Abstract
Vesicular glutamate transporters (VGLUTs) are responsible for vesicular glutamate storage and exocytotic glutamate release in neurons and astrocytes. Here we selectively and efficiently over-expressed individual VGLUT proteins (VGLUT1, 2 or 3) in solitary astrocytes and studied their effects on mechanical stimulation-induced Ca 2+- dependent glutamate release. Neither VGLUT1 nor VGLUT2 over-expression changed the amount of glutamate release, while over-expression of VGLUT3 significantly enhanced Ca 2+- dependent glutamate release from astrocytes. None of the VGLUT over-expression affected mechanically-induced intracellular Ca2+ increase. Inhibition of glutamine synthetase activity by L-methionine sulfoximine in astrocytes, which leads to increased cytosolic glutamate concentration, greatly increased their mechanically-induced Ca 2+- dependent glutamate release, without affecting intracellular Ca2+ dynamics. Taken together these data indicate that both VGLUT3 and the cytosolic concentration of glutamate are key limiting factors in regulating the Ca 2+- dependent release of glutamate from astrocytes.
Keywords: Ca 2+- dependent glutamate release, VGLUTs, glutamine synthetase, cytosolic glutamate concentration, MSO, signaling
Introduction
Transport of glutamate from the cytosol into secretory vesicles is necessary for vesicular glutamate release and is mediated by a group of vesicle membrane-bound proteins, the vesicular glutamate transporters. Three isoforms of VGLUTs (1, 2 and 3) have been cloned and characterized in the brain (Bellocchio et al. 1998; Fremeau et al. 2002; Fremeau et al. 2001; Gras et al. 2002; Takamori et al. 2000; Takamori et al. 2001). Differential centrifugation of synaptosomal extracts from the brain, in combination with immunoblotting analysis, indicates that VGLUTs are predominantly located on synaptic vesicles and co-sediment with other vesicle-associated synaptic proteins (Bellocchio et al. 1998; Fremeau et al. 2001; Takamori et al. 2000; Takamori et al. 2001). At the ultrastructural level, electron microscopy shows VGLUT localization on synaptic vesicles of asymmetric synapses (Bellocchio et al. 2000; Fremeau et al. 2001; Gras et al. 2002). Functionally, VGLUTs exhibit selective glutamate uptake activity dependent on the electrochemical proton gradient across the vesicular membrane (Bellocchio et al. 2000; Fremeau et al. 2001; Gras et al. 2002).
All three VGLUTs essentially account for all the glutamatergic synaptic transmission in the adult mammalian brain. Their distribution displays a complementary and mutually exclusive pattern of expression, suggesting that each protein may have a distinct role in the operation of the brain. VGLUT1 is expressed mainly by excitatory neurons in cerebral cortex, hippocampus and cerebellar cortex, while VGLUT2 is largely expressed in thalamus, hypothalamus and cerebellar nuclei (Bellocchio et al. 1998; Fremeau et al. 2001; Gras et al. 2002; Herzog et al. 2001). In addition to glutamatergic neurons, VGLUT2 has also been found in dopaminergic neurons and peptidergic neurons of the supraoptic nucleus (Dal Bo et al. 2004; Kawano et al. 2006; Ponzio et al. 2006). In contrast, VGLUT3 can be found in a small group of glutamatergic neurons in the brain (Fremeau et al. 2002; Gras et al. 2002; Seal et al. 2008), although its expression is usually within non-glutamatergic neurons such as cholinergic, serotoninergic, GABAergic, and peptidergic neurons (Fremeau et al. 2002; Gras et al. 2002; Ponzio et al. 2006). VGLUT3 has also be found in other organs, most notably in liver, kidneys and muscles (Boulland et al. 2002; Fremeau et al. 2002; Gras et al. 2002; Schafer et al. 2002). Interestingly, VGLUT expression may influence the phenotype of neurons. Targeted expression of VGLUT1 or 2 in GABAergic neurons, converted these neurons into glutamatergic neurons (Takamori et al. 2000; Takamori et al. 2001).
Indeed, VGLUTs may play a critical role in synaptic transmission. Genetic manipulations resulting in VGLUT1 knockout mice revealed that, during early development, a group of neurons in the hippocampus co-expressed VGLUT1 and 2 at distinct synaptic sites with different release properties (Fremeau et al. 2004). Moreover, while VGLUT2 expression was turned-off at a later time in development, VGLUT1 expression persisted. Deletion of the VGLUT1 gene resulted in a significant reduction in the amplitude of excitatory postsynaptic currents in hippocampal neurons (Fremeau et al. 2004; Wojcik et al. 2004), while the over-expression of VGLUT1 in glutamatergic neurons increased the amplitude of these currents (Wilson et al. 2005). More recently, it was found that mice with VGLUT3 gene deletion lacked synaptic transmission at the first synapse in the auditory pathway resulting in deafness (Seal et al. 2008).
Astrocytes can release glutamate in a Ca2+ -dependent manner (Parpura et al. 1994) and signal to adjacent neurons causing a variety of physiological consequences [reviewed in (Ni et al. 2007)]. As a functional component of the tripartite synapse, along with pre- and post-synaptic neuronal elements, astrocytes actively participate in synaptic transmission (Araque et al. 1999; Halassa et al. 2007). Here, vesicular/exocytotic release is employed by astrocytes to carry out their glutamate release. Indeed, all three isoforms of VGLUTs have been detected in astrocytes [reviewed in (Montana et al. 2006)]. These transporters are functional within astrocytes since Rose Bengal, a broad spectrum modulator of the allosteric site of VGLUTs, greatly reduced glutamate release (Montana et al. 2004). Interestingly, VGLUTs are developmentally regulated in astrocytes with a gradual age-dependent decrease in the expression of all three VGLUT isoforms in astrocytes from the visual cortices of rats (Montana et al. 2004). Hence, in freshly-isolated astrocytes originating from 1- to 2-day-old rat pups, there was a high likelihood that VGLUTs 1, 2, and 3 were co-expressed in a single cell. Although there was no direct demonstration of the presence of these proteins in single cells, the proportion of cells expressing individual proteins (86% for VGLUT 1, 99% for VGLUT 2 and 74% for VGLUT 3) supports this inference. Similarly, VGLUT protein expression in cultured astrocytes originating from 0-to 1-day-old animals was similar (each isoform in 94% of astrocytes), indicating that astrocytes in the culture system do not (de)differentiate (Montana et al. 2004).
To study the roles of VGLUT isoforms in exocytotic release of glutamate from astrocytes, we selectively over-expressed individual isoforms of VGLUT proteins in astrocytes and examined the effects on the amount of exocytotic glutamate release from these glial cells. Neither VGLUT1 nor VGLUT2 over-expression changed the amount of glutamate release, while over-expression of VGLUT3 significantly enhanced exocytotic glutamate release from astrocytes. Cytosolic glutamate concentration in astrocytes, estimated at 0.1–5 mM (Attwell et al. 1993), is lower than in neurons due to the presence of glutamine synthetase (GS), a major glutamate metabolizing enzyme concentrated in astrocytes (Norenberg and Martinez-Hernandez 1979). To address whether the availability of cytosolic glutamate for filling vesicles plays a role in exocytotic release of glutamate from astrocytes, we increased the cytosolic glutamate concentration by blocking GS activity with L-methionine sulfoximine (Weisbrod and Meister 1973). Inhibition of GS greatly increased exocytotic glutamate release. Taken together these data indicate that both VGLUT3 and cytosolic concentration of glutamate regulate vesicular release of glutamate from astrocytes. This finding points to a distinct role of VGLUT3 in packaging cytosolic glutamate into astrocytic secretory vesicles. Additionally, since astrocytic GS plays a role in several brain pathological conditions, such as hepatic encephalopathy [reviewed in (Butterworth 2008)] and epilepsy [reviewed in (Binder and Steinhäuser 2008)], the findings that the inhibition of this enzyme can lead to an increase of exocytotic glutamate release from astrocytes may represent a novel site for therapeutic intervention in these conditions. Some of these data have appeared in preliminary form (Ni and Parpura 2008).
Materials and Methods
Cell Culture
All procedures were in strict accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the University of Alabama Birmingham Institutional Animal Care and Use Committee. We prepared enriched astrocytic cultures from 0- to 2-day-old Sprague-Dawley rats as previously described (Hua et al. 2004; Montana et al. 2004). Briefly, dissociated cells were initially plated into tissue culture flasks and maintained at 36.8°C in a humidified 5% CO2/95% air atmosphere in a culture medium, that consisted of α-MEM (without phenol red) supplemented with fetal bovine serum (10% v/v; Hyclone, Logan, UT), L-glutamine (2 mM), D-glucose (20 mM), sodium pyruvate (1 mM), penicillin (100 I.U./ml), streptomycin (100 μg/ml), and sodium bicarbonate (14 mM)(pH=7.4). After 5–19 days in culture, purified astrocytes were obtained using a shaking procedure described elsewhere (Montana et al. 2004). The cells that remained attached to the bottom of the flask were re-plated onto 12 mm (in diameter) round glass coverslips pre-coated with polyethyleneimine (1mg/mL). Four coverslips seeded with purified cells were inlayed in each 35 mm (in diameter) culture dish, and kept in culture for 1–7 days until used in experiments. A subset of cultured astrocytes was treated with L-methionine sulfoximine (MSO; Sigma-Aldrich) for 4–9 h, before being used in experiments. The purity (>99%) of astrocytic culture was confirmed by two methods: (i) by anti-glial fibrillary acidic protein antibody and indirect immunocytochemistry; and (ii) by visualization of accumulation of the fluorescently conjugated dipeptide β-Ala-Lys, as previously described (Montana et al. 2004).
Transfection
We transfected purified astrocytes grown in culture dishes as previously described (Montana et al. 2004). The transfection mixture (less than 120 μL), containing a transfection reagent (6 μl/dish, TransIT-293, Mirus, Madison, WI) and (co)complexed plasmid(s), encoding the individual VGLUT isoforms (2 μg/dish) and enhanced green fluorescent protein (EGFP; 1 μg/dish), was applied into a culture dish populated with astrocytes bathed in culture medium (1 mL). Following incubation (3.5 hours at 36.8°C in a 5% CO2/95% air atmosphere incubator) and wash out of transfection agent, cells were returned to the incubator for 2–4 days, until they were used in experiments. Based on the EGFP expression ~18% of astrocytes were successfully transfected using this procedure. Plasmids (pcDNA3) encoding for VGLUT1 (Herzog et al. 2001), VGLUT2 and VGLUT3 (Gras et al. 2002) were provided by Dr. Salah El Mestikawy, INSERM U513, Créteil Cedex, France. Plasmid (pcDNA3) encoding EFGP was provided by Dr. Philip G. Haydon, Tufts University, Boston.
RNA interference
A target sequence of 23 nucleotides in length beginning with AA and ending with TT contained within the coding sequence region of genes were selected using VectorNTI software. Three target sequences were specified for each VGLUT isoform.
VGLUT1: 5′-AACTTTTGCCGCAGCTGGACTTT-3′, 5′-AAATTCGCAGCCAACAGGGTCTT-3′ and 5′-AAAGCCCAGTTCAACTGGGATTT-3′
VGLUT 2: 5′-AACCAGCTTATTTCGAGGAGGTT-3′, 5′-AAACTTCTGCAGGAGTTGGACTT-3′ and 5′-AAGCCACACTGCTTCTGGTTGTT-3′
VGLUT 3; 5′-AACCCTGAACATGTTCATCCCTT-3′, 5′-AAGTCGTCTAGCCACGGCCTCTT-3′ and 5′-AACAGAACTCAACCACGAGGCTT-3′
For EGFP we designed siRNA using its 5′-AAGACCCGCGCCGAGGTGAAGTT-3′ sequence. In addition to using above described AA(N19)TT template approach, we also used the online siRNA design service (Qiagen; Valencia, CA) to obtain two more targeted sequences for VGLUT 1: 5′-TGGAGGATTTATCTGCCAAAA-3′ and 5′-GTGGCTACCTCCACCCTAAAT-3′. Annealed double stranded RNA with over-hangs based on each of the target sequences listed above were purchased from Qiagen. This siRNA was co-applied with plasmids encoding the individual VGLUT isoforms and EGFP (or in some cases EGFP alone) in the transfection mixture and then followed the transfection protocol outlined above; the final concentration of siRNA in each dish was 90–100 nM. When assessing the efficacy of interference targeting the EGFP gene, EGFP siRNA was co-applied with the mixture of plasmids, pEGFP and pDsRed at 1:1 ratio (Clontech, Mountain View, CA). Validity of this method for co-delivery of siRNA and DNA was assessed using Alexa Fluor® 488 tagged siRNA (Qiagen; Catalog No. 102-25-63) and the plasmid encoding DsRed. Based on fluorescence detection, siRNA was delivered to all astrocytes and retained intracellulary throughout duration of experiments (2–4 days); thus this method shows 100% efficacy in delivery of siRNA, far exceeding DNA delivery (~18%) into astrocytes.
Immunocytochemistry
Astrocytes were fixed with paraformaldehyde (4% w/v, 30 min, room temperature). Rabbit polyclonal antibodies against three VGLUT isoforms, previously characterized for their use in astrocytes (Montana et al. 2004), were utilized: anti-VGLUT1 [(Bellocchio et al. 1998), provided by Dr. Robert H. Edwards, University of California, San Francisco, 1:1000 dilution; or Synaptic Systems, Goettingen, Germany, cat. No. 135 002, 1: 500 dilution]; anti-VGLUT2 (Synaptic Systems, cat. No. 135 102, 1:500 dilution); anti-VGLUT3 [(Fremeau et al. 2002), provided by Dr. Robert H. Edwards, 1:1000 dilution). After washout of the primary antibody, cells were incubated with TRITC conjugated secondary antibody (1 hour at room temperature). In all experiments, we performed controls in which primary antibodies were omitted to test for non-specific binding of secondary antibodies. All imaging data were background subtracted using fluorescence emission from a region of the coverslip containing no cells. Immunoreactivity for individual VGLUTs and expression of EGFP were measured as the average fluorescence intensity for a probed protein, within astrocytic somata. For detailed quantitative assessment of individual VGLUT (over)expression we additionally generated the ratio of VGLUT over EGFP fluorescence emissions for each cell. Ratio is expressed as a mean ± SEM.
Glutamate measurements
We optically monitored extracellular glutamate concentrations using an L-glutamate dehydrogenase (GDH)-linked assay (Hua et al. 2004; Innocenti et al. 2000). GDH generates NADH from NAD+ in presence of glutamate, which released into the extracellular space can be detected as an increase in NADH fluorescence. Astrocytes were bathed in an enzymatic assay solution containing external solution supplemented with NAD+ (1 mM; Sigma-Aldrich, cat. No. N6522) and GDH (~53–137 I.U./ml; Sigma-Aldrich, cat. No. G2626) (pH=7.4). External solution contained (in mM) 140 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 5 glucose and 10 HEPES (pH 7.4). Every experiment was preceded by a sham run on astrocytes bathed in solution lacking GDH and NAD+, which was used to correct for photo-bleaching and background subtraction. All imaging data, corrected for photo-bleaching and background subtraction, were expressed as dF/Fo (%), in which dF represents the change of fluorescence, while Fo represents the fluorescence level surrounding solitary astrocytes at rest. This allowed day-to-day comparisons between experiments acquired with the same lot of enzyme. Due to the variability of GDH activity in the different lots of enzymes, however, in a subset of experiments we additionally normalized dF/Fo. Glutamate release from individual cells was ranked within the experimental groups and then expressed as ratio of the dF/Fo responses in the tested over rank-matched cells in the control group. Ratio is expressed as a mean ± SEM.
Ca2+ measurements
The intracellular Ca2+ levels in solitary astrocytes were recorded using the Ca2+ indicator X-rhod-1 (Invitrogen). Astrocytes were loaded with acetoxymethyl (AM) form of X-rhod-1 (1μg/ml), facilitated by pluronic acid (0.025% w/v; Invitrogen), in external solution for 20 min at room temperature. After wash, the indicator was permitted to de-esterify for 20 minutes at room temperature in external solution before experiments. All imaging data were background subtracted using regions of the coverslip field containing no cells. Data are expressed as dF/Fo ± SEM (%) in which dF represents the change of fluorescence, while Fo represents the fluorescence of cells at rest.
Imaging acquisition and processing
All experiments were done at room temperature (20–24°C) using an inverted microscope (TE 300; Nikon, Melville, NY). Visualization of indirect immunocytochemistry of VGLUTs was accomplished using a standard rhodamine/TRITC filter set, whereas for EFGP and Alexa Fluor® 488 conjugated to siRNA we used a standard fluorescein/FITC filter set. X-rhod-1 and DsRed fluorescence were visualized using a standard Texas Red filter set. Images were captured through a 60x PlanApo oil-immersion objective using a CoolSNAP-HQ cooled charge-coupled device (CCD) camera (Photometrics, Tucson, AZ) driven by V++ imaging software (Digital Optics, Auckland, New Zealand). For glutamate imaging experiments, we used a 40x SFluor objective and a DAPI filter set. All filter sets were from Chroma Technology, Rockingham, VT. For time-lapse image acquisition, a camera and an electronic shutter (Vincent Associates, Rochester, NY) inserted in the excitation pathway were controlled by software. A Xenon arc lamp (100 W) was used as a light source. All images shown in figures represent raw data with their pixel intensities within the camera’s (CoolSNAP-HQ) dynamic range (0–4095).
Stimulation of astrocytes
To evoke an increase in the internal Ca2+ concentrations in astrocytes and consequential glutamate release, we mechanically stimulated astrocytes using patch pipettes (Araque et al. 2000; Parpura et al. 1994). To control the establishment of the contact between the pipette and an astrocyte we monitored pipette resistance (Hua et al. 2004). When pipette tips were immersed in external solution, their resistances measured 1.7–1.9 MΩ, which increased to 2.0–2.2 MΩ (15–22 % increase) during transient contacts with astrocytes, lasting less than one second.
Results
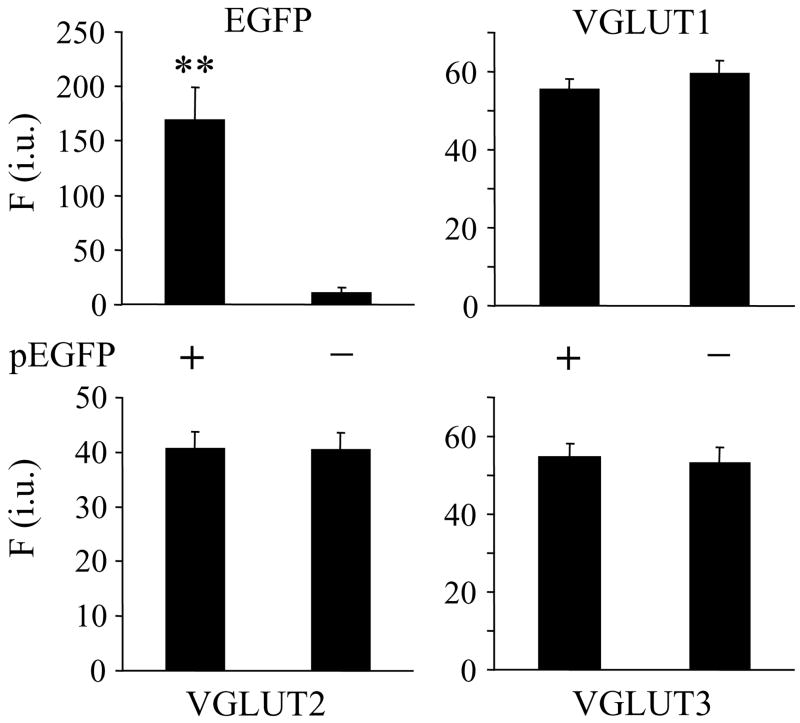
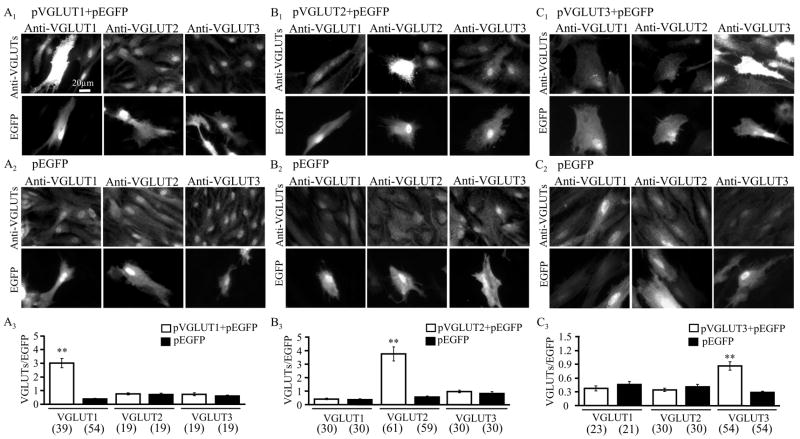
Cultured as well as freshly-isolated astrocytes originating from visual cortices of newborn rat pups co-express VGLUTs 1, 2 and 3 in a single cell (Montana et al. 2004). Pharmacological inhibition of VGLUTs in astrocytes greatly reduces the agonist- and mechanically-induced glutamate release from astrocytes without affecting their Ca2+ elevations, indicating that VGLUTs mediate exocytotic release from astrocytes (Montana et al. 2004). As a first step towards addressing the role of individual VGLUT isoforms in vesicular glutamate release from astrocytes, we selectively over-expressed each VGLUT isoform in cultured astrocytes. We co-transfected purified astrocytic cultures with two plasmids, one encoding for an individual VGLUT isoform (pVGLUT) and another for EGFP (pEGFP) at a 2:1 ratio. As a control for the transfection procedure, we also transfected astrocytes with pEGFP alone. In astrocytes expressing EGFP alone, the labeling against VGLUT isoforms displayed similar levels of expression to those seen for all three VGLUTs in untransfected astrocytes (Figure 1). Having determined that EGFP does not affect endogenous VGLUT expression, we used EGFP as an expression marker to identify transfected cells and quantify VGLUT (over)expression. We examined the expression levels of all three VGLUTs in astrocytes as a consequence of introduction of only one VGLUT isoform gene (Figure 2). Using indirect immunocytochemistry to label astrocytes with antibodies directed against each VGLUT isoform, we found that astrocytes transfected to over-express VGLUT 1, 2 or 3 along with co-expressing EGFP showed qualitatively higher expression levels of the respective VGLUT isoforms than astrocytes expressing EGFP alone (Figure 2A1 – C1). To quantify the apparent isoform-specific over-expression of VGLUTs in astrocytes, we used a ratiometric approach. We calculated the ratio of VGLUT (TRITC) over EGFP (FITC) fluorescence emissions for each cell (Figure 2A3 – C3). Based on such fluorescence measurements VGLUT1 expression was increased by ~ 7.5 fold when astrocytes were co-transfected with pVGLUT1+pEGFP as compared to group-matched transfection with pEGFP alone (Figure 2A3; Student t-test, p<0.01), while VGLUT 2 and 3 expression levels remained unaltered when compared to their matching controls. Similarly, only the expression level of VGLUT2, but not VGLUT 1 and 3, was enhanced by ~ 6.8-fold (Student t-test, p<0.01) in pVGLUT2+pEGFP co-transfected astrocytes when compared to matching controls transfected with pEGFP alone (Figure 2B3). Finally, pVGLUT3+pEGFP co-transfection in astrocytes specifically increased the expression level of VGLUT3 by ~ 3-fold (Student t-test, p<0.01), but not of the other two VGLUT isoforms (Figure 2C3). Taken together these data indicate that we can achieve the isoform specific over-expression of each VGLUT along with the co-expression of a fluorescent marker which can be used for visualization in live cells, both features being prerequisites to further study the effects of the expressed gene products on exocytotic release of glutamate from astrocytes.
Figure 1.
Expression of the fluorescent marker EGFP does not affect endogenous expression of VGLUTs in astrocytes. Astrocytes transfected with a plasmid encoding EGFP (pEGFP +) were identified based on the fluorescence of this protein. Expression of EGFP does not affect expression VGLUT1, 2, or 3, as assessed by indirect immunocytochemistry. Bars represent mean ± SEM of fluorescence intensity (F) measurements from individual astrocytes (each condition contains 30 cells) expressed as intensity units (i.u.). Asterisks indicate a significant increase in fluorescence intensity (Student t-test, p<0.01) as a consequence of EGFP expression in transfected cells when compared to control (un-transfected; pEGFP -) matching astrocytes that did not receive the plasmid.
Figure 2.
VGLUT isoform-specific over-expression in astrocytes. Astrocytes co-transfected with plasmids encoding for a single VGLUT gene and enhanced green fluorescent protein (EGFP) (pVGLUT+pEGFP), but not astrocytes transfected with plasmid encoding for EGFP (pEGFP) alone, selectively over-expressed a single VGLUT isoform. (A1) Astrocytes successfully co-transfected with pVGLUT1+pEGFP were identified by their EGFP expression. Immunocytochemical labeling of astrocytes with antibodies against each VGLUT isoform (anti-VGLUT1, anti-VGLUT2, or anti-VGLUT3) indicated that the transfected astrocytes had much higher expression of the specifically over-expressed VGLUT (A1, VGLUT1; B1, VGLU2; C1, VGLUT3) than the other two VGLUTs, which appeared qualitatively similar to neighboring un-transfected astrocytes. All three VGLUTs exhibited a qualitatively similar level of expression in astrocytes expressing EGFP alone as in neighboring un-transfected astrocytes (A2–C2). (A3–C3) Quantitative assessment of the isoform-specific over-expression. Comparison of the ratio of individual VGLUT (TRITC) fluorescence intensity to EGFP (FITC) fluorescence intensity (VGLUT/EGFP) between pVGLUT+pEGFP co-transfected (open bars) astrocytes and matching astrocytes transfected with pEGFP alone (black bars). Bars in the graphs represent mean ± SEM of measurements from individual astrocytes (numbers in parentheses). Asterisks indicate a significant change in measurement when compared with matching astrocytes expressing EGFP alone (Student t-test; ** p<0.01). Scale bar, 20 μm.
We used solitary astrocytes and mechanical stimulation to asses the role of VGLUT isoforms in Ca2+-dependent exocytotic glutamate release. Solitary astrocytes have been used to minimize the effects of intercellular astrocytic communication that could affect calcium ion and glutamate measurements (Hua et al. 2004; Malarkey et al. 2008; Montana et al. 2004). Mechanical stimulation of astrocytes causes Ca2+ -dependent glutamate release that can be inhibited by buffering cytoplasmic Ca2+ with the membrane permeable Ca2+ chelator, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester (BAPTA-AM) (Hua et al. 2004; Innocenti et al. 2000; Montana et al. 2004). This mechanically-induced Ca2+ -dependent glutamate release can also be blocked bafilomycin A1, a vacuolar-type H+-ATPase inhibitor, Clostridial tetanus and botulinum type B toxins which specifically cleave synaptobrevin 2, and Rose Bengal, a VGLUT inhibitor (Araque et al. 2000; Hua et al. 2004; Montana et al. 2004); thus, a vesicular mechanism underlies mechanically-induced glutamate release from astrocytes. Almost all astrocytes (94% express each isoform) in culture originating from newborn rats endogenously express all three isoforms of VGLUTs (Montana et al. 2004).
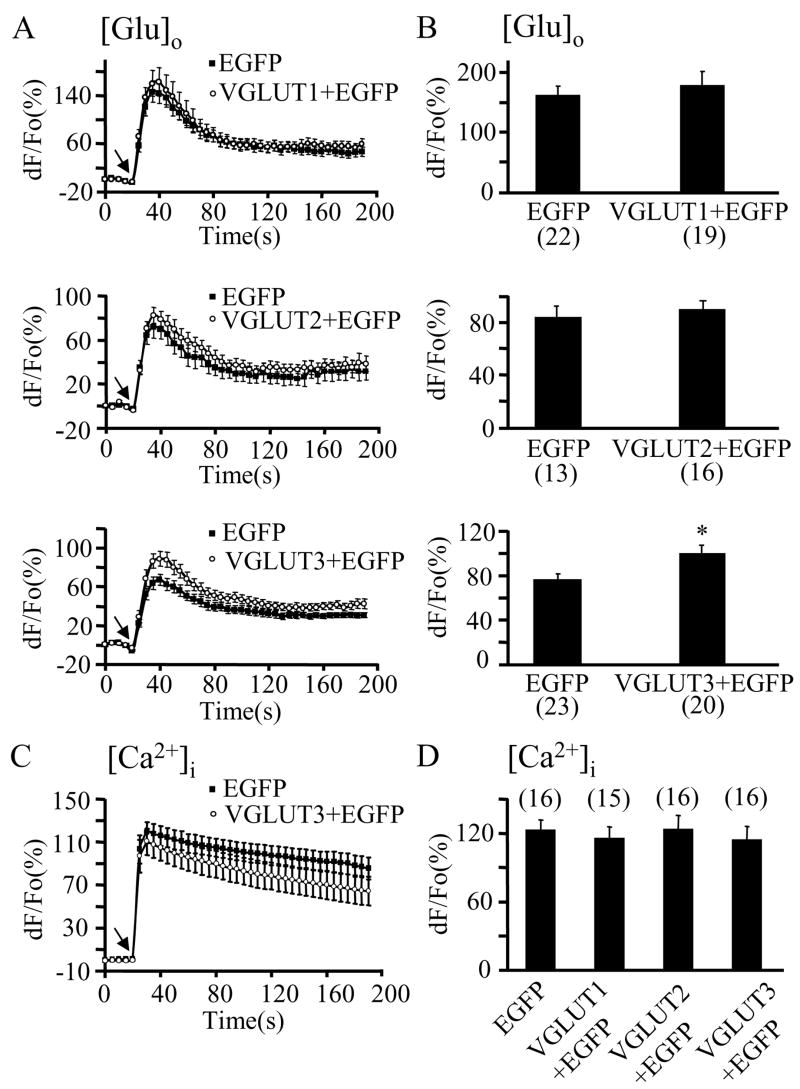
To investigate the effects of isoform-specific VGLUT over-expression on vesicular glutamate release from astrocytes, we located the astrocytes of interest based on their EGFP expression. Solitary astrocytes were then mechanically stimulated while measuring extracellular glutamate concentration changes using an enzyme-linked assay (Innocenti et al. 2000). The time-courses of mechanically-induced glutamate release from astrocytes and their peak values indicated that over-expressing VGLUT 1 or 2 along with EGFP did not significantly affect glutamate release from astrocytes (Figure 3A–B, top and middle). However, astrocytes over-expressing VGLUT3, along with the transfection marker EGFP, displayed a significant increase in glutamate release (~35 %; Student t-test, p<0.05) when compared to astrocytes expressing EGFP alone (Figure 3A,B; bottom).
Figure 3.
Over-expression of VGLUT3, but not VGLUT1 or VGLUT2, regulates glutamate release. A-B) Mechanical stimulation evokes the release of glutamate into the extracellular space ([Glu]o) caused by intracellular Ca2+ elevations (Ca2+]i) in astrocytes (C–D). A) Time lapse of NADH fluorescence, reporting on glutamate release from solitary astrocytes either transfected with pEGFP alone (black squares) or co-transfected with pEGFP+pVGLUT1, 2, or 3 (top, middle, or bottom, respectively; open circles). Mechanical stimulation caused glutamate release that was significantly augmented in cells over-expressing VGLUT3 (open circles; bottom graph). Over-expression of VGLUT1 or VGLUT2 did not significantly affect the astrocytic ability to release glutamate (top and middle). B) Peak values of mechanically-induced glutamate release. Changes in NADH fluorescence are shown as dF/Fo (%) after background subtraction and correction for bleaching. C–D) In experiments parallel to those in A–B), astrocytes where mechanically stimulated, while measuring intracellular Ca2+ levels using X-rhod-1. B) Time lapse of X-rhod-1 fluorescence, reporting on Ca2+ levels in astrocytes. Mechanical stimulation caused increases in astrocytic intracellular Ca2+ levels (black squares) that were unaffected when cells were co-expressing VGLUT3 (open circles). Only the time-course for VGLUT3 over-expression is shown for simplicity. D) Peak values of mechanically-induced Ca2+ elevations were not affected when astrocytes over-expressed VGLUT isoforms in addition to EGFP. Changes in X-rhod-1 fluorescence are expressed as dF/Fo (%) after background subtraction. Arrows (A,C) indicate the time when the pipette-astrocyte contact occurred. Points and bars represent means and SEMs of measurements from solitary astrocytes (numbers in parentheses). Asterisks indicate significant change in measurements as compared with the control group (cells expressing EGFP alone; Student t-test; * p<0.05).
Since intracellular Ca2+ increases are a pre-requisite for VGLUT-dependent exocytotic release of glutamate from astrocytes, we tested the preservation of intracellular Ca2+ dynamics in astrocytes over-expressing VGLUT isoforms. We monitored the intracellular Ca2+ levels using the Ca2+ indicator X-rhod-1. The levels of mechanically-induced Ca2+ elevations in astrocytes were not affected by VGLUT over-expression (Figure 3C, D; one way ANOVA, p(3,59)=0.9). These data indicate that only the isoform VGLUT3 is likely a rate-limiting factor in the regulation of exocytotic release of glutamate from astrocytes.
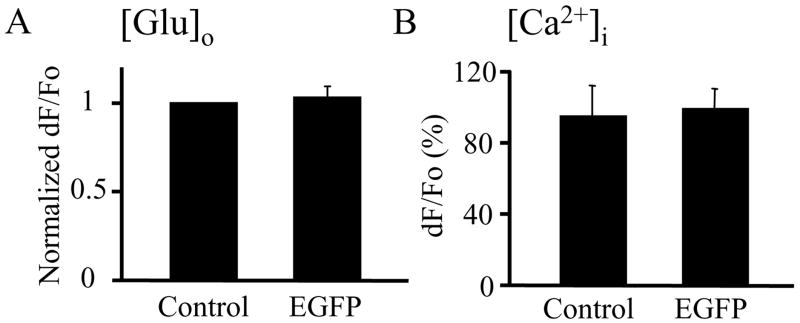
Since astrocytes over-expressing VGLUT gene were co-expressing EGFP, we performed an additional set of control experiments to address whether the expression of EGFP alone affected astrocytic glutamate release and Ca2+ dynamics. Mechanically-induced glutamate release from un-transfected (control) astrocytes showed similar peak values to those of EGFP-expressing solitary astrocytes (Figure 4A). Similarly, the Ca2+ dynamics and peak Ca2+ elevation in response to mechanical stimulation were unaltered by the expression of EGFP (Figure 4B). Thus, EGFP expression in astrocytes does not change the exocytotic glutamate release and Ca2+ response evoked by mechanical stimulation. Taken together, these data indicate that VGLUT3 over-expression in astrocytes augments exocytotic glutamate release without affecting intracellular Ca2+ dynamics.
Figure 4.
Expression of EGFP in astrocytes does not change their ability to release glutamate and display Ca2+ dynamics in response to mechanical stimulation. A) The normalized peak dF/Fo values of glutamate release into the extracellular space ([Glu]o) were similar between control (un-transfected) and EGFP-expressing solitary astrocytes (each group n=12). B). Peak values of mechanically-induced astrocytic Ca2+ increases ([Ca2+]i) expressed as dF/Fo(%) indicate no difference between the un-transfected and EGFP expressing solitary astrocytes (each group n=8). Bars in the graphs represent mean ± SEM.
To further address the role of VGLUT isoforms in glutamate release from astrocytes, we used an RNA interference approach in an attempt to acutely knock-down VGLUT expression. We initially tested our siRNA approach using ratiometric imaging of EGPF (FITC) and DsRed (TexasRed), which were co-expressed in astrocytes. Based on such fluorescence measurements, the EGFP expression was decreased by 41% when astrocytes were treated with siRNA targeting EGFP gene (n=82; EGFP/DsRed= 1.1 ± 0.2) when compared to their matching controls (n= 81; EGFP/DsRed=1.9 ± 0.2; Student t-test, p<0.05). Next we applied siRNA targeting individual VGLUT isoforms. As in Figure 2, we used immunocytochemistry of individual VGLUTs (TRITC) and the expression of EGFP (FITC), to generate VGLUT/EGFP ratio. Based on this ratiometric approach none of siRNA (5 for VGLUT1, 3 for VGLUT2 and 3 for VGLUT3; see Materials and Methods) caused significant reduction of any of VGLUT isoforms within 2–4 days of treatment when compared to their matching controls (Student t-tests, p= 0.41 to 0.82; each group containing between 15–59 astrocytes). This finding, combined with success in down-regulating EGFP, may indicate that VGLUTs are stable proteins based on results showing unchanged expression of individual VGLUTs 2–4 days (in some cases up to 14 days) after siRNA treatment.
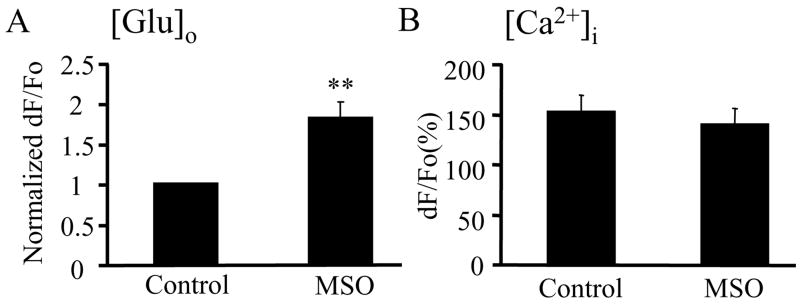
Having determined that VGLUT3, at heightened levels of expression, is a key regulatory component of glutamate release via exocytotic pathways in astrocytes, we addressed whether cytosolic glutamate, which VGLUTs use to fill vesicles, regulates glutamate release as well. The cytosolic glutamate concentration in astrocytes is lower than neuronal due to the expression of GS and its activity in astrocytes. To increase the cytosolic glutamate concentration in astrocytes we blocked GS activity with L-methionine sulfoximine (MSO) (Weisbrod and Meister 1973). We examined the effects of this blockade on astrocytic exocytotic glutamate release. The pre-treatment of astrocytes with MSO (1.5mM; 36.8°C; 4–9 h) greatly increased the exocytotic glutamate release in response to mechanical stimulation when compared to the release from untreated astrocytes in the control group (Mann-Whitney U-test, p<0.01) (Figure 5A), while Ca2+ dynamics were unaffected (Figure 5B). Unfortunately, co-expression of individual isoforms of VGLUT 2 or 3 and EGFP in astrocytes when combined with MSO treatment caused a significant reduction in the intracellular Ca2+ increases upon stimulation, thus hampering any interpretation of glutamate release data in such conditions (One-way ANOVA followed by Fisher’s least significant difference (LSD) test; **p<0.01). The exception was VGLUT1+EGFP co-expression followed by MSO treatment, where astrocytes showed only a trend in the reduction of peak intracellular Ca2+ levels due to mechanical stimulation and a trend in the increase in the consequential glutamate release (data not shown). Nonetheless, the above findings using MSO to block GS activity implicate that the increase of cytosolic glutamate concentration provided more glutamate for undetermined isoforms of VGLUT to transport across the vesicular membrane into the vesicular lumen, thus increasing the amount of glutamate in vesicles.
Figure 5.
The availability of cytosolic glutamate contributes to the regulation of exocytotic glutamate release from astrocytes. A) Pre-treatment of astrocytes with L-methionine sulfoximine (MSO; 1.5mM; 36.8°C; 4–9 h) to block glutamine synthetase activity enhanced mechanically-induced glutamate release into the extracellular space (A; [Glu]o) without affecting intracellular Ca2+ dynamics (B; (Ca2+]i). Bars represent means and SEMs of measurements from solitary astrocytes (each group n=17 in A, while n=12 in B). Peak glutamate release is expressed as the normalized dF/Fo values, while peak Ca2+ increase is expressed as dF/Fo (%). Asterisks indicate a significant change in measurements as compared with the control group (untreated cells) (Mann-Whitney U-test, **p<0.01).
Discussion
Although neuronal and astrocytic populations of cells express all three VGLUTs, they may function differently in neurons and astrocytes. Some of the differences may originate from: (i) substantially different cytosolic concentrations of glutamate within these two types of cells, (ii) different protein expression and, consequently, the possibility for different protein-protein interactions, and (iii) the expression of all three isoforms of VGLUT in individual astrocytes isolated from newborn rats. It was found previously that over-expression of VGLUT1 in glutamatergic neurons increased the quantal release of glutamate from presynaptic terminals (Daniels et al. 2004; Wilson et al. 2005). Here we showed that over-expression of VGLUT3, but not VGLUT1 or 2, in astrocytes endogenously expressing all three VGLUT isoforms, greatly enhanced their exocytotic glutamate release. Although our over-expression method increased VGLUT1 and 2 expression by ~ 7-fold, neither of these proteins affected the amount of exocytotic glutamate release from the astrocytes. In contrast, VGLUT3 over-expressed by ~3-fold significantly enhanced (~35 %) glutamate release from astrocytes.
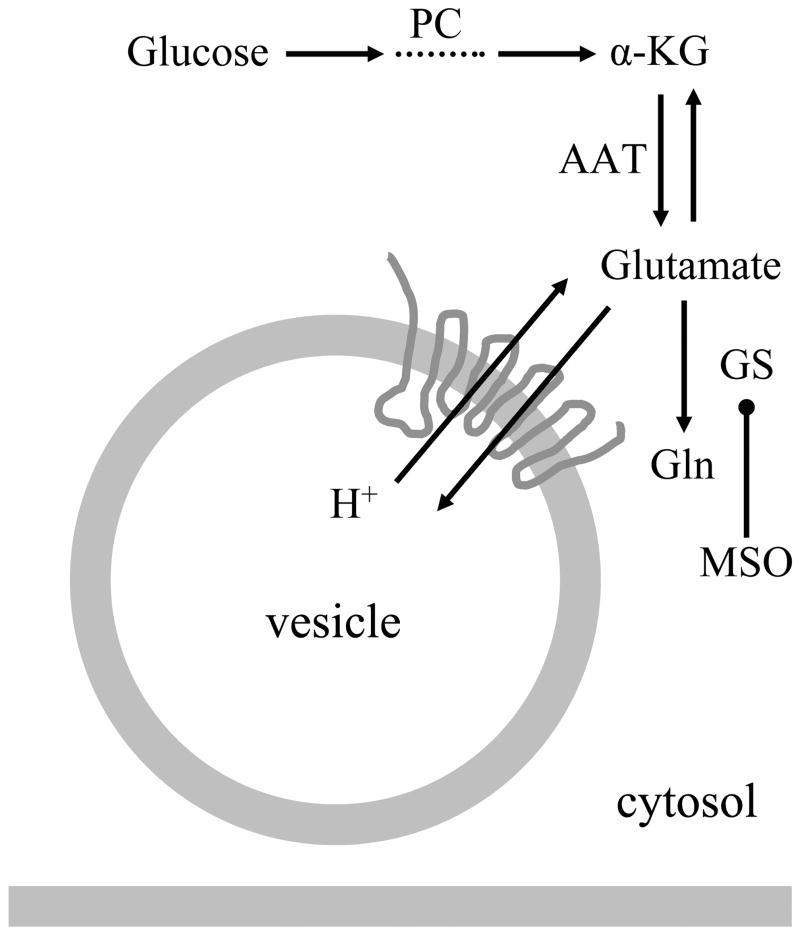
The cytosolic glutamate concentrations in neurons and astrocytes are differentially maintained [for reviews see (Danbolt 2001; Montana et al. 2006)]. In the cytosol of synaptic terminals, the glutamate concentration reaches 10–15 mM (Attwell et al. 1993), while astrocytic cytosol, due to the presence of GS, has a lower glutamate concentration at 0.1–5 mM (Attwell et al. 1993). Such cytosolic glutamate concentrations would allow VGLUTs (Km ~0.6–4.7 mM) (Bellocchio et al. 2000; Fremeau et al. 2002; Fremeau et al. 2001; Gras et al. 2002; Herzog et al. 2001) to operate at higher rates to concentrate glutamate into synaptic vesicles than in astrocytic vesicles, reaching an estimated intravesicular glutamate concentration of ~60 mM in neurons and ~ 20 mM in astrocytes [reviewed in (Montana et al. 2006)]. The Michaelis-Menten kinetics indicates that VGLUT3 has the lowest values for Km and Vmax among all three VGLUTs. Hence, Km and Vmax for VGLUT3 were 0.56 mM and 19 pmol/mg protein/min (Gras et al. 2002), while for VGLUT1 they measured 3.4 mM and 500 pmol/mg protein/min and for VGLUT2 1.9 mM and 470 pmol/mg protein/min (Herzog et al. 2001); it should be noted that we used here results from the same research group for direct comparison. Thus, VGLUT3 should fill vesicles more efficiently than the other two VGLUTs under the cytosolic glutamate concentrations found in astrocytes, which is consistent with our results that overexpressing VGLUT3, but not VGLUT1 or 2, increases the exocytotic release of glutamate from astrocytes. Taken together, the level of VGLUT3 expression appears to be a major limiting factor in regulating the amount of exocytotic glutamate release from astrocytes (Figure 6).
Figure 6.
Regulation of glutamate in exocytotic glutamate release from astrocytes. Glutamate can be synthesized in astrocytes de novo from glucose entry to the tricarboxylic acid cycle via pyruvate carboxylase (PC). Glutamate is converted from the cycle intermediate, α-ketoglutarate (α-KG), usually by transamination of aspartate via aspartate amino transferase (AAT). The synthesized glutamate once in the cytosol can then be converted to glutamine (Gln) by glutamine synthetase (GS), or transported into vesicles via proton-dependent vesicular glutamate transporters (VGLUTs), especially isoform 3 (VGLUT3). Pharmacological inhibition of GS activity with L-methionine sulfoximine L-methionine sulfoximine (MSO) can be used to increase the cytosolic glutamate concentration in astrocytes.
Besides the level of VGLUT3 expression in astrocytes, the low cytosolic glutamate concentration in these cells is another limiting factor in regulating exocytotic glutamate release from astrocytes. While astrocytes can synthesize glutamate de novo from glucose entry into the tricarboxylic acid cycle via pyruvate carboxylase, the levels of glutamate in the cytosol are lower than in neurons due to the powerful conversion of glutamate to glutamine by the action of GS expressed in astrocytes (Figure 6). The increase of cytosolic glutamate concentration in astrocytes because of pharmacological inhibition of GS with MSO resulted in enhanced (by ~82%) exocytotic release of glutamate from these glial cells, indicating that the availability of cytosolic glutamate directly affected vesicular glutamate storage and release from astrocytes. This finding may have consequences for both the physiology and pathophysiology in the brain.
The consequences of astrocytic Ca2+-dependent exocytotic glutamate release on neurons has been observed in multiple studies: an elevation of neuronal [Ca2+]i, a slow inward current, an increase of excitability, modulation of synaptic transmission, synchronization of synaptic events, or some combination of these [reviewed in (Ni et al. 2007)]. Since such effects have been observed in neurons of different brain regions, this signaling pathway might be a wide-spread phenomenon throughout the brain with astrocytes having a modulatory role in many functions of the CNS. Increased concentration of cytosolic glutamate resulting in increased glutamate release from astrocytes could affect any of the outcomes of the glutamate-mediated astrocyte-neuron signaling pathway. This notion may be evident in the finding that GS can be down-regulated in time of female puberty [(Roth et al. 2006); also reviewed in (Lomniczi and Ojeda 2008). Namely, mammalian puberty requires activation of luteinizing hormone-releasing hormone (LHRH) neurons of hypothalamus. Underlying changes in astrocytic glutamate metabolism, i.e., reduction of GS expression, in puberty lead to increased excitatory input to the LHRH neurons. Thus, this glutamate-mediated astrocyte-neuron signaling, enhanced by increased astrocytic cytosolic concentration, contributes to pubertal activation of LHRH secretion required for sexual development. Whether an exocytotic mechanism, as opposed to other mechanisms, of glutamate release from astrocytes mediates this signaling awaits further experimentation.
In several pathophysiological conditions there have been reports of changes of GS expression/activity and cytosolic glutamate levels in astrocytes. For example, in ischemia, the cytosolic glutamate concentration in rat hippocampal astrocytes increased significantly perhaps due to their inability to convert glutamate to glutamine (Torp et al. 1993). During ischemia, most cells experience swelling and can compensate for this volume increase by opening volume-regulated anion channels (VRAC) [reviewed in (Kimelberg et al. 2006)], which are permeable to glutamate (and aspartate, among other organic anions) (Mongin and Orlov 2001) and can lead to release of this amino acid (Kimelberg et al. 1990). Perturbed ionic conditions (e.g. increased extracellular K+ levels) that occur during ischemia can also lead to the release of glutamate by the reversal of the plasma membrane glutamate transporters (Rossi et al. 2000; Seki et al. 1999). The possible contribution of vesicular release of astrocytes have not been established in these conditions, however, release of aspartate via VRACs can be modulated in a Ca2+-dependent manner (Mongin and Kimelberg 2005).
In patients with mesial temporal lobe epilepsy there is a pronounced loss of GS in the hippocampus (Eid et al. 2004). A rat kainate model of temporal lobe epilepsy revealed that dynamics in GS expression are more complex than just a simple loss of this enzyme. Nonetheless, the reduction in GS expression underlie the susceptibility of animals to seizures (Hammer et al. 2008). Indeed, recent lines of evidence suggest the involvement of astrocytic Ca2+ dynamics and consequential glutamate release in epilepsy [reviewed in (Binder and Steinhäuser 2008; Reyes and Parpura 2008).
The studies of GS expression in a range of human brain tumors implicate that the expression level of GS decreases as the malignancy of the tumor increases (Pilkington and Lantos 1982). Glutamate excitotoxicity has long been known to contribute to the pathology of aggressive gliomas [reviewed in (Sontheimer 2008)]. In these tumors, glutamate is mainly released via the plasma membrane Na+-independent cystine-glutamate exchanger (system xc-). Since glioma cells can display intracellular Ca2+ dynamics (Lyons et al. 2007), they could also release glutamate in a Ca2+-dependent vesicular manner if they posses the appropriate secretory machinery.
Human immunodeficiency virus (HIV)1 coat protein gp120 can reduce the expression of GS and can also stimulate CXC Chemokine receptor 4 (CXCR4). Activation of these receptors caused release of tumor necrosis factor α that can then stimulate exocytotic glutamate release from astrocytes which subsequently acted on neuronal glutamate receptors (Bezzi et al. 2001). Thus, this signaling may have importance for HIV infections. In the central nervous system, only microglia are productively infected by HIV-1. Activated microglia can release HIV-1 coat protein gp120 (that can activate CXCR4 and also reduce GS expression) and lead to apoptotic neuronal death (Kaul et al. 2001). Thus, the outlined signaling pathway may provide fertile ground for therapeutic intervention in the prevention of dementia that can develop in HIV infected individuals as a result of neuronal loss. In addition, astrocytes play a key role in hepatic encephalopathy and the associated ammonia-induced neurotoxicity (Butterworth 2008). Ammonia can cause tyrosine nitration of, among other proteins, GS, which is associated with the loss of its enzymatic activity (Schliess et al. 2002). Additionally, ammonia can cause Ca2+-dependent exocytotic glutamate release in rat cortical astrocytes (Rose 2006; Rose et al. 2005).
As alluded to above, glutamate release from astrocytes can occur by several different mechanisms [reviewed in (Malarkey and Parpura 2008)]. Future work will be necessary to determine whether the same glutamate release mechanisms that operate under physiological conditions operate during pathophysiological conditions or whether there are specific release mechanisms that operate under particular conditions. An understanding of conditions that underlie glutamate release will provide information on astrocytcic functions in heath and disease and may also introduce opportunities for medical intervention. Our findings that VGLUT3 expression levels and cytosolic glutamate concentrations are limiting factors in regulation of exocytotic release of glutamate from astrocytes could serve as the initial step towards such a goal.
Acknowledgments
The authors’ work is supported by a grant from the National Institute of Mental Health (MH 069791). We thank Reno C. Reyes, Erik B. Malarkey and Randy F. Stout, Jr. for comments on previous versions of this manuscript; and William Lee for help with siRNA and data management.
Abbreviations
- BAPTA-AM
1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester
- GS
glutamine synthetase
- CXCR4
CXC Chemokine receptor 4
- EGFP
enhanced green fluorescent protein
- GDH
L-glutamate dehydrogenase
- HIV1
Human immunodeficiency virus 1
- MSO
L-methionine sulfoximine
- PBS
phosphate buffered saline
- VGLUT
Vesicular glutamate transporter
- VRAC
volume-regulated anion channels
References
- Araque A, Li N, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. J Neurosci. 2000;20(2):666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22(5):208–15. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11(3):401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Bellocchio EE, Hu H, Pohorille A, Chan J, Pickel VM, Edwards RH. The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission. J Neurosci. 1998;18(21):8648–59. doi: 10.1523/JNEUROSCI.18-21-08648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio EE, Reimer RJ, Fremeau RT, Jr, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289(5481):957–960. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, et al. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4(7):702–10. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Binder DK, Steinhäuser A. Role of astrocytes in epilepsy. In: Parpura V, Haydon PG, editors. Astrocytes in (patho)physiology of the nervous system. Boston, MA: Springer; 2008. [Google Scholar]
- Boulland JL, Osen KK, Levy LM, Danbolt NC, Edwards RH, Storm-Mathisen J, Chaudhry FA. Cell-specific expression of the glutamine transporter SN1 suggests differences in dependence on the glutamine cycle. Eur J Neurosci. 2002;15(10):1615–31. doi: 10.1046/j.1460-9568.2002.01995.x. [DOI] [PubMed] [Google Scholar]
- Butterworth RF. Hepatic encephalopathy: A primary astrocytopathy. In: Parpura V, Haydon PG, editors. Astrocytes in (patho)physiology of the nervous system. Boston, MA: Springer; 2008. [Google Scholar]
- Dal Bo G, St-Gelais F, Danik M, Williams S, Cotton M, Trudeau LE. Dopamine neurons in culture express VGLUT2 explaining their capacity to release glutamate at synapses in addition to dopamine. J Neurochem. 2004;88(6):1398–405. doi: 10.1046/j.1471-4159.2003.02277.x. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Daniels RW, Collins CA, Gelfand MV, Dant J, Brooks ES, Krantz DE, DiAntonio A. Increased expression of the Drosophila vesicular glutamate transporter leads to excess glutamate release and a compensatory decrease in quantal content. J Neurosci. 2004;24(46):10466–74. doi: 10.1523/JNEUROSCI.3001-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid T, Thomas MJ, Spencer DD, Runden-Pran E, Lai JC, Malthankar GV, Kim JH, Danbolt NC, Ottersen OP, de Lanerolle NC. Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet. 2004;363(9402):28–37. doi: 10.1016/s0140-6736(03)15166-5. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, et al. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci U S A. 2002;99(22):14488–93. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004;304(5678):1815–9. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31(2):247–60. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22(13):5442–51. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13(2):54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Hammer J, Alvestad S, Osen KK, Skare O, Sonnewald U, Ottersen OP. Expression of glutamine synthetase and glutamate dehydrogenase in the latent phase and chronic phase in the kainate model of temporal lobe epilepsy. Glia. 2008;56(8):856–68. doi: 10.1002/glia.20659. [DOI] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21(22):181RC-. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Malarkey EB, Sunjara V, Rosenwald SE, Li WH, Parpura V. Ca2+-dependent glutamate release involves two classes of endoplasmic reticulum Ca2+ stores in astrocytes. J Neurosci Res. 2004;76(1):86–97. doi: 10.1002/jnr.20061. [DOI] [PubMed] [Google Scholar]
- Innocenti B, Parpura V, Haydon PG. Imaging extracellular waves of glutamate during calcium signaling in cultured astrocytes. J Neurosci. 2000;20(5):1800–8. doi: 10.1523/JNEUROSCI.20-05-01800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–94. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, Nogami H, Hisano S. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J Comp Neurol. 2006;498(5):581–92. doi: 10.1002/cne.21054. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990;10(5):1583–91. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK, Macvicar BA, Sontheimer H. Anion channels in astrocytes: biophysics, pharmacology, and function. Glia. 2006;54(7):747–57. doi: 10.1002/glia.20423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi A, Ojeda SR. A role for glial cells of the neuroendocrine brain in the central control of female sexual development. In: Parpura V, Haydon PG, editors. Astrocytes in (patho)physiology of the nervous system. Boston, MA: Springer; 2008. [Google Scholar]
- Lyons SA, Chung WJ, Weaver AK, Ogunrinu T, Sontheimer H. Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res. 2007;67(19):9463–71. doi: 10.1158/0008-5472.CAN-07-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarkey EB, Ni Y, Parpura V. Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia. 2008;56(8):821–35. doi: 10.1002/glia.20656. [DOI] [PubMed] [Google Scholar]
- Malarkey EB, Parpura V. Mechanisms of transmitter release from astrocytes. In: Parpura V, Haydon PG, editors. Astrocytes in (patho)physiology of the nervous system. Boston, MA: Springer; 2008. [Google Scholar]
- Mongin AA, Kimelberg HK. ATP regulates anion channel-mediated organic osmolyte release from cultured rat astrocytes via multiple Ca2+-sensitive mechanisms. Am J Physiol Cell Physiol. 2005;288(1):C204–13. doi: 10.1152/ajpcell.00330.2004. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Orlov SN. Mechanisms of cell volume regulation and possible nature of the cell volume sensor. Pathophysiology. 2001;8(2):77–88. doi: 10.1016/s0928-4680(01)00074-8. [DOI] [PubMed] [Google Scholar]
- Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. Vesicular transmitter release from astrocytes. Glia. 2006;54(7):700–15. doi: 10.1002/glia.20367. [DOI] [PubMed] [Google Scholar]
- Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24(11):2633–42. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Y, Malarkey EB, Parpura V. Vesicular release of glutamate mediates bidirectional signaling between astrocytes and neurons. J Neurochem. 2007;103(4):1273–84. doi: 10.1111/j.1471-4159.2007.04864.x. [DOI] [PubMed] [Google Scholar]
- Ni Y, Parpura V. Regulation of exocytotic release of glutamate by vesicular glutamate transporters and cytoplasmic glutamate in astrocytes. Liver Int. 2008;28:744. [Google Scholar]
- Norenberg MD, Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979;161(2):303–10. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369(6483):744–7. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Pilkington GJ, Lantos PL. The role of glutamine synthetase in the diagnosis of cerebral tumours. Neuropathol Appl Neurobiol. 1982;8(3):227–36. doi: 10.1111/j.1365-2990.1982.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Ponzio TA, Ni Y, Montana V, Parpura V, Hatton GI. Vesicular glutamate transporter expression in supraoptic neurones suggests a glutamatergic phenotype. J Neuroendocrinol. 2006;18(4):253–65. doi: 10.1111/j.1365-2826.2006.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes RC, Parpura V. Models of astrocytic Ca2+ dynamics and epilepsy. Drug Discov Today Dis Models. 2008 doi: 10.1016/j.ddmod.2008.07.002. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose C. Effect of ammonia on astrocytic glutamate uptake/release mechanisms. J Neurochem. 2006;97 (Suppl 1):11–15. doi: 10.1111/j.1471-4159.2006.03796.x. [DOI] [PubMed] [Google Scholar]
- Rose C, Kresse W, Kettenmann H. Acute insult of ammonia leads to calcium-dependent glutamate release from cultured astrocytes, an effect of pH. J Biol Chem. 2005;280(22):20937–44. doi: 10.1074/jbc.M412448200. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403(6767):316–21. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Roth CL, McCormack AL, Lomniczi A, Mungenast AE, Ojeda SR. Quantitative proteomics identifies a change in glial glutamate metabolism at the time of female puberty. Mol Cell Endocrinol. 2006;254–255:51–9. doi: 10.1016/j.mce.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Schafer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem. 2002;277(52):50734–48. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- Schliess F, Gorg B, Fischer R, Desjardins P, Bidmon HJ, Herrmann A, Butterworth RF, Zilles K, Haussinger D. Ammonia induces MK-801-sensitive nitration and phosphorylation of protein tyrosine residues in rat astrocytes. Faseb J. 2002;16(7):739–41. doi: 10.1096/fj.01-0862fje. [DOI] [PubMed] [Google Scholar]
- Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Clause A, Kandler K, Noebels JL, Glowatzki E, et al. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57(2):263–75. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki Y, Feustel PJ, Keller RW, Jr, Tranmer BI, Kimelberg HK. Inhibition of ischemia-induced glutamate release in rat striatum by dihydrokinate and an anion channel blocker. Stroke. 1999;30(2):433–40. doi: 10.1161/01.str.30.2.433. [DOI] [PubMed] [Google Scholar]
- Sontheimer H. Role of ion channels and amino-acid transporters in the biology of astrocytic tumors. In: Parpura V, Haydon PG, editors. Astrocytes in (patho)physiology of the nervous system. Boston, MA: Springer; 2008. [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407(6801):189–94. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of differentiation-associated brain-specific phosphate transporter as a second vesicular glutamate transporter (VGLUT2) J Neurosci. 2001;2(22):RC182. doi: 10.1523/JNEUROSCI.21-22-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torp R, Arvin B, Le Peillet E, Chapman AG, Ottersen OP, Meldrum BS. Effect of ischaemia and reperfusion on the extra- and intracellular distribution of glutamate, glutamine, aspartate and GABA in the rat hippocampus, with a note on the effect of the sodium channel blocker BW1003C87. Exp Brain Res. 1993;96(3):365–76. doi: 10.1007/BF00234106. [DOI] [PubMed] [Google Scholar]
- Weisbrod RE, Meister A. Studies on glutamine synthetase from Escherichia coli. Formation of pyrrolidone carboxylate and inhibition by methionine sulfoximine. J Biol Chem. 1973;248(11):3997–4002. [PubMed] [Google Scholar]
- Wilson NR, Kang J, Hueske EV, Leung T, Varoqui H, Murnick JG, Erickson JD, Liu G. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci. 2005;25(26):6221–34. doi: 10.1523/JNEUROSCI.3003-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci U S A. 2004;101(18):7158–63. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]