Abstract
Acetylcholine (ACh) is a neurotransmitter/neuromodulator in the nematode nervous system and induces its effects through interaction with both ligand-gated ion channels (LGICs) and G protein-coupled receptors (GPCRs). The structure, pharmacology and physiological importance of LGICs have been appreciably elucidated in model nematodes, including parasitic species where they are targets for anthelmintic drugs. Significantly less, however, is understood about nematode ACh GPCRs, termed GARs (G protein-linked ACh receptors). What is known comes from the free-living Caenorhabditis elegans as no GARs have been characterized from parasitic species. Here we clone a putative GAR from the pig gastrointestinal nematode Ascaris suum with high structural homology to the C. elegans receptor GAR-1. Our GPCR, dubbed AsGAR-1, isalternatively spliced and expressed in the head and tail of adult worms but not in dorsal or ventralbody wall muscle, or the ovijector. ACh activated AsGAR-1 in a concentration-dependent manner but the receptor was not activated by other small neurotransmitters. The classical muscarinic agonists carbachol, arecoline, oxotremorine M and bethanechol were also AsGAR-1 agonists but pilocarpine was ineffective. AsGAR-1 activation by ACh was partially antagonized by the muscarinic blocker atropine but pirenzepine and scopolamine were largely ineffective. Certain biogenic amine GPCR antagonists were also found to block AsGAR-1. Our conclusion is that Ascaris possesses G protein-coupled ACh receptors that are homologous in structure to thosepresent in C. elegans, and that although they have some sequence homology to vertebrate muscarinic receptors, their pharmacology is atypically muscarinic.
Keywords: Nematode, G protein-coupled receptor, Acetylcholine, Muscarinic, Ascaris, Yeast
1. Introduction
Nematode cholinergic neurotransmission and neuromodulation are mediated by both ionotropic and metabotropic receptors. Non-selective cation permeable nicotinic acetylcholine receptors (nAChR) and the recently identified ACh-gated chloride channels (Putrenko, et al., 2005), belong to the family of cys-loop ligand-gated ion channels (LGICs) whereas the G protein-linked acetylcholine receptors (GARs) are rhodopsin-like G protein-coupled receptors (GPCRs). Relatively more research attention has been given to nAChRs as they mediate fast, excitatory neurotransmission and are the site of action of a number of anti-nematodal drugs including levamisole and pyrantel (Robertson and Martin, 1993; Robertson et al., 1994). Comparatively little is known of the structure, pharmacology and physiological significance of nematode GARs, particularly in parasitic species.
Caenorhabditis elegans offers some insight into the nematode GAR complement with three GARs having been identified (Hwang et al., 1999; Lee et al., 1999, 2000). These receptors have sequence homology with the five known classes of vertebrate muscarinic receptors; GAR-1 and GAR-2 are similar to the mammalian sub-types M2 and M4, which preferentially couple to Gi/o class G-protein α sub-units, whereas GAR-3 has more homology with M1, M3 and M5, which couple to Gq/11 proteins. While the pharmacology of these GAR receptors has not been strenuously interrogated, they are in some ways similar to but in other ways different from, vertebrate muscarinic receptors (Lee et al., 1999, 2000). Finally, each C. elegans GAR modulates some key nematode behaviours. The expression of GAR-1 in head ciliated sensory neurons and the posterior ventral microtubule (PVM) mechanosensory neuron (Lee et al., 2000) suggest a role in sensory perception and GAR-1 RNA interference (RNAi)-silenced worms have a “sluggish” locomotory phenotype (Keating et al., 2003). GAR-2 is expressed in ventral nerve cord motorneurons, cooperatively modulating worm locomotion with GABAB receptors (Dittman and Kaplan, 2008), and is also expressed in the hermaphroditic specific motorneuron (HSN) vulval motorneuron, where its activation inhibits egg-laying (Bany et al., 2003). GAR-3 has two known roles; it serves to regulate normal pharyngeal function, allowing appropriate excitation-contraction coupling (Steger and Avery, 2004) and it provides a mechanism for male spicule protraction during reproduction (Liu et al., 2007). Therefore, in nematodes, GARs function both in the central and peripheral nervous systems. This is also true for vertebrate muscarinic receptors, which are involved in a plethora of physiological activities, amongst them: memory (Hamilton and Nathanson, 2001); thermoregulation (Gomeza et al., 1999); regulating contractility of the heart, urinary bladder, trachea and stomach (Stengel et al., 2000); constriction of the pupils and control of salivation (Matsui et al., 2000); cerebral vasodilation (Yamada et al., 2001) and modulation of dopaminergic neurotransmission in the CNS (Gerber et al., 2001; Zhang et al., 2002).
Evidence for GARs in parasitic nematodes is less explicit and is based on the responses of parasite tissue preparations to classical muscarinic ligands. Colquhoun et al. (1991) initially described a mixed cholinergic pharmacology in Ascaris somatic musculature; the muscarinic agonists muscarone, furtrethonium and arecoline produced muscle depolarization but the majority tested were either weak or ineffective. Also, the archetypal muscarinic antagonist, atropine, was found to be a poor antagonist of the parasite “muscarinic” receptor, a finding confirmed by others (Segerberg and Stretton, 1993; Martin and Valkanov, 1996). Segerberg and Stretton (1993) also found that the muscarinic antagonist N-methyl-scopolamine was somewhat ineffective. These studies indicate that parasitic nematodes possess GARs albeit with pharmacology that, although similar to mammalian muscarinic receptors, is clearly not identical.
Involvement in key nematode behaviours such as sensory perception, locomotion, pharyngeal pumping and reproduction earmarks GARs for consideration as potential drug targets for controlling nematode parasites but specific knowledge of GAR form and function in parasitic species is lacking. Here we address this by identifying a transcript that encodes a putative GAR from the gastrointestinal roundworm Ascaris suum. This receptor appears analogous to C. elegans GAR-1 in terms of structure and perhaps function, as it is expressed in a manner generally conserved to that of GAR-1 in the head and tail of adult worms. Functional expression of the receptor with a yeast-based system revealed AsGAR-1 has atypical muscarinic pharmacology that may make it therapeutically discernable from host muscarinic receptors.
2. Materials and methods
2.1. Parasite Material
Adult Ascaris suum were collected from a local abattoir, transported to the laboratory and maintained at 33°C, in Locke's solution (NaCl, 155 mM; KCl, 5 mM; CaCl2, 2 mM; NaHCO3, 1.5 mM; glucose, 5 mM).
2.2. Rapid Amplification of cDNA Ends (RACE)
Total RNA was extracted from an adult female Ascaris fresh tissue preparation using TRI Reagent (Sigma); mRNA was then purified from this extract using the Dynabeads mRNA Purification Kit (Dynal Biotech). cDNA suitable for rapid amplification of cDNA ends (RACE) PCR was constructed from the Ascaris mRNA using the SMART RACE cDNA Amplification Kit (Clontech) and used as a template in RACE PCR with either a 5′ RACE primer (5′ GCAATAAGCGTTGTCCAATAGTAAACAAC 3′) or 3′ RACE primer (5′ CATTGGCAATGCGATGGTCATTGTGG 3′) designed from expressed sequence tag (EST) sequence information (see Results). The components for this reaction were as suggested by the manufacturer and the touchdown PCR cycling conditions were as follows: 94°C for 30 s, 72°C for 3 min (5 cycles); 94°C for 30 s, 70°C for 30 s, 72°C for 3 min (5 cycles); 94°C for 30 s, 68°C for 30 s, 72°C for 3 min (30 cycles). Reactions were performed with a Thermo Hybaid Px2 thermal cycler and visualized on 1.2% agarose gel containing ethidium bromide. Discrete amplicons were gel excised, purified (PureLink Gel Extraction Kit, Invitrogen) and ligated into the pGEM-T Easy vector (Promega) prior to subcloning and sequence analysis. DNA sequencing was edited and aligned using VectorNTI v10.3 software (Invitrogen).
2.3. Reverse transcription (RT)-PCR
Adult female Ascaris were dissected and a number of tissue-specific preparations obtained. Sectioning the parasite posterior to the circumpharyngeal nerve ring generated a ‘head’ preparation consisting of said neuropile, some pharyngeal musculature, body wall muscle and anterior sensory structures. Sectioning the parasite anterior to the perianal nerve ring generated a ‘tail’ preparation that included the perianal nerve ring and associated posterior sensory structures, body wall muscle and a small amount of intestinal tract. Dorsal and ventral body wall preparations were composed of muscle strips and associated sections of the dorsal and ventral nerve cords, respectively. Finally, the ovijector was dissected and flushed to remove residual eggs.
Total RNA was extracted from each preparation using TRI Reagent and reverse transcribed into cDNA using the RETROscript Kit (Ambion). A relative semi-quantitative multiplex RT-PCR was performed on each cDNA template. We amplified both of the putative receptor isoforms in this reaction and compared their intensity with normalized 18S rRNA. The QuantumRNA 18S Internal Standards Kit (Ambion) was used as a source of primers to amplify the 18S target combined with receptor-specific primers flanking the deletion site in the third intracellular loop (As18SF: 5′ ACCGAACGAAGCAGCGTTGATATGTTAAG 3′; As18SR: 5′ TGACCTGAGCGCATGCATCA 3′). We used Platinum Taq DNA polymerase (Invitrogen) and manufacturer's buffer system for that enzyme in the reaction, which had the following cycle profile: 94°C for 2 min, then 94°C for 30 s, 57°C for 30 s, 72°C for 1 min (35 cycles). Reactions were visualized on a 1.2% agarose gel containing ethidium bromide.
2.4. Yeast functional expression
Receptor isoforms were PCR-amplified using primers that added NcoI (5′ GCCATACCATGGACGATTCTTACATCCCTAACG 3′) and BamHI (5′ GCCATAGGATCCTTAAATTGCTTTATTAAAATTTCCAC 3′) to the 5′ and 3′ ends of the amplicon, respectively. These primers were sufficient to amplify both isoforms, clones of which were verified upon later sequencing. Purified amplicons were digested and ligated into NcoI/BamHI linearized yeast expression vector Cp4258, before transformation into JM109 competent Escherichia coli (Promega). Individual clones were selected and Luria-Bertani (LB) broth cultured, the plasmid DNA purified using HiSpeed Plasmid Midi Kit (QIAGEN) and sequenced to confirm receptor orientation and fidelity.
The receptor functional assay is an adaptation of that described by Wang et al. (2006). The Saccharomyces cerevisiae strains used (CY19043, CY10560, CY13393, CY13395, CY13399 and YEX 108; kindly provided by J. Broach, Princeton University, USA) differed in their expression of G-protein α subunits, allowing differential coupling of receptor activation to a pheromone responsive cell growth pathway. Initial studies indicated that the two AsGAR isoforms expressed most effectively in strain YEX 108 (MATa PFUS1-HIS3 PGPA1-Gαq(41)-GPA1-Gαq(5) can1 far1D1442 his3 leu2 lys2 sst2D2 ste14∷trp1∷LYS2 ste18g6-3841 ste3D1156 tbt1-1 trp1 ura3) and therefore this strain was used throughout the study. YEX 108 is identical to the strain used by Wang et al. (2006) except that it expresses a chimeric Gα subunit in which the last five amino acids of yeast Gα (gpa1) are replaced with those of rat Gαq. Mid-log phase cells were transformed with 1 μg receptor/vector construct or 1 μg empty vector (mock-transfected control) in the presence of 200 μg salmon sperm DNA (Invitrogen), according to standard protocols. Positive transformants were selected in leucine-deficient media [1 X YNB (Difco), 1 X yeast synthetic drop-out medium supplement without leucine (Sigma), 10 mM ammonium sulphate (Sigma), 2% glucose] and those colonies expressing the receptor were verified by PCR prior to the functional assay.
For the agonist assay, 4 mL leucine-deficient media was inoculated with receptor-expressing yeast or the mock controls and grown at 30°C until the OD600 was approximately 1. The cells were washed three times with leucine and histidine deficient medium [1 X YNB (Difco), 1 X yeast synthetic drop out medium supplement without leucine, tryptophan, uracil and histidine (Sigma) supplemented with uracil (Sigma) and L-tryptophan (Sigma), 10 mM ammonium sulphate (Sigma), 2% glucose, 50 mM 4-Morpholinepropanesulfonic acid (MOPS), pH 6.8] then resuspended in 1 mL leucine and histidine-deficient media containing 1.5 mM 3-Amino-1,2,4-triazole (Sigma) to a density of ≈ 5-20 cells/μL. Approximately 3,000 cells were added to each well of a black-walled, clear bottom 96 well plate containing the same medium and test agonist in a total volume of 200 μl. Cells were grown at 30°C for approximately 30 h before addition of 20 μL Alamar Blue (Invitrogen). Alamar blue is a cell growth indicator and its reduction results in a blue to pink colour change. Fluorescence (520 nm excitation/560 nm emission) was measured at 30 min intervals for up to 4 h using a FLEXStation plate fluorometer (Molecular Devices) preset at 30°C. For the antagonist assays the same protocol was used but antagonists were also included when cells and agonist were added to the 96 well plate. All drugs used in the study were obtained from Sigma (St. Louis, MO). Assay data analysis and dose-response curve fits were performed using Prizm 5.0 (GraphPad Software, San Diego, CA).
3. Results
3.1. A homolog of GAR-1 is expressed in Ascaris suum
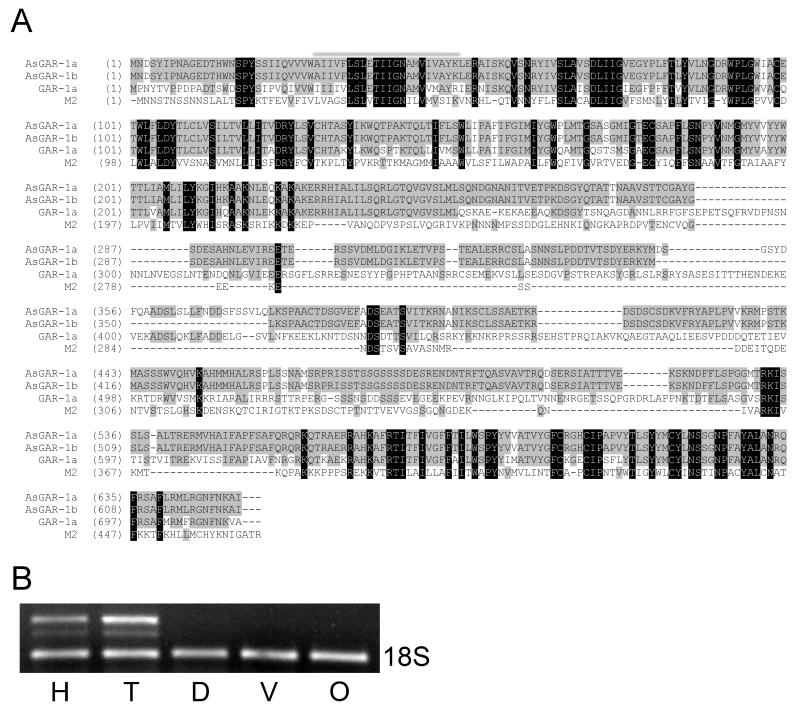
Our hypothesis was that GARs are present in animal parasitic nematodes and that given the degree of structural conservation amongst nematodes, animal parasitic nematodes would share a similar GAR complement to that of C. elegans. To examine this hypothesis, we searched the Ascaris EST data set for GAR orthologs with the tBLASTn algorithm (www.ncbi.nlm.nih.gov/blast) using C. elegans GAR sequences as queries. This approach returned a number of ESTs with varying degrees of similarity to the C. elegans receptors. The EST with highest homology was chosen for further examination. RACE PCR identified a transcript possessing the SL1 spliced leader sequence and a predicted 1,953 nucleotide open reading frame. The 651 amino acid encoded peptide possesses features typical of a rhodopsin class G protein-coupled receptor (GPCR) including seven predicted transmembrane helices with rhodopsin signatures. A comparison of our putative GPCR with a non-redundant protein dataset using the blastp algorithm (http://blast.ncbi.nlm.nih.gov/Blast.cgi) revealed the peptide encoded on our transcript most closely aligns with acetylcholine GPCRs and, in particular, invertebrate GARs. The most closely related molecule is C. elegans GAR-1, which is 50% identical to our putative Ascaris receptor at the amino acid level (see Fig.1A) and when the variable third intracellular (i3) loop is subtracted, this homology rises to 78% identity. Thus, we believe the putative Ascaris receptor constitutes a GAR-1 ortholog and have designated it AsGAR-1 accordingly. The most similar muscarinic receptor is M2, which is 21% identical to AsGAR-1. Further RT-PCR and sequence analysis of the AsGAR-1 open reading frame indicated the presence of an alternatively spliced isoform of the receptor, identical to the first save for a 27 amino acid deletion in the predicted i3 loop. Examination of this deletion did not reveal any motifs that would denote predictable functionality other than a possible conserved tyrosine phosphorylation site that is not present in the shorter isoform (see Fig. 1A). Caenorhabditis elegans GARs also display alternate splicing, resulting in multiple receptor isoforms that, like AsGAR-1, vary with respect to i3 loop deletions (Park et al., 2000, 2003; Suh et al., 2001). In keeping with the adopted C. elegans nomenclature, the longer Ascaris isoform was designated AsGAR-1a and the shorter isoform AsGAR-1b.
Fig. 1.
A G protein-linked acetylcholine receptor (GAR), dubbed AsGAR-1, is expressed in the pig gastrointestinal roundworm, Ascaris suum. (A) AsGAR-1 has high homology with GAR-1, a Caenorhabditis elegans G protein-coupled ACh receptor. Alignment of the two AsGAR-1 isoforms, a and b, with C. elegans GAR-1 (Swiss-Prot ID Q18007) and the most closely related muscarinic receptor, M2 (Swiss-Prot ID P08172). The seven predicted transmembrane domains of AsGAR-1 are indicated with bars. A possible tyrosine phosphorylation site present in AsGAR-1a but not AsGAR-1b is marked (*). Shading indicates the degree of conservation. Alignment was constructed using the blosum62mt2 matrix and Vector NTI v10.3 software (Invitrogen). (B) AsGAR-1 is expressed in a tissue-specific manner. A multiplexed relative semi-quantitative reverse transcription (RT)-PCR reaction resulted in AsGAR-1a and b amplification from specific RNA preparations. AsGAR-1a (upper amplicon) and AsGAR-1b (middle amplicon) were observed in head (H) and tail (T) preparations but not in dorsal (D) or ventral (V) body wall muscle preparations or an ovijector preparation (O). 18S rRNA was used as our normalized internal standard (lower amplicon).
3.2. AsGAR-1 displays tissue-specific distribution
Caenorhabditis elegans GARs are expressed in a tissue-specific manner; for example, GAR-1 is expressed in a set of ciliated sensory neurons in the head of C. elegans and in the PVM mechanosensory neuron (Lee et al., 2000). Also, although all GAR splice variants are expressed in a temporally similar manner, typically through all life cycle stages, one isoform is expressed athigher levels than the others (Park et al., 2000, 2003; Suh et al., 2001). To examine the gross spatial distribution of AsGAR-1, and also to examine the relative expression levels of the two AsGAR-1 isoforms, we used a multiplexed relative semi-quantitative RT-PCR protocol, with 18S rRNA as our internal standard, performed on RNA extracted from tissue-specific preparations.
AsGAR-1 could be amplified from two of our RNA preparations, the ‘head’ and ‘tail’ (Fig. 1B). The ‘head’ preparation was generated from the circumpharyngeal nerve ring and anterior sensory structures and included some body wall and pharyngeal muscle. Amplification of AsGAR-1 from this preparation indicates expression in one or more of these structures. The ‘tail’ preparation contained RNA from the perianal nerve ring and posterior sensory structures as well as body wall muscle and a small section of gut. A positive PCR result also indicates AsGAR-1 expression in one or more of these structures. There was no amplification of the receptor from the dorsal or ventral body wall muscle or ovijector RNA preparations. Both AsGAR-1 isoforms were amplified from the ‘head’ and ‘tail’ RNA although the longer isoform (AsGAR-1a) was expressed more strongly in both cases, approximately nine-fold more than the shorter isoform.
3.3. AsGAR-1 is an acetylcholine receptor
AsGAR-1 has high sequence homology to known ACh GPCRs. To determine whether AsGAR-1 is an ACh receptor we monitored the response of the putative receptor to ACh using a histidine auxotrophic yeast functional expression assay (Wang et al., 2006). Briefly, the GPCR of interest is expressed in a modified auxotrophic yeast strain that carries the His3 reporter gene under the transcriptional control of the pheromone-responsive FUS1 promoter. His3 confers the ability to synthesize histidine and the FUS1 promoter links this synthesis to an endogenous pheromone-signaling pathway. It is this pathway that is stimulated by activation of the heterologous GPCR. Thus, in histidine-deficient media, receptor expression and activation will induce yeast growth. We were able to output yeast growth as fluorescence through the addition of Alamar Blue (Invitrogen). Reduction of this dye by the reproducing yeast produces a colour change from dark blue to hot pink that can be measured fluorometrically. Receptor activation was calculated as increased yeast growth relative to mock-transfected control tested on the same 96-well plate.
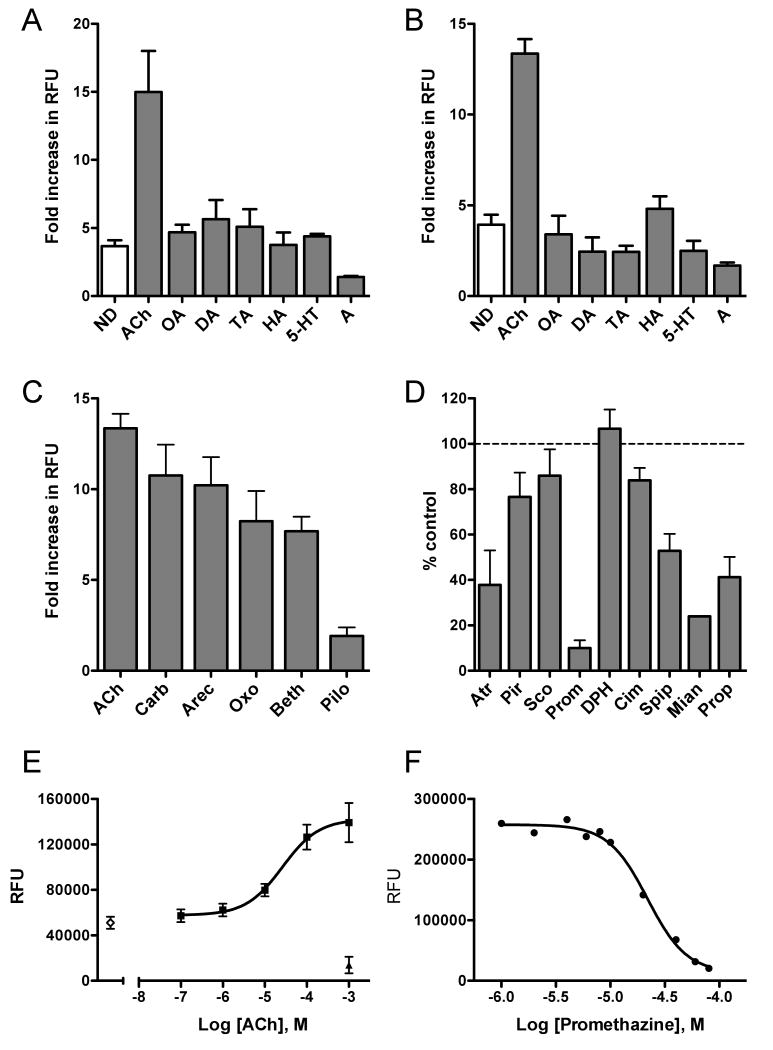
AsGAR-1 shows some constitutive activity when expressed in yeast. Cultures transformed with AsGAR-1a or -1b plasmids showed approximately three to four times more cell growth than the mock-transfected control in the absence of test ligand (Fig. 2A). The receptor was further activated, however, when the cells were treated with ACh. ACh (10-4 M) activated AsGAR-1, producing a 15 ± 3 -fold growth increase in yeast expressing AsGAR-1a (n = 8, P = < 0.05), and a 13.4 ± 0.8 -fold increase in growth in yeast expressing AsGAR-1b (n = 9, P = < 0.05)(see Fig. 2A and B). The response to ACh between the two strains was not significantly different. To confirm this response was ACh-specific we screened the two receptors with other potential ligands. Phylogenetic analysis indicated that AsGAR-1 was similar to both GARs identified from other invertebrates and vertebrate muscarinic receptors but the next most related cluster of GPCRs were invertebrate biogenic amine receptors. We therefore screened AsGAR-1 with a panel of aminergic neurotransmitters. None of these small signaling molecules significantly activated either AsGAR-1 isoform, suggesting ACh specifically activates these receptors (Fig. 2A and B).
Fig. 2.
AsGAR-1, an Ascaris suum homolog of the Caenorhabditis elegans G protein-linked acetylcholine receptor, GAR-1, has atypical muscarinic pharmacology. (A) AsGAR-1a is activated by 10-4 M acetylcholine (ACh), but not by a panel of other neurotransmitters. Receptor activation is expressed as a fold increase in fluorescence (RFU) over mock-transfected yeast. Octopamine (OA), dopamine (DA), tyramine (TA), histamine (HA), serotonin (5-HT), adrenaline (A) all at 10-4 M, no drug (ND). The data are the means and S.E.M. of at least three separate experiments, each with three to six replicates. (B) AsGAR-1b is activated by ACh, but not by other neurotransmitters, drugs as in A. (C) AsGAR-1 activation by a panel of muscarinic agonists: carbachol (Carb), arecoline (Arec), oxotremorine M (Oxo), bethanechol (Beth) and pilocarpine (Pilo) all at 10-4 M. (D) Antagonism of AsGAR-1 by muscarinic antagonists, expressed as a percentage of AsGAR-1 activation by ACh (10-4 M). Atropine (Atr), pirenzepine (Pir), scopolamine (Sco), promethazine (Prom), diphenhydramine (DPH), cimetidine (Cim), spiperone (Spip), mianserin (Mian) and propranolol (Prop) all 10-4 M. (E) ACh activates AsGAR-1b in a concentration-dependent manner with an EC50 of 20.3 ± 1.7 μM. ◇, no agonist and ▲, mock-transfected yeast. (F) A representative dose response curve showing the dose-dependent antagonism of ACh induced AsGAR-1b activation by promethazine. Promethazine was the most potent AsGAR-1 blocker with an IC50 of 17.6 ± 4.2 μM based on three separate experiments, each with three to six replicates.
Subsequent experiments to pharmacologically profile AsGAR-1 used the b isoform in the YEX 108 yeast strain. AsGAR-1b was chosen because although both isoforms responded in a similar manner to potential ligands, in combination with YEX 108 it gave the most consistent responses. Using this pairing, the AsGAR-1 response to ACh was found to be concentration-dependent (see Fig. 2E) with a half maximal effective concentration (EC50) of 20.3 ± 1.7 μM.
3.4. AsGAR-1 displays atypical “muscarinic” pharmacology
Invertebrate GARs have notable sequence homology to vertebrate muscarinic receptors but there is evidence that GARs are pharmacologically distinct. In terms of agonists for example, C. elegans GAR-2 was found to be unresponsive to the muscarinic agonists arecoline and oxotremorine (Lee et al., 2000; Suh et al., 2001). Similarly for antagonists, the same receptor was not blocked by atropine (Lee et al., 2000), an antagonist that classically defines muscarinic-like pharmacology. Our hypothesis was AsGAR-1 would show a pharmacological profile similar to C. elegans GARs and different from vertebrate muscarinic receptors. To test this hypothesis we first screened AsGAR-1 with a panel of cholinergic agonists at 10-4 M (see Fig. 2C). The most potent of these compounds was carbachol, a non-selective cholinergic agonist, which elicited a 10.8 ± 1.7 fold increase in yeast growth relative to mock-transfected yeast (n = 4, P ≤0.05). Arecoline increased yeast growth 10.2 ± 1.6 -fold (n = 4, P ≤0.05). The vertebrate muscarinic agonists oxotremorine M and bethanechol were less potent, producing an 8.2 ± 1.7 -fold increase (n = 3, P ≤0.05) and a 7.7 ± 0.8 -fold increase (n = 3, P ≤0.05) in yeast growth, respectively. Finally, the muscarinic agonist pilocarpine produced a modest 1.9 ± 0.5 -fold increase in yeast growth, which was not significant (n = 3, P = 0.07). In summary, the relative rank order potency of cholinergic agonists at AsGAR-1 was ACh > carbachol = arecoline > oxotremorine M > bethanechol ≫pilocarpine.
Next we examined the ability of some muscarinic antagonists to block AsGAR-1 activation by ACh. To do this, antagonists were tested at 10-4 M in the presence of 10-4 M ACh. Yeast growth in the presence of antagonist with ACh was expressed as a percentage of growth in Ach alone. Further, antagonists were tested in media supplemented with histidine to confirm that any observed growth inhibition was due to receptor antagonism and not cytotoxicity. Atropine reduced yeast growth to 37.8 ± 15.1% of control, blocking AsGAR-1 activation by 62.2%. Two other muscarinic antagonists were less effective. Pirenzepine reduced yeast growth to 76.6 ± 10.7% of control (a 23.4% decrease in AsGAR-1 activation) and scopolamine reduced yeast growth to 85.9 ± 11.7% of control (14.1% inhibition).
As described earlier, we screened AsGAR-1 with biogenic amines due to shared sequence homology between AsGAR-1 and biogenic amine GPCRs but found that none of the amines activated our receptor. Extending this rationale, we also tested some antagonists of biogenic amine receptors at 10-4 M for their ability to block AsGAR-1 (Fig. 2D). We first tested some known histamine receptor antagonists and found that promethazine, a H1 receptor blocker, reduced AsGAR-1 activation to 10 ± 3.5% of control. This was the most potent AsGAR-1 blocker identified in our study; promethazine inhibition was concentration-dependent with a half maximal inhibitory concentration (IC50) of 17.6 ± 4.2 μM (Fig. 2F). Another H1 receptor blocker, diphenhydramine, was much less effective and actually increased yeast growth slightly (106.6 ± 8.5% of control). The H2 receptor antagonist cimetidine elicited a mild blockade of AsGAR-1 to 83.9 ± 5.5% of control. Spiperone, a potent dopamine and serotonin receptor antagonist also partially blocked ACh activation of AsGAR-1, reducing yeast growth to 52.8 ± 7.6% of control. Our final antagonists tested were adrenergic receptor blockers and both were effective at antagonizing ACh activation of AsGAR-1. Mianserin, a α2-adrenergic and serotonergic receptor antagonist, reduced yeast growth to 24 ± 0.1% of control while propranolol, a non-selective β-adrenergic receptor blocker, reduced yeast growth to 41.2 ± 8.9% of control levels. To summarize, the rank order potency of those antagonists tested was promethazine > mianserin > atropine > propranolol > spiperone > pirenzepine > cimetidine >scopolamine > diphenhydramine. None of the compounds impaired yeast growth in the presence of histidine indicating the inhibition observed was receptor-specific.
4. Discussion
Here we found that the pig gastrointestinal roundworm, A. suum, has a GAR that is structurally and likely functionally orthologous to a GAR present in C. elegans. In terms of structural homology, AsGAR-1 possesses features consistent with GARs beyond that of sequence identity, for example, the expression of multiple isoforms of the receptor. Vertebrate muscarinic receptors do not display alternative splicing whereas this appears to be a feature of invertebrate GARs; all three C. elegans GARs are alternatively spliced (Park et al., 2000, 2003; Suh et al., 2001) and the Drosophila melanogaster ACh GPCR characterized by Millar et al. (1995) also shows splicing (NCBI Reference Sequences NP 523844.2 and NP 726440.1). Each of these variants differs from their canonical receptors by deletions in the i3 loops, more specifically, towards the middle of these highly variable regions. The physiological significance of these deletions is unclear. Even between receptors with high overall homology such as AsGAR-1 and C. elegans GAR-1, the i3 loops share little similarity and the deletions here do not appear to be conserved either in terms of their locations or the amino acids deleted. One possibility is that because the i3 loop plays a role in G-protein coupling, these deletions may have an effect on receptor/G-protein interaction or the specificity of that interaction. It seems more likely, however, that this is not the case as no isoform-specific G-protein preference was noted during the functional expression of C. elegans GARs, which also have deletions in the middle of the i3 loop (Park et al., 2000, 2003; Suh et al., 2001). Further, the key muscarinic receptor motifs determining GPCR/G-protein interaction and the selectivity of that interaction are located in the i2 loop and at the N- and C-termini of the i3 loop (Kunkel and Peralta, 1993; Blüml et al., 1994a, b; Zhu et al., 1994; Blin et al., 1995; Burstein et al., 1995; Högger et al., 1995; Jones et al., 1995; Liu et al., 1995), not in the middle of the i3 loop where the GAR deletions typically manifest. A more likely physiological consequence of these deletion events may be an alteration in agonist-induced receptor desensitization and internalization. Muscarinic receptor desensitization occurs via receptor phosphorylation (Haga and Haga, 1990), typically by protein kinase C (PKC) and/or G protein-receptor kinases (GRK) at conserved serine and threonine sites in the i3 loop (Haga et al., 1990, 1996; Kameyama et al., 1994; Nakata et al., 1994; Pals-Rylaarsdam et al., 1995; Pals-Rylaarsdam and Hosey, 1997). Deletion events removing the major phosphorylation sites could alter the kinetics of receptor desensitization. The 27 amino acid deletion in AsGAR-1b removes a conserved tyrosine phosphorylation site (see Fig. 1) that, although different to the serine and threonine residues that typically serve as substrates for PKC and GRK, may alter the signaling properties of AsGAR-1b.
Demonstrating functional GAR homology between A. suum and C. elegans is more difficult but we provide preliminary evidence that suggests AsGAR-1 could serve a similar functional role to GAR-1 in C. elegans. This is based on a broadly conserved expression pattern. Lee et al. (2000) used a GAR-1 promoter-GFP fusion approach to demonstrate the expression of GAR-1 in a subset of C. elegans amphidial sensory neurons, and also in the PVM mechanosensory (stretch) neuron. This strongly supports a role for GAR-1 in mediating or modulating nematode sensory perception. We demonstrated AsGAR-1 expression in the head and tail of Ascaris. These were fairly complex tissue preparations and being more explicit about cellular expression is difficult. Both head and tail preparations contained RNA from sensory structures (anterior amphids, posterior phasmids), nerve rings, somatic and visceral musculature but a lack of receptor expression in body wall muscle supports the hypothesis that AsGAR-1 is expressed in areas where sensory and neuronal elements are enriched. In broad terms the AsGAR-1 expression pattern is similar to that of GAR-1 in C. elegans and a conserved functional role in sensory perception could be hypothesized. In addition to this presumed role in sensory perception, Keating et al. (2003) observed a “sluggish” GAR-1 RNAi phenotype with a decrease in the number of body bends per minute compared with the normal nematode sinusoidal waveform. It may be that GAR-1, like GAR-2, is also a component of the circuits that regulate nematode locomotion adding to its attractiveness as a possible drug target. Further study using a more visual in situ approach such as immunocytochemistry or in situ hybridization will help delineate AsGAR-1 expression in Ascaris and shed light on its functionality in this parasite.
Using a yeast expression assay we were able to develop a pharmacological profile of AsGAR-1. Our hypothesis was that this profile would resemble that of C. elegans GAR-1 and be different to classical muscarinic pharmacology. In terms of agonist activation, this was generally found to be the case. Our rank order of agonist potency at AsGAR-1 correlated with what is known of GAR-1, but importantly was different to that of vertebrate muscarinic receptors. Specifically, we found pilocarpine to be an ineffective agonist of AsGAR-1 whereas it is an effective agonist at the M2 receptor, albeit less potent than other classical muscarinic agonists (McKinney et al., 1991; Lazareno et al., 1993; Bräuner-Osborne and Brann, 1996). Examination of the antagonist profile also supports our hypothesis, underlining the pharmacological differences between GARs and muscarinic receptors. Atropine inhibited ACh activation of AsGAR-1 by 62% but inhibited the M2 muscarinic receptor by 90% (Lee et al., 1999). Pirenzepine inhibited ACh activation of AsGAR-1 by 23%, but M2 by 84% (Lee et al., 1999). Finally, scopolamine inhibited ACh activation of AsGAR-1 by 14%, but M2 by 98% (Lee et al., 1999). Clearly the classical muscarinic antagonists have much reduced efficacy at the parasite receptor. The pharmacological differences between GARs and muscarinic receptors may lie in the conservation, or lack thereof, of key amino acids that define the ligand receptor interaction. Numerous amino acids in the transmembrane domains and extracellular loops of muscarinic receptors have been defined as important for ligand binding and stabilization of the active state of the receptor (Wess et al., 1991; Heitz et al., 1999; Hulme et al., 2001). Many of these residues are conserved between muscarinic receptors and AsGAR-1 but, importantly, several are not, including the critical tryptophan at position 155 of the M2 receptor (W155), N404, T187 and T190. Such amino acid variation could result in the altered pharmacological profile we observed.
Further evidence for the pharmacological distinction between GARs and muscarinic receptors was provided by the response of AsGAR-1 to select amine receptor antagonists. Some histamine receptor antagonists are widely known to have anticholinergic effects but we found their activity at AsGAR-1 to be different to that at muscarinic receptors. Promethazine, an H1 receptor antagonist, diphenhydramine, an H1 antagonist, and cimetidine, an H2 antagonist, all display varying degrees of vertebrate muscarinic receptor antagonism (Kubo et al., 1987; Gwee and Cheah, 1990; 1991; Orzechowski et al., 2005; Liu et al., 2006). Only promethazine was an effective antagonist of AsGAR-1. Mianserin is an antagonist at α2 adrenergic receptors and peripheral 5-HT and histamine receptors (for review, see Marshall, 1983) and although it is a very weak muscarinic receptor antagonist (Richelson and Nelson, 1984) it is an effective AsGAR-1 antagonist at the concentration tested. Finally, propranolol and spiperone were somewhat effective AsGAR-1 antagonists but have not been reported to have any anti-muscarinic actions. Ultimately, assessment of the ligand activity at AsGAR-1 observed in this study leads to the conclusion that AsGAR-1 displays, at best, atypical muscarinic pharmacology.
In this study we used the yeast, Saccharomyces cerevisiae, as a host for the heterologous expression of AsGAR-1. Saccharomyces cerevisiae is a relatively familiar vehicle for GPCR expression and has been used previously to study muscarinic receptors (Schmidt et al., 2003; Scarselli et al., 2007), but its utility for invertebrate GPCR expression is less well reported (Minic et al., 2005). We believe that the technique employed here is a useful addition to the parasitologist's toolbox. In our experience this application has several advantages over receptor expression in alternative cell lines. Yeasts are highly amenable to genetic transformation and obtaining stably transfected lines is more straightforward and rapid than for mammalian or insect cell lines. Once optimized, we found both agonist and antagonist assays to be robust and highly suited to our high-throughput platform (FLEXStation, Molecular Devices). The yeast system was more cost effective than other assays we have used as expensive media and cultureware were generally not required. Finally, this yeast assay was amenable to AsGAR-1 expression when other cell lines were not; we unsuccessfully attempted AsGAR-1 expression in two commonly used mammalian cell lines (CHO and HEK-293) and the insect cell line, Sf9, and in each case, although we successfully transfected each cell line and observed mRNA transcription, no functional protein could be detected. In our hands, only two downsides to this assay were noted. Firstly, we observed an overall decrease in the potency of ligands, both agonists and antagonists, compared with other functional expression formats. For example, the AsGAR-1 ACh EC50 was 20.3 ± 1.7 μM, between one and two orders of magnitude higher than the approximate EC50 for C. elegans GAR-1 expressed in Xenopus oocytes (Park et al., 2000). Others have observed similar potency decreases compared with other assay formats (Wang et al., 2006) which may be attributable either to accessibility of the receptor to ligand (a factor of the thick yeast cell wall), or to imperfect interaction between the exogenous receptor and the endogenous signaling mechanism. For this reason, high concentrations of agonist and antagonist were used in this study. A second downside to the assay was a degree of AsGAR-1 constitutive activity, which was manifest as yeast growth in response to no drug (see Fig. 2A). Relative to receptor activation by ACh, the level of constitutive activity encountered was small and not a hindrance to AsGAR-1 deorphanization. Previous investigators have not reported consistent constitutive receptor activity using this expression system so this activity may not be a problem inherent to the assay platform but rather, particular to AsGAR-1.
Two criteria are important in the validation of a molecule as a potential novel drug target for nematode control. One is that the molecule plays an important biological role, such that interference with that molecule is detrimental to the parasite. A second is that the parasite molecule can be selectively targeted relative to the host. Further work will be required to define the physiological role of AsGAR-1 but we provide some evidence that its function is conserved with that of the C. elegans ortholog, GAR-1. Thus we may find that Ascaris GARs are also involved in sensory perception, regulation of locomotion, pharyngeal contractility and reproduction; all are attractive targets for chemotherapeutic intervention. We have also demonstrated that AsGAR-1 is pharmacologically different to vertebrate muscarinic receptors. This opens the door to the possibility of drugs acting selectively on parasite GARs and not host G protein-coupled Ach receptors. On this basis, parasitic nematode GARs warrant further investigation as potential novel drug targets.
Acknowledgments
The authors with to thanks Dr. James Broach, Princeton University, for the yeast strains used here and Cheryl Clark, Iowa State University, for her assistance with worm collection. Pfizer, Inc. (MK), NIH grant number AI049162 (TAD) and the Natural Sciences and Engineering Research Council of Canada (PR) supported this research.
Footnotes
Note: Nucleotide sequence data reported in this paper are available in the GenBank™ database under the accession numbers FJ609743 and FJ609744.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bany IA, Dong MQ, Koelle MR. Genetic and cellular basis for acetylcholine inhibition of Caenorhabditis elegans egg-laying behavior. J Neurosci. 2003;23:8060–8059. doi: 10.1523/JNEUROSCI.23-22-08060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N, Yun J, Wess J. Mapping of single amino acid residues required for selective activation of Gq/11 by the M3 muscarinic acetylcholine receptor. J Biol Chem. 1995;270:17741–17748. doi: 10.1074/jbc.270.30.17741. [DOI] [PubMed] [Google Scholar]
- Blüml K, Mutschler E, Wess J. Identification of an intracellular tyrosine residue critical for muscarinic receptor-mediated stimulation of phosphatidyl inositol hydrolysis. J Biol Chem. 1994a;269:402–405. [PubMed] [Google Scholar]
- Blüml K, Mutschler E, Wess J. Functional role of a cytoplasmic aromatic amino acid in muscarinic receptor-mediated activation of phospholipase C. J Biol Chem. 1994b;269:11537–11541. [PubMed] [Google Scholar]
- Bräuner-Osborne H, Brann MR. Pharmacology of muscarinic acetylcholine receptor subtypes (M1-M5): high throughput assays in mammalian cells. Eur J Pharmacol. 1996;295:93–102. doi: 10.1016/0014-2999(95)00639-7. [DOI] [PubMed] [Google Scholar]
- Burstein ES, Spalding TA, Hill-Eubanks D, Brann MR. Structure-function of muscarinic receptor coupling to G proteins: random saturation mutagenesis identifies a critical determinant of receptor affinity for G proteins. J Biol Chem. 1995;270:3141–3146. doi: 10.1074/jbc.270.7.3141. [DOI] [PubMed] [Google Scholar]
- Colquhoun L, Holden-Dye L, Walker RJ. The pharmacology of cholinoceptors on the somatic muscle cells of the parasitic nematode Ascaris suum. J Exp Biol. 1991;158:509–530. doi: 10.1242/jeb.158.1.509. [DOI] [PubMed] [Google Scholar]
- Dittman JS, Kaplan JM. Behavioral impact of neurotransmitter-activated G-protein-coupled receptors: muscarinic and GABAB receptors regulate Caenorhabditis elegans locomotion. J Neurosci. 2008;28:7104–7112. doi: 10.1523/JNEUROSCI.0378-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber DJ, Sotnikova TD, Gainetdinov RR, Huang SJ, Caron MG, Tonegawa S. Hyperactivity, elevated dopaminergic transmission and response to amphetamine in M1 muscarinic acetylcholine receptor-deficient mice. Proc Natl Acad Sci. 2001;98:15312–15317. doi: 10.1073/pnas.261583798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J. Pronounced pharmacological deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:1692–1697. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwee MCE, Cheah LS. Selectivity of cimetidine and ranitidine actions at some cholinergic sites in the anaesthetised cat. Eur J Pharmacol. 1990;183:2004–2005. [Google Scholar]
- Gwee MCE, Cheah LS. Antimuscarinic and anticholinesterase activity of cimetidine and ranitidine: clinically significant? Br J Clin Pharmac. 1991;32:260–261. doi: 10.1111/j.1365-2125.1991.tb03895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga K, Haga T. Dual regulation by G proteins of agonist-dependent phosphorylation of muscarinic acetylcholine receptors. FEBS Lett. 1990;268:43–47. doi: 10.1016/0014-5793(90)80968-o. [DOI] [PubMed] [Google Scholar]
- Haga K, Haga T, Ichiyama A. Phosphorylation by protein kinase C of the muscarinic acetylcholine receptor. J Neurochem. 1990;54:1639–1644. doi: 10.1111/j.1471-4159.1990.tb01216.x. [DOI] [PubMed] [Google Scholar]
- Haga K, Kameyama K, Haga T, Kikkawa U, Shiozaki K, Uchiyama H. Phosphorylation of human M1 muscarinic acetylcholine receptors by G protein-coupled receptor kinase 2 and protein kinase C. J Biol Chem. 1996;271:2776–2782. doi: 10.1074/jbc.271.5.2776. [DOI] [PubMed] [Google Scholar]
- Hamilton SE, Nathanson NM. The M1 receptor is required for muscarinic activation of mitogen-activated protein (MAP) kinase in murine cerebral cortical neurons. J Biol Chem. 2001;276:15850–15853. doi: 10.1074/jbc.M011563200. [DOI] [PubMed] [Google Scholar]
- Heitz F, Holzwarth JA, Gies JP, Pruss RM, Trumpp-Kallmeyer S, Hibert MF, Guenet C. Site-directed mutagenesis of the putative human muscarinic M2 receptor binding site. Eur J Pharmacol. 1999;380:183–195. doi: 10.1016/s0014-2999(99)00439-2. [DOI] [PubMed] [Google Scholar]
- Högger P, Shockley MS, Lameh J, Sadee W. Activating and inactivating mutations in N- and C-terminal i3 loop junctions of muscarinic acetylcholine Hm1 receptors. J Biol Chem. 1995;270:7405–7410. doi: 10.1074/jbc.270.13.7405. [DOI] [PubMed] [Google Scholar]
- Hulme EC, Lu ZL, Bee M, Curtis CAM, Saldanha J. The conformational switch in muscarinic acetylcholine receptors. Life Sci. 2001;68:2495–2500. doi: 10.1016/s0024-3205(01)01044-x. [DOI] [PubMed] [Google Scholar]
- Hwang JM, Chang DJ, Kim US, Lee YS, Park YS, Kaang BK, Cho NJ. Cloning and functional characterization of a Caenorhabditis elegans muscarinic acetylcholine receptor. Receptor Channel. 1999;6:415–424. [PubMed] [Google Scholar]
- Jones PG, Curtis CAM, Hulme EC. The function of a highly-conserved arginine residue in activation of the muscarinic M1 receptor. Eur J Pharmacol. 1995;288:251–257. doi: 10.1016/0922-4106(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Kameyama K, Haga K, Haga T, Moro O, Sadee W. Activation of a GTP-binding-protein-coupled receptor kinase ((β-adrenergic-receptor kinase-1) by a muscarinic receptor M2 mutant lacking phosphorylation sites. Eur J Biochem. 1994;226:267–276. doi: 10.1111/j.1432-1033.1994.tb20050.x. [DOI] [PubMed] [Google Scholar]
- Keating CD, Kriek N, Daniels M, Ashcroft NR, Hopper NA, Siney EJ, Holden-Dye L, Burke JF. Whole-genome analysis of 60 G Protein-coupled receptors in Caenorhabditis elegans by gene knockout with RNAi. Curr Biol. 2003;13:1715–1720. doi: 10.1016/j.cub.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Kubo N, Shirakawa O, Kuno T, Tanaka C. Antimuscarinic effects of antihistamines: quantitative evaluation by receptor-binding assay. Jpn J Pharmacol. 1987;43:277–282. doi: 10.1254/jjp.43.277. [DOI] [PubMed] [Google Scholar]
- Kunkel MT, Peralta EG. Charged amino acids required for signal transduction by the M3 muscarinic acetylcholine receptor. EMBO J. 1993;12:3809–3815. doi: 10.1002/j.1460-2075.1993.tb06059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazareno SS, Farries T, Birdsall NJM. Pharmacological characterization of guanine nucleotide exchange reactions in membranes from CHO cells stably transfected with human muscarinic receptors M1-M4. Life Sci. 1993;52:449–456. doi: 10.1016/0024-3205(93)90301-i. [DOI] [PubMed] [Google Scholar]
- Lee YS, Park YS, Chang DJ, Hwang JM, Min CK, Kaang BK, Cho NJ. Cloning and expression of a G protein linked acetylcholine receptor from Caenorhabditis elegans. J Neurochem. 1999;72:58–65. doi: 10.1046/j.1471-4159.1999.0720058.x. [DOI] [PubMed] [Google Scholar]
- Lee YS, Park YS, Nam S, Suh SJ, Lee J, Kaang BK, Cho NJ. Characterization of GAR-2, a novel G protein-linked acetylcholine receptor from Caenorhabditis elegans. J Neurochem. 2000;75:1800–1809. doi: 10.1046/j.1471-4159.2000.0751800.x. [DOI] [PubMed] [Google Scholar]
- Liu H, Zheng Q, Farley JM. Antimuscarinic actions of antihistamines on the heart. J Biomed Sci. 2006;13:395–401. doi: 10.1007/s11373-005-9053-7. [DOI] [PubMed] [Google Scholar]
- Liu J, Conklin BR, Blin N, Yun J, Wess J. Identification of a receptor/G-protein contact site critical for signaling specificity and G-protein activation. Proc Natl Acad Sci USA. 1995;92:11642–11646. doi: 10.1073/pnas.92.25.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, LeBoeuf B, Garcia LR. Gαq-coupled muscarinic acetylcholine receptors enhance nicotinic acetylcholine receptor signaling in Caenorhabditis elegans mating behavior. J Neurosci. 2007;27:1411–1421. doi: 10.1523/JNEUROSCI.4320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney M, Miller JH, Gibson VA, Nickelson L, Aksoy S. Interactions of agonists with M2 and M4 muscarinic receptor subtypes mediating cyclic AMP inhibition. Mol Pharmacol. 1991;40:1014–1022. [PubMed] [Google Scholar]
- Marshall RJ. The pharmacology of mianserin - an update. Br J Clin Pharmacol. 1983;15:263S–268S. doi: 10.1111/j.1365-2125.1983.tb05874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RJ, Valkanov MA. Effects of acetylcholine on a slow voltage-activated non-selective cation current mediated by non-nicotinic receptors on isolated Ascaris muscle bags. Exp Physiol. 1996;81:909–925. doi: 10.1113/expphysiol.1996.sp003992. [DOI] [PubMed] [Google Scholar]
- Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, Takahashi S, Taketo MM. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci. 2000;97:9579–9584. doi: 10.1073/pnas.97.17.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar NS, Baylis HA, Reaper C, Bunting R, Mason WT, Sattelle DB. Functional expression of a cloned Drosophila muscarinic acetylcholine receptor in a stable Drosophila cell line. J Exp Biol. 1995;198:1843–1850. doi: 10.1242/jeb.198.9.1843. [DOI] [PubMed] [Google Scholar]
- Minic J, Persuy MA, Godel E, Aioun J, Connerton I, Salesse R, Pajot-Augy E. Functional expression of olfactory receptors in yeast and development of a bioassay for odorant screening. FEBS J. 2005;272:524–537. doi: 10.1111/j.1742-4658.2004.04494.x. [DOI] [PubMed] [Google Scholar]
- Nakata H, Kameyama K, Haga K, Haga T. Location of agonist-dependent- phosphorylation sites in the third intracellular loop of muscarinic acetylcholine receptors (M2 subtype) Eur J Biochem. 1994;220:29–36. doi: 10.1111/j.1432-1033.1994.tb18595.x. [DOI] [PubMed] [Google Scholar]
- Orzechowski RF, Currie DS, Valancius CA. Comparative anticholinergic effects of 10 histamine H1 receptor antagonists in two functional models. Eur J Pharmacol. 2005;506:257–264. doi: 10.1016/j.ejphar.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Pals-Rylaarsdam R, Xu Y, Witt-Enderby P, Benovic JL, Hosey MM. Desensitization and internalization of the M2 muscarinic acetylcholine receptor are directed by independent mechanisms. J Biol Chem. 1995;270:29004–29011. doi: 10.1074/jbc.270.48.29004. [DOI] [PubMed] [Google Scholar]
- Pals-Rylaarsdam R, Hosey MM. Two homologous phosphorylation domains differentially contribute to desensitization and internalization of the M2 muscarinic acetylcholine receptor. J Biol Chem. 1997;272:14152–14158. doi: 10.1074/jbc.272.22.14152. [DOI] [PubMed] [Google Scholar]
- Park YS, Lee YS, Cho NJ, Kaang BS. Alternative splicing of GAR-1, a Caenorhabditis elegans G protein-linked acetylcholine receptor gene. Biochem Biophys Res Comm. 2000;268:354–358. doi: 10.1006/bbrc.2000.2108. [DOI] [PubMed] [Google Scholar]
- Park YS, Kim S, Shin Y, Choi B, Cho NJ. Alternative splicing of the muscarinic acetylcholine receptor GAR-3 in Caenorhabditis elegans. Biochem Biophys Res Comm. 2003;308:961–965. doi: 10.1016/s0006-291x(03)01508-0. [DOI] [PubMed] [Google Scholar]
- Putrenko I, Zakikhani M, Dent JA. A family of acetylcholine-gated chloride channel subunits in Caenorhabditis elegans. J Biol Chem. 2005;280:6392–6398. doi: 10.1074/jbc.M412644200. [DOI] [PubMed] [Google Scholar]
- Richelson E, Nelson A. Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro. J Pharmacol Exp Ther. 1984;230:94–102. [PubMed] [Google Scholar]
- Robertson SJ, Martin RJ. Levamisole-activated single channel currents from the muscle of the nematode parasite, Ascaris suum. Br J Pharmacol. 1993;108:70–178. doi: 10.1111/j.1476-5381.1993.tb13458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SJ, Pennington AJ, Evans AM, Martin RJ. The action of pyrantel as an agonist and open channel blocker at acetylcholine receptors in isolated Ascaris suum muscle vesicles. Eur J Pharmacol. 1994;271:273–282. doi: 10.1016/0014-2999(94)90784-6. [DOI] [PubMed] [Google Scholar]
- Scarselli M, Li B, Kim SK, Wess L. Multiple residues in the second extracellular loop are critical for M3 muscarinic acetylcholine receptor activation. J Biol Chem. 2007;282:7385–7396. doi: 10.1074/jbc.M610394200. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Li B, Bloodworth L, Erlenbach I, Zheng FY, Wess J. Random mutagenesis of the M3 muscarinic acetylcholine receptor expressed in yeast. J Biol Chem. 2003;278:30248–30260. doi: 10.1074/jbc.M304991200. [DOI] [PubMed] [Google Scholar]
- Segerberg MA, Stretton AOW. Actions of cholinergic drugs in the nematode Ascaris suum. J Gen Physiol. 1993;101:271–296. doi: 10.1085/jgp.101.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger KA, Avery L. The GAR-3 muscarinic receptor cooperates with calcium signals to regulate muscle contraction in the Caenorhabditis elegans pharynx. Genetics. 2004;167:633–643. doi: 10.1534/genetics.103.020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel PW, Gomeza J, Wess J, Cohen ML. M2 and M4 receptor knockout mice: muscarinic receptor function in cardiac and smooth muscle in vitro. J Pharmacol Exp Ther. 2000;292:877–885. [PubMed] [Google Scholar]
- Suh SJ, Park YS, Lee YS, Cho TJ, Kaang BK, Cho NJ. Three functional isoforms of GAR-2, a Caenorhabditis elegans G-protein-linked acetylcholine receptor, are produced by alternate splicing. Biochem Biophys Res Comm. 2001;288:1238–1243. doi: 10.1006/bbrc.2001.5909. [DOI] [PubMed] [Google Scholar]
- Wang ZX, Broach JR, Peiper SC. Functional expression of CXCR4 in Saccharomyces cerevisiae in the development of powerful tools for the pharmacological characterization of CXCR4. In: Ali H, Haribabu B, editors. Methods in Molecular Biology, vol 332: Transmembrane Signaling Protocols. Second. Humana Press Inc.; Totowa, NJ: 2006. pp. 115–127. [DOI] [PubMed] [Google Scholar]
- Wess J, Gdula D, Brann MR. Site-directed mutagenesis of the M3 muscarinic receptor: identification of a series of threonine and tyrosine residues involved in agonist but not antagonist binding. EMBO J. 1991;10:3729–3734. doi: 10.1002/j.1460-2075.1991.tb04941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Lamping KG, Duttaroy A, Zhang W, Cui Y, Bymaster FP, McKinzie DL, Felder CC, Deng CX, Faraci FM, Wess J. Cholinergic dilation of cerebral blood vessels in abolished M5 muscarinic receptor knockout mice. Proc Natl Acad Sci. 2001;98:14096–14101. doi: 10.1073/pnas.251542998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yamada M, Gomeza J, Basile AS, Wess J. Multiple muscarinic receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knockout mice. J Neurosci. 2002;22:6347–6352. doi: 10.1523/JNEUROSCI.22-15-06347.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu SZ, Wang SZ, Hu J, El-Fakahany EE. An arginine residue conserved in most G protein-coupled receptors is essential for the function of the M1 muscarinic receptor. Mol Pharmacol. 1994;45:517–523. [PubMed] [Google Scholar]