Abstract
Oral high-dose glycine administration has been used as an adjuvant treatment for schizophrenia to enhance glutamate neurotransmission and mitigate glutamate system hypofunction thought to contribute to the disorder. Prior studies in schizophrenia subjects documented clinical improvements after 2 weeks of oral glycine administration, suggesting that brain glycine levels are sufficiently elevated to evoke a clinical response within that time frame. However, no human study has reported on brain glycine changes induced by its administration. We utilized a noninvasive proton magnetic resonance spectroscopy (1H-MRS) technique termed echo time-averaged (TEAV) 1H-MRS, which permits noninvasive quantification of brain glycine in vivo, to determine whether 2 weeks of oral glycine administration (peak dose of 0.8g/kg/day) increased brain glycine/creatine (Gly/Cr) ratios in 11 healthy adult men. In scans obtained 17 hours after the last glycine dose, brain (Gly/Cr) ratios were significantly increased. The data indicate that it is possible to measure brain glycine changes with proton spectroscopy. Developing a more comprehensive understanding of human brain glycine dynamics may lead to optimized use of glycine site agonists and glycine transporter inhibitors to treat schizophrenia, and possibly to treat other disorders associated with glutamate system dysfunction.
Keywords: Schizophrenia, antipsychotic, glutamate receptors, substance abuse
1. INTRODUCTION
Schizophrenia is a brain disorder associated with glutamatergic N-methyl-D-aspartate (NMDA) system hypofunction (Javitt and Zukin, 1991; Olney and Farber 1995). Since glycine enables optimum NMDA receptor activity (Johnson and Ascher, 1987; Kleckner and Dingledine, 1988; Kessler et al., 1989), one therapeutic approach to treat schizophrenia has been to administer glycine orally to increase synaptic glycine levels. Early evidence supporting that strategy came from rodent oral glycine administration studies documenting increased brain glycine concentration (Toth and Lajtha, 1981) and glycine-normalized behavior in a phencyclidine (PCP) model of schizophrenia (Toth and Lajtha, 1986). Subsequent studies in humans with schizophrenia provided clinical support for this approach (Waziri, 1988; Rosse et al., 1989; Costa et al., 1990; Javitt et al., 1994). High glycine dose (0.8 g/kg/day) studies replicated and extended initial findings by demonstrating improvements in positive, negative, and cognitive symptoms of the disorder (Heresco-Levy et al., 1996; Leiderman et al., 1996; Heresco-Levy et al., 1999; Javitt et al., 2001; Heresco-Levy et al., 2004).
The current literature linking abnormal glutamate neurotransmission to schizophrenia remains active, with a number of studies documenting that the NMDA receptor glycine site is likely to play a role in schizophrenia and its treatment. For example, oral glycine-induced behavioral improvements were reported in a nonhuman primate PCP model of schizophrenia (Linn et al., 2007). Genetically engineered mice with abnormal glutamatergic NMDA receptor glycine site affinity exhibit aberrant behaviors paralleling some behaviors observed in schizophrenia subjects (Labrie et al., 2008). In animal models of schizophrenia involving NMDA receptor antagonists, glycine transporter inhibitors can mitigate schizophrenia-like symptoms, improve behavioral deficits, and blunt PCP-induced functional MRI activations (Boulay et al., 2008; Gozzi et al., 2008; Hashimoto et al., 2008; Kanahara et al., 2008; Karasawa et al., 2008). Together, these findings lend continuing support to the concepts that abnormal glutamate neurotransmission is an important component of schizophrenia and that glycine, an NMDA receptor co-agonist that can augment glutamatergic NMDA receptor neurotransmission (Johnson and Ascher 1987; Kleckner and Dingledine 1988; Kessler et al. 1989), may have therapeutic potential for treating schizophrenia. By contrast, a recent clinical trial documented minimal efficacy for oral high-dose glycine to improve negative symptoms in schizophrenia subjects (Buchanan et al., 2007). Yet, the study authors concluded that “it is not known if efficacy would have been achieved at substantially higher serum glycine levels” (Buchanan et al., 2007).
One important limitation of glycine adjuvant therapy observed in all studies to date is its highly variable clinical efficacy; in studies reporting an overall beneficial effect, coefficients of variation for negative symptom improvements ranged from 20 to 70% (Heresco-Levy et al., 1996; Leiderman et al., 1996; Heresco-Levy et al., 1999; Javitt et al., 2001; Heresco-Levy et al., 2004). In those studies, part of that variability may have been due to inclusion of subjects treated with antipsychotics that inhibit glycine transporters such as clozapine (Williams et al., 2004; Javitt et al., 2005; Konradsson et al., 2006). Such subjects typically do not benefit from added glycine or D-serine treatment (Goff et al., 1996; Potkin et al., 1999; Tsai et al., 1999; Evins et al., 2000), or from sarcosine (a glycine congener and glycine transporter inhibitor) treatment (Lane et al., 2005). However, variations in gut glycine absorption, which in human and animal studies is extensive and variable (Silk et al., 1974; Stoll et al., 1998; Wu, 1998), could explain why, despite dosing glycine by weight, plasma (and presumably brain) glycine levels vary substantially, even in studies sampling plasma at pharmacokinetic troughs (Heresco-Levy et al., 1996; Heresco-Levy et al., 1999; Heresco-Levy et al., 2004). Plasma and cerebrospinal fluid glycine increments also were variable in studies involving intravenous glycine administration (D’Souza et al., 2004; Neumeister et al., 2006), suggesting that glycine metabolism and blood brain barrier uptake also vary between subjects.
How plasma glycine variations are manifest synaptically is unknown since it currently is not possible to measure synaptic glycine noninvasively. However, neurons and glia, which are structural elements of synapses, not only accumulate glycine (Zafra et al., 1995) but also release it (Galli et al., 1993; Roux and Supplisson, 2000; Harsing et al., 2001; Billups and Attwell, 2003; Huang et al., 2004; Dopico et al., 2006; Hayashi et al., 2006; Wojcik et al., 2006). Thus, both cell types regulate synaptic glycine and may play roles in the therapeutic response to glycine treatment. As an initial step toward characterizing relationships between brain glycine levels and glycine’s therapeutic efficacy in schizophrenia and perhaps other disorders, we measured brain glycine changes induced by oral high-dose glycine administration in healthy men, using proton magnetic resonance spectroscopy (1H-MRS).
High-resolution 1H-MRS can detect glycine in cultured neurons and glia (Urenjak et al., 1993; Flogel et al., 1995). In vivo, 1H-MRS brain glycine detection is complicated because proton resonances for myo-inositol (mI), present at several-fold higher concentration than glycine, have a similar chemical shift (3.61 ppm) as glycine’s 3.55 ppm methylene protons (Govindaraju et al., 2000). Thus, in vivo 1H-MRS glycine detection has been limited primarily to rare glycine excess disorders such as nonketotic hyperglycinemia (Viola et al., 2002). However, a method termed echo time averaged 1H-MRS (TEAV) (Hurd et al., 2004), which selectively resolves glycine at 4.0 Tesla by eliminating most of the overlapping mI proton resonance at 3.55 ppm, (Prescot et al., 2006), can be used to measure brain glycine changes. In healthy human brain, the method has good reliability for measuring glycine/creatine ratios, with a test-retest coefficient of variation of 15% (Prescot et al., 2006). Presently, we used TEAV 1H-MRS to measure brain glycine/creatine ratio changes in healthy adult men following 2 weeks of glycine dosing. That treatment duration was selected because schizophrenia subjects administered glycine for 2 weeks exhibited statistically significant clinical improvements, suggesting that brain glycine levels were increased (Heresco-Levy et al., 1996; Leiderman et al., 1996; Heresco-Levy et al., 1999; Javitt et al., 2001; Heresco-Levy et al., 2004). Based on rodent studies documenting brain glycine increases after oral glycine administration (Toth and Lajtha, 1981; Toth and Lajtha, 1986), we hypothesized that glycine administration would increase occipital cortex glycine/creatine ratios.
2. METHODS
2.1. Subjects
This study was conducted after review and approval by the McLean Hospital Institutional Review Board. We enrolled 14 healthy adult men who provided written informed consent and who were compensated for their participation, including receiving incentives for reporting times they consumed each glycine drink, as described below. Prior to being admitted into the study, potential subjects underwent a complete physical screening including urinalysis, blood work, and electrocardiogram, and all had normal 1.5 Tesla clinical MRI brain scans. They also were screened for histories of psychiatric disorders or substance abuse by one of the two study physicians (DO and PFR), both of whom are board-certified psychiatrists. A SCID was not conducted. Individuals with current medical or psychiatric disorders, or substance abuse were excluded from study participation. No restrictions were placed on caffeine or nicotine use, although all subjects reported being nonsmokers and they were instructed to not drink coffee on study days in which scans occurred. Individuals taking prescription or over the counter medications, or those with histories of glycine supplement use, were not accepted into the study. Subjects were screened for recent alcohol and drug exposure using breath and urine screens, and were excluded from participation if testing positive at any time. Data are reported from 11 men (8 caucasians, 2 asians, and 1 african american). Data from 2 subjects who developed gastrointestinal side effects and were unable to consume all glycine doses are not reported. In addition, data was excluded from one subject whose day 14 brain glycine measurement exhibited low reliability (see below).
2.2. Magnetic Resonance Imaging, Spectroscopy, and Plasma Sampling
Magnetic resonance imaging and TEAV 1H-MRS were performed as described previously (Prescot et al., 2006) on a 4.0 Tesla Varian Unity/Inova whole-body scanner (Varian, Inc., Palo Alto, CA, USA). A transverse electromagnetic resonator head coil was used for radiofrequency transmission and reception. After manual shimming, high-contrast, 3D fast low angle shot (FLASH) T1-weighted axial MRI images (TR/TE = 11.4/6.2 ms, field-of-view = 24 × 24 cm, matrix = 256 × 256 × 32, slice thickness = 2.5 mm) were obtained to enable spectroscopy voxel positioning on midline occipital cortex (predominantly gray matter). Imaging data also were used for voxel repositioning in repeat scans, as described below. The occipital cortex was our region of interest for these studies because it exhibits good magnetic field homogeneity, resulting in narrow and reproducible metabolite resonance linewidths.
A point-resolved spectroscopy (PRESS) pulse sequence modified for TEAV 1H-MRS along with a four-pulse WET (water suppression enhanced through T1-effects) sequence (Ogg et al., 1994) used for water suppression (WET flip angles: θ(1)= 81.4°; θ(2)= 101.4°; θ(3)= 69.3°; θ(4) =161.0°) were applied. Occipital cortex TEAV 1H-MRS spectra were acquired from midline 2 cm × 2 cm × 2 cm (8-ml) voxels using the following parameters: TR = 2000 ms, TE range = 30–284 ms, ΔTE = 2 ms, NEX = 4, measurement time = 18 minutes. Signal acquisition duration was 1024 ms, 2048 spectral points were acquired, and acquisition bandwidth was 2000 Hz. Transmitter pulse power and global water suppression were optimized using automated methods, whereas B0 homogeneity was manually adjusted for each study. The localized unsuppressed water signal line width was ≤ 9 Hz for all measurements.
At the time of each TEAV 1H-MRS spectrum acquisition, a 5 ml venous blood sample was obtained for plasma glycine measurements. Samples were centrifuged to separate plasma from red blood cells. Plasma was obtained and aliquoted into plastic sample tubes and stored frozen at −80°C. Samples were analyzed for glycine levels by the Massachusetts General Hospital Clinical Laboratory (Boston, MA). The laboratory did not provide assay coefficients of variation as part of data reports. However, assay precision information was provided for the period during which study sample assays were performed (January to August, 2006); the glycine inter-assay coefficient of variation was 5.2% for a 250 μM standard value, and the intra-assay coefficients of variation were 2.5, 5, and 10% for standard values of 120, 250, and 480 μM, respectively.
2.3. Data Analysis
Spectroscopy data were transferred to a personal computer for processing with FELIX 2002 (Accelrys, Inc., San Diego, CA, USA). For illustration purposes, TEAV data were processed by averaging all 128 echo times with Gauss–Lorentz apodization (exponential broadening = −2 Hz, Gaussian broadening coefficient = 0.05 centering the Gaussian at point 102), fast Fourier transformation, and automated signal phase correction. LC-model (Provencher, 1993) (version 6.0–1) was used to provide an unbiased fitting method for TEAV 1H-MRS data via a simulated TEAV basis set (Prescot et al., 2006). No apodization filters were applied to the TEAV data prior to LC-model analysis. The output provided by LC-model corresponded to the raw integral for each metabolite resonance. Spectral fitting was performed in the frequency domain from 1.4 to 4.4 ppm and the raw integrals were measured for the Gly methylene protons (3.55 ppm), mI protons (3.61 ppm), an almost pure Glu peak (2.35 ppm) and the Cr methyl peak (3.0 ppm). As the Gly peak contains a small residual mI contribution in vivo (Prescot et al., 2006), we refer to it as Gly* to reflect that it is not a pure glycine resonance. TEAV measurements in phantoms simulating a 3-fold water T2 relaxation time difference exhibited only a 5% difference in the Gly*/Cr ratio (unpublished data), indicating that our TEAV measurement and spectrum quantification methods are relatively insensitive to T2 effects on metabolites of interest (Gly, mI, Cr, and Glu). Further, there is no appreciable macromolecule resonance near 3.55 ppm either in short-TE proton spectra (Behar et al., 1994) or in metabolite-nulled TEAV spectra (unpublished data), suggesting that macromolecule resonances do not contribute to the glycine peak in TEAV spectra. In addition to these measurements, spectral fitting also was performed for the unsuppressed water (UW) peak to determine whether Cr/UW ratios were stable during the study.
The Cramér-Rao lower bound (CRLB) of each metabolite peak was used to determine LC-model fit reliability (Provencher, 2005). The CRLBs for Cr, mI, and Glu did not exceed 3, 13, and 9%, respectively. The CRLBs for Gly* peaks were higher, and one day 14 spectrum had a very high CRLB (40%). By contrast, Gly* CRLBs for all other subjects in baseline and day 14 scans averaged 20 ± 2.9 and 17 ± 5.1%, respectively (means ± SD). As the outlying spectrum had a Gly* CRLB exceeding 3 standard deviations of the day 14 Gly* mean CRLB value, that spectrum was excluded from further analysis. The remaining data were used to calculate metabolite/Cr ratios. Since metabolite ratios are sensitive to metabolite resonance changes in the numerator and denominator (Cr), we extracted Cr T2 values for all subjects on both scan days to determine whether metabolite ratio changes could be attributable to Cr T2 changes. We fitted Cr methyl proton T2 relaxation time data to mono-exponential decay curves using Origin (V 8.0, OriginLab Corp., Northampton, MA, USA).
2.4. Glycine Administration and Subject Dosing Confirmation
High-dose oral glycine administration was accomplished with a modification of the protocol developed by Evins et al., (2000), which increased plasma glycine levels (>3.5-fold). Our 2-week protocol involved twice daily dosing with a glycine-enriched beverage. This treatment period was selected since two weeks of high-dose oral glycine administration was sufficient to evoke a clinical response in schizophrenia subjects (Heresco-Levy et al., 1996; Leiderman et al., 1996; Heresco-Levy et al., 1999; Javitt et al., 2001; Heresco-Levy et al., 2004). Glycine powder (U.S.P.) was obtained from Spectrum Chemical and Manufacturing Corp. (New Brunswick, NJ) and mixed with lemon juice concentrate and water to form 250 ml beverages. Initial glycine doses were 10g/day (administered as a divided dose in the morning and evening) for 2 days. Doses were gradually increased every 2 days during the course of the study to 0.2, 0.4, and 0.6 g/kg/day, and the terminal dose of 0.8 g/kg/day was maintained for 5 days. Subjects consumed the first 5g glycine drink in the laboratory and were sent home with the first week of drinks labeled with dosing date and time (e.g., morning or evening). Subjects were instructed to refrigerate drinks until consumed and to provide phone reports after consuming each dose. Phone reports permitted us to confirm dose compliance and timing, and to assess relationships between the time elapsed since glycine administration and brain metabolite ratios. Subjects also were instructed to report any side effects they experienced, but they were not asked to keep track of mood or mood changes. To increase compliance, subjects were compensated $5 for consuming and reporting each scheduled glycine dose. Subjects returned to the laboratory on study day 7 or 8 to pick up remaining freshly-prepared glycine doses and for a brief side-effects interview. All subjects were scanned at baseline (day 0, subsequently referred to as D0), prior to glycine administration, and on day 14 (D14), the day after completing glycine dosing.
2.5. Head Repositioning in Repeat Scans
We utilized an internal landmark head alignment procedure to register brain and TEAV 1H-MRS voxel positions within subjects between scan days (Kaufman et al., 2003). On midsagittal high-resolution FLASH images, the scanner monitor cursor was used to connect corpus callosum genu and splenium centroids. The angle subtended by that line with respect to the superior-inferior (S/I, or z-axis) normal was recorded during the baseline scan for alignment on subsequent scans. Similarly, in the axial image slice through the occipital cortex voxel midpoint, longitudinal cerebral fissure extremes were connected. The angle subtended by that line with respect to the right-left (R/L, or y-axis) normal also was recorded for matching on subsequent scans. Deviations of ≤ 5° in either S/I or R/L axes when compared to base line measures were considered acceptable brain/voxel registration. Deviations exceeding that value in either axis resulted in manual head repositioning.
2.6. Statistical Analyses
Statistical analyses were performed with Prism software (v4.0c), GraphPad Software Inc. (San Diego, CA) and Statview 5.0.1 (SAS Institute Inc. Cary, NC). The tests conducted for each analysis are noted in text sections describing statistical findings. Statistical significance was defined as P < 0.05.
3. RESULTS
Study subjects were 30 ± 7.3 years old (mean ± SD, range: 22 – 41), had a body mass index of 25 ± 2.1 kg/m2 (range: 22 – 29), and had D0 plasma glycine levels averaging 240 ± 84 μmol/l (range: 190 – 460). Twelve of the 14 men initially enrolled in the study tolerated oral glycine administration well and reported minimal side effects. Two subjects experienced nausea and emesis after beginning the 0.8 g/kg/day dose, and were not able to complete the glycine dosing protocol. Data from those two subjects, as well as data from one completer with a high D14 CRLB value are not included in overall study findings.
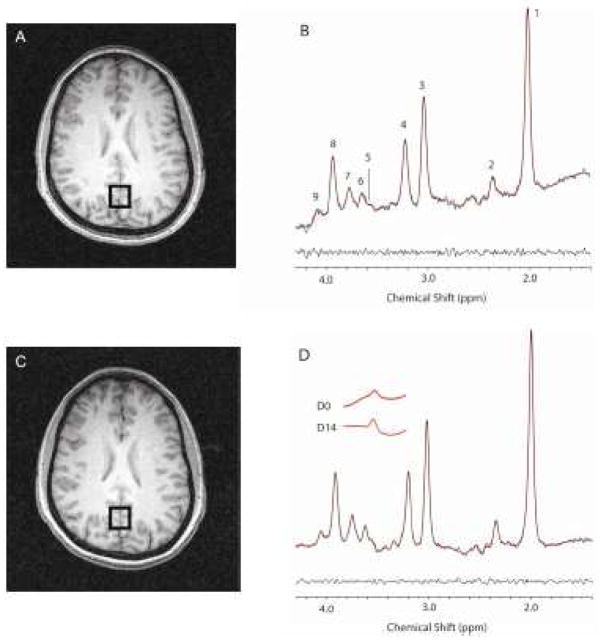
Brain TEAV spectra exhibit Gly* resonances at 3.55 ppm (Figure 1). Occipital cortex D0 Gly*/Cr metabolite ratios averaged 0.022 ± 0.004 (range: 0.011 – 0.027). D0 mI/Cr and Glu/Cr ratios averaged 0.221 ± 0.022 (range 0.170 – 0.250) and 0.270 ± 0.020 (range 0.240 – 0.320), respectively. We found no associations between D0 brain Gly*/Cr ratios and either plasma glycine levels (R = 0.00, P > 0.98, data not shown) or brain mI/Cr or Glu/Cr ratios (R < 0.32, P > 0.33).
Figure 1.
Top Panels: D0 TEAV 1H-MRS voxel placement and resultant spectra from one study subject (Panels A–B). Bottom Panels: D14 TEAV 1H-MRS voxel placement and resultant spectrum (Panels C–D). Panel D inset: LC-Model Gly* fit spectrum extractions for D0 and D14. Resonance peak assignments are as follows: 1) N-acetylasparate, 2) glutamate, 3) total creatine, 4) choline, 5) Gly*, 6) myo-inositol, 7) glutamate/glutamine, 8) total creatine, 9) myo-inositol. Unsuppressed water line widths, which reflect metabolite line widths, for D0 and D14 averaged 7.84 ± 0.66 and 7.84 ± 0.44 Hz, respectively, indicating comparable water and metabolite line widths on the different scan days. Panels A and C demonstrate good voxel overlap in repeat scans. Panels B and D document the Gly* resonance and its change after glycine administration.
Our head repositioning protocol for repeat scan voxel registration resulted in a high degree of voxel overlap, as illustrated by Figure 1. Brain position on D14 scans deviated from D0 by 4.2 ± 4.0 and 1.7 ± 1.7° in the axial and sag ittal planes, respectively.
On D14, TEAV MRS was performed 17 ± 3.8 (range: 11–20) hours after the last glycine dose, when plasma glycine levels averaged 640 ± 340 μmol/l (260% baseline level). The D14 Gly*/Cr ratio was 0.030 ± 0.006 (range: 0.018 – 0.038) and it was significantly increased to 138 ± 37% of D0 (ANOVA F1,10 = 18.8, P < 0.002, Figure 2). The D14 ratio was larger than the D0 ratio in 10 of 11 subjects (Figures 1 and 2). Group analyses revealed a trend toward a statistically significant association between brain Gly*/Cr ratio increments and elapsed time since last glycine dose (R = −0.42, P > 0.19), but found no association between Gly*/Cr ratio increments and plasma glycine levels (R = 0.02, P > 0.95). The D14 mI/Cr and Glu/Cr ratios averaged 0.227 ± 0.028 (range: 0.190 – 0.270) and 0.271 ± 0.017 (range: 0.250 – 0.310), respectively, and were 103 ± 13 and 100 ± 9.3% D0 levels, respectively. We found no associations between D14 Gly*/Cr ratio changes and either mI/Cr (R = 0.05, P > 0.88) or Glu/Cr ratio (R = 0.41, P > 0.20) changes. On D0 and D14, Cr T2s averaged (mean ± SD) 128 ± 25 ms and 128 ± 23 ms, respectively, and were statistically equivalent. Further, Cr/UW ratios averaged (mean ± SD) 3.0 ± 3.9 (×10−7) and 2.8 ± 3.6 (×10−7) on D0 and D14, respectively, and also were statistically equivalent.
Figure 2.
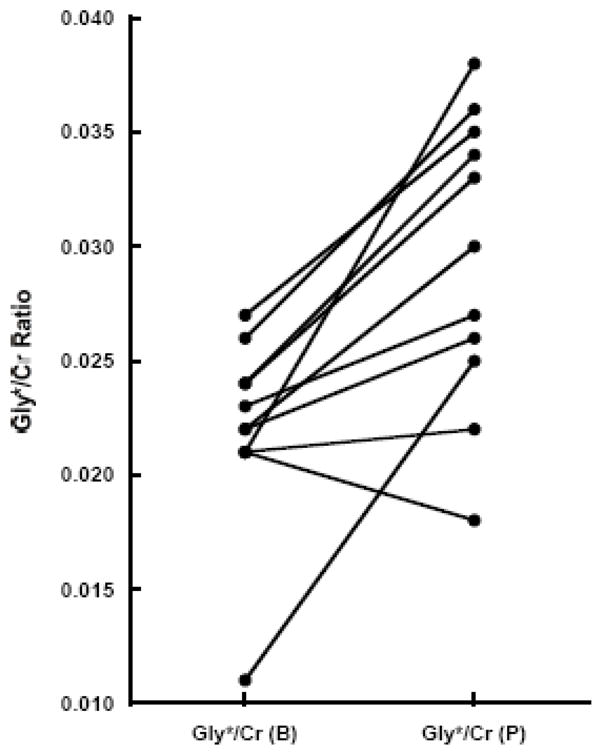
Brain Gly*/Cr ratios at D0 and D14 for all study subjects. The Gly*/Cr ratio increased in 10 of 11 subjects who completed the glycine dosing protocol (ANOVA F1,10=18.8, P < 0.002).
4. DISCUSSION
These data document that 2 weeks of oral high-dose glycine administration increased occipital lobe brain Gly*/Cr ratios in healthy men. The Gly*/Cr ratio changes we detected likely are a result of brain glycine changes, since glycine treatment did not alter mI/Cr ratios, indicating that the residual mI contribution to the Gly* resonance at 3.55 ppm was unchanged, and glycine treatment did not alter either Cr resonance T2 values or concentrations (estimated as Cr/UW ratios). Although we detected a brain glycine ratio increase, there was a considerable degree of intersubject variability in brain glycine ratio increments, (coefficient of variation for baseline-normalized Gly*/Cr change exceeding 25%). That variability could be a result of study subjects experiencing highly variable plasma glycine increments despite dosing glycine by weight, a finding consistent with previous high glycine dose studies in schizophrenia subjects (Heresco-Levy et al., 1996; Leiderman et al., 1996; Heresco-Levy et al., 1999; Javitt et al., 2001; Heresco-Levy et al., 2004). The general finding of plasma glycine variability after oral dosing likely is attributable to intersubject variations in gut glycine absorption (Silk et al., 1974; Stoll et al., 1998; Wu, 1998). While some might interpret our data variability as indicating imprecision of the MRS method, the 38% increase in brain glycine ratio we detected is substantially larger in magnitude than the 15% precision of our brain glycine ratio measurement (Prescot et al., 2006). This suggests that our study was more than adequately powered to detect a statistically significant effect of glycine administration on brain glycine ratio increases. Measurement precision likely would be improved with inclusion of more subjects.
Another point that should be emphasized when considering the present findings is that the magnitude brain glycine ratio increase we detected is small in comparison to peak brain glycine levels reported in rodent glycine administration studies, which more than doubled brain glycine levels (Toth and Lajtha, 1981; Toth and Lajtha, 1986). This apparent discrepancy could result from several factors including study differences in glycine dosing methods, species differences in brain glycine uptake, and differences in the precision of glycine ratio detection methods (e.g., analytical techniques for rodent studies versus TEAV 1H-MRS for humans). Measurement timing differences between studies also could account for the apparent discrepancy. In this regard, peak brain glycine levels were detected 1 hour after intragastric glycine administration in mice and brain glycine levels declined by nearly 30% within 3 hours (Toth and Lajtha, 1986). Presently, we measured brain Gly*/Cr ratios 17 ± 3.8 hours after the last glycine dose. Accordingly, if human and mouse brain glycine pharmacokinetics are similar, then our measurements would have been acquired long after peak brain levels had been achieved and after a prolonged period of brain glycine efflux. While we detected only a trend effect for a time-related brain glycine ratio decline in our group analysis, such an effect could have been obscured by the intersubject variability we observed both for plasma and brain glycine ratio increments. Clearly, additional high-dose glycine administration studies will be necessary to better characterize human brain glycine dynamics. Yet, our findings along with those from rodent studies (Toth and Lajtha, 1986) suggest that there is substantial interindividual variability in brain uptake after oral dosing.
These findings may have relevance for interpreting results from glycine treatment studies in schizophrenia subjects. In this regard, treatment studies published to date appear not to have accounted for intersubject differences in glycine bioavailability, reflected presently and in prior studies as highly variable plasma glycine increments despite dosing glycine by weight (Heresco-Levy et al., 1996; Leiderman et al., 1996; Heresco-Levy et al., 1999; Javitt et al., 2001; Heresco-Levy et al., 2004; Buchanan et al., 2007). It is conceivable that schizophrenia subjects experiencing minimal beneficial effects from glycine might have sustained inadequate plasma (and brain) glycine increases to improve clinical state. This could have resulted in smaller effect sizes for, and an apparent underappreciation of, glycine’s therapeutic efficacy. Further, if, as our data suggest there is large intersubject variability in brain glycine dynamics, it is conceivable that the elapsed time since glycine dosing also may be important to consider when assessing clinical efficacy, particularly for cognitive components sensitive to glutamate system function at the time of assessment (e.g., affect, attention). In studies published to date, none appear to have controlled for elapsed time since glycine dosing when performing clinical assessments. Accounting for these sources of brain glycine variability could reveal larger clinical effects that emerge earlier in treatment time courses.
While glycine administration is not capable of serving as a stand-alone treatment for schizophrenia, developing a better understanding of brain glycine dynamics may enhance glycine’s potential for use as an adjuvant treatment. In this regard, glycine is effective when combined with certain antipsychotics (Heresco-Levy et al. 2004). It also may be useful when combined with glycine transport inhibitors currently being developed to treat psychotic disorders, either by increasing therapeutic efficacy or by facilitating use of lower drug doses to achieve therapeutic effects (Bergeron et al. 1998; Depoortere et al. 2005). The ability to administer lower antipsychotic doses could be especially beneficial for agents that promote side effects.
Understanding brain glycine dynamics also may be useful for developing novel treatments for other disorders associated with abnormal glutamatergic NMDA receptor system function including substance abuse (Bisaga and Popik, 2000; Coyle, 2006). The NMDA receptor system is involved in mediating acute or chronic effects of nicotine, alcohol, cannabinoids, cocaine, and opiates (Martin et al., 2004; Roberto et al., 2004; Vengeliene et al., 2005; Backstrom and Hyytia, 2006; Coyle, 2006; Hejazi et al., 2006). Accordingly, NMDA receptor glycine site agonists or antagonists have the potential to be useful treatments for a wide range of substance abuse disorders as well as for comorbid schizophrenia/substance abuse.
4.1. Limitations
There are several limitations to this study to consider when interpreting its findings. Metabolite ratios can change either when their numerator (e.g., Gly*) or denominator (Cr) change. We were able to estimate Cr T2 from TEAV data and concentration from Cr/UW ratios, and we found that glycine treatment did not alter either the Cr T2 or the Cr/UW ratio. Thus, we can rule out a denomimator effect as a contributor to the Gly*/Cr ratio changes we detected. The Cr peak stability is consistent with our observation of stable mI/Cr and Glu/Cr ratios. Since we found no evidence for covariation between the mI/Cr and Gly*/Cr ratios either at baseline or after glycine administration, and since we determined that the Cr resonance was not affected by glycine treatment, the Gly*/Cr ratio increases we detected after glycine treatment likely are due to brain Gly resonance changes. The TEAV method does not completely eliminate the mI resonance near 3.55 ppm at all echo times, which prevents accurate quantification of the Gly T2. It is conceivable that glycine T2 increases could have contributed to the effects we observed, however, the magnitude change required to fully account for the 38% increase we detected in the Gly*/Cr ratio is on the order of 100 ms, an extremely large change in vivo. Although we cannot rule out a glycine T2 change as contributing to our findings, we believe it is highly unlikely to be a dominant effect.
Newly developed proton spectroscopy methods that virtually eliminate the neighboring mI resonance (Choi et al., 2008) may be better suited for in vivo brain glycine quantification in future studies. Our midline occipital lobe findings may not be applicable to other brain areas. However, glycine concentration is close to 1 mM throughout the forebrain (Aprison et al., 1969; Toth and Lajtha, 1981; Gundlach and Beart, 1982) and recent work suggests that glycine levels as well as activity levels of the enzyme primarily responsible for brain glycine synthesis, serine hydroxymethyl transferase, are comparable in gray and white matter (Hejnum and Hassel, 2007). This suggests that glycine homeostatic mechanisms are similar in different forebrain areas. Thus, we believe that the occipital lobe Gly*/Cr ratios and changes following glycine administration we detected should generalize to other brain areas. In addition, while brain and plasma glycine levels tended to covary in a rodent study (Toth and Lajtha, 1986), we did not detect a similar relationship. As noted above, we attribute this to intersubject differences in glycine absorption and bioavailability after oral glycine dosing (Silk et al., 1974; Stoll et al., 1998; Wu, 1998), which would limit how much glycine can get into brain. Two subjects in this study exhibited outlying values. One, subject had a very low D0 Gly* level and he exhibited the highest percent increase in his D14 Gly*/Cr ratio, strongly influencing the D14 Gly*/Cr ratio increase effect size. His data were included because they exhibited acceptable CRLB values and there were no independent criteria (e.g., outlying values for demographic factors) supporting exclusion of his data. Had his data been excluded, the group D14 Gly*/Cr ratio increase would have remained statistically significant (129 ± 24% D0, F1,9=15.0, P < 0.005). The other subject’s data were excluded because his D14 Gly* peak had a very high CRLB value of 40, more than 4 standard deviations (5.1) higher than the mean D14 CRLB for study subjects (16.9). Had his data been included, the group D14 Gly*/Cr ratio increase would have been smaller but remained statistically significant (130 ± 44% D0, F1,11 = 6.7, P < 0.03). Lastly, this study was conducted on an outpatient basis and lifestyle factors that have the potential to influence study findings such as glycine dose administration times, diet, exercise, and sleep patterns, were not standardized. Also, subjects were not monitored to confirm that they consumed glycine doses when they reported doing so, and the study did not assess mood or mood changes. Accordingly, any conclusions we advance must be considered preliminary in nature. We are planning additional within-subjects repeated-measures studies to assess plasma and brain glycine time courses following high-dose glycine administration, which should help to characterize different sources of brain glycine measurement variability and their behavioral and clinical relevance.
Acknowledgments
We thank John Brown for his contributions to this study. This study was supported in part by the following grants: DA017324 (MJK), DA014674 (MJK), DA022276 (AEE), DA019378 (MF), RR013938 (PFR), NARSAD (APP), the Counterdrug Technology Assessment Center–an office within the Office of National Drug Control Policy (PFR), GlaxoSmithKline (PFR), and John and Virginia Taplin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of these data were presented previously at the 44th and 46th annual meetings of the American College of Neuropsychopharmacology (Waikoloa, HI, December, 2005 and Boca Raton, FL, December 2007, respectively).
References
- Aprison MH, Shank RP, Davidoff RA. A comparison of the concentration of glycine, a transmitter suspect, in different areas of the brain and spinal cord in seven different vertebrates. Comparative Biochemistry and Physiology. 1969;28:1345–1355. doi: 10.1016/0010-406x(69)90571-4. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- Behar KL, Rothman DL, Spencer DD, Petroff OA. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magnetic Resonance in Medicine. 1994;32:294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Meyer TM, Coyle JT, Greene RW. Modulation of N-methyl-D-aspartate receptor function by glycine transport. Proceedings of the National Academy of Sciences USA. 1998;95:15730–15734. doi: 10.1073/pnas.95.26.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billups D, Attwell D. Active release of glycine or D-serine saturates the glycine site of NMDA receptors at the cerebellar mossy fibre to granule cell synapse. European Journal of Neuroscience. 2003;18:2975–2980. doi: 10.1111/j.1460-9568.2003.02996.x. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Popik P. In search of a new pharmacological treatment for drug and alcohol addiction: N-methyl-D-aspartate (NMDA) antagonists. Drug and Alcohol Dependence. 2000;59:1–15. doi: 10.1016/s0376-8716(99)00107-6. [DOI] [PubMed] [Google Scholar]
- Boulay D, Pichat P, Dargazanli G, Estenne-Bouhtou G, Terranova JP, Rogacki N, Stemmelin J, Coste A, Lanneau C, Desvignes C, Cohen C, Alonso R, Vigé X, Biton B, Steinberg R, Sevrin M, Oury-Donat F, George P, Bergis O, Griebel G, Avenet P, Scatton B. Characterization of SSR103800, a selective inhibitor of the glycine transporter-1 in models predictive of therapeutic activity in schizophrenia. Pharmacology Biochemistry and Behavior. 2008;91:47–58. doi: 10.1016/j.pbb.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, Heresco-Levy U, Carpenter WT. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. American Journal of Psychiatry. 2007;164:1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- Choi C, Bhardwaj PP, Seres P, Kalra S, Tibbo PG, Coupland NJ. Measurement of glycine in human brain by triple refocusing 1H-MRS in vivo at 3.0T. Magnetic Resonance in Medicine. 2008;59:59–64. doi: 10.1002/mrm.21450. [DOI] [PubMed] [Google Scholar]
- Costa J, Khaled E, Sramek J, Bunney W, Jr, Potkin SG. An open trial of glycine as an adjunct to neuroleptics in chronic treatment-refractory schizophrenics. Journal of Clinical Psychopharmacology. 1990;10:71–72. doi: 10.1097/00004714-199002000-00027. [DOI] [PubMed] [Google Scholar]
- Coyle JT. Substance use disorders and Schizophrenia: a question of shared glutamatergic mechanisms. Neurotoxicity Research. 2006;10:221–233. doi: 10.1007/BF03033359. [DOI] [PubMed] [Google Scholar]
- Depoortere R, Dargazanli G, Estenne-Bouhtou G, Coste A, Lanneau C, Desvignes C, Poncelet M, Heaulme M, Santucci V, Decobert M, Cudennec A, Voltz C, Boulay D, Terranova JP, Stemmelin J, Roger P, Marabout B, Sevrin M, Vigé X, Biton B, Steinberg R, Françon D, Alonso R, Avenet P, Oury-Donat F, Perrault G, Griebel G, George P, Soubrié P, Scatton B. Neurochemical, electrophysiological and pharmacological profiles of the selective inhibitor of the glycine transporter-1 SSR504734, a potential new type of antipsychotic. Neuropsychopharmacology. 2005;30:1963–1985. doi: 10.1038/sj.npp.1300772. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Gil R, Cassello K, Morrissey K, Abi-Saab D, White J, Sturwold R, Bennett A, Karper LP, Zuzarte E, Charney DS, Krystal JH. IV glycine and oral D-cycloserine effects on plasma and CSF amino acids in healthy humans. Biological Psychiatry. 2000;47:450–462. doi: 10.1016/s0006-3223(99)00133-x. [DOI] [PubMed] [Google Scholar]
- Dopico JG, Gonzalez-Hernandez T, Perez IM, Garcia IG, Abril AM, Inchausti JO, Rodríguez Díaz M. Glycine release in the substantia nigra: Interaction with glutamate and GABA. Neuropharmacology. 2006;50:548–557. doi: 10.1016/j.neuropharm.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Evins AE, Fitzgerald SM, Wine L, Rosselli R, Goff DC. Placebo-controlled trial of glycine added to clozapine in schizophrenia. American Journal of Psychiatry. 2000;157:826–828. doi: 10.1176/appi.ajp.157.5.826. [DOI] [PubMed] [Google Scholar]
- Flogel U, Niendorf T, Serkowa N, Brand A, Henke J, Leibfritz D. Changes in organic solutes, volume, energy state, and metabolism associated with osmotic stress in a glial cell line: a multinuclear NMR study. Neurochemical Research. 1995;20:793–802. doi: 10.1007/BF00969691. [DOI] [PubMed] [Google Scholar]
- Galli A, Mori F, Bargellini M, Coppini L. Sodium-dependent release of exogenous glycine from preloaded rat hippocampal synaptosomes. Journal of Neural Transmission General Section. 1993;93:167–179. doi: 10.1007/BF01244994. [DOI] [PubMed] [Google Scholar]
- Goff DC, Tsai G, Manoach DS, Flood J, Darby DG, Coyle JT. D-cycloserine added to clozapine for patients with schizophrenia. American Journal of Psychiatry. 1996;153:1628–1630. doi: 10.1176/ajp.153.12.1628. [DOI] [PubMed] [Google Scholar]
- Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR in Biomedicine. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Herdon H, Schwarz A, Bertani S, Crestan V, Turrini G, Bifone A. Pharmacological stimulation of NMDA receptors via co-agonist site suppresses fMRI response to phencyclidine in the rat. Psychopharmacology (Berlin) 2008;201:273–284. doi: 10.1007/s00213-008-1271-z. [DOI] [PubMed] [Google Scholar]
- Gundlach AL, Beart PM. Neurochemical studies of the mesolimbic dopaminergic pathway: glycinergic mechanisms and glycinergic-dopaminergic interactions in the rat ventral tegmentum. Journal of Neurochemistry. 1982;38:574–581. doi: 10.1111/j.1471-4159.1982.tb08665.x. [DOI] [PubMed] [Google Scholar]
- Harsing LG, Jr, Solyom S, Salamon C. The role of glycineB binding site and glycine transporter (GlyT1) in the regulation of [3H]GABA and [3H]glycine release in the rat brain. Neurochemical Research. 2001;26:915–923. doi: 10.1023/a:1012328300037. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fujita Y, Ishima T, Chaki S, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the glycine transporter-1 inhibitor NFPS and D-serine. European Neuropsychopharmacology. 2008;18:414–421. doi: 10.1016/j.euroneuro.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Ishibashi H, Hashimoto K, Nakanishi H. Potentiation of the NMDA receptor-mediated responses through the activation of the glycine site by microglia secreting soluble factors. Glia. 2006;53:660–668. doi: 10.1002/glia.20322. [DOI] [PubMed] [Google Scholar]
- Hejazi N, Zhou C, Oz M, Sun H, Ye JH, Zhang L. Delta9-tetrahydrocannabinol and endogenous cannabinoid anandamide directly potentiate the function of glycine receptors. Molecular Pharmacology. 2006;69:991–997. doi: 10.1124/mol.105.019174. [DOI] [PubMed] [Google Scholar]
- Hejnum S, Hassel B. High-affinity glycine and glutamate transport in pig forebrain white and gray matter: a quantitative study. Neurochemistry International. 2007;50:696–702. doi: 10.1016/j.neuint.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Ermilov M, Lichtenberg P, Bar G, Javitt DC. High-dose glycine added to olanzapine and risperidone for the treatment of schizophrenia. Biological Psychiatry. 2004;55:165–171. doi: 10.1016/s0006-3223(03)00707-8. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Horowitz A, Kelly D. Double-blind, placebo-controlled, crossover trial of glycine adjuvant therapy for treatment-resistant schizophrenia. British Journal of Psychiatry. 1996;169:610–617. doi: 10.1192/bjp.169.5.610. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M. Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Archives of General Psychiatry. 1999;56:29–36. doi: 10.1001/archpsyc.56.1.29. [DOI] [PubMed] [Google Scholar]
- Huang H, Barakat L, Wang D, Bordey A. Bergmann glial GlyT1 mediates glycine uptake and release in mouse cerebellar slices. Journal of Physiology. 2004;560:721–736. doi: 10.1113/jphysiol.2004.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd R, Sailasuta N, Srinivasan R, Vigneron DB, Pelletier D, Nelson SJ. Measurement of brain glutamate using TE-averaged PRESS at 3T. Magnetic Resonance in Medicine. 2004;51:435–440. doi: 10.1002/mrm.20007. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Duncan L, Balla A, Sershen H. Inhibition of system A-mediated glycine transport in cortical synaptosomes by therapeutic concentrations of clozapine: implications for mechanisms of action. Molecular Psychiatry. 2005;10:276–287. doi: 10.1038/sj.mp.4001552. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Silipo G, Cienfuegos A, Shelley AM, Bark N, Park M, Lindenmayer JP, Suckow R, Zukin SR. Adjunctive high-dose glycine in the treatment of schizophrenia. International Journal of Neuropsychopharmacology. 2001;4:385–391. doi: 10.1017/S1461145701002590. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. American Journal of Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zylberman I, Zukin SR, Heresco-Levy U, Lindenmayer JP. Amelioration of negative symptoms in schizophrenia by glycine. American Journal of Psychiatry. 1994;151:1234–1236. doi: 10.1176/ajp.151.8.1234. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kanahara N, Shimizu E, Ohgake S, Fujita Y, Kohno M, Hashimoto T, Matsuzawa D, Shirayama Y, Hashimoto K, Iyo M. Glycine and D: -serine, but not D: -cycloserine, attenuate prepulse inhibition deficits induced by NMDA receptor antagonist MK-801. Psychopharmacology (Berlin) 2008;198:363–374. doi: 10.1007/s00213-008-1151-6. [DOI] [PubMed] [Google Scholar]
- Karasawa J, Hashimoto K, Chaki S. D-Serine and a glycine transporter inhibitor improve MK-801-induced cognitive deficits in a novel object recognition test in rats. Behavioural Brain Research. 2008;186:78–83. doi: 10.1016/j.bbr.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Kaufman MJ, Henry ME, Frederick deB B, Hennen J, Villafuerte RA, Stoddard EP, Schmidt ME, Cohen BM, Renshaw PF. Selective serotonin reuptake inhibitor discontinuation syndrome is associated with a rostral anterior cingulate choline metabolite decrease: a proton magnetic resonance spectroscopic imaging study. Biological Psychiatry. 2003;54:534–539. doi: 10.1016/s0006-3223(02)01828-0. [DOI] [PubMed] [Google Scholar]
- Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. Journal of Neurochemistry. 1989;52:1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Konradsson A, Marcus MM, Hertel P, Svensson TH, Jardemark KE. Inhibition of the glycine transporter GlyT-1 potentiates the effect of risperidone, but not clozapine, on glutamatergic transmission in the rat medial prefrontal cortex. Synapse. 2006;60:102–108. doi: 10.1002/syn.20286. [DOI] [PubMed] [Google Scholar]
- Labrie V, Lipina T, Roder JC. Mice with reduced NMDA receptor glycine affinity model some of the negative and cognitive symptoms of schizophrenia. Psychopharmacology (Berlin) 2008;200:217–230. doi: 10.1007/s00213-008-1196-6. [DOI] [PubMed] [Google Scholar]
- Lane HY, Chang YC, Liu YC, Chiu CC, Tsai GE. Sarcosine or D-serine add-on treatment for acute exacerbation of schizophrenia: a randomized, double-blind, placebo-controlled study. Archives of General Psychiatry. 2005;62:1196–1204. doi: 10.1001/archpsyc.62.11.1196. [DOI] [PubMed] [Google Scholar]
- Leiderman E, Zylberman I, Zukin SR, Cooper TB, Javitt DC. Preliminary investigation of high-dose oral glycine on serum levels and negative symptoms in schizophrenia: an open-label trial. Biological Psychiatry. 1996;39:213–215. doi: 10.1016/0006-3223(95)00585-4. [DOI] [PubMed] [Google Scholar]
- Linn GS, O’Keeffe RT, Lifshitz K, Schroeder C, Javitt DC. Behavioral effects of orally administered glycine in socially housed monkeys chronically treated with phencyclidine. Psychopharmacology (Berlin) 2007;192:27–38. doi: 10.1007/s00213-007-0771-6. [DOI] [PubMed] [Google Scholar]
- Martin G, Guadano-Ferraz A, Morte B, Ahmed S, Koob GF, De Lecea L, Siggins GR. Chronic morphine treatment alters N-methyl-D-aspartate receptors in freshly isolated neurons from nucleus accumbens. Journal of Pharmacology and Experimental Therapeutics. 2004;311:265–273. doi: 10.1124/jpet.104.067504. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Carson R, Henry S, Planeta-Wilson B, Binneman B, Maguire RP, Luckenbaugh DA, D’Souza C, Krystal JH, Frost JJ. Cerebral metabolic effects of intravenous glycine in healthy human subjects. Journal of Clinical Psychopharmacology. 2006;26:595–599. doi: 10.1097/01.jcp.0000245558.14284.aa. [DOI] [PubMed] [Google Scholar]
- Ogg RJ, Kingsley PB, Taylor JS. WET, a T1- and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. Journal of Magnetic Resonance B. 1994;104:1–10. doi: 10.1006/jmrb.1994.1048. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Archives of General Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Jin Y, Bunney BG, Costa J, Gulasekaram B. Effect of clozapine and adjunctive high-dose glycine in treatment-resistant schizophrenia. American Journal of Psychiatry. 1999;156:145–147. doi: 10.1176/ajp.156.1.145. [DOI] [PubMed] [Google Scholar]
- Prescot AP, Frederick deB B, Wang L, Brown J, Jensen JE, Kaufman MJ, Renshaw PF. In vivo detection of brain glycine with echo-time-averaged (1)H magnetic resonance spectroscopy at 4.0 T. Magnetic Resonance in Medicine. 2006;55:681–686. doi: 10.1002/mrm.20807. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic Resonance in Medicine. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Provencher SW. LCModel and LCMgui User’s Manual. 2005. p. 163. [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. Journal of Neuroscience. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse RB, Theut SK, Banay-Schwartz M, Leighton M, Scarcella E, Cohen CG, Deutsch SI. Glycine adjuvant therapy to conventional neuroleptic treatment in schizophrenia: an open-label, pilot study. Clinical Neuropharmacology. 1989;12:416–424. doi: 10.1097/00002826-198910000-00006. [DOI] [PubMed] [Google Scholar]
- Roux MJ, Supplisson S. Neuronal and glial glycine transporters have different stoichiometries. Neuron. 2000;25:373–383. doi: 10.1016/s0896-6273(00)80901-0. [DOI] [PubMed] [Google Scholar]
- Silk DB, Kumar PJ, Perrett D, Clark ML, Dawson AM. Amino acid and peptide absorption in patients with coeliac disease and dermatitis herpetiformis. Gut. 1974;15:1–8. doi: 10.1136/gut.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll B, Henry J, Reeds PJ, Yu H, Jahoor F, Burrin DG. Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets. Journal of Nutrition. 1998;128:606–614. doi: 10.1093/jn/128.3.606. [DOI] [PubMed] [Google Scholar]
- Toth E, Lajtha A. Elevation of cerebral levels of nonessential amino acids in vivo by administration of large doses. Neurochemical Research. 1981;6:1309–1317. doi: 10.1007/BF00964352. [DOI] [PubMed] [Google Scholar]
- Toth E, Lajtha A. Antagonism of phencyclidine-induced hyperactivity by glycine in mice. Neurochemical Research. 1986;11:393–400. doi: 10.1007/BF00965013. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Yang P, Chung LC, Tsai IC, Tsai CW, Coyle JT. D-serine added to clozapine for the treatment of schizophrenia. American Journal of Psychiatry. 1999;156:1822–1825. doi: 10.1176/ajp.156.11.1822. [DOI] [PubMed] [Google Scholar]
- Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. Journal of Neuroscience. 1993;13:981–989. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Bachteler D, Danysz W, Spanagel R. The role of the NMDA receptor in alcohol relapse: a pharmacological mapping study using the alcohol deprivation effect. Neuropharmacology. 2005;48:822–829. doi: 10.1016/j.neuropharm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Viola A, Chabrol B, Nicoli F, Confort-Gouny S, Viout P, Cozzone PJ. Magnetic resonance spectroscopy study of glycine pathways in nonketotic hyperglycinemia. Pediatric Research. 2002;52:292–300. doi: 10.1203/00006450-200208000-00024. [DOI] [PubMed] [Google Scholar]
- Waziri R. Glycine therapy of schizophrenia. Biological Psychiatry. 1988;23:210–211. doi: 10.1016/0006-3223(88)90093-5. [DOI] [PubMed] [Google Scholar]
- Williams JB, Mallorga PJ, Conn PJ, Pettibone DJ, Sur C. Effects of typical and atypical antipsychotics on human glycine transporters. Schizophrenia Research. 2004;71:103–112. doi: 10.1016/j.schres.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Wojcik SM, Katsurabayashi S, Guillemin I, Friauf E, Rosenmund C, Brose N, Rhee JS. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron. 2006;50:575–587. doi: 10.1016/j.neuron.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Wu G. Intestinal mucosal amino acid catabolism. Journal of Nutrition. 1998;128:1249–1252. doi: 10.1093/jn/128.8.1249. [DOI] [PubMed] [Google Scholar]
- Zafra F, Aragon C, Olivares L, Danbolt NC, Gimenez C, Storm-Mathisen J. Glycine transporters are differentially expressed among CNS cells. Journal of Neuroscience. 1995;15:3952–3969. doi: 10.1523/JNEUROSCI.15-05-03952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]