Abstract
There are distinct differences in the accessibility, purity, dosing, and misuse associated with illicit gamma-hydroxybutyrate (GHB) compared to pharmaceutical sodium oxybate. Gamma-hydroxybutyrate sodium and sodium oxybate are the chemical and drug names, respectively, for the pharmaceutical product Xyrem® (sodium oxybate) oral solution. However, the acronym GHB is also used to refer to illicit formulations that are used for non-medical purposes. This review highlights important differences between illicit GHB and sodium oxybate with regard to their relative abuse liability, which includes the likelihood and consequences of abuse. Data are summarized from the scientific literature; from national surveillance systems in the U.S., Europe, and Australia (for illicit GHB); and from clinical trials and post-marketing surveillance with sodium oxybate (Xyrem). In the U.S., the prevalence of illicit GHB use, abuse, intoxication, and overdose has declined from 2000, the year that GHB was scheduled, to the present and is lower than that of most other licit and illicit drugs. Abuse and misuse of the pharmaceutical product, sodium oxybate, has been rare over the 5 years since its introduction to the market, which is likely due in part to the risk management program associated with this product. Differences in the accessibility, purity, dosing, and misuse of illicit GHB and sodium oxybate suggest that risks associated with illicit GHB are greater than those associated with the pharmaceutical product sodium oxybate.
Keywords: abuse, dependence, GHB, narcolepsy, sodium oxybate, Xyrem
1. Introduction
Gamma-hydroxybutyric acid is an endogenous compound and putative neurotransmitter that differs from the primary inhibitory neurotransmitter gamma-aminobutyric acid (GABA) by the substitution of a hydroxyl group in place of the amino group of the GABA molecule (Maitre, 1997; Pardi and Black, 2006). Sodium gamma-hydroxybutyrate or sodium 4-hydroxybutyrate (GHB) is the International Union of Pure and Applied Chemistry chemical name for the sodium salt of gamma-hydroxybutyric acid, whereas sodium oxybate is the international drug name for the identical compound (Hillebrand et al., 2008). Sodium oxybate is marketed as Xyrem® in the United States (U.S.), Canada, and Europe by Jazz Pharmaceuticals, Valeant Pharmaceuticals International, and UCB, respectively. It is approved for the treatment of excessive daytime sleepiness and cataplexy in patients with narcolepsy in the U.S., for the treatment of narcolepsy with cataplexy in adult patients in Europe, and for the treatment of cataplexy with narcolepsy in Canada. Sodium oxybate is approved in Germany as an anesthetic, Somsanit® (Dr. F. Köhler Chemie), and is approved in Austria and Italy for the treatment of opioid and alcohol withdrawal as Alcover® (Laboratorio Farmaceutico; Hillebrand et al., 2008). Clinical development programs are also under way to study the clinical efficacy and safety of sodium oxybate for the treatment of conditions such as fibromyalgia (Russell et al., 2009) and essential tremor (Frucht et al., 2005). For the purposes of this report, sodium oxybate will be used to refer to the government-approved drug or pharmaceutical product. GHB will be used to refer to endogenous gamma-hydroxybutyric acid and chemical grade gamma-hydroxybutyrate. Illicit GHB will be used to refer to illicitly manufactured gamma-hydroxybutyric acid or gamma-hydroxybutyrate and street drug products that are purported to be GHB and might contain GHB or other compounds of unknown dose and purity.
GHB was legally manufactured and widely available as a nutritional supplement (to induce sleep or increase muscle mass) in the 1980s until reports of abuse as a “club drug” (a drug used in a club or party setting for its euphoric effects; e.g., Sumnall et al., 2008) and “date-rape drug” (a drug used for drug-facilitated sexual assault; e.g., Chin et al. 1992) led to the scheduling of GHB as a controlled substance. As of March 2000, GHB and sodium oxybate were placed in a unique bifurcated Federal schedule in the U.S.: GHB for non-medical use is Schedule I, the most restrictive schedule of the U.S. Controlled Substances Act. When used as prescribed for medical purposes (e.g., the treatment of narcolepsy), it is a Schedule III substance.
In March 2001, the Commission on Narcotic Drugs of the United Nations, at the recommendation of the World Health Organization, added GHB to Schedule IV of the 1971 Convention on Psychotropic Substances, with GHB subject to scheduling or control in all Member States of the European Union. Some Member States (e.g., Italy, Latvia, and Sweden) subsequently also placed controls on one or both of the GHB precursors gamma-butyrolactone (GBL) and 1,4-butanediol (1,4-BD; Hillebrand et al., 2008). In the U.S., GBL is a List I chemical (a chemical that is used in, and important to, the manufacture of a controlled substance) and is subject to regulatory controls; 1,4-BD is neither controlled nor listed at the Federal level (U.S. Controlled Substances Act), but is controlled in some U.S. states under State Law (e.g., Hawaii, Nevada; Hawaii Revised Statute, Chapter 329; Nevada Administrative Code, Chapter 453). Canada lists sodium oxybate/GHB and all salts as Schedule III.
Distinguishing between illicit GHB and licit GHB or sodium oxybate from clinical case reports of abuse and dependence is difficult. Many of the epidemiological studies or case reports that describe the effects of illicit GHB refer to the molecule simply as GHB (e.g., Kim et al., 2008). Without forensic analysis of the substance consumed or of a biological sample from the consumer, extrapolating effects reported after the administration of illicit GHB or a GHB precursor to the effects of chemical grade GHB or sodium oxybate requires several assumptions to be made, such as the illicit formulation contained GHB, the illicit formulation was not contaminated or adulterated by other chemicals, and the effects were not caused by a co-administered drug or chemical.
However, studies examining the effects of GHB are applicable to sodium oxybate. All pharmaceutical products have chemical names (e.g., sodium gamma-hydroxybutyrate), non-proprietary pharmaceutical names (e.g., sodium oxybate), and trade names (e.g., Xyrem). Clinical studies that were conducted with pharmaceutical grade GHB prior to the development of sodium oxybate as a commercial product (e.g., Broughton and Mamelak, 1979; Scharf et al., 1985) use the chemical name GHB and are applicable to the pharmaceutical product. The use of the chemical name in the scientific literature has likely persisted because of the availability and use of chemical grade GHB for non-human studies (e.g., Carter et al., 2003; Goodwin et al., 2005)
It is important, however, to recognize that illicit GHB and sodium oxybate have different risks or liabilities of abuse and using “GHB” to refer to both illicit GHB and sodium oxybate has blurred this distinction in the scientific literature and in the popular press. The purpose of this review is to summarize the differences between the relative abuse liability of sodium oxybate and that of illicit GHB, with a specific focus on the availability and prevalence of non-medical use, and the risks and consequences of misuse and abuse. Relative abuse liability includes both a drug's liability for abuse (likelihood that the drug will be abused) and its liability of abuse (consequences of abuse; Griffiths et al., 2003). Information on sodium oxybate, GHB, and illicit GHB from three types of sources are presented in this review: data from the peer-reviewed scientific literature; data from national surveys of drug use, abuse, and law enforcement activity; and data from Jazz Pharmaceuticals on the rates of abuse, diversion, drug-facilitated sexual assault, and deaths associated with sodium oxybate.
2. Characteristics of illicit GHB compared to those of sodium oxybate
2.1 Availability
2.1.1 Availability of illicit GHB
After GHB was scheduled in the U.S. in 2000 and became an illegal drug, it continued to be sold as a dietary supplement under a variety of different names, as a GHB alternative, or more covertly, as a solvent not recommended for human consumption (Maxwell and Spence, 2005). Chemistry kits, reagents, and recipes to convert GHB precursors into GHB also became available for purchase over the internet; however, the availability of these kits, reagents, and recipes is thought to have diminished in recent years (Nicholson and Balster, 2001; Mason and Kerns, 2002; European Monitoring Centre for Drugs and Drug Addiction Annual Report, 2007; National Drug Intelligence Center U.S. Department of Justice, 2008). Epidemiological data show that illicit GHB remains accessible to individuals in the U.S., Europe, and Australia (Degenhardt et al., 2005; Barker et al., 2007; Sumnall et al., 2008). International restrictions on the production and sale of GHB are thought to have shifted recreational use from GHB toward the GHB precursors GBL and 1,4 BD in the U.S., Europe, and Australia (Winickoff et al., 2000; Zvosec et al., 2001; Dupont and Thornton, 2001; Caldicott et al., 2004; Palmer, 2004; European Monitoring Centre for Drugs and Drug Addiction Annual Report, 2008; Hillebrand et al., 2008; Knudsen et al., 2008; Wood et al., 2008). GBL and 1,4-BD are ingested for recreational use presumably because they are converted to GHB in the body (Doherty and Roth, 1978; Lettieri and Fung, 1978), but they can also be chemically converted to illicit GHB prior to consumption.
Several surveys and data collection methods have been used in different countries to assess the relative availability and reported prevalence of use of illicit GHB (Table 1). In the U.S., the relative availability of illicit GHB can be assessed by examining the reported prevalence of use by individuals and the prevalence of drug confiscations by law enforcement. Data regarding the use of illicit GHB by adults in the U.S. are limited since GHB was not added to the U.S. National Survey on Drug Use and Health (formerly the National Household Survey on Drug Abuse) until 2006 (Substance Abuse and Mental Health Services Administration, Office of Applied Studies, 2008b). However, data from the Monitoring the Future Study indicate that the estimated annual rates of non-medical use of GHB by 8th, 10th, and 12th grade students are low (0.5-1.2%), and have declined from 2000 (the first year in which GHB use was queried in the survey) to 2008 (Johnston et al., 2008). Reported rates of non-medical use of GHB among each of the grade levels in the Monitoring the Future Study in 2007 were lower than those of other sedatives, tranquilizers, Vicodin®, Oxycontin®, Ritalin®, and over-the-counter cough and cold medicines (Johnston et al., 2008). Similarly, the rate of confiscations of illicit GHB/GBL by U.S. law enforcement is also low, relative to that of other drugs, and has declined in recent years. Data from the U.S. Drug Enforcement Administration's National Forensic Laboratory Information System (NFLIS) indicate that the percentage of items that tested positive for GHB or GBL decreased by 83% from 2000 to 2007 (Figure 1, top panel; The National Forensic Laboratory Information System, 2001; The National Forensic Laboratory Information System, 2002; Strom et al., 2003; Strom et al., 2004; Weimer et al., 2004; Weimer et al., 2006; Weimer et al., 2007; Office of Diversion Control, 2008). Indeed, the 2009 National Drug Threat Assessment authored by the U.S. Department of Justice states that GHB has been a very low threat and low priority for law enforcement for the last several years (National Drug Intelligence Center U.S. Department of Justice, 2008).
Table 1.
Sources of data for reported availability and use of illicit GHB
| US |
U.S. National Survey on Drug Use and Health (formerly the National Household Survey on Drug Abuse)
|
Monitoring the Future Study
| |
National Forensic Laboratory Information System (NFLIS)
| |
Drug Abuse Warning Network (DAWN)
| |
National Poison Database
| |
| Europe |
European Monitoring Center for Drugs and Drug Addiction (EMCDDA)
|
| Australia |
National Drug Strategy Household Survey
|
Figure 1.
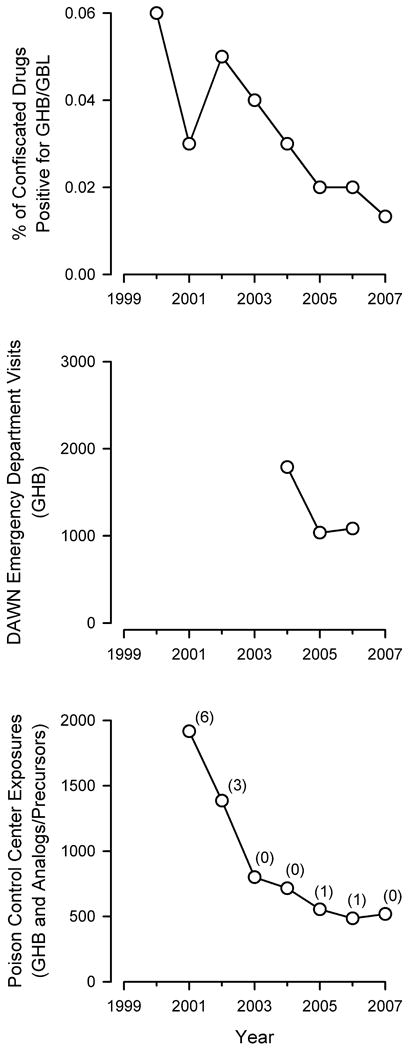
National estimates of the availability, use, and abuse of GHB in the U.S. Top panel: Percent of items confiscated in law enforcement operations in the U.S. from 2000 through 2007 that were positive for GHB or GBL. Data are from the annual reports from The National Forensic Laboratory Information System (NFLIS). Data are not available before the year 2000 because GHB was not illegal until 2000. Middle panel: Number of emergency department visits in which GHB was mentioned. Data are from the annual reports from the Drug Abuse Warning Network (DAWN) coordinated by the Substance Abuse and Mental Health Services Administration (SAMHSA) of the U.S. Department of Health and Human Services. Note: trends prior to 2003 are not shown due to methodological changes made to the DAWN surveillance system that preclude the comparison of DAWN data from before and after 2003. Bottom panel: Number of exposures (circles) and deaths (numbers in parentheses) attributed to illicit GHB, GHB analogs, and GHB precursors in each of the following years. Data are from the Annual Reports from the American Association of Poison Control Centers National Poison Database (formerly named the Toxic Exposure Surveillance System).
In Europe, the European Monitoring Centre for Drugs and Drug Addiction is the central source of comprehensive information on drugs and drug addiction. The European Monitoring Centre for Drugs and Drug Addiction 2007 and 2008 Annual Reports and thematic paper on GHB and GBL showed that the prevalence of illicit GHB use in Europe is low, with levels of use limited to specific subpopulations of drug users (Bellis et al., 2003; European Monitoring Centre for Drugs and Drug Addiction Annual Report, 2007; European Monitoring Centre for Drugs and Drug Addiction Annual Report, 2008; Hillebrand et al., 2008). In Australia, data from the National Drug Strategy Household Survey conducted by the Australian Institute of Health and Welfare estimated that in 2007 a total of 0.1% of the Australian population over 14 years of age reported using GHB in the previous 12 months (Australian Institute of Health and Welfare, 2008). This figure is the same as that reported in 2004 for GHB (Degenhardt and Dunn, 2008), and is considerably lower than the reported use of other illicit drugs, such as marijuana/cannabis (9.1%) and ecstasy (3.5%), as well as non-medical use of pain-killers (2.5%) and sleeping pills during the same year (1.4%; Australian Institute of Health and Welfare, 2008). In contrast to the national reports, a recent retrospective study from a metropolitan area in Australia reported that GHB-related, non-fatal ambulance calls increased from 2001 to 2005, suggesting that use of illicit GHB in Australia might also be limited to specific subpopulations of drug users (Dietze et al., 2008).
2.1.2 Availability of sodium oxybate
Sodium oxybate is available by prescription in the U.S., Canada, and Europe. In the U.S., an extensive risk management program called the Xyrem Success Program® was specifically designed to prevent diversion and misuse of sodium oxybate by limiting distribution of the drug and by educating physicians and patients on proper use of the drug. In the U.S., Xyrem is manufactured at a single source and is distributed through a central pharmacy. The Xyrem Success Program also includes physician and patient registries, whereby prescriptions are verified before being filled (in addition to confirming that the prescriber and the prescription are valid, the central pharmacy calls the physician to identify the physician's name and DEA and state license numbers, confirms that the physician's DEA number is valid, and confirms that the physician is registered with the central pharmacy, a one-time process in which the physician must attest that he/she has read specific educational materials that include information regarding the approved indications and doses). The central pharmacy also calls each patient to confirm that they have received and understood the Patient Success Program materials explaining the risks and proper use of Xyrem (and sends or re-sends the materials if they have not been received). Once it is documented that the patient has read and understood the educational materials, the drug is shipped overnight to the patient; if a patient or a patient's designee does not sign for the drug, it is returned to the central pharmacy (see Fuller et al., 2004 and Wedin et al., 2006).
The central pharmacy tracks all instances of potential diversion, theft, and loss of drug (e.g., reports of misplaced, spilt, or damaged bottles; delivery of drug to an incorrect address). From the approximately 26,000 patients that have received drug and the approximately 600,000 bottles of Xyrem distributed worldwide (approximately 5 million defined daily doses, based on the modal prescribed dose of 9 g/night) from market introduction through March 2008, there have been 5 reported instances of drug diversion (drug was used or intended to be used by someone other than the patient), 6 instances of possible diversion (drug was reported to have been stolen with no information about its subsequent use), 9 instances of coincidental theft (drug was part of a general theft including other items; e.g., burglary, theft of a backpack), and 22 instances in which the drug was lost or missing and theft was not suspected (e.g., drug was delivered to the wrong address and was not recovered, drug was left in a hotel room by the patient). Given the 5 reported instances of drug diversion, the estimated rate of diversion of Xyrem is approximately 1 instance per 5,200 patients treated (approximately 0.019%), or 1 instance per 120,000 bottles of drug shipped (less than 0.0009%; Wang et al., 2009).
2.2 Product identity, purity, and dosing
2.2.1 Identity, purity, and dosing of illicit GHB
As with any illicit drug, there are no standards governing the production or sale of illicit GHB. A powder or solution sold as GHB might or might not contain GHB or other substances. Similarly, illicitly synthesized GHB might contain toxic contaminants or residual reagents from the synthesis. As such, case reports that describe instances of illicit GHB intoxication and unintentional overdose without forensic confirmation that GHB and not other drugs was present in a biological sample, assume that GHB was actually ingested and that illicit GHB, and not a contaminant of synthesis or another drug(s) or chemical(s), was responsible for the observed effects.
GHB used for non-medical purposes is most commonly synthesized illicitly from GBL. As such, less than careful chemistry can result in a final product that contains GBL. GBL is metabolized to GHB in the body (Lettieri and Fung, 1978); however, in a number of different species, including mice, rats, pigeons, and baboons, GBL has been shown to be more potent than GHB (cf., Weerts et al., 2005 and Goodwin et al., 2006), which is likely due to pharmacokinetic differences between GHB and GBL (see Carter et al., 2009 for review). Thus, the use of illicitly synthesized GHB can result in greater harm if GBL is present since GBL is more potent than GHB across species and might increase the risk of unintentional overdose.
In addition, GBL or other drugs such as 1,4-BD might be sold as illicit GHB. For example, reported use of GHB by patients admitted to the emergency department in a recent study in the U.K. was substantially higher than data from analyzed substances would suggest. In one study that included 158 patients who reported use of either GHB, GBL, or 1,4-BD after being admitted to the emergency department, 95% of the patients reported using GHB. However, an analysis of substances seized from clubs in the same area showed that 38% of seized substances contained GHB, whereas 62% contained GBL (Wood et al., 2008).
Although both GBL and 1,4-BD are metabolized to GHB in vivo following oral administration (Doherty and Roth, 1978; Lettieri and Fung, 1978), the toxic effects of these compounds might differ from those of GHB. In one study in mice, 1,4-BD produced loss of righting and resulted in 25% lethality (LD25) at a dose of 1,780 mg/kg, whereas lethality was not observed after doses of GHB up to 3,200 mg/kg (Carter et al., 2005). A study in human subjects reported that there were no serious adverse effects observed after a single relatively low dose of 1,4-BD (25 mg/kg; Thai et al., 2007); however, the relative toxicity of 1,4-BD in humans has been suggested to be quite high (Zvosec et al., 2001).
Recreational users of illicit GHB have typically reported self-administering one or more “capfuls” of liquid. Depending on the size of the cap and the concentration of the solution, a capful of liquid could contain 5 g of GHB, or approximately 70 mg/kg for a 70 kg person (based on an approximate concentration of 1 g/mL of pure GHB solution; Miotto et al., 2001; Barker et al., 2007; Sumnall et al., 2008). In a few studies, users of illicit GHB reported taking the drug 1 to 6 times per week, 1 to 3 times per day, often in the evenings (but not immediately before bed), and most frequently on the weekends (Miotto et al., 2001; Barker et al., 2007; Sumnall et al., 2008). In addition, case reports of illicit GHB abuse and withdrawal often describe use that has escalated to larger doses and frequent dosing throughout the day and night (e.g., Craig et al., 2000; Wojtowicz et al., 2008).
The adverse consequences of illicit GHB abuse have been postulated to be worse than those of other sedative/hypnotic drugs because the dose-effect curve for GHB appears to be steep and there is a narrow range between doses that are used for recreational purposes and those that can result in loss of consciousness (Griffiths and Johnson, 2005). As noted in the following section (2.2.2), studies conducted with sodium oxybate under controlled conditions have shown that the pharmacokinetics are nonlinear, with, for instance, a doubling of the dose resulting in a greater than 2-fold increase in blood levels of the drug. In surveys of illicit GHB users in the U.S. and Australia, more than half of the respondents reported experiencing some degree of unintentional loss of consciousness as a result of their illicit GHB use (Miotto et al,. 2001; Degenhardt et al., 2003).
2.2.2 Identity, purity, and dosing of sodium oxybate
Sodium oxybate is manufactured in liquid form to precise specifications (500 mg sodium oxybate per mL of solution), and doses are measured by patients using a calibrated device. Under controlled conditions and using known doses of sodium oxybate, the dose-effect curve, across a wide range of doses, was found to be relatively steep and the relative potency of the sedative effects somewhat variable across participants (Abanades et al., 2006; Carter et al., 2006; Abanades et al., 2007). This finding is consistent with the nonlinear pharmacokinetics of sodium oxybate, in which blood levels increased 3.7-fold as the dose was doubled from 4.5 to 9 g per day (Palatini et al., 1993; Scharf et al., 1998).
The recommended starting dose of sodium oxybate for treating cataplexy and excessive daytime sleepiness associated with narcolepsy is 4.5 g/night divided into two equal doses of 2.25 g to be taken at bedtime and 2.5 to 4 hours later. The dose can be increased to a maximum of 9 g/night (two doses of 4.5 g/night) in increments of 1.5 g/night (0.75 g per dose; Xyrem Prescribing Information). The doses under evaluation in Phase II and Phase III trials for fibromyalgia are 4.5 and 6 g/night (Russell et al., 2009; data on file, Jazz Pharmaceuticals).
3. Substance Abuse, Substance Dependence, and misuse with illicit GHB compared to sodium oxybate
3.1 Substance Abuse and Substance Dependence with illicit GHB
In cases of intoxication or unintentional overdose, the dose or doses of illicit GHB are often unknown; however, surveys of users of illicit GHB provide some information about the effects of acute illicit GHB intoxication. In surveys of recreational users of illicit GHB in the U.S., U.K., and Australia, individuals reported using GHB for euphoric-, sociable-, and aphrodisiac-like effects (Miotto et al., 2001; Degenhardt et al., 2002; Barker et al., 2007; Sumnall et al., 2008). In some of the same studies, more than half of the respondents also reported that acute adverse effects of illicit GHB intoxication included confusion, dizziness, blurred vision, hot/cold flushes, profuse sweating, vomiting, and loss of consciousness (Miotto et al., 2001; Degenhardt et al., 2002). In one survey, users who had experienced an unintentional, non-fatal overdose with illicit GHB reported that the most frequent reason for the unintentional overdose was taking “too much GHB” (37% of respondents), followed by a concentration of illicit GHB “stronger than usual” (21% of respondents; Degenhardt et al., 2003).
Epidemiological information on the prevalence of illicit GHB abuse and intoxication includes data from the Drug Abuse Warning Network (DAWN) and the American Association of Poison Control Centers, which receive reports of drug-related hospital emergency department visits and calls or case reports of poisonings, respectively (Table 1). Data from the DAWN surveillance system estimate that the number of emergency department visits in which GHB or a GHB precursor was mentioned decreased from 2004 to 2006 (Figure 1, middle panel); however, the number of emergency department visits reported in 2006 was not statistically different than those reported in 2004 or 2005 (Substance Abuse and Mental Health Services Administration, Office of Applied Studies, 2008b; Dr. Elizabeth Crane, personal communication). Annual Reports of the American Association of Poison Control Centers also suggest that poisonings attributed to illicit GHB, GHB analogs, and GHB precursors have declined in recent years, with the number of GHB exposures decreasing by 73% from a total of 1916 exposures (including 6 deaths) in 2001 to 518 exposures (including no deaths) in 2007 (Figure 1, bottom panel; Litovitz et al., 2002; Watson et al., 2003; Watson et al., 2004; Watson et al., 2005; Lai et al., 2006; Bronstein et al., 2007; Bronstein et al., 2008). Similarly, a retrospective review of cases presented to the California Poison Control System showed that from 1999 to 2003 there was a 76% decrease in case reports of GHB exposure to the California Poison Control System (Anderson et al., 2006).
The actual prevalence of death from illicit GHB overdose is difficult to determine because of the lack of objective assessments, such as sensitive, specific assays for measuring GHB in biological matrices; the high prevalence of concomitant drug use; and differences in reporting practices within the medical community. Nausea and vomiting can occur after GHB administration and increase the risk for aspiration if an individual is unconscious. Cardiac and respiratory depression can lead to bradycardia, bradypnea, and apnea. Myoclonic seizure-like activity and loss of consciousness have also been described (Centers for Disease Control 1991; Dyer, 1991; Chin et al., 1992). The clinical course of illicit GHB overdose is thought to be relatively short, with most people awakening within 3 to 4 hours and recovering within 5 to 8 hours of ingestion (Chin et al., 1998; Van Sassenbroeck et al., 2007; but see Strickland et al., 2005). The concomitant use of other sedative-hypnotics, including alcohol, has been reported to prolong the time it takes to recover (Williams, 1998; Thai et al., 2006).
Fatalities due to illicit GHB intoxication both alone and together with other drugs have been reported (Figure 1, bottom panel; also see Ferrara et al., 1995; Caldicott et al., 2004; Knudsen et al., 2008). The relationship between adverse events and the consumption of illicit GHB is often not straightforward. Studies conducted in the U.S., Europe, and Australia have shown that the majority of individuals co-administer illicit GHB with at least one other substance (Miotto et al., 2001; Degenhardt et al., 2002; Degenhardt et al., 2003; Barker et al., 2007; Kim et al., 2007; Dietze et al., 2008; Sumnall et al., 2008). In addition, cases of intentional or unintentional overdose from drugs other than GHB might be mistaken for illicit GHB overdose (Couper et al., 2004; Wood et al., 2008).
There are case reports that describe individuals who appear to fulfill DSM-IV criteria for a diagnosis of Substance Dependence upon (i.e., addiction to) illicit GHB (Galloway et al., 1997; Craig et al., 2000; McDaniel and Miotto, 2001; Degenhardt et al., 2002). There are also case reports (including those above) that describe individuals who have become physically dependent (evidenced by a withdrawal syndrome) upon illicit GHB as a result of frequent administration of the drug (every 1-3 hours), resulting in daily doses of 43-144 g/day (e.g., Price, 2000; Dyer et al., 2001; Miotto et al., 2001; Glasper et al., 2005). In many cases, DSM-IV Substance Dependence and physical dependence on illicit GHB are associated with administration of supratherapeutic doses of GHB in an around-the-clock manner (Dyer et al., 2001; Tarabar and Nelson, 2004). Surveys of recreational users of illicit GHB have reported a prevalence of physical dependence ranging from 4-21%, with a higher prevalence in surveys in which participants reported more frequent use of illicit GHB (Miotto et al., 2001; Degenhardt et al., 2002).
3.2 Substance Abuse and Substance Dependence with sodium oxybate
Human abuse liability studies of supratherapeutic doses of sodium oxybate have shown that although subjects report positive subjective effects comparable to those of alcohol or a benzodiazepine, they also report greater negative subjective effects such as nausea and gastrointestinal distress (Carter et al., 2006; Abanades et al., 2007). Data from post-marketing surveillance indicate that abuse of sodium oxybate by patients and/or recreational drug users is rare. All reported cases of sodium oxybate abuse from spontaneous reporting and the central pharmacy were reviewed for fulfilling the DSM-IV criteria for Substance Abuse. Ten cases fulfilling DSM-IV criteria for Substance Abuse with sodium oxybate have been reported from market introduction through March 2008 in the U.S., Europe, and Canada (Wang et al., 2009). As shown in Table 2, there has been approximately one case of Substance Abuse reported for every 2,600 patients treated (a rate of approximately 0.039%). Also noted in Table 2, the majority of cases that fulfilled DSM-IV criteria for Substance Abuse involved use of sodium oxybate in a potentially hazardous situation.
Table 2.
DSM-IV criteria fulfilled in the post-marketing cases of DSM-IV Substance Abuse and Substance Dependence on Xyrem. a
| Cases of DSM-IV Substance Abuse b | DSM-IV Substance Abuse Criteria Fulfilled c | Physical Dependence | Patient History of Substance Abuse |
| Case #2 | B – use in physically hazardous situation | No | Yes |
| Case #3 | B – use in physically hazardous situation | No | Yes |
| Case #4 | D – continued use despite interpersonal problems | No | Yes |
| Case #5 | B – use in physically hazardous situation | No | Yes |
| Case #7 | A – failure to fulfill major role obligations | No | Unknown |
| Case #8 | B – use in physically hazardous situation | No | Unknown |
| Case #10 | B – use in physically hazardous situation | No | No |
| Case #12 | B – use in physically hazardous situation | No | Yes |
| Case #13 | B – use in physically hazardous situation | No | No |
| Case #40 | B – use in physically hazardous situation | No | Unknown |
| Cases of DSM-IV Substance Dependence d | DSM-IV Substance Dependence Criteria Fulfilled e | ||
| Case #9 | E – tolerance; F – withdrawal; G – larger amounts | Yes | No |
| Case #21 | E – tolerance; F – withdrawal; G – larger amounts | Yes | Yes |
| Case #22 | E – tolerance; G – larger amounts; K – use despite problems | No | Yes |
| Case #31 | E – tolerance; F – withdrawal; G – larger amounts | Yes | Yes |
The ten cases of Substance Abuse and the four cases of Substance Dependence were identified using case reports from the approximately 26,000 patients that received Xyrem worldwide from its introduction to the market in the U.S. in 2002, in Europe in 2005, and in Canada in 2007, through March 31, 2008. Thus, the rate of Substance Abuse and Substance Dependence in this population of approximately 26,000 patients is 0.039% and 0.015%, respectively.
A case was designated as DSM-IV Substance Abuse if one or more or of four criteria for Substance Abuse were fulfilled in the same 12 month period and symptoms did not meet criteria for DSM-IV Substance Dependence.
Letters designate which of the four criteria for Substance Abuse listed below were fulfilled in each case. In all ten cases, only one criterion was fulfilled.
A) recurrent substance use resulting in a failure to fulfill major role obligations at work, school, or home (e.g., repeated absences or poor work performance related to substance use; substance-related absences, suspensions, or expulsions from school; neglect of children or household)
B) recurrent substance use in situation in which it is physically hazardous (e.g., driving an automobile or operating a machine when impaired by substance use)
C) recurrent substance-related legal problems (e.g., arrests for substance-related disorderly conduct)
D) continued substance use despite having persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of the substance (e.g., arguments with spouse about consequences of intoxication, physical fights)
A case was designated as DSM-IV Substance Dependence if three or more of the seven Substance Dependence diagnostic criteria were fulfilled in the same 12 month period.
Letters designate which of the seven criteria for Substance Dependence listed below were fulfilled in each case. In all four cases, only three criteria were fulfilled.
E) tolerance, as defined by either of the following: a need for markedly increased amounts of the substance to achieve intoxication or desired effect or markedly diminished effect with continued use of the same amount of the substance
F) withdrawal, as manifested by either of the following: the characteristic withdrawal syndrome for the substance or the same (or a closely related) substance is taken to relieve or avoid withdrawal symptoms
G) the substance is often taken in larger amounts or over a longer period than was intended
H) there is a persistent desire or unsuccessful efforts to cut down or control substance use
I) a great deal of time is spent in activities necessary to obtain the substance (e.g., visiting multiple doctors or driving long distances), use the substance (e.g., chain-smoking), or recover from its effects
J) important social, occupational, or recreational activities are given up or reduced because of substance use
K) the substance use is continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by the substance (e.g., current cocaine use despite recognition of cocaine-induced depression, or continued drinking despite recognition that an ulcer was made worse by alcohol consumption)
A total of 21 deaths have been reported in patients who were likely or known to be currently taking sodium oxybate (out of 26,000 patients who took sodium oxybate) since market introduction in the U.S., Europe, and Canada (Wang et al., 2009). In 6 of the 21 cases, a physician deemed that the cause of death was not related to sodium oxybate. The causes of death in the remaining 15 cases include unknown causes (7 cases), drug overdose (3 cases total, in 2 of the 3 cases, the overdose itself did not involve sodium oxybate; all 3 overdoses involved more than one drug), accidental drowning (1 case), suicide (1 case), renal failure (1 case), cardiac arrest (1 case), and metastatic lung cancer (1 case).
Since market introduction in the U.S., Europe, and Canada, 4 cases fulfilling a DSM-IV diagnosis of Substance Dependence (i.e., addiction) on sodium oxybate (3 cases also fulfilled the criterion for physical dependence) have been documented by the central pharmacy and from spontaneous reporting (Table 2). Of the cases that fulfilled DSM-IV criteria for Substance Dependence, the criteria of tolerance (4 cases), withdrawal (3 cases), and using larger amounts of the drug than intended (4 cases) were the most common criteria endorsed (Table 2). An additional 5 cases fulfilled the criterion for physical dependence, but did not fulfill DSM-IV criteria for a Substance Dependence diagnosis. Thus, reported rates of Substance Dependence per DSM-IV criteria and physical dependence, respectively, are 1 case for every 6,500 patients treated and 1 case for every 3,300 patients treated, or approximately 0.015% and 0.031% (Wang et al., 2009).
The effects of abrupt discontinuation of therapeutic doses of sodium oxybate were examined in a retrospective analysis of clinical trial data. In a study in which 29 of 55 narcoleptic patients who had been taking nightly doses of 3-9 g of sodium oxybate for 7-44 months were (re)randomized to placebo treatment under double-blind conditions, 5 instances of possible withdrawal symptoms (e.g., anxiety, dizziness, insomnia, and somnolence) were reported (U.S. Xyrem Multi-Center Study Group, 2003).
3.3 Drug-facilitated sexual assault with illicit GHB
A major concern in the area of illicit GHB misuse and abuse has been its use for incapacitating victims for the purpose of sexual assault or “date rape.” It has been suggested that some effects attributed to illicit GHB, such as a rapid onset of sedation, anterograde amnesia, and increased libido and suggestibility, might specifically lend illicit GHB for use in drug-facilitated sexual assault, particularly when combined with alcohol (Marwick, 1997; ElSohly and Salamone, 1999; Varela et al., 2004). This aspect of illicit GHB abuse has drawn much attention from the media and criminal justice systems in the U.S., Europe, and Australia. As is the case with voluntary non-medical use of illicit GHB, determining whether illicit GHB was associated with drug-facilitated sexual assault is often confounded by the presence of other drugs of abuse (e.g., cannabis and/or alcohol), the difficulty in confirming that GHB was in fact administered due to its relatively short (40-50 min) half-life (Scharf et al., 1998; Borgen et al., 2004; Abanades et al., 2006), the lack of readily available specific assays for detecting GHB, and the difficulty of differentiating between levels of endogenous GHB and exogenously administered illicit GHB (Slaughter, 2000).
GHB is an endogenous compound and human urine and other biological specimens often contain measurable amounts of GHB in the absence of exogenous GHB administration (Doherty and Roth, 1978; LeBeau et al., 2006; LeBeau et al., 2007). Thus, failure to distinguish between exogenous and endogenous concentrations of GHB in biological specimens, as well as the documented post-mortem increase of GHB levels in biological specimens (LeBeau et al., 2001; LeBeau et al., 2007), might have resulted in the reporting of false-positive test results for illicit GHB prior to the development of sensitive and specific analytical methods and established cut-off values for normal endogenous levels of GHB. Specific and sensitive analytical methods for measuring GHB in biological matrices were developed during the clinical development of sodium oxybate as a pharmaceutical product (Scharf et al., 1998) and subsequent methodology has continued to address the need for more accurate assessments in forensic toxicology (e.g., LeBeau et al., 2006).
Forensic analyses of cases of suspected drug-facilitated sexual assault have typically reported a lower prevalence of illicit GHB use compared to other drugs. In two studies conducted in the U.S., forensic analysis showed that one or more drugs were detected in approximately 40-60% of biological samples from cases of suspected drug-facilitated sexual assault (ElSohly and Salamone, 1999; Slaughter, 2000; Juhascik et al., 2007). Of the samples in which only one drug was detected, GHB was found in 0-3%, whereas alcohol, tetrahydrocannabinol (THC; the primary active compound in marijuana), or a benzodiazepine was found in 8-69% of samples (ElSohly and Salamone, 1999; Slaughter, 2000). Of the samples in which one or more drugs were detected, GHB was found in 3-4%, whereas alcohol, THC, or a benzodiazepine was found in 19-56% of samples (ElSohly and Salamone, 1999; Slaughter, 2000). The ability to detect a drug in a biological sample depends on the rate of elimination of a drug or drug metabolite, and the window of detection for some drugs (e.g., THC, benzodiazepines) is typically longer than for others (e.g., alcohol, GHB). Such differences can reduce the likelihood of detecting drugs with a shorter half-life in blood or urine. Each of the studies mentioned above included only samples collected within 72 hours of the assault and acknowledged that the detection of GHB in urine or plasma is limited by the relatively rapid elimination of GHB from the body and its metabolism to carbon dioxide and water (ElSohly and Salamone, 1999; Slaughter, 2000; Juhascik et al., 2007). These issues complicate the interpretation of these forensic data and have prompted the development of toxicological analyses able to detect GHB or specific GHB metabolites in biological specimens for greater periods of time after administration.
3.4 Drug-facilitated sexual assault with sodium oxybate
In ten clinical trials evaluating the effects of sodium oxybate in different disease states in a total of 781 patients, one instance of drug-facilitated sexual assault was reported (∼0.128%), which involved the assault of a patient (Wang et al., 2009). Two confirmed cases (∼0.008%) of drug-facilitated sexual assault were reported from the approximately 26,000 patients treated with sodium oxybate (one case involved a patient, one case did not) since market introduction through March 2008 in the U.S., Europe, and Canada. One additional suspected case of drug-facilitated sexual assault not included in the numbers above involved a report of a drug-facilitated sexual assault of a patient made to the central pharmacy by a prescribing physician; however, the central pharmacy had neither received a prescription for, nor shipped Xyrem to, this physician's patient (Wang et al., 2009).
Administration of known supratherapeutic doses of sodium oxybate can produce a state in which the patient is largely unresponsive, and with larger doses, a state of unconsciousness (Carter et al., 2006). However, in human studies with drug users (Carter et al., 2006) and non-drug users (Carter et al., 2007), administration of sodium oxybate did not produce the same magnitude of anterograde amnestic effects on the encoding of episodic memory that were observed after comparable doses of the benzodiazepine triazolam.
4. Discussion and Conclusions
There are important differences between illicit GHB and the pharmaceutical product sodium oxybate in accessibility, purity, and dosing, as well as in the prevalence and potential consequences of misuse and abuse. Illicit GHB and GHB precursors are more widely available than sodium oxybate, which is manufactured and distributed under strict conditions that allow the prospective monitoring of potential cases of abuse and dependence. Illicit GHB is synthesized and sold in a manner in which the purity and dose of the illicit formulation are often unknown to the consumer, whereas sodium oxybate is produced and stored according to Good Manufacturing Practice standards. Illicit GHB is often consumed at frequent intervals and together with other illicit drugs, whereas sodium oxybate is specifically prescribed to be taken in bed and not to be taken with alcohol or other CNS depressants.
Despite confounding factors (e.g., co-administration of other substances, misreporting, challenges in detection, and generic use of the term GHB to refer to any drug that has caused sedation or has been used for sexual assault), data from national surveys of drug use and abuse, law enforcement activity, emergency department visits, and poison control center exposures and deaths suggest that the rate of GHB abuse has remained low over the past several years, even as sodium oxybate was introduced to the market. These data, together with the extremely low rates of diversion of sodium oxybate, support the conclusion that market introduction has not substantially contributed to the rates of GHB abuse or misuse. There are several possible reasons for the decreases in production, availability, and use of illicit GHB. International restrictions on the production and sale of illicit GHB have been suggested to have shifted use away from illicit GHB and toward GHB precursors GBL and 1,4 BD (Zvosec et al., 2001; Wood et al., 2008). The relative risk of overdose with illicit GHB, possibly due to a narrow dose range for abuse, impurities of illicit synthesis, or consumption with other drugs, is thought to lessen its desirability as a drug of abuse. Moreover, in studies conducted in the U.S. and Australia more than half of recreational GHB users report that the acute effects of illicit GHB intoxication include confusion, dizziness, blurred vision, hot/cold flushes, profuse sweating, vomiting, and loss of consciousness (Miotto et al., 2001; Degenhardt et al., 2002).
Rates of DSM-IV Substance Abuse and Substance Dependence, physical dependence, and diversion of sodium oxybate have been extremely low since its introduction to the market in the U.S., Europe, and Canada. The product's risk management program has played an important role in prospectively identifying instances of sodium oxybate misuse, abuse, or diversion and discontinuing access to the drug in those cases. Prospective monitoring of reports of lost or stolen drug and requests for early refills by the central pharmacy reduces the likelihood that patients can develop a chronic problem undetected. In fact, in 2007 Barker and colleagues reported that in a survey of 51 individuals in the U.S. who reported using illicit GHB for an average of 4.3 years, “only a handful of people in the study, those working in healthcare, had ever heard of the drug Xyrem…. No one had ever received or attempted to procure a prescription for this substance from a physician” (Barker et al., 2007). Data also suggest that the likelihood of developing physical dependence to sodium oxybate at therapeutic doses is low (Tarabar and Nelson, 2004). For the relatively small population of narcoleptic patients, prospective monitoring by the central pharmacy is feasible and has likely limited and prevented potential cases of abuse, dose escalation, and dependence.
Some of the surveillance systems described in this review are limited by their reliance on the spontaneous reporting of events (i.e., events might go unreported or different types of cases might be more likely to be reported than others) and the inability to specifically differentiate between the abuse of illicit GHB and the abuse of the pharmaceutical product. Prospective post-marketing surveillance methodologies have been developed that monitor the non-medical use of drugs in drug-using populations and that can distinguish between the abuse of illicit and licit formulations of drugs (e.g., Arfken et al., 2003). Future investigations that prospectively differentiate abuse of illicit GHB from abuse of sodium oxybate would speak directly to some of the issues presented in this review.
Data from the scientific literature, national surveillance systems, and the manufacturer support the conclusion that there are substantial differences in the availability, purity, and dosing, as well as in the prevalence and potential consequences of misuse and abuse of illicit GHB compared to the pharmaceutical product sodium oxybate. These differences result in a greater liability of abuse of illicit GHB compared to sodium oxybate, which is reflected in the additional controls, restrictions, and penalties associated with illicit GHB in the U.S.. Although GHB and sodium oxybate are the chemical and non-proprietary drug names for the identical compound, recognition of the different liabilities of abuse for illicit GHB and sodium oxybate will help to ensure that patients who benefit from the pharmaceutical product will have continued unimpeded access to their medication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abanades S, Farré M, Barral D, Torrens M, Closas N, Langohr K, Pastor A, de la Torre R. Relative abuse liability of gamma-hydroxybutyric acid, flunitrazepam, and ethanol in club drug users. J Clin Psychopharmacol. 2007;27:625–638. doi: 10.1097/jcp.0b013e31815a2542. [DOI] [PubMed] [Google Scholar]
- Abanades S, Farré M, Segura M, Pichini S, Barral D, Pacifici R, Pellegrini M, Fonseca F, Langohr K, De La Torre R. Gamma-hydroxybutyrate (GHB) in humans: pharmacodynamics and pharmacokinetics. Ann N Y Acad Sci. 2006;1074:559–576. doi: 10.1196/annals.1369.065. [DOI] [PubMed] [Google Scholar]
- Anderson IB, Kim SY, Dyer JE, Burkhardt CB, Iknoian JC, Walsh MJ, Blanc PD. Trends in gamma-hydroxybutyrate (GHB) and related drug intoxication: 1999 to 2003. Ann Emerg Med. 2006;47:177–83. doi: 10.1016/j.annemergmed.2005.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfken CL, Schuster CR, Johanson CE. Postmarketing surveillance of abuse liability of sibutramine. Drug Alcohol Depend. 2003;69:169–173. doi: 10.1016/s0376-8716(02)00311-3. [DOI] [PubMed] [Google Scholar]
- Australian Institute of Health and Welfare. Canberra: AIHW; 2008. [Oct 8, 2008]. 2007 National Drug Strategy Household Survey: first results. Drug Statistics Series number 20. Cat. no. PHE 98. http://www.aihw.gov.au/publications/index.cfm/title/10579. [Google Scholar]
- Barker JC, Harris SL, Dyer JE. Experiences of gamma hydroxybutyrate (GHB) ingestion: a focus group study. J Psychoactive Drugs. 2007;39:115–29. doi: 10.1080/02791072.2007.10399870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellis MA, Hughes K, Bennett A, Thomson R. The role of an international nightlife resort in the proliferation of recreational drugs. Addiction. 2003;98:1713–1721. doi: 10.1111/j.1360-0443.2003.00554.x. [DOI] [PubMed] [Google Scholar]
- Borgen LA, Okerholm RA, Lai A, Scharf MB. The pharmacokinetics of sodium oxybate oral solution following acute and chronic administration to narcoleptic patients. J Clin Pharmacol. 2004;44:253–257. doi: 10.1177/0091270003262795. [DOI] [PubMed] [Google Scholar]
- Bronstein AC, Spyker DA, Cantilena LR, Green J, Rumack BH, Heard SE. 2006 Annual Report of the American Association of Poison Control Centers' National Poison Data System. Clin Toxicol. 2007;45:815–917. doi: 10.1080/15563650701754763. [DOI] [PubMed] [Google Scholar]
- Bronstein AC, Spyker DA, Cantilena JR, Louis R, Green JL, Rumack BH, Heard SE. 2007 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 25th Annual Report. Clin Toxicol. 2008;46:927–1057. doi: 10.1080/15563650802559632. [DOI] [PubMed] [Google Scholar]
- Broughton R, Mamelak M. The treatment of narcolepsy-cataplexy with nocturnal gamma-hydroxybutyrate. Can J Neurol Sci. 1979;6:1–6. doi: 10.1017/s0317167100119304. [DOI] [PubMed] [Google Scholar]
- Caldicott DG, Chow FY, Burns BJ, Felgate PD, Byard RW. Fatalities associated with the use of γ-hydroxybutyrate and it analogues in Australasia. Med J Aust. 2004;181:310–313. doi: 10.5694/j.1326-5377.2004.tb06295.x. [DOI] [PubMed] [Google Scholar]
- Carter LP, Flores LR, Wu H, Chen W, Unzeitig AW, Coop A, France CP. The role of GABAB receptors in the discriminative stimulus effects of γ-hydroxybutyrate in rats: time course and antagonism studies. J Pharmacol Exp Ther. 2003;305:668–674. doi: 10.1124/jpet.102.047860. [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR, Mintzer MZ. Psychomotor, subjective, and cognitive effects of GHB and triazolam in healthy volunteers. Presented at the 69th Annual Meeting of the College on Problems of Drug Dependence; 2007. [Jul 17, 2008]. http://www.cpdd.vcu.edu/Pages/Meetings/MeetProgAbSearchAll.html. [Google Scholar]
- Carter LP, Koek W, France CP. Behavioral analyses of GHB: Receptor mechanisms. Pharmacol Ther. 2009;121:100–114. doi: 10.1016/j.pharmthera.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Richards BD, Mintzer MZ, Griffiths RR. Relative abuse liability of GHB in humans: A comparison of psychomotor, subjective, and cognitive effects of supratherapeutic doses of triazolam, pentobarbital, and GHB. Neuropsychopharmacology. 2006;31:2537–2551. doi: 10.1038/sj.npp.1301146. [DOI] [PubMed] [Google Scholar]
- Carter LP, Wu H, Chen W, Matthews MM, Mehta AK, Hernandez RJ, Thomson JA, Ticku MK, Coop A, Koek W, France CP. Novel gamma-hydroxybutyric acid (GHB) analogs share some, but not all behavioral effects of GHB and GABAB receptor agonists. J Pharmacol Exp Ther. 2005;313:1314–1323. doi: 10.1124/jpet.104.077578. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. Multistate outbreak of poisonings associated with illicit use of gamma hydroxy butyrate. JAMA. 1991;265:447–448. [PubMed] [Google Scholar]
- Chin MY, Kreutzer RA, Dyer JE. Acute poisoning from γ-hydroxybutyrate in California. West J Med. 1992;156:380–384. [PMC free article] [PubMed] [Google Scholar]
- Chin RL, Sporer KA, Cullison B, Dyer JE, Wu TD. Clinical course of gamma-hydroxybutyrate overdose. Ann Emerg Med. 1998;31:716–722. [PubMed] [Google Scholar]
- Couper FJ, Thatcher JE, Logan BK. Suspected GHB overdoses in the emergency department. J Anal Toxicol. 2004;28:481–484. doi: 10.1093/jat/28.6.481. [DOI] [PubMed] [Google Scholar]
- Craig K, Gomez HF, McManus JL, Bania TC. Severe gamma-hydroxybutyrate withdrawal: a case report and literature review. J Emerg Med. 2000;18:65–70. doi: 10.1016/s0736-4679(99)00163-8. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Copeland J, Dillon P. Recent trends in the use of “club drugs”: an Australian review. Subst Use Misuse. 2005;40:1241–1256. doi: 10.1081/JA-200066777. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Darke S, Dillon P. GHB use among Australians: characteristics, use patterns and associated harm. Drug Alcohol Depend. 2002;67:89–94. doi: 10.1016/s0376-8716(02)00017-0. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Darke S, Dillon P. The prevalence and correlates of gamma-hydroxybutyrate (GHB) overdose among Australian users. Addiction. 2003;98:199–204. doi: 10.1046/j.1360-0443.2003.00265.x. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Dunn M. The epidemiology of GHB and ketamine use in an Australian household survey. Int J Drug Policy. 2008;19:311–316. doi: 10.1016/j.drugpo.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Dietze PM, Cvetkovski S, Barratt MJ, Clemens S. Patterns and incidence of gamma-hydroxybutyrate (GHB)-related ambulance attendances in Melbourne, Victoria. Med J Aust. 2008;188:709–711. doi: 10.5694/j.1326-5377.2008.tb01851.x. [DOI] [PubMed] [Google Scholar]
- Doherty JD, Roth RH. Metabolism of γ-hydroxy-[1-14C] butyrate by rat brain: relationship to the Krebs cycle and metabolic compartmentation of amino acids. J Neurochem. 1978;30:1305–1309. doi: 10.1111/j.1471-4159.1978.tb10460.x. [DOI] [PubMed] [Google Scholar]
- Dupont P, Thornton J. Near-fatal gamma-butyrolactone intoxication--first report in the UK. Hum Exp Toxicol. 2001;20:19–22. doi: 10.1191/096032701666142043. [DOI] [PubMed] [Google Scholar]
- Dyer JE. γ-Hydroxybutyrate: a health-food product producing coma and seizurelike activity. Am J Emerg Med. 1991;9:321–324. doi: 10.1016/0735-6757(91)90050-t. [DOI] [PubMed] [Google Scholar]
- Dyer JE, Roth B, Hyma BA. Gamma-hydroxybutyrate withdrawal syndrome. Ann Emerg Med. 2001;37:147–153. doi: 10.1067/mem.2001.112985. [DOI] [PubMed] [Google Scholar]
- ElSohly MA, Salamone SJ. Prevalence of drugs used in cases of alleged sexual assault. J Anal Toxicol. 1999;23:141–146. doi: 10.1093/jat/23.3.141. [DOI] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. Luxembourg: Office for Official Publications of the European Communities; [Jul 17, 2008]. Annual report 2007: the state of the drugs problem in Europe. http://www.emcdda.europa.eu/html.cfm/index44682EN.html. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. Luxembourg: Office for Official Publications of the European Communities; [Feb 6, 2009]. Annual report 2008: the state of the drugs problem in Europe. http://www.emcdda.europa.eu/attachements.cfm/att_64227_EN_EMCDDA_AR08_en.pdf. [Google Scholar]
- Ferrara SD, Tedeschi L, Frison G, Rossi A. Fatality due to gamma-hydroxybutyric acid (GHB) and heroin intoxication. J Forensic Sci. 1995;40:501–504. [PubMed] [Google Scholar]
- Frucht SJ, Houghton WC, Bordelon Y, Greene PE, Louis ED. A single-blind, open-label trial of sodium oxybate for myoclonus and essential tremor. Neurology. 2005;65:1967–1969. [PubMed] [Google Scholar]
- Fuller DE, Hornfeldt CS, Kelloway JS, Stahl PJ, Anderson TF. The Xyrem risk management program. Drug Saf. 2004;27:293–306. doi: 10.2165/00002018-200427050-00002. [DOI] [PubMed] [Google Scholar]
- Galloway GP, Frederick SL, Staggers FE, Jr, Gonzales M, Stalcup SA, Smith DE. Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence. Addiction. 1997;92:89–96. [PubMed] [Google Scholar]
- Glasper A, McDonough M, Bearn J. Within-patient variability in clinical presentation of gamma-hydroxybutyrate withdrawal: a case report. Eur Addict Res. 2005;11:152–154. doi: 10.1159/000085551. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Froestl W, Weerts EM. Involvement of gamma-hydroxybutyrate (GHB) and GABA-B receptors in the acute behavioral effects of GHB in baboons. Psychopharmacology. 2005;180:342–351. doi: 10.1007/s00213-005-2165-y. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Griffiths RR, Brown PR, Froestl W, Jakobs C, Gibson KM, Weerts EM. Chronic intragastric administration of gamma-butyrolactone produces physical dependence in baboons. Psychopharmacology. 2006;189:71–82. doi: 10.1007/s00213-006-0534-9. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Ator NA. Principles of initial experimental drug abuse liability assessment in humans. Drug Alcohol Depend. 2003;70(3 Suppl):S41–54. doi: 10.1016/s0376-8716(03)00098-x. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry. 2005;66(Suppl):31–41. [PubMed] [Google Scholar]
- Hawaii Revised Statute, Chapter 329 – Uniformed Controlled Substances Act. [Oct 1, 2008]; http://hawaii.gov/dcca/areas/pvl/main/hrs/
- Hillebrand J, Olszewski D, Sedefov R. European Monitoring Centre for Drugs and Drug Addiction; 2008. [Jul 17, 2008]. EMCDDA Thematic Papers — GHB and its precursor GBL: an emerging trend case study. http://www.emcdda.europa.eu/html.cfm/index50526EN.html. [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. University of Michigan News Service; Ann Arbor, MI: 2008. [Jan 23, 2009]. Various stimulant drugs show continuing gradual declines among teens in 2008, most illicit drugs hold steady. http://monitoringthefuture.org/ [Google Scholar]
- Juhascik MP, Negrusz A, Faugno D, Ledray L, Greene P, Lindner A, Haner B, Gaensslen RE. An estimate of the proportion of drug-facilitation of sexual assault in four U.S. localities. J Forensic Sci. 2007;52:1396–400. doi: 10.1111/j.1556-4029.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- Kim SY, Anderson IB, Dyer JE, Barker JC, Blanc PD. High-risk behaviors and hospitalizations among gamma hydroxybutyrate (GHB) users. Am J Drug Alcohol Abuse. 2007;33:429–438. doi: 10.1080/00952990701312316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Barker JC, Anderson IB, Dyer JE, Earnest G, Blanc PD. Systematic Assessment of Gamma Hydroxybutyrate (GHB) Effects During and After Acute Intoxication. Am J Addict. 2008;17:312–318. doi: 10.1080/10550490802138988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen K, Greter J, Verdicchio M. High mortality rates among GHB abusers in Western Sweden. Clin Toxicol (Phila) 2008;46:187–192. doi: 10.1080/15563650701263633. [DOI] [PubMed] [Google Scholar]
- Lai MW, Klein-Schwartz W, Rodgers GC, Abrams JY, Haber DA, Bronstein AC, Wruk KM. 2005 Annual Report of the American Association of Poison Control Centers' National Poisoning and Exposure Database. Clin Toxicol. 2006;44:803–932. doi: 10.1080/15563650600907165. [DOI] [PubMed] [Google Scholar]
- LeBeau MA, Miller ML, Levine B. Effect of storage temperature on endogenous GHB levels in urine. Forensic Sci Int. 2001;15:161–167. doi: 10.1016/s0379-0738(00)00426-6. [DOI] [PubMed] [Google Scholar]
- LeBeau MA, Montgomery MA, Morris-Kukoski C, Schaff JE, Deakin A. Further evidence of in vitro production of gamma-hydroxybutyrate (GHB) in urine samples. Forensic Sci Int. 2007;169:152–156. doi: 10.1016/j.forsciint.2006.08.007. [DOI] [PubMed] [Google Scholar]
- LeBeau MA, Montgomery MA, Morris-Kukoski C, Schaff JE, Deakin A, Levine B. A comprehensive study on the variations in urinary concentrations of endogenous gamma-hydroxybutyrate (GHB) J Anal Toxicol. 2006;30:98–105. doi: 10.1093/jat/30.2.98. [DOI] [PubMed] [Google Scholar]
- Lettieri J, Fung H. Improved pharmacological activity via pro-drug modification: comparative pharmacokinetics of sodium γ-hydroxybutyrate and γ-butyrolactone. Res Commun Chem Pathol Pharmacol. 1978;22:107–118. [PubMed] [Google Scholar]
- Litovitz TL, Klein-Schwartz W, Rodgers GC, Cobaugh DJ, Youniss J, Omslaer JC, May ME, Woolf AD, Benson BE. 2001 Annual Report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med. 2002;20:391–452. doi: 10.1053/ajem.2002.34955. [DOI] [PubMed] [Google Scholar]
- Maitre M. The γ-hydroxybutyrate signaling system in brain: organization and functional implications. Prog Neurobiol. 1997;51:337–361. doi: 10.1016/s0301-0082(96)00064-0. [DOI] [PubMed] [Google Scholar]
- Marwick C. Coma-inducing drug GHB may be reclassified. JAMA. 1997;277:1505–1506. doi: 10.1001/jama.277.19.1505. [DOI] [PubMed] [Google Scholar]
- Mason PE, Kerns WP., 2nd Gamma hydroxybutyric acid (GHB) intoxication. Acad Emerg Med. 2002;9:730–739. doi: 10.1111/j.1553-2712.2002.tb02154.x. [DOI] [PubMed] [Google Scholar]
- Maxwell JC, Spence RT. Profiles of club drug users in treatment. Substance Use & Misuse. 2005;40:1409–1426. doi: 10.1081/JA-200066968. [DOI] [PubMed] [Google Scholar]
- McDaniel CH, Miotto KA. Gamma hydroxybutyrate (GHB) and gamma butyrolactone (GBL) withdrawal: five case studies. J Psychoactive Drugs. 2001;33:143–149. doi: 10.1080/02791072.2001.10400479. [DOI] [PubMed] [Google Scholar]
- Miotto KA, Darakjian J, Basch J, Murray S, Zogg J, Rawson R. Gamma-hydroxybutyric acid: patterns of use, effects and withdrawal. Am J Addict. 2001;10:232–241. doi: 10.1080/105504901750532111. [DOI] [PubMed] [Google Scholar]
- National Drug Intelligence Center U.S. Department of Justice. National Drug Threat Assessment 2009. [Jan 23, 2009];2008 http://www.usdoj.gov/ndic/pubs31/31379/index.htm.
- Nevada Administrative Code, Chapter 453 – Controlled Substances. [Oct 1, 2008]; http://www.leg.state.nv.us/NAC/NAC-453.html#NAC453Sec510.
- Nicholson KL, Balster RL. GHB: a new and novel drug of abuse. Drug Alcohol Depend. 2001;63:1–22. doi: 10.1016/s0376-8716(00)00191-5. [DOI] [PubMed] [Google Scholar]
- Office of Diversion Control. Washington, DC: U.S. Drug Enforcement Administration; 2008. [Feb 6, 2009]. National Forensic Laboratory Information System: Year 2007 Annual Report. http://www.deadiversion.usdoj.gov/nflis/index.html. [Google Scholar]
- Palatini P, Tedeschi L, Frison G, Padrini R, Zordan R, Orlando R, Gallimberti L, Gessa GL, Ferrara SD. Dose-dependent absorption and elimination of gamma-hydroxybutyric acid in healthy volunteers. Eur J Clin Pharmacol. 1993;45:353–356. doi: 10.1007/BF00265954. [DOI] [PubMed] [Google Scholar]
- Palmer RB. Gamma-butyrolactone and 1,4-buandiol: abused analogues of gamma-hydroxybutyrate. Toxicol Rev. 2004;23:21–31. doi: 10.2165/00139709-200423010-00003. [DOI] [PubMed] [Google Scholar]
- Pardi D, Black J. gamma-Hydroxybutyrate/sodium oxybate: neurobiology, and impact on sleep and wakefulness. CNS Drugs. 2006;20:993–1018. doi: 10.2165/00023210-200620120-00004. [DOI] [PubMed] [Google Scholar]
- Price G. In-patient detoxification after GHB dependence. Br J Psychiatry. 2000;177:181. doi: 10.1192/bjp.177.2.181. [DOI] [PubMed] [Google Scholar]
- Russell IJ, Perkins AT, Michalek JE. Oxybate SXB-26 Fibromyalgia Syndrome Study Group. Sodium oxybate relieves pain and improves function in fibromyalgia syndrome: a randomized, double-blind, placebo-controlled, multicenter clinical trial. Arthritis Rheum. 60:299–309. doi: 10.1002/art.24142. [DOI] [PubMed] [Google Scholar]
- Scharf MB, Brown D, Woods M, Brown L, Hirschowitz J. The effects and effectiveness of gamma-hydroxybutyrate in patients with narcolepsy. J Clin Psychiatry. 1985;46:222–225. [PubMed] [Google Scholar]
- Scharf MB, Lai AA, Branigan B, Stover R, Berkowitz DB. Pharmacokinetics of gammahydroxybutyrate (GHB) in narcoleptic patients. Sleep. 1998;21:507–514. doi: 10.1093/sleep/21.5.507. [DOI] [PubMed] [Google Scholar]
- Slaughter L. Involvement of drugs in sexual assault. J Reprod Med. 2000;45:425–430. [PubMed] [Google Scholar]
- Strickland RM, Felgate P, Caldicott DG. Survival of massive gamma-hydroxybutyrate/1,4-butanediol overdose. Emerg Med Australas. 2005;17:281–283. doi: 10.1111/j.1742-6723.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- Strom KJ, Wong L, Fornnarino L, Eicheldinder C, Bethke A, Ancheta J, Rachal V. Washington, DC: U.S. Drug Enforcement Administration; 2003. [Jul 17, 2008]. The National Forensic Laboratory Information System: 2002 Annual Report. http://www.deadiversion.usdoj.gov/nflis/index.html. [Google Scholar]
- Strom KJ, Wong L, Fornnarino L, Eicheldinger C, Bethke A, Ancheta J, Rachal V. Washington, DC: U.S. Drug Enforcement Administration; 2004. [Jul 17, 2008]. The National Forensic Laboratory Information System: 2003 Annual Report. http://www.deadiversion.usdoj.gov/nflis/index.html. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Rockville, MD: 2008a. [Feb 6, 2009]. Drug Abuse Warning Network, 2006: National Estimates of Drug-Related Emergency Department Visits. DAWN Series D-30, DHHS Publication No. (SMA) 08-4339. http://dawninfo.samhsa.gov/pubs/edpubs/default.asp. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Rockville, MD: 2008b. [Feb 6, 2009]. Results from the 2007 National Survey on Drug Use and Health: National Findings. NSDUH Series H-34, DHHS Publication No. (SMA) 08-4343. http://oas.samhsa.gov/nsduh/2k7nsduh/2k7Results.pdf. [Google Scholar]
- Sumnall HR, Woolfall K, Edwards S, Cole JC, Beynon CM. Use, function, and subjective experiences of gamma-hydroxybutyrate (GHB) Drug Alcohol Depend. 2008;92:286–290. doi: 10.1016/j.drugalcdep.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Tarabar AF, Nelson LS. The gamma-hydroxybutyrate withdrawal syndrome. Toxicol Rev. 2004;23:45–49. doi: 10.2165/00139709-200423010-00005. [DOI] [PubMed] [Google Scholar]
- Thai D, Dyer JE, Benowitz NL, Haller CA. Gamma-hydroxybutyrate and ethanol effects and interactions in humans. J Clin Psychopharmacol. 2006;26:524–529. doi: 10.1097/01.jcp.0000237944.57893.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai D, Dyer JE, Jacob P, Haller CA. Clinical pharmacology of 1,4-butandiol and gamma-hydroxybutyrate after oral 1,4-butanediol administration in healthy volunteers. Clin Pharmacol Ther. 2007;81:178–184. doi: 10.1038/sj.clpt.6100037. [DOI] [PubMed] [Google Scholar]
- The National Forensic Laboratory Information System. U.S. Drug Enforcement Administration, Office of Diversion Control; Washington, D.C.: 2001. [Jul 17, 2008]. Year 2000 Annual Report. http://www.deadiversion.usdoj.gov/nflis/index.html. [Google Scholar]
- The National Forensic Laboratory Information System. U.S. Drug Enforcement Administration, Office of Diversion Control; Washington, D.C.: 2002. [Jul 17, 2008]. Year 2001 Annual Report. http://www.deadiversion.usdoj.gov/nflis/index.html. [Google Scholar]
- U.S. Controlled Substances Act. Title 21, U.S.C. 802(34) [Sept 4, 2008]; http://www.deadiversion.usdoj.gov/21cfr/21usc/802.htm#34.
- U.S. Xyrem Multi-Center Study Group. The abrupt cessation of therapeutically administered sodium oxybate (GHB) does not cause withdrawal symptoms. J Toxicol Clin Toxicol. 2003;41:131–135. doi: 10.1081/clt-120019128. [DOI] [PubMed] [Google Scholar]
- Van Sassenbroeck DK, De Neve N, De Paepe P, Belpaire FM, Verstraete AG, Calle PA, Buylaert WA. Abrupt awakening phenomenon associated with gamma-hydroxybutyrate use: a case series. Clin Toxicol (Phila) 2007;45:533–538. doi: 10.1080/15563650701365818. [DOI] [PubMed] [Google Scholar]
- Varela M, Nogue S, Oros M, Miro O. Gamma hydroxybutirate use for sexual assault. Emerg Med J. 2004;21:255–256. doi: 10.1136/emj.2002.002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL. Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem®): abuse, misuse, dependence, and diversion. J Clin Sleep Med. 2009 in press. [PMC free article] [PubMed] [Google Scholar]
- Watson WA, Litovitz TL, Rodgers GC, Klein-Schwartz W, Reid N, Youniss J, Flanagan A, Wruk KM. 2004 Annual Report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med. 2005;23:589–666. doi: 10.1016/j.ajem.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Watson WA, Litovitz TL, Rodgers GC, Klein-Schwartz W, Youniss J, Reid N, Rouse WG, Rembert RS, Borys D. 2003 Annual Report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med. 2004;22:335–404. doi: 10.1016/j.ajem.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Watson WA, Litovitz TL, Rodgers GC, Klein-Schwartz W, Youniss J, Rose SR, Borys D, May ME. 2002 Annual Report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med. 2003;21:353–421. doi: 10.1016/s0735-6757(03)00088-3. [DOI] [PubMed] [Google Scholar]
- Wedin GP, Hornfeldt CS, Ylitalo LM. The Clinical Development of γ-Hydroxybutyrate (GHB) Current Drug Safety. 2006;1:99–106. doi: 10.2174/157488606775252647. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Goodwin AK, Griffiths RR, Brown PR, Froestl W, Jakobs C, Gibson KM. Spontaneous and precipitated withdrawal after chronic intragastric administration of gamma-hydroxybutyrate (GHB) in baboons. Psychopharmacology. 2005;179:678–687. doi: 10.1007/s00213-004-2079-0. [DOI] [PubMed] [Google Scholar]
- Weimer BJ, Peters D, Sannerud C, Eicheldinger C, Ancheta J, Strom K, Rachal V. Washington, DC: U.S. Drug Enforcement Administration; 2007. [Jul 17, 2008]. The National Forensic Laboratory Information System: 2006 Annual Report. http://www.deadiversion.usdoj.gov/nflis/index.html. [Google Scholar]
- Weimer BJ, Wong L, Sannerud C, Eicheldinger C, Ancheta J, Strom K, Rachal V. Washington DC: U.S. Drug Enforcement Administration; 2006. [Jul 17, 2008]. The National Forensic Laboratory Information System: 2005 Annual Report. http://www.deadiversion.usdoj.gov/nflis/index.html. [Google Scholar]
- Weimer BJ, Wong L, Strom K, Forti A, Eicheldinger C, Bethke A, Ancheta J, Rachal V. Washington, DC: U.S. Drug Enforcement Administration; 2004. [Jul 17, 2008]. The National Forensic Laboratory Information System: Year 2004 Annual Report. http://www.deadiversion.usdoj.gov/nflis/index.html. [Google Scholar]
- Williams SR. gamma-Hydroxybutyric acid poisoning. West J Med. 1998;168:187–8. [PMC free article] [PubMed] [Google Scholar]
- Winickoff JP, Houck CS, Rothman EL, Bauchner H. Verve and Jolt: deadly new internet drugs. Pediatrics. 2000;106:829–830. doi: 10.1542/peds.106.4.829. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Yarema MC, Wax PM. Withdrawal from gamma-hydroxybutyrate, 1,4-butanediol and gamma-butyrolactone: a case report and systematic review. CJEM. 2008;10:69–74. doi: 10.1017/s1481803500010034. [DOI] [PubMed] [Google Scholar]
- Wood DM, Warren-Gash C, Ashraf T, Greene SL, Shather Z, Trivedy C, Clarke S, Ramsey J, Holt DW, Dargan PI. Medical and legal confusion surrounding gamma-hydroxybutyrate (GHB) and its precursors gamma-butyrolactone (GBL) and 1,4-butanediol (1,4BD) QJM. 2008;101:23–29. doi: 10.1093/qjmed/hcm117. [DOI] [PubMed] [Google Scholar]
- Jazz Pharmaceuticals, Inc.; 2008. [Jul 17, 2008]. Xyrem Prescribing Information. http://www.xyrem.com/prescribing-information.php. [Google Scholar]
- Zvosec DL, Smith SW, McCutcheon JR, Spillane J, Hall BJ, Peacock EA. Adverse events, including death, associated with the use of 1,4-butandiol. New England J Med. 2001;544:87–93. doi: 10.1056/NEJM200101113440202. [DOI] [PubMed] [Google Scholar]


