Abstract
Background
To determine the risk of thyroid dysfunction and subsequent thyroid cancer among childhood acute lymphoblastic leukemia (ALL) survivors.
Procedure
Rates of self-reported thyroid dysfunction and thyroid cancer were determined among 3,579 ALL survivors participating in the Childhood Cancer Survivor Study, a cohort of 5-year survivors of pediatric cancers diagnosed from 1970–1986, and compared with 3,846 siblings and population rates, respectively.
Results
The cumulative incidence of hypo- and hyperthyroidism among survivors 15 years following leukemia diagnosis was 1.6% (95% CI 1.1, 2.1) and 0.6% (95% CI 0.3, 1.1), respectively, both significantly increased compared with siblings. In multivariate analysis, survivors who received ≥20 Gy cranial radiotherapy plus any spinal radiotherapy had the highest risk of subsequent hypothyroidism (HR 8.3, 95% CI 3.3, 20.5) compared with those treated with chemotherapy alone. Craniospinal radiotherapy also was associated with an increased risk of subsequent hyperthyroidism (HR 6.1, 95% CI 1.1, 34.2) compared with chemotherapy alone, as well as an increased risk of subsequent thyroid cancers (SIR 30.3, 95% CI 14.5, 55.7) compared with population rates. In radiation dosimetry analysis, pituitary doses ≥20 Gy combined with thyroid doses ≥10 Gy were associated with hypothyroidism, whereas pituitary doses ≥20 Gy combined with thyroid doses ≥15 Gy were associated with hyperthyroidism.
Conclusions
The risk of thyroid dysfunction and thyroid cancer was increased among childhood ALL survivors treated with craniospinal radiotherapy. In these individuals, long-term surveillance is warranted as no obvious plateau in risk was seen, even after 25 years of follow-up.
Keywords: late effects, leukemia, radiation, hypothyroidism, hyperthyroidism, thyroid cancer
INTRODUCTION
Hypothyroidism, and to a lesser degree, hyperthyroidism and thyroid cancer, occur at increased rates following radiotherapy exposure to the thyroid. Children treated for acute lymphoblastic leukemia (ALL) may receive cranial or craniospinal radiotherapy as part of therapy, placing them at risk of thyroid dysfunction due to disruption of the hypothalamic-pituitary axis and/or direct injury to the thyroid gland. While the risk of thyroid dysfunction has been described following higher dose radiotherapy given for brain tumors(1;2) and Hodgkin lymphoma(3), the effect of lower doses given to ALL patients has not been as well characterized. Studies that have examined thyroid dysfunction following ALL therapy have either reported no significant thyroid function abnormalities(4;5), or more subtle compensated hypothyroidism(6–8). These studies all typically feature small numbers of survivors, often with limited follow-up post-treatment. A few larger studies have examined the risk of thyroid malignancies following childhood cancers but typically have not examined ALL survivors in detail(9;10). Although many ALL treatment protocols have now transitioned away from using radiotherapy in favor of more intensive chemotherapy, an estimated 10–15% of patients with ALL currently still are treated upfront with some form of central nervous system (CNS) radiotherapy(11). Additional children are exposed to CNS radiotherapy while being treated for recurrent disease and/or as part of hematopoietic cell transplantation.
The Childhood Cancer Survivor Study (CCSS) is a large retrospective cohort of children treated for common childhood cancers between 1970 and 1986 and who are being followed on an on-going basis. Detailed treatment and late-effects information were collected on this cohort, which provides an opportunity to study relatively rare outcomes with a degree of precision and detail not possible in smaller studies or those with shorter follow-up. The goal of this study was to determine the risk of thyroid dysfunction and subsequent thyroid cancer among childhood ALL survivors stratified by both cranial and craniospinal radiotherapy doses, as well as by specific doses of radiation to the pituitary and thyroid glands.
METHODS
CCSS Overview
Specifics concerning the methodology and subject accrual for the CCSS have been reported in detail(12). Briefly, the cohort (n=14,372) was constructed from rosters of all children treated for most forms of childhood cancer at 26 institutions in the U.S. and Canada (see Supplemental Appendix). Inclusion criteria included diagnosis before age 21, initial treatment at one of the collaborating institutions between 1970 and 1986, and survival for at least 5 years following diagnosis. As a comparison group, 5,857 siblings were randomly selected from all eligible CCSS cases. If a cancer survivor had more than one sibling, the sibling of closest age was selected for participation. At the time of this analysis, 3,846 siblings had agreed to participate and were recruited to serve as a comparison group.
The CCSS protocol was approved by the Human Subjects Committee at each participating institution. Beginning in 1994, all participants (or parents of participants <18 years of age) completed a baseline questionnaire covering demographic characteristics, health conditions, health related behaviors, family cancer history, and reproductive history. For those participants who survived for 5 years following the initial cancer diagnosis and subsequently died, a family member completed the baseline questionnaire. Specifically, the questionnaire asked participants whether they had been diagnosed with an underactive (hypothyroid) or overactive (hyperthyroid) thyroid gland, if they ever developed a thyroid nodule, if their thyroid gland had been removed, whether they had subsequently developed any other malignancies, and their age at first occurrence for any of these outcomes. Participants could answer “yes”, “no”, or “not sure” to any question. Participants also were asked to list all prescription medications they consistently used in the prior 2 years. Medical records were reviewed and abstracted only for cancer diagnosis and treatment data including chemotherapy and radiotherapy exposures. In instances of self-reported second malignancies, copies of pathology reports were reviewed centrally and verified for inclusion(13); such reports were not available for self-reported thyroid nodules.
Cancer Treatment Exposures
4,151 ALL survivors were enrolled as part of the CCSS at the time of this analysis. After excluding survivors with incomplete or unknown radiotherapy exposure data associated with their initial ALL diagnosis, 3,579 participants were available for analysis. Cranial radiotherapy doses were categorized into <20/≥20 Gy, given that among the 2,325 survivors who received any cranial radiotherapy only 1.6% of survivors received doses <15 Gy and 2.8% received doses >30 Gy; 41.9% and 40.1% received 18 and 24 Gy respectively. Of the 330 survivors who also received spinal radiotherapy, 90.0% received doses between 10 and 30 Gy, with equal proportions receiving doses between 10–19 Gy and 20–30 Gy, with a mean of 18 Gy. However, given the relative rarity of thyroid dysfunction, spinal radiotherapy exposure was analyzed as a “yes/no” variable. Radiation doses to the pituitary gland and lobe-specific doses to the thyroid gland were estimated for each case, based on individual radiotherapy treatment records. Total absorbed doses to each lobe of the thyroid and the pituitary gland were estimated by applying water phantom measurements to a 3-dimensional mathematical phantom that can simulate a patient of any age or size(14). Among the 2,351 survivors with thyroid gland exposure, only 9 had >1 Gy discrepancies between the right and left lobes. Therefore the total thyroid gland dose was calculated as the average of the 2 lobes.
Statistical Analysis
The primary outcomes of interest in this study were hypothyroidism and hyperthyroidism. Participants who were “unsure” in their self-report were classified as unaffected. Cox proportional hazards models were constructed to examine the relative likelihood (hazard ratios, HR) of developing the outcomes of interest following ALL diagnosis among different radiotherapy exposure groups (STATA, version 9). Survivors were censored at the time of thyroidectomy, leukemia recurrence, hematopoietic cell transplant, second malignancy, or death. This was done because treatment data associated with recurrences, hematopoietic cell transplant, and second malignancy were not uniformly captured by the CCSS. Furthermore, in the hypothyroidism analysis, participants also were censored if they developed hyperthyroidism, and vice versa in the analysis of hyperthyroidism. All analyses were adjusted for sex, race/ethnicity, age and year of diagnosis, unless otherwise indicated. In comparisons between survivors and siblings, models estimated the HRs for selected outcomes since birth, adjusted for sex and race/ethnicity alone. To account for potential within-family correlation between survivors and siblings, robust variance estimators clustering on kinship were used. Proportional hazards assumptions for all models were tested using Schoenfeld residuals. Using data censored as above, cumulative incidence curves for hypo- and hyperthyroidism were plotted and differences between groups tested using Log Rank tests. Although these thyroid conditions likely do not impact survival, given that the CCSS cohort is defined by 5-year survivorship, a sensitivity analysis that excluded survivors reporting these outcomes within 5 years of diagnosis was performed and showed similar results. Lastly, we calculated the expected age and sex-specific incidence rates of thyroid cancer for the survivor cohort based on data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. These rates then were compared with the observed rates in the survivor population, and standardized incidence ratios (SIR) estimated(15). For this analysis, survivors were censored at the time of thyroidectomy, leukemia recurrence, hematopoietic cell transplant, other second malignancy, and death. All cases of thyroid cancer were diagnosed ≥5 years following ALL diagnosis.
RESULTS
Demographic and treatment characteristics of the survivor and sibling cohorts are summarized in Table I. Compared with siblings, survivors were younger and more likely male, but similar in the proportion of self-reported White, non-Hispanic race/ethnicity. Compared with survivors analyzed in this study, survivors with incomplete or unknown radiotherapy exposure data (n=572) were more often male (59.3%), non-White (25.7%), and treated before 1981 (53.5%), but were similar in the proportion who relapsed.
TABLE I.
Characteristics of 5-year acute lymphoblastic leukemia survivors and a sibling comparison group.
| Survivors n=3579 | Siblings n=3846 | |
|---|---|---|
| Female, % | 47.7 | 52.0 |
| Race/ethnicity, % | ||
| White, non-Hispanic | 86.6 | 87.5 |
| Black | 3.3 | 2.6 |
| Hispanic | 5.8 | 3.5 |
| Asian/Native/Pacific Islander | 2.3 | 1.6 |
| Other/unknown | 2.0 | 4.8 |
| Median age at follow-up, years (range) | 19 (6–42) | 26 (1–56) |
| Median age at diagnosis, years (range) | 4 (0–20) | |
| Year of diagnosis, % | ||
| 1970–75 | 20.3 | |
| 1976–80 | 27.8 | |
| 1981–86 | 51.8 | |
| Radiotherapy exposure, % | ||
| None, chemotherapy only | 35.0 | |
| Cranial <20 Gy, no spinal radiotherapy | 28.4 | |
| Cranial ≥20 Gy, no spinal radiotherapy | 27.3 | |
| Cranial <20 Gy, any spinal radiotherapy | 1.8 | |
| Cranial ≥20 Gy, any spinal radiotherapy | 7.4 | |
| Secondary event* | ||
| Relapse, % | 17.0 | |
| Hematopoietic cell transplant, % | 4.6 | |
| Second malignancy | 6.1 | |
In analysis of thyroid dysfunction, survivors were censored at time of secondary event.
Fifty-six survivors reported developing hypothyroidism compared with 50 siblings during follow-up. However, among these individuals only 26 survivors and 25 siblings reported recently taking thyroid hormone replacement. Twenty-three survivors reported a history of hyperthyroidism compared with 19 siblings. Two of these survivors and 3 of these siblings had subsequent thyroidectomies; 1 survivor and 3 siblings with hyperthyroidism also reported recently taking anti-thyroid medications.
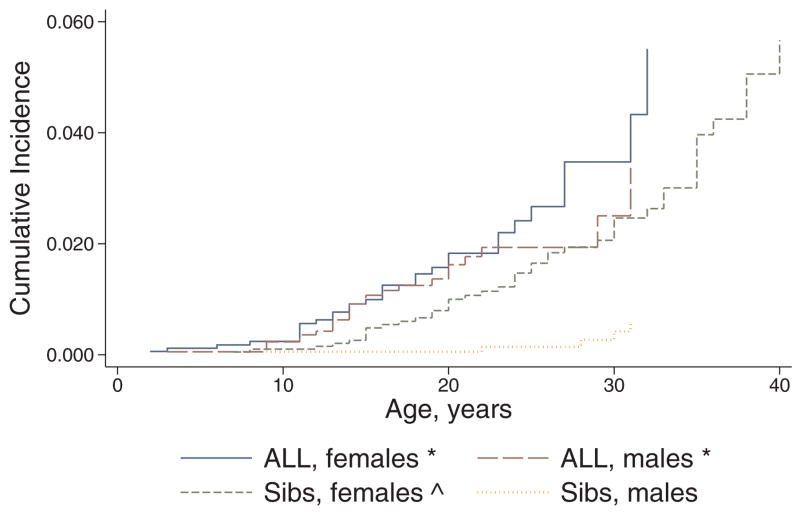
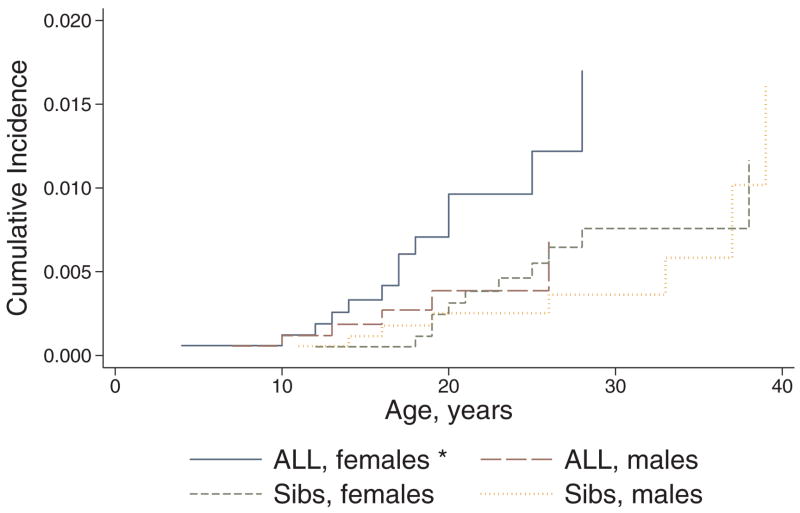
The overall cumulative incidence of hypo- and hyperthyroidism among survivors 15 years following original ALL diagnosis was 1.6% (95% CI 1.1, 2.1) and 0.6% (95% CI 0.3, 1.1), respectively. Compared with siblings, survivors were significantly more likely to report both hypo- (HR 2.6, 95% CI 1.8, 3.8) and hyperthyroidism (HR 2.0, 95% CI 1.1, 3.9). Both male and female survivors were more likely to report hypothyroidism compared with same-sex siblings, and as expected, female siblings were more likely to report hypothyroidism compared with male siblings (Figure 1). However, only female survivors were more likely than same-sex siblings to report hyperthyroidism; male survivors and male siblings had similar risk (Figure 2). Overall, no apparent plateau in risk of either hypo- or hyperthyroidism was seen among survivors during the follow-up period.
FIGURE 1.
Cumulative incidence of hypothyroidism among 5-year acute lymphoblastic leukemia (ALL) survivors versus siblings, stratified by sex. Outcomes censored by alternative thyroid conditions, leukemia recurrence, second malignancy, hematopoietic cell transplant, and death. * Log rank test, p<0.05 when survivors compared with same-sex siblings; ^ log rank test, p<0.05 when females compared with males, restricted to either survivors or siblings.
FIGURE 2.
Cumulative incidence of hyperthyroidism among 5-year acute lymphoblastic leukemia (ALL) survivors versus siblings, stratified by sex. Outcomes censored by alternative thyroid conditions, leukemia recurrence, second malignancy, hematopoietic cell transplant, and death. * Log rank test, p<0.05 when survivors compared with same-sex siblings; no differences when females compared with males, restricted to either survivors or siblings.
Compared with survivors who received only chemotherapy, those who received ≥20 Gy cranial radiotherapy in addition to any dose of spinal radiotherapy had the highest risk of reporting subsequent hypothyroidism (HR 8.3, 95% CI 3.3, 20.5; Table II). Survivors who received <20 Gy cranial radiotherapy with or without spinal radiotherapy and those who received ≥20 Gy cranial radiotherapy alone were not at significantly increased risk of subsequent hypothyroidism. There was no difference in risk between female survivors treated without radiotherapy and female siblings; however, male survivors treated without radiotherapy had a borderline increased risk versus male siblings (p=0.053). Results were similar if analysis was restricted only to those survivors who reported being on thyroid hormone supplementation (Table II). The radiation exposures to the thyroid and pituitary glands were similar between survivors with hypothyroidism who reported being on thyroid hormone compared to those who were not on hormonal treatment (data not shown).
TABLE II.
Pituitary and thyroid dosimetry and corresponding risk of hypothyroidism, stratified by radiotherapy exposure and adjusted for diagnosis year and age, race/ethnicity, and sex.
| Treatment group | Pituitary dose, Gy median (IQR) | Thyroid dose, Gy median (IQR) | Hypothyroid – all individuals | Hypothyroid – on supplementation | ||||
|---|---|---|---|---|---|---|---|---|
| n | HR | (95% CI) | n | HR | (95% CI) | |||
| Chemotherapy only | 0 (-) | 0 (-) | 10 | 1 | ref | 4 | 1 | ref |
| Cranial <20 Gy, no spinal radiotherapy | 16.8 (8.8–17.0) | 0.7 (0.5–0.8) | 12 | 1.5 | (0.7, 3.6) | 2 | 0.6 | (0.1, 3.2) |
| Cranial ≥20 Gy, no spinal radiotherapy | 22.5 (21.6–24.0) | 0.8 (0.6–1.1) | 16 | 1.7 | (0.7, 4.2) | 10 | 2.1 | (0.6, 7.9) |
| Cranial <20 Gy, any spinal radiotherapy | 17.8 (12.5–18.2) | 12.0 (10.1–14.4) | 1 | 2.0 | (0.2, 16.0) | 1 | 4.5 | (0.5, 43.3) |
| Cranial ≥20 Gy, any spinal radiotherapy | 24.3 (23.9–27.0) | 16.6 (11.0–19.8) | 17 | 8.3 | (3.3, 20.5) | 9 | 11.1 | (2.9, 41.7) |
| Siblings, males* | 5 | 0.2 | (0.04, 1.02) | 2 | 0.2 | (0.01, 2.5) | ||
| Siblings, females* | 45 | 0.7 | (0.3, 1.7) | 23 | 1.2 | (0.2, 6.3) | ||
IQR, interquartile range; HR, hazard ratio;
adjusted only for race/ethnicity and compared with same-sex survivors who received chemotherapy only.
Survivors exposed to craniospinal radiotherapy also were at increased risk of subsequent hyperthyroidism compared with those treated with chemotherapy alone (HR 6.1, 95% CI 1.1, 34.2; Table III). Those treated with cranial radiotherapy alone had a 3-fold increased risk compared with those treated with chemotherapy only, but estimates were imprecise. In both hypo- and hyperthyroid analyses, other treatment and demographic characteristics such as age and year of diagnosis, and race/ethnicity were not independently associated with an altered risk of either thyroid condition among ALL survivors.
TABLE III.
Risk of hyperthyroidism, stratified by radiotherapy exposure and adjusted for diagnosis year and age, race/ethnicity, and sex.
| Treatment group | Hyperthyroid |
||
|---|---|---|---|
| n | HR | (95% CI) | |
| Chemotherapy only | 2 | 1 | ref |
| Cranial radiotherapy only | 15 | 2.7 | (0.6, 12.6) |
| Craniospinal radiotherapy | 6 | 6.1 | (1.1, 34.2) |
| Siblings* | 19 | 1.3 | (0.3, 5.2) |
HR, hazard ratio;
adjusted only for race/ethnicity and sex, with no evidence for effect modification by sibling sex.
When treatment exposures were re-categorized by pituitary and thyroid gland-specific radiation dosimetry, only pituitary doses ≥20 Gy combined with thyroid doses ≥10 Gy were associated with a significantly increased risk of hypothyroidism (HR 9.9, 95% CI 4.0, 24.8) compared with survivors treated with chemotherapy only; there was no difference in risk if 10–14 Gy and ≥15 Gy thyroid doses were analyzed separately. In analysis of hyperthyroidism, only survivors exposed to pituitary doses ≥20 Gy combined with thyroid doses ≥15 Gy were at increased risk (HR 8.4, 95% CI 1.3, 53.5) versus chemotherapy only.
Twenty (0.6%) survivors reported developing a thyroid nodule at a mean of 14 years (range, 0–22 years) after ALL diagnosis; 18 had received some form of cranial or craniospinal radiotherapy. Among these 20 survivors, 4 reported subsequent hyperthyroidism, 3 reported subsequent hypothyroidism, and 3 reported subsequent thyroid cancer. Overall, 13 survivors developed thyroid cancer as their first subsequent malignancy: 11 papillary carcinomas (including 3 with the follicular variant type), 1 follicular adenocarcinoma, and 1 unknown histology. On average, these 13 survivors developed thyroid cancer at a median age of 25 years (range 14–40) and a median interval of 22 years (range 9–29) following ALL diagnosis. All 13 had received some form of CNS radiation with 10 having received spinal radiation. The median radiation dose to the thyroid gland was 13.4 Gy (range 0.1–21.4), and among patients who had received spinal radiotherapy, all received at least 10 Gy to the thyroid. Overall, the SIR of thyroid cancer among survivors was 4.8 (95% CI 2.6, 8.2; Table IV)), but this was primarily associated with craniospinal radiotherapy (SIR 30.3, 95% CI 14.5, 55.7). Survivors who received only cranial radiotherapy were not at increased risk and there was no difference in risk by gender.
TABLE IV.
Risk of thyroid cancer among 5-year acute lymphoblastic leukemia survivors without prior relapse, hematopoietic cell transplant, thyroidectomy, or other secondary malignancies.
| Observed | Expected* | SIR | 95% CI | |
|---|---|---|---|---|
| All survivors | 13 | 2.7 | 4.8 | (2.6, 8.2) |
| Chemotherapy only | 0 | 0.7 | 0 | (0, 5.1) |
| Cranial radiotherapy only | 3 | 1.7 | 1.8 | (0.4, 5.3) |
| Craniospinal radiotherapy | 10 | 0.3 | 30.3 | (14.5, 55.7) |
SIR, standardized incidence ratio;
based on age and sex-specific SEER incidence data.
DISCUSSION
Cancer survivors treated with radiotherapy may experience central and/or primary hypothyroidism as a consequence of injury to the hypothalamic-pituitary axis and thyroid gland, respectively. The risk of central hypothyroidism is greatest after treatment for brain tumors where cranial radiotherapy ≥30 Gy is often used(1;2;16). In contrast, the literature has been conflicting regarding the effect of cranial radiotherapy doses used in ALL (<30 Gy) on the risk of hypothyroidism with some studies reporting an increased risk(16) and others not(5). A number of other studies have reported subclinical thyroid hormone abnormalities following cranial radiotherapy, but had insufficient power to estimate risk precisely(6–8). The thyroid gland also may receive some degree of radiation scatter in the course of cranial radiotherapy(17;18). However, our results would suggest that cranial radiotherapy alone was insufficient to induce reportable hypothyroidism, whether central or primary in origin. In contrast, craniospinal radiotherapy used in ALL is associated with a higher amount of thyroid gland exposure and was strongly associated with subsequent hypothyroidism in our analysis and in other studies(19). Of note, the interval between cancer diagnosis and subsequent hypothyroidism is often more prolonged (≥10 years) in ALL survivors compared with patients treated for CNS tumors or Hodgkin lymphoma, where dysfunction is often diagnosed within 5 years(19). Finally, although an increased risk of hypothyroidism among survivors treated with chemotherapy alone has been reported(19), this was not found in our study and several others(3;4;20).
Hyperthyroidism has been less commonly reported following radiotherapy. Among childhood Hodgkin lymphoma survivors enrolled in the CCSS, after approximately 15 years of follow-up, 5% of survivors reported overactive thyroid(3). However, in studies of childhood leukemia patients treated with or without hematopoietic cell transplantation(4;21;22) or in cohorts inclusive of all cancer diagnoses(20;23), hyperthyroidism has been reported only rarely. In the current study we report a low incidence of hyperthyroidism (0.6%, based on 23 cases). Nonetheless, our results suggest that thyroid absorbed doses ≥15 Gy were associated with increased risk of hyperthyroidism. As all patients who received thyroid gland radiation also received cranial radiotherapy in our study, it was impossible to separate out any treatment effects on the hypothalamic-pituitary area from direct effects on the thyroid gland. Since hyperthyroidism has not been reported as a consequence of hypothalamic-pituitary axis injury, most cases of hyperthyroidism were likely the result of direct radiation damage to the thyroid gland.
Consistent with our results, prior analyses have suggested that risk of subsequent thyroid cancer is greatest following 10–30 Gy exposure to the thyroid gland with risk diminishing at doses ≥30 Gy(24;25). Our overall estimate of risk following leukemia therapy (SIR ~5, with patients diagnosed between 1970–1986) is intermediate to recent US national survey data from 1973–2002(26) and that from a international (primarily European) registry covering the years 1943–2000(10) which reported significantly increased SIRs of 2 and 19, respectively (both estimates based on 9 cases). However, neither of these 2 studies had access to detailed treatment data, and risk differences may reflect evolving treatment practices as well as statistical imprecision.
A limitation of our study was our use of self-reported thyroid dysfunction A study of hematopoietic cell transplant survivors that used a similar questionnaire as this study found that the validity of self-reported hypothyroidism compared with medical records was excellent with sensitivity, specificity, and overall agreement ≥95%(27). However, an earlier survey of American households found lower sensitivity (64%) but similar specificity (99%) for thyroid conditions(28). These differences may reflect a greater awareness of medical conditions among individuals who have had a history of serious illness. It also was possible that there may be some misclassification between over and underactive thyroid conditions as treatment for the former often results in the latter. Nevertheless, our rates of hyperthyroidism mirror those reported in previous studies and no patient with hypothyroidism also reported taking anti-thyroid medications.
Our study was limited also by lack of hormonal data, which made it impossible for us to establish whether those diagnosed with hypothyroidism suffered from primary or central hypothyroidism or both. Survivors treated with CNS radiotherapy may develop more subtle compensated hypothyroidism where thyroxine levels may be normal but thyroid stimulating hormone (TSH; thyrotropin) levels either are elevated at baseline or there is a blunted TSH response following thyrotropin releasing hormone stimulation(5;7;8;16). The former suggests a component of primary thyroid gland insufficiency while the latter suggests a component of central hypothyroidism. It is possible survivors in our study who reported underactive thyroid but were not on thyroid supplementation may have these more mild or subtle forms of hypothyroidism, which not all clinicians routinely treat(29). Overall, our results were similar if hypothyroidism was restricted to those individuals who also reported being on current supplementation.
In summary, with access to a large number of ALL survivors and detailed radiotherapy exposure information, we found that after a median follow-up of 15 years, cranial radiotherapy alone at the doses typically given to treat ALL (<30 Gy) was not associated with a significantly increased risk of either hypo- or hyperthyroidism. The risk of self-reported thyroid dysfunction and thyroid cancer was increased only among those treated with craniospinal radiotherapy, particularly at thyroid absorbed doses ≥10 Gy. In these high risk patients, continued long-term surveillance for thyroid dysfunction and subsequent thyroid cancer is warranted as no obvious plateau in risk was seen, even after 25 years.
Supplementary Material
Acknowledgments
This study was supported by grant U24-CA55727 from the National Institutes of Health awarded to St. Jude Children’s Research Hospital (LLR, Principal Investigator). EJC was supported by an American Society of Clinical Oncology/Lance Armstrong Foundation Young Investigator Award.
Footnotes
The authors disclose no potential conflicts of interest.
References
- 1.Ogilvy-Stuart AL, Shalet SM, Gattamaneni HR. Thyroid function after treatment of brain tumors in children. J Pediatr. 1991;119:733–737. doi: 10.1016/s0022-3476(05)80288-4. [DOI] [PubMed] [Google Scholar]
- 2.Schmiegelow M, Feldt-Rasmussen U, Rasmussen AK, et al. A population-based study of thyroid function after radiotherapy and chemotherapy for a childhood brain tumor. J Clin Endocrinol Metab. 2003;88:136–140. doi: 10.1210/jc.2002-020380. [DOI] [PubMed] [Google Scholar]
- 3.Sklar C, Whitton J, Mertens A, et al. Abnormalities of the thyroid in survivors of Hodgkin’s disease: data from the Childhood Cancer Survivor Study. J Clin Endocrinol Metab. 2000;85:3227–3232. doi: 10.1210/jcem.85.9.6808. [DOI] [PubMed] [Google Scholar]
- 4.Nygaard R, Bjerve KS, Kolmannskog S, et al. Thyroid function in children after cytostatic treatment for acute leukemia. Pediatr Hematol Oncol. 1988;5:35–38. doi: 10.3109/08880018809031249. [DOI] [PubMed] [Google Scholar]
- 5.Lando A, Holm K, Nysom K, et al. Thyroid function in survivors of childhood acute lymphoblastic leukaemia: the significance of prophylactic cranial irradiation. Clin Endocrinol (Oxf) 2001;55:21–25. doi: 10.1046/j.1365-2265.2001.01292.x. [DOI] [PubMed] [Google Scholar]
- 6.Shalet SM, Beardwell CG, Twomey JA, et al. Endocrine function following the treatment of acute leukemia in childhood. J Pediatr. 1977;90:920–923. doi: 10.1016/s0022-3476(77)80559-3. [DOI] [PubMed] [Google Scholar]
- 7.Pasqualini T, McCalla J, Berg S, et al. Subtle primary hypothyroidism in patients treated for acute lymphoblastic leukemia. Acta Endocrinol (Copenh) 1991;124:375–380. doi: 10.1530/acta.0.1240375. [DOI] [PubMed] [Google Scholar]
- 8.Mohn A, Chiarelli F, Di Marzio A, et al. Thyroid function in children treated for acute lymphoblastic leukemia. J Endocrinol Invest. 1997;20:215–219. doi: 10.1007/BF03346906. [DOI] [PubMed] [Google Scholar]
- 9.Sigurdson AJ, Ronckers CM, Mertens AC, et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): a nested case-control study. Lancet. 2005;365:2014–2023. doi: 10.1016/S0140-6736(05)66695-0. [DOI] [PubMed] [Google Scholar]
- 10.Maule M, Scelo G, Pastore G, et al. Risk of second malignant neoplasms after childhood leukemia and lymphoma: an international study. J Natl Cancer Inst. 2007;99:790–800. doi: 10.1093/jnci/djk180. [DOI] [PubMed] [Google Scholar]
- 11.Margolin JF, Steuber CP, Poplack DG. Acute lymphoblastic leukemia. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 5. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 538–590. [Google Scholar]
- 12.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 13.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 14.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 15.Breslow NE, Day NE. Volume II - the design and analysis of cohort studies. Lyon: IARC Sci Publ; 1987. Statistical methods in cancer research; p. 406p. [PubMed] [Google Scholar]
- 16.Rose SR, Lustig RH, Pitukcheewanont P, et al. Diagnosis of hidden central hypothyroidism in survivors of childhood cancer. J Clin Endocrinol Metab. 1999;84:4472–4479. doi: 10.1210/jcem.84.12.6097. [DOI] [PubMed] [Google Scholar]
- 17.Bessho F, Ohta K, Akanuma A, Sakata K. Dosimetry of radiation scattered to thyroid gland from prophylactic cranial irradiation for childhood leukemia. Pediatr Hematol Oncol. 1994;11:47–53. doi: 10.3109/08880019409141900. [DOI] [PubMed] [Google Scholar]
- 18.Stevens G, Downes S, Ralston A. Thyroid dose in children undergoing prophylactic cranial irradiation. Int J Radiat Oncol Biol Phys. 1998;42:385–390. doi: 10.1016/s0360-3016(98)00222-3. [DOI] [PubMed] [Google Scholar]
- 19.Madanat LM, Lahteenmaki PM, Alin J, Salmi TT. The natural history of thyroid function abnormalities after treatment for childhood cancer. Eur J Cancer. 2007;43:1161–1170. doi: 10.1016/j.ejca.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 20.van Santen HM, Vulsma T, Dijkgraaf MG, et al. No damaging effect of chemotherapy in addition to radiotherapy on the thyroid axis in young adult survivors of childhood cancer. J Clin Endocrinol Metab. 2003;88:3657–3663. doi: 10.1210/jc.2003-030209. [DOI] [PubMed] [Google Scholar]
- 21.Berger C, Le-Gallo B, Donadieu J, et al. Late thyroid toxicity in 153 long-term survivors of allogeneic bone marrow transplantation for acute lymphoblastic leukaemia. Bone Marrow Transplant. 2005;35:991–995. doi: 10.1038/sj.bmt.1704945. [DOI] [PubMed] [Google Scholar]
- 22.Ishiguro H, Yasuda Y, Tomita Y, et al. Long-term follow-up of thyroid function in patients who received bone marrow transplantation during childhood and adolescence. J Clin Endocrinol Metab. 2004;89:5981–5986. doi: 10.1210/jc.2004-0836. [DOI] [PubMed] [Google Scholar]
- 23.Miyoshi Y, Ohta H, Hashii Y, et al. Endocrinological Analysis of 122 Japanese Childhood Cancer Survivors in a Single Hospital. Endocr J. 2008 Aug 22; doi: 10.1507/endocrj.k08e-075. [DOI] [PubMed] [Google Scholar]
- 24.Acharya S, Sarafoglou K, LaQuaglia M, et al. Thyroid neoplasms after therapeutic radiation for malignancies during childhood or adolescence. Cancer. 2003;97:2397–2403. doi: 10.1002/cncr.11362. [DOI] [PubMed] [Google Scholar]
- 25.Ronckers CM, Sigurdson AJ, Stovall M, et al. Thyroid cancer in childhood cancer survivors: a detailed evaluation of radiation dose response and its modifiers. Radiat Res. 2006;166:618–628. doi: 10.1667/RR3605.1. [DOI] [PubMed] [Google Scholar]
- 26.Inskip PD, Curtis RE. New malignancies following childhood cancer in the United States, 1973–2002. Int J Cancer. 2007;121:2233–2240. doi: 10.1002/ijc.22827. [DOI] [PubMed] [Google Scholar]
- 27.Louie AD, Robison LL, Bogue M, et al. Validation of self-reported complications by bone marrow transplantation survivors. Bone Marrow Transplant. 2000;25:1191–1196. doi: 10.1038/sj.bmt.1702419. [DOI] [PubMed] [Google Scholar]
- 28.National Centers for Health Statistics. Net differences in interview data on chronic conditions and information derived from medical records. Series 2, Number 57. Rockville: US Department of Health, Education, and Welfare; 1973. [accessed on 3/27/2009]. Available at http://www.cdc.gov/nchs/data/series/sr_02/sr02_057.pdf. [PubMed] [Google Scholar]
- 29.Darzy KH, Shalet SM. Circadian and stimulated thyrotropin secretion in cranially irradiated adult cancer survivors. J Clin Endocrinol Metab. 2005;90:6490–6497. doi: 10.1210/jc.2005-1593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.