Summary
Objective
Age-related changes in multiple components of the musculoskeletal system may contribute to the well established link between aging and osteoarthritis (OA). This review focused on potential mechanisms by which age-related changes in the articular cartilage could contribute to the development of OA.
Methods
The peer-reviewed literature published prior to February 2009 in the PubMed database was searched using pre-defined search criteria. Articles, selected for their relevance to aging and articular chondrocytes or cartilage, were summarized.
Results
Articular chondrocytes exhibit an age-related decline in proliferative and synthetic capacity while maintaining the ability to produce pro-inflammatory mediators and matrix degrading enzymes. These findings are characteristic of the senescent secretory phenotype and are most likely a consequence of extrinsic stress-induced senescence driven by oxidative stress rather than intrinsic replicative senescence. Extracellular matrix changes with aging also contribute to the propensity to develop OA and include the accumulation of proteins modified by non-enzymatic glycation.
Conclusion
The effects of aging on chondrocytes and their matrix result in a tissue that is less able to maintain homeostasis when stressed, resulting in breakdown and loss of the articular cartilage, a hallmark of osteoarthritis. A better understanding of the basic mechanisms underlying senescence and how the process may be modified could provide novel ways to slow the development of osteoarthritis.
Keywords: aging, cell senescence, chondrocyte, cartilage, oxidative stress
Introduction
The prevalence of osteoarthritis (OA) rises directly with age and it the most common cause of chronic disability in older adults1,2. However, it is important to note that OA is not an inevitable consequence of aging; it is not a simple “wearing out” of the joints; and aging-related changes in the joint can be distinguished from those due to disease. Not all older adults develop OA and not all joints are equally affected. Although the relationship between aging and the development of OA is incompletely understood, it is becoming apparent that aging changes in the musculoskeletal system contribute to the development of OA by working in conjunction with other factors such as obesity, joint injury, and genetics. From studies of surgically-induced OA in young animals3, it is also apparent that OA-like changes in the joint can develop without a significant contribution of aging. Thus aging and OA are inter-related but not inter-dependent.
OA is best characterized as joint failure due to progressive changes in several components of the musculoskeletal system that include, but are not limited to, the articular cartilage. Other joint structures, including the bone, muscle, synovium, and soft tissues (ligaments, tendons, and in the knee the menisci) are altered in OA but have not been as extensively studied as the articular cartilage, especially in regards to aging. This review focuses on how aging affects the articular cartilage but many of the concepts discussed will likely apply to other joint tissues as well.
Methods
The PubMed database until February 1, 2009 was searched using the search terms: aging, cell senescence, chondrocytes, cartilage, or osteoarthritis. Articles, published in English, were selected for review based on their relevance to the topic of aging-changes in chondrocytes or cartilage that might contribute to the development of osteoarthritis.
Results
CELL SENESCENCE
The term senescence is derived from the Latin word senescere which means to grow old or to wane. Classical descriptions of cell senescence most often refer to the loss of the ability of mitotic cells to further divide in culture after a period of 30-40 population doublings, often referred to as the “Hayflick limit”4. It could be argued that this form of “replicative senescence”, resulting from an arrest in cell-cycle progression, is an in vitro artifact of cell culture. However, in vivo relevance of replicative senescence, for at least some tissues such as skin, is supported by findings that fibroblasts isolated from older humans or animals reach replicative senescence sooner than cells isolated from younger individuals5. In addition, at least some of the changes exhibited by cells that have undergone replicative senescence can be found in cells in older adults, such as the findings of shortened telomeres and the formation of senescence-associated heterochromatin5.
Cell senescence may have evolved as a mechanism to prevent cells with damaged DNA from being replicated and thus to prevent tumor formation. Replicative senescence is associated with changes in DNA structure and function including a shortening in the telomeres accompanied by telomere dysfunction6,7. Telomeres are found at the ends of chromosomes and are incompletely replicated during mitosis such that with each cell division a portion of the end of the telomere is lost resulting in telomere shortening. The discovery of telomere shortening with each cell division and the finding that a loss in telomere function could cause cell-cycle arrest provided a mechanism for a biological clock that over time would result in replicative senescence.
However, cell senescence appears to be much more complex than simple cell-cycle arrest occurring after a finite number of cell divisions. Progressive telomere shorting due to repeated cycles of cell division does not explain senescence in post-mitotic cells such as neurons, or quiescent cells such as chondrocytes. More recently, attention has been drawn to other forms of cell senescence sometimes referred to as “extrinsic” or “stress-induced” senescence as opposed to the intrinsic senescence resulting from replication. Stress-induced senescence can occur from diverse stimuli including ultraviolet radiation, oxidative damage, activated oncogenes, and chronic inflammation7,8. Oxidative damage to DNA can directly contribute to stress-induced senescence and, because the ends of chromosomes are particularly sensitive to oxidative damage, can result in telomere shortening similar to that seen with replicative senescence6,7.
Stress-induced senescence due to oxidative stress fits quite well with one of the longstanding theories of aging first proposed by Harman in the 1950s that invoked free radicals, or reactive oxygen species (ROS), as mediators of aging9. Oxidative stress has been found to induce cell senescence in vitro and there is in vivo evidence for age-related oxidative stress in many tissues5. As additional evidence for a role of ROS in aging, increased expression of the antioxidant enzyme catalase in mitochondria of transgenic mice can extend life-span and reduce age-related changes in tissues such as the heart10,11. However, extension of life-span could not be reproduced in transgenic mice overexpressing catalase in peroxisomes12, suggesting that the source of ROS may be important in aging.
The concept that reactive oxygen species (ROS) contribute to cell senescence by causing direct damage to proteins, lipids, and DNA is evolving to include the role of ROS in regulating cell signaling pathways that promote senescence13. ROS are generated by intracellular enzymes such as NADPH oxidase and 5-lipoxygenase in response to activation of specific cell signaling pathways. These ROS serve as secondary messengers that regulate signal transduction by activating redox-sensitive kinases and inhibiting redox-sensitive phosphatases13,14. Insufficient levels of ROS can be detrimental to certain signaling pathways, such as the EGF pathway that regulates cell proliferation, while excessive levels of ROS may inhibit pathways, such the insulin signaling pathway, through activation of the stress-induced kinase JNK13,15. A direct role for ROS in mediating senescence has been demonstrated through a positive feedback loop where mitogenic signaling that includes activation of PKCδ by ROS cooperates with the p16INK4A pathway to promote senescence16.
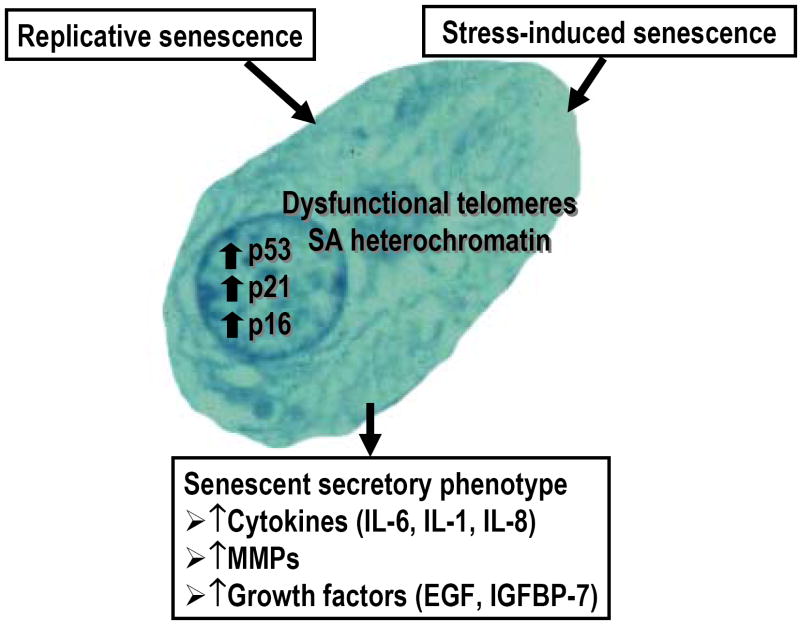
Senescent cells exhibit altered activity and expression of regulatory proteins that control growth and proliferation (Fig.1). These include p53 and the cyclin-dependent kinase inhibitors p21CIP1, and p16INK4A 5,8. Activation of p53 occurs from DNA damage or from telomere shortening and serves to inhibit cell-cycle progression. Activated p53 increases the expression of p21 which contributes to senescence. As p21 declines in senescent cells, p16 is increased which appears to serve a more long-term role in the inhibition of cell-cycle progression through inhibition of retinoblastoma protein5. The permanent state of cell-cycle arrest is also related to epigenetic changes that include the formation of foci of heterochromatin referred to as senescence-associated heterochromatin foci or SAHFs that include histone variants such as the macro-H2A17. SAHFs and macroH2A are used as markers for senescent cells as are findings of increased p16 expression17,18.
Fig. 1. Cell senescence.
There are two major types of cell senescence-replicative (intrinsic) and stress-induced (extrinsic). Senescence is associated with telomere dysfunction, formation of senescence-associated (SA) heterochromatin, and increased expression of p53, p21, and p16. The senescent secretory phenotype is characterized by increased production of cytokines, matrix metalloproteinases (MMPs) and growth factors such as epidermal growth factor (EGF) or growth factor binding proteins such as insulin-like growth factor binding protein-7 (IGFBP-7).
Senescent cells have also been found to have increased levels of the lysosomal enzyme β-galactosidase that is detectable at pH 6 rather than the normal pH 4.519. Detection of β-galactosidase activity at pH 6 has been referred to as senescence-associated (SA) β-galactosidase. Detection of activity at pH 6 is thought to be due to an increase in lysosomal mass and is not specific to cell senescence since it has been noted in immortalized cells, tumor cell lines, and even in normal cells under certain cell culture conditions5,19,20. We have noted positive staining for SA-β-galactosidase in the immortalized chondrocyte cell line C28I2 indicating it is not a specific marker for chondrocyte senescence (unpublished observation).
In addition to causing cell-cycle arrest due to an increase in expression of genes that inhibit proliferation, the changes that occur in senescent cells can also result in the increased production of cytokines, growth factors, and matrix metalloproteinases (Fig.1). Sometimes referred to as the senescent secretory phenotype8,18, this form of cell senescence may be particularly relevant to the development of osteoarthritis. The senescent secretory phenotype is characterized by the increased production of cytokines including IL-6 and IL-1, matrix metalloproteinases, and growth factors such as EGF. Recent studies have also provided evidence for a role of the IL-8 receptor CXCR221 and insulin-like growth factor binding protein 722 in senescence, suggesting autocrine loops of secreted proteins contribute to cell senescence. The accumulation of cells expressing the senescent secretory phenotype can also contribute to tissue aging through damage to the extracellular matrix, such as seen with the degradation of dermal collagen due to an age-related increase in collagenase8,18.
CHONDROCYTE SENESCENCE
Chondrocytes from older adults exhibit many of the changes that are typical of cell senescence (Table 1). Chondrocytes will divide in cell culture and after multiple passages will exhibit telomere shortening characteristic of replicative senescence23. Evidence of telomere shortening in chondrocytes has also been reported in cells isolated from older adults24. But because adult articular chondrocytes rarely, if ever, divide in normal tissue in vivo25,26, it would seem unlikely that they would experience telomere shortening due to classical replicative senescence in vivo.
Table I.
Features of Chondrocyte Senescence
| Senescence Feature | References |
|---|---|
| Telomere shortening | 23-24,27-28 |
| ↑ SA-βgal, p53, p21, p16 | 24,35-36 |
| ↑ Cytokine and MMP production | 37-38,41-43 |
| ↑ Oxidative stress/damage | 30-31,35,75 |
| ↓ Growth factor response | 45-52 |
| ↓ Growth factor production | 53-54 |
| ↑ Cell death | 26,31,53,59-62,64 |
SA-βgal = Senescence-associated β-galactosidase; MMP = Matrix Metalloproteinase
It is much more likely that chondrocyte senescence is the extrinsic type induced by chronic stress. There is evidence that telomere shortening noted in chondrocytes could be due to DNA damage from reactive oxygen species27,28. Interestingly, shorter telomere length in peripheral leucocytes was associated with radiographic hand OA in a cross-sectional study29. This association might also suggest a link between systemic oxidative stress, telomere erosion, and OA.
As discussed further below, an increase in chondrocyte ROS levels can be aging-related30,31. However, ROS generated from excessive mechanical loading and/or stimulation by cytokines could also contribute to DNA damage and subsequent telomere shortening32,33. The lack of cell division in normal adult articular cartilage and the lack of a ready supply of local progenitor cells in cartilage suggest that the chondrocytes present in the cartilage of an older adult are likely to be the very same cells that were present decades earlier. In contrast to many other tissues in the body that experience a regular turnover of cells, the long lifetime of chondrocytes would make these cells particularly susceptible to the accumulation of changes from both aging and extrinsic stress.
Evidence of cell senescence in tissues from older adults can be obtained by examining for the presence of senescence markers. These markers currently include histological staining for senescence-associated (SA) β-galactosidase (SA-βgal), SA heterochromatin, increased p53, p21, and p16 and reduced Wnt218. Staining for SA-βgal has been shown to be present in articular chondrocytes from older adults24 and in OA chondrocytes34. Chondrocyte SA-βgal staining, as well as telomere shortening, has also been noted after treatment in vitro with IL-1β or H2O2 consistent with stress-induced senescence35. Staining for SA-βgal needs to be interpreted with caution since this marker of cell senescence is not specific and can be influenced by factors such as cell culture20. As noted above, we have seen positive staining in immortalized cells that would not be considered senescent.
Importantly, Dai et al35 have provided evidence that stress-induced senescence in vitro is also accompanied by an increase in chondrocyte p53 and p21 expression as additional markers of the senescent phenotype. The senescence marker p16INK4A has also been examined and found to be present at greater levels in OA chondrocytes relative to age-matched normal tissue which in turn had higher levels than fetal tissue36. In the latter study, siRNA knockdown of p16INK4A was found to promote chondrocyte proliferation and matrix gene expression consistent with a link between senescence and the reduction in the ability of chondrocytes to proliferate and repair the matrix.
There is mounting evidence that chondrocytes can exhibit features of the senescent secretory phenotype which has important implications for the role of chondrocyte senescence in the development and progression of OA. When compared to cells isolated from young tissue donors, human articular chondrocytes from older adults were found to secrete more MMP-13 into the media after stimulation with either IL-1β or fibronectin fragments37. Isolated human chondrocytes were also found to produce more IL-137 and more IL-738 with increasing donor age and, like IL-1 and fibronectin fragments, IL-7 can also induce MMP-13 production38. MMP-13 serves as a major mediator of type II collagen cleavage39,40. Studies have shown increased immunostaining for MMP-3 and MMP-13 in cartilage with aging41 as well as an age-related accumulation of collagen neoepitopes representing denatured or cleaved collagen42,43. Development of the senescent secretory phenotype also might explain the cross-sectional observation of an increase in type II collagen degradation products in the urine of healthy adults after about age 50 years44.
Another feature of chondrocyte senescence is a decline in the proliferative and anabolic response of chondrocytes to growth factor stimulation. An age-related loss in the normal mitogenic response to several different growth factors has been noted including TGFβ, bFGF, PDGF, IGF-I, as well as those contained in fetal bovine serum45. IGF-I is well known to stimulate cartilage matrix synthesis and there is substantial evidence for an age-related decline in the ability of IGF-I to stimulate proteoglycan and collagen production46-48. TGF-β is another important cartilage anabolic factor and studies in equine chondrocytes have noted an age-related decline in response49. While the anabolic response to TGF-β was maintained in cartilage from aging mice, the ability of TGF-β to counteract the anti-anabolic affects of IL-1 was lost50. The ability of BMP-6 to stimulate proteoglycan synthesis has also been shown to decline with age in human chondrocytes51. Finally, a comparison of young and old bovine chondrocytes revealed that the cells from older animals produce less functional matrix when cultured with 10% serum52.
Besides a loss in the ability of chondrocytes to respond to growth factor stimulation, there is also evidence for an age-related reduction in the levels of certain growth factors in cartilage. In the mouse, levels of TGF-β2 and TGF-β3 but not TGF-β1 decline with age as does the level of the TGF-β receptor I and II53. It has been shown that the expression and amount of OP-1 (BMP-7) present in human cartilage declines significantly with age54. The reduction in OP-1 levels may be due to a recently discovered age-related increase in methylation of the chondrocyte OP-1 promoter55. DNA methylation and histone acetylation are two common epigenetic mechanisms that serve to regulate the level of gene transcription. The extent of DNA methylation in gene promoters that are rich in CpG sequences (such as the OP-1 promoter) can be altered with aging or in disease states and result in altered gene expression56. In general, an increase in promoter methylation will result in reduced gene expression.
The potential for epigenetic regulation of chondrocyte gene expression is just beginning to be explored. Although changes in DNA methylation were not found to directly regulate aggrecan expression in normal aged or OA cartilage57, inhibition of OP-1 promoter methylation in vitro resulted in an increase in both IGF-I and aggrecan expression, most likely from autocrine stimulation from an increase in OP-1 expression55. In contrast to the age-related increase in chondrocyte OP-1 promoter methylation, Roach et al58 found that in OA chondrocytes there was a decrease in the methylation of promoter sites in several matrix degrading enzymes including MMP-3, MMP-9, MMP-13, and ADAMTS-4 that could explain their increased expression in OA. Most recently, levels of the high-mobility group box (HMGB) protein 2, which is expressed in the superficial zone of cartilage, have been shown to decline with age in human and mouse articular cartilage59. HMGB2 is a nonhistone chromatin protein that can serve as a transcriptional regulator. Taniguchi et al59 demonstrated that deletion of HMGB2 in transgenic mice resulted in the early onset of OA-like changes in the superficial zone of cartilage that were associated with an increase in susceptibility of chondrocytes to cell death. Further studies on epigenetic regulation of chondrocyte gene expression may provide novel insights into the changes in chondrocyte gene expression noted in aging and OA.
Chondrocyte senescence can contribute to a decline in chondrocyte numbers due to increased cell death, although the extent of cell death with aging or in OA has varied among studies60-62. In human hip cartilage, a 30% fall in cell density between the ages of 30 and 70 years has been reported63. In femoral head cartilage from rats, a 46% decline in cell numbers was noted in old compared to young adult rats31and an age-related increase in apoptotic chondrocytes in rat cartilage has been reported as well64. In mice a similar age-related reduction in cell numbers in the medial tibial cartilage was seen53. However, a study of human knees found less than 5% cell loss with aging26.
There are certainly reasons to expect an age-related increase in death of chondrocytes including the decline in growth factor activity, the loss of survival promoting matrix proteins, and the increase in oxidative damage. As noted above, the response of chondrocytes to IGF-I declines with age and IGF-I is an important autocrine survival factors in cartilage65. Although matrix alterations occur with aging, it is not known if these affect the ability of either type II collagen66 or fibronectin signaling through the α5β1 integrin67 to promote chondrocyte survival. Oxidative damage from reactive oxygen species (ROS) could also contribute to chondrocyte death. Levels of ROS increase in cartilage with aging and chondrocytes from older adults are more susceptible to ROS-mediated cell death30. There is also evidence, at least in mouse cartilage, for an age-related decline in the anti-apoptotic protein Bcl-2 and the Bcl-2 associated-athanogene-1 (Bag-1)68 that could increase the susceptibility of chondrocytes to cell death. Finally, the age-related decline in HMGB2, discussed above, may also make chondrocytes more susceptible to cell death59. Because of a lack of replacement cells in cartilage, any loss of cells due to cell death could have important negative consequences.
THE ROLE OF OXIDATIVE STRESS IN CHONDROCYTE SENESCENCE
Oxidative stress may play a major role in the link between aging and the development of OA (Fig.2). Oxidative stress results when the amount of ROS exceeds the anti-oxidant capacity of the cell. This can be due to either increased production of ROS or decreased levels of antioxidantsand in aging both are often responsible13. Glutathione is a major intracellular antioxidant that also participates in regulating redox signaling events. An increase in levels of oxidized glutathione can be a sign of oxidative stress5. Evidence for an age-related increase in oxidative stress in human chondrocytes was obtained by finding an increase in the ratio of oxidized to reduced glutathione in isolated cells30. Increased levels of intracellular ROS were also detected in cartilage from old rats when compared to young adults31. Importantly, age-related oxidative stress was found to make human chondrocytes30 and rat chondrocytes31 more susceptible to cell death mediated by oxidants. As additional evidence for oxidative stress playing a role in chondrocyte senescence, chondrocyte senescence in vitro was associated with oxidative stress69 and exogenous addition of ROS to cultured chondrocytes was found to induce markers of the senescent phenotype35.
Fig. 2. Theoretical model for the relationships of aging, oxidative stress, and the development of osteoarthritis.

Aging-related oxidative stress as well as abnormal biomechanical stress results in increased levels of reactive oxygen species (ROS) in chondrocytes. The increase in ROS modulates anabolic and catabolic signaling pathways resulting in reduced matrix synthesis, inhibition of growth factor expression, and increased production of matrix metalloproteinases (MMPs) and cytokines that lead to matrix loss and osteoarthritis. Aging also results in increased formation of advanced glycation end-products (AGEs) which causes increased fatigue failure of the cartilage that when stressed also contributes to the development of osteoarthritis.
There is also evidence for reduced levels of anti-oxidant enzymes in cartilage with aging and in OA that would contribute to chondrocyte oxidative stress. In chondrocytes from aged rats, catalase, but not superoxide dismutase or glutathione peroxidase, was found at lower levels than in young adults31. Proteomic studies of human articular chondrocytes found a decrease in mitochondrial superoxide dismutase with aging70 as well as a decrease in OA cells when compared to cells from normal tissue71. Although not studied in aging, cartilage from adults with OA also had less extracellular superoxide dismutase than normal cartilage72 and gene array studies performed with RNA isolated from OA cells revealed a decreased expression of superoxide dismutase and glutathione peroxidase73.
Increased levels of ROS can contribute to aging changes in cells and tissues by causing oxidative damage to proteins, lipids, and DNA. One marker of protein oxidation is the presence of nitrotyrosine which can be detected using anti-nitrotyrosine antibodies. Nitrotyrosine is created by the reaction of protein tyrosine residues with peroxynitrite (ONOO−) formed when the ROS superoxide (O2•−) and nitric oxide (NO•) react74. Increased immunostaining for nitrotyrosine has been noted with aging in normal human and monkey cartilage75. Nitrotyrosine has also been detected in OA tissue28, 72, 75. In monkey cartilage, the presence of positive immunostaining for nitrotyrosine correlated with a reduced anabolic response to IGF-I in chondrocytes isolated from nearby tissue, suggesting that oxidative damage may be one mechanism for the reduced growth factor response75. In addition, excess levels of NO, a reactive nitrogen species, have also been found to reduce the chondrocyte response to IGF-I76. Likewise, earlier studies noted that treatment with H2O2 inhibits chondrocyte proteoglycan synthesis77.
The source of ROS contributing to oxidative stress and oxidative damage can include both free radicals generated as by-products of aerobic metabolism as well as ROS generated in response to specific stimuli such as growth factors and cytokines. Although chondrocytes live in an environment with a low oxygen tension, they do consume oxygen and therefore exhibit aerobic metabolism78. ROS have been shown to be produced by chondrocytes in response to stimulation by cytokines and growth factors, including IL-1, TNF-α, FGF and TGF-β79-82 as well as by integrin stimulation with fibronectin fragments83. IL-1 stimulation of ROS has been associated with chondrocyte DNA damage33. Production of ROS by chondrocytes may also have an important physiologic role in vivo. A recent study showed that in the growth plate ROS regulate proliferation and the initiation of hypertrophy84. The latter finding suggests a potential connection between ROS production in articular cartilage and chondrocyte hypertrophy observed in OA.
The underlying mechanisms by which oxidative stress contributes to chondrocyte senescence have not been well defined. Studies in other cell types have provided evidence that oxidative stress contributes to senescence through modulation of the activity of specific cell signaling pathways5,85. As noted above, this can be due to modulation of the activity of a number of redox-sensitive kinases and phosphatases. The activity of MAP kinase pathways, which include ERK, JNK, and p38, may be particularly important. Caveolin-1 is an integral membrane protein that serves as a scaffold and can regulate cell signaling pathways involved in senescence5. Caveolin-1 has been found to play a role in chondrocyte senescence induced by IL-1 and H2O2 through activation of the p38 MAP kinase35. We have recent evidence that ROS can contribute to chondrocyte IGF-I resistance and reduced proteoglycan synthesis by causing an imbalance in the activity of the PI-3 kinase-Akt pathway, which is necessary for chondrocyte proteoglycan synthesis, and the MEK-ERK MAP kinase pathway, which inhibits proteoglycan synthesis (Yin et al, manuscript under review). Because IGF-I can also stimulate chondrocyte anti-oxidant capacity82, resistance to IGF-I could further contribute to a redox imbalance.
Oxidative stress may also contribute to chondrocyte senescence by promoting endoplasmic reticulum (ER) stress. ER stress has been shown to down-regulate expression of cartilage matrix proteins including collagen type II and aggrecan and to increase chondrocyte apoptosis86,87. Whether ER stress increases with age in cartilage has not been determined. Further studies on redox regulation of cell signaling in chondrocytes, as well as on ER stress, should help to better define the mechanism of oxidative stress-induced chondrocyte senescence and may provide new targets for slowing the aging process in cartilage.
AGING IN THE CARTILAGE MATRIX
Age-related changes in the cartilage matrix have been reported that could be important in contributing to the development of OA (Table 2). There is evidence from MRI studies that the articular cartilage in the knee thins with aging, particularly at the femoral side of the joint88 and at the patella89. Cartilage thinning is consistent with a gradual loss of cartilage matrix with aging as well as a decrease in cartilage hydration and cellularity. A recent study of human femoral cartilage demonstrated an age-related decrease in cellularity and glycosaminoglycan content that could contribute to weakening of the tissue90. Age-related changes in the size, structure, and sulfation pattern of aggrecan have also been reported91-94. Aggrecan’s abundant negatively charged sulfates, which are very hydrophilic, are responsible for maintaining the high content (about 70-80%) of water in cartilage. Aging changes in aggrecan likely contribute to a loss in cartilage resiliency and hydration95. There is also evidence for an age-related accumulation of aggrecan fragments containing the hyaluronic acid binding region96. The aggrecan fragments that remain bound to hyaluronic acid can occupy the space where a newly synthesized complete aggrecan molecule would normally bind and thus result in smaller proteoglycan aggregates being present with increasing age91.
Table II.
Aging Changes in the Cartilage Matrix
| Aging change | References |
|---|---|
| ↑ AGE formation | 97-101,103 |
| ↓ Hydration | 95 |
| ↓ Aggrecan size | 91-94 |
| ↑ Collagen cleavage | 42-44 |
| ↑ Fatigue failure | 90,95,100 |
| ↓ Growth factor levels | 53-54 |
| ↑ Matrix calcification | 108-111 |
AGE = Advanced Glycation End-products
The aging cartilage matrix appears to be particularly susceptible to the accumulation of advanced glycation end-products (AGEs). AGEs are formed by reducing sugars such as glucose, fructose or ribose, reacting with lysine or arginine residues in a process of nonenzymatic glycation97. The low turnover rate of type II collagen, calculated to be over 100 years98, allows for the accumulation of AGEs that have been noted in human knee tissue97,99. AGE formation in collagen can result in increased cross-linking. The most common AGE-related cross link is pentosidine which has been found to be present in cartilage in increasing amounts with age98,100,101. Formation of excessive collagen cross-links affects the biomechanical properties of cartilage resulting in increased stiffness making the cartilage more brittle102 and increasing the susceptibility of the tissue to fatigue failure100.
In addition to altering the biomechanical properties of cartilage, increased levels of AGEs in cartilage may also affect chondrocyte function. An association has been noted between AGE formation and a decline in chondrocyte anabolic activity103. The mechanism by which AGEs affect chondrocyte function may include a direct interaction with cell receptors such as RAGE (the Receptor for Advanced Glycation End-products). RAGE is expressed by chondrocytes and RAGE levels in cartilage increase with both aging and the development of OA104. Stimulation of chondrocyte RAGE by AGE-albumin, produced in vitro105, or by S100 proteins, which can also bind RAGE and are present in vivo104,106,107, results in increased production of MMPs as well as a modulation of the chondrocyte phenotype to hypertrophy. Because an increase in MMP production and chondrocyte hypertrophy are hallmarks of OA, signaling through RAGE could play an important role in connecting age-related changes in the matrix to the development of OA. Chondrocyte RAGE signaling requires ROS107, providing another link between oxidative stress, aging, and OA.
Despite the fact that type II collagen has a very long half-life in cartilage, there is evidence for an age-related increase in collagen turnover. Type II collagen degradation was noted to increase with age in macroscopically normal ankle cartilage43. In that study, the ratio of cleaved/denatured type II collagen was examined and revealed a highly significant positive correlation with age (r=0.78, p<0.0001) suggesting a disassociation between cleavage and denaturation with aging. An increase in cleavage without a similar level of denaturation could be due to the age-related increase in collagen cross-linking from AGEs.
An age-related increase in calcification of the articular cartilage, and the menisci in the knee, has been demonstrated radiographically108,109. This may be related to an increase in the activity of transglutaminase, an enzyme involved in the biomineralization process110 and to an increase in inorganic pyrophosphate production in response to transforming growth factor-ß stimulation111. Despite a strong association among age, chondrocalcinosis, and the presence of OA108,109, the precise role of cartilage matrix calcification in the development of OA is not clear, in part due to the number of older people with asymptomatic chondrocalcinosis112.
AGING AND THE LOSS OF HOMEOSTASIS
If aging does not directly cause OA, how does it contribute to the development and/or progression of the disease? It is now fairly well accepted that in OA, at least in the articular cartilage, an imbalance exists in anabolic and catabolic pathways that favors matrix degradation113-115. We have proposed116, that aging changes including excessive levels of ROS could play an important role in tipping the balance of anabolic and catabolic signaling (Fig.2). An alteration in the level of anabolic and catabolic activity represents a loss in homeostasis. Many of the chronic degenerative conditions associated with aging appear to result from an age-related loss in the ability of cells and tissues in the body to maintain homeostasis, particularly when put under stress117.
The obvious stress for joint tissues is the mechanical stress that results from joint loading and motion. It is clear that excessive or abnormal mechanical stresses play a central role in the development of OA118. Under conditions where an anatomically normal joint is stressed, healthy joint tissues appear to be very capable of adapting to stress. As an example of successful adaptation, the chronic repetitive loads endured by long distance runners do not appear to result in OA later in life119,120. The development of OA occurs in joints that are unable to maintain homeostasis. Excessive loads, particularly when placed on a malaligned joint, overwhelm the homeostatic mechanisms leading to OA118,121,122.
OA is rare in young adults and even serious joint injuries usually don’t manifest as OA until years later, suggesting that young joint tissues can compensate, to some degree, to abnormal mechanical stress. But with aging, the ability to compensate and maintain homeostasis declines. Older adults who experience a joint injury develop OA much more rapidly than younger adults with a similar injury123. Likewise, older adults who develop inflammatory arthritis, such as rheumatoid arthritis, exhibit more rapid joint destruction relative to younger adults124. The age-related changes in the chondrocyte and cartilage matrix described above likely result in a tissue that cannot adequately maintain homeostasis when stressed resulting in matrix destruction and loss.
Conclusions
As we learn more about the basic biology of aging and how aging affects joint tissues such as the articular cartilage, the links between aging and the development of OA are becoming more apparent. It is unlikely that OA is a direct consequence of aging joints but rather aging affects the ability of the articular cartilage, and likely other joint tissues as well, to maintain homeostasis when stressed. The aging chondrocyte’s ability to produce and repair the extracellular matrix is compromised due to a decline in growth factor activity. This appears to be related to both a decline in the local availability of growth factors, including BMP-7 and TGF-β, as well as a decline in the chondrocyte’s response to stimulation with growth factors such as IGF-I. The latter findings suggest that growth factor therapy, being developed as a way to stimulate cartilage matrix production and repair, may not work well in older adults.
Chondrocyte senescence is associated with an increased production of inflammatory mediators and matrix degrading enzymes characteristic of the senescent secretory phenotype. The aging cartilage matrix likely contributes to these changes in chondrocyte function and also contributes to a loss in homeostasis due to altered biomechanical properties. Age-related oxidative stress and damage may play a central role in cartilage aging through modulation of cell signaling pathways that regulate anabolic and catabolic activity. Although the use of general anti-oxidants as therapies for aging-related diseases has not met with much success to date, it is possible that modulating the activity of a specific set of redox-regulated pathways may be more efficacious. Further studies aimed at elucidating the mechanisms that contribute to chondrocyte and cartilage aging should uncover new ways to slow the aging process in joint tissues and postpone the development of OA.
Acknowledgments
Dr. Loeser’s work was supported by the National Institute on Aging (RO1 AG16697 and the Wake Forest University Claude D. Pepper Older Americans Independence Center P30 AG021332), the National Institute on Arthritis, Musculoskeletal and Skin Diseases (RO1 AR49003), the American Federation for Aging Research, and the Dorothy Rhyne Kimbrell and Willard Duke Kimbrell Professorship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prevalence of disabilities and associated health conditions among adults--United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50:120–125. [PubMed] [Google Scholar]
- 2.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33:2271–2279. [PubMed] [Google Scholar]
- 3.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Hayflick L. Intracellular determinants of cell aging. Mech Ageing Dev. 1984;28:177–185. doi: 10.1016/0047-6374(84)90018-6. [DOI] [PubMed] [Google Scholar]
- 5.Muller M. Cellular senescence: molecular mechanisms, in vivo significance, and redox considerations. Antioxid Redox Signal. 2009;11:59–98. doi: 10.1089/ars.2008.2104. [DOI] [PubMed] [Google Scholar]
- 6.Goyns MH. Genes, telomeres and mammalian ageing. Mech Ageing Dev. 2002;123:791–799. doi: 10.1016/s0047-6374(01)00424-9. [DOI] [PubMed] [Google Scholar]
- 7.Itahana K, Campisi J, Dimri GP. Mechanisms of cellular senescence in human and mouse cells. Biogerontology. 2004;5:1–10. doi: 10.1023/b:bgen.0000017682.96395.10. [DOI] [PubMed] [Google Scholar]
- 8.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 10.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 11.Treuting PM, Linford NJ, Knoblaugh SE, Emond MJ, Morton JF, Martin GM, et al. Reduction of age-associated pathology in old mice by overexpression of catalase in mitochondria. J Gerontol A Biol Sci Med Sci. 2008;63:813–822. doi: 10.1093/gerona/63.8.813. [DOI] [PubMed] [Google Scholar]
- 12.Perez VI, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The Overexpression of Major Antioxidant Enzymes Does Not Extend the Lifespan of Mice. Aging Cell. 2008 doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 14.Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 15.Nishikawa T, Kukidome D, Sonoda K, Fujisawa K, Matsuhisa T, Motoshima H, et al. Impact of mitochondrial ROS production in the pathogenesis of insulin resistance. Diabetes Res Clin Pract. 2007;77(Suppl 1):S161–164. doi: 10.1016/j.diabres.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi A, Ohtani N, Yamakoshi K, Iida S, Tahara H, Nakayama K, et al. Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat Cell Biol. 2006;8:1291–1297. doi: 10.1038/ncb1491. [DOI] [PubMed] [Google Scholar]
- 17.Zhang R, Adams PD. Heterochromatin and its relationship to cell senescence and cancer therapy. Cell Cycle. 2007;6:784–789. doi: 10.4161/cc.6.7.4079. [DOI] [PubMed] [Google Scholar]
- 18.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 19.Itahana K, Campisi J, Dimri GP. Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol Biol. 2007;371:21–31. doi: 10.1007/978-1-59745-361-5_3. [DOI] [PubMed] [Google Scholar]
- 20.Severino J, Allen RG, Balin S, Balin A, Cristofalo VJ. Is beta-galactosidase staining a marker of senescence in vitro and in vivo? Exp Cell Res. 2000;257:162–171. doi: 10.1006/excr.2000.4875. [DOI] [PubMed] [Google Scholar]
- 21.Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 22.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsch D, Brummendorf TH, Richter W, Fellenberg J. Replicative aging of human articular chondrocytes during ex vivo expansion. Arthritis Rheum. 2002;46:2911–2916. doi: 10.1002/art.10626. [DOI] [PubMed] [Google Scholar]
- 24.Martin JA, Buckwalter JA. Telomere erosion and senescence in human articular cartilage chondrocytes. J Gerontol A Biol Sci Med Sci. 2001;56:B172–179. doi: 10.1093/gerona/56.4.b172. [DOI] [PubMed] [Google Scholar]
- 25.Mankin HJ. Localization of tritiated thymidine in articular cartilage of rabbits. J Bone Joint Surg. 1963;45-A:529–540. [Google Scholar]
- 26.Aigner T, Hemmel M, Neureiter D, Gebhard PM, Zeiler G, Kirchner T, et al. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 2001;44:1304–1312. doi: 10.1002/1529-0131(200106)44:6<1304::AID-ART222>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 27.Martin JA, Klingelhutz AJ, Moussavi-Harami F, Buckwalter JA. Effects of oxidative damage and telomerase activity on human articular cartilage chondrocyte senescence. J Gerontol A Biol Sci Med Sci. 2004;59:324–337. doi: 10.1093/gerona/59.4.b324. [DOI] [PubMed] [Google Scholar]
- 28.Yudoh K, Nguyen T, Nakamura H, Hongo-Masuko K, Kato T, Nishioka K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Ther. 2005;7:R380–391. doi: 10.1186/ar1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhai G, Aviv A, Hunter DJ, Hart DJ, Gardner JP, Kimura M, et al. Reduction of leucocyte telomere length in radiographic hand osteoarthritis: a population-based study. Ann Rheum Dis. 2006;65:1444–1448. doi: 10.1136/ard.2006.056903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Del Carlo M, Jr, Loeser RF. Increased oxidative stress with aging reduces chondrocyte survival: Correlation with intracellular glutathione levels. Arthritis Rheum. 2003;48:3419–3430. doi: 10.1002/art.11338. [DOI] [PubMed] [Google Scholar]
- 31.Jallali N, Ridha H, Thrasivoulou C, Underwood C, Butler PE, Cowen T. Vulnerability to ROS-induced cell death in ageing articular cartilage: the role of antioxidant enzyme activity. Osteoarthritis Cartilage. 2005;13:614–622. doi: 10.1016/j.joca.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Kurz B, Lemke AK, Fay J, Pufe T, Grodzinsky AJ, Schunke M. Pathomechanisms of cartilage destruction by mechanical injury. Ann Anat. 2005;187:473–485. doi: 10.1016/j.aanat.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Davies CM, Guilak F, Weinberg JB, Fermor B. Reactive nitrogen and oxygen species in interleukin-1-mediated DNA damage associated with osteoarthritis. Osteoarthritis Cartilage. 2008;16:624–630. doi: 10.1016/j.joca.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price JS, Waters JG, Darrah C, Pennington C, Edwards DR, Donell ST, et al. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002;1:57–65. doi: 10.1046/j.1474-9728.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- 35.Dai SM, Shan ZZ, Nakamura H, Masuko-Hongo K, Kato T, Nishioka K, et al. Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: possible involvement of caveolin 1-induced downregulation of articular chondrocytes in the pathogenesis of osteoarthritis. Arthritis Rheum. 2006;54:818–831. doi: 10.1002/art.21639. [DOI] [PubMed] [Google Scholar]
- 36.Zhou HW, Lou SQ, Zhang K. Recovery of function in osteoarthritic chondrocytes induced by p16INK4a-specific siRNA in vitro. Rheumatology (Oxford) 2004;43:555–568. doi: 10.1093/rheumatology/keh127. [DOI] [PubMed] [Google Scholar]
- 37.Forsyth CB, Cole A, Murphy G, Bienias JL, Im HJ, Loeser RF., Jr Increased matrix metalloproteinase-13 production with aging by human articular chondrocytes in response to catabolic stimuli. J Gerontol A Biol Sci Med Sci. 2005;60:1118–1124. doi: 10.1093/gerona/60.9.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long D, Blake S, Song XY, Lark M, Loeser RF. Human articular chondrocytes produce IL-7 and respond to IL-7 with increased production of matrix metalloproteinase-13. Arthritis Res Ther. 2008;10:R23. doi: 10.1186/ar2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99:1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu W, Billinghurst RC, Pidoux I, Antoniou J, Zukor D, Tanzer M, et al. Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Arthritis Rheum. 2002;46:2087–2094. doi: 10.1002/art.10428. [DOI] [PubMed] [Google Scholar]
- 42.Hollander AP, Pidoux I, Reiner A, Rorabeck C, Bourne R, Poole AR. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995;96:2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aurich M, Poole AR, Reiner A, Mollenhauer C, Margulis A, Kuettner KE, et al. Matrix homeostasis in aging normal human ankle cartilage. Arthritis Rheum. 2002;46:2903–2910. doi: 10.1002/art.10611. [DOI] [PubMed] [Google Scholar]
- 44.Mouritzen U, Christgau S, Lehmann HJ, Tanko LB, Christiansen C. Cartilage turnover assessed with a newly developed assay measuring collagen type II degradation products: influence of age, sex, menopause, hormone replacement therapy, and body mass index. Ann Rheum Dis. 2003;62:332–336. doi: 10.1136/ard.62.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guerne PA, Blanco F, Kaelin A, Desgeorges A, Lotz M. Growth factor responsiveness of human articular chondrocytes in aging and development. Arthritis Rheum. 1995;38:960–968. doi: 10.1002/art.1780380712. [DOI] [PubMed] [Google Scholar]
- 46.Martin JA, Ellerbroek SM, Buckwalter JA. Age-related decline in chondrocyte response to insulin-like growth factor-I: the role of growth factor binding proteins. J Orthop Res. 1997;15:491–498. doi: 10.1002/jor.1100150403. [DOI] [PubMed] [Google Scholar]
- 47.Loeser RF, Shanker G, Carlson CS, Gardin JF, Shelton BJ, Sonntag WE. Reduction in the chondrocyte response to insulin-like growth factor 1 in aging and osteoarthritis: studies in a non-human primate model of naturally occurring disease. Arthritis Rheum. 2000;43:2110–2120. doi: 10.1002/1529-0131(200009)43:9<2110::AID-ANR23>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 48.Messai H, Duchossoy Y, Khatib A, Panasyuk A, Mitrovic DR. Articular chondrocytes from aging rats respond poorly to insulin-like growth factor-1: an altered signaling pathway. Mech Ageing Dev. 2000;115:21–37. doi: 10.1016/s0047-6374(00)00107-x. [DOI] [PubMed] [Google Scholar]
- 49.Iqbal J, Dudhia J, Bird JL, Bayliss MT. Age-Related Effects of TGF-beta on Proteoglycan Synthesis in Equine Articular Cartilage. Biochem Biophys Res Commun. 2000;274:467–471. doi: 10.1006/bbrc.2000.3167. [DOI] [PubMed] [Google Scholar]
- 50.Scharstuhl A, van Beuningen HM, Vitters EL, van der Kraan PM, van den Berg WB. Loss of transforming growth factor counteraction on interleukin 1 mediated effects in cartilage of old mice. Ann Rheum Dis. 2002;61:1095–1098. doi: 10.1136/ard.61.12.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bobacz K, Gruber R, Soleiman A, Erlacher L, Smolen JS, Graninger WB. Expression of bone morphogenetic protein 6 in healthy and osteoarthritic human articular chondrocytes and stimulation of matrix synthesis in vitro. Arthritis Rheum. 2003;48:2501–2508. doi: 10.1002/art.11248. [DOI] [PubMed] [Google Scholar]
- 52.Tran-Khanh N, Hoemann CD, McKee MD, Henderson JE, Buschmann MD. Aged bovine chondrocytes display a diminished capacity to produce a collagen-rich, mechanically functional cartilage extracellular matrix. J Orthop Res. 2005;23:1354–1362. doi: 10.1016/j.orthres.2005.05.009.1100230617. [DOI] [PubMed] [Google Scholar]
- 53.Blaney Davidson EN, Scharstuhl A, Vitters EL, van der Kraan PM, van den Berg WB. Reduced transforming growth factor-beta signaling in cartilage of old mice: role in impaired repair capacity. Arthritis Res Ther. 2005;7:R1338–1347. doi: 10.1186/ar1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chubinskaya S, Kumar B, Merrihew C, Heretis K, Rueger DC, Kuettner KE. Age-related changes in cartilage endogenous osteogenic protein-1 (OP-1) Biochim Biophys Acta. 2002;1588:126–134. doi: 10.1016/s0925-4439(02)00158-8. [DOI] [PubMed] [Google Scholar]
- 55.Loeser RF, Im HJ, Richardson B, Lu Q, Chubinskaya S. Methylation of the OP-1 promoter: potential role in the age-related decline in OP-1 expression in cartilage. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richardson B. Impact of aging on DNA methylation. Ageing Res Rev. 2003;2:245–261. doi: 10.1016/s1568-1637(03)00010-2. [DOI] [PubMed] [Google Scholar]
- 57.Poschl E, Fidler A, Schmidt B, Kallipolitou A, Schmid E, Aigner T. DNA methylation is not likely to be responsible for aggrecan down regulation in aged or osteoarthritic cartilage. Ann Rheum Dis. 2005;64:477–480. doi: 10.1136/ard.2004.022509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roach HI, Yamada N, Cheung KS, Tilley S, Clarke NM, Oreffo RO, et al. Association between the abnormal expression of matrix-degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 2005;52:3110–3124. doi: 10.1002/art.21300. [DOI] [PubMed] [Google Scholar]
- 59.Taniguchi N, Carames B, Ronfani L, Ulmer U, Komiya S, Bianchi ME, et al. Aging-related loss of the chromatin protein HMGB2 in articular cartilage is linked to reduced cellularity and osteoarthritis. Proc Natl Acad Sci U S A. 2009;106:1181–1186. doi: 10.1073/pnas.0806062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horton WE, Jr, Feng L, Adams C. Chondrocyte apoptosis in development, aging and disease. Matrix Biol. 1998;17:107–115. doi: 10.1016/s0945-053x(98)90024-5. [DOI] [PubMed] [Google Scholar]
- 61.Aigner T, Kim HA, Roach HI. Apoptosis in osteoarthritis. Rheum Dis Clin North Am. 2004;30:639–653. xi. doi: 10.1016/j.rdc.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 62.Kuhn K, D’Lima DD, Hashimoto S, Lotz M. Cell death in cartilage. Osteoarthritis Cartilage. 2004;12:1–16. doi: 10.1016/j.joca.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 63.Vignon E, Arlot M, Patricot LM, Vignon G. The cell density of human femoral head cartilage. Clin Orthop. 1976;121:303–308. [PubMed] [Google Scholar]
- 64.Adams CS, Horton WE., Jr Chondrocyte apoptosis increases with age in the articular cartilage of adult animals. Anat Rec. 1998;250:418–425. doi: 10.1002/(SICI)1097-0185(199804)250:4<418::AID-AR4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 65.Loeser RF, Shanker G. Autocrine stimulation by insulin-like growth factor 1 and insulin-like growth factor 2 mediates chondrocyte survival in vitro. Arthritis Rheum. 2000;43:1552–1559. doi: 10.1002/1529-0131(200007)43:7<1552::AID-ANR20>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 66.Yang C, Li SW, Helminen HJ, Khillan JS, Bao Y, Prockop DJ. Apoptosis of chondrocytes in transgenic mice lacking collagen II. Exp Cell Res. 1997;235:370–373. doi: 10.1006/excr.1997.3692. [DOI] [PubMed] [Google Scholar]
- 67.Pulai JI, Del Carlo M, Jr, Loeser RF. The alpha5beta1 integrin provides matrix survival signals for normal and osteoarthritic human articular chondrocytes in vitro. Arthritis Rheum. 2002;46:1528–1535. doi: 10.1002/art.10334. [DOI] [PubMed] [Google Scholar]
- 68.Kinkel MD, Yagi R, McBurney D, Nugent A, Horton WE., Jr Age-related expression patterns of Bag-1 and Bcl-2 in growth plate and articular chondrocytes. Anat Rec A Discov Mol Cell Evol Biol. 2004;279:720–728. doi: 10.1002/ar.a.20063. [DOI] [PubMed] [Google Scholar]
- 69.Pfeuty A, Gueride M. Peroxide accumulation without major mitochondrial alteration in replicative senescence. FEBS Lett. 2000;468:43–47. doi: 10.1016/s0014-5793(00)01188-1. [DOI] [PubMed] [Google Scholar]
- 70.Ruiz-Romero C, Lopez-Armada MJ, Blanco FJ. Mitochondrial proteomic characterization of human normal articular chondrocytes. Osteoarthritis Cartilage. 2006;14:507–518. doi: 10.1016/j.joca.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 71.Ruiz-Romero C, Calamia V, Mateos J, Carreira V, Martinez-Gomariz M, Fernandez M, et al. Mitochondrial dysregulation of osteoarthritic human articular chondrocytes analyzed by proteomics: A decrease in mitochondrial superoxide dismutase points to a redox imbalance. Mol Cell Proteomics. 2008 doi: 10.1074/mcp.M800292-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Regan E, Flannelly J, Bowler R, Tran K, Nicks M, Carbone BD, et al. Extracellular superoxide dismutase and oxidant damage in osteoarthritis. Arthritis Rheum. 2005;52:3479–3491. doi: 10.1002/art.21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aigner T, Fundel K, Saas J, Gebhard PM, Haag J, Weiss T, et al. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum. 2006;54:3533–3544. doi: 10.1002/art.22174. [DOI] [PubMed] [Google Scholar]
- 74.Reiter CD, Teng RJ, Beckman JS. Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. J Biol Chem. 2000;275:32460–32466. doi: 10.1074/jbc.M910433199. [DOI] [PubMed] [Google Scholar]
- 75.Loeser RF, Carlson CS, Carlo MD, Cole A. Detection of nitrotyrosine in aging and osteoarthritic cartilage: Correlation of oxidative damage with the presence of interleukin-1beta and with chondrocyte resistance to insulin-like growth factor 1. Arthritis Rheum. 2002;46:2349–2357. doi: 10.1002/art.10496. [DOI] [PubMed] [Google Scholar]
- 76.Studer RK, Levicoff E, Georgescu H, Miller L, Jaffurs D, Evans CH. Nitric oxide inhibits chondrocyte response to IGF-I: inhibition of IGF-IRbeta tyrosine phosphorylation. Am J Physiol Cell Physiol. 2000;279:C961–C969. doi: 10.1152/ajpcell.2000.279.4.C961. [DOI] [PubMed] [Google Scholar]
- 77.Baker MS, Feigan J, Lowther DA. Chondrocyte antioxidant defences: the roles of catalase and glutathione peroxidase in protection against H2O2 dependent inhibition of proteoglycan biosynthesis. J Rheumatol. 1988;15:670–677. [PubMed] [Google Scholar]
- 78.Zhou S, Cui Z, Urban JP. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: a modeling study. Arthritis Rheum. 2004;50:3915–3924. doi: 10.1002/art.20675. [DOI] [PubMed] [Google Scholar]
- 79.Rathakrishnan C, Tiku K, Raghavan A, Tiku ML. Release of oxygen radicals by articular chondrocytes: a study of luminol-dependent chemiluminescence and hydrogen peroxide secretion. J Bone Miner Res. 1992;7:1139–1148. doi: 10.1002/jbmr.5650071005. [DOI] [PubMed] [Google Scholar]
- 80.Lo YY, Cruz TF. Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes. J Biol Chem. 1995;270:11727–11730. doi: 10.1074/jbc.270.20.11727. [DOI] [PubMed] [Google Scholar]
- 81.Lo YY, Conquer JA, Grinstein S, Cruz TF. Interleukin-1 beta induction of c-fos and collagenase expression in articular chondrocytes: involvement of reactive oxygen species. J Cell Biochem. 1998;69:19–29. doi: 10.1002/(sici)1097-4644(19980401)69:1<19::aid-jcb3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 82.Jallali N, Ridha H, Thrasivoulou C, Butler P, Cowen T. Modulation of intracellular reactive oxygen species level in chondrocytes by IGF-1, FGF, and TGF-beta1. Connect Tissue Res. 2007;48:149–158. doi: 10.1080/03008200701331516. [DOI] [PubMed] [Google Scholar]
- 83.Del Carlo M, Schwartz D, Erickson EA, Loeser RF. Endogenous production of reactive oxygen species is required for stimulation of human articular chondrocyte matrix metalloproteinase production by fibronectin fragments. Free Radic Biol Med. 2007;42:1350–1358. doi: 10.1016/j.freeradbiomed.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morita K, Miyamoto T, Fujita N, Kubota Y, Ito K, Takubo K, et al. Reactive oxygen species induce chondrocyte hypertrophy in endochondral ossification. J Exp Med. 2007;204:1613–1623. doi: 10.1084/jem.20062525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 86.Yang L, Carlson SG, McBurney D, Horton WE., Jr Multiple signals induce endoplasmic reticulum stress in both primary and immortalized chondrocytes resulting in loss of differentiation, impaired cell growth, and apoptosis. J Biol Chem. 2005;280:31156–31165. doi: 10.1074/jbc.M501069200. [DOI] [PubMed] [Google Scholar]
- 87.Yang L, McBurney D, Tang SC, Carlson SG, Horton WE., Jr A novel role for Bcl-2 associated-athanogene-1 (Bag-1) in regulation of the endoplasmic reticulum stress response in mammalian chondrocytes. J Cell Biochem. 2007;102:786–800. doi: 10.1002/jcb.21328. [DOI] [PubMed] [Google Scholar]
- 88.Hudelmaier M, Glaser C, Hohe J, Englmeier KH, Reiser M, Putz R, et al. Age-related changes in the morphology and deformational behavior of knee joint cartilage. Arthritis Rheum. 2001;44:2556–2561. doi: 10.1002/1529-0131(200111)44:11<2556::aid-art436>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 89.Ding C, Cicuttini F, Scott F, Cooley H, Jones G. Association between age and knee structural change: a cross sectional MRI based study. Ann Rheum Dis. 2005;64:549–555. doi: 10.1136/ard.2004.023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Temple MM, Bae WC, Chen MQ, Lotz M, Amiel D, Coutts RD, et al. Age- and site-associated biomechanical weakening of human articular cartilage of the femoral condyle. Osteoarthritis Cartilage. 2007 doi: 10.1016/j.joca.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 91.Buckwalter JA, Roughley PJ, Rosenberg LC. Age-related changes in cartilage proteoglycans: quantitative electron microscopic studies. Microsc Res Tech. 1994;28:398–408. doi: 10.1002/jemt.1070280506. [DOI] [PubMed] [Google Scholar]
- 92.Dudhia J, Davidson CM, Wells TM, Vynios DH, Hardingham TE, Bayliss MT. Age-related changes in the content of the C-terminal region of aggrecan in human articular cartilage. Biochem J. 1996;313(Pt 3):933–940. doi: 10.1042/bj3130933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bayliss MT, Osborne D, Woodhouse S, Davidson C. Sulfation of chondroitin sulfate in human articular cartilage. The effect of age, topographical position, and zone of cartilage on tissue composition. J Biol Chem. 1999;274:15892–15900. doi: 10.1074/jbc.274.22.15892. [DOI] [PubMed] [Google Scholar]
- 94.Wells T, Davidson C, Morgelin M, Bird JL, Bayliss MT, Dudhia J. Age-related changes in the composition, the molecular stoichiometry and the stability of proteoglycan aggregates extracted from human articular cartilage. Biochem J. 2003;370:69–79. doi: 10.1042/BJ20020968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grushko G, Schneiderman R, Maroudas A. Some biochemical and biophysical parameters for the study of the pathogenesis of osteoarthritis: a comparison between the processes of ageing and degeneration in human hip cartilage. Connect Tissue Res. 1989;19:149–176. doi: 10.3109/03008208909043895. [DOI] [PubMed] [Google Scholar]
- 96.Maroudas A, Bayliss MT, Uchitel-Kaushansky N, Schneiderman R, Gilav E. Aggrecan turnover in human articular cartilage: use of aspartic acid racemization as a marker of molecular age. Arch Biochem Biophys. 1998;350:61–71. doi: 10.1006/abbi.1997.0492. [DOI] [PubMed] [Google Scholar]
- 97.Verzijl N, Bank RA, TeKoppele JM, DeGroot J. AGEing and osteoarthritis: a different perspective. Curr Opin Rheumatol. 2003;15:616–622. doi: 10.1097/00002281-200309000-00016. [DOI] [PubMed] [Google Scholar]
- 98.Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, et al. Effect of collagen turnover on the accumulation of advanced glycation endproducts. J Biol Chem. 2000;275:39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 99.DeGroot J, Verzijl N, Wenting-van Wijk MJ, Jacobs KM, Van El B, Van Roermund PM, et al. Accumulation of advanced glycation end products as a molecular mechanism for aging as a risk factor in osteoarthritis. Arthritis Rheum. 2004;50:1207–1215. doi: 10.1002/art.20170. [DOI] [PubMed] [Google Scholar]
- 100.Bank RA, Bayliss MT, Lafeber FP, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998;330(Pt 1):345–351. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Verzijl N, DeGroot J, Ben ZC, Brau-Benjamin O, Maroudas A, Bank RA, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46:114–123. doi: 10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 102.Chen AC, Temple MM, Ng DM, Verzijl N, DeGroot J, TeKoppele JM, et al. Induction of advanced glycation end products and alterations of the tensile properties of articular cartilage. Arthritis Rheum. 2002;46:3212–3217. doi: 10.1002/art.10627. [DOI] [PubMed] [Google Scholar]
- 103.DeGroot J, Verzijl N, Bank RA, Lafeber FP, Bijlsma JW, TeKoppele JM. Age-related decrease in proteoglycan synthesis of human articular chondrocytes: the role of nonenzymatic glycation. Arthritis Rheum. 1999;42:1003–1009. doi: 10.1002/1529-0131(199905)42:5<1003::AID-ANR20>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 104.Loeser RF, Yammani RR, Carlson CS, Chen H, Cole A, Im HJ, et al. Articular chondrocytes express the receptor for advanced glycation end products: Potential role in osteoarthritis. Arthritis Rheum. 2005;52:2376–2385. doi: 10.1002/art.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Steenvoorden MM, Huizinga TW, Verzijl N, Bank RA, Ronday HK, Luning HA, et al. Activation of receptor for advanced glycation end products in osteoarthritis leads to increased stimulation of chondrocytes and synoviocytes. Arthritis Rheum. 2006;54:253–263. doi: 10.1002/art.21523. [DOI] [PubMed] [Google Scholar]
- 106.Cecil DL, Johnson K, Rediske J, Lotz M, Schmidt AM, Terkeltaub R. Inflammation-induced chondrocyte hypertrophy is driven by receptor for advanced glycation end products. J Immunol. 2005;175:8296–8302. doi: 10.4049/jimmunol.175.12.8296. [DOI] [PubMed] [Google Scholar]
- 107.Yammani RR, Carlson CS, Bresnick AR, Loeser RF. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: Role of the receptor for advanced glycation end products. Arthritis Rheum. 2006;54:2901–2911. doi: 10.1002/art.22042. [DOI] [PubMed] [Google Scholar]
- 108.Wilkins E, Dieppe P, Maddison P, Evison G. Osteoarthritis and articular chondrocalcinosis in the elderly. Ann Rheum Dis. 1983;42:280–284. doi: 10.1136/ard.42.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Felson DT, Anderson JJ, Naimark A, Kannel W, Meenan RF. The prevalence of chondrocalcinosis in the elderly and its association with knee osteoarthritis: the Framingham Study. J Rheumatol. 1989;16:1241–1245. [PubMed] [Google Scholar]
- 110.Rosenthal AK, Derfus BA, Henry LA. Transglutaminase activity in aging articular chondrocytes and articular cartilage vesicles. Arthritis Rheum. 1997;40:966–970. doi: 10.1002/art.1780400526. [DOI] [PubMed] [Google Scholar]
- 111.Rosen F, McCabe G, Quach J, Solan J, Terkeltaub R, Seegmiller JE, et al. Differential effects of aging on human chondrocyte responses to transforming growth factor beta: increased pyrophosphate production and decreased cell proliferation. Arthritis Rheum. 1997;40:1275–1281. doi: 10.1002/1529-0131(199707)40:7<1275::AID-ART12>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 112.Doherty M, Dieppe P. Clinical aspects of calcium pyrophosphate dihydrate crystal deposition. Rheum Dis Clin North Am. 1988;14:395–414. [PubMed] [Google Scholar]
- 113.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 114.Aigner T, Zien A, Gehrsitz A, Gebhard PM, McKenna L. Anabolic and catabolic gene expression pattern analysis in normal versus osteoarthritic cartilage using complementary DNA-array technology. Arthritis Rheum. 2001;44:2777–2789. doi: 10.1002/1529-0131(200112)44:12<2777::aid-art465>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 115.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 116.Loeser RF. Molecular mechanisms of cartilage destruction: Mechanics, inflammatory mediators, and aging collide. Arthritis Rheum. 2006;54:1357–1360. doi: 10.1002/art.21813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ferrucci L, Cavazzini C, Corsi A, Bartali B, Russo CR, Lauretani F, et al. Biomarkers of frailty in older persons. J Endocrinol Invest. 2002;25:10–15. [PubMed] [Google Scholar]
- 118.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32:447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 119.Lane NE, Bloch DA, Wood PD, Fries JF. Aging, long-distance running, and the development of musculoskeletal disability. A controlled study. Am J Med. 1987;82:772–780. doi: 10.1016/0002-9343(87)90014-3. [DOI] [PubMed] [Google Scholar]
- 120.Lane NE, Michel B, Bjorkengren A, Oehlert J, Shi H, Bloch DA, et al. The risk of osteoarthritis with running and aging: a 5-year longitudinal study. J Rheumatol. 1993;20:461–468. [PubMed] [Google Scholar]
- 121.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188–195. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 122.Felson DT, Goggins J, Niu J, Zhang Y, Hunter DJ. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum. 2004;50:3904–3909. doi: 10.1002/art.20726. [DOI] [PubMed] [Google Scholar]
- 123.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarth Cartilage. 1995;3:261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 124.Bukhari M, Lunt M, Barton A, Bunn D, Silman A, Symmons D. Increasing age at symptom onset is associated with worse radiological damage at presentation in patients with early inflammatory polyarthritis. Ann Rheum Dis. 2007;66:389–393. doi: 10.1136/ard.2006.055657. [DOI] [PMC free article] [PubMed] [Google Scholar]