM, Keating GM. Basiliximab: a review of its use as induction therapy in renal transplantation. Drugs 2003;63:2803–2835. [DOI] [PubMed] [Google Scholar]
- 19.Kuypers DR, Vanrenterghem YF. Monoclonal antibodies in renal transplantation: old and new. Nephrol Dial Transplant 2004;19:297–300. [DOI] [PubMed] [Google Scholar]
- 20.Platz KP, Braun F, Fandrich F, Kremer B, Mueller AR. IL-2 antagonists: the European perspective. Transplant Proc 2005;37:1783–1784. [DOI] [PubMed] [Google Scholar]
- 21.Usuda M, Fujimori K, Koyamada N, Fukumori T, Sekiguchi S, Kawagishi N, Akamatsu Y, Enomoto Y, Satoh K, Satoh A, et al. Successful use of anti-CD20 monoclonal antibody (rituximab) for ABO-incompatible living-related liver transplantation. Transplantation 2005;79:12–16. [DOI] [PubMed] [Google Scholar]
- 22.Ciancio G, Burke GW, Gaynor JJ, Mattiazzi A, Roohipour R, Carreno MR, Roth D, Ruiz P, Kupin W, Rosen A, et al. The use of Campath-1H as induction therapy in renal transplantation: preliminary results. Transplantation 2004;78:426–433. [DOI] [PubMed] [Google Scholar]
- 23.Watson CJ, Bradley JA, Friend PJ, Firth J, Taylor CJ, Bradley JR, Smith KG, Thiru S, Jamieson NV, Hale G, et al. Alemtuzumab (CAMPATH 1H) induction therapy in cadaveric kidney transplantation: efficacy and safety at five years. Am J Transplant 2005;5:1347–1353. [DOI] [PubMed] [Google Scholar]
- 24.Kunzendorf U, Ziegler E, Kabelitz D. FTY720: the first compound of a new promising class of immunosuppressive drugs. Nephrol Dial Transplant 2004;19:1677–1681. [DOI] [PubMed] [Google Scholar]
- 25.Tedesco-Silva H, Mourad G, Kahan BD, Boira JG, Weimar W, Mulgaonkar S, Nashan B, Madsen S, Charpentier B, Pellet P, et al. FTY720, a novel immunomodulator: efficacy and safety results from the first phase 2A study in de novo renal transplantation. Transplantation 2005;79:1553–1560. [PubMed] [Google Scholar]
- 26.Murray JF, Nadel JA. Textbook of respiratory medicine, 3rd ed. Philadelphia: Saunders;2000.
- 27.Zhang P, Summer WR, Bagby GJ, Nelson S. Innate immunity and pulmonary host defense. Immunol Rev 2000;173:39–51. [DOI] [PubMed] [Google Scholar]
- 28.Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med 1998;338:1741–1751. [DOI] [PubMed] [Google Scholar]
- 29.Snydman DR. Epidemiology of infections after solid-organ transplantation. Clin Infect Dis 2001;33:S5–S8. [DOI] [PubMed] [Google Scholar]
- 30.Cisneros JM, Munoz P, Torre-Cisneros J, Gurgui M, Rodriguez-Hernandez MJ, Aguado JM, Echaniz A. Pneumonia after heart transplantation: a multi-institutional study. Spanish Transplantation Infection Study Group. Clin Infect Dis 1998;27:324–331. [DOI] [PubMed] [Google Scholar]
- 31.Chao NJ, Emerson SG, Weinberg KI. Stem cell transplantation (cord blood transplants). Hematology (Am Soc Hematol Educ Program) 2004. pp. 354–371. [DOI] [PubMed]
- 32.Dykewicz CA. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis 2001;33:139–144. [DOI] [PubMed] [Google Scholar]
- 33.Kotloff RM, Ahya VN, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2004;170:22–48. [DOI] [PubMed] [Google Scholar]
- 34.Montoya JG, Giraldo LF, Efron B, Stinson EB, Gamberg P, Hunt S, Giannetti N, Miller J, Remington JS. Infectious complications among 620 consecutive heart transplant patients at Stanford University Medical Center. Clin Infect Dis 2001;33:629–640. [DOI] [PubMed] [Google Scholar]
- 35.Singh N, Paterson DL, Chang FY, Gayowski T, Squier C, Wagener MM, Marino IR. Methicillin-resistant Staphylococcus aureus: the other emerging resistant gram-positive coccus among liver transplant recipients. Clin Infect Dis 2000;30:322–327. [DOI] [PubMed] [Google Scholar]
- 36.Torres A, Ewig S, Insausti J, Guergue JM, Xaubet A, Mas A, Salmeron JM. Etiology and microbial patterns of pulmonary infiltrates in patients with orthotopic liver transplantation. Chest 2000;117:494–502. [DOI] [PubMed] [Google Scholar]
- 37.Speich R, van der Bij W. Epidemiology and management of infections after lung transplantation. Clin Infect Dis 2001;33:S58–S65. [DOI] [PubMed] [Google Scholar]
- 38.Glanville AR, Gencay M, Tamm M, Chhajed P, Plit M, Hopkins P, Aboyoun C, Roth M, Malouf M. Chlamydia pneumoniae infection after lung transplantation. J Heart Lung Transplant 2005;24:131–136. [DOI] [PubMed] [Google Scholar]
- 39.Singh N, Paterson DL. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis 1998;27:1266–1277. [DOI] [PubMed] [Google Scholar]
- 40.Alexander BD, Tapson VF. Infectious complications of lung transplantation. Transpl Infect Dis 2001;3:128–137. [DOI] [PubMed] [Google Scholar]
- 41.Pereyra F, Rubin RH. Prevention and treatment of cytomegalovirus infection in solid organ transplant recipients. Curr Opin Infect Dis 2004;17:357–361. [DOI] [PubMed] [Google Scholar]
- 42.Zamora MR. Cytomegalovirus and lung transplantation. Am J Transplant 2004;4:1219–1226. [DOI] [PubMed] [Google Scholar]
- 43.Dickenmann MJ, Cathomas G, Steiger J, Mihatsch MJ, Thiel G, Tamm M. Cytomegalovirus infection and graft rejection in renal transplantation. Transplantation 2001;71:764–767. [DOI] [PubMed] [Google Scholar]
- 44.Ramsey PG, Fife KH, Hackman RC, Meyers JD, Corey L. Herpes simplex virus pneumonia: clinical, virologic, and pathologic features in 20 patients. Ann Intern Med 1982;97:813–820. [DOI] [PubMed] [Google Scholar]
- 45.Billings JL, Hertz MI, Wendt CH. Community respiratory virus infections following lung transplantation. Transpl Infect Dis 2001;3:138–148. [DOI] [PubMed] [Google Scholar]
- 46.Khalifah AP, Hachem RR, Chakinala MM, Schechtman KB, Patterson GA, Schuster DP, Mohanakumar T, Trulock EP, Walter MJ. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med 2004;170:181–187. [DOI] [PubMed] [Google Scholar]
- 47.Vilchez RA, Dauber J, McCurry K, Iacono A, Kusne S. Parainfluenza virus infection in adult lung transplant recipients: an emergent clinical syndrome with implications on allograft function. Am J Transplant 2003;3:116–120. [DOI] [PubMed] [Google Scholar]
- 48.Wendt CH. Community respiratory viruses: organ transplant recipients. Am J Med 1997;102:31–36 (Discussion: 42–33). [DOI] [PubMed] [Google Scholar]
- 49.Bag R. Fungal pneumonias in transplant recipients. Curr Opin Pulm Med 2003;9:193–198. [DOI] [PubMed] [Google Scholar]
- 50.Hagerty JA, Ortiz J, Reich D, Manzarbeitia C. Fungal infections in solid organ transplant patients. Surg Infect (Larchmt) 2003;4:263–271. [DOI] [PubMed] [Google Scholar]
- 51.Nicod LP, Pache JC, Howarth N. Fungal infections in transplant recipients. Eur Respir J 2001;17:133–140. [DOI] [PubMed] [Google Scholar]
- 52.Oner-Eyuboglu F, Karacan O, Akcay S, Arslan H, Demirhan B, Haberal M. Invasive pulmonary fungal infections in solid organ transplant recipients: a four-year review. Transplant Proc 2003;35:2689–2691. [DOI] [PubMed] [Google Scholar]
- 53.Patterson JE. Epidemiology of fungal infections in solid organ transplant patients. Transpl Infect Dis 1999;1:229–236. [DOI] [PubMed] [Google Scholar]
- 54.Fishman JA. Prevention of infection caused by Pneumocystis carinii in transplant recipients. Clin Infect Dis 2001;33:1397–1405. [DOI] [PubMed] [Google Scholar]
- 55.Brown JM. Fungal infections in bone marrow transplant patients. Curr Opin Infect Dis 2004;17:347–352. [DOI] [PubMed] [Google Scholar]
- 56.Chouaki T, Lavarde V, Lachaud L, Raccurt CP, Hennequin C. Invasive infections due to Trichoderma species: report of 2 cases, findings of in vitro susceptibility testing, and review of the literature. Clin Infect Dis 2002;35:1360–1367. [DOI] [PubMed] [Google Scholar]
- 57.Nunley DR, Gal AA, Vega JD, Perlino C, Smith P, Lawrence EC. Saprophytic fungal infections and complications involving the bronchial anastomosis following human lung transplantation. Chest 2002;122:1185–1191. [DOI] [PubMed] [Google Scholar]
- 58.Teachey DT, Russo P, Orenstein JM, Didier ES, Bowers C, Bunin N. Pulmonary infection with microsporidia after allogeneic bone marrow transplantation. Bone Marrow Transplant 2004;33:299–302. [DOI] [PubMed] [Google Scholar]