Summary
Objective
Imaging modalities available for the localization of pheochromocytoma (PHEO) include computed tomography (CT), magnetic resonance imaging (MRI), [123I]-or [131I]-labeled metaiodobenzylguanidine (123/131I-MIBG) scintigraphy and 6-[18F]-fluorodopamine (18F-FDA) positron emission tomography (PET). Our aim was to investigate the yield of 18F-FDA PET versus biochemical testing and other imaging techniques to establish the diagnosis and location of PHEO.
Patients and measurements
The study included 99 consecutive patients (35 M, 64 F, mean±SD age 46.4±13.4 years), who underwent 18F-FDA PET, biochemical testing (plasma catecholamines and free metanephrines) and CT and/or MRI. The majority (78%) also underwent 123/131I-MIBG.
Results
26 patients had non-metastatic PHEO, 34 patients had metastatic PHEO, and PHEO was ruled out in 39 patients. Investigations to rule out or confirm PHEO yielded the following sensitivity/specificity: plasma metanephrines 97/95%, 18F-FDA 92/90%, 123I-MIBG 83/100%, 123/131I-MIBG 70/100%, CT 100/41%, MRI 98/60%. Sensitivities for localizing non-metastatic PHEO on a per-lesion base were: CT 97%, MRI 92%, 18F-FDA 78%, 123I-MIBG 78% and 123/131I-MIBG 76%. Sensitivities for detecting metastases on a per-patient base were: CT and MRI 100%, 18F-FDA 97%, 123I-MIBG 85%, and 123/131I-MIBG 65%.
Conclusion
For tumor localization, 18F-FDA PET and 123/131I-MIBG scintigraphy perform equally well in patients with non-metastatic PHEO, but metastases are better detected by 18FFDA PET than by 123/131I-MIBG.
Keywords: paraganglioma, positron emission tomography, imaging, fluorodopamine, metanephrines
Introduction
Pheochromocytoma (PHEO) is a chromaffin cell tumor that produces catecholamines. Most PHEOs develop from the adrenal medulla, but they may also derive from sympathetic paraganglia in extra-adrenal abdominal or thoracic locations. 1 Although PHEO is a rare cause of hypertension, the diagnosis is considered frequently by clinicians in patients with refractory hypertension or symptoms or signs of catecholamine excess. Plasma levels of fractionated free metanephrines are considered the gold standard to confirm or rule out the presence of PHEO. 2 Biochemical evidence of PHEO is followed by efforts to localize the tumor. Widely available imaging modalities for localization of PHEO include computed tomography (CT), magnetic resonance imaging (MRI), and [123I] or [131I]-labeled metaiodobenzylguanidine (123/131I-MIBG) scintigraphy. CT and MRI offer high sensitivity but low specificity for unequivocally identifying PHEO. Distorted anatomy or surgical clips can also compromise results of anatomical imaging. 123/131I-MIBG is a highly specific agent that is transported into neurosecretory granules of catecholamine-producing cells via the vesicular monoamine transporters after uptake into chromaffin cells by the norepinephrine transporter. 3 However, the sensitivity of 123/131I-MIBG is somewhat limited, especially for metastatic PHEO. 4–6 PHEO cells are targeted through a similar mechanism by 6-[18F]-fluorodopamine (18F-FDA), a recently developed agent available for positron emission tomography (PET) 7 and also by 11C-hydroxyephedrine and 11C-epinephrine. 8;9 18F-FDA PET offers excellent sensitivity and specificity for the localization of both non-metastatic and metastatic PHEO. 4;7;10;11 Besides 123/131I-MIBG and 18F-FDA, agents that accumulate in PHEO cells through different mechanisms such as 6-[18F]-fluoro-L-3,4-dihydroxyphenylalanine (18F-DOPA) and -[18F]-fluoro-2-deoxy-D-glucose (18F-FDG) PET are increasingly recognized as alternatives to localize certain types of PHEO. 4;12–14
In the Europe and the United States and, patients with a high clinical suspicion of PHEO are typically referred to specialized physicians, usually endocrinologists, who practice in a secondary or tertiary health care setting. In such clinical settings, including the National Institutes of Health (NIH), the a priori chance of finding PHEO in referred patients is relatively high. Accurate and swift establishment of the diagnosis and localization of the tumor is critical for directing appropriate treatment. Considering the excellent sensitivity and specificity of 18F-FDA PET, we hypothesized, that performing 18F-FDA PET as first line investigation in such patients would provide a good alternative to biochemical testing to confirm or rule out the presence of PHEO, and to localize the tumor at the same time. We therefore performed a prospective study in a large group of patients with known or suspected PHEO and compared the yield of 18F-FDA PET with biochemical testing, 123/131I-MIBG scintigraphy, and anatomical imaging with respect to establishing the diagnosis and location of PHEO. For non-metastatic PHEO, surgical pathology of the removed tumor served as the “gold standard” diagnostic criterion. Reviews of anatomical and functional scanning results were performed by investigators who were blinded to any associated clinical findings.
Materials and methods
Subjects
The study included 99 patients (35 M, 64 F, mean±SD age 46.4±13.4 years) who were consecutively referred to the National Institutes of Health (NIH) for known or suspected PHEO, and underwent 18F-FDA PET scanning without CT co-localization between August 2001 and February 2005. Most patients were evaluated because of symptoms and signs suggestive of PHEO (n=60), including 10 with a previous history of PHEO and 5 with a (possible) hereditary form of PHEO. Ten asymptomatic patients underwent PHEO screening because of a (possible) hereditary disorder. Others were evaluated for the presence of an adrenal “incidentaloma” (n=8), or known or suspected metastatic PHEO (n=21). The study protocol was approved by the Institutional Review Board of the National Institutes of Child Health and Development at the NIH. All patients provided written informed consent. All patients underwent biochemical testing, anatomical imaging (CT and/or MRI), and 18F-FDA PET. In addition, most patients underwent 123I-MIBG or 131I-MIBG scintigraphy. Imaging was performed contemporaneously within three months of each other.
Biochemical testing
Plasma was assayed by HPLC for concentrations of free metanephrines and catecholamines, as described previously. 15;16 All patients were instructed to abstain from caffeine overnight, avoid taking acetaminophen for 5 days before blood sampling, or other medications that could interfere with biochemical tests. 17;18
Anatomical imaging
CT from the neck to the pelvis (‘whole body’) was performed with a Hi Speed Advantage Scanner (General Electric Medical Systems, Milwaukee, WI) after rapid infusion of nonionic water-soluble contrast agent as well as oral contrast material. Section thickness was 5–10 mm through the chest and abdomen. MRI was conducted using 1.5 Tesla equipment (General Electric Healthcare Technologies, Waukesha, WI) using phased-array coils for the neck, and either quadrature body or phased-array torso coils for imaging of chest, abdomen, and pelvis, with injection of gadolinium-DTPA unless contraindicated.
18F-FDA PET
PET was performed with a General Electric Advance scanner (General Electric, Milwaukee, WI). Patients were studied after having refrained from caffeine, tobacco, and alcohol for at least 12 hours prior to the scan. 18F-FDA was synthesized as previously described 19 given at specific activity of 7400 to 37000 MBq per millimol. Patients received an activity of 37 Mbq intravenously over 3 minutes. Immediately following infusion, 8–15 minute/level emission images were obtained in 2-or 3-dimensional mode from the pelvis to the neck (typically 5–7 levels). At each level, a transmission scan (3–5 minutes) was obtained for attenuation correction.
123/131I-MIBG scintigraphy
Scintigraphy was initially performed using 131I-labeled MIBG. When 123I-MIBG became available during the course of the study, 131I-MIBG was abandoned and replaced by 123I-MIBG scintigraphy. For whole-body 123I-MIBG and 131I-MIBG scintigraphy, patients were imaged 24 and occasionally 48 h following iv administration of 370 MBq 123I-MIBG or 48 h and occasionally 72 h following 684.5 MBq 131I-MIBG. To protect the thyroid from accumulation of free radioactive iodine, patients took 100 mg of saturated solution of potassium iodide by mouth twice a day for 4 or 8 days with 123I-and 131I-MIBG, respectively, starting the night before 123I-or 131I-MIBG administration. Both planar and single photon emission computed tomography (SPECT) images were obtained with all 123I-MIBG scans.
Data analysis
CT and MRI were read by one radiologist (AL) who was blinded to the biochemical and functional imaging findings. Tumor suspicious lesions were documented in the following locations: adrenal glands, lung, liver, spleen, kidneys, bone, subcutaneous fat and muscle. Region-specific morphological and size criteria for pathologically versus physiologically enlarged lymph nodes were applied as previously suggested. 20–22 If lesions were identified as probably benign, such as renal cysts, liver hemangiomas, colon diverticula and gallstones, they were discarded from analysis.
18F-FDA PET and 123/131I-MIBG images were independently reviewed by two nuclear medicine investigators (JAC, CCC) who were blinded to the biochemical and anatomical imaging findings. Areas of non-physiologic uptake were graded using a scale of 1 to 5, where 1 was considered negative for PHEO, 2 was probably negative, 3 equivocal, 4 probably positive and 5 definitely positive. Lesions rated 4 and 5 were considered positive. Any degree of uptake in the region of the adrenal glands on FDA PET scans was considered positive. All differences between the two readers were resolved by consensus review. Since the imaging properties of 123I-MIBG are clearly superior to 131I-MIBG, 23 results of 123I-MIBG and pooled 123/131I-MIBG were analyzed.
Biochemical and imaging results were compared between patients with non-metastatic and metastatic PHEO, and those ultimately shown not to have PHEO. The diagnosis of PHEO required histopathological confirmation. After successful surgical resection of non-metastatic PHEO, patients were discharged from follow-up at the NIH and referred back to their local physician. Metastatic PHEO was defined as histological or radiological evidence of lesions at sites where chromaffin tissue is normally absent. 24 In patients lacking PHEO, the diagnosis was ruled out by a combination of (additional) biochemical investigation, imaging, and clinical follow-up.
To determine the usefulness of plasma free metanephrines versus anatomical and functional imaging as first-line diagnostic tools to confirm or rule out the presence of PHEO, we calculated sensitivity, specificity, and positive and negative predictive values for each modality. In patients with non-metastatic PHEO, the accuracy of different imaging techniques for tumor localization was assessed in reference to lesions that were histopathologically identified as PHEO. Radiologically suspect lesions on anatomical imaging that were also visualized by 123/131I-MIBG scintigraphy and/or 18F-FDA PET were selected for surgical resection. If surgical resection of the culprit lesion(s) resulted in normalization of plasma free metanephrines without signs of recurrence during follow-up, additional lesions on pre-operative imaging were presumed to be false-positive.
In patients with metastastic PHEO, comprehensive histopathological confirmation of all lesions to serve as gold standard for imaging results was not feasible. Therefore, the sensitivity of different imaging modalities in patients with metastatic PHEO was calculated on a per-patient basis only, i.e. a technique was scored positive if any lesion(s) were detected in a patient.
Results are given as mean±SD unless stated otherwise. The McNemar test was used to compare sensitivity and specificity between different imaging modalities. A two-sided P<0.05 was considered significant. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS for Windows 12; SPSS Inc., Chicago, Illinois, USA).
Results
Diagnosis
Twenty-six patients had non-metastatic PHEO (11 M, 15 F, age 49.1±13.1 y). PHEO locations were left adrenal (N=7), right adrenal (N=12), bilateral adrenal (N=7), and extraadrenal retroperitoneal (N=3). Six of these patients had an underlying mutation of the RET proto-oncogene, one of the succinate dehydrogenase subunit D (SDHD) gene, one of the von Hippel-Lindau (VHL) gene, and one of the neurofibromatosis type 1 (NF1) gene. Fourteen patients had sporadic PHEO, and in 3, genetic testing results are pending. Thirty-four patients had metastatic PHEO (17 M, 17 F, age 43.7±14.4 y) with the following genetic testing results: succinate dehydrogenase subunit B (SDHB) (N=13), RET (N=2), VHL (N=1), sporadic (N=17), results pending (N=1). PHEO was ruled out in 39 patients (6 M, 33 F, age 46.9±12.7 y), including one with a VHL mutation and 3 with a RET mutation (1 proven, 2 clinically inferred).
Confirming or ruling out PHEO
Plasma metanephrines were determined in all patients. FDA was performed in all patients, and CT, MRI, and either 123/131I-MIBG was performed in most. The numbers of scans performed in the different patient categories are given in Table 1. Plasma metanephrines were elevated in all 26 patients with non-metastatic PHEO, and 32 of 34 with metastatic PHEO. The two patients with normal plasma metanephrines had extensive metastatic disease originating from extra-adrenal tumors and were carriers of an SDHB mutation: one of them had an elevated plasma dopamine level of 600 pg/mL (URL 36 pg/mL), whereas in the other (m33), plasma catecholamines and their 0-methylated metabolites were consistently normal.
Table 1.
Number of scans performed
| Nr. of patients | CT | MRI | CT and/or MRI | [18F]-FDA | [123I]-MIBG | [131I]-MIBG | [123I] or [131I]-MIBG | |
|---|---|---|---|---|---|---|---|---|
| Non-metastatic | ||||||||
| PHEO | 26 | 26 (100%) | 20 (77%) | 26 (100%) | 26 (100%) | 21 (81%) | 2 (8%) | 23 (88%) |
| Metastatic PHEO | 34 | 32 (94%) | 31 (91%) | 34 (100%) | 34 (100%) | 21 (62%) | 10 (29%) | 31 (91%) |
| PHEO total | 60 | 58 (97%) | 51 (85%) | 60 (100%) | 60 (100%) | 42 (70%) | 12 (20%) | 54 (90%) |
| No PHEO | 39 | 29 (74%) | 25 (64%) | 31 (79%) | 39 (100%) | 21 (54%) | 2 (5%) | 23 (59%) |
| Total | 99 | 87 (88%) | 76 (77%) | 91 (92%) | 99 (100%) | 63 (64%) | 14 (14%) | 77 (78%) |
Overall, plasma metanephrines provided a sensitivity of 97% (58/60) and a specificity of 95% (37/39) (Table 2). Plasma metanephrines were compared with imaging studies as first-line investigations to rule out or confirm PHEO (Table 2). Imaging yielded the following per-patient sensitivities and specificities, respectively: 18F-FDA 92% (55/60) and 90% (35/39), 123I-MIBG 83% (34/41) and 100% (22/22), 123I-or 131I-MIBG 70% (38/54) and 100% (24/24), CT 100% (58/58) and 41% (12/29), MRI 98% (50/51) and 60% (15/25). The three false-positive foci on 18F-FDA PET were located in the adrenal regions (2 right, 1 left) and probably represented physiological uptake. 123/131I-MIBG scintigraphy did not show any false-positive lesions.
Table 2.
Plasma metanephrines versus imaging as first-line investigations to confirm or rule out PHEO
| plasma NMN/MN | [18F]-FDA | [123I]-MIBG | [123I] or [131I]-MIBG | whole body CT | whole body MRI | |
|---|---|---|---|---|---|---|
| Sensitivity | 97% (58/60) | 92% (55/60) | 83% (34/41) | 83% (34/41) | 100% (58/58) | 98% (50/51) |
| Specificity | 95% (37/39) | 90% (35/39) | 100% (22/22) | 100% (24/24) | 41% (12/29) | 60% (15/25) |
| Pos. predictive value | 97% (58/60) | 93% (55/59) | 100% (34/34) | 100% (38/38) | 77% (58/75) | 83% (50/60) |
| Neg. predictive value | 95% (37/39) | 88% (35/40) | 76% (22/29) | 60% (24/40) | 100% (12/12) | 94% (15/16) |
Localizing non-metastatic PHEO
The sensitivity and specificity of imaging techniques in non-metastatic PHEO are given in Table 3. In 26 patients, a total of 45 foci were detected by anatomical and functional imaging, 32 of which were histologically confirmed PHEOs and 13 were false positives. CT and MRI provided high sensitivity of 97% (31/32) and 92% (24/26), respectively. Sensitivity was similar between 18F-FDA (78%, 25/32), 123I-MIBG (78%, 21/27) and 123I-or 131I-MIBG (76%, 22/29). One right adrenal PHEO was missed by CT and two left adrenal PHEOs by MRI. 18F-FDA failed to detect 7 PHEOs in 6 patients: 1 right adrenal and 2 left adrenal PHEOs with mild 18F-FDA uptake, 2 right adrenal PHEOs without any 18F-FDA uptake, and 2 right adrenal PHEOs that were masked by tracer accumulation in the biliary system and the rim of a renal cyst, respectively. 18F-FDA-negative PHEOs (range 1.5–5 cm) did not differ in size from 18F-FDA-positive PHEOs. Six of the seven PHEOs (three right and three left adrenal) that were missed by 18F-FDA were also missed by 123I-MIBG (4 without and 1 with mild 123I-MIBG uptake) and 131I-MIBG (1 without 123I-MIBG uptake). One additional left adrenal PHEO was missed by 123IMIBG but positive on 18F-FDA.
Table 3.
Localization of PHEO
| [18F]-FDA | [123I]-MIBG | [123I] or [131I]-MIBG | CT | MRI | |
|---|---|---|---|---|---|
| Non-metastatic PHEO, per lesion analysis | |||||
| Sensitivity | 78% (25/32) | 78% (21/27) | 76% (22/29) | 97% (31/32) | 92% (24/26) |
| Specificity | 77% (10/13) | 92% (12/13) | 92% (12/13) | 38% (5/13) * | 58% (7/12) |
| Metastatic PHEO, per patient analysis | |||||
| Sensitivity | 97% (33/34) | 85% (17/20) | 65% (20/31) # | 100% (32/32) | 100% (31/31) |
P=0.039 versus [123I], and [123]/[131] MIBG
P=0.002 versus [18F]-FDA, whole body CT, and whole body MRI
The specificity of CT and MRI was relatively poor (Table 3). CT showed 8 false positive foci in the following locations: periportal (n=1), liver (n=1), cervical (n=1), left adrenal (n=2), retroperitoneal (n=2), cervical (n=1) and bone (n=1). MRI showed five false positive foci: retroperitoneal (n=1), liver (n=1), cervical (n=2), mediastinal (n=1). Two out of 3 false positive foci on 18F-FDA PET were located in the left adrenal bed and probably represented physiological uptake, whereas one appeared to be located in the uterus. 123I-MIBG showed one false positive left adrenal focus, again possibly physiologic.
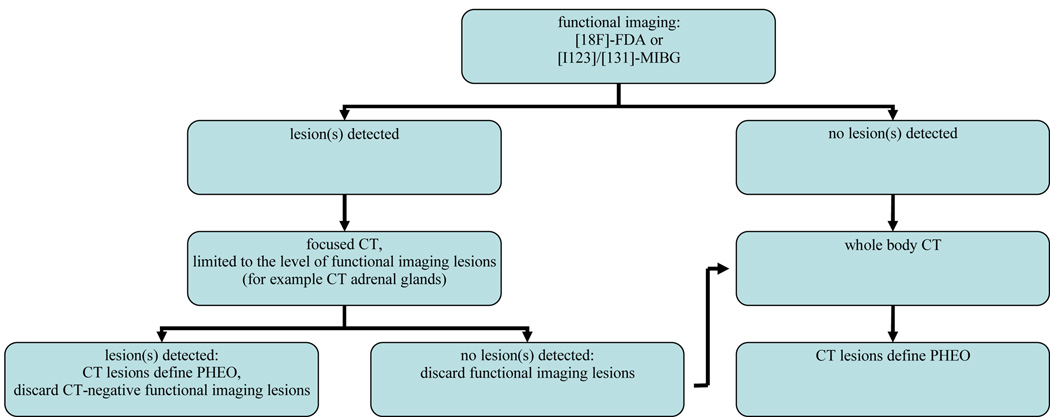
Because of the large number of false-positive findings on CT and MRI, an additional analysis was performed in patients with non-metastatic PHEO. The aim of this secondary analysis was to simulate the diagnostic strategy depicted in Figure 1. According to this strategy, patients with a biochemically established diagnosis of PHEO would first undergo functional imaging, followed by focused CT scanning limited to the level(s) of lesions on functional imaging. Whole body CT scanning would be restricted to patients with negative findings on functional imaging or focused CT. In patients with positive functional imaging results, CT lesions were included in the analysis only if they were located at a similar level as the lesion(s) on functional imaging. Results were extrapolated to 100 patients. This analysis suggests that such a strategy could reduce the field of CT scanning (and thereby radiation exposure and costs) in most patients, yielding a very high sensitivity and specificity, especially when using 18F-FDA PET (Table 4).
Figure 1.
Imaging flow-chart for patients with a biochemically established diagnosis of PHEO
Table 4.
Localization of non-metastatic PHEO
| Results of imaging as performed | Projected results for strategy depicted in Figure 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| whole body CT | [18F]-FDA | [123I]-MIBG | [123I] or [131I] MIBG |
[18F]-FDA and focused CT |
[123I]-MIBG and focused CT |
[123I] or [131I] MIBG and focused CT |
||
| Nr. of patients available for analysis | 26 | 23 | 22 | 24 | 26 | 21 | 23 | |
| % of patients with: | ||||||||
| true positive lesions only | 65% | 70% | 82% | 79% | 88% | 81% | 83% | |
| true positive and false positive lesions | 31% | 4% | 0% | 0% | 8% | 14% | 13% | |
| true positive and false negative lesions | 4% | 9% | 0% | 0% | 4% | 5% | 4% | |
| false positive lesions only | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| false positive and false negative lesions | 0% | 4% | 5% | 4% | 0% | 0% | 0% | |
| no lesions (false negative scan) | 0% | 13% | 14% | 17% | 0% | 0% | 0% | |
| Projected nr. of scans needed for 100 patients: | ||||||||
| whole body CT | 100 | - | - | - | 15 | 19 | 22 | |
| focused CT | - | - | - | - | 88 | 81 | 78 | |
| [18F]-FDA | - | 100 | - | - | 100 | - | - | |
| [123I]-MIBG | - | - | 100 | - | - | 100 | - | |
| [123I] or [131I]-MIBG | - | - | - | 100 | - | - | 100 | |
Localizing metastatic PHEO
The sensitivity for the detection of metastatic PHEO was 100% for CT and MRI, 97% (33/34) for 18F-FDA, 85% (17/20) for 123I-MIBG, and 65% (20/31) for 123I-or 131I-MIBG (P=0.002 versus 18F-FDA, CT and MRI, Table 2).
Discussion
The usefulness of 18F-FDA PET versus biochemical testing, 123/131I-MIBG scintigraphy, and anatomical imaging to establish the diagnosis of PHEO and to localize the tumor was prospectively studied in a large group of patients with non-metastatic or metastatic PHEO, and in patients in whom PHEO was suspected but ruled out. Both plasma metanephrines and 18F-FDA PET provided high sensitivity and specificity (>90%) to confirm or rule out the diagnosis of PHEO. For tumor localization in patients with non-metastatic PHEO, 18F-FDA PET and 123/131IMIBG scintigraphy had a similar sensitivity of 78%. For the localization of metastases, however, the sensitivity of 18F-FDA PET was clearly superior to 123/131I-MIBG scintigraphy, i.e. 97% versus 65%, respectively.
123/131I-MIBG is the most widely used tracer in the first-line functional imaging of PHEO. 123I-MIBG is preferred over 131I-MIBG because of its higher sensitivity, lower radiation exposure, and improved imaging quality with SPECT. 23 123I-MIBG scintigraphy was previously shown to have a high sensitivity of 92–98% for non-metastatic PHEO, 5 whereas a lower sensitivity of 57%–79% was reported for metastatic lesions. 4;5;25 In the present study, the superiority of 123I-over 131I-MIBG scintigraphy was confirmed, as was its relatively poor sensitivity for metastases. In search of a better functional imaging agent for the sympathetic nervous system and PHEO, 18F-FDA was developed at the NIH. In our initial studies on 18F-FDA PET in patients with PHEO, all tumors were visualized in nine patients with non-metastatic PHEO, and the presence of metastases was confirmed in all eight patients with malignant disease. 7 In 16 patients with metastatic PHEO, 18F-FDA PET showed a large number of foci that were negative on 131I-MIBG 10. The current study confirms these initial findings of high sensitivity of 18F-FDA PET for the localization of both primary PHEO and metastatic lesions, and it’s superiority over 123/131I-MIBG scintigraphy in detecting metastases.
Aside from its valuable role in tumor localization in patients with biochemically established PHEO, we show that 18F-FDA PET can also serve as a first-line study to confirm or rule out the diagnosis in patients with a strong clinical suspicion of PHEO in the context of a specialized referral center. Obviously, replacement of biochemical testing by functional imaging is not feasible or cost-effective as a screening method for all patients with possible secondary hypertension or symptoms of catecholamine excess, particularly in a primary health care setting. However, in selected patients with a high a priori chance of PHEO as in the present study, such an approach offers immediate establishment of the diagnosis and localization of PHEO. A delay in tumor localization and treatment until after laboratory results become available could be avoided by performing 18F-FDA PET as initial investigation. It also allows focused anatomic imaging, with associated cost saving and decreased radiation dose to patients.
18F-FDA PET currently entails several practical limitations. Relatively few centers have the capacity to synthesize 18F -labeled radiopharmaceuticals, although this is likely to expand now that Medicare covers diagnostic 18F-fluorodeoxyglucose PET. In calculating the relative costs of different approaches for diagnostic localization of PHEO, a center should weigh the size of the patient load and length and labor-intensiveness of the radiopharmaceutical synthesis, versus the shorter scanning duration (60 minutes in a single visit) and resulting higher throughput. Advantages of 18F-FDA PET are high resolution and the ability to acquire quantitative information, which might prove valuable in follow-up studies or assessing response to therapy. Because of the short physical half-life of 18F, absorbed radiation doses are smaller than those for typical 123I-MIBG scanning—37 MBq of 18F-FDA corresponds to an effective dose of 0.10 mSv versus 0.71 mSv for 370 MBq 123I-MIBG. The use of combined PET/CT scanners to further improve tumor localization with 18F-FDA, and facilitates the distinction between PHEO and physiological tracer accumulation in normal adrenal tissue, a source of false-positive results in this study. 26
The mainstay in PHEO treatment is surgical resection. Pre-surgical imaging serves three purposes: 1. To specifically diagnose PHEO 2. To detect or rule out metastases, and 3. To determine the tumor’s anatomical relationship to surrounding structures. The major drawback of anatomical imaging in this context is lack of specificity. CT and MRI commonly detect coincidental adrenal non-chromaffin tumors, as well as many false-positive lesions outside the adrenal beds. On the other hand, functional imaging offers specificity but not the detailed anatomical information required by the surgeon. Our analysis suggests that the favorable characteristics of anatomical and functional imaging are best combined by performing whole body functional imaging first, followed by focused anatomical imaging, restricted to the body region(s) that are positive on 18F-FDA PET or 123I-MIBG scintigraphy.
18F-FDA is only one of a number of functional PET imaging agents used in PHEO. In this study, we did not investigate 18F-DOPA, 18F-FDG, 11C-hydroxyephedrine or 11C-epinephrine, which also yield promising results. 4;8;12–14;27 Previously, we have shown that for the localization of metastases in patients with SDHB mutation, 18F-FDG PET is clearly superior to 123I-MIBG scintigraphy and 18F-FDA PET, 4 an observation that may be explained by loss of the cell membrane norepinephrine transporter or the vesicular monoamine transporter, or alternatively by altered glucose metabolism due to SDHB–related mitochondrial dysfunction.
In conclusion, for tumor localization, 18F-FDA PET and 123/131I-MIBG scintigraphy perform equally well in patients with non-metastatic PHEO, but metastases are better detected by 18F-FDA PET than by 123/131I-MIBG. After establishing a biochemical diagnosis of PHEO, 18F-FDA PET is an excellent tool to confirm the diagnosis and localize the tumor(s) at the same time. In patients with a high a priori chance of PHEO. Considering the limited availability of 18F-FDA PET, however, 123I-MIBG scintigraphy is still the best available modality in most centers.
Acknowledgements
This research was supported by the Intramural Research Program of the NICHD/NIH.
Reference List
- 1.DeLellis RA, Lloyd RV, Heitz PU, Eng C. Pathology and Genetics: World Health Organization classisfication of tumours of endocrine organs. Oxford, UK: Oxford University Press; 2004. [Google Scholar]
- 2.Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, et al. Biochemical diagnosis of pheochromocytoma: which test is best? Journal of the American Medical Association. 2002;287(11):1427–1434. doi: 10.1001/jama.287.11.1427. [DOI] [PubMed] [Google Scholar]
- 3.Eisenhofer G. The role of neuronal and extraneuronal plasma membrane transporters in the inactivation of peripheral catecholamines. Pharmacological Therapy. 2001;91(1):35–62. doi: 10.1016/s0163-7258(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 4.Timmers HJ, Kozupa A, Chen CC, Carrasquillo JA, Ling A, Eisenhofer G, et al. Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. Journal of Clinical Oncology. 2007;25(16):2262–2269. doi: 10.1200/JCO.2006.09.6297. [DOI] [PubMed] [Google Scholar]
- 5.Van Der Horst-Schrivers AN, Jager PL, Boezen HM, Schouten JP, Kema IP, Links TP. Iodine-123 metaiodobenzylguanidine scintigraphy in localising phaeochromocytomas--experience and meta-analysis. Anticancer Research. 2006;26(2B):1599–1604. [PubMed] [Google Scholar]
- 6.van der Harst E, De Herder WW, Bruining HA, Bonjer HJ, de Krijger RR, Lamberts SW, et al. [(123)I]metaiodobenzylguanidine and [(111)In]octreotide uptake in benign and malignant pheochromocytomas. Journal of Clinical Endocrinology and Metabolism. 2001;86(2):685–693. doi: 10.1210/jcem.86.2.7238. [DOI] [PubMed] [Google Scholar]
- 7.Pacak K, Eisenhofer G, Carrasquillo JA, Chen CC, Li ST, Goldstein DS. 6-[18F]fluorodopamine positron emission tomographic (PET) scanning for diagnostic localization of pheochromocytoma. Hypertension. 2001;38(1):6–8. doi: 10.1161/01.hyp.38.1.6. [DOI] [PubMed] [Google Scholar]
- 8.Ilias I, Shulkin B, Pacak K. New functional imaging modalities for chromaffin tumors, neuroblastomas and ganglioneuromas. Trends in Endocrinology and Metabolism. 2005;16(2):66–72. doi: 10.1016/j.tem.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Shulkin BL, Wieland DM, Schwaiger M, Thompson NW, Francis IR, Haka MS, et al. PET scanning with hydroxyephedrine: an approach to the localization of pheochromocytoma. Journal of Nuclear medicine. 1992;33(6):1125–1131. [PubMed] [Google Scholar]
- 10.Ilias I, Yu J, Carrasquillo JA, Chen CC, Eisenhofer G, Whatley M, et al. Superiority of 6-[18F]-fluorodopamine positron emission tomography versus [131I]-metaiodobenzylguanidine scintigraphy in the localization of metastatic pheochromocytoma. Journal of Clinical Endocrinology and Metabolism. 2003;88(9):4083–4087. doi: 10.1210/jc.2003-030235. [DOI] [PubMed] [Google Scholar]
- 11.Mamede M, Carrasquillo JA, Chen CC, Del CP, Whatley M, Ilias I, et al. Discordant localization of 2-[18F]-fluoro-2-deoxy-D-glucose in 6-[18F]-fluorodopamineand [(123)I]-metaiodobenzylguanidine-negative metastatic pheochromocytoma sites. Nuclear Medicine Communications. 2006;27(1):31–36. doi: 10.1097/01.mnm.0000189780.54658.e8. [DOI] [PubMed] [Google Scholar]
- 12.Hoegerle S, Nitzsche E, Altehoefer C, Ghanem N, Manz T, Brink I, et al. Pheochromocytomas: detection with 18F DOPA whole body PET--initial results. Radiology. 2002;222(2):507–512. doi: 10.1148/radiol.2222010622. [DOI] [PubMed] [Google Scholar]
- 13.Brink I, Schaefer O, Walz M, Neumann HP. Fluorine-18 DOPA PET imaging of paraganglioma syndrome. Clinical Nucearl Medicine. 2006;31(1):39–41. doi: 10.1097/01.rlu.0000191577.39458.a0. [DOI] [PubMed] [Google Scholar]
- 14.Timmers HJ, Hadi M, Carrasquillo JA, Chen CC, Martiniova L, Whatley M, et al. The effects of carbidopa on uptake of 6-18F-Fluoro-L-DOPA in PET of pheochromocytoma and extraadrenal abdominal paraganglioma. Journal of Nuclear medicine. 2007;48(10):1599–1606. doi: 10.2967/jnumed.107.042721. [DOI] [PubMed] [Google Scholar]
- 15.Lenders JW, Eisenhofer G, Armando I, Keiser HR, Goldstein DS, Kopin IJ. Determination of metanephrines in plasma by liquid chromatography with electrochemical detection. Clinical Chemistry. 1993;39(1):97–103. [PubMed] [Google Scholar]
- 16.Eisenhofer G, Goldstein DS, Stull R, Keiser HR, Sunderland T, Murphy DL, et al. Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clinical Chemistry. 1986;32(11):2030–2033. [PubMed] [Google Scholar]
- 17.Eisenhofer G, Goldstein DS, Walther MM, Friberg P, Lenders JW, Keiser HR, et al. Biochemical diagnosis of pheochromocytoma: how to distinguish true-from false-positive test results. Journal of Clinical Endocrinology and Metabolism. 2003;88(6):2656–2666. doi: 10.1210/jc.2002-030005. [DOI] [PubMed] [Google Scholar]
- 18.Pacak K. Preoperative management of the pheochromocytoma patient. Journal of Clinical Endocrinology and Metabolism. 2007;92(11):4069–4079. doi: 10.1210/jc.2007-1720. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein DS, Chang PC, Smith CB, Herscovitch P, Austin SM, Eisenhofer G, et al. Dosimetric estimates for clinical positron emission tomographic scanning after injection of [18F]-6-fluorodopamine. Journal of Nuclear medicine. 1991;32(1):102–110. [PubMed] [Google Scholar]
- 20.Dorfman RE, Alpern MB, Gross BH, Sandler MA. Upper abdominal lymph nodes: criteria for normal size determined with CT. Radiology. 1991;180(2):319–322. doi: 10.1148/radiology.180.2.2068292. [DOI] [PubMed] [Google Scholar]
- 21.Glazer GM, Gross BH, Quint LE, Francis IR, Bookstein FL, Orringer MB. Normal mediastinal lymph nodes: number and size according to American Thoracic Society mapping. American Journal of Roentgenology. 1985;144(2):261–265. doi: 10.2214/ajr.144.2.261. [DOI] [PubMed] [Google Scholar]
- 22.Mancuso AA, Harnsberger HR, Muraki AS, Stevens MH. Computed tomography of cervical and retropharyngeal lymph nodes: normal anatomy, variants of normal, and applications in staging head and neck cancer. Part I: normal anatomy. Radiology. 1983;148(3):709–714. doi: 10.1148/radiology.148.3.6878691. [DOI] [PubMed] [Google Scholar]
- 23.Lynn MD, Shapiro B, Sisson JC, Beierwaltes WH, Meyers LJ, Ackerman R, et al. Pheochromocytoma and the normal adrenal medulla: improved visualization with I-123 MIBG scintigraphy. Radiology. 1985;155(3):789–792. doi: 10.1148/radiology.155.3.4001380. [DOI] [PubMed] [Google Scholar]
- 24.Linnoila RI, Keiser HR, Steinberg SM, Lack EE. Histopathology of benign versus malignant sympathoadrenal paragangliomas: clinicopathologic study of 120 cases including unusual histologic features. Human Pathology. 1990;21(11):1168–1180. doi: 10.1016/0046-8177(90)90155-x. [DOI] [PubMed] [Google Scholar]
- 25.van der HE, De Herder WW, Bruining HA, Bonjer HJ, de Krijger RR, Lamberts SW, et al. [(123)I]metaiodo benzylguanidine and [(111)In]octreotide uptake in begnign and malignant pheochromocytomas. Journal of Clinical Endocrinology and Metabolism. 2001;86(2):685–693. doi: 10.1210/jcem.86.2.7238. [DOI] [PubMed] [Google Scholar]
- 26.Timmers HJ, Carrasquillo JA, Whatley M, Eisenhofer G, Chen CC, Ling A, et al. Usefulness of standardized uptake values for distinguishing adrenal glands with pheochromocytoma from normal adrenal glands by use of 6-18F-fluorodopamine PET. Journal of Nuclear medicine. 2007;48(12):1940–1944. doi: 10.2967/jnumed.107.043281. [DOI] [PubMed] [Google Scholar]
- 27.Shulkin BL, Wieland DM, Schwaiger M, Thompson NW, Francis IR, Haka MS, et al. PET scanning with hydroxyephedrine: an approach to the localization of pheochromocytoma. Journal of Nuclear medicine. 1992;33(6):1125–1131. [PubMed] [Google Scholar]