Abstract
Our studies of the brain microvascular system have focused on some aspects not commonly studied by other research groups because we use some techniques not often used by others. Our observations tend to add new details to the pathological picture rather than contradict the mainstream findings. We use large, thick celloidin sections which provide a three dimensional view of vascular networks, and alkaline phosphatase (AP) staining which allows one to differentiate between afferent and efferent vessels. We found millions of lipid microemboli in the brains of patients after cardiac surgery, and concluded that they caused vascular dementia in many patients. We previously proposed an animal model of vascular dementia using brain irradiation, which induces capillary loss. Lipid emboli might also be used to create an animal model of vascular dementia. The deep white matter is vulnerable to chronic hypoperfusion because the blood vessels supplying this region arise from the border-zone and have the longest course of all vessels penetrating the cerebrum. In cases with leukoaraiosis (LA), we found periventricular venous collagenosis (PVC), resulting in stenosis. Thirteen of 20 subjects older than 60 years had PVC, and 10 of 13 subjects with severe PVC had LA. Vascular stenosis might induce chronic ischemia and/or edema in the deep white matter, leading to LA. We suggest three mechanisms for a possible genetic predisposition to PVC: i) a predisposition to excessive venous collagenosis: ii) an indirect effect that causes chronic periventricular ischemia with a reactive over-production of collagen; and iii) mechanical damage to small vessels due to increased pulsatile motion. We found tortuous arterioles supplying the deep white matter beginning at about age 50. We also found a trend toward an increase in tortuosity in LA. If tortuousity is a factor in LA, it is probably significant in only a subset of cases. String vessels, remnants of capillaries, occur commonly in the brain, and are increased in ischemia, AD, and irradiation. Capillary injury or shutdown of blood flow can lead to capillary loss and string vessel formation. We found string vessels in brains from preterm babies to the very old. They seem to disappear after some months or years. We found an early loss of capillaries in LA, followed in a few years by the disappearance of string vessels. LA lesions do not progress to cortical cavitating lesions. Our findings raise three questions. 1. Why is the capillary loss arrested before infarction? 2. Why is there a floor below which the vascular density will not fall? 3. Why does the process which initiates string vessels shut down? We explain the vascular changes in LA as follows. LA induces apoptosis with loss of oligodendrocytes. Capillaries and neuropil are lost. Increased oxygen extraction from the blood in the deep white matter in LA implies that there are too many cells for the remaining capillaries. Thus, the capillaries appear to die first. But why do they stop dying? Perhaps a minimum number of capillaries are needed to transport the arterial blood to the venous system. Once the capillaries stop dying, no more string vessels are formed, and the string vessels gradually disappear.
Keywords: Leukoaraiosis, Capillary loss, String vessels, Tortuous vessels, Vascular dementia, Periventricular venous collagenosis, Cerebrovascular lipid emboli
Body of Review
A good look at the vessels
Our studies of the brain microvascular system over the past 20 years have featured two technical methods; cutting thick sections from large tissue blocks embedded in the plastic material celloidin, and staining the vessels for the endogenous enzyme alkaline phosphatase (AP). The large, thick celloidin sections allow one to study relatively large portions of the vascular network in three dimensions, and the key benefit of AP staining is that one can differentiate between afferent and efferent vessels. With these and other techniques, we have made important findings on microvascular changes associated with vascular dementia; not only the vascular dementia found in age-related neurodegenerative conditions such as leukoaraiosis (LA) and Alzheimer’s disease (AD), but also vascular dementia associated with brain complications of cardiac surgery.
Vascular dementia from cardiac surgery
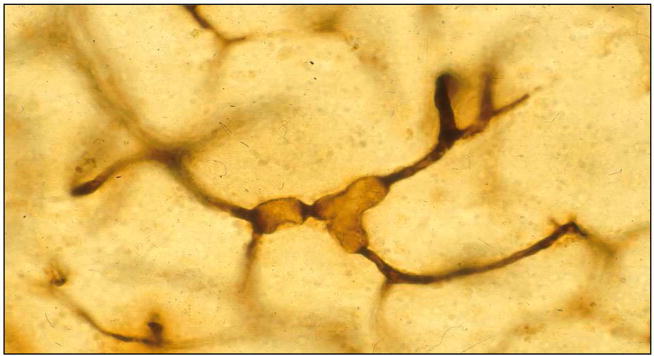
The brain complications of cardiac surgery appear to be a type of vascular dementia resulting from the brain being flooded with millions of lipid microemboli during cardiac surgery assisted by cardiopulmonary bypass (CPB) [1]. In patients who die within days after cardiac surgery, these lipid microemboli can be seen in brain tissue sections as SCADs (small capillary and arteriolar dilatations) (Fig. 1) [2]. Our findings suggest that SCADs come mostly from the patient’s fat that drips from the chest wound (especially the fatty marrow of the sternum) into the blood around the heart during surgery [1]. That lipid-laden blood is often suctioned and returned to the patient via the CPB circuit. The fat can slip through the filters into the left-sided circulation to the brain and other organs. Lipid emboli may be the major contributor to the post-surgical encephalopathy. As a result of more than 200 experimental dog surgeries in 17 years of studies, our group has devised strategies of blood return and temperature management that are being employed to improve outcome in many cardiac surgery centers. Post-CPB neurological dysfunction has become a topic of considerable interest and the manner in which field-aspirated shed blood is handled has been modified. Persistent neurological dysfunction in modern medical centers has decreased from about 50% 15 years ago to perhaps as low as 10% presently.
Fig. 1.
Lipid microemboli in brain capillaries in a patient after cardiac surgery assisted by cardiopulmonary bypass. The capillaries and arterioles are stained black by AP. Thick celloidin section. Reprinted from Brown et al. Echocardiography 1996;13:559–65, with permission.
Animal models of vascular dementia
If, as we suggest, lipid microemboli can cause vascular dementia, then this may form the basis for the development of an animal model of vascular dementia. We have previously proposed another animal model of vascular dementia, brain irradiation [3]. Each model has advantages and disadvantages. Brain irradiation causes capillary damage resulting in capillary loss [4]. The radiation-induced decrease in vascular density is similar to what we have found in LA [5, 6]. This may be a key element in causing chronic global brain ischemia and subsequent neuronal dysfunction. Lipid microemboli tend to pump through the vascular system by breaking into smaller emboli at vascular branch-points [1]. Most of these emboli pass through the brain in a few hours to a few days, but some remain impacted for weeks or longer [7]. The resulting shutdown of blood flow through the blocked capillaries likely leads to capillary loss. This suggests that there may be a decrease in vascular density after lipid emboli, but this has not yet been demonstrated, or perhaps even investigated. (It is technically difficult to demonstrate small to moderate decreases in vascular density.) However, there is ample evidence that brain irradiation and lipid microemboli can cause vascular injury (including endothelial dysfunction and increased blood-brain barrier permeability), chronic ischemia, and neuronal dysfunction. Thus, such animal models may be useful for testing the responses to drugs aimed at restoring the vascular network in the brain or improving the survival or function of neurons and glia undergoing chronic ischemia.
Perilous blood supply
An arterial network covers the surface of the brain and gives off vessels that penetrate the brain in the form of end arteries, i.e., they terminate in a capillary bed and do not have shunts to arterioles or veins within the brain parenchyma [8]. The vascular supply to the cerebrum is not uniform: some areas can be predicted to be vulnerable to chronic hypoperfusion, most obviously the deep white matter supplied by long medullary arterioles arising from the border-zone [9]. Those areas supplied by short penetrating vessels do not exhibit LA. Even when adjacent arterioles share a capillary bed the possibility of rescue is limited because collateral flow is weak. For blood to flow from one arteriole to an adjacent one by way of a capillary, it would first have to pass the entrance to a venule.
Periventricular venous collagenosis
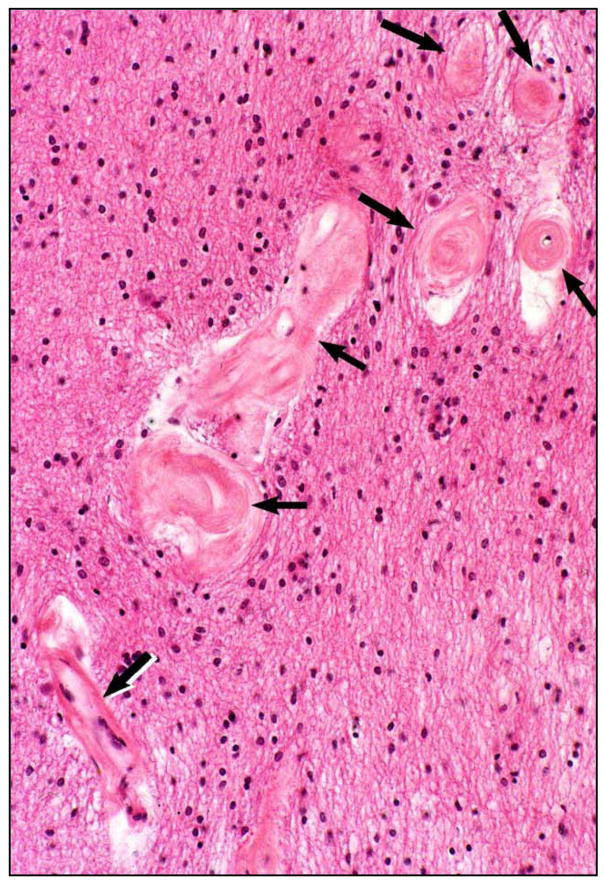
In 1995, we identified a new type of cerebral vascular pathology, periventricular venous collagenosis (PVC) in subjects with LA (Fig. 2) [10, 11]. We found a gradual increase in the thickness of the walls of veins and venules near the lateral ventricles with aging. In cases with LA, we found a striking degree of vascular wall thickening, resulting in narrowed lumina and even occlusion. In H&E sections these vessels could be mistaken for hyalinized arterioles, but with AP staining we can see that the affected vessels are exclusively venous. The material in the thickened walls stains strongly for collagens I and III. There is an outer membrane of collagen IV, possibly representing the glial basement membrane at the brain parenchyma. These observations have not been directly repeated by other laboratories, but somewhat similar findings described as perivascular collagen deposits, microvascular fibrosis, and basement membrane thickening, in brain capillaries have been reported in studies of aging rats and hypertensive rats (see review by Farkas and Luiten [12]). Note however, that our studies provide additional information because we distinguished between capillaries and post-capillary venules by the AP staining method. Also, our studies were in aging human subjects, some with LA, not in rats.
Fig. 2.
Periventricular venous collagenosis (arrows) in the brain of a subject with leukoaraiosis. Thin paraffin section stained with H&E. Reprinted from reference 10, with permission.
In a study of 22 subjects, we found that 13 of 20 subjects older than 60 years had PVC, and 10 of 13 subjects with severe PVC had LA [10]. These were statistically significant associations. We did not find an association with hypertension, although hypertension has often been associated with vascular dementia and LA. As discussed in the review of leukoaraiosis by Pantoni and Garcia [13], hypertension is not always found in LA cases.
Recently, Gao et al [14] presented a poster which included some of our findings on PVC, and they showed their MRI findings that demonstrate tapering or occlusion of veins at the margin of confluent LA, suggesting that LA may be etiologically related to venous insufficiency. Increased resistance to blood flow resulting from the venous stenosis might induce chronic ischemia and/or edema in the deep white matter, leading to LA. The collagenous thickening of the venous walls might impair passage into the veins of fluids, solutes, and toxins for removal from the brain via the blood stream.
Rennels [15] proposed that CSF is pumped from the subarachnoid space down into the brain in the periarteriolar spaces by the pulsations of the arterioles. He showed a tracer flowing into the basement membrane around the capillaries and then along the veins back to the subarachnoid space. PVC might negatively affect this flow. Also, age-related arteriolar sclerosis might impede the pulsatile pumping along the arterioles. Rennels demonstrated that vasogenic edema in the brain can inhibit the flow of interstitial fluid. It would be interesting to know if interstitial fluid flow is abnormal in LA.
It is unknown why the veins become thickened in this particular part of the brain and why PVC is associated with LA. We suggest that some people may have a genetic predisposition to develop PVC, and this might result in ischemia, leading to LA. We suggest three possible mechanisms that might explain the development of PVC: i) a genetic predisposition directly affecting excessive collagen production and deposition in veins; ii) an indirect genetic effect that causes chronic periventricular ischemia that triggers a reactive over-production of collagen in the veins; and iii) mechanical damage to small vessels due to abnormally high pulsatile motion, particularly in the periventricular white matter, as proposed by Marie-Cecile Henry-Feugeas [16] This mechanical damage could also involve unknown genetic susceptibilities. These three mechanisms need not be mutually exclusive. The most severe cases of PVC may be due to the additive or synergistic effects of multiple mechanisms and/or genetic susceptibilities.
Tortuous vessels
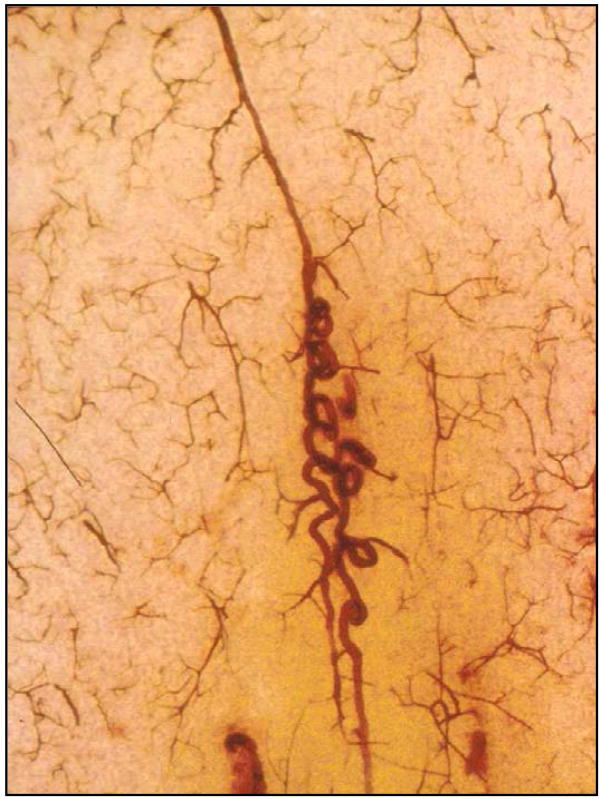
The arterioles nourishing the deep white matter arise from the border-zone, have the longest course through the brain, and are often tortuous (Fig. 3) [9, 11]. Tortuosity increases the vessel length and each change in direction causes a loss of kinetic energy so that increased blood pressure would be needed to maintain flow in these vessels [17]. In a recent study, we found large amounts of tortuosity after age 50 [18]. Although there was a trend toward an increase in tortuosity in LA, the numbers of control and LA subjects were not sufficient to show a statistically significant increase. (That we lacked sufficient cases was shown by a power analysis of the data from 13 LA cases and 17 controls [18].) We conclude that if tortuous vessels are a contributing factor to the development of LA, it is probably significant in only a subset of cases.
Fig. 3.
Tortuous vessel in a thick celloidin section stained with AP.
String vessels
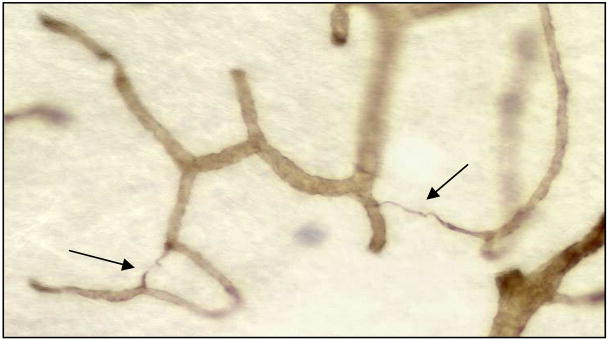
String vessels (Fig. 4) are composed of vascular basement membrane components such as collagen IV, laminin, and heparin sulfate. We think they are remnants of capillaries that have lost their endothilium. They occur commonly in the normal brain, spinal cord, and eye of humans and other animals [19], and they are increased in pathologies including ischemia [20], AD [21–26], and irradiation [27–30]. It appears that capillary injury can lead to capillary loss and string vessel formation. The shutdown of flow in a capillary for a critical period of time may, by itself, result in the loss of the capillary and the formation of a string vessel. We found string vessels in normal brains at all ages from preterm babies to the very old [31]. Because the numbers of string vessels were fewer at older ages than at birth, we concluded that they disappear after some months or years. In contrast to other brain pathologies, we found decreased rather than increased string vessels in the white matter of subjects with LA (unpublished). We also found a decreased density of capillaries in the deep white matter in LA [5, 32]. However, the decrease in string vessels was greater than the decrease in capillaries. It seems that the early loss of capillaries in LA is followed by string vessel formation and then perhaps a few years later by the disappearance of the string vessels. By the time of death, most of the strings in LA lesions have disappeared.
Fig. 4.
String vessels (arrows) in a thick celloidin section stained with antibody to collagen IV.
Capillary loss
We found a statistically significant decrease in vascular density in LA lesions compared to nearby white matter. It is interesting to note that in LA lesions vascular density does not fall below a certain level. Our observation that many subjects with striking LA do not have cortical infarcts or cavitating lesions in the deep white matter, along with our observations on capillary loss and string vessel loss in LA, raises three related questions regarding vascular pathology in LA. 1. Why is the capillary loss arrested before infarction occurs in LA lesions? 2. Why is there a floor below which the vascular density will not fall in LA lesions? 3. Why does the process which initiates string vessels shut down in LA lesions?
LA is a progressive disease characterized by spongiosis, gliosis, demyelination, and degeneration of the capillary bed (including endothelial dysfunction and increased blood-brain barrier permeability [13, 33, 34]. If the white matter is being progressively asphyxiated by loss of vessels, in the advanced cases, why do we not see an admixture of hyperintensity and cavitation? This is the mechanistic scheme we propose to explain the vascular changes in LA. In the classic infarct, there is cavitation, glial scar, and macrophages, which are indicative of necrotic cell loss. In contrast, in LA we find no macrophages or inflammation; instead we find apoptosis with loss of oligodendrocytes [35, 36]. As the disease progresses, capillaries and neuropil are lost. But which comes first? It could be that the loss of neuropil results in a decreased requirement for oxygen and glucose, and this leads to a loss of capillaries, as they are no longer needed. However, there have been two reports suggesting that the capillary loss occurs first [37, 38]. These reports show increased oxygen extraction from the blood in the deep white matter in LA, which implies that there are too many cells for the existing oxygen supply. Thus, something appears to be causing the capillaries to die first. But why do they stop dying? Perhaps a hemodynamic imperative is reached, i.e., the number of capillaries needed to simply transport the arterial blood to the venous system is reached. Once the capillaries reach a minimum number and stop dying, no more string vessels are formed, and the string vessels gradually disappear.
Acknowledgments
We thank Patricia Wood and Carolyn Cox for technical assistance. This work was supported by NIH grants NS20618 and NS 36780 to D. M. Moody and CA113321 to W. R. Brown.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown WR, Moody DM, Challa VR. Cerebral fat embolism from cardiopulmonary bypass. J Neuropathol Exp Neurol. 1999;58:109–19. doi: 10.1097/00005072-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Moody DM, Bell MA, Challa VR, Johnston WE, Prough DS. Brain microemboli during cardiac surgery or aortography. Ann Neurol. 1990;28:477–86. doi: 10.1002/ana.410280403. [DOI] [PubMed] [Google Scholar]
- 3.Brown WR, Blair RM, Moody DM, Thore CR, Ahmed S, Robbins ME, Wheeler KT. Capillary loss precedes the cognitive impairment induced by fractionated whole-brain irradiation: A potential rat model of vascular dementia. J Neurol Sci. 2007;257:67–71. doi: 10.1016/j.jns.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Brown WR, Thore CR, Moody DM, Robbins ME, Wheeler KT. Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res. 2005;164:662–68. doi: 10.1667/rr3453.1. [DOI] [PubMed] [Google Scholar]
- 5.Moody DM, Thore CR, Anstrom JA, Challa VR, Langefeld CD, Brown WR. Quantification of afferent vessels shows reduced brain vascular density in subjects with leukoaraiosis. Radiology. 2004;223:883–90. doi: 10.1148/radiol.2333020981. [DOI] [PubMed] [Google Scholar]
- 6.Brown WR, Moody DM, Thore CR, Challa VR, Anstrom JA. Vascular dementia in leukoaraiosis may be a consequence of capillary loss not only in the lesions, but in normal-appearing white matter and cortex as well. J Neurol Sci. 2007;257:62–6. doi: 10.1016/j.jns.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown WR, Moody DM, Challa VR, Stump DA, Hammon JW. Longer duration of cardiopulmonary bypass is associated with greater numbers of cerebral microemboli. Stroke. 2000;31:707–13. doi: 10.1161/01.str.31.3.707. [DOI] [PubMed] [Google Scholar]
- 8.Anstrom JA, Brown WR, Moody DM, Thore CR, Challa VR, Block SM. Anatomical analysis of the developing cerebral vasculature in premature neonates: absence of precapillary arteriole-to-venous shunts. Pediatr Res. 2002;52:554–60. doi: 10.1203/00006450-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. AJNR Am J Neuroradiol. 1990;11:431–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Moody DM, Brown WR, Challa VR, Anderson RL. Periventricular venous collagenosis: association with leukoaraiosis. Radiology. 1995;194:469–76. doi: 10.1148/radiology.194.2.7824728. [DOI] [PubMed] [Google Scholar]
- 11.Brown WR, Moody DM, Challa VR, Thore CR, Anstrom JA. Venous collagenosis and arteriolar tortuosity in leukoaraiosis. J Neurol Sci. 2002;203–204:159–63. doi: 10.1016/s0022-510x(02)00283-6. [DOI] [PubMed] [Google Scholar]
- 12.Farkas E, Luiten PGM. Cerbral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 13.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–9. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 14.Gao FQ, Levy N, Scott C, Ramirez R, Bilbao J, Black SE. White matter hyperintensities a sign of cerebral venous insufficiency. Presented at VasCog 2007; San Antonio, Texas. [Google Scholar]
- 15.Rennels ML, Blaumanis OR, Grady PA. Rapid solute transpot throughout the brain via paravascular fluid pathways. Adv Neurol. 1990;52:431–9. [PubMed] [Google Scholar]
- 16.Henry-Feugeas MC. Alzheimer’s disease in late-life dementia: A minor toxic consequence of devastating cerebrovascular dysfunction. Med Hypotheses. 2007 doi: 10.1016/j.mehy.2007.07.027. in press. [DOI] [PubMed] [Google Scholar]
- 17.Moody DM, Santamore WP, Bell MA. Does tortuosity in cerebral arterioles impair down-autoregulation in hypertensives and elderly normotensives? A computer model Clin Neurosurg. 1991;37:372–87. [PubMed] [Google Scholar]
- 18.Thore CR, Anstrom JA, Moody DM, Challa VR, Marion MC, Brown WR. Morphometric analysis of arteriolar tortuosity in human cerebral white matter of preterm, young, and aged subjects. J Neuropathol Exp Neurol. 2007;66:337–45. doi: 10.1097/nen.0b013e3180537147. [DOI] [PubMed] [Google Scholar]
- 19.Cammermeyer J. A comparative study of intravascular connective tissue strands in the central nervous system. J Comp Neurol. 1960;114:189–208. doi: 10.1002/cne.901140206. [DOI] [PubMed] [Google Scholar]
- 20.Reinecke RD, Kuwabara T, Cogan DG. Retinal vascular patterns. Part V. Experimental ischemia of the cat eye. Arch Ophthalmol. 1962;67:470–5. doi: 10.1001/archopht.1962.00960020470015. [DOI] [PubMed] [Google Scholar]
- 21.Challa VR, Thore CR, Moody DM, Anstrom JA, Brown WR. Increase of white matter string vessels in Alzheimer’s disease. J Alzheimer’s Disease. 2004;6:379–83. doi: 10.3233/jad-2004-6404. [DOI] [PubMed] [Google Scholar]
- 22.Buee L, Hof PR, Bouras C, Delacourte A, Perl DP, Morrison JH, Fillit HM. Pathological alterations of the cerebral microvasculature in Alzheimer’s disease and related dementing disorders. Acta Neuropathol. 1994;87:469–80. doi: 10.1007/BF00294173. [DOI] [PubMed] [Google Scholar]
- 23.Kalaria RN, Kroon SN. Expression of leukocyte antigen CD34 by brain capillaries in Alzheimer’s disease and neurologically normal subjects. Acta Neuropathol. 1992;84:606–12. doi: 10.1007/BF00227737. [DOI] [PubMed] [Google Scholar]
- 24.Kalaria RN, Hedera P. Differential degeneration of the cerebral microvasculature in Alzheimer’s disease. Neuroreport. 1995;6:477–80. doi: 10.1097/00001756-199502000-00018. [DOI] [PubMed] [Google Scholar]
- 25.McGeer PL, Zhu SG, Dedhar S. Immunostaining of human brain capillaries by antibodies to very late antigens. J Neuroimmunol. 1990;26:213–8. doi: 10.1016/0165-5728(90)90003-6. [DOI] [PubMed] [Google Scholar]
- 26.Perlmutter LS, Chui HC. Microangiopathy, the vascular basement membrane and Alzheimer’s disease: a review. Brain Res Bull. 1990;24:677–86. doi: 10.1016/0361-9230(90)90007-m. [DOI] [PubMed] [Google Scholar]
- 27.Archer DB, Amoaku WMK, Gardiner TA. Radiation retinopathy – clinical, histopathological, ultrastructural and experimental correlations. Eye. 1991;5:239–51. doi: 10.1038/eye.1991.39. [DOI] [PubMed] [Google Scholar]
- 28.Archer DB. Doyne lecture: Responses of retinal and choroidal vessels to ionizing radiation. Eye. 1993;7:1–13. doi: 10.1038/eye.1993.3. [DOI] [PubMed] [Google Scholar]
- 29.Archambeau JO, Mao XW, McMillan PJ, Gouloumet VL, Oeinck SC, Grove R, Yonemoto LT, Slater JD, Slater JM. Dose response of rat retinal microvessels to proton dose schedules used clinically: A pilot study. Int J Radiat Oncol Biol Phys. 2000;48:1155–66. doi: 10.1016/s0360-3016(00)00754-9. [DOI] [PubMed] [Google Scholar]
- 30.Mao XW, Archambeau JO, Kubinova L, Boyle S, Petersen G, Grove R. Quantification of rat retinal growth and vascular population changes after single and split doses of proton irradiation: translational study using stereology methods. Radiat Res. 2003;160:5–13. doi: 10.1667/rr3007. [DOI] [PubMed] [Google Scholar]
- 31.Challa VR, Thore CR, Moody DM, Brown WR, Anstrom JA. A three-dimensional study of brain string vessels using celloidin sections stained with anti-collagen antibodies. J Neurol Sci. 2002;203–4:165–7. doi: 10.1016/s0022-510x(02)00284-8. [DOI] [PubMed] [Google Scholar]
- 32.Brown WR, Moody DM, Thore CR, Challa VR, Anstrom JA. Vascular dementia in leukoaraiosis may be a consequence of capillary loss not only in the lesions, but in normal-appearing white matter and cortex as well. J Neurol Sci. 2007;257:62–6. doi: 10.1016/j.jns.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hassan A, Hunt BJ, O’Sullivan M, Parmar K, Bamford JM, Briley D, Brown MM, Thomas DJ, Markus HS. Markers of endothelial dysfunction in lacunar infarction and ischemic leukoaraiosis. Brain. 2003;126:424–32. doi: 10.1093/brain/awg040. [DOI] [PubMed] [Google Scholar]
- 34.Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease – systematic review and meta-analysis. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Brown WR, Moody DM, Thore CR, Challa VR. Apoptosis in leukoaraiosis. AJNR Am J Neuroradiol. 2000;21:79–82. [PMC free article] [PubMed] [Google Scholar]
- 36.Brown WR, Moody DM, Challa VR, Thore CR, Anstrom JA. Apoptosis in leukoaraiosis lesions. J Neurol Sci. 2002;203–204:169–71. doi: 10.1016/s0022-510x(02)00285-x. [DOI] [PubMed] [Google Scholar]
- 37.Meguro K, Hatazawa J, Yamaguchi T, et al. Cerebral circulation and oxygen metabolism associated with subclinical periventricular hyperintensity as shown by magnetic resonance imaging. Ann Neurol. 1990;28:378–83. doi: 10.1002/ana.410280313. [DOI] [PubMed] [Google Scholar]
- 38.Yamauchi H, Fukuyama H, Yamaguchi S, Miyoshi T, Kimura J, Konishi J. High-intensity area in the deep white matter indicating hemodynamic compromise in internal carotid artery occlusive disorders. Arch Neurol. 1991;48:1067–71. doi: 10.1001/archneur.1991.00530220089024. [DOI] [PubMed] [Google Scholar]