Abstract
The SonoPrep® ultrasonic skin permeation system is used clinically to increase skin permeability for rapid, non-invasive delivery of local anaesthetics. This study tested the hypothesis that sonication can generate a long-lived increase of skin permeability for continuous transdermal drug delivery and diagnostic metabolite extraction. In order to accomplish this, the volar forearm skin of ten healthy adult subjects was sonicated. As a surrogate measure of skin permeability, skin electrical impedance was measured at occluded and non-occluded sites every hour over a period of 48 h. Sonication dramatically increased skin permeability, as demonstrated by a large drop in skin impedance. Under occlusion, sonicated skin remained highly permeable during the entire 42 h period of occlusion, which was followed by an immediate decrease in permeability upon removal of occlusion. Without occlusion, sonicated skin retained elevated permeability throughout the 48 h experiment, but regained its barrier function more quickly. Therefore, sonication can increase skin permeability for prolonged periods of time, especially under the effect of occlusion, and has potential to facilitate continuous transdermal drug delivery and diagnostic metabolite extraction.
Keywords: Electrical impedance, skin permeability, sonoporation, transdermal drug delivery, ultrasound
Introduction
Transdermal drug delivery is severely limited by the low permeability of stratum corneum (Prausnitz et al. 2004). Although transdermal delivery has grown to a multi-billion dollar industry over the past 25 years, this industry is based on fewer than 20 drugs that can currently be delivered across the skin (Prausnitz et al. 2004). Sonication using low-frequency ultrasound has been shown to create openings in the stratum corenum (Wu et al. 1998) and to increase skin permeability for transdermal delivery of several classes of drugs including insulin, lidocaine, tetanus toxoid antigen, and low molecular weight heparin, as well as extraction of interstitial fluid metabolites for glucose sensing (Katz et al. 2004, Kost et al. 2000, Mitragotri and Kost 2004). To further assess the clinical utility of this approach, this study tested the hypothesis that sonication can generate a long-lived increase of skin permeability for continuous transdermal drug delivery and diagnostic metabolite extraction. This is the first human study to assess permeability of sonicated skin for more than one day and the first to compare skin recovery with and without occlusion.
Sonication was carried out in this study using the SonoPrep® ultrasonic skin permeation system, which is the only low-frequency ultrasound device that is FDA-approved for transdermal drug delivery (Farinha et al. 2006). This device transmits ultrasonic energy to the skin through an aqueous medium, which generates cavitation bubbles at the skin surface that create transport pathways across the stratum corneum. SonoPrep® has been approved by the FDA as a pre-treatment to permeabilize the skin for rapid delivery of local anaesthetics.
In this study, skin permeability was monitored after sonication by measuring electrical skin impedance. Skin impedance is a well-accepted measure of the skin’s barrier properties and has been shown to correlate with skin permeability for a variety of compounds over a broad range of impedance values (Karande et al. 2005).
Materials and Methods
Ten healthy adult human subjects (3 female, 7 male, age: 24–52) with no history of dermatological disease provided informed consent to participate in the study, which was approved by the Georgia Tech Institutional Review Board.
Two sites were identified on the right volar forearm of each subject, one of which was occluded (skin made impermeable to air and moisture by covering with surgical tape and dressings) during the study and the other was non-occluded (uncovered skin). After measuring pre-treatment skin impedances, the identified sites were sonicated by briefly applying low-frequency (55 kHz) ultrasound (Intensity = 15 W/cm2) until the skin was permeabilized, as determined by the inbuilt mechanism in the device (SonoPrep®; Sontra Corporation, Franklin, MA). The occluded sites were un-occluded during the sonication procedure followed by immediate occlusion after treatment. Hourly impedance measurements were taken at each site over a period of 48 h. Occluded sites remained covered during the impedance measurement process. After the 42nd hour reading, the occlusive dressings were removed and all sites remained non-occluded for the final 6 h.
Subjects were divided into two groups of five individuals each. The first group provided data for time points 1–11 and 23–35 h and the second group provided data for time points 12–22 and 36–48 h. Both groups also provided data for time points −1, −0.5, and 0 h (immediately after treatment). Throughout each data collection period, subjects remained seated in the isolated study room maintained at 20–22°C and 40% relative humidity.
Impedance measurements (EIM-105 Prep-Check, 30 Hz, modified with a 200 kΩ resistor in parallel; General Devices, Ridgefield, NJ) were made using Ag/AgCl dry electrodes (Thought Technology T-3404, Stens Corporation, San Rafael, CA) placed at each treatment site with a Ag/AgCl gelled reference electrode (Superior Silver electrode with PermaGel, Tyco Healthcare Uni-Patch, Wabasha, MN) placed approximately 5 mm away.
Results and Discussion
Skin on the volar forearm of ten human subjects was exposed to low-frequency ultrasound using a SonoPrep® sonication device (Fig. 1a). Visual inspection of the skin immediately before (Fig. 1b) and after (Fig. 1c) sonication showed no differences other than formation of a slight circular indentation due to pressing the sonication hand piece against the skin. This indentation disappeared within 1 h and no erythema or edema was observed at any time on the skin surface for both occluded and non-occluded skin (Fig. 1d). There were no adverse events and the treatment was well tolerated by all subjects.
Fig 1.
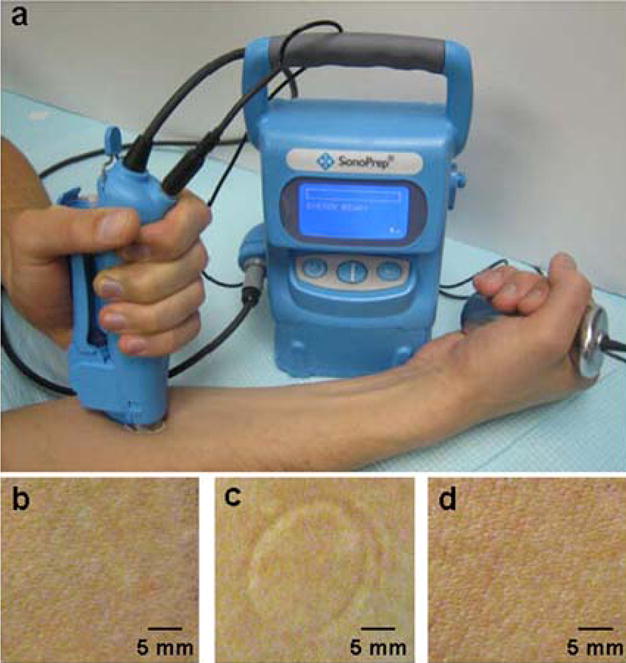
Sonication of the skin. (a) Sonication was carried out by applying a hand piece containing ultrasound coupling medium to the subject’s volar forearm skin. A reference sensor clasped in the subject’s hand helped determine the duration of sonication by interacting with the main control console. Photographs of a representative sonicated skin site are shown (b) immediately prior to sonication, (c) immediately after sonication and (d) 24 h after sonication. No erythema, edema or adverse events were associated with sonication at any time. Only a transient skin indentation due to application of the hand piece was observed immediately after sonication (c).
As a surrogate measure of skin permeability, the electrical impedance of skin was measured before, immediately after, and hourly for 48 h after sonication. During this time, the skin was either non-occluded or was occluded for 42 h and then non-occluded for the remaining 6 h. Immediately after sonication, skin impedance dropped dramatically from pre-treatment values (>200 kΩ-cm2) to less than 10 kΩ-cm2 (Fig. 2). This impedance drop signifies a large increase in skin permeability. Although no drugs were delivered in this study, previous studies suggest that skin with such a low impedance corresponds to skin permeability increased by orders of magnitude that should be sufficient for delivery of hydrophilic molecules and even macromolecules including proteins (Tezel et al. 2003).
Fig 2.
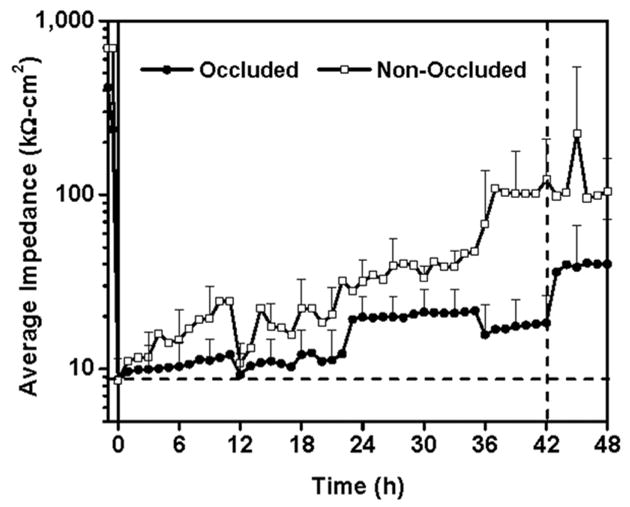
Average skin impedance for occluded (●) and non-occluded (□) skin before and after ultrasound treatment, which was carried out at t = 0. Non-occluded skin remained non-occluded throughout the experiment. Occluded skin remained occluded until t = 42 h, after which skin was non-occluded for the final 6 h. Immediately after sonication, skin impedance dropped dramatically from pre-treatment values (>200 k Ω-cm2) to less than 10 k Ω-cm2 indicating increase in skin permeability. Skin impedance recovered slowly over time, but the occluded sites recovered more slowly than non-occluded sites. Upon removal of occlusion at t = 42 h, skin impedance rapidly jumped higher. Data are presented as the average of n = 10 samples (t < 0) or n = 5 samples (t ≥ 0) with standard deviation error bars.
The primary goal of this study was to assess the longevity of increased skin permeability after sonication. Over the 48 h experiment, skin impedance values steadily increased for both the occluded and non-occluded sites, although the increase was slower for the occluded case (Fig. 2). Statistical analysis at each time point using a paired t-test with 95% confidence interval revealed that impedance of the occluded sites remained indistinguishable from the initial post-sonication impedance value for up to 42 h. These kinetics are similar to those reported in a previous 24 h study examining the suitability of sonication as a skin pre-treatment for electrophysiology measurements (Farinha et al. 2006) and another 24 h study measuring extraction of interstitial glucose after sonication (Kost et al. 2000). This long-lived effect suggests that transdermal delivery from an occlusive patch applied to sonicated skin could persist at high levels for at least 42 h. Upon removal of occlusion after the 42nd hour, the impedance jumped and was significantly different from the initial post-sonication impedance. This suggests that upon removal of an occlusive transdermal patch from sonicated skin, the skin barrier can rapidly begin to recover. We hypothesise that this slow skin barrier recovery under occlusion is caused by the reduced transepidermal water loss under occlusive skin conditions. Previous studies have shown that stratum corneum barrier repair is mediated by the formation of a water gradient in the skin caused by increased transepidermal water loss through the compromised skin barrier (Grubauer et al. 1989). In this way, occluded skin is more slowly repaired and removal of occlusion creates a water gradient allowing normal repair to occur.
In contrast, the non-occluded sites regained their impedance more rapidly and were significantly different from the initial post-sonication impedance within 7 h. However, even after the 7 h period, the impedance was still much lower than that of intact skin and thus, the sites could potentially be permeable to, for example, smaller molecules. Experimental and theoretical studies of transport across sonicated cadaver skin indicate that as skin impedance increases, permeability decreases more steeply for larger molecules (Tang et al. 2001, Tezel et al. 2003). Thus, even though macromolecule delivery may be reduced beyond 7 h post-sonication, non-occluded skin could still be used for delivery of small molecules. This presents opportunities for long-term delivery, but also presents safety concerns after short-term delivery, due to residual permeability after a transdermal delivery procedure is completed.
These results can guide the use of sonication for both short-term and long-term transdermal delivery applications. SonoPrep® is currently approved as a pre-treatment before short-term delivery of local anaesthetic over a timecourse of a few minutes under typically non-occluded conditions, although the anaesthetic formulation itself may provide partial occlusion. Our data showing a large initial drop in skin impedance, corresponding to a large increase in skin permeability, are consistent with this demonstrated application. However, the persistence of increased skin permeability for hours after delivery of the local anaesthetic is complete suggests that patients and clinical personnel should take care to prevent unintentional absorption of exogenous materials during that time.
For long-term delivery from an occlusive transdermal patch, our data suggest that drug delivery can persist at relatively steady levels for at least 42 h. Animal studies have demonstrated delivery of a variety of drugs, including of insulin (Mitragotri and Kost 2004, Park et al. 2007), heparin (Mitragotri and Kost 2004), and tetanus toxoid antigen (Tezel et al. 2005). Thus, a sonication pre-treatment could be followed by sustained-release delivery of drugs and vaccines from a patch. Similarly, non-invasive glucose monitoring has been demonstrated in diabetic subjects via interstitial glucose extraction from sonicated skin (Kost et al. 2000). Our data suggest that continuous monitoring for at least one day may be possible after a single ultrasound pre-treatment.
In conclusion, skin impedance measurements in this study showed that skin permeability can be dramatically increased by sonication and that elevated skin permeability can persist for at least 42 h under occlusion. Skin permeability recovered more rapidly without occlusion. This suggests that sonication can be used as a pre-treatment to generate a long-lived increase of skin permeability for continuous transdermal drug delivery and diagnostic metabolite extraction.
Acknowledgments
We thank Harvinder Gill and Samantha Andrews for assistance in carrying out experiments and Scott Kellogg for help with experimental design. This work was supported in part by Sontra Medical Corporation and the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Farinha A, Kellogg S, Dickinson K, Davidson T. Skin impedance reduction for electrophysiology measurements using ultrasonic skin permeation: initial report and comparison to current methods. Biomed Instrum Technol. 2006;40:72–77. doi: 10.2345/0899-8205(2006)40[72:SIRFEM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Grubauer G, Elias PM, Feingold KR. Transepidermal water loss: the signal for recovery of barrier structure and function. Journal of Lipid Research. 1989;30:323–333. [PubMed] [Google Scholar]
- Karande P, Jain A, Mitragotri S. Relationships between skin’s electrical impedance and permeability in the presence of chemical enhancers. Journal of Controlled Release. 2005;110:307–13. doi: 10.1016/j.jconrel.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Katz NP, Shapiro DE, Herrmann TE, Kost J, Custer LM. Rapid onset of cutaneous anesthesia with EMLA cream after pretreatment with a new ultrasound-emitting device. Anesth Analg. 2004;98:371–76. doi: 10.1213/01.ANE.0000099716.02783.C4. [DOI] [PubMed] [Google Scholar]
- Kost J, Mitragotri S, Gabbay R, Pishko M, Langer R. Transdermal monitoring of glucose and other analytes using ultrasound. Nat Med. 2000;6:347–50. doi: 10.1038/73213. [DOI] [PubMed] [Google Scholar]
- Mitragotri S, Kost J. Low-frequency sonophoresis: A review. Advanced Drug Delivery Reviews. 2004;56:589–601. doi: 10.1016/j.addr.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Park EJ, Werner J, Smith NB. Ultrasound mediated transdermal insulin delivery in pigs using a lightweight transducer. Pharm Res. 2007;24:1396–401. doi: 10.1007/s11095-007-9306-4. [DOI] [PubMed] [Google Scholar]
- Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nature Reviews Drug Discovery. 2004;3:115–24. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- Tang H, Mitragotri S, Blankschtein D, Langer R. Theoretical description of transdermal transport of hydrophilic permeants: application to low-frequency sonophoresis. J Pharm Sci. 2001;90:545–68. doi: 10.1002/1520-6017(200105)90:5<545::aid-jps1012>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Tezel A, Sens A, Mitragotri S. Description of transdermal transport of hydrophilic solutes during low-frequency sonophoresis based on a modified porous pathway model. J Pharm Sci. 2003;92:381–93. doi: 10.1002/jps.10299. [DOI] [PubMed] [Google Scholar]
- Tezel A, Paliwal S, Shen Z, Mitragotri S. Low-frequency ultrasound as a transcutaneous immunization adjuvant. Vaccine. 2005;23:3800–07. doi: 10.1016/j.vaccine.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Wu J, Chappelow J, Yang J, Weimann L. Defects generated in human stratum corneum specimens by ultrasound. Ultrasound Med & Biol. 1998;24:705–10. doi: 10.1016/s0301-5629(98)00049-0. [DOI] [PubMed] [Google Scholar]


