Abstract
Phenotypic remodeling of Schwann cells is required to ensure successful regeneration of damaged peripheral axons. After nerve damage, Schwann cells produce an over 100-fold increase in metalloproteinase-9 (MMP-9), and therapy with an MMP inhibitor increases the number of resident (but not infiltrating) cells in injured nerve. Here, we demonstrate that MMP-9 regulates proliferation and trophic signaling of Schwann cells. Using in vivo BrdU incorporation studies of axotomized sciatic nerves of MMP-9−/− mice, we found increased Schwann cell mitosis in regenerating (proximal) stump relative to wild-type mice. Treatment of cultured primary Schwann cells with recombinant MMP-9 suppressed their growth, mitogenic activity and produced a dose-dependent, biphasic and selective activation of ERK1/2, but not JNK and p38 MAPK. MMP-9 induced ERK1/2 signaling in both undifferentiated and differentiated (using dbcAMP) Schwann cells. Using inhibitors to MEK and trophic tyrosine kinase receptors, we established that MMP-9 regulates Ras/Raf/MEK - ERK pathways through IGF-1, ErbB and PDGF receptors. We also report on the early changes of MMP-9 mRNA expression (within 24 h) after axotomy. These studies establish that MMP-9 controls critical trophic signal transduction pathways and phenotypic remodeling of Schwann cells.
Keywords: Schwann cell, EGF, IGF, PDGF, glia, proliferation, nerve injury, mitosis, MMP
INTRODUCTION
To ensure successful peripheral nerve regeneration Schwann cells strive to survive through vigorous proliferation, controlled by a well-coordinated network of mitogens. For example, neuregulins, such as neuregulin-1 (NRG-1), interact with the tyrosine kinase receptors of the erbB family to regulate Schwann cell proliferation (Corfas et al. 2004; Jessen and Mirsky 2005). Interference with NRG-1-erbB signaling results in excessive Schwann cell proliferation, as indicated by the increase in 5-bromo-2-deoxyuridine (BrdU) incorporation in Schwann cells in injured nerves of transgenic mice expressing dominant-negative ErbB4 receptor (Chen et al. 2003). Sustained activation of the Ras/Raf/MEK extracellular signal-regulated kinase (ERK) pathway, a downstream event of NRG-1-erbB and other trophic systems, induces cell cycle arrest as a protective checkpoint mechanism to prevent excessive mitosis (Lloyd et al. 1997; Marshall 1995). Because trophic systems activate ERK to both, initiate and terminate cell mitosis, identifying their upstream modulators is critical to elucidating the mechanisms of Schwann cell survival after nerve injury.
Metalloproteases (MPs) are extracellular proteases that include related families of matrix metalloproteinases (MMPs) and a dysintegrin and metalloproteases (ADAMs) (Werb 1997), and regulate activation of trophic systems through proteolytic cleavage of ligand and/or exracellular domains of their tyrosine kinase receptors (Page-McCaw et al. 2007). For example, ADAM-17 (or TNF converting enzyme, TACE) is required for processing and subsequent nuclear translocation of ErbB4 receptor (Rio et al. 2000; Vecchi and Carpenter 1997), whereas MMP-9 and MMP-12 regulate IGF-1 release from its binding protein in the CNS (Larsen et al. 2006). Upregulation of MPs has been attributed to the pathogenesis of experimental and clinical peripheral nerve damage (Demestre et al. 2004; Leppert et al. 1999; Platt et al. 2003; Shubayev and Myers 2002), but their role in Schwann cell survival or regulation of trophic signaling is not well understood.
MMP-9 (or gelatinase B) is an intriguing MMP family member found in adult nerve only after injury and predominantly in Schwann cells (Chattopadhyay et al. 2007; Demestre et al. 2004; La Fleur et al. 1996; Shubayev et al. 2006; Shubayev and Myers 2000; Shubayev and Myers 2002). Dominant-negative MMP-9 gene knockout (MMP-9−/−) mice demonstrate remarkable protection from peripheral Wallerian degeneration due to MMP-9 control of myelin protein degradation and macrophage migration into the injured sciatic nerves (Shubayev et al. 2006)Chattopadhyay et al. 2007; Kobayashi et al. 2008). Because MMP inhibition increases the number of resident but not infiltrating immune cells at the nerve injury site (Kobayashi et al. 2008), we hypothesized that MMPs suppress survival of resident cells (predominantly, Schwann cells in population).
This study aimed to determine the role of MMP-9 in Schwann cell mitosis, and establish whether MMP-9 regulates trophic signaling in Schwann cells. We found increased BrdU incorporation in the proximal (regenerating) but not distal (degenerating) stumps of axotomized sciatic nerves of MMP-9−/− mice. Treatment of cultured primary Schwann cells with exogenous MMP-9 suppressed BrdU incorporation and induced the Ras/Raf/MEK-ERK pathway via IGF-1, an ErbB and PDGF tyrosine kinase receptors. This study established that MMP-9 activates critical trophic systems in Schwann cells and signals to suppress Schwann cell mitosis in vivo and in vitro, and pointed at differential roles of MMP-9 in the processes of peripheral nerve regeneration and degeneration.
MATERIALS AND METHODS
Reagents
Dulbecco’s modified Eagle’s medium (DMEM) and DMEM Ham’s F12 (Gibco), poly-D-lysine hydrobromide (PDL, Sigma), forskolin (Calbiochem), cytosine-D-arabino-furanoside (AraC), anti-Thy1.1 antibody and rabbit complement from Sigma, fetal bovine serum (FBS, Hyclone), 5-bromo-2-deoxyuridine (BrdU, Calbiochem), bovine pituitary extract (Clonetics), N2 supplement (Gibco), 6, O2′-dibutyryl adenosine 3′,5′-monophosphate (dibutyryl cyclic AMP, dbcAMP, Sigma), nuclear stain 4′-6-diamidino-2-phenylindole (DAPI, Molecular Probes, 1:20,000). Bovine serum albumin (BSA, 100 μg/ml, Sigma), recombinant rat tumor necrosis factor alpha (TNF-α, 10 ng/ml, R&D), lipopolysaccharide (LPS, 100 ng/ml, Sigma), recombinant murine 7S nerve growth factor (NGF, 100 ng/ml, Invitrogen), recombinant human neuregulin 1 (NRG-1, 10 ng/ml, R&D Systems) and recombinant human active MMP-9 (rhMMP-9, Calbiochem). Polyclonal anti-phospho- and total ERK, JNK and p38 were all obtained from Cell Signaling (Danvers, MA), polyclonal anti-MMP-9 (Chemicon), polyclonal anti-S100 (Dako, Carpinteria, CA), rabbit anti-myelin protein zero (P0, Protein Tech Group, IL) and mouse anti-β-actin (Sigma).
Pharmacologic Inhibitors were obtained from Calbiochem and their concentrations are selected based on their respective IC50 values and previous use in similar experiments: ErbB1/2/4 Receptor Inhibitor (ErbB-I, 10 μM), ErbB2 Receptor Inhibitor (ErbB2-I, 10–50 μM), PDGF Receptor Tyrosine Kinase Inhibitor (AG 1296, 10 μM), IGF-1R inhibitor PPP (IGF1R-I, 10 μM), MEK1 inhibitor (PD98059, 10 μM), MEK1/2 inhibitor (U0126, 10 μM) and PI3K inhibitor (LY294002, 50 μM). The broad-spectrum MMP inhibitor, GM6001 (Ilomastat, 10 μM, Chemicon), has Ki of 0.4 nM for MMP-1, 27 nM for MMP-3, 0.5 nM for MMP-2, 0.1 nM for MMP-8, and 0.2 nM for MMP-9.
Animals and Surgeries
Adult female Sprague-Dawley rats (N = 48; 250 g, Harlan Labs, San Diego, CA), adult female FVB.Cg-Mmp9tm1Tvu/J mice (MMP-9−/−, N = 10; 20 g) and age-matched female wild-type FVB/NJ mice (WT, N = 10; 20 g, Jackson Labs, Bar Harbor, ME) were used. Animals were housed at 22°C under a 12 h light/dark cycle with ad libitum access to food and water. FVB.Cg-Mmp9tm1Tvu/J originated on a B6;129 background was mated to Black Swiss mice for an unknown number of generations and crossed to FVB/N mice for five generations before being made homozygous. Anesthesia was achieved with 4% isofluorane (IsoSol; Vedco, St. Joseph, MO). The rat or mouse sciatic nerve was exposed unilaterally at the mid-thigh level, and transected to produce a sciatic nerve axotomy. Animals were sacrificed using intraperitoneal injection of a deep anesthesia cocktail of pentobarbital (Nembutal, 50 mg/ml; Abbott Labs, North Chicago, IL), diazepam (5 mg/ml, Steris Labs, Phoenix, AZ) and saline (0.9%, Steris Labs), followed by lethal intracardiac injection of Euthasol (Virbac, Fort Worth, TX, 100–150 mg/kg). Nerve sections proximal and distal to transection were collected for analysis at 10 min - 4 d after axotomy. Contralateral to injury and sham-operated (unilaterally exposured) nerves were collected for controls. Animal protocols were approved by the VA Healthcare System Committee on Animal Research, and conform to the NIH Guidelines for Animal Use.
In vivo BrdU labeling and detection
In vivo BrdU incorporation studies in sciatic nerve were done as published (Cheng and Zochodne 2002). FVB/MMP-9−/− (N= 5/group) or wild type FVB/NJ mice (N= 5/group) underwent axotomy. BrdU (100 mg/kg) or vehicle (1 mM Tris, 0.8% NaCl, 0.25 mM EDTA, pH 7.4) was injected intraperitoneally 4 h and 2 h before sacrifice. Four days after axotomy, animals were perfused with 4% PFA and sacrificed, as described above. Sciatic nerves were isolated, post-fixed in 4% PFA overnight, rinsed, cryoprotected in graded sucrose, embedded into OCT compound in liquid N2 and cut into 10-μm-thick transverse sections. For BrdU detection, the sections were rinsed in PBS, hydrolyzed in 2N HCl in PBS for 30 min, digested with 0.01% Trypsin for 30 min at 37°C and washed with PBS. Non-specific binding was blocked with 10% normal goat serum. Mouse anti-BrdU antibody (Sigma, 1:1000) was applied for 2 h at 37°C, followed with a PBS rinse and goat anti-mouse Alexa 488 antibody (Invitrogen) treatment for 1 h at RT.
Morphometry
DAPI- and BrdU-positive profiles were quantified in transverse sham and axotomized proximal and distal sciatic nerve sections at objective magnification × 40 in 4 mice per group, 2 sections per animal, 3 randomly selected fields per section by a blinded experimenter using Openlab 4.0 software (Improvision) followed by statistical analyses (SPSS 16.0 software).
Schwann cell cultures
Primary Schwann cells were cultured as described (Brockes et al. 1979). Briefly, sciatic nerves of post-natal day 1 Sprague Dawley rats (Harlan Labs) were isolated and purified using AraC, an anti-fibronectin Thy1.1 antibody and rabbit complement (Shubayev et al. 2006). Schwann cell purity was confirmed by over 99% pure S-100-positive profiles. Schwann cells were plated on PDL-coated dishes in DMEM containing 10% FBS, 100 units/ml penicillin and 100 μg/ml streptomycin, 21 μg/ml bovine pituitary extract and 4 μM forskolin (referred to as “complete media”) at 37°C under humidified 5.0% CO2. Cells were passaged upon confluency and used at 3–7 passages. Schwann cell differentiation was induced with 500 μM dbcAMP for 48 h. Successful differentiation was confirmed by myelin protein zero (P0) expression. For inhibition studies, cells were maintained in DMEM containing 0.5% FBS for 16 h, washed and inhibitors (specified above) were added 15 min before rhMMP-9 treatment for 6 h.
Growth kinetics assay
Kinetics of Schwann cell growth was determined using crystal violet staining. Cells were plated in triplicate at 1 × 104 cells/well in a 24-well plate and allowed to grow in complete medium with or without 100nM rhMMP-9. Then fixed with 1% glutaraldehyde for 20 min and stained by 0.1% crystal violet for 45 min at room temperature. Unbound dye was washed away with water, while bound dye was eluted with 10% acetic acid and measured the absorbance at 590 nm. The value of relative increase of absorbance versus time ± S.D. was plotted for each time-point relative to absorbance values of cells attached overnight (representing a 0 h time-point).
BrdU cell proliferation assay
Cells were plated in 96-well PDL-coated plates at 2 × 105 cells/ml in 1% FBS DMEM overnight. The inhibitors were applied for 30 min, followed by treatment with 100 nM rhMMP-9 and BrdU labeling for 8 h. Newly synthesized DNA was detected using the BrdU Cell Proliferation Assay (Calbiochem), following the manufacturer’s protocol for cell denaturation, mouse anti-BrdU antibody and HRP-tagged goat anti-mouse IgG applications. Chromogenic tetramethylbenzidine substrate was applied for 15 min and the reaction was stopped with 2.5N sulfuric acid. Absorbance was measured at 450–595 nm using a Spectramax plate reader (Molecular Devices, Downingtown, PA). All samples were analyzed in quadruplicate in three independent experiments. Statistical analyses were done by ANOVA and Tukey-Kramer post-hoc test using SPSS 16.0 software.
Western blotting
Sciatic nerves were isolated, snap frozen in liquid N2 and stored at −80°C. Proteins were extracted using lysis buffer (50 mM Tris-HCl, pH 7.4, 1% NP 40, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 eg/mL aprotinin and leupeptin, 1 mM sodium orthovanadate). Schwann cells were lysed in a buffer containing 10 mM Tris-HCl, 1% NP-40, 0.1% sodium deoxycholate, 1 mM EDTA, 2 mM sodium orthovanadate, 10 mM sodium fluoride and 10 g/ml aprotinin (Chattopadhyay et al. 2006). Total protein was measured using Pierce BCA Protein Assay. Samples containing equal amounts of protein (10 μg for cells and 30–50 μg for tissues) were run on SDS-PAGE and transferred to Immobilon-P (Millipore, Bedford, MA) in Tris-Glycine transfer buffer (Invitrogen) at 200 mA for 1 h. The membranes were blocked with 5% non-fat milk (Biorad), incubated with a primary antibody (identified above) in 5% BSA in TBS overnight at 4°C, washed in TBS containing 0.05% Tween 20 and incubated for 1 h at RT with HRP-conjugated anti-rabbit or anti-mouse secondary antibody (Cell Signaling; 1:10,000). The blots were developed using ECL (Amersham), followed by densitometry with NIH Image J 1.38 software. All blots represent at least 3 independent in vitro experiments or N=3 animals per group.
Real-time qPCR
Primers and Taqman probes for rat MMP-9 (Biosearch Technologies, Novato, CA) were optimized as described (Shubayev et al. 2006). Nerve samples were stored in RNA-later (Ambion) at −20°C. RNA was extracted from sciatic nerves and cell lysates with Trizol (Invitrogen) and treated with RNAse-free DNAse (Qiagen). The RNA purity was verified by OD260/280 absorption ratio of ~ 2.0. cDNA was synthesized using a SuperScript first-strand RT-PCR kit (Invitrogen). Gene expression was measured by quantitative real-time qPCR (MX4000, Stratagene, La Jolla, CA) using 50 ng of cDNA and 2x Taqman Universal PCR Master Mix (Applied Biosystems) with a one-step program: 95°C for 10 min, 95°C for 30 seconds and 60°C for 1 min for 50 cycles. Duplicate samples without cDNA (no-template control) showed no contaminating DNA. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a normalizer gene. Relative mRNA levels were quantified using the comparative Ct method (Livak and Schmittgen 2001). A fold change was determined by the MX4000 software using the described methods (Pfaffl 2001).
Immunofluorescence
Nerves were perfused and post-fixed in 4% PFA overnight, rinsed, cryoprotected in graded sucrose, embedded into OCT compound in liquid N2 and cut into 10-μm-thick transverse sections. Cells were fixed with 4% PFA in TBS for 10 min, washed with TBS and permeabilized with 0.1% Triton X in TBS. Nonspecific binding was blocked with 10% goat serum. In tissue sections, endogenous aldehydes were blocked with 0.5% sodium borohydride in 1% dibasic sodium phosphate for 5 min and Dako antigen retrieval (Carpinteria, CA) was applied for 5 min at 95°C, then for 20 min at RT. Primary antibodies were diluted in TBS containing 1% FBS and applied sequentially, the first primary antibody for 1 h at RT, then rinsed, followed by Alexa 564 conjugated (red) first secondary goat antibody for 1 h at RT and second primary antibody application overnight at 4°C. Slides were rinsed in PBS containing 0.1% Tween 20 and incubated with second Alexa 488 conjugated (green) secondary goat antibody for 1 h at 22°C. DAPI (1:20,000) was applied for 5 min. Replacement of primary antibody with the respective normal IgG was done to control signal specificity. Imaging was performed using a Leica DMR bright-light and fluorescence microscope using Openlab 4.0 software (Improvision). All micrographs represent at least 3 independent in vitro experiments and N=3 animals per group.
RESULTS
MMP-9 is induced in Schwann cells by proinflammatory but not trophic factors
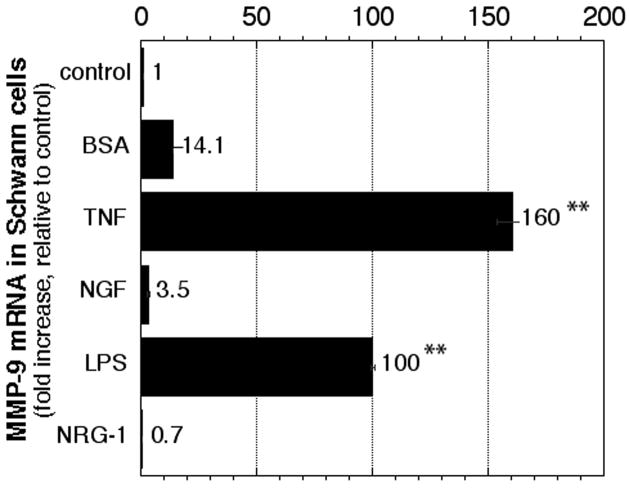
To identify the stimuli for MMP-9 mRNA expression primary Schwann cells were treated with TNF-α, LPS, NGF or NRG-1. MMP-9 mRNA expression was significantly induced 100-fold by LPS and 160-fold by TNF-α, whereas the changes in NGF and NRG-1 were not significant (Fig. 1). BSA, used as a control TNF-α carrier, increased MMP-9 mRNA by 14-fold, consistent with our observations in nerve (Chattopadhyay et al. 2007). This data suggests that Schwann cells produce MMP-9 after pro-inflammatory but not trophic stimulation.
Figure 1. MMP-9 expression in primary Schwann cells.
Taqman qPCR for MMP-9 in primary Schwann cells after 24 h of stimulation with BSA, TNF-α, NGF, LPS or NRG-1. Expressed as mRNA fold increase compared to low serum media, using GAPDH as normalizer. Data represents the mean ± SEM of N=4/group, by one-way ANOVA and Tukey-Kramer post-hoc test (*, p<0.05; **, p<0.01).
MMP-9 gene deletion promotes Schwann cell mitosis in vivo
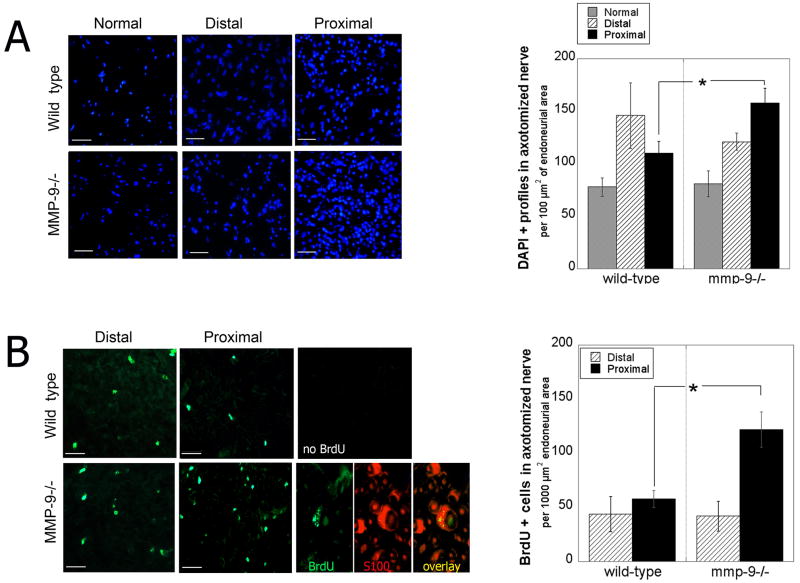
Sciatic nerve axotomy was used to spatially separate events of Wallerian degeneration (distal stump) and regeneration (proximal stump). Nerves were analyzed at 4 d post-injury, when Schwann cell proliferation occurs in wild-type nerve injury models (Cheng and Zochodne 2002; Clemence et al. 1989) and when contribution of infiltrating immune cells in MMP-9−/− mice is minimal (Shubayev et al. 2006). A statistically significant increase in DAPI-positive profiles was observed in the proximal but not distal nerve stumps of MMP-9−/− compared to wild-type mice (Fig. 2, A). In fact, the distal stump demonstrated a 17% decline in cell number in knockout vs. wild-type mice that was not quite statistically significant (p = 0.0594).
Figure 2. Schwann cell proliferation in axotomized nerves of MMP-9 −/− knockout mice.
A, DAPI profiles (blue) in proximal and distal sciatic nerve stumps 4 d after axotomy. Mean ± SEM per 100 μm2 endoneurial area, N = 4/group, 2 sections/N, 3 areas/section, by unpaired Student’s t-test (*, p<0.05). Objective magnification × 40 (scale bar = 50 μm). B, BrdU incorporation (green) in proximal and distal sciatic nerve stumps 4 d after axotomy. Mean ± SEM 1000 μm2 endoneurial area, N=5/group, 2 sections/N, 3 areas/section at objective magnification × 40 (scale bar = 50 μm), by unpaired Student’s t-test (*, p<0.05). Dual-immunofluorescence for BrdU (green) and S100 (Schwann cell marker, red) in MMP-9−/− nerves (scale bar = 20 μm).
In vivo BrdU incorporation studies in axotomized MMP-9−/− mice were performed to assess the role of MMP-9 in cell mitosis. A 2.1-fold increase in cell proliferation was found in proximal nerve stumps of MMP-9−/− compared to wild-type mice (Fig. 2B), whereas no difference was observed in the distal stump. BrdU-positive cells co-localized with S100, a phenotypic marker for Schwann cells, suggesting that MMP-9 suppresses Schwann cell proliferation in vivo.
MMP-9 activates ERK1/2 and reduces growth of primary Schwann cells
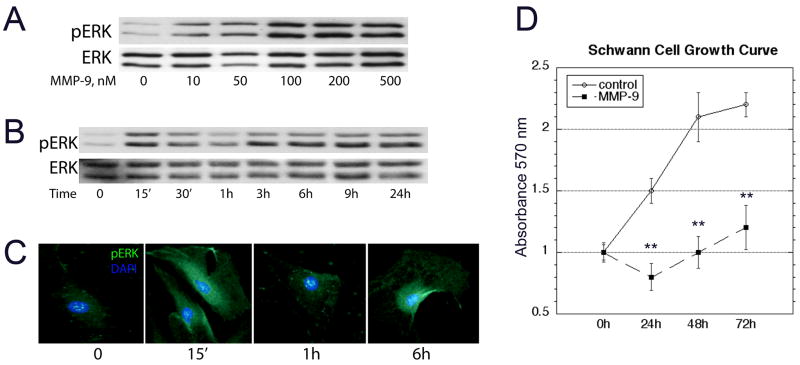
Because ERK MAPK signals cell cycle arrest in Schwann cells (Harrisingh et al. 2004; Lloyd et al. 1997), we studied the effect of rhMMP-9 on ERK1/2 activation, and its correlation to Schwann cell growth. rhMMP-9 produced a dose-dependent activation of ERK1/2 (Fig. 3A). Based on this data, rhMMP-9 of 100 nM was selected for subsequent experiments. Temporal analyses of rhMMP-9 effect in Schwann cells demonstrated biphasic pERK1/2 activation with acute (15 min) and sustained (3–12 h) phases (Fig. 3B). Immunofluorescence for pERK1/2 (Fig. 3C) demonstrates its cytosolic distribution at 15 min and 6 h after rhMMP-9 stimulation, consistent with its phosphorylated state in Schwann cell lysates seen by western blot.
Figure 3. MMP-9 activates ERK1/2 and suppresses Schwann cell growth.
A, MMP-9 activates ERK1/2 in a dose-dependent manner. 100 nM rhMMP-9 was selected for use in the subsequent experiments. B, A time-course of rhMMP-9 treatment demonstrating a biphasic activation of ERK1/2. C, Immunofluorescence for pERK (green) and DAPI (blue) confirms the biphasic reactivity of cytosolic pERK at 15 min and 6 h, objective magnification × 100 (representative micrographs of 3 independent experiments). D, Schwann cell growth curve studies using crystal violet, with and without daily rhMMP-9 stimulation for 72 h. Data represents the mean ± SD of N=3/group, analyzed by one-way ANOVA (*, p<0.05).
The effect of rhMMP-9 on ERK1/2 activation was correlated to Schwann cell growth kinetics. A significantly reduced Schwann cell growth was observed after daily rhMMP-9 treatment over a course of 72 h (Fig. 3D).
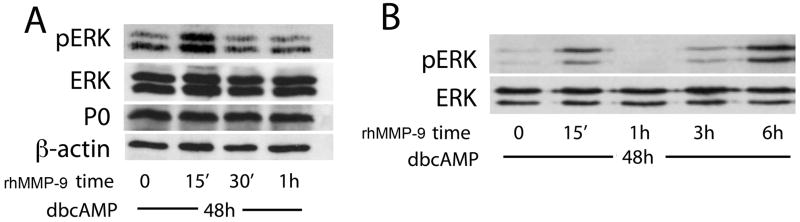
To test whether MMP-9-induced ERK1/2 activation depends on the state of Schwann cell differentiation, the latter was stimulated with dibutyryl cyclic AMP (dbcAMP) for 48 h, as suggested (Harrisingh et al. 2004), followed by treatment with rhMMP-9 (Fig. 4A). Successful differentiation was confirmed by the expression of myelin protein zero (P0). Biphasic activation of pERK, peaking at 15 min and 6 h of rhMMP-9 stimulation in myelinating Schwann cells (Fig. 4B), was consistent with the findings in undifferentiated cells.
Figure 4. rhMMP-9 stimulates biphasic ERK1/2 activation in myelinating Schwann cells.
Schwann cell differentiation was induced with dbcAMP (500 μM) for 48 h, followed by treatment with rhMMP-9 (100 nM) for 15 min. A, rhMMP-9 stimulates transient ERK1/2 activation over a 1 h period. Successful Schwann cell differentiation was confirmed by myelin protein zero (P0) expression, β-actin was used as loading control. B, An extended time-course of rhMMP-9 stimulation displayed biphasic activation of ERK1/2, as seen in undifferentiated cells in Fig. 3.
Correlation of MMP-9 and ERK1/2 expression in injured nerve
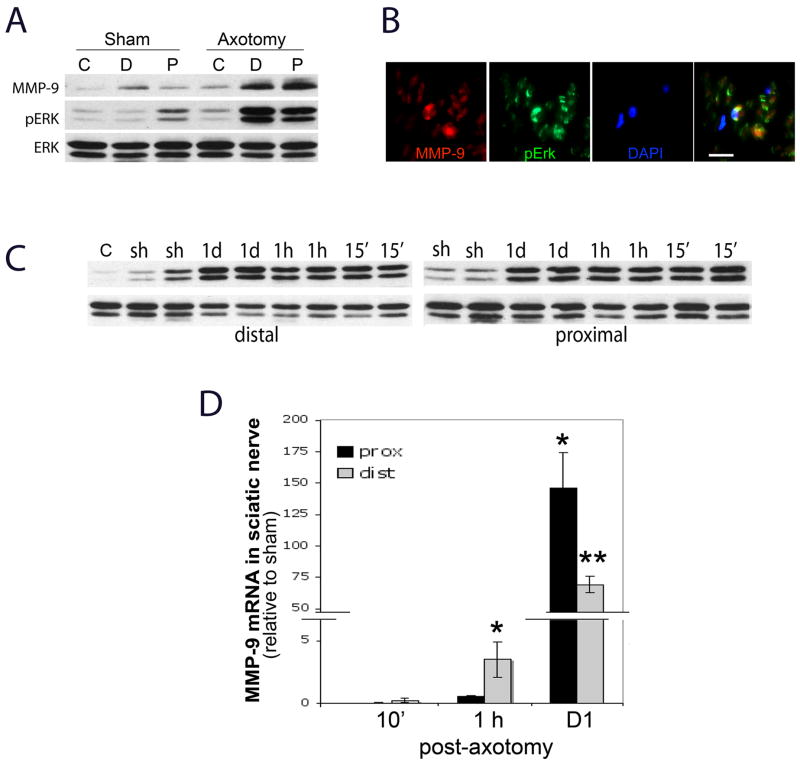
Exogenous MMP-9 can activate ERK1/2 in injured sciatic nerve (Mantuano et al. 2008). Here, we correlated the patterns of endogenous MMP-9 expression and ERK1/2 activation after axotomy, with the focus on immediate changes. At 24 h after nerve injury both MMP-9 and pERK are coordinately expressed in distal and proximal stumps of axotomized sciatic nerves (Fig. 5A), co-localizing in Schwann cells, as determined by a characteristic crescent morphology (Fig. 5B). There was no visible difference between the stumps observed and, thus, only one representative micrograph is shown (Fig. 5B). While ERK1/2 activation has been shown to sustain from 30 min to 8 days of axotomy in both stumps (Sheu et al. 2000), we established its initiation within 15 min of axotomy in both distal and proximal stumps (Fig. 5C).
Figure 5. Endogenous ERK1/2 and MMP-9 in axotomized rat sciatic nerve.
A, Western blot for ERK1/2 and MMP-9 in the proximal (P) and distal (D) stumps 1 d after rat sciatic nerve axotomy relative to contralateral (C) nerves; representative of N=4/group. B, MMP-9 (red) and pERK (green) co-localize in Schwann cells of a proximal stump, a representative micrograph of N=3 (scale bar = 20 μm). C, Sustained ERK1/2 activation in distal and proximal stumps at 15 min, 1 h and 1 d after axotomy, relative to sham (sh) and contralateral (c) nerves. Duplicate representative of N=4/group. D, Real-time Taqman qPCR for MMP-9 in axomotized rat nerves normalized to GAPDH and calibrated to sham. Data represents the mean ± SEM of N=4/group, by one-way ANOVA and Tukey-Kramer post-hoc test (*, p<0.05; **, p<0.01).
Early changes in MMP-9 mRNA expression after any sciatic nerve axotomy have not been reported. Using Taqman qPCR (Fig. 5D), we found no significant change in MMP-9 mRNA at 10 min (i.e., preceding ERK activation), a 3.5-fold induction at 1 h in the distal stump, and a 70-fold increase in distal and a 146-fold increase in proximal stumps at 1 d post-axotomy, representing an about 2-fold higher MMP-9 expression level in proximal vs. distal stumps.
MMP-9 activates MEK/ERK1/2 pathway via ErbB, IGF-1 and PDGF tyrosine kinase receptors
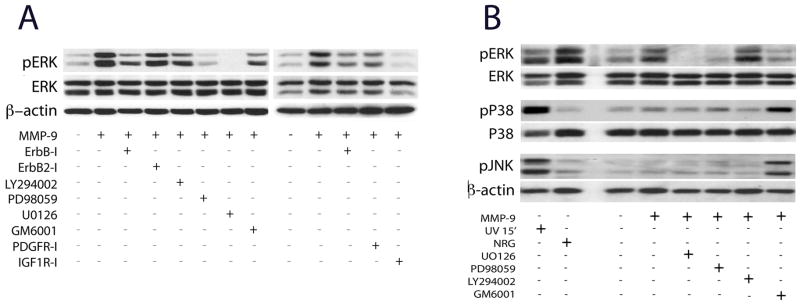
MMPs activate trophic signaling in various cells (Page-McCaw et al. 2007). We analyzed whether MMP-9 induced ERK1/2 signaling by activation of trophic tyrosine kinase receptors involved in regulation of Schwann cell mitosis, including ErbB (Corfas et al. 2004), PDGF and IGF-1 receptors (Delaney et al. 1999; Meier et al. 1999) and/or the MEK/ERK pathway (Fig. 6A). The results are summarized in a schematic diagram (Fig. 8). Focus on sustained and not transient ERK1/2 activation (i.e., 6 h after MMP-9 stimulation) is based on its role in suppression of cell mitosis (Lloyd et al. 1997; Marshall 1995).
Figure 6. rhMMP9 activates MEK-ERK pathway via IGF-1, ErbB and PDGF receptors.
A, Western blot for ERK1/2 in primary Schwann cells 6 h after 100 nM rhMMP-9 stimulation, with or without kinase 15 min of pre-treatment with the inhibitors ErbB1/2/4 receptor (ErbB-I, 10 μM), ErbB2 receptor (ErbB2-I, 10–50 μM), PDGF receptor (PDGFR-I, 10 μM), IGF1 receptor (IGFR-I, 10 μM), MEK (PD98059, 10 μM), MEK1/2 (U0126, 10 μM), PI3K (LY294002, 50 μM), and MMP inhibitor (GM6001, 10 μM). MMP-9-induced ERK activation was inhibited by to MEK ErbB1/2/4, IGF-1R and PDGFR, but not ErbB2 or PI3K inhibitors. B, Western blot for MAPKs of Schwann cells lysates 6 h after treatment with 100 nM rhMMP-9. MMP-9 produced no effect on activation of p38 and JNK, whereas pretreatment with GM6001 induced it. β-actin was used as loading control. Data is representative of three independent experiments.
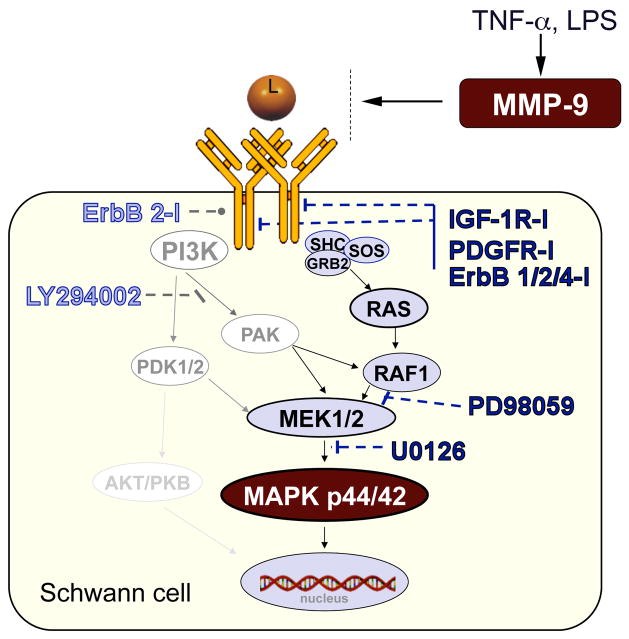
Figure 8. MMP-9 activation of trophic signaling in Schwann cells (a schematic diagram).
MMP-9 expression is induced by proinflammatory stimuli (see Fig. 1). MMP-9 stimulates MAPK p44/42 (or ERK1/2) signaling in Schwann cells via activation IGF-1, ErbB4, and PDGF tyrosine kinase receptor and Ras/Raf/MEK pathway (shown in black), but not PI3K/PDK/MEK pathway (shown in grey), as determined using the specified pharmacologic inhibitors (see Fig. 6).
MMP-9-stimulated pERK1/2 levels declined after treatment with general ErbB1/2/4 inhibitor by 49%, but not the specific ErbB2 inhibitor. PDGF receptor inhibitor (PDGFR-I) blocked MMP-9-stimulated pERK activation by 36%, while IGF-1 receptor inhibitor (IGF1R-I) virtually ablated it. MEK (PD98059) and MEK1/2 (U0126) inhibitors reduced MMP-9-stimulated pERK increase by 79% and 93%, respectively, while control PI3K inhibitor (LY294002) produced little effect. The latter also indicates that MMP-9 does not activate ERK via the PI3K/PDK/MEK pathway (Fig. 8). GM6001 (10 μM), a broad-spectrum MMP inhibitor, reduced MMP-9 effect.
To evaluate selectivity of rhMMP-9-induced ERK1/2 activation, we analyzed the changes in JNK or p38 at 6 h of rhMMP-9 stimulation (Fig. 6B). No change in phospho-JNK or phospho-p38 activation was observed. UV (250 J/m2) stimulation for 15 min was used as positive controls for p38 and JNK activation, and NRG-1 (10 ng/ml) as positive controls for ERK1/2 activation. Note that pre-treatment with GM6001 (a broad-spectrum MMP inhibitor) stimulated JNK or p38 MAPK activation above MMP-9 or basal levels.
ErbB inhibition reversed MMP-9-induced suppression of Schwann cell mitosis
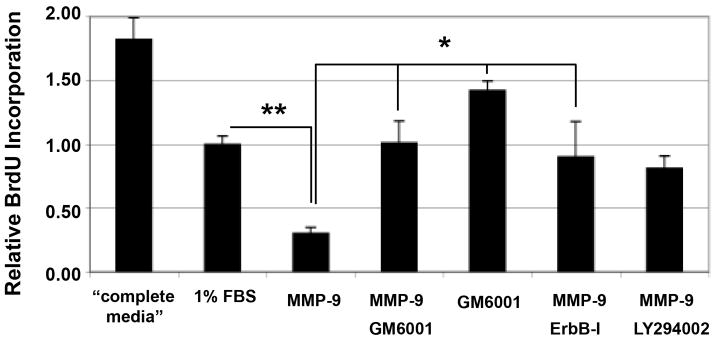
Using an in vitro BrdU incorporation assay, we assessed rhMMP-9 effect on Schwann cell mitosis and its relationship to an MMP-9-dependent ErbB trophic pathway (Fig. 7) that participates in suppression of Schwann cell proliferation (Chen et al. 2003). rhMMP-9 stimulation inhibited Schwann cell mitosis relative to low-serum media. Both ErbB-I and control MMPi, GM6001, reversed anti-mitogenic action of MMP-9. GM6001 treatment without MMP-9 stimulation further promoted mitosis. PI3K inhibitor (LY294002) produced no effect on rhMMP-9-stimulated reduction in mitosis.
Figure 7. MMP-9 inhibits Schwann cell proliferation in vitro.
BrdU incorporation is measured 8 h after treatment with 100 nM rhMMP-9. The GM6001 (50 μM), ErbB-I (10 μM) or LY294002 (50 μM) were applied 15 min before stimulation with rhMMP-9. Complete media containing 10% FBS and pro-mitotic bovine pituitary extract were used as a positive control. Media containing 1% FBS was used for all experimental treatments. Data shown represents the mean ± SEM of 3 independent experiments performed in quadruplicate, analyzed by one-way ANOVA and Tukey-Kramer post-hoc test (**, p<0.05, *, p<0.01).
DISCUSSION
These data are the first to implicate MMP-9 in regulation of Schwann cell proliferation or trophic signaling. We find that MMP-9 can activate the Ras/Raf/MEK - ERK1/2 signal transduction pathway via ErbB, IGF-1 and PDGF tyrosine kinase receptors, as summarized in Fig. 8. The exact mechanisms need to be clarified, as MMPs control cell signaling via regulatory proteolysis of latent signaling factors localized in the extracellular matrix or by (non-proteolytic) direct receptor binding.
Evidence for proteolytic MMP function in activation of trophic systems is sound. For example, release of trophic factors from their regulatory proteins depends on catalytic activity of MMPs (Page-McCaw et al. 2007), such as IGF-1 release from IGF binding protein, IGFBP-6 in CNS (Larsen et al. 2006). IGF-1 can stimulate MMP-mediated release of the EGF ligand from its heparin-bound form (HB-EGF), leading to cumulative transactivation of its own and EGF receptors and Ras/Raf/MEK signaling (El-Shewy et al. 2004; Roudabush et al. 2000). In other cells, MMP-9 controls ERK1/2 via activation of the EGF receptor (Roelle et al. 2003). Of the EGF receptor family, Schwann cells express ErbB2, 3 and 4 (Corfas et al. 2004) and ErbB2 is dispensable in Schwann cell survival after nerve injury (Atanasoski et al. 2006). Considering that ErbB3 has no functional kinase domain (Pearson and Carroll 2004) and ErbB2-I was ineffective in blocking MMP-9-induced ERK1/2 activation, the effects of the general ErbB-I seen here is likely to result from ErbB4 block. Proteolytic processing of ErbB4 and its subsequent nuclear translocation depend on metalloproteases (Vecchi and Carpenter 1997), identified in various cells as ADAM-17, MMP-3, MMP-7 and MMP-9 (Dempsey et al. 2002; Ii et al. 2006; Rio et al. 2000; Sanderson et al. 2006).
Supporting data for MMP-mediated signaling by direct receptor binding is only surfacing. A recent study suggests that hemopexin (substrate-binding) MMP-9 domain fused with a GST protein (GST-MMP-9-PEX) activates ERK1/2 in Schwann cells via low-density lipoprotein receptor related protein 1 (LRP-1) (Mantuano et al. 2008), a hybrid scavenger and signaling receptor (Herz and Strickland 2001). Although the study provides no evidence for its direct LRP-1 binding, GST-MMP-9-PEX contains a binding site for LRP-1, among other surface receptors and substrates (Burg-Roderfeld et al. 2007; Roeb et al. 2002). Consistently, GST-MMP-9-PEX was sufficient to activate ERK in other cells (Dufour et al. 2008). Thus, MMP-9 utilizes several Schwann cell receptor systems to activate ERK signaling, including trophic tyrosine kinase and other signaling receptors, such as LRP-1. Because proteolytic and receptor agonist MMP actions are not mutually exclusive, either or both potentially relate to the trophic systems. Yet, MMPs intrinsically lacking the hemopexin domain, such as MMP-7, are potent inducers of trophic signaling, including that of IGF, EGF and ErbB (Ii et al. 2006; Sanderson et al. 2006), presumably through a proteolytic mechanism.
MMP-9-induced activation of trophic signaling and suppression of Schwann cell mitosis are independent findings. Their relationship was evidenced by ErbB receptor block of MMP-9-induced Schwann cell mitosis. ErbB4−/− in Schwann cells can lead to excessive mitosis (Chen et al. 2003). Because state of Schwann cell differentiation and microenvironment influence the functional outcome of NRG-1/ErbB system action (Corfas et al. 2004; Jessen and Mirsky 2005) and MMP-9 stimulates ERK1/2 in undifferentiated and differentiated (myelinating) Schwann cells, it is important to establish how propensity to myelinate and axonal contact affect the outcomes of MMP-9-induced ERK1/2 activation. In our study, it correlates with reduced Schwann cell growth and suppressed mitosis, consistent with its role in pro-differentiating functions of migration (Mantuano et al. 2008) and myelin protein maintenance (Kobayashi et al. 2008).
MMP-9 selectively activates ERK but not p38 or JNK signaling in Schwann cells, as seen in other cells (Roelle et al. 2003). Because growth arrest and suppression of mitosis is signaled through sustained and not transient Ras/Raf/ERK activation (Lloyd et al. 1997; Marshall 1995), sustained ERK activation (i.e., 6 h after MMP-9 stimulation) was the focus of this study. It will be important to determine the mechanisms of MMP-9 induced transient (15 min) ERK1/2 activation or ability of MMP-9 to induce p38 or JNK pathways in Schwann cells at other time-points in future studies. Interestingly, GM6001 (specific and broad-spectrum MMP inhibitor) activated JNK and p38 and stimulated in vitro BrdU incorporation above basal levels, implicating endogenous MMPs in suppressing these signaling pathways and Schwann cell mitosis. For example, MMP-3 can generate anti-mitogenic fibronectin fragments in Schwann cells (Muir and Manthorpe 1992).
Increased Schwann cell mitosis in axotomized MMP-9−/− nerves was consistent with anti-mitogenic properties of MMP-9 in primary Schwann cells (both were determined by BrdU incorporation). While other cells types might have contributed to the increased number of mitotic cells, neuronal cell bodies were excluded from analyses and MMP-9−/− nerves were deficient in macrophages (Shubayev et al. 2006). An interesting finding is that excessive mitosis was uncompensated only in the proximal (regenerating) but not distal (degenerating) stump of axotomized MMP-9−/− nerves. The failure of distal MMP-9−/− nerves to recruit macrophages (Shubayev et al. 2006) is consistent with the diminished contribution of macrophage-released factors to promote Schwann cell mitosis (Baichwal et al. 1988). Besides, the patterns of Schwann cell proliferation are intrinsically different between the stumps. It spans from 48 h to 14 d after axotomy in the proximal stump, accompanied by de-differentiation of myelinating Schwann cells (Cheng and Zochodne 2002), also regulated by the Ras/Raf/MEK pathway (Harrisingh et al. 2004). In the distal stump, myelinating Schwann cells remain mitotic longer than non-myelinating phenotype (Clemence et al. 1989), owing to pro-mitogenic action of myelin degradation products (Clemence et al. 1989; Murinson et al. 2005). Thus, reduced degradation of myelin basic protein in distal MMP-9−/− nerves (Kobayashi et al. 2008) can also explain their reduced mitogenic activity. Overall, these differential responses of axotomized stumps to MMP-9 gene deletion support a model of distinct MMP-9 actions in nerve degeneration and regeneration.
Although Schwann cells express low levels of MMP-9 in normal nerve (Shubayev and Myers 2000; Shubayev and Myers 2002), they induce MMP-9 expression in denervated Schwann cells within 1 h after axotomy. We have already demonstrated an over 200-fold increase in MMP-9 mRNA by 6 h of sciatic nerve damage (Shubayev et al. 2006). Cytokines induce MMP-9 mRNA in nerves to promote neuroinflammatory remodeling (Chattopadhyay et al. 2007; Shubayev et al. 2006). Thus, induction of MMP-9 mRNA in response to proinflammatory (LPS and TNF-α), but not trophic (NGF and NRG-1) stimuli in cultured Schwann cells is consistent with this earlier developed paradigm. rhMMP-9-induced ERK activation seen in vitro correlates with MMP-9 ability to activate ERK in injured sciatic nerve (Mantuano et al. 2008). But because ERK1/2 activation precedes endogenous MMP-9 expression, we suggest that MMP-9 is not the initial stimulus to ERK1/2 activation after axotomy. This is not surprising given that ERK1/2 signals for a plethora of cytokines and trophic factors after nerve damage (Ji and Woolf 2001). Moreover, our results do not rule out the possibility that ERK signaling is utilized (by cytokines) to induce MMP-9, as found in cortical astrocytes (Arai et al. 2003).
In conclusion, MMP-9 emerges as a potent modulator of Schwann cell signaling and phenotypic remodeling after nerve injury. It suppresses Schwann cell mitosis and supports functions of differentiation, such as migration and myelin protein maintenance (Kobayashi et al. 2008).
Acknowledgments
We thank Jennifer Dolkas and Julie Janes for technical assistance, and Amber Millen for help in editing the manuscript. This work is supported by the Department of Veterans Affairs Merit Review Award and NIH/NINDS R21 NS060307-01 Award to V.I.S.
References
- Arai K, Lee SR, Lo EH. Essential role for ERK mitogen-activated protein kinase in matrix metalloproteinase-9 regulation in rat cortical astrocytes. Glia. 2003;43(3):254–64. doi: 10.1002/glia.10255. [DOI] [PubMed] [Google Scholar]
- Atanasoski S, Scherer SS, Sirkowski E, Leone D, Garratt AN, Birchmeier C, Suter U. ErbB2 signaling in Schwann cells is mostly dispensable for maintenance of myelinated peripheral nerves and proliferation of adult Schwann cells after injury. J Neurosci. 2006;26(7):2124–31. doi: 10.1523/JNEUROSCI.4594-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baichwal RR, Bigbee JW, DeVries GH. Macrophage-mediated myelin-related mitogenic factor for cultured Schwann cells. Proc Natl Acad Sci U S A. 1988;85(5):1701–5. doi: 10.1073/pnas.85.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165(1):105–18. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Burg-Roderfeld M, Roderfeld M, Wagner S, Henkel C, Grotzinger J, Roeb E. MMP-9-hemopexin domain hampers adhesion and migration of colorectal cancer cells. Int J Oncol. 2007;30(4):985–92. doi: 10.3892/ijo.30.4.985. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Machado-Pinilla R, Manguan-Garcia C, Belda-Iniesta C, Moratilla C, Cejas P, Fresno-Vara JA, de Castro-Carpeno J, Casado E, Nistal M, et al. MKP1/CL100 controls tumor growth and sensitivity to cisplatin in non-small-cell lung cancer. Oncogene. 2006;25(23):3335–45. doi: 10.1038/sj.onc.1209364. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Myers RR, Janes J, Shubayev V. Cytokine regulation of MMP-9 in peripheral glia: implications for pathological processes and pain in injured nerve. Brain Behav Immun. 2007;21(5):561–8. doi: 10.1016/j.bbi.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Rio C, Ji RR, Dikkes P, Coggeshall RE, Woolf CJ, Corfas G. Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat Neurosci. 2003;6(11):1186–93. doi: 10.1038/nn1139. [DOI] [PubMed] [Google Scholar]
- Cheng C, Zochodne DW. In vivo proliferation, migration and phenotypic changes of Schwann cells in the presence of myelinated fibers. Neuroscience. 2002;115(1):321–9. doi: 10.1016/s0306-4522(02)00291-9. [DOI] [PubMed] [Google Scholar]
- Clemence A, Mirsky R, Jessen KR. Non-myelin-forming Schwann cells proliferate rapidly during Wallerian degeneration in the rat sciatic nerve. J Neurocytol. 1989;18(2):185–92. doi: 10.1007/BF01206661. [DOI] [PubMed] [Google Scholar]
- Corfas G, Velardez MO, Ko CP, Ratner N, Peles E. Mechanisms and roles of axon-Schwann cell interactions. J Neurosci. 2004;24(42):9250–60. doi: 10.1523/JNEUROSCI.3649-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney CL, Cheng HL, Feldman EL. Insulin-like growth factor-I prevents caspase-mediated apoptosis in Schwann cells. J Neurobiol. 1999;41(4):540–8. doi: 10.1002/(sici)1097-4695(199912)41:4<540::aid-neu9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Demestre M, Wells GM, Miller KM, Smith KJ, Hughes RA, Gearing AJ, Gregson NA. Characterisation of matrix metalloproteinases and the effects of a broad-spectrum inhibitor (BB-1101) in peripheral nerve regeneration. Neuroscience. 2004;124(4):767–79. doi: 10.1016/j.neuroscience.2003.12.037. [DOI] [PubMed] [Google Scholar]
- Dempsey PJ, Garton K, Raines EW. Emerging roles of TACE as a key protease in ErbB ligand shedding. Mol Interv. 2002;2(3):136–41. doi: 10.1124/mi.2.3.136. [DOI] [PubMed] [Google Scholar]
- Dufour A, Sampson NS, Zucker S, Cao J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. J Cell Physiol. 2008;217(3):643–51. doi: 10.1002/jcp.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shewy HM, Kelly FL, Barki-Harrington L, Luttrell LM. Ectodomain shedding-dependent transactivation of epidermal growth factor receptors in response to insulin-like growth factor type I. Mol Endocrinol. 2004;18(11):2727–39. doi: 10.1210/me.2004-0174. [DOI] [PubMed] [Google Scholar]
- Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. Embo J. 2004;23(15):3061–71. doi: 10.1038/sj.emboj.7600309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108(6):779–84. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med (Maywood) 2006;231(1):20–7. doi: 10.1177/153537020623100103. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6(9):671–82. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8(1):1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Chattopadhyay S, Kato K, Dolkas J, Kikuchi S, Myers RR, Shubayev VI. MMPs initiate Schwann cell-mediated MBP degradation and mechanical nociception after nerve damage. Mol Cell Neurosci. 2008;39(4):619–27. doi: 10.1016/j.mcn.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fleur M, Underwood JL, Rappolee DA, Werb Z. Basement membrane and repair of injury to peripheral nerve: defining a potential role for macrophages, matrix metalloproteinases, and tissue inhibitor of metalloproteinases-1. J Exp Med. 1996;184(6):2311–26. doi: 10.1084/jem.184.6.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PH, DaSilva AG, Conant K, Yong VW. Myelin formation during development of the CNS is delayed in matrix metalloproteinase-9 and -12 null mice. J Neurosci. 2006;26(8):2207–14. doi: 10.1523/JNEUROSCI.1880-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert D, Hughes P, Huber S, Erne B, Grygar C, Said G, Miller KM, Steck AJ, Probst A, Fuhr P. Matrix metalloproteinase upregulation in chronic inflammatory demyelinating polyneuropathy and nonsystemic vasculitic neuropathy. Neurology. 1999;53(1):62–70. doi: 10.1212/wnl.53.1.62. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lloyd AC, Obermuller F, Staddon S, Barth CF, McMahon M, Land H. Cooperating oncogenes converge to regulate cyclin/cdk complexes. Genes Dev. 1997;11(5):663–77. doi: 10.1101/gad.11.5.663. [DOI] [PubMed] [Google Scholar]
- Mantuano E, Inoue G, Li X, Takahashi K, Gaultier A, Gonias SL, Campana WM. The hemopexin domain of matrix metalloproteinase-9 activates cell signaling and promotes migration of schwann cells by binding to low-density lipoprotein receptor-related protein. J Neurosci. 2008;28(45):11571–82. doi: 10.1523/JNEUROSCI.3053-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80(2):179–85. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Meier C, Parmantier E, Brennan A, Mirsky R, Jessen KR. Developing Schwann cells acquire the ability to survive without axons by establishing an autocrine circuit involving insulin-like growth factor, neurotrophin-3, and platelet-derived growth factor-BB. J Neurosci. 1999;19(10):3847–59. doi: 10.1523/JNEUROSCI.19-10-03847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir D, Manthorpe M. Stromelysin generates a fibronectin fragment that inhibits Schwann cell proliferation. J Cell Biol. 1992;116(1):177–85. doi: 10.1083/jcb.116.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murinson BB, Archer DR, Li Y, Griffin JW. Degeneration of myelinated efferent fibers prompts mitosis in Remak Schwann cells of uninjured C-fiber afferents. J Neurosci. 2005;25(5):1179–87. doi: 10.1523/JNEUROSCI.1372-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RJ, Jr, Carroll SL. ErbB transmembrane tyrosine kinase receptors are expressed by sensory and motor neurons projecting into sciatic nerve. J Histochem Cytochem. 2004;52(10):1299–311. doi: 10.1177/002215540405201006. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt CI, Krekoski CA, Ward RV, Edwards DR, Gavrilovic J. Extracellular matrix and matrix metalloproteinases in sciatic nerve. J Neurosci Res. 2003;74(3):417–29. doi: 10.1002/jnr.10783. [DOI] [PubMed] [Google Scholar]
- Rio C, Buxbaum JD, Peschon JJ, Corfas G. Tumor necrosis factor-alpha-converting enzyme is required for cleavage of erbB4/HER4. J Biol Chem. 2000;275(14):10379–87. doi: 10.1074/jbc.275.14.10379. [DOI] [PubMed] [Google Scholar]
- Roeb E, Schleinkofer K, Kernebeck T, Potsch S, Jansen B, Behrmann I, Matern S, Grotzinger J. The matrix metalloproteinase 9 (mmp-9) hemopexin domain is a novel gelatin binding domain and acts as an antagonist. J Biol Chem. 2002;277(52):50326–32. doi: 10.1074/jbc.M207446200. [DOI] [PubMed] [Google Scholar]
- Roelle S, Grosse R, Aigner A, Krell HW, Czubayko F, Gudermann T. Matrix metalloproteinases 2 and 9 mediate epidermal growth factor receptor transactivation by gonadotropin-releasing hormone. J Biol Chem. 2003;278(47):47307–18. doi: 10.1074/jbc.M304377200. [DOI] [PubMed] [Google Scholar]
- Roudabush FL, Pierce KL, Maudsley S, Khan KD, Luttrell LM. Transactivation of the EGF receptor mediates IGF-1-stimulated shc phosphorylation and ERK1/2 activation in COS-7 cells. J Biol Chem. 2000;275(29):22583–9. doi: 10.1074/jbc.M002915200. [DOI] [PubMed] [Google Scholar]
- Sanderson MP, Dempsey PJ, Dunbar AJ. Control of ErbB signaling through metalloprotease mediated ectodomain shedding of EGF-like factors. Growth Factors. 2006;24(2):121–36. doi: 10.1080/08977190600634373. [DOI] [PubMed] [Google Scholar]
- Sheu JY, Kulhanek DJ, Eckenstein FP. Differential patterns of ERK and STAT3 phosphorylation after sciatic nerve transection in the rat. Exp Neurol. 2000;166(2):392–402. doi: 10.1006/exnr.2000.7508. [DOI] [PubMed] [Google Scholar]
- Shubayev VI, Angert M, Dolkas J, Campana WM, Palenscar K, Myers RR. TNFalpha-induced MMP-9 promotes macrophage recruitment into injured peripheral nerve. Mol Cell Neurosci. 2006;31(3):407–15. doi: 10.1016/j.mcn.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubayev VI, Myers RR. Upregulation and interaction of TNFalpha and gelatinases A and B in painful peripheral nerve injury. Brain Res. 2000;855(1):83–9. doi: 10.1016/s0006-8993(99)02321-5. [DOI] [PubMed] [Google Scholar]
- Shubayev VI, Myers RR. Endoneurial remodeling by TNFalpha- and TNFalpha-releasing proteases. A spatial and temporal co-localization study in painful neuropathy. J Peripher Nerv Syst. 2002;7(1):28–36. doi: 10.1046/j.1529-8027.2002.02003.x. [DOI] [PubMed] [Google Scholar]
- Vecchi M, Carpenter G. Constitutive proteolysis of the ErbB-4 receptor tyrosine kinase by a unique, sequential mechanism. J Cell Biol. 1997;139(4):995–1003. doi: 10.1083/jcb.139.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91(4):439–42. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]